Abstract
Objective
To detail the scope, nature, and disclosure of financial conflicts of interest (COI) between the pharmaceutical and medical device industries (Industry) and authors in high-impact clinical neurology journals.
Methods
Using the Centers for Medicare and Medicaid Services Open Payments Database (OPD), we retrieved information on payments from Industry to 2,000 authors from randomly selected 2016 articles in 5 journals. We categorized payments by type (research, general, and associated research/institutional), sponsoring entity, and year (from 2013 to 2016). Each author's self-disclosures were compared to OPD-listed Industry relationships to measure discordance. Payments were manually reviewed to identify those from manufacturers of products that were directly tested or discussed in the article. We also quantified the prevalence and value of these nondisclosed, relevant COI.
Results
Two hundred authors from 158 articles had at least 1 OPD payment. Median/mean annual payments per author were $4,229/$19,586 (general); $1,702/$5,966 (research); and $67,512/$362,102 (associated research). Most neurologists received <$1,000/y (74.6%, 93.0%, and 79.5% for general, research, and associated research, respectively), but a sizeable minority (>10% of authors) received more than $10,000 per year, and several received over $1 million. Of 3,013 payments deemed directly relevant to the article, 50.9% were not self-disclosed by the authors, totaling $5,782,197 ($1,665,603 general; $25,532 research; $4,091,062 associated research).
Conclusion
Industry-related financial relationships are prevalent among United States–based physicians publishing in major neurology journals, and incomplete self-disclosure is common. As a profession, academic and other neurologists must work to establish firm rules to ensure and manage disclosure of financial COI.
Neurologists have extensive financial relationships with pharmaceutical and medical device companies (Industry).1 From 2013 to 2016, over half of all United States–based neurologists received at least 1 payment from Industry each year, totaling over $354 million.2 While academic–industrial collaborations support scientific discoveries,3 they also introduce conflicts of interest (COI), or secondary interests that may compromise an individual's ability to place patients' interests first.4 For example, Industry funding has been associated with higher prescription rates of sponsored products5–7 and increased publication of favorable articles that report positive effects.8,9
Recognizing these ethical implications, full COI disclosure has been advocated as a way of contextualizing research findings and mitigating potential bias.10,11 Physicians are required to disclose COI for publication in major academic journals and drug and device manufacturers are mandated to report all payments to physicians and academic medical centers according to the Physician Payments Sunshine Act of 2010.12,13 These transactions are publicly available in the Centers for Medicare and Medicaid Services (CMS)' Open Payments Database (OPD).14
Studies comparing voluntary author disclosures with Industry-reported OPD payments have identified discordant disclosures in orthopedics, otolaryngology, cardiac surgery, hematology, pulmonology, sports medicine, plastic surgery, vascular surgery, and general surgery.15–19 To date, the adequacy of author self-disclosure of Industry-related COI has not been studied within neurology. The current study aims to determine the nature and scope of Industry payments to authors of major neurology journal articles and to characterize the discordance between self-disclosed and Industry-reported COI for payments that are directly related to the articles.
Methods
This cross-sectional, retrospective study reviewed articles published in 2016 in major clinical neurology journals and compared disclosures against 2013–2016 OPD data. Data collection occurred from July 2018 to May 2019.
Standard Protocol Approvals, Registrations, and Patient Consents
This descriptive study used publicly available nonclinical data. Institutional review board approval and informed consent were not required.
The Open Payments Database
Information about the OPD and collection methods can be found in the CMS OPD online “data dictionary”20 and has been discussed previously.2 Briefly, applicable manufacturers and group purchasing organizations are legally required to submit annual data listing all payments and other transfers of value made to physicians and teaching hospitals. Financial relationships are categorized into “research payments,” “general (nonresearch) payments,” “associated research payments” (made to an institution, not an individual), and “ownership or investment” interests.
Research payments are payments to individuals “made in connection with a research agreement or research protocol” and “can include direct compensation to physicians, funding for research study coordination and implementation, or payments to study participants to cover expenses associated with the study.” Research grants and other funds (e.g., clinical trial support) paid directly to the institution are classified as associated research. General payments are “payments or other transfers of value made that are not in connection with a research agreement or research protocol.” Examples of general payment categories include food and beverage payments, travel expenses, honoraria, consulting fees, and other (nonconsulting speaking or faculty payments). Detailed descriptions of each category of general payments, including examples, can be found on the CMS website14 and are copied verbatim in table e-1 (doi.org/10.5061/dryad.547d7wm78). Prior to publication of data on the OPD Web site, physicians and teaching hospitals are offered a 3-week period during which to review their data and challenge inaccurate reports. Thereafter, the data are published on the OPD website for online browsing and downloading. Data submitted to OPD that are eligible for publication are published twice annually. Data from nonphysicians (e.g., PhDs) and physicians based outside the United States are not included in the database, nor are physicians with no reported payments of any kind.
Author Selection and Payment Data Collection
We randomly selected a total of 2,000 authors of articles published in 2016 in 5 journals considered by the authors, SCImago Journal Rank,21 and peer opinion22 to be the highest impact, clinically oriented general neurology journals: Neurology®, JAMA Neurology, The Lancet Neurology, Brain, and Annals of Neurology. The most recent year with OPD data when this study commenced was 2016. We randomly selected articles using an online number generator, starting with month (1–12), issue (number varied based on publication), and article within the issue. We included only reviews and original research articles. Authors based outside the United States and nonphysician authors (e.g., advance practice providers, PhDs) were excluded. We recorded the self-disclosed Industry relationships, defined as payments from commercial entities such as pharmaceutical or medical device companies, for all remaining authors, regardless of authorship role. We excluded disclosures of academic and governmental entities, as well as nongovernment organizations (e.g., foundations, societies). We then manually entered each author in OPD and recorded all individual transactions made between January 1, 2013, and December 31, 2016, along with the paying organization. We separated and totaled payments by year and type: general, research, associated research, or ownership. Only 1 author had ownership interests, so we omitted this payment category from further discussion. We further subcategorized general payments.
Determination of Relevance
We reviewed all payments disclosed by the author or listed in OPD for relevance to the manuscript. A payment was deemed relevant if the sponsoring Industry entity manufactured a drug, device, or other product that was directly tested or discussed in the study or if the paying company sponsored the study. This narrow definition of relevance does not encompass all potential sources of bias (e.g., relationships with manufacturers of competing products or manufacturers in the same therapeutic class), but identifies a selection of obviously relevant payments for analysis.
Determination of Disclosure Status
We determined disclosure status for both individual payments and authors. We considered payments author-disclosed if the author listed a relationship to a sponsoring entity (or its parent company or subsidiary) or if that company funded the study. For the disclosure analysis, we omitted relationships with Industry entities that only supplied food and beverage payments to the author, as physicians are sometimes unaware of these payments; for example, Industry might fund a meal at a conference at which a physician signs in for attendance and report a payment even if the physician is never aware of the sponsoring organization.23
As has been done previously,16 we categorized disclosure status as follows: “no disclosures” (authors with no OPD- or author-disclosed payments); “full disclosure” (authors whose disclosed relationships completely match those found in the OPD); “incomplete self-disclosure” (authors with payments listed in OPD but not self-disclosed); and “incomplete Industry disclosure” (authors with author-disclosed relationship not listed in OPD). A single author with multiple relationships could have both Industry and author underdisclosures. When comparing disclosure status, we did not take the disclosure policies of the journal into account; for publication in Brain, Annals of Neurology, and JAMA Neurology, authors were not required to disclose all relationships unless they were considered relevant to the manuscript. Therefore, authors could still follow journal disclosure policies and be categorized as having incomplete disclosures. All journals required that authors disclose COI relevant to the manuscript. See supplementary Appendix e-1 for journal disclosure policies as of 2016 (doi.org/10.5061/dryad.547d7wm78).
Data Analysis
We tabulated descriptive data in Microsoft Excel. Quantitative information for number, nature, and value of payments was only available for OPD-listed relationships. Only the name of the Industry payer was noted for author-disclosed relationships.
Two authors (J.E.S., C.W.) reviewed all data in duplicate. We assessed interrater reliability with an intraclass correlation coefficient (ICC) (SPSS v25.0, IBM, Armonk, NY) for all relevant payments to ensure consistency of the methods. When data were discordant, results were reviewed by consensus and reconciled for final reporting. A third author (N.M.R.) then reviewed data and disclosure status for authors receiving relevant payments to ensure agreement.
Data Availability
Payments from Industry are publicly accessible on the CMS Open Payments database (openpaymentsdata.cms.gov/). Our anonymized relevance data will be shared by request from any qualified investigator.
Results
The sample selection process is summarized in figure 1. We screened 2,000 authors from 158 journal articles published in 2016 in Neurology (n = 34), JAMA Neurology (n = 33), Lancet Neurology (n = 25), Annals of Neurology (n = 31), and Brain (n = 35). Among these, 423 authors were eligible for OPD inclusion. Excluded authors were those outside the United States (n = 1,171), non-MD/DO authors (n = 381), and duplicate authors (n = 25). A total of 126/423 authors (29.8%) disclosed Industry-related payments and 200/423 authors (47.3%) had OPD-listed payments.
Figure 1. Sample Selection.
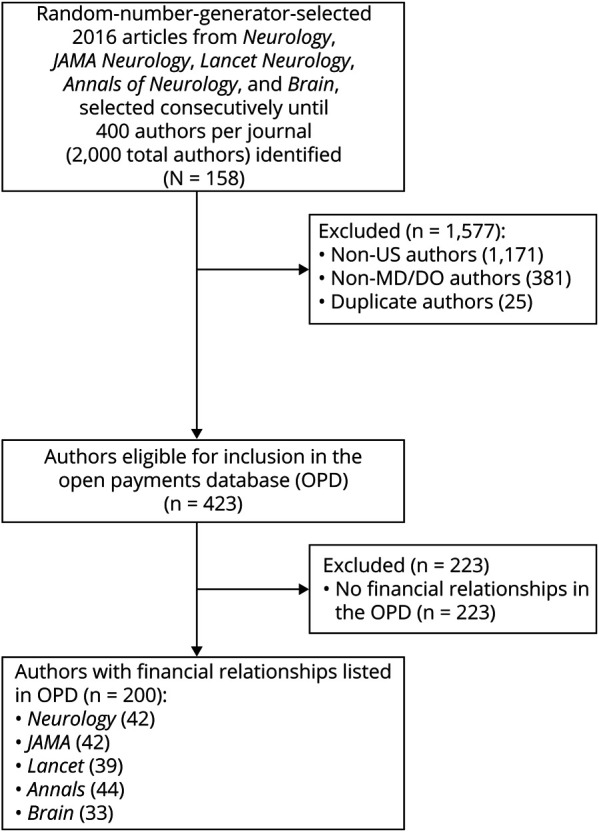
OPD = Open Payments Database.
Reliability of Data Ascertainment
We assessed interrater agreement on 56 pairs of observations using 2-way mixed, average-measures ICC using an absolute agreement definition. We considered the complete payment information for each author with relevant payments as a single observation. ICC was good to excellent (ICC 0.89, 95% confidence interval 0.81–0.94).
Scope of Payments
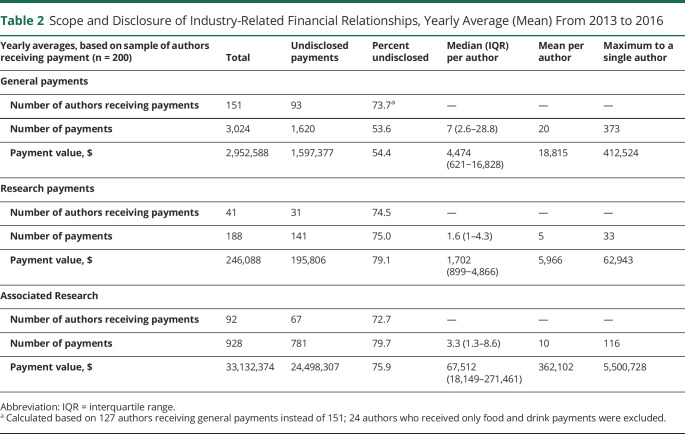
The number of authors receiving payments and the total payment values were calculated for each year and then averaged. In the average year, only a minority of authors received general payments (151/423 [35.6%]), research payments (41/423 [9.8%]), or associated research payments (91/423 [21.5%]). Most authors (223/423 [52.7%]) had no Industry relationships (table 1). Most payments were small (<$1,000 per year), but some neurologists received substantial compensation. Six authors (1.4%) averaged over $1,000,000 in associated research-related earnings each year, and 76 (17.6%) received $10,000 or more (see table 1). Mean and median annual payments to authors by category are shown in table 2. These values are broken down by individual year in supplementary table e-3 (doi.org/10.5061/dryad.547d7wm78).
Table 1.
Stratified Values of Mean Annual Industry Payments to Neurologists, 2013–2016, n (% of Physicians)
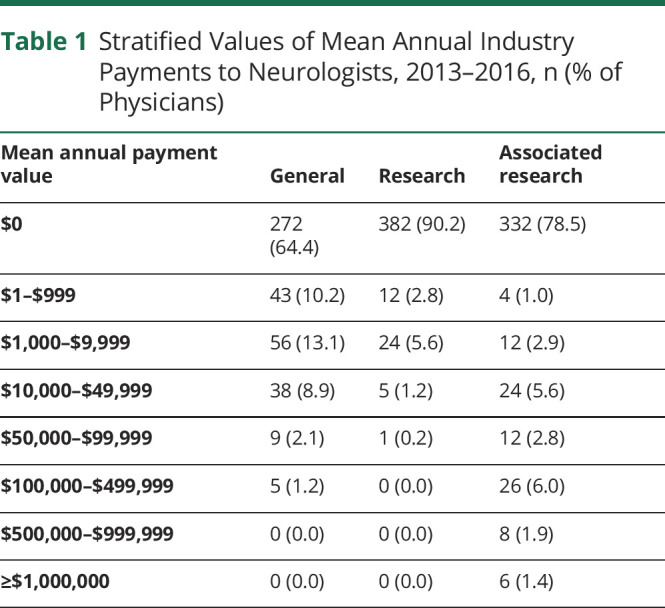
Table 2.
Scope and Disclosure of Industry-Related Financial Relationships, Yearly Average (Mean) From 2013 to 2016
General payment value mostly comprised consulting fees ($4,163,208 [36.0%]) and compensation for services other than consulting, including serving as faculty or as a speaker at a venue other than a continuing education program ($4,390,866 [38.0%]). Food and beverage payments were the most common general payment type, but contributed least to the payment value ($306,051 [2.6%]), followed by honoraria ($810,008 [7.0%]) and travel expenses ($1,898,532 [16.4%]).
COI Disclosure Completeness
A total of 49.9% (n = 211) of the authors had discordant COI disclosure: self-disclosure was incomplete for 42.8% (181/423) of authors; Industry disclosure was incomplete for 18.2% (77/423) of authors; 11.1% (47/423) had both incomplete industry and author disclosures. Among all authors, 48.2% (204/423) had no Industry-related COI and 1.9% (8/423) had perfectly aligned (“matching”) Industry and author disclosures. For authors with OPD-listed payments, disclosures were missing (i.e., author disclosed “no conflicts” in the article) among 54.0% (108/200) of authors. Disclosure and disclosure status is further categorized by payment type in table 2. Supplementary figure e-1 depicts disclosure status by author (doi.org/10.5061/dryad.547d7wm78).
Disclosure Status for Payments Directly Relevant to the Article
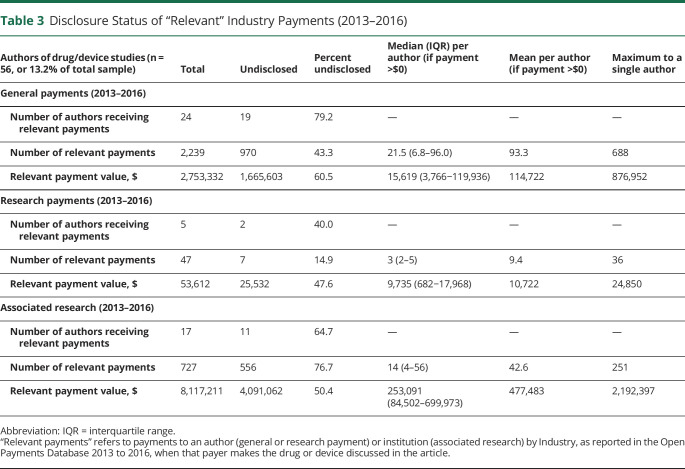
While only 13.2% (56/423) of authors wrote a manuscript about a drug or device, 48.2% (27/56) of these authors received payment from an Industry entity that manufactured the drug or device directly discussed in that article. Despite journal policies mandating disclosure of relevant COI, most of these authors (25/27) did not completely disclose their relevant relationships; table 3 summarizes the scope and disclosure status of relevant payments. Academic neurologists in this sample failed to disclose almost $1.7 million in relevant general payments, $25,500 in relevant research, and over $4 million in relevant associated research payments. These values are the sum of all relevant payments received during the 4-year lookback period, not yearly averages, since authors are required to report all previous relevant relationships up until the time of publication.
Table 3.
Disclosure Status of “Relevant” Industry Payments (2013–2016)
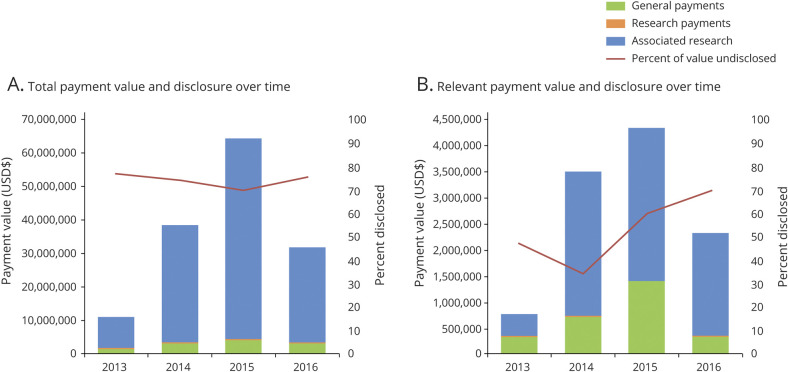
Additional yearly information on value and disclosure status of total and relevant payments from Industry to neurology authors, including 2013 to 2016 trends, is shown in figure 2. The percentage of undisclosed relevant payments is greatest for recent payments; authors were most likely to disclose relevant payments from 2014 and least likely to disclose 2016 relevant payments. Whereas associated research comprises the majority of overall payment value, general payments made directly to the individual author make up a disproportionate percentage of the relevant payment value, compared with total payment value.
Figure 2. Value and Nature of Industry-Related Payment to Neurologist Authors and Rates of Payment Underdisclosure (2013–2016).
Stacked bars for total associated research payments (blue), research payments (orange), and general payments (green) depict the total value of payments from Industry to select authors of neurology journals. The red line depicts the percentage of these payments that were undisclosed in the manuscript by authors. (A) All payments reported in the Open Payments Database to included authors (n = 200). (B) Payments reported in the Open Payments Database to authors from companies that manufacture drugs or devices directly relevant to the article (n = 56).
Discussion
This is the first study to comprehensively survey financial relationships between Industry and academic neurologists using the OPD. Our central findings are that the majority of Industry payments go to a well-paid minority of authors in high-impact clinical neurology journals, many of whom fail to disclose these relationships, even when directly relevant to the articles and required to do so according to journal policies. These findings raise several points that warrant further discussion.
A minority of authors accounts for the majority of Industry relationships in this sample. More than 70% of authors in this sample received few, if any, payments (less than $999 per year), but 10% of authors received in excess of $10,000 per year on average (combined general and research payments) and more when considering institutional associated research payments. Notably, the mean annual general payment value in this sample of academic neurologists was much greater than that of the general population of US neurologists ($19,586 compared to ∼$6,000), as was the median general payment value ($4,229 compared to ∼$400), suggesting more payments in the form of speaking and consulting fees. This differential remuneration may reflect the fact that academic leaders, as a result of their expertise, are better positioned to synergistically advance neurologic science through Industry cooperation. It may also reflect a concerted effort from Industry to shape medical opinion and practice; by paying key opinion leaders to speak at company events, Industry pays for leaders' widespread influence on the prescribing patterns of other physicians.24 Future studies should examine the influence of these Industry payments on shaping (1) neurologic scholarly literature and neurologic education; (2) the prevalence of Industry relationships among academic neurologists holding influential positions, such as journal editors, conference speakers, and authors of clinical practice guidelines; and (3) the proportion of Industry payments that go towards advancing neurologic science as opposed to promoting Industry interests when the 2 outcomes conflict.
The disclosure discordance found in this study mirrors that found in diverse subspecialties, highlighting that this problem is not unique to neurologists.16,19 A large proportion of the underdisclosure we found could be explained by the journal disclosure policies; disclosure of nonrelevant relationships was not required for publication in Brain, Annals of Neurology, and JAMA Neurology. Therefore, author disclosures could be categorized as incomplete in the current study while still adhering to the journal disclosure policy. An unresolved question for further study is where to draw the line in determining whether COI are related and which COI should always be disclosed. Regardless, all 5 journals we reviewed required authors to disclose relevant COI, though this provision was often not met (see supplementary Appendix e-1 for journal disclosure policies as of 2016, doi.org/10.5061/dryad.547d7wm78).
The high rates of underdisclosure of relevant, Industry-related financial relationships, as illustrated in table 3, are particularly problematic and should be highlighted. To state simply, the majority of authors who wrote about a drug or device, while receiving payments from the company that makes that drug or device, did not voluntarily disclose these relevant payments. In this sample, 19 authors failed to disclose nearly $1.7 million in relevant individual payments, and 11 authors underdisclosed over $4 million in relevant associated research payments to their institutions. Whereas associated research payments are not paid directly to the authors, the benefits of winning grants are often passed along to the physician as salary support or incentives, reduced clinical responsibilities, and promotion. Recall also that the risk of Industry bias from institutional payments is seen empirically—Industry-funded drug and device studies report more favorable efficacy results and conclusions than studies sponsored by other sources9—and therefore these relationships should be disclosed to the readers as COI. In addition, our narrow definition of relevance did not capture payments indirectly related to a product being tested in the study, such as payments from a company manufacturing a competitor drug, which may also represent COI. Therefore, the true extent of undisclosed financial relationships with Industry remains unknown and may be larger than the numbers detailed here.
This study was not designed to answer the important question of why authors failed to disclose relevant Industry-related payments. One possibility is that the authors were unaware of the payments. Prior work has highlighted that physicians may be unaware of small meal payments reported in OPD.23 However, we addressed this concern by removing meal payments from our analysis of relevant payment disclosure. The highest paid authors received over 650 general payments valued over $800,000, so it is understandable if those authors could not keep track of all of their payments, or include all of them on the COI disclosure forms. However, the sheer magnitude of Industry relationships should not preclude accurate reporting of COI, and it remains imperative to transparently disclose relevant payments.
Another possibility is inaccurate Industry reporting in the OPD. There are provisions to monetarily fine companies for failure to report payments, but neither disincentives for overreporting or penalties for erroneous reporting. These factors create a system that encourages overreporting.23,25 Reliance on the OPD as the gold standard is a categorical limitation of our study, because we cannot exclude the possibility of overreporting or erroneous reporting. However, prior work in a diverse array of fields has confirmed the overall validity and utility of the OPD as a repository for Industry-associated payments to physicians.16,26–29 Also, no payments in our sample were successfully disputed by authors during the OPD comment period. A recent case study supported the accuracy and validity of the OPD and its usefulness in cross-checking large COI disclosures. One neurologist failed to disclose over $8 million received from Industry (listed in OPD) when writing an editorial in a premier journal that negatively discussed a competitor's product.30 The author apologized for the oversight in a correction issued several years later.
Given the accumulated evidence that a problematic minority of physicians fails to accurately and voluntarily disclose COI, the question becomes: What should we do about it? We do not advocate prohibiting Industry–academic collaboration, which runs counter to the mutual goal of improving health. Many Industry–physician relationships are healthy, promote both the interests of physicians and Industry, and do not constitute COI.23 Whether disclosure actually does mitigate bias and safeguard scientific integrity in cases of COI remains an open debate, as does the optimal level of disclosure; too many disclosures can be distracting and detract from the relevant ones, while underdisclosure limits the reader's ability to contextualize an author's conclusions.2 As a first step, institutions can perform internal comparisons of voluntary disclosures to the OPD, as we have done at Dartmouth Hitchcock Medical Center. Ensuring accurate disclosure does not mitigate COI, but it publicizes it to those who need to know about it, and can be followed by subsequent administrative review and action. Simultaneously, OPD must be improved in order to ensure accuracy and allow for better dispute resolution in instances of inaccurate Industry reporting. Accurate reporting can also benefit from standardized disclosure policies. Whereas all 5 journals required disclosure of “relevant” payments, relevance was not clearly defined in their disclosure policies. Specifying criteria for relevance within journal policies would help authors accurately list their disclosures, and including the reason for relevance would help readers understand the potential biases.
We note several limitations of our study. First, we examined a relatively small sample of authors of the large number of articles published in neurology journals each year. We limited our sample to only 5 high-impact, clinically focused general neurology journals, and only articles published in 2016, limiting generalizability. In addition, our sample was limited to US physicians eligible for OPD inclusion. Future studies should strive to investigate COI in nonclinicians and investigators based outside the United States. Second, whereas prior studies have detailed how COI bias the neurologic literature,9,31–35 this study did not investigate whether the financial relationships detailed above, disclosed or undisclosed, resulted in bias. Finally, this study was limited to Industry-related financial COI. There are other nonfinancial COI that can introduce bias and negatively impact neurologic practice and research,10 but were beyond this project's scope.
Despite its limitations, this study had several strengths. It is the first to comprehensively examine Industry-related COI among authors in neurology journals using the OPD and the first to highlight the prevalence of undisclosed relevant financial relationships. Despite its shortcomings, the use of the OPD is a strength because it relies on payment reporting that is required by law (as opposed to failure to self-disclosure, which carries a risk of censure but no formal legal punishment). Our use of duplicate data collection ensured reliability.
Industry-related COI are widespread among authors in high-impact neurology journals, and underdisclosure is common. The extent to which these COI produce bias remains unknown, though prior research suggests that COI can produce bias and influence medical education, research, and clinical practice. Future studies can explore the effects of these COI in neurology, and also expand studies of COI and disclosure to additional settings such as subspecialty journals, lower impact journals, editorial staff, clinical practice guideline authors, and grant review committee members. Given the prevalence of COI underdisclosure, institutions should consider performing internal comparisons of voluntary disclosures to the OPD, while simultaneously working towards improving the accuracy of the OPD and clarifying journal policies. Finally, given the profound and important contributions of Industry towards improving health and society, future research must determine how to best encourage Industry collaboration without compromising the ethical practice of neurology.
Glossary
- CMS
Centers for Medicare and Medicaid Services
- COI
conflicts of interest
- ICC
intraclass correlation coefficient
- OPD
Open Payments Database
Appendix. Authors
Footnotes
Podcast: NPub.org/v84dmc
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc 2016;91:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins NM, Meyer MJ, Bernat JL. Scope and nature of financial conflicts of interest between neurologists and industry: 2013-2016. Neurology 2019;93:438–449. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Hauser SL, Desmond-Hellmann S. Enhancing ties between academia and industry to improve health. Nat Med 2011;17:434–436. [DOI] [PubMed] [Google Scholar]
- 4.Rubin EB, Bernat JL. Resident and Fellow Section: conflicts of interest between physicians and the pharmaceutical industry: focus on headache medicine. Headache 2008;48:1545–1549. [DOI] [PubMed] [Google Scholar]
- 5.DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical industry-sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med 2016;176:1114–1122. [DOI] [PubMed] [Google Scholar]
- 6.Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim AS. Association of industry payments to physicians with the prescribing of brand-name statins in Massachusetts. JAMA Intern Med 2016;176:763–768. [DOI] [PubMed] [Google Scholar]
- 7.Fleischman W, Agrawal S, King M, et al. Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study. BMJ 2016;354:i4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003;289:454–465. [DOI] [PubMed] [Google Scholar]
- 9.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins NM. Ethical issues pertaining to conflicts of interest between neurologists and the pharmaceutical and medical device industries. Semin Neurol 2018;38:589–598. [DOI] [PubMed] [Google Scholar]
- 11.Ethics and Humanities Subcommittee. American Academy of Neurology policy on conflicts of interest. Neurology 1998;50:332–334. [DOI] [PubMed] [Google Scholar]
- 12.Braillon A, Wilson M. The Sunshine Act: moving forward. Am J Med 2016;129:e201. [DOI] [PubMed] [Google Scholar]
- 13.United States Congress. Compilation of Patient Protection and Affordable Care Act: As Amended Through November 1, 2010 Including Patient Protection and Affordable Care Act Health-Related Portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 14.Centers for Medicare and Medicaid Services. Open Payments Data [online]. Available at: cms.gov/OpenPayments [Google Scholar]
- 15.Boddapati V, Fu MC, Nwachukwu BU, Ranawat AS, Zhen WY, Dines JS. Accuracy between AJSM author-reported disclosures and the centers for Medicare and Medicaid services open payments database. Am J Sports Med 2018;46:969–976. [DOI] [PubMed] [Google Scholar]
- 16.Cherla DV, Olavarria OA, Holihan JL, et al. Discordance of conflict of interest self-disclosure and the centers of Medicare and Medicaid services. J Surg Res 2017;218:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes TL. Author disclosure of conflict of interest in vascular surgery journals. J Vasc Surg 2011;54:55S–58S. [DOI] [PubMed] [Google Scholar]
- 18.Luce EA, Jackman CA. Disclosure of financial conflicts of interest in plastic and reconstructive surgery. Plast Reconstr Surg 2017;140:635–639. [DOI] [PubMed] [Google Scholar]
- 19.Olavarria OA, Holihan JL, Cherla D, et al. Comparison of conflicts of interest among published hernia researchers self-reported with the centers for Medicare and Medicaid services open payments database. J Am Coll Surg 2017;224:800–804. [DOI] [PubMed] [Google Scholar]
- 20.Open Payments Methodology Overview & Data Dictionary [online]. Available at: cms.gov/OpenPayments/Downloads/OpenPaymentsDataDictionary.pdf [Google Scholar]
- 21.SCImago. SJR: SCImago Journal & Country Rank [Portal] [online]. Available at: scimagojr.com. Accessed June. [Google Scholar]
- 22.Yue W, Wilson CS, Boller F. Peer assessment of journal quality in clinical neurology. J Med Libr Assoc 2007;95:70–76. [PMC free article] [PubMed] [Google Scholar]
- 23.Bernat JL, Swash M. Relationships between neurologists and industry. Neurology 2018;90:1047–1048. [DOI] [PubMed] [Google Scholar]
- 24.Sismondo S. Key opinion leaders and the corruption of medical knowledge: what the Sunshine Act will and won't cast light on. J L Med Ethics 2013;41:635–643. [DOI] [PubMed] [Google Scholar]
- 25.Saver RS. Deciphering the Sunshine Act: transparency regulation and financial conflicts in health care. Am J L Med 2017;43:303–343. [DOI] [PubMed] [Google Scholar]
- 26.Wayant C, Turner E, Meyer C, Sinnett P, Vassar M. Financial conflicts of interest among oncologist authors of reports of clinical drug trials. JAMA Oncol 2018;4:1426–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn J, Checketts JX, Jawhar O, Vassar M. Evaluation of industry relationships among authors of otolaryngology clinical practice guidelines. JAMA Otolaryngol Head Neck Surg 2018;144:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combs TR, Scott J, Jorski A, Heavener T, Vassar M. Evaluation of industry relationships among authors of clinical practice guidelines in gastroenterology. JAMA Intern Med 2018;178:1711–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreatos N, Zacharioudakis IM, Zervou FN, Muhammed M, Mylonakis E. Discrepancy between financial disclosures of authors of clinical practice guidelines and reports by industry. Medicine 2017;96:e5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osorio I. The NeuroPace trial: missing knowledge and insights. Epilepsia 2014;55:1469–1470. [DOI] [PubMed] [Google Scholar]
- 31.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer's disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis 2010;19:465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilstad JR, Finucane TE. Results, rhetoric, and randomized trials: the case of donepezil. J Am Geriatr Soc 2008;56:1556–1562. [DOI] [PubMed] [Google Scholar]
- 34.Koepp R, Miles SH. Meta-analysis of tacrine for Alzheimer disease: the influence of industry sponsors. JAMA 1999;281:2287–2288. [DOI] [PubMed] [Google Scholar]
- 35.Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med 2009;361:1963–1971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Payments from Industry are publicly accessible on the CMS Open Payments database (openpaymentsdata.cms.gov/). Our anonymized relevance data will be shared by request from any qualified investigator.