Abstract
Objective
To determine whether patients with Lewy body dementia (LBD) with likely Alzheimer disease (AD)–type copathology are more impaired on confrontation naming than those without likely AD-type copathology.
Methods
We selected 57 patients with LBD (dementia with Lewy bodies [DLB], n = 38; Parkinson disease dementia [PDD], n = 19) with available AD CSF biomarkers and neuropsychological data. CSF β-amyloid1-42 (Aβ42), phosphorylated-tau (p-tau), and total-tau (t-tau) concentrations were measured. We used an autopsy-validated CSF cut point (t-tau:Aβ42 ratio > 0.3, n = 43), or autopsy data when available (n = 14), to categorize patients as having LBD with (LBD + AD, n = 26) and without (LBD − AD, n = 31) likely AD-type copathology. Analysis of covariance tested between-group comparisons across biologically defined groups (LBD + AD, LBD − AD) and clinical phenotypes (DLB, PDD) on confrontation naming (30-item Boston Naming Test [BNT]), executive abilities (letter fluency [LF], reverse digit span [RDS]), and global cognition (Mini-Mental State Examination [MMSE]), with adjustment for age at dementia onset, time from dementia onset to test date, and time from CSF to test date. Spearman correlation related cognitive performance to CSF analytes.
Results
Patients with LBD + AD performed worse on BNT than patients with LBD − AD (F = 4.80, p = 0.03); both groups performed similarly on LF, RDS, and MMSE (all p > 0.1). Clinically defined PDD and DLB groups did not differ in performance on any of these measures (all p > 0.05). A correlation across all patients showed that BNT score was negatively associated with CSF t-tau (ρ = −0.28, p < 0.05) and p-tau (ρ = −0.26, p = 0.05) but not Aβ42 (p > 0.1).
Conclusion
Markers of AD-type copathology are implicated in impaired language performance in LBD. Biologically based classification of LBD may be advantageous over clinically defined syndromes to elucidate clinical heterogeneity.
Parkinson disease (PD) dementia (PDD) and dementia with Lewy bodies (DLB) are Lewy body dementias (LBDs) characterized by progressive cognitive and motor deficits.1,2 Historically, LBD has been partitioned into PDD or DLB on the basis of relative onset of cognitive and motor features,3 but this approach is not well supported in large autopsy studies.4 Other work has attempted to distinguish clinically defined PDD and DLB by neuropsychological profiles.5,6 However, findings are inconsistent, with some emphasizing memory and language difficulties in DLB,7,8 while others show these deficits in PDD.9,10
One important source of heterogeneity in LBD may be differences in underlying neuropathology.4,11,12 LBD is defined by the aggregation of pathologic α-synuclein protein; however, ≈50% of LBD autopsy cases have additional plaque and tangle pathology consistent with a secondary diagnosis of Alzheimer disease (AD),12,13 which has been linked to worse prognosis.4,14–16 Few studies have examined directly the specific cognitive impairments associated with AD-type copathology in LBD, but recent postmortem work has found evidence of greater temporal lobe tau burden17 associated with antemortem confrontation naming deficits in patients with LBD with AD-type copathology.17–19
This study compares neuropsychological profiles between patients with LBD with (LBD + AD) and without (LBD − AD) likely AD-type copathology, using CSF biomarkers or autopsy data to biologically define groups. We hypothesize that patients with LBD + AD will be more impaired on temporal lobe–mediated confrontation naming than patients with LBD − AD and that this deficit will be linked to markers of tau pathology. We do not expect biologically defined groups to differ on frontal lobe–mediated executive functioning or clinically defined groups (PDD/DLB) to have differentiated neuropsychological profiles.
Methods
Patients
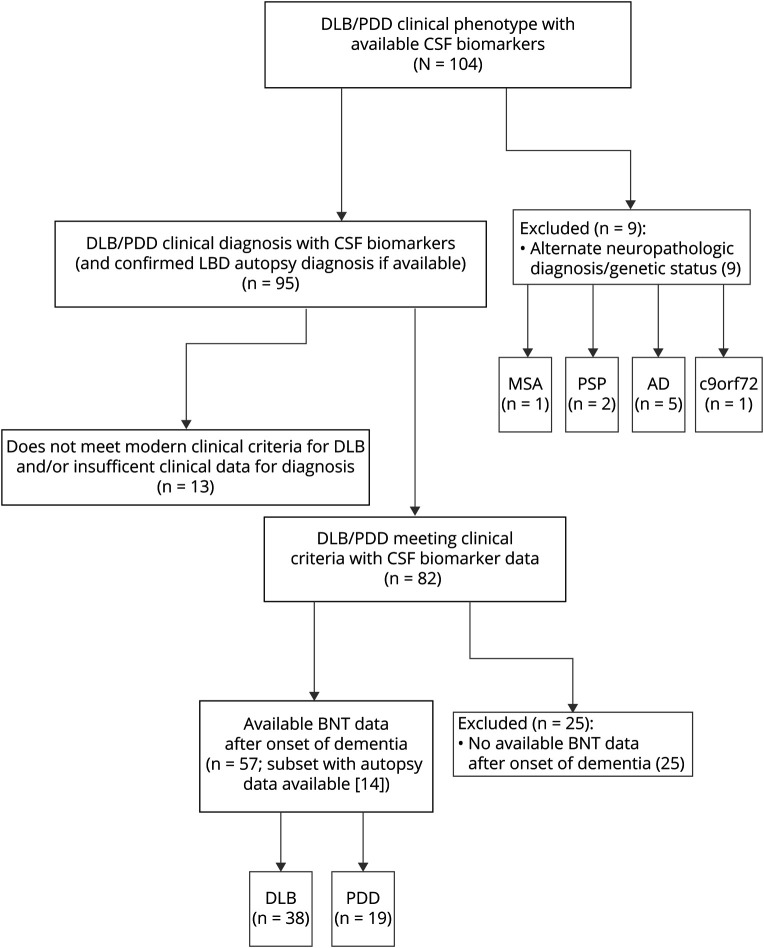
Figure 1 outlines the inclusion criteria for 57 patients with LBD retrospectively selected from the University of Pennsylvania Integrated Neurodegenerative Disease Database20 as of October 7, 2019. Patients were followed up and evaluated at the University of Pennsylvania's Frontotemporal Degeneration Center, Parkinson's Disease and Movement Disorders Clinic, or Alzheimer's Disease Core Center. To investigate the effects of AD-type copathology in LBD, with a specific emphasis on the temporal lobe–mediated task of confrontation naming, patients were included according to clinical diagnosis of cognitively impaired LBD (DLB or PDD)1,2 and availability of CSF biomarkers or autopsy diagnosis and Boston Naming Test (BNT) data collected after the onset of dementia. We performed a chart review of medical records of patients with DLB to ensure that they met current clinical criteria, using the 1-year interval between parkinsonism motor symptoms and dementia onset to differentiate PDD from DLB,2 and to confirm the year of dementia onset on the basis of the year of earliest noted cognitive symptom with a functional deficit in the record. Clinical diagnoses of PDD and DLB were rendered in a manner blinded to CSF data. Of our final cohort of patients, 14 (24.6%) had autopsy data and confirmed LBD neuropathologic diagnosis. Exclusion criteria included PD without cognitive impairment and autopsy or genetic evidence21 of nonsynucleinopathy neuropathologic diagnoses. Thirty-eight patients with a clinical diagnosis of DLB and 19 patients with a clinical diagnosis of PDD fulfilled these criteria (figure 1). Because these data are retrospective, a subset of patients with BNT scores (n = 4) were included in a previous autopsy study of LBD.17
Figure 1. Flowchart of Inclusion/Exclusion Criteria Used to Select Our Cohort.
AD = Alzheimer disease; BNT = Boston Naming Test; c9orf72 = chromosome 9 open reading frame 72 mutation; DLB = dementia with Lewy bodies; LBD = Lewy body disease; MSA = multiple system atrophy; PDD = Parkinson disease dementia; PSP = progressive supranuclear palsy.
Biological Classification
CSF was collected for AD biomarkers, including total tau (t-tau), phosphorylated tau (p-tau), and β-amyloid1-42 (Aβ42), and measured with the Luminex xMAP immunoassay platform (Luminex Corp, Austin, TX) as described.22 To establish biologically defined groups, we used an autopsy-validated CSF cut point as a biomarker of concomitant AD-type pathology in LBD (t-tau:Aβ42 ratio >0.3)23 to categorize patients as having LBD + AD (n = 19) or LBD − AD (n = 24). For the small subset of patients with autopsy data available (n = 14), we used neuropathologic criteria24 to define patients with LBD with concomitant AD. Patients with LBD with intermediate or high AD neuropathologic change were considered to have LBD + AD (n = 7), and patients with LBD with no or low AD neuropathologic change were considered to be LBD − AD (n = 7). We note that 3 of the 31 total patients with LBD − AD (9.68%) had a CSF collection date before the onset of dementia (maximum interval 1.4 years); these patients were included in our main analysis because accumulation of AD-type pathology is thought to precede cognitive impairment,25 and longitudinal AD CSF biomarker data in LBD show minimal change over this time interval of <2 years.26 Even so, because we cannot be certain that the biological status of these 3 patients with CSF collection before dementia onset fully reflected their underlying disease state at the time of testing, we also repeated all analyses excluding these 3 cases.
Neuropsychological Testing
To test the association between AD-type copathology in LBD and confrontation naming early in the symptomatic course of dementia, we retrospectively selected available neuropsychological data collected at the earliest time point after the onset of dementia. The 30-item BNT and 32-item Multi-Lingual Naming Test were used to assess confrontation naming. Correct responses given after semantic cues were included in the total score. For patients given the Multi-Lingual Naming Test, we used a previously normed conversion developed by the National Alzheimer's Coordinating Center to make these scores comparable to BNT scores.27 To test the specificity of AD-type copathology in LBD to deficits in language, we included other neuropsychological tests assessing global cognition and executive functioning collected within a year of BNT. The majority (97.24%) were restricted to a 9-month interval; to maximize available data, a small number (2.76%) had a slightly longer 12-month interval. Neuropsychological tasks in which <50% of our main cohort completed the task were excluded from analysis; thus, available data included letter fluency (LF), reverse digit span (RDS), and Mini-Mental State Examination (MMSE).28 LF, or the number of unique words beginning with the letter F named in a minute, and RDS, or the longest span of digits that can be reproduced in reverse order, were used to measure executive functioning. The MMSE total score was used as a measure of global cognition; all MMSE scores were collected within 3 months of BNT. All standardized instructions for neuropsychological examinations were administered to participants by trained research personnel. Of note, these standardized research neuropsychological assessments were distinct from clinical examinations used for diagnosis.
Statistical Analyses
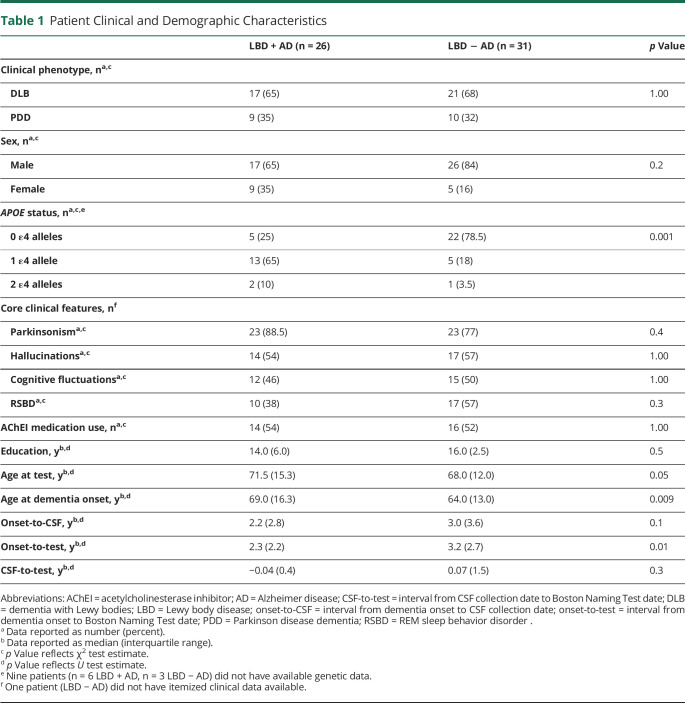
Demographic variables, neuropsychological scores, and CSF analytes were not normally distributed according to the Shapiro-Wilk test. Mann-Whitney U tests compared education, age at dementia onset, and the intervals from dementia onset to CSF collection date (onset-to-CSF), from CSF collection date to cognitive test date (CSF-to-test), and from dementia onset to cognitive test date (onset-to-test) between the LBD + AD and LBD − AD groups (table 1). The χ2 tests compared categorical variables of sex, phenotype, APOE genetic status, core clinical features, and acetylcholinesterase inhibitor (AChEI) medication use across groups. Analysis of covariance (ANCOVA) performed between-group comparisons on all neuropsychological tests across biologically defined groups (LBD + AD, LBD − AD). To test whether phenotype could explain the neuropsychological differences observed, ANCOVAs were repeated across clinical phenotype (DLB, PDD). To perform nonparametric comparisons, we rank-transformed the neuropsychological data before performing these analyses.29 Covariates in each model included age at dementia onset, the interval from onset-to-test, and education (formula = test ∼ group + age + onset-to-test + education). In addition, because CSF used to determine biologically defined groups was not necessarily acquired at the same time as cognitive test data, we included the CSF-to-test interval in biologically defined models.
Table 1.
Patient Clinical and Demographic Characteristics
Spearman correlation tested associations between CSF analytes (t-tau, p-tau, Aβ42) and BNT, as well as other neuropsychological measures, within the whole patient cohort. We retested associations after removing high leverage data points to ensure robustness. Mann-Whitney U test compared BNT performance between APOE ε4 carriers (1 or 2 alleles) and APOE ε4 noncarriers (0 alleles).
All analyses were performed with R programming (version 1.2.5033; R Foundation for Statistical Computing, Vienna, Austria) and used a 2-tailed test with α = 0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
All procedures were performed with written informed consent from all patients under guidelines established and approved by the Institutional Review Board at the University of Pennsylvania.
Data Availability
Anonymized data will be shared with qualified investigators who have Institutional Review Board approval and a Material Transfer Agreement on request.
Results
Demographic and clinical characteristics are described in table 1. The LBD + AD group was older at the time of dementia onset, while the LBD − AD group had a longer time interval between dementia onset and test date. The LBD + AD group also had a greater frequency of APOE ε4 alleles, indicating a heightened risk of AD. The LBD + AD group otherwise did not differ from the LBD − AD group in clinical features or AChEI use. When our cohort was compared by clinical phenotype, the PDD group was older at the time of dementia onset and test date, and the DLB group had a longer time interval between dementia onset and test date (both p < 0.01). Clinical groups also differed in core clinical features: the PDD group had more parkinsonism motor symptoms, whereas the DLB group had a greater frequency of hallucinations, cognitive fluctuations, and REM sleep behavior disorder (all p < 0.05). The PDD and DLB groups did not significantly differ in sex, education, APOE genetic status, or AChEI use. There was no clear association between clinical phenotype (DLB, PDD) and biologically based grouping (LBD + AD, LBD − AD) in this cohort.
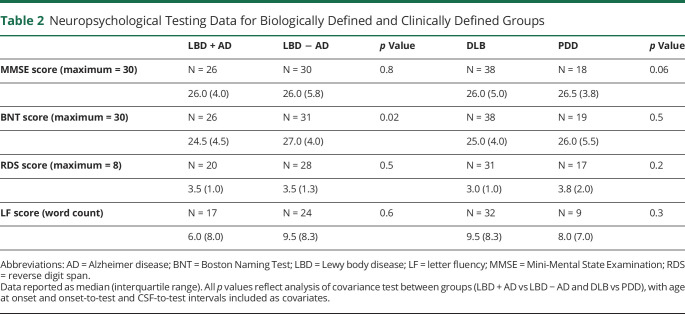
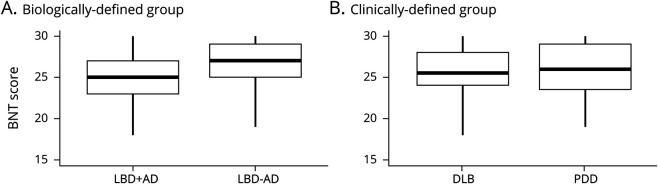
Table 2 summarizes neuropsychological data and ANCOVA comparisons across biologically based (LBD + AD, LBD − AD) and clinical (DLB, PDD) subgroups. Patients with LBD + AD performed worse than patients with LBD − AD on confrontation naming (BNT: F = 4.80, p = 0.03) (figure 2). There was no difference between the performance of patients with LBD + AD and LBD − AD on measures of global cognition (MMSE) or executive (RDS, LF) functioning (all p > 0.1). When our cohort was divided by phenotype, the PDD and DLB groups performed similarly on all neuropsychological tasks (all p > 0.05).
Table 2.
Neuropsychological Testing Data for Biologically Defined and Clinically Defined Groups
Figure 2. Confrontation Naming Performance Across Biologically and Clinically Defined Groups.
Patients with Lewy body dementia (LBD) + Alzheimer disease pathology (AD) perform worse than patients with LBD − AD on the Boston Naming Test (BNT) (A), but the dementia with Lewy bodies (DLB) and Parkinson disease dementia (PDD) groups perform similarly (B). Boxplots illustrate the median, interquartile range, and range of scores for each group independently.
Covariates examined additional factors that might contribute to confrontation naming performance in LBD + AD and LBD − AD. Education was significantly associated with BNT performance between biologically defined groups (F = 7.62, p = 0.008). Despite a positive correlation between BNT score and education (ρ= 0.36, p = 0.007), the groupwise difference between patients with LBD + AD and those with LBD − AD seen in BNT score remained robust. Age and the onset-to-test and CSF-to-test intervals had no effect on BNT performance (all p > 0.1). Mann-Whitney U test showed no difference in BNT score according to APOE ε4 status (p > 0.1).
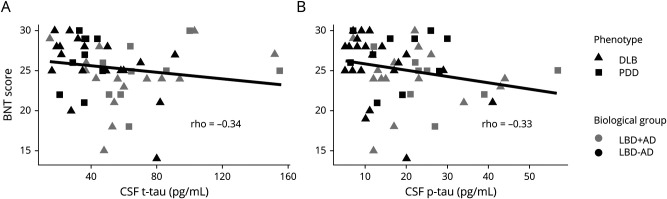
Spearman correlation tested the relationship between CSF analyte concentrations and BNT score impairments across all patients with LBD. We found an inverse correlation between BNT score and CSF t-tau (ρ = −0.28, p = 0.04) and p-tau (ρ = −0.26, p = 0.05) levels. To ensure robustness of this observation, we repeated this correlation after removing high leverage points according to the Cook distance30; the correlation remained for both t-tau (ρ = −0.34, p = 0.01) and p-tau (ρ = −0.33, p = 0.01) (figure 3). There was no correlation between BNT score and Aβ42 (p > 0.1). Finally, we tested whether the t-tau and p-tau associations were specific to BNT score and found no correlation between CSF tau measures and the remaining neuropsychological measures (MMSE, RDS, LF; all p > 0.1).
Figure 3. Confrontation Naming Correlates With CSF t-Tau and p-Tau Levels.
Scatterplots depict individual patients' CSF total tau (t-tau) (A) and phosphorylated tau (p-tau) (B) measurements plotted against Boston Naming Test (BNT) score value with a line of best fit. High leverage points are excluded. BNT score tends to decrease as t-tau and p-tau accumulation in the brain increases.
When analyses excluding the 3 patients with CSF collection before dementia onset were repeated, our main findings were consistent, showing that BNT performance was significantly different between the LBD + AD and LBD − AD groups (F = 5.50, p = 0.02) but not between clinical phenotypes (p = 0.4). No other group-wise comparisons were significant (all p > 0.05). Although the trend of the correlation between BNT and t-tau was nonsignificant when these 3 patients were removed, the correlation between BNT score and p-tau (ρ = −0.29, p = 0.03) remained. When we omitted high leverage points, similar to our main analyses, BNT was significantly associated with both t-tau (ρ = −0.31, p = 0.03) and p-tau (ρ = −0.39, p = 0.005) but not Aβ42.
Discussion
There is considerable evidence that AD-type copathology presents in up to one-half of patients with LBD at autopsy12,13 and markedly affects disease in LBD.14 While biologically based in vivo diagnostic strategies are emerging that use CSF biomarkers to identify AD,31 these approaches are seldom explored to address clinical heterogeneity in the context of LBD. Here, we use a biologically based approach, partitioning LBD into subgroups with and without likely AD-type copathology based on CSF biomarkers and autopsy data when available, to examine cognitive impairment in the full spectrum of LBD. We compare these biological groupings to a clinically based approach and examine the same patients partitioned according to PDD and DLB clinical diagnostic criteria.1,2 We find evidence of a selective, domain-specific cognitive impairment in language in patients with LBD with markers of likely AD-type copathology compared to those lacking significant markers of AD-type copathology. This finding is consistent with autopsy data demonstrating relatively greater tau burden in the temporal lobe of patients with LBD with AD-type copathology.17 In contrast, classic clinical groupings of PDD and DLB do not reveal robust differences in cognitive performance. These observations may have important implications for the clinical utility of stratifying LBD patient cohorts on the basis of biomarkers of AD-type copathology, leading to more biologically meaningful patient groupings compared to clinically based syndromic diagnoses such as PDD and DLB.
Both retrospective autopsy series4,14 and prospective studies using CSF biomarkers as a surrogate marker for AD-type copathology15,16 relate the increasing severity of plaque and tangle pathology to faster cognitive decline and reduced survival during life. Despite this growing body of evidence that AD-type copathology is associated with detrimental outcomes and clinical heterogeneity in LBD,11,16 there are limited data on the specific neuropsychological features associated with AD-type copathology in LBD. This is due in part to the infrequent harmonization of clinical evaluations coordinated with biomarker or autopsy data across cognitive and movement disorders centers32 needed to effectively study the full spectrum of LBD.3,5 Indeed, the clinical criteria for both PDD1 and DLB2 are largely overlapping and acknowledge heterogeneity of cognitive impairment with frequent attentional and executive deficits. Moreover, there is ongoing debate about whether these syndromes are distinct clinicopathologic entities1,3,33 and whether therapeutic trials should include both PDD and DLB due to heterogeneous underlying biology and partially overlapping symptomatology.3,34 To address this issue, we use a unique approach of biologically based stratification in a cohort of patients with PDD and DLB to directly examine clinically and biologically based groupings of LBD.
We hypothesize that the impaired confrontation naming observed in LBD + AD may be related to increased pathologic burden in the temporal lobe, grounded in anatomic data from previous histology and neuroimaging. Confrontation naming is a cognitive task that relies heavily on temporal lobe resources to recognize and retrieve the name of a line drawing.35 Indeed, recent autopsy studies have linked confrontation naming to AD-type copathology in LBD.18,19 In vivo molecular tracer studies in LBD have also found tau36,37 and amyloid38 tracer uptake largely in temporoparietal regions, often implicated in confrontation naming tasks, and our recent PET study associated tau tracer uptake in the temporal lobe with naming difficulty in LBD.39 MRI data comparing LBD + AD and LBD − AD directly are rare, and the few existing studies have focused on medial temporal lobe atrophy40,41 associated with tau tangles and episodic memory difficulty.41 Nonetheless, we recently found greater antemortem MRI neocortical temporal lobe atrophy in LBD + AD vs LBD − AD cohorts.42 Moreover, our group's previous autopsy study showed that antemortem naming deficits in LBD + AD were related to regional tau pathologic burden in the temporal neocortex.17 In the current study, we extend these findings using a largely independent dataset, suggesting that a relative predominance of AD-type tau pathology in the temporal neocortex may indeed play a role in impaired BNT performance seen in patients with LBD + AD. Future work is needed to examine the relationship of other neocortical temporal lobe–mediated domains such as ideomotor apraxia and other features of language impairment associated with frontal disease in LBD such as narrative organization,43 which have been related to AD biomarkers in LBD.
In addition to group-wise comparisons using dichotomized CSF biomarker cut points of AD-type pathology, we test continuous measures of CSF Aβ42, p-tau, and t-tau. We find a significant, negative correlation between BNT scores and CSF t-tau and p-tau levels (figure 3), which have been shown to have a direct linear relationship with postmortem tau pathology in LBD.23 These findings further link confrontation naming with tau pathology in LBD. While amyloid pathology is also implicated in LBD + AD, the association of cognitive difficulty with tau pathology appears to be relatively specific because we find no correlations with CSF Aβ42. These findings are in accordance with our postmortem work that did not find an association of BNT scores with amyloid-plaque pathology17 and others' work showing that tau correlates more closely with cognitive functioning.36,39,44 Still, others have found that CSF Aβ42 is also linked to atrophy patterns and cognitive deficits in LBD.15,40,45 The reasons for these discrepancies remain unclear and could be due in part to the fact that it is difficult to determine the impact of α-synuclein46 and other copathologies in a clinical LBD cohort defined on the basis of CSF biomarkers.12,13,47 Additional work is needed to investigate the relative contributions of tau, amyloid, α-synuclein, and other copathologies to cognitive impairment and clinical heterogeneity seen in LBD.
Aside from confrontation naming, our analysis finds no differences in performance on measures of executive functioning (RDS, LF) or global cognition (MMSE) between the LBD + AD and LBD − AD patient groups. There is also no correlation between these measures and continuous CSF analytes, further suggesting a relatively specific cognitive association of confrontation naming with tau pathology in LBD. These findings are in contrast to previous studies that show that low CSF Aβ42 relates to executive performance in PD.48 The reasons for these discrepancies are unclear and may be related in part to differences in patient cohorts; we focus on dementia in PDD and DLB, while the previous study focused on PD without dementia. One interpretation is that executive dysfunction may be an earlier phenomenon in LBD more closely linked to amyloidosis, α-synuclein, or another pathology. Nonetheless, future prospective work is needed to study longitudinal changes from presymptomatic to end-stage LBD with autopsy.
In this study, we find no evidence for cognitive domain–specific distinctions across clinically defined groups of PDD and DLB. These data contrast with a recent autopsy-confirmed study that found greater decline in memory and language domains in DLB vs PDD7; however, the authors found that when a subgroup of DLB with relative pure α-synuclein pathology was examined, the cognitive profile was more similar to PDD. Thus, it is not entirely clear how the clinical diagnoses of PDD and DLB correspond to LBD subgroups defined on the basis of biomarkers and pathology. Our study supports the consideration of AD-type pathology in LBD and the use of AD CSF biomarker profiles to help define LBD patient cohorts.34 In the setting of LBD, a disease-modifying treatment focused on coexistent AD-type pathology could potentially benefit from the ability to identify a biologically defined subgroup such as LBD + AD.31 Future prospective work following patients to autopsy should explore these and other biological influences on clinical heterogeneity in LBD such as genetic factors49 to better address these complex issues.
Limitations of this study include the retrospective nature of available neuropsychological data and limited testing in specific cognitive domains. While these data reflect a unique effort to harmonize clinical assessments across cognitive and movement disorders researchers32 that facilitates comprehensive study of the full LBD spectrum rather than focused study of clinically defined subgroups alone, lack of completely harmonized data prevented testing of other domains such as episodic memory recall and visuo-construction. Some comparisons were unbalanced and may have lacked power to observe existing group differences, with small numbers of patients with PDD with LF score. Future efforts should focus on collecting prospective, harmonized, complete datasets with molecular imaging for anatomic localization. A second important caveat to consider is our definition of groups. While our study supports the use of CSF cut points23 to provide a biologically meaningful diagnosis in LBD, CSF samples in autopsy-confirmed cases are rare and need further replication before widespread clinical use of AD CSF biomarkers in LBD. Moreover, most patients in this study lacked autopsy data, so we could not assess for other copathologies, such as TAR DNA-binding protein 43, which may influence cognitive performance in the aging brain.50 Third, LBD and AD-type pathology may exist along more of a spectrum5 that is not represented by our CSF-based dichotomization of groups. To test this possibility, we also examined CSF measures as continuous biomarker variables and found an association between CSF tau and BNT performance. Fourth, we could not study the potential contribution of α-synuclein pathology to differences seen in confrontation naming performance between groups because most of our participants had only CSF biomarkers. AD-type pathology may co-occur with greater α-synuclein46 and overall pathologic burden,12,17 but this can be determined only in an autopsy cohort, and validated CSF biomarkers to directly measure α-synuclein in CSF-defined cohorts are lacking. Finally, the greater impairment seen in LBD + AD could have been attributed to more general impairment from a more advanced stage of disease compared to LBD − AD. Although we saw no evidence for differences in global cognition between groups, we included measures of disease severity in our analyses to account for this potential confound. Future studies should investigate longitudinal cognitive data in autopsy- or biomarker-defined cohorts to explore potential effects of AD-type copathology in patients with PD without dementia.
We show that biological markers of AD-type copathology in LBD have a relatively specific association with cognition in the language domain, where reduced confrontation naming appears to be related in part to markers of AD-type copathology and the burden of tau in particular. While cognition in clinically defined LBD syndromes (DLB, PDD) remains heterogeneous, we observe more interpretable cognitive profiles in biologically defined cohorts. Defining LBD by markers of AD-type copathology may have advantages over solely clinically defined syndromes (DLB, PDD) because the antemortem identification of both AD-type and synuclein pathologies appears to have a strong impact on prognosis and treatment. Incorporating biomarker stratification of LBD + AD vs LBD − AD into clinical trials will allow the development of more targeted, disease-modifying drug therapies.
Acknowledgment
Data contributed by current project Center on Alpha-Synuclein Strains in Alzheimer Disease & Related Dementias at the Perelman School of Medicine at the University of Pennsylvania (U19 AG062418, Trojanowski JQ-PI) and former Morris K. Udall Center at the Perelman School of Medicine at the University of Pennsylvania (P50 NS053488, Trojanowski JQ-PI). K.A.Q. Cousins is a recipient of the Alzheimer's Association Research Fellowship to Promote Diversity (AARF-D-10072703). The authors thank all of their patients and caregivers for their participation in our research.
Glossary
- Aβ
β-amyloid1-42
- AChEI
acetylcholinesterase inhibitor
- AD
Alzheimer disease
- ANCOVA
analysis of covariance
- BNT
Boston Naming Test
- DLB
dementia with Lewy bodies
- LBD
Lewy body dementia
- LF
letter fluency
- MMSE
Mini-Mental State Examination
- p-tau
phosphorylated tau
- PD
Parkinson disease
- PDD
PD dementia
- RDS
reverse digit span
- t-tau
total tau
Appendix. Authors
Study Funding
Study funding by U19 AG062418 & P50 NS053488.
Disclosure
E. Howard, D.J. Irwin, and K. Rascovsky report no disclosures. N. Nevler is supported by grants from the Alzheimer's Association (AACSF-18-567131), Institute on Aging, and Department of Defense (PR192041). S Shellikeri, T.F. Tropea, and M. Spindler report no disclosures. A. Deik has participated in advisory boards for Acorda Therapeutics and has received research funding from TeVa Pharmaceuticals, Revance Therapeutics, Sunovion Pharmaceuticals, and Biohaven Pharmaceuticals. A. Chen-Plotkin reports no disclosures. A. Siderowf has been a consultant to the following companies in the past year: Biogen, Wave Life Sciences, Axovant, Prevail, and Prilenia Therapeutics. He has received grant funding from the Michael J. Fox Foundation for Parkinson's Research (MJFF) and National Institute of Neurological Disorders and Stroke. N. Dahodwala reports no disclosures. D. Weintraub has received research funding or support from MJFF, Alzheimer's Therapeutic Research Initiative, Alzheimer's Disease Cooperative Study, the International Parkinson and Movement Disorder Society, and National Institute on Aging (NIA); honoraria for consultancy from Acadia, Aptinyx, Biogen, CHDI Foundation, Clintrex LLC, Eisai, Enterin, F. Hoffmann-La Roche Ltd, Ferring, Janssen, Otsuka, Promentis, Sage, Signant Health, Sunovion, and Takeda; and license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS. L.M. Shaw receives research support from NIH/NIA, Alzheimer's Disease Neuroimaging Initiative (ADNI) (AG024904), University of Pennsylvania Alzheimer's Disease Coordinating Center Biomarker Core (AG010124), MJFF, and Roche; provides quality control oversight for Roche Elecsys CSF AD biomarker immunoassays for ADNI; is a consultant for Roche, Lilly, Biogen, and Novartis; and is a member of the Biogen teaching program. J.Q. Trojanowski may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is coinventor, and he received revenue from the sale of Avid to Eli Lily as coinventor of imaging-related patents submitted by the University of Pennsylvania. S.N. Vaishnavi, ,D.A. Wolk, and D. Mechanic-Hamilton report no disclosures. J.F. Morley receives research funding from the Department of Veterans Affairs and has received honoraria from MJFF. J.E. Duda reports no disclosures. M. Grossman receives support from the NIH (AG066597, AG054519, AG052943, AG017586, AG062418), Department of Defense (PR192041), Biogen, Eisai, Avid Radiopharmaceuticals, and Life Molecular Imaging. K.A.Q. Cousins is a recipient of the Alzheimer's Association Research Fellowship to Promote Diversity (AARF-D-10072703). Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011699 for full disclosures.
References
- 1.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippa C, Duda J, Grossman M, et al. DLB and PDD boundary issues diagnosis, treatment, molecular pathology, and biomarkers. Neurology 2007;68:812–819. [DOI] [PubMed] [Google Scholar]
- 4.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017;16:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman JG, Williams-Gray C, Barker RA, et al. The spectrum of cognitive impairment in Lewy body diseases. Mov Disord 2014;29:608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troster AI. Lewy bodies and Parkinson's disease with dementia: differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol Rev 2008;18:103–119. [DOI] [PubMed] [Google Scholar]
- 7.Smirnov DS, Galasko D, Edland SD, Filoteo JV, Hansen LA, Salmon DP. Cognitive decline profiles differ in Parkinson disease dementia and dementia with Lewy bodies. Neurology 2020;94:e2076–e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao AW, Racine CA, Quitania LC, et al. Cognitive and neuropsychiatric profile of the synucleinopathies: Parkinson disease, dementia with Lewy bodies, and multiple system atrophy. Alzheimer Dis Assoc Disord 2009;23:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson's disease with and without dementia. J Clin Exp Neuropsychol 1999;21:435–443. [DOI] [PubMed] [Google Scholar]
- 10.Dunet V, Joao-Fartaria M, Deverdun J, et al. Episodic memory decline in Parkinson's disease: relation with white matter hyperintense lesions and influence of quantification method. Brain Imag Behav 2019;13:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Censo R, Abdelnour C, Blanc F, et al. CSF tau proteins correlate with an atypical clinical presentation in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2020;91:109–110. [DOI] [PubMed] [Google Scholar]
- 12.Irwin DJ, Hurtig HI. The contribution of tau, amyloid-beta and alpha-synuclein pathologies to dementia in Lewy body disorders. J Alzheimers Dis Parkinsonism 2018;8:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018;141:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol 2008;115:427–436. [DOI] [PubMed] [Google Scholar]
- 15.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology 2010;75:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemstra AW, de Beer MH, Teunissen CE, et al. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2017;88:113–118. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin D, Xie S, Liang M, et al. Cognitive and pathological influences of tau pathology in Lewy Body disorders. Ann Neurol 2019;85:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraybill ML, Larson EB, Tsuang D, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 2005;64:2069–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peavy GM, Edland SD, Toole BM, Hansen LA, Galasko DR, Mayo AM. Phenotypic differences based on staging of Alzheimer's neuropathology in autopsy-confirmed dementia with Lewy bodies. Parkinsonism Relat Disord 2016;31:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: the University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement 2014;10:477.e1–484.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011;72:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's Disease Neuroimaging Initiative subjects. Ann Neurol 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin D, Xie S, Coughlin D, et al. CSF tau and amyloid-beta predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 2018;90:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beason-Held LL, Goh JO, An Y, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci 2013;33:18008–18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathol 2013;126:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monsell SE, Dodge HH, Zhou XH, et al. Results from the NACC Uniform Data Set neuropsychological battery crosswalk study. Alzheimer Dis Assoc Disord 2016;30:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 29.Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. Am Statistician 1981;35:124–129. [Google Scholar]
- 30.Cook RD. Detection of influential observations in linear regression. Technometrics 1977;1:15–18. [Google Scholar]
- 31.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson GS, Cholerton BA, Gross RG, et al. Neuropsychologic assessment in collaborative Parkinson's disease research: a proposal from the National Institute of Neurological Disorders and Stroke Morris K. Udall Centers of Excellence for Parkinson's Disease Research at the University of Pennsylvania and the University of Washington. Alzheimer Dement 2013;9:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeve BF, Dickson DW, Duda JE, et al. Arguing against the proposed definition changes of PD. Mov Disord 2016;31:1619–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coughlin DG, Hurtig HI, Irwin DJ. Pathological influences on clinical heterogeneity in Lewy body disease. Mov Disord 2019;35:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obler LK, Rykhlevskaia E, Schnyer D, et al. Bilateral brain regions associated with naming in older adults. Brain Lang 2010;113:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomperts SN, Locascio JJ, Makaretz SJ, et al. Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol 2016;73:1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kentarci K, Lowe VJ, Boeve BF, et al. AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 2017;81:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology 2008;71:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coughlin DG, Phillips JS, Roll E, et al. Multimodal in vivo and post-mortem assessments of tau in Lewy body disorders. Neurobiol Aging 2020;96:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelnour C, Ferreria D, Oppedal K, et al. The combined effect of amyloid-β and tau biomarkers on brain atrophy in dementia with Lewy bodies. Neuroimage Clin 2020;27:102333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedelska Z, Ferman TJ, Boeve BF, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging 2015;36:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spotorno N, Coughlin DG, Olm CA, et al. Tau pathology associates with in vivo cortical thinning in Lewy body disorders. Ann Neurol 2020;7:2342–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grossman M, Irwin DJ, Jester C, et al. Narrative organization deficit in Lewy body disorders is related to Alzheimer pathology. Front Neurosci 2017;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dumurgier J, Hanseeuw BJ, Hatling FB, et al. Alzheimer's disease biomarkers and future decline in cognitive normal older adults. J Azheimers Dis 2017;60:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira D, Przybelski SA, Lesnick TG, et al. Amyloid-beta and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology 2020;95:e3257–e3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferman TJ, Aoki N, Crook JE, et al. The limbic and neocortical contribution of alpha-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement 2018;14:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapasi A, DeCarli C, Schneider JA, et al. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017;134:171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leverenz JB, Watson GS, Shofer J. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson's disease. Parkinsonism Relat Disord 2011;17:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cilia R, Tunesi S, Marotta G, et al. Survival and dementia in GBA-associated Parkinson's disease: the mutation matters. Ann Neurol 2016;80:662–673. [DOI] [PubMed] [Google Scholar]
- 50.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 2019;142:1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared with qualified investigators who have Institutional Review Board approval and a Material Transfer Agreement on request.