Abstract
BACKGROUND & AIMS:
Obese patients with nonalcoholic steatohepatitis (NASH) are at risk for cirrhosis if significant weight loss is not achieved. The single fluid-filled intragastric balloon (IGB) induces meaningful weight loss and might be used in NASH treatment. We performed an open-label prospective study to evaluate the effects of IGB placement on metabolic and histologic features of NASH.
METHODS:
Twenty-one patients with early hepatic fibrosis (81% female; mean age, 54 years; average body mass index, 44 kg/m2) underwent magnetic resonance elastography (MRE) and endoscopic ultrasound with core liver biopsy collection at time IGB placement and removal at a single center from October 2016 through March 2018. The primary outcome measure was the changes in liver histology parameters after IGB, including change in nonalcoholic fatty liver disease activity score (NAS) and fibrosis score. We also evaluated changes in weight, body mass index, waist to hip ratio, aminotransaminases, fasting levels of lipids, fasting glucose, glycosylated hemoglobin, and MRE-detected liver stiffness.
RESULTS:
Six months after IGB, patients’ mean total body weight loss was 11.7% ± 7.7%, with significant reductions in HbA1c (1.3% ± 0.5%) (P = .02). Waist circumference decreased by 14.4 ± 2.2 cm (P = .001). NAS improved in 18 of 20 patients (90%), with a median decrease of 3 points (range, 1–4 points); 16 of 20 patients (80%) had improvements of 2 points or more. Fibrosis improved by 1.17 stages in 15% of patients, and MRE-detected fibrosis improved by 1.5 stages in 10 of 20 patients (50%). Half of patients reached endpoints approved by the Food and Drug Administration of for NASH resolution and fibrosis improvement. Percent total body weight loss did not correlate with reductions in NAS or fibrosis. Other than post-procedural pain (in 5% of patients), no serious adverse events were reported.
CONCLUSION:
In a prospective study, IGB facilitated significant metabolic and histologic improvements in NASH. IGB appears to be safe and effective for NASH management when combined with a prescribed diet and exercise program. ClinicalTrials.gov no: NCT02880189
Keywords: Bariatric Endoscopy, Obesity, Nonalcoholic fatty liver disease, NAFLD, Diabetes
As obesity soars to unprecedented heights, afflicting almost every other adult and nearly one-fifth of children,1 its associated comorbid conditions, including nonalcoholic fatty liver disease (NAFLD), rise unabated, striking 33% of Western adult population, including 70% and 50% of obese adults and children, respectively.2
On the one hand, lifestyle changes and dietary interventions, which are considered the backbone of NAFLD management, rarely permit ≥10% total body weight loss (TBWL), which constitutes the threshold for meaningful improvement in NASH.3 On the other hand, bariatric surgery, the most effective therapy for obesity, is not appropriate for those with mild-to-moderate obesity, nor is it a desired intervention for the majority of patients who qualify for it.4 Further, concern exists if NAFLD has resulted in advanced liver disease, in which major weight loss achieved by bariatric surgery may induce further destabilizing metabolic perturbations, such as sarcopenia, or lead to decompensated liver disease.
In this context, endoscopic bariatric therapies are garnering more attention as potential strategies to address these shortcomings in obesity care and its comorbidities5; however, their influences on the driving and prognostic parameters of NAFLD remain unclear.6
To address some of these gaps, we prospectively evaluated the most effective and widely used intragastric balloon (IGB), the single fluid-filled IGB,7 to determine its impact on metabolic and histologic parameters of NAFLD through the gold standard of liver biopsy, as well as noninvasive biochemical and magnetic resonance-based evaluations.
Materials and Methods
A single-center, prospective, open-label Institutional Review Board (15–009262) and Food and Drug Administration (FDA) investigational device exemption (G160077) approved single-blinded study was conducted at a tertiary care center from October 2016 to March 2018.
Inclusion criteria included men and women between 18 and 65 years of age, with obesity (defined as a body mass index [BMI] >30 kg/m2) and radiologically proven hepatic steatosis and early fibrosis (mean stiffness <5 kPa by magnetic resonance elastography [MRE]). Patients must have failed conservative and lifestyle interventions to accomplish weight loss for at least 6 months before participating in the study.
Exclusion criteria are relayed in the Supplementary Materials.
Once enrolled, patients underwent paired endoscopic ultrasound (EUS)–guided core biopsy of the liver, and IGB placement using the single fluid-filled Orbera balloon system (Apollo Endosurgery, Austin TX). Upon IGB removal at 6 months, repeat core biopsy and MRE were conducted (Supplementary Figure 1). The NASH Clinical Research Network (CRN) method was applied to stage and grade all liver tissue samples (Supplementary Table 1).
The Supplementary Materials and Methods relays details regarding IGB placement, sojourn, and removal, MRE technique, EUS liver biopsy technique, sample processing details, and EUS liver biopsy histological evaluation details.
Study Outcomes and Statistical Methods
The primary outcome measure was liver histology change after the intervention, including a change in NAFLD Activity Score (NAS) and fibrosis score. An evaluation of changes in weight, BMI, waist-to-hip ratio, aminotransaminases, fasting levels of lipids, fasting glucose, hemoglobin A1C (HbA1c), and MRE-detected liver stiffness was done before and after treatment (Supplementary Materials).
Results
A total of 21 consecutive patients were prospectively enrolled, with 41 core biopsies performed. One patient did not receive an exit liver biopsy due to the initiation of antithrombotics during the study period. No patients were lost to follow-up during the study period. Baseline characteristics of age; sex; BMI; waist-to-hip ratio; proportions of diabetes mellitus, hypertension, and dyslipidemia; mean alanine aminotransferase and aspartate aminotransferase levels; aspartate aminotransferase-to-platelet ratio index; steatosis, ballooning, and inflammation scores; NAS; and fibrosis scores are shown in Table 1 and Figures 1 and 2.
Table 1.
Baseline Characteristics
| Total enrolled patients | 21 |
| Total placement biopsies | 21 |
| Total exit biopsies | 20 |
| Total liver biopsies | 41 |
| Age, y | 54 (34–65) |
| Female | 17/21 (81) |
| BMI, kg/m2 | 44 (32–55) |
| Obesity class | |
| 1 | 2/21 (10) |
| 2 | 6/21 (28) |
| 3 | 13/21 (62) |
| Waist, cm | 129 (106–155) |
| Hip, cm | 131 (107–174) |
| Waist-to-hip ratio | 0.96 (0.81–1.26) |
| Diabetes | 11/21 (52) |
| Impaired glucose tolerance | 6/21 (29) |
| Hypertension, % | 57 |
| Dyslipidemia, % | 71 |
| Fasting glucose, mg/dL | 116 (86–245) |
| Hemoglobin A1c, % | 7.4 (5.7–11.1) |
| Cholesterol, mg/dL | 198 (109–268) |
| LDL, mg/dL | 121 (45–220) |
| HDL, mg/dL | 46 (30–61) |
| Triglycerides, mg/dL | 159 (67–241) |
| AST, IU/L | 55 (14–228) |
| ALT, IU/L | 91 (22–255) |
| APRI | 0.87 (0.2–5.2) |
| Steatosis | 1 (0–2) |
| 0 | 1/21 (5) |
| 1 | 14/21 (67) |
| 2 | 6/21 (28) |
| Ballooning | 1 (1–2) |
| 0 | None |
| 1 | 14/21 (67) |
| 2 | 7/21 (33) |
| Inflammation | 1 (1–2) |
| 0 | None |
| 1 | 17/21 (81) |
| 2 | 4/21 (19) |
| NAS | 4 (2–5) |
| 0 | None |
| 1 | None |
| 2 | 1/21 (5) |
| 3 | 9/21 (43) |
| 4 | 5/21 (24) |
| 5 | 6/21 (28) |
| Fibrosis score | 2 (1.25–3) |
| 1A | 6/21 (29) |
| 1B | 1/21 (5) |
| 1C | 2/21 (10) |
| 2 | 7/21 (32) |
| 3 | 5/21 (24) |
Values are n, n/n (%), or median (range).
ALT, alanine aminotransferase; APRI, aspartate aminotransferase-to-platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MRE, magnetic resonance elastography; NAS, NAFLD Activity Score.
Figure 1.

Change in NAS and fibrosis pre and post IGB therapy.
Figure 2.
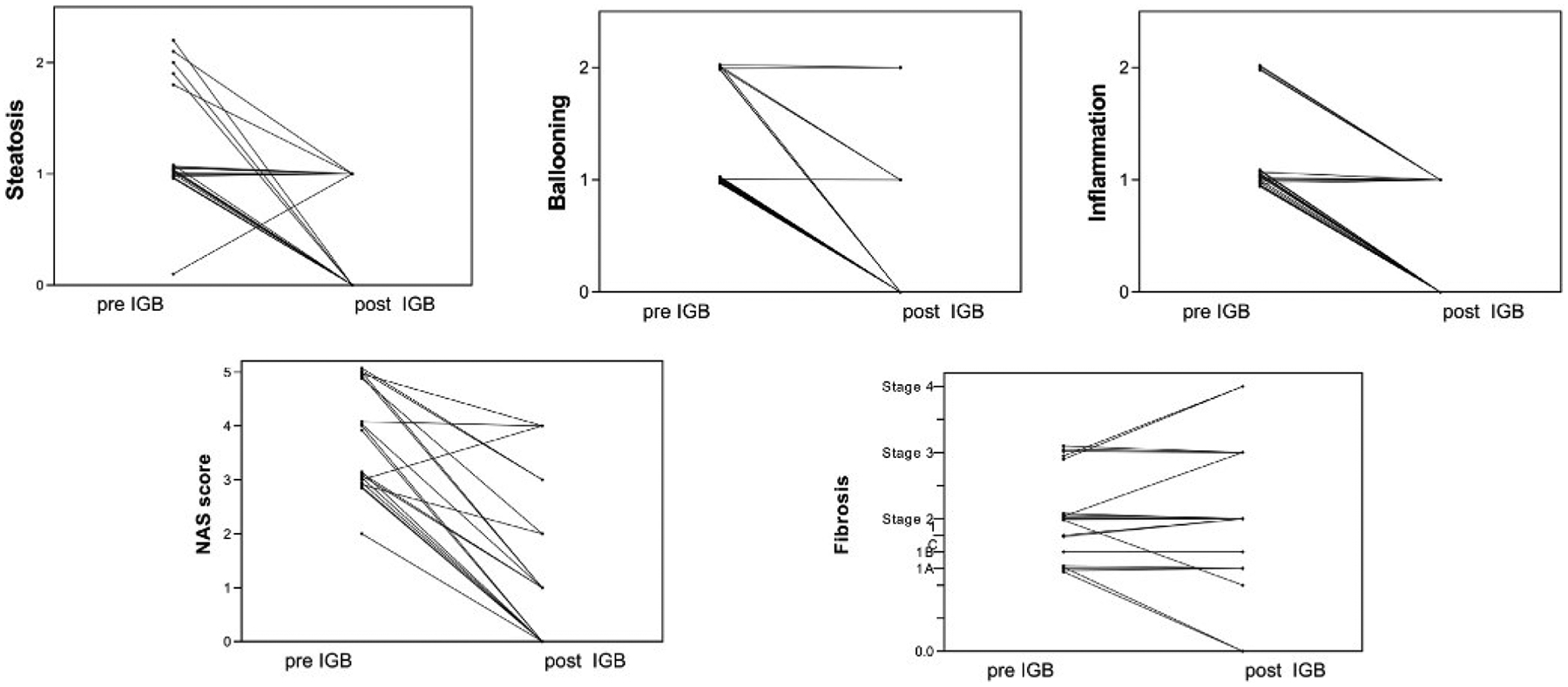
Individual patient change in NAS and fibrosis pre- and post-IGB therapy.
Baseline NAS and Fibrosis Scores
Preintervention liver biopsies revealed NAS as shown in Table 1 and Figures 1 and 2. All patients demonstrated histologic fibrosis, and none were cirrhotic (Supplementary Table 2 and Figures 1 and 2).
Figure 3 shows baseline MRE. In the total cohort (pre- and postintervention), when compared with MRE, the EUS-guided core biopsy technique identified 17 of 41 samples as harboring fibrosis, while being described as normal or as mildly elevated liver stiffness on MRE. In the preintervention cohort, 9 of 21 samples described as normal or mildly elevated liver stiffness on MRE harbored fibrosis on liver biopsy.
Figure 3.
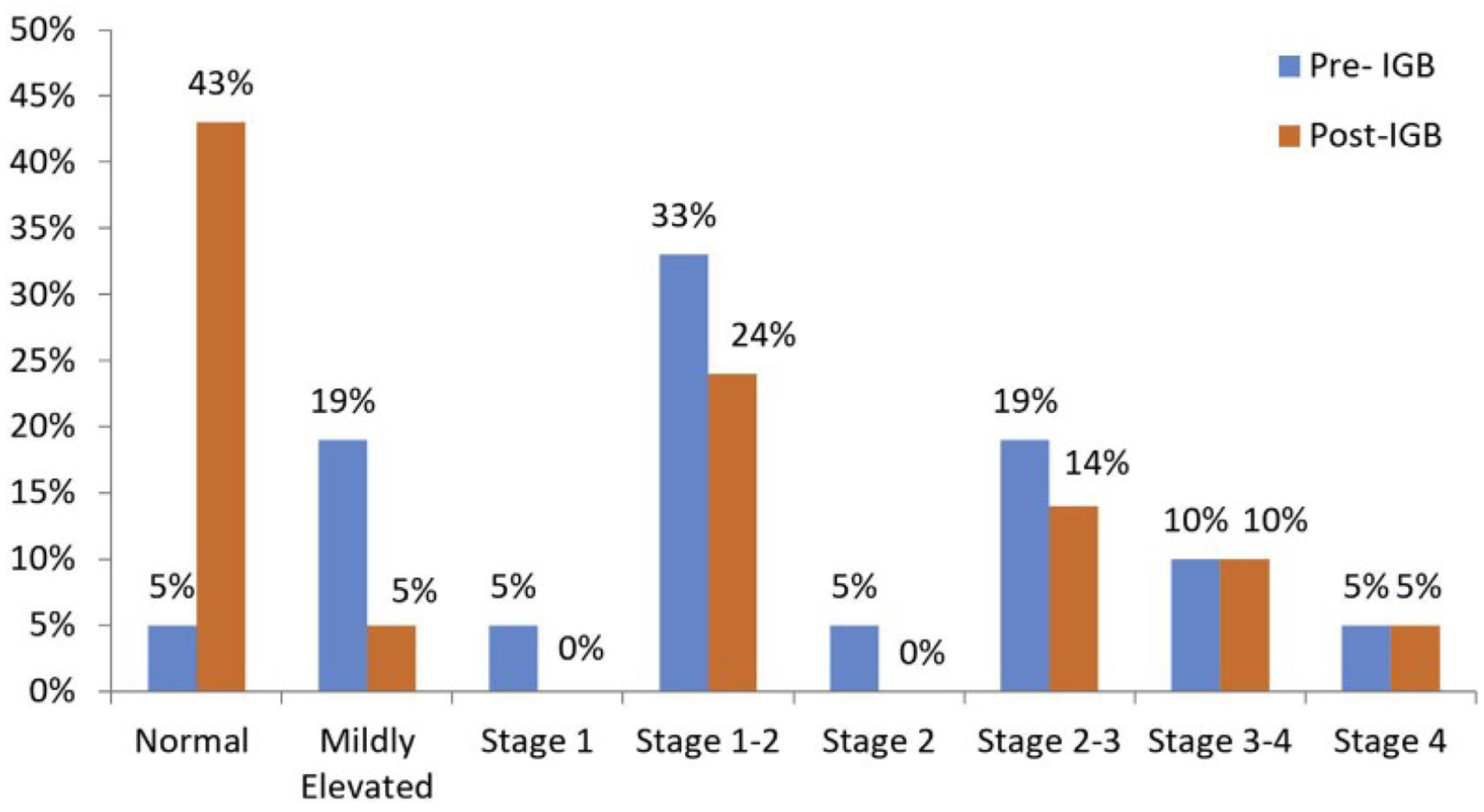
Pre- and post-IGB MRE fibrosis stage.
Postintervention Changes
At the time of balloon removal, there was a significant decrease in BMI, weight, waist circumference, hip circumference, HbA1c, fasting glucose, aspartate aminotransferase, alanine aminotransferase, aspartate aminotransferase-to-platelet ratio index, NAS, and MRE findings. There was no difference in total cholesterol or low-density lipoprotein postintervention (Table 2 and Figure 4). Furthermore, there was a significant decrease in NAS and fibrosis stage after the intervention (Figures 1 and 2). NAS improved from a median of 4 (range, 2–5) points at baseline vs postintervention at 1 (range, 0–4) point (P < .001) (Supplementary Table 3 and Figures 1 and 2). Seventy-three percent of patients achieved ≥2-point improvement in NAS. Individual NAS components—steatosis, hepatocellular ballooning, and lobular inflammation—are relayed in Supplementary Table 3.
Table 2.
Changes in Anthropomorphic Parameters, Laboratory Values, and NAS
| Variable | Pre-IGB | Post-IGB | Mean Difference | P Value |
|---|---|---|---|---|
| BMI, kg/m2 | 43.2 ± 6.8 | 37.9 ± 6.6 | −5.2 ± 0.75 | .01 |
| Weight, kg | 122.3 ± 26.4 | 107.9 ± 24.9 | −14.4 ± 7.9 | .01 |
| Waist, cm | 128.9 ± 15.4 | 119.7 ± 16.9 | −14.4 ± 2.2 | .001 |
| Hip, cm | 132.9 ± 16.2 | 124.7 ± 16.7 | −8.3 ± 1.6 | .001 |
| Waist to hip | 0.97 ± 0.1 | 0.96 ± 0.06 | −0.01 ± 0.01 | .37 |
| Hemoglobin A1c, % | 7.7 ± 1.6 | 6.5 ± 1.2 | −1.2 ± 0.5 | .02 |
| Fasting glucose, mg/dL | 150.4 ± 49.6 | 127.5 ± 37 | −22.9 ± 8.7 | .01 |
| Cholesterol, mg/dL | 200.8 ± 45.3 | 193.1 ± 46.7 | −7.7 ± 7.7 | .33 |
| LDL, mg/dL | 126.6 ± 45.1 | 115.1 ± 43.9 | −11.5 ± 6.72 | .11 |
| AST, IU/L | 67.5 ± 48.8 | 31.32 ± 20 | −36.2 ± 9.8 | .002 |
| ALT, IU/L | 91.6 ± 59.9 | 39.4 ± 25.4 | −52 ± 17.3 | .01 |
| APRI | 1.35 ± 1.38 | 0.62 ± 0.49 | −0.73 ± 1.03 | .005 |
| NAS | 4 (2–5) | 1 (0–4) | N/A | <.001a |
| MRE, kPa | 3.52 ± 1.1 | 3.2 ± 1.1 | −0.3 ± 0.16 | .03 |
Values are mean ± SD or median (range).
ALT, alanine aminotransferase; APRI, aspartate aminotransferase-to-platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; IGB, intragastric balloon; LDL, low-density lipoprotein; MRE, magnetic resonance elastography; N/A, not applicable; NAS, NAFLD Activity Score.
Wilcoxon rank sum test.
Figure 4.
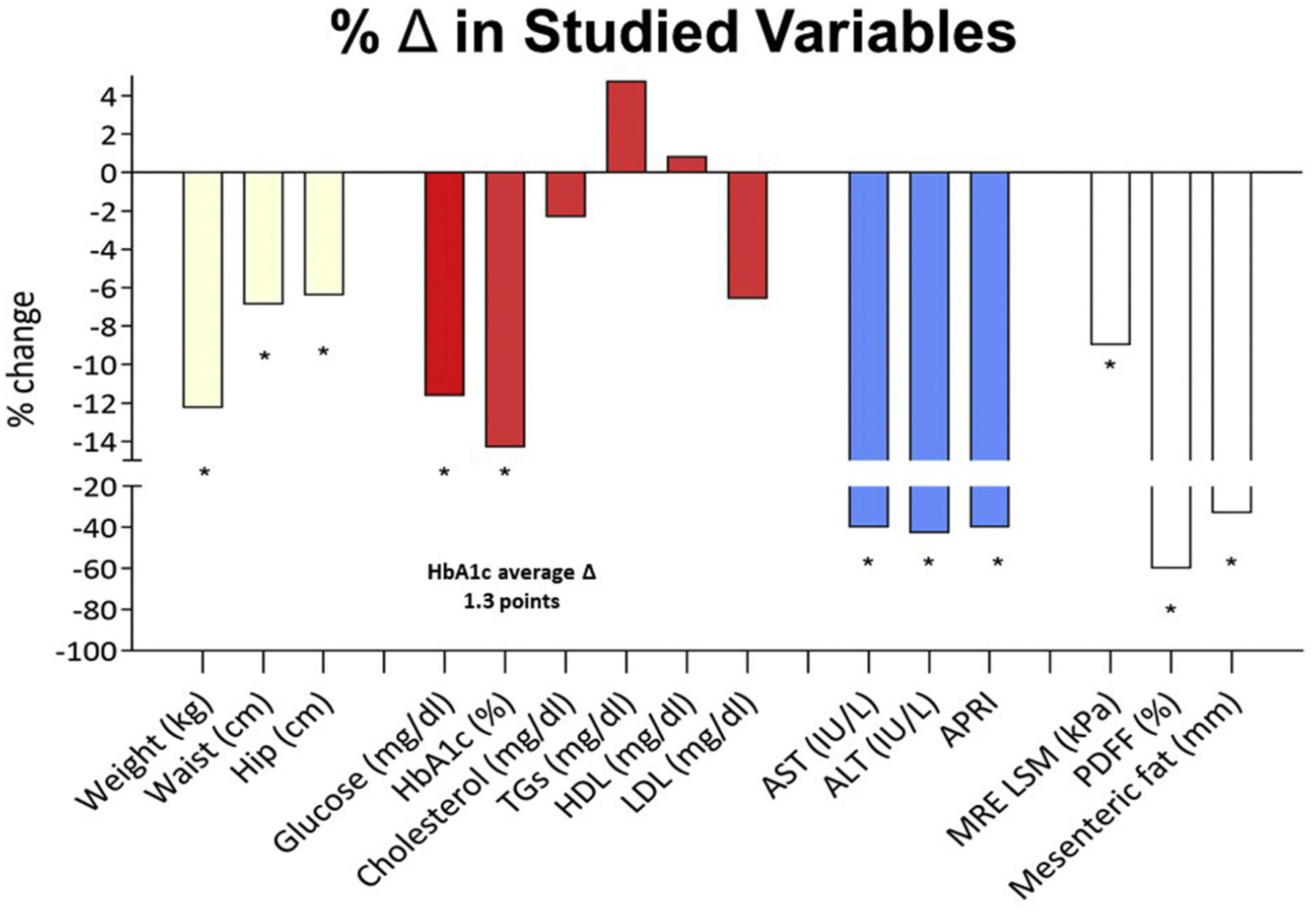
Changes in anthropomorphic parameters, laboratory values, and NAS. ALT, alanine aminotransferase; AST, aspartate aminotransferase; LSM, liver stiffness measurement; PDFF, proton density fat-fraction; TGs, triglycerides.
Postintervention, histologic fibrosis improved in 3 of 20 subjects (2 patients improved from stage 1A to become free of fibrosis, and 1 patient from stage 2 to stage 1A). Histologic fibrosis unchanged in 12 of 20 subjects. Fibrosis stage worsened in 5 of 20 subjects (Figure 2): by 1 stage in 3 of 20 subjects and by 0.25 stage in 2 of 20 subjects. Two patients progressed from stage 1C to stage 2 fibrosis, 1 patient progressed from stage 2 to stage 3 fibrosis, and 2 progressed from stage 3 to stage 4 fibrosis (n = 5).
Among patients who experienced worsening of 1 fibrosis stage, 2 had suboptimal weight loss response (0% and 7% TBWL), and 1 had an exaggerated weight loss response, relative to the study period (20% TBWL, and who progressed from stage 3 to stage 4).
Interestingly, MRE worsening was seen in the extreme weight loss patients, but it showed improvement form stage 3 to normal in the patient who did not experience any %TBWL (this was in tandem of improvement in NAS from 3 to 0).
The FDA has defined endpoints for NASH resolution and fibrosis improvement in pharmacologic therapy trials.8 Resolution of steatohepatitis is defined as lack of fatty liver disease or isolated or simple steatosis without steatohepatitis and a NAS score of 0–1 point for inflammation and 0 points for ballooning, and any degree for steatosis and no progression of hepatic fibrosis on NASH CRN fibrosis score. Improvement in liver fibrosis ≥1 stage (NASH CRN fibrosis score) and no worsening of steatohepatitis (defined as no increase in NAS for ballooning, inflammation, or steatosis). By applying these criteria, 50% (10 of 20) of patients reached both FDA optimal endpoints at 6 months.
The mean %TBWL in those whose fibrosis progressed during the study was 8.2% TBWL compared with 12.5% TBWL in those whose fibrosis did not progress (P = .27).
By MRE, after IGB removal, 42% of patients had normal liver stiffness, 5% of patients with mildly elevated liver stiffness, 24% of patients had stage 1–2 fibrosis, 14% patients had stage 2–3 fibrosis, 10% of patients had stage 3–4 fibrosis, and 5% of patients had stage 4 fibrosis (Figure 3).
Of note, the MRE values were classified as normal or mildly elevated liver stiffness in 19 of 41 instances, 17 of which had corresponding liver biopsies that harbored fibrosis. Four of the 5 subjects whose fibrosis progressed were classified as normal (3 of 5 patients) or as unchanged stage 1–2 fibrosis (1 of 5 patients), based on MRE.
Predictors of NAS and Fibrosis Improvement
This is relayed in the Supplementary Materials. Importantly, baseline weight or BMI and changes in weight (%TBWL) or HbA1c were not associated with improvement in NAS, but the reduction in waist circumference was correlated with it (β = 0.12, P = .001).
Discussion
This is the first prospective study to show not only NAS improvement, but also fibrosis regression in patients treated with IGB therapy. The use of the IGB with a prescribed diet and exercise program accomplished the expected weight loss over 6 months, similar to prior reports.9 Intriguingly, this significant weight loss did not correlate with the improvement in NAS and fibrosis score. The weight loss did, however, translate into a reduction in waist circumference, hip circumference, aminotransferases, and HbA1c, which collectively resulted in impactful amelioration in NASH liver histology parameters. It is physiologically plausible to extrapolate that this improvement is predominately a consequence of the robust improvement in insulin resistance and glucose metabolism.
The IGB has dual weight-dependent and weight-independent mechanisms that confer its metabolic benefit and result in significant improvement in the fibroinflammatory consequences of liver steatosis.10 The weight-dependent pathways lead to an improvement in insulin action at the level of peripheral organs, such as skeletal muscle. Weight-independent pathways include downregulation of the orexigenic gastric hormone (ghrelin),11 a delay in gastric emptying, reducing post-prandial hyperglycemia, and improved SIRT-1 action.12
Based on the published literature, weight loss >10% TBWL is the threshold beyond which NASH resolves and fibrosis regresses in a significant percentage of patients.13 This threshold is only reached in the minority of patients subjected to high-intensity lifestyle intervention.14 Interestingly, additional weight loss beyond this threshold may not confer a further benefit for fibrosis as measured by surrogate markers: for instance, in one study, although significant improvements of %TBWL postoperatively was observed at 1 year (28.9 ± 7.7% TBWL), and although liver stiffness, a surrogate of liver fibrosis, improved significantly (12.9 ± 10.4 kPa to 7.1 ± 3.7 kPa; P < .001) at 1 year, no correlation was observed between pre- and postoperative changes of liver stiffness and changes in %TBWL.15
In our study, significant improvement in NASH histology occurred in the context of IGB, consistent with that seen with bariatric surgery. Second, both NASH resolution rates and fibrosis regression reported in our study surpassed those seen with investigated NASH pharmacotherapies, including vitamin E, pioglitazone, liraglutide,16 and liver-directed agents such as obeticholic acid, elafibranor, and cenicriviroc.13,17,18
By applying the FDA criteria for NASH pharmacological therapy endpoints, 50% of patients reached both FDA optimal endpoints at 6 months. To put these numbers in perspective, obeticholic acid was able to reach NASH resolution and fibrosis improvement endpoint in only 12% and 23% of patients at 18 months, respectively.19
Given the lack of approved NASH pharmacotherapies and their limited efficacy, using the IGB in this cohort represents an important clinical advancement that bridges the therapeutic gap between medical and surgical therapies. It should be noted that fibrosis worsened by more than 1 stage in 3 patients. Two of these patients had suboptimal weight loss (<10% TBWL), and 1 of these patients had exaggerated weight loss (20% TBWL) and had stage 3 fibrosis at baseline. It is possible that the suboptimal and exaggerated response in these patients accounted for this observed worsening of fibrosis. However, at least in 1 patient, the histology results were not congruent with the MRE results that showed improvement in fibrosis from stage 3 to normal in tandem with NAS improvement on histology. Although the limited sample in our cohort precludes full understanding of these patterns, progression in fibrosis observed in some patients on histology could be secondary to a sampling error of a patchy disease, such as NAFLD.20,21 Future use of the IGB to achieve NASH resolution followed by the addition of pharmacotherapeutic agents, such as liraglutide, that have a synergistic effect for long-term maintenance may represent a paradigm shift that can significantly impact the management of NAFLD within the framework of a chronic disease model. IGB therapy cannot be viewed in isolation, but rather as part of a comprehensive multidisciplinary program that includes at least moderate-intensity lifestyle interventions with adjunct therapeutics to maintain a durable response and appropriately manage a chronic disease such as NALFD in the context of excess adiposity. In a large postmarketing U.S. study of IGB, weight loss was 13.3 ± 10% at 3 months after balloon removal.22 In addition, dietary interventions, antiobesity pharmacotherapies, sequential IGBs, and subsequent bariatric surgery have been shown to be successful weight maintenance strategies after initial IGB removal.6 The IGB alone without additional interventions is likely incapable of maintaining remission of obesity and NASH in the long term. The paradigm shift in this proof-of-concept trial is that the IGB resulted in remission of the disease (NAS <2) as determined by gold-standard histology in most patients. The durability of response is not investigated in this trial; however, strategies that combines the use of IGB to induce remission followed by pharmacotherapies, such as liraglutide, to maintain remission should be studied in the future and have shown early promise clinically. A recent study suggested the weight loss advantage was still significantly higher 1 year after balloon implantation (and 6 months after IGB removal), when liraglutide was started 1 month after IGB implant and maintained only for 1 month after balloon removal, and not during the entire follow-up.23
This meaningful improvement in critical NAFLD parameters, conjugated with the novel modality by which histologic information was obtained (ie, EUS-guided core biopsy), opens new horizons for the role of endoscopy to enhance the management of patients with NAFLD.
In this paradigm, patients not only receive an impactful therapy for both the triggering disease (obesity) and the resultant disease (NAFLD), but also are additionally permitted, during the same session, to undergo minimally invasive risk stratification by EUS assessment of visceral adiposity (data not shown),24,25 or through demonstrably safe tissue sampling by EUS-guided core biopsy technique on which next-generation lipidomics and metabolomics testing can be pursued.26 This opportunity for safe, reliable, and simultaneous liver sampling and IGB placement is powerful, given that the present noninvasive diagnostic and risk-stratification landscape includes several tests (eg, NAFLD fibrosis score, Fibrosis-4 index, Enhanced Liver Fibrosis test, FibroTest/FibroSure) that, while validated, have a lower sensitivity and specificity for earlier stages of fibrosis than for advanced fibrosis.27 This is also a limitation of radiology-based testing.26
The multifaceted and parsimonious approach to NAFLD diagnosis and risk stratification allowed by an endoscopic paradigm may provide an opportunity to overcome these challenges and limitations. The “metabolic endoscopic bariatric therapy bundle” introduced in this study further provides means of closing the widening NAFLD and obesity management gaps between less effective pharmacologic or behavioral strategies and effective but invasive bariatric surgical procedures.
From a patient perspective, it has been shown that motivation is a major limiting factor in NAFLD patients, who may not perceive their condition as a life-threatening disease, which reflects on their efforts to lose weight.28 Accurate risk stratification may help such patients gain motivation by witnessing the end-organ damage that has already taken place.
The lack of correlation with %TBWL we observed in the present study was also described in other studies, whether in terms of correlation with liver stiffness measurement change29 or diabetes mellitus improvement.30 This observation suggests that the amount of weight loss may be a poor surrogate and predictor for NASH Improvement, which may instead be induced by the metabolic changes associated with weight loss. It is possible that other factors might be in play, such as visceral fat changes, as was suggested by our findings.25
Importantly, we show that fibrosis exists even in those patients who may not have evidence of its presence on noninvasive MR-based testing, and this has implications for early detection and risk stratification. In another study probing the specifics of EUS-guided core biopsy in this cohort of patients, we have shown that MRE classified 32% of included samples as not harboring liver fibrosis, although biopsy later demonstrated the presence of fibrosis.26
These data are congruent with another multicenter international study examining apparently healthy individuals, in which we have shown that certain factors significantly increased LSM (eg, diabetes, waist circumference), albeit not reaching the threshold for fibrosis on transient elastography.31 Despite being more sensitive than transient elastography, MRE still underperformed compared with biopsies in this cohort of obese patients undergoing IGB therapy.26
Our study is an open-label single-arm study, and the lack of a control group renders us unable to determine the degree of placebo effect contribution to the observed findings. A recent systematic review and network meta-analysis of randomized controlled trials for NASH pharmacological therapies evaluated the association between placebo treatments and changes in NASH histology and MR-based fat improvements.32 The authors showed that a placebo was associated with an improvement of ≥2 in NAS, on average, in 25% of patients.32 Given the moderate-intensity lifestyle intervention in our study, the magnitude of histologic response, and high responder rate, the likelihood that our observed findings are attributed in total to a placebo effect is low. This issue would have been a significant concern had nonadherent patients been excluded, or if the results were skewed with a low responders rate. Furthermore, there are plausible physiological and tangible downstream effects that resulted from the intervention; indeed, it becomes conceptually difficult, if that magnitude of placebo response is presumed, to distinguish or define what constitutes a placebo. Our study contains other inherent limitations that warrant acknowledgment. First, the study population is neither ethnically diverse (most were Caucasians) nor representative of all obesity classes. This lack of diversity limits the generalizability of results, given that NAFLD behavior may differ according to the ethnicity33 and may very well have a different response in greater or lesser BMI. Second, the limited duration of follow-up does not allow the evaluation of long-term outcomes on liver histology. Third, as with any study aiming to improve NAFLD outcomes, the ultimate outcome is all-cause mortality, and our study is not designed to address that question. Despite these limitations, there are many strengths of our study, bolstered by a prospective rigorous design, blinded approach during adjudication of liver histology and MRE, and the comprehensive evaluation of many synergistic parameters, such as laboratory testing, noninvasive radiologic testing, and invasive gold standard tissue histology, all corroborating the parallel metabolic, biochemical, and histologic improvement of the disease.
In conclusion, the IGB is a safe and effective treatment for NAFLD, which may allow a reversal in the natural history of NAFLD and NASH, despite the short duration of the intervention. The logistics of IGB placement will enable accurate risk stratification of these patients in a safe and reproducible manner, obviating the need for additional investigations, and clarifying the real risk of patients afflicted with NAFLD.
Materials and Methods
All authors had access to the study data and reviewed and approved the final manuscript. Subjects were identified and recruited from the liver disease clinic. Clinical history, physical exam, laboratory, and magnetic resonance elastography (MRE) imaging data were documented.
Exclusion criteria included other primary causes of liver disease, foregut organic disease, and persistent use of chronic anti-inflammatory drugs, anticoagulants, or chronic steroids. A hiatal hernia >5 cm, active peptic ulcer disease, prior foregut surgery, inflammatory bowel disease of the foregut, esophagogastric neoplasms, esophageal dysphagia, dysmotility, or eosinophilic esophagitis were all considered contraindications to intragastric balloon (IGB) placement.
Complications were captured at all points, were defined a priori, and included anticipated and unanticipated complications related to IGB placement and sojourn. Adverse events related to anesthesia, endoscopy, or liver sampling, in general, were captured as an emergency department visit, hospitalization in a general ward, or hospitalization in a critical care unit.
The study protocol was authorized by the Mayo Clinic Institutional Review Board (July 16, 2016), and registered at ClinicalTrials.gov (NCT02880189). All patients gave informed consent. All authors had access to the study data and reviewed and approved the final paper.
IGB Placement, Sojourn, and Removal
Under general anesthesia, patients underwent a complete diagnostic upper endoscopy to rule out any occult abnormalities that would preclude the patient from IGB placement, as described previously. The IGB (Orbera; Apollo Endosurgery, Austin, TX), was introduced by using the oral placement of the IGB assembly and then filling it with 650 mL of saline solution. Triple antiemetic therapy and a dose of intravenous corticosteroids were given intraoperatively.1 The patient was then extubated and, after recovery, was discharged from the endoscopy suite. In addition to scheduled proton pump inhibitor administration, patients were given antiemetic drugs, antispasmodic drugs, and laxatives as needed. A scheduled anxiolytic at bedtime was also prescribed for the first 2–3 days following IGB placement. Patients were placed on a translational diet and given standard recommendations for physical activity.1 No obesity pharmacological therapy medications were prescribed. A low-calorie diet (1200–1500 calories/d) was recommended. The exercise recommendations consisted of cardiovascular activity for 30 minutes daily at least 5 days a week. All patients underwent a moderate intensity lifestyle intervention consisting of 2 phone calls in the first week (nurse and or study coordinator), then bimonthly visits for the first 6 months with a healthcare team either in person or virtually for healthy living coaching, nutritional counseling, and capture of adverse events. The final comprehensive in-person visit was conducted at 6 months for balloon retrieval, liver biopsy, and measurements. Phone calls were conducted 1 week after balloon removal to ensure no adverse events. Daily caloric intake and exercise minutes were not captured, and therefore determination of strict adherence cannot be established. At 6 months, the patient underwent repeat esophagogastroduodenoscopy or endoscopic ultrasound liver biopsy (EUS-LB) under general anesthesia, with balloon removal. MRE was repeated the next day. The patients were followed up for an additional 1 month on the protocol.
EUS-LB Technique
Prior to IGB placement or explant, a curvilinear-array echoendoscope (Pentax EG-3870UTK; Pentax Medical, Montvale, NJ) was used to obtain biopsies from the left hepatic lobe using a 22-gauge fork-tip core biopsy needle (SharkCore; Medtronic, Sunnyvale, CA). The details of the technique were previously described.2
Two passes were acquired in all patients to collect histopathology samples. Two additional passes were then obtained with a second 22-gauge fork-tip core biopsy needle for liver lipidomic or metabolomic testing (data not shown). The samples were preserved in formaldehyde for histologic evaluation.
Biopsy Processing
Blood clots, if present, were separated from the pearly white liver core tissue, and then this core was placed in formalin. The formalin bottle contents were transferred into a plate, and a blinded surgical pathology assistant identified the core fragments. Post–pathology laboratory processing, total aggregate sample length (TASL) was measured. These pieces were gathered to form a line and measured with a ruler to record TASL. Tissue processing occurred according to standard procedures. Hematoxylin and eosin, trichrome, and reticulin stains were used to create the slides examined by a blinded pathologist.
MRE Technique
All MRE studies imaging were performed on a clinical 1.5T MR imaging system (Signa; GE Healthcare, Waukesha, WI) using a phased array torso coil, and standard clinical protocol. An expert in MRE, blinded to the patient’s clinical status, manually drew the largest possible region of interest on the stiffness maps including both right and left lobes of the liver in each of the 4 slices obtained. The mean stiffness from the 4 slices was used for statistical analysis.
EUS-LB Histological Evaluation
All EUS-LBs were examined by an experienced liver pathologist, blinded to the clinical status of the subjects, their tests, and their MRE or EUS results. TASL and complete portal triads (CPT) were evaluated. The adequacy of EUS-LB for nonalcoholic fatty liver disease (NAFLD) evaluation has been shown elsewhere.2 Other parameters assessed included the ability to calculate the NAFLD Activity Score (NAS) (Supplementary Table 1), determine the fibrosis stage, and determine sample adequacy as defined by standard published criteria.3 Histologic fibrosis stages were quantified on an ordinal scale, wherein stage 1A designated as 1.25, stage 1B as 1.5, stage 1C as 1.75, stage 2 as 2, stage 3 as 3, and stage 4 as 4. Supplement Figure 1 relays EUS-LB technique and sample processing details.
Statistical Methods
Baseline subject characteristics were described by their means, medians, or frequencies. For our primary study endpoint of improvement in NAS on paired liver biopsies at baseline and after 6 months, we used a paired t test with 2-sided alpha <0.05. A power calculation was performed, and with a sample size of 20 subjects, it was determined that 80% power will be present to detect a medium to a large difference in activity scores. A P value of <.05 was considered significant. Linear regression was employed to examine the associations between change in NAS and changes in weight, changes in hemoglobin A1c (HbA1c) and weight, changes in aspartate aminotransferase-to-platelet ratio index and weight, and changes in MRE and weight. The association between improvement or no change in fibrosis and weight loss, changes in NAS, waist circumference, and HbA1c was examined in a post hoc fashion using logistic regression.
EUS-LB Results
Forty-one EUS-LB specimens were acquired. The median TASL was 2.4 (interquartile range, 2.00–2.75) cm. The median number of complete portal triads per TASL was 26 (interquartile range, 7–62). In terms of specimen adequacy, 78% of specimens had TASL ≥20 mm, and 95% had ≥11 CPT; with 76% of specimens satisfying both conditions. Moreover, 100% of samples had TASL ≥10 mm, and 100% had ≥6 CPT; with 98% achieving both benchmarks. Of the samples, 100% were satisfactory to describe NAFLD activity score and fibrosis stage.
Results
Baseline MRE
By baseline MRE, 3 of 21 of patients had results interpreted as normal liver stiffness (however, they still fulfilled the inclusion criteria) (all with fibrosis on biopsies, stages 1A and 2), 6 of 21 as mildly elevated liver stiffness (all with fibrosis on biopsies, stages 1A, 1B, 1C, and 2), 5 of 21 as stage 1–2 fibrosis (histology stages 1A, 1C, 2, and 3), 4 of 21 as stage 2–3 fibrosis (histology stages 1A, and 2), 2 of 21 as stage 3–4 fibrosis (histology stage 3), and 1 of 21 as stage 4 fibrosis (histology stage 3) (Figure 3 in main text).
Predictors of NAS and Fibrosis Improvement
Patients’ baseline weight and body mass index and changes in weight, or HbA1c were not associated with improvement in NAS. Reduction in waist circumference (β = 0.12, P = .001) and increasing age of the patient (β = 0.4, p=<0.001) in unadjusted analyses were both significant predictors of NAS improvement. Using mixed stepwise selection linear regression, increasing age (β = 0.43, P < .0001) and waist reduction (β = 0.06, P = .03) were the only independent predictors of NAS improvement. On unadjusted analysis, the change in NAS (P = .44), changes in weight (P = .2), and waist reduction (P = .99) were not associated with improvement in fibrosis (P = .44).
Supplementary Material
What You Need to Know.
Background
The single fluid-filled intragastric balloon (IGB) induces meaningful weight loss and might be used in the treatment of nonalcoholic steatohepatitis (NASH).
Findings
In a prospective study, IGB therapy resulted in NASH remission (NAFLD Activity Scores below 2 in most patients), and half of patients reached Food and Drug Administration endpoints for therapy benchmarks at 6 months. Fibrosis, measured by magnetic resonance elastography, improved by 1.5 stages in half of the patients.
Implications for patient care
In this obesity pandemic, in which bariatric surgery penetrance is below 2% of qualified patients, IGB placement (a short-term intervention) produces substantial and meaningful improvements in NASH.
Acknowledgments
The authors acknowledge support from Apollo Endosurgery (provided intragastric balloons) and Medtronic (provided SharkCore needles).
Funding
This work was supported by the John Barry and Mayo Gastroenterology and Hepatology Fund for the conduction of the study, Mayo Clinic Center for Translational Science Activities grant number UL1 TR000135, and National Institute of Diabetes and Digestive and Kidney Diseases R01–029953 (to Rita Basu).
Abbreviations used in this paper:
- BMI
body mass index
- CRN
Clinical Research Network
- EUS
endoscopic ultrasound
- FDA
Food and Drug Administration
- HbA1c
hemoglobin A1c
- IGB
intragastric balloon
- MRE
magnetic resonance elastography
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- TBWL
total body weight loss
Footnotes
Supplementary Materials
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.04.068.
Conflicts of interest
These authors disclose the following: Barham K. Abu Dayyeh has served as a consultant for Metamodix, BFKW, DyaMx, Boston Scientific, and USGI medical; received research support from Apollo Endosurgery, USGI, Spatz Medical, Boston Scientific, GI Dynamics, Cairn Diagnostics, Aspire Bariatrics, and Medtronic; and served as a speaker for Johnson and Johnson, Endogastric Solutions, and Olympus. Rita Basu has received research support from AstraZeneca and served as a consultant for Genfit. Andres Acosta is founder and stockholder in Gila Therapeutics, Phenomix Sciences, and Lipiquester; has served as a consultant for Rhythm Pharmaceuticals, General Mills, Gila Therapeutics, and Phenomix Sciences; has received funding from the National Institutes of Health, Satiogen Pharmaceuticals, Vivus Pharmaceuticals, Novo Nordisk, Rhythm Pharmaceuticals, and One Ome; and has participated as co-investigator in research funded by Apollo Endosurgery. Andrew C. Storm has served as a consultant for Apollo Endosurgery; and received research support from Apollo Endosurgery and Boston Scientific. The remaining authors disclose no conflicts.
References
- 1.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief No. 288 2017. Available at: https://www.cdc.gov/nchs/data/databriefs/db288.pdf. Accessed January 23, 2020. [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 3.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.English WJ, DeMaria E, Hutter M, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis 2020;16:457–463. [DOI] [PubMed] [Google Scholar]
- 5.Bazerbachi F, Vargas Valls EJ, Abu Dayyeh BK. Recent clinical results of endoscopic bariatric therapies as an obesity intervention. Clin Endosc 2017;50:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic bariatric therapy: a guide to the intragastric balloon. Am J Gastroenterol 2019;114:1421–1431. [DOI] [PubMed] [Google Scholar]
- 7.Bazerbachi F, Haffar S, Sawas T, et al. Fluid-filled versus gas-filled intragastric balloons as obesity interventions: a network meta-analysis of randomized trials. Obes Surg 2018;28:2617–2625. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment: guidance for industry. 2018. Available at: https://www.fda.gov/media/119044/download. Accessed January 23, 2020.
- 9.Kumar N, Bazerbachi F, Rustagi T, et al. The influence of the orbera intragastric balloon filling volumes on weight loss, tolerability, and adverse events: a systematic review and meta-analysis. Obes Surg 2017;27:2272–2278. [DOI] [PubMed] [Google Scholar]
- 10.Abu Dayyeh BK, Bazerbachi F, Graupera I, et al. Endoscopic bariatric and metabolic therapies for non-alcoholic fatty liver disease. J Hepatol 2019;71:1246–1248. [DOI] [PubMed] [Google Scholar]
- 11.Mion F, Napoleon B, Roman S, et al. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obes Surg 2005;15:510–516. [DOI] [PubMed] [Google Scholar]
- 12.Mariani S, Fiore D, Persichetti A, et al. Circulating SIRT1 increases after intragastric balloon fat loss in obese patients. Obes Surg 2016;26:1215–1220. [DOI] [PubMed] [Google Scholar]
- 13.Hannah WN Jr, Harrison SA. Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clin Liver Dis 2016;20:339–350. [DOI] [PubMed] [Google Scholar]
- 14.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367–378.e5; quiz e14–e15. [DOI] [PubMed] [Google Scholar]
- 15.Nickel F, Tapking C, Benner L, et al. Bariatric surgery as an efficient treatment for non-alcoholic fatty liver disease in a prospective study with 1-year follow-up. Obes Surg 2018;28:1342–1350. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–690. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165:305–315. [DOI] [PubMed] [Google Scholar]
- 19.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184–2196. [DOI] [PubMed] [Google Scholar]
- 20.Bhandari M, Fobi MAL, Buchwald JN, et al. Standardization of bariatric metabolic procedures: World Consensus Meeting statement. Obes Surg 2019;29:309–345. [DOI] [PubMed] [Google Scholar]
- 21.Keleidari B, Mahmoudieh M, Gorgi K, et al. Hepatic failure after bariatric surgery: a systematic review. Hepat Mon 2019;19:e86078. [Google Scholar]
- 22.Vargas EJ, Pesta CM, Bali A, et al. Single fluid-filled intragastric balloon safe and effective for inducing weight loss in a real-world population. Clin Gastroenterol Hepatol 2018;16:1073–1080.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosli MM, Elyas M. Does combining liraglutide with intragastric balloon insertion improve sustained weight reduction? Saudi J Gastroenterol 2017;23:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazerbachi F, Vargas EJ, Mounajjed T, et al. 795 Impact of single fluid-filled intragastric balloon on metabolic parameters and nonalcoholic steatohepatitis: a prospective paired endoscopic ultrasound guided core liver biopsy at the time of balloon placement and removal. Gastrointest Endosc 2018;87:AB118–AB119. [Google Scholar]
- 25.Bazerbachi F, Vargas E, Maselli DB, et al. Su1273 endosonographic estimation of the celiac artery mesenteric fat thickness (cameus) is a novel reliable anthropomorphic correlate of obesity and obesity-associated steatohepatitis: a proof-of-concept prospective controlled study and technique description. Gastrointestinal Endoscopy 2020;91:AB305. [Google Scholar]
- 26.Bazerbachi F, Vargas EJ, Matar R, et al. EUS-guided core liver biopsy sampling using a 22-gauge fork-tip needle: a prospective blinded trial for histologic and lipidomic evaluation in nonalcoholic fatty liver disease. Gastrointest Endosc 2019;90:926–932. [DOI] [PubMed] [Google Scholar]
- 27.Stefan N, Häring H-U, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019;7:313–324. [DOI] [PubMed] [Google Scholar]
- 28.Centis E, Moscatiello S, Bugianesi E, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol 2013;58:771–777. [DOI] [PubMed] [Google Scholar]
- 29.Naveau S, Lamouri K, Pourcher G, et al. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes Surg 2014;24:1693–1701. [DOI] [PubMed] [Google Scholar]
- 30.Kenngott HG, Clemens G, Gondan M, et al. DiaSurg 2 trial–surgical vs. medical treatment of insulin-dependent type 2 diabetes mellitus in patients with a body mass index between 26 and 35 kg/m2: study protocol of a randomized controlled multicenter trial–DRKS00004550. Trials 2013;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazerbachi F, Haffar S, Wang Z, et al. Range of normal liver stiffness and factors associated with increased stiffness measurements in apparently healthy individuals. Clin Gastroenterol Hepatol 2019;17:54–64.e1. [DOI] [PubMed] [Google Scholar]
- 32.Han MAT, Altayar O, Hamdeh S, et al. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17:616–629.e26. [DOI] [PubMed] [Google Scholar]
- 33.Szanto KB, Li J, Cordero P, et al. Ethnic differences and heterogeneity in genetic and metabolic makeup contributing to nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes 2019;12:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic bariatric therapy: a guide to the intragastric balloon. Am J Gastroenterol 2019;114:1421–1431. [DOI] [PubMed] [Google Scholar]
- 2.Bazerbachi F, Vargas EJ, Matar R, et al. EUS-guided core liver biopsy sampling using a 22-gauge fork-tip needle: a prospective blinded trial for histologic and lipidomic evaluation in nonalcoholic fatty liver disease. Gastrointest Endosc 2019;90:926–932. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009;49:1017–1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


