Abstract
Objective
To evaluate the effect of intensive rehabilitation on the modified Rankin Scale (mRS), a measure of activities limitation commonly used in acute stroke studies, and to define the specific changes in body structure/function (motor impairment) most related to mRS gains.
Methods
Patients were enrolled >90 days poststroke. Each was evaluated before and 30 days after a 6-week course of daily rehabilitation targeting the arm. Activity gains, measured using the mRS, were examined and compared to body structure/function gains, measured using the Fugl-Meyer (FM) motor scale. Additional analyses examined whether activity gains were more strongly related to specific body structure/function gains.
Results
At baseline (160 ± 48 days poststroke), patients (n = 77) had median mRS score of 3 (interquartile range, 2–3), decreasing to 2 [2–3] 30 days posttherapy (p < 0.0001). Similarly, the proportion of patients with mRS score ≤2 increased from 46.8% at baseline to 66.2% at 30 days posttherapy (p = 0.015). These findings were accounted for by the mRS score decreasing in 24 (31.2%) patients. Patients with a treatment-related mRS score improvement, compared to those without, had similar overall motor gains (change in total FM score, p = 0.63). In exploratory analysis, improvement in several specific motor impairments, such as finger flexion and wrist circumduction, was significantly associated with higher likelihood of mRS decrease.
Conclusions
Intensive arm motor therapy is associated with improved mRS in a substantial fraction (31.2%) of patients. Exploratory analysis suggests specific motor impairments that might underlie this finding and may be optimal targets for rehabilitation therapies that aim to reduce activities limitations.
Clinical Trial
Clinicaltrials.gov identifier: NCT02360488.
Classification of Evidence
This study provides Class III evidence that for patients >90 days poststroke with persistent arm motor deficits, intensive arm motor therapy improved mRS in a substantial fraction (31.2%) of patients.
US Food and Drug Administration (FDA) approval of a new therapy requires evidence for clinical benefit to the patient,1 using a clinical end point that measures how a patient feels, functions, or survives,2,3 where function is ability to perform daily activities.4 Trials of acute stroke therapies often assess benefit using the modified Rankin Scale (mRS), which covers functional outcomes,5 is categorized by the WHO International Classification of Function (ICF) as a measure of activities limitations6,7 (previously called “disability”8), and is accepted by the FDA as the primary end point for acute stroke trials.5 Indeed, the mRS is included with the definition of stroke disability codeveloped by the FDA.9
In contrast, trials of stroke recovery therapies often assess benefit using measures categorized as loss of body structure/function (previously called “impairment”8), such as the Fugl-Meyer (FM) motor scale, and often do not even measure the mRS.10–13 The relationship between improved body structure/function (e.g., FM scale) and gains in measures of activities limitations (e.g., mRS) is not always evident,6,14–17 but a better understanding of the relationship between these 2 dimensions could be useful to emerging stroke recovery therapeutics.18
This issue was addressed by examining results from a trial of intensive rehabilitation therapy targeting the arm that reported body structure/function gains (FM score),19 here hypothesizing that intensive rehabilitation therapy also improves activities limitations (mRS scores). To further probe this issue, mRS gains were explored in relation to improvements in body structure/function, both at the summary level (change in total FM score) and at the level of specific motor impairments.
Methods
Study Summary
This report provides a novel post hoc analysis of outcomes data from a prior 11-site, assessor-blind study,19 conducted in the NIH StrokeNet clinical trials network,20,21 which provided intensive arm motor therapy to 124 adult patients who had a stroke 4–36 weeks prior to enrollment and had persistence of arm motor deficits, a common22 and often devastating6,23,24 consequence of stroke.
Participants were serially evaluated over 1 week at baseline, then approximately 1 week later were randomized to receive 6 weeks of intensive therapy, either in the home using telerehabilitation or in the clinic, 70 min/d, 6 d/wk. The therapy approach was based on an upper-extremity task-specific training manual25 and the Accelerated Skill Acquisition Program.26 Therapy was supervised by a licensed occupational therapist or physical therapist, included arm exercises and functional training, and was matched in intensity, duration, and frequency across the 2 treatment groups. Therapists performing outcome assessments underwent online training and formal certification on the FM27 and mRS28 scales. Both patient groups had a stable motor examination over 1 week at baseline and showed clinically and statistically significant gains on the primary endpoint: change in FM score from baseline to 30 days posttreatment (7.9–8.4 points on this 66-point scale, for which the minimal clinically important difference is 5.25 points).29 Telerehabilitation was found to be noninferior compared to in-clinic therapy. Given that behavioral gains were near-identical across groups, data from the 2 groups are combined in the current analysis.
Standard Protocol Approvals, Registrations, and Patient Consents
Ethics approval was obtained from the local institutional review board and written informed consent was obtained from all enrollees. Data from this study are listed on Clinicaltrials.gov (NCT02360488).
Statistics
The purpose of the current analysis is to examine treatment effects on a secondary outcome measure, the mRS, and to determine whether patients who showed a treatment-related change in mRS differ from those who did not with respect to changes in body structure/function, both at the summary level (change in total FM score) and at the level of specific motor impairments (individual FM subscores). The population of interest consists of patients who were enrolled >90 days poststroke, a time when spontaneous motor recovery after stroke is largely complete,30–32 and so observed behavioral gains are more likely attributable to the intervention rather than spontaneous motor recovery.
Analyses were 2-tailed, used α = 0.05, did not adjust for covariates, and were calculated using JMP v13.2.0 (SAS, Cary, NC). To examine within-patient changes over time, paired t tests were used when data were normally distributed or could be transformed; otherwise, the Wilcoxon signed rank test was used; nonparametric statistics were used for analyses of FM scores. When comparing differences between those who had improvements on mRS and those who did not, the t test was used for continuous variables when data were normally distributed or could be transformed; otherwise, the Wilcoxon rank sum test was used. χ2 testing was used to compare categorical variables. Logistic regression was used when mRS score change status (changed or not) was the dependent variable and linear regression was used when the change in mRS score was the dependent variable. The de-identified publicly available National Institute of Neurological Disorders and Stroke (NINDS) tissue plasminogen activator (tPA) trial33 mRS outcome data were downloaded and evaluated for comparison with current findings.34
The FM motor score for the arm is the sum of 33 assessments of specific motor impairment, each scored as 0 (not done at all), 1 (partly done), or 2 (faultless performance),27,35 with higher values indicating better arm motor status. After examining mRS score change status in relation to change in the total FM score, mRS change status was then examined in relation to each of the 33 specific motor impairments assessed by the FM; no correction was made for multiple comparisons, as the goal was to identify which specific impairment improvements are most related to gains in global function from baseline to 30 days posttherapy.
Classification of Evidence
The 2 primary research questions were (1) What is the effect of intensive rehabilitation on the mRS, a measure of activities limitation? and (2) What are the specific changes in body structure/function (motor impairment) that are most related to mRS gains? This study provides level III evidence because these questions were addressed analyzing data from a controlled trial, where the control was an active comparator: in-home telerehabilitation vs in-clinic therapy, matched in intensity, duration, and frequency across the 2 randomly assigned treatment groups.
Data Availability
The complete de-identified study data, as well as the study protocol and statistical analysis plan, are available indefinitely through the NINDS Archived Clinical Research Datasets website.
Results
Patients
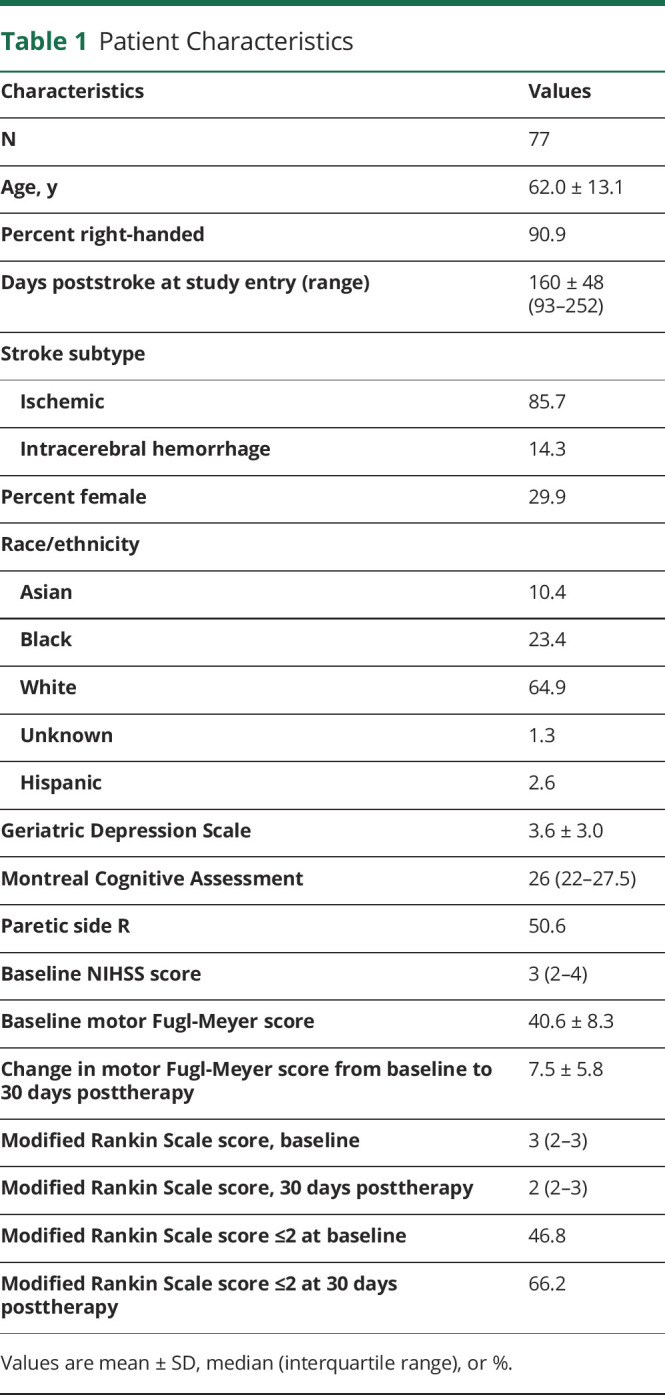
Of the 124 patients in the clinical trial of intensive rehabilitation therapy, 85 were enrolled >90 days (range 93–252) after stroke onset. Of these, 8 dropped out prior to measurement of mRS score change; the main reasons were the patient was lost to follow-up, was removed from the study by local physician, or withdrew consent. This left 77 patients who are the primary focus of this report (table 1) and who did not differ (p > 0.05) from the 8 who dropped out in age, days from stroke to randomization, baseline Geriatric Depression Scale score, Montreal Cognitive Assessment score, FM motor score, NIHSS score, or mRS score. These 77 patients were on average 5.3 months poststroke at study entry, mostly had stroke that was ischemic in origin, had mild cognitive and moderate motor deficits, and were compliant with 97.2% of the assigned therapy sessions. There were 3 serious adverse events, all unrelated to study procedures. Nonserious adverse events considered reasonably or definitely related to study procedures occurred in 9 patients and consisted of fatigue or arm/shoulder pain; none interrupted study therapy. Weight did not change from baseline to 30 days posttherapy (median change, 0 pounds, p = 0.75). Baseline FM score was 40.6 ± 8.3 points (mean ± SD) and increased by 7.5 ± 5.8 points at 30 days posttherapy (p < 0.0001).
Table 1.
Patient Characteristics
Does Intense Rehabilitation Therapy Improve Activities Limitations, Measured as a Decrease in mRS Score?
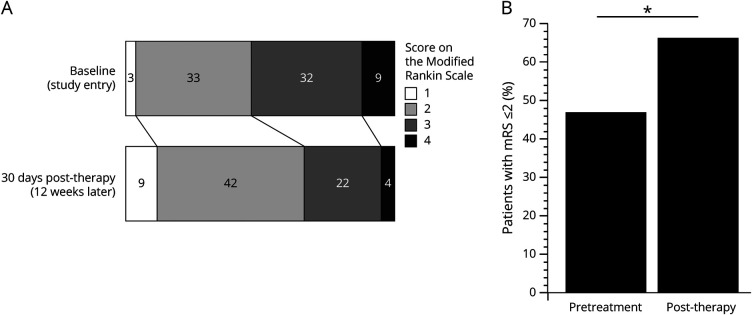
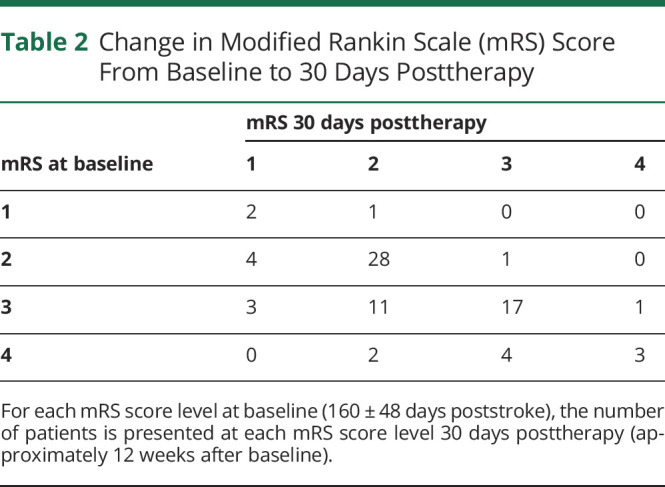
At baseline (160 ± 48 days poststroke), median mRS score was 3 (interquartile range, 2–3), with scores ranging from 1 to 4. At 30 days posttherapy (approximately 12 weeks postbaseline), median mRS decreased to 2 (2–3) (range 1–4), a significant change (p < 0.0001, figure 1A). Similarly, the proportion of patients with mRS score ≤2, a metric commonly used in acute stroke studies,5 increased from 46.8% at baseline to 66.2% at 30 days posttherapy (p = 0.015, figure 1B). These findings (table 2) are accounted for by 24 patients (31.2%) with an mRS score decrease (5 with a 2-point drop and 19 with a 1-point drop), 50 patients (64.9%) with no change, and 3 (3.9%) patients with an mRS score increase (all 1 point). The proportion of patients showing an mRS score decrease was similar (p = 0.07) in the telerehabilitation group (17/43, 39.5%) and the in-clinic therapy group (7/34, 20.6%). Also, the proportion of patients showing an mRS score decrease was similar (p = 0.73) in patients with ischemic stroke (20/66, 30.3%) compared to patients with intracerebral hemorrhage (4/11, 36.4%).
Figure 1. Intensive Arm Therapy Improves Modified Rankin Scale (mRS) Scores.
The number of patients at each mRS level is shown at baseline (at study entry, 160 ± 48 days poststroke) and 30 days after end of a 6-week course of intense rehabilitation therapy (approximately 12 weeks postbaseline). Intense rehabilitation therapy targeting the arm was associated with activities gains as measured by the mRS. (A) At baseline, median mRS score was 3 (2–3), decreasing to 2 (2–3) at 30 days posttherapy (p < 0.0001). This decrease reflects 24 patients (31.2%) with an mRS score decrease, 50 patients (64.9%) with no change, and 3 (3.9%) patients with an mRS score increase. (B) The proportion of patients with mRS score ≤2 increased from 46.8% at baseline to 66.2% at 30 days posttherapy (*p = 0.015).
Table 2.
Change in Modified Rankin Scale (mRS) Score From Baseline to 30 Days Posttherapy
How Do Patients Who Did Show Improvement in Activities Limitations (mRS) Differ From Those Who Did Not?
The 24 patients who had an mRS score decrease from baseline to 30 days posttherapy, compared with the 53 patients who did not, had higher baseline mRS score (3 [3–3.75] vs 2 [2–3], p = 0.0002). The 2 groups did not differ, however, in the number of study-provided treatment sessions (p = 0.70) or number of hours of any rehabilitation therapy received outside of study procedures (p = 0.92). In addition, the likelihood of an mRS score decrease was not related to the number of days poststroke at study entry (range odds ratio 1.49, 95% confidence interval 0.3–7.3, p = 0.62).
When patients with mRS gains, vs those without, were examined in relation to improvements in body structure/function, the 2 groups did not differ in the change in the total FM motor score over the same period (7.9 ± 6.4 vs 7.3 ± 5.6 points, p = 0.63). Furthermore, change in total FM score was not related to change in mRS score (p = 0.25) or to the likelihood of an mRS score decrease (p = 0.70).
Specific Motor Impairments That Improved in Relation to mRS Score Decrease
Although the change in total FM score change did not differ when comparing patients with mRS gains vs those without, there were nonetheless differences between these 2 groups in the specific motor impairments that improved over time.
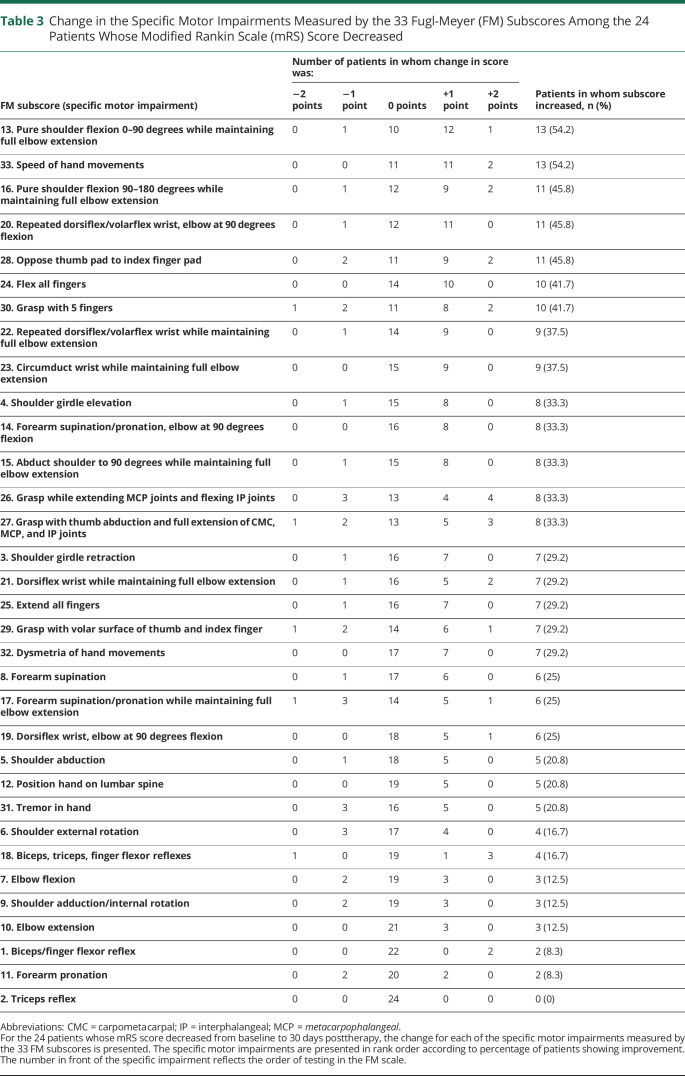
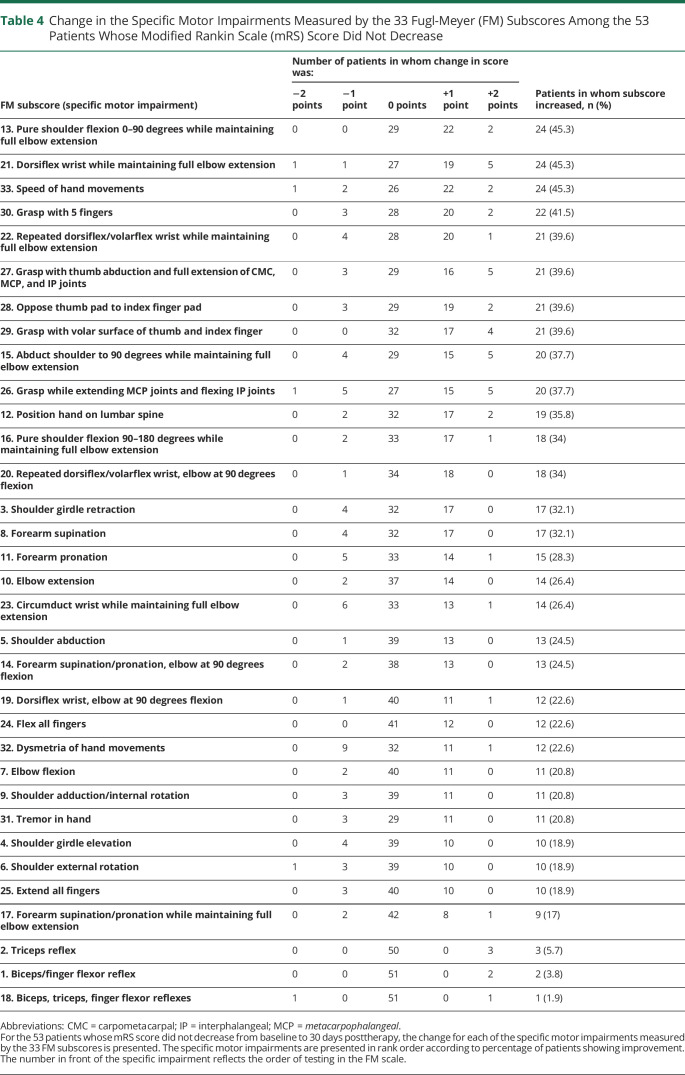
Change in the specific motor impairments measured by each of the 33 FM subscores is listed for the 24 patients who had an mRS score decrease from baseline to 30 days posttherapy (table 3) and for the 53 patients who did not (table 4). Some of these specific motor impairments improved in more than a third of patients, while others improved in fewer than 10% of patients. The motor impairments that improved most often were not the same in the 2 groups.
Table 3.
Change in the Specific Motor Impairments Measured by the 33 Fugl-Meyer (FM) Subscores Among the 24 Patients Whose Modified Rankin Scale (mRS) Score Decreased
Table 4.
Change in the Specific Motor Impairments Measured by the 33 Fugl-Meyer (FM) Subscores Among the 53 Patients Whose Modified Rankin Scale (mRS) Score Did Not Decrease
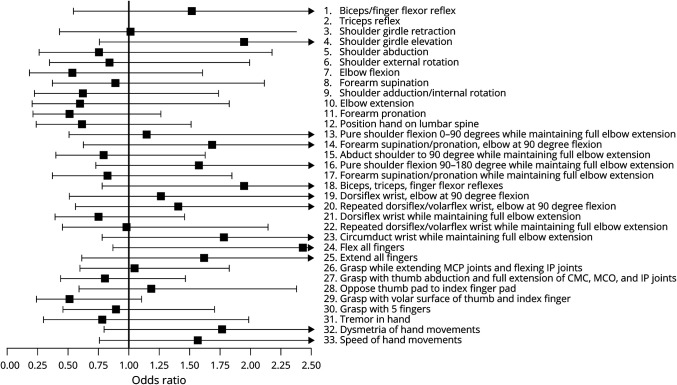
None of the 33 specific impairments measured by FM subscores improved to an extent that it could, as a single independent variable in bivariate analysis, predict the likelihood of mRS score decrease from baseline to 30 days posttherapy: figure 2 presents the odds ratio relating gains in the 33 specific motor impairments to likelihood of mRS improvement, and in each case, the 95% confidence interval crosses 1, indicating prediction of likelihood of mRS improvement was not significant. However, examining the 4 specific motor impairments with the highest odds ratio (figure 2) in a multiple logistic regression analysis did predict the likelihood of mRS improvement (p = 0.034). These 4 are (1) flexing all fingers at the metacarpophalangeal and interphalangeal joints, (2) full shoulder girdle elevation, (3) no reflex hyperactivity in the arm, and (4) circumduction of the wrist through full range of motion in a smooth manner.
Figure 2. Odds Ratio for Modified Rankin Scale (mRS) Score Improvement in Relation to Gains in Specific Motor Impairments.
The forest plot displays the odds ratio (95% confidence interval) for mRS decrease in relation to improvement in each of the specific motor impairments measured by the 33 FM subscores; no data are presented for the second subscore (triceps reflex) because calculation of the odds ratio was unstable due to 74 of 77 patients showing no change over time. CMC = carpometacarpal; IP = interphalangeal; MCP = metacarpophalangeal.
Discussion
Acute stroke trials often rely on the mRS, a measure of activities limitations that covers functional outcomes.5 However, stroke recovery trials typically do not measure the mRS10–13 and instead focus on measures capturing loss of body structure/function (such as the FM scale that covers motor impairments), which have an uncertain relationship to measure of activities limitations.6,14–17 The current report therefore examined change in mRS scores in patients receiving intense rehabilitation therapy targeting the arm >90 days poststroke, hypothesizing that significant improvements in mRS scores would be seen, and exploring how such improvements are related to improvements in body structure/function. Intense therapy was associated with a significant (p < 0.0001) drop in mRS score. In an exploratory analysis, these gains were explained in part by improvement in several specific motor impairments such as finger flexion and wrist circumduction. The results indicate that a 6-week course of intense rehabilitation can improve activities limitations (mRS score) in a substantial fraction of patients with stroke, and suggest that there may be specific motor impairments that underlie mRS improvement that may be evaluated as optimal targets for rehabilitation therapies aiming to improve function in hemiparetic patients.
In patients an average of 5.3 months poststroke, 6 weeks of intensive arm rehabilitation was associated with a decrease in median mRS score, from 3 to 2 (p < 0.0001; figure 1A), and an increase in the proportion of patients with final mRS score ≤2 from 46.8% to 66.2% (figure 1B). The absence of an inactive comparator group makes it difficult to know whether some component of the observed mRS change is due to spontaneous recovery rather than study-provided therapy. That the main driver of mRS gains was study therapy, rather than spontaneous recovery, is suggested by the absence of a relationship between likelihood of mRS gain and time poststroke (p = 0.62); a significant negative relationship might be expected if spontaneous recovery was a substantial contributor to mRS gains given that spontaneous recovery declines with time poststroke. Also, an mRS improvement in 31.2% of patients compares favorably with literature reports of patient trajectories during similar time periods: most data suggest that spontaneous functional improvement is uncommon after 3 months poststroke.32 For example, functional recovery was completed ≤12.5 weeks poststroke in 95% of Copenhagen Study enrollees31 and mRS score improved from day 90 to month 6 poststroke in only 18.0% of 471 surviving NINDS tPA study enrollees,34 although 25% of Oxford Vascular Study enrollees with 3-month mRS ≥1 showed mRS improvement by 1 year.36 Moreover, in the current study, only 3.9% of enrollees experienced mRS score worsening, which also compares favorably with literature reports: 11.5% declined in the NINDS tPA study,34 20.2% declined in the Oxford Vascular Study, and Ullberg et al.37 found that 16.3% of 28,683 patients who were activities of daily living–independent at 3 months deteriorated to activities of daily living dependency at 12 months poststroke. A patient-level meta-analysis of 1,822 patients38 found that from 3 to 12 months poststroke, the number of mRS improvements was counterbalanced with the number of mRS declines. Whether 6 weeks of intense rehabilitation therapy initiated >90 days poststroke contributes to functional improvement or prevents functional decline, or both, can be more clearly understood in future studies that include an inactive comparator group.
To understand the finding that mRS improved in 31.2% of patients, patients who showed a treatment-related change in mRS were contrasted with those who did not. Patients with, vs those without, an mRS score decrease from baseline to 30 days posttherapy did not differ in the change in total FM score over the same time period. In addition, change in total FM score was not related to change in mRS score or to the likelihood of an mRS score decrease. On the one hand, degree of weakness is a key influence on functional outcomes after stroke,39–41 with cross-sectional mRS scores showing a significant albeit incomplete relationship with motor status.23,42 On the other hand, a limited relationship exists across WHO ICF dimensions, such as loss of body function/structure (here measured using change in total FM score) and activities limitations (here measured using change in mRS score),6,14–17 although exceptions have been suggested.43 The absence of a relationship between change in total FM score and change in mRS score in the current study may reflect the fact that numerous factors have a greater influence on outcome as one moves from body function/structure to activity limitations,6 or that the FM captures numerous arm impairments and only some of these are relevant to functional gains as measured by the mRS.
The latter possibility was explored by comparing specific motor impairments between patients who showed a treatment-related change in mRS and patients who did not. Thus while change in total FM score was not related to likelihood of mRS score decrease, change in specific motor impairments did show a link to likelihood of mRS score decrease. Whereas no single specific motor impairment improved to an extent that significantly predicted likelihood of mRS improvement, combining the 4 strongest predictors did. Although based on an exploratory analysis, these findings suggest that certain specific motor impairments might be optimal targets of therapies that aim to improve the mRS in hemiparetic patients who are >90 days poststroke. For example, therapies that target finger flexion or wrist circumduction might be evaluated to see if they increase the likelihood of functional gains; lack of hyperactive deep tendon reflexes is a marker of a healthy nervous system and not likely a treatment target per se.
This report has several limitations. The sample size of 77 patients is modest. Evaluation of the 33 specific motor impairments from the FM scale did not correct for multiple comparisons and is an exploratory analysis. Across 11 US sites, only 29.9% of enrollees were female. Six of the patients with mRS improvement had a baseline mRS score of 4 (table 2), indicating inability to walk without assistance, and so the contribution of gait improvement is unclear for a therapy that targeted the arm.
Among patients >90 days poststroke, intensive arm rehabilitation was associated with mRS improvement in 31% of patients. In patients with, vs those without, a treatment-related mRS gain, the change in total FM score change was not different. However, patients with a treatment-related mRS gain were more likely to show improvement in specific motor impairments, such as finger flexion and wrist circumduction. This suggests that not all motor impairments have the same effect on functional outcome, and therefore improvement in motor impairments that have a stronger link to function could be targeted as a useful component of a broader rehabilitation therapy strategy. These findings emphasize the importance of understanding the relationship between changes in activities limitations and loss of body structure/function after stroke. This would be aided by including both types of measures in acute stroke trials and in stroke recovery trials, as has been recommended.44 Such knowledge would foster a more cohesive system for understanding the benefit of stroke therapeutics, from acute to recovery targets.
Glossary
- FDA
US Food and Drug Administration
- FM
Fugl-Meyer
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- NINDS
National Institute of Neurological Disorders and Stroke
- tPA
tissue plasminogen activator
Appendix. Authors
Footnotes
Editorial, page 643
Class of Evidence: NPub.org/coe
Study Funding
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) as well as NINDS (U01 NS091951), K24 HD074722, and T32 AR047752.
Disclosure
Dr. Cramer is a consultant for AbbVie, Constant Therapeutics, MicroTransponder, Neurolutions, SanBio, Fujifilm Toyama Chemical Co., and TRCare; V. Le, for TRCare. No other authors have any disclosures to report. Go to Neurology.org/N for full disclosures.
References
- 1.Katz R. FDA: evidentiary standards for drug development and approval. NeuroRx 2004;1:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA-NIH Biomarker Working Group. BEST (Biomarkers, Endpoints, and Other Tools). Silver Spring: Food and Drug Administration; 2016. Available at: ncbi.nlm.nih.gov/books/n/biomarkers/pdf/ [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Expedited Programs for Serious Conditions: Drugs and Biologics. 2017. Available at: fda.gov/media/86377/download. Accessed August 5, 2020. [Google Scholar]
- 4.Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med 2012;31:2973–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin Scale and its use in future stroke trials. Stroke 2017;48:2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98–e169. [DOI] [PubMed] [Google Scholar]
- 7.Shirley Ryan Ability Lab. Rehabilitation Measures Database. Available at: sralab.org/rehabilitation-measures/modified-rankin-handicap-scale. Accessed August 5, 2020. [Google Scholar]
- 8.Ustun TB, Chatterji S, Bickenbach J, Kostanjsek N, Schneider M. The International Classification of Functioning, Disability and Health: a new tool for understanding disability and health. Disabil Rehabil 2003;25:565–571. [DOI] [PubMed] [Google Scholar]
- 9.Hicks KA, Mahaffey KW, Mehran R, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018;137:961–972. [DOI] [PubMed] [Google Scholar]
- 10.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006;296:2095–2104. [DOI] [PubMed] [Google Scholar]
- 11.McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil 2015;96:981–990. [DOI] [PubMed] [Google Scholar]
- 12.Daly JJ, McCabe JP, Holcomb J, Monkiewicz M, Gansen J, Pundik S. Long-dose intensive therapy is necessary for strong, clinically significant, upper limb functional gains and retained gains in severe/moderate chronic stroke. Neurorehabil Neural Repair 2019;33:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry 2019;90:498–506. [DOI] [PubMed] [Google Scholar]
- 14.Burridge JH, Turk R, Notley SV, Pickering RM, Simpson DM. The relationship between upper limb activity and impairment in post-stroke hemiplegia. Disabil Rehabil 2009;31:109–117. [DOI] [PubMed] [Google Scholar]
- 15.Lemmens RJM, Timmermans AAA, Janssen-Potten YJM, Smeets RJEM, Seelen HAM. Valid and reliable instruments for arm-hand assessment at ICF activity level in persons with hemiplegia: a systematic review. BMC Neurol 2012;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewthwaite R, Winstein CJ, Lane CJ, et al. Accelerating stroke recovery: body structures and functions, activities, participation, and quality of life outcomes from a large rehabilitation trial. Neurorehabil Neural Repair 2018;32:150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth EJ, Heinemann AW, Lovell LL, Harvey RL, McGuire JR, Diaz S. Impairment and disability: their relation during stroke rehabilitation. Arch Phys Med Rehabil 1998;79:329–335. [DOI] [PubMed] [Google Scholar]
- 18.Lin DJ, Finklestein SP, Cramer SC. New directions in treatments targeting stroke recovery. Stroke 2018;49:3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cramer SC, Dodakian L, Le V, et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurol 2019;76:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broderick JP, Palesch YY, Janis LS; National Institutes of Health StrokeNet. The National Institutes of Health StrokeNet: a user's guide. Stroke 2016;47:301–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer SC, Wolf SL, Adams HP, Jr., et al. Stroke recovery and rehabilitation research: issues, opportunities, and the National Institutes of health StrokeNet. Stroke 2017;48:813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathore S, Hinn A, Cooper L, Tyroler H, Rosamond W. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke 2002;33:2718–2721. [DOI] [PubMed] [Google Scholar]
- 23.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke 2013;44:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyller TB, Sveen U, Sodring KM, Pettersen AM, Bautz-Holter E. Subjective well-being one year after stroke. Clin Rehabil 1997;11:139–145. [DOI] [PubMed] [Google Scholar]
- 25.Lang C, Birkenmeier R. Upper-Extremity Task-specific Training after Stroke or Disability. Bethesda: AOTA Press; 2013. [Google Scholar]
- 26.Winstein CJ, Wolf SL, Dromerick AW, et al. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA 2016;315:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair 2013;27:732–741. [DOI] [PubMed] [Google Scholar]
- 28.Saver JL, Filip B, Hamilton S, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke 2010;41:992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 2012;92:791–798. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama H, Jorgensen H, Raaschou H, Olsen T. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 1994;75:394–398. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen H, Nakayama H, Raaschou H, Vive-Larsen J, Stoier M, Olsen T. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil 1995;76:406–412. [DOI] [PubMed] [Google Scholar]
- 32.Rejno A, Nasic S, Bjalkefur K, Bertholds E, Jood K. Changes in functional outcome over five years after stroke. Brain Behav 2019;9:e01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 34.National Institute of Neurological Disorders and Stroke. Archived clinical research datasets. Available at: ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets. Accessed August 5, 2020.
- 35.Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 36.Ganesh A, Luengo-Fernandez R, Wharton RM, et al. Time course of evolution of disability and cause-specific mortality after ischemic stroke: implications for trial design. J Am Heart Assoc 2017;6:e005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ullberg T, Zia E, Petersson J, Norrving B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke 2015;46:389–394. [DOI] [PubMed] [Google Scholar]
- 38.de Havenon A, Tirschwell D, Heitsch L, et al. Variability of the modified Rankin Scale score between day 90 and 1 year after ischemic stroke. Neurol Clin Pract Epub 2020 Sep 11. [DOI] [PMC free article] [PubMed]
- 39.Kwakkel G, Wagenaar RC, Kollen BJ, Lankhorst GJ. Predicting disability in stroke: a critical review of the literature. Age Ageing 1996;25:479–489. [DOI] [PubMed] [Google Scholar]
- 40.Shelton F, Volpe B, Reding M. Motor impairment as a predictor of functional recovery and guide to rehabilitation treatment after stroke. Neurorehabil Neural Repair 2001;15:229–237. [DOI] [PubMed] [Google Scholar]
- 41.Weimar C, Ziegler A, Konig IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. J Neurol 2002;249:888–895. [DOI] [PubMed] [Google Scholar]
- 42.Kongsawasdi S, Klaphajone J, Wivatvongvana P, Watcharasaksilp K. Prognostic factors of functional outcome assessed by using the modified Rankin scale in subacute ischemic stroke. J Clin Med Res 2019;11:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 2018;9:CD006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair 2017;31:784–792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete de-identified study data, as well as the study protocol and statistical analysis plan, are available indefinitely through the NINDS Archived Clinical Research Datasets website.