Abstract
Locomotion is a vital motor function for both animals and humans. Epidural electrical stimulation of the spinal cord (ES) is used to restore/improve locomotor movements in patients. However, operation of locomotor networks during ES has never been studied. Here we compared activity of individual spinal neurons recorded in decerebrate cats of either sex during locomotion initiated by supraspinal commands (caused by stimulation of the mesencephalic locomotor region, MLR) and by ES. We found that under both conditions, the same neurons had modulation of their activity related to the locomotor rhythm, suggesting that the network generating locomotion under the two conditions is formed by the same neurons. About 40% of these neurons had stable modulation (i.e., small dispersion of their activity phase in sequential cycles), as well as similar phase and shape of activity burst in MLR- and ES-evoked locomotor cycles. We suggest that these neurons form a part of the locomotor network that operates similarly under the two conditions, and are critical for generation of locomotion. About 23% of the modulated neurons had stable modulation only during MLR-evoked locomotion. We suggest that these neurons are responsible for some differences in kinematics of MLR- and ES-evoked locomotor movements. Finally, 25% of the modulated neurons had unstable modulation during both MLR- and ES-evoked locomotion. One can assume that these neurons contribute to maintenance of the excitability level of locomotor networks necessary for generation of stepping, or belong to postural networks, activated simultaneously with locomotor networks by both MLR stimulation and ES.
Keywords: decerebrate cat, locomotion, spinal neurons, sensory feedback, mesencephalic locomotor region, epidural stimulation
Introduction
Forward locomotion is vitally important for animals and humans as it is their main form of progression. The neuronal system controlling forward locomotion is one of the most studied motor systems. In vertebrates, the basic neuronal networks controlling forward locomotion are located at the spinal-brainstem-cerebellum level. The locomotor movements of an individual limb are generated by spinal neuronal networks. Sensory feedback from the limb adapts the activity of the spinal locomotor networks to different environmental conditions by affecting them both directly and through supraspinal systems (Grillner, 1975; Grillner & Zangger, 1979; Orlovsky et al. 1999; Rossignol et al. 2006). Spinal locomotor networks are activated by supraspinal commands caused by activation of mesencephalic (MLR; Shik et al. 1966; Shik & Orlovsky, 1976; Caggiano et al. 2018; Ferreira-Pinto et al. 2018) or subthalamic (Waller, 1940; Orlovsky, 1969) locomotor regions and transmitted by reticulospinal neurons (Orlovsky, 1970a,b; Baev et al. 1988; Capelli et al. 2017; Ferreira-Pinto et al. 2018).
Forward locomotion can also be evoked by epidural electrical stimulation (ES) of the spinal cord (Iwahara et al. 1992; Dimitrijevic et al. 1998; Musienko et al. 2007, 2012). Since ES can also evoke locomotion in spinal subjects (Gerasimenko et al. 2003; Minassian et al. 2004; Barthelemy et al. 2007; Courtine et al. 2009), it was suggested that this stimulation directly activates the spinal locomotor mechanisms through the myelinated sensory fibers of the dorsal roots and dorsal column (Gerasimenko et al. 2005; Capogrosso et al. 2013). However, one cannot rule out, however, that SC stimulation in subjects with an intact spinal cord through the ascending pathways, can activate the brainstem circuits forming supraspinal commands activating spinal locomotor networks. Nevertheless, it seems likely that MLR- and ES-evoked locomotion differ in the contribution of spinal and supraspinal mechanisms to the activation and modulation of spinal locomotor networks. However, operation of locomotor networks activated by ES has never been studied.
Earlier, we demonstrated that locomotor mechanisms activated by MLR stimulation and ES, as well as their operation, differ to some extent (Musienko et al. 2012). In particular, stimulation of MLR selects and activates locomotor networks generating exclusively forward stepping. By contrast, during ES, the network generating the horizontal component of a step in a particular direction is activated by sensory input from the limb signaling the direction of the limb movement during stance (determined by the direction of the treadmill belt movement). It is not clear whether this difference is the result of some changes in operation of the same network or whether substantially different neuronal groups are involved in generation of locomotor movements under the two conditions.
The spinal cord can generate stepping-like motor output in the absence of movement-related afferent signals from the limbs (Goldberger, 1977; Grillner & Zanger, 1984; Giuliani & Smith, 1987). Under such conditions, the timing of different events in the step cycle is determined by the central mechanism (the central pattern generator, CPG; Grillner & Zanger, 1979; Kiehn, 2006), with the leading rhythm generating networks residing in the rostral segments of the lumbosacral enlargement (Deliagina et al. 1983; Cazalets et al. 1995; Kjaerulff & Kiehn, 1996; Langlet et al. 2005; Barthélemy et al. 2007; Gerasimenko et al. 2019). By contrast, during “real” locomotion when the limbs are actually stepping, the events in the step cycle are not determined exclusively by CPG but, to a large extent, by sensory inputs signaling the limb position, limb loading, etc. (Grillner & Rossignol, 1978; Duysens & Pearson, 1980; Conway et al. 1987; Gossard et al. 1994; Rossignol et al. 2006). While spinal neuronal networks constituting the CPG have been extensively studied during the last decade and considerable progress in understanding their operation in some animal models has been achieved (e.g., Grillner & El Manira, 2015), our knowledge of neuronal mechanisms of real locomotion, with reflex control of the step cycle, is very limited. In particular, it is not clear to what extent sensory feedback contributes to modulation of individual neurons during real locomotion, as well as whether operation of locomotor networks located in different segments of the spinal cord differs.
Here, to address the unanswered questions mentioned above, first, we recorded the activity of individual spinal neurons in the L4 and L6 spinal segments during real locomotion evoked by both ES and MLR stimulation. Second, the activity modulation of individual neurons during real locomotion was compared with that caused by passive movements of the limbs along the locomotor trajectory.
A brief account of this study has been published in abstract form (Zelenin et al. 2015).
Methods
Ethical approval
Experiments were carried out on five wild-type adult cats (Felis catus) of either sex (weighing 2.5–4.0 kg). All five cats exhibited well-coordinated forward walking during both MLR stimulation and ES. The animals were obtained from the cat’s colony of Pavlov Institute of Physiology Animal House, where they were kept in cages with enriched environments and had free access to food and water.
All procedures were conducted in accordance with protocols (#01a/2017 and #01a/2018) approved by the Animal Care Committee of the Pavlov Institute of Physiology, St. Petersburg, Russia, and adhered to the European Community Council Directive (2010/63EU) and the guidelines of the National Institute of Health (Guide for the Care and Use of Laboratory Animals).
Surgical procedures
The surgical procedures were similar to those in the previous study (Merkulyeva et al. 2018). The cats were deeply anesthetized with isoflurane (2–4%) delivered in O2. For the induction of anesthesia, xylazine (0.5 mg/kg, i.m.) was injected and then the animal was placed in a hermetic plastic box, which was gradually filled with isoflurane (2–4%) delivered in O2. The surgery was started when the animal was deeply anesthetized. The level of anesthesia was monitored based on applying pressure to a paw (to detect limb withdrawal), as well as by checking the size and reactivity of the pupils. The trachea was cannulated and the carotid arteries were ligated. The animals were decerebrated at the precollicular-postmammillary level. The brain tissue rostral to the transection was removed. The carotid artery was cannulated to measure the blood pressure. A skin incision above the lumbar area was made, the paravertebral muscles were deflected, and laminectomy was performed between the L4 and L7 segments for ES and recording of neurons. The exposed dorsal surface of the spinal cord was covered with warm paraffin oil. Bipolar electromyographic (EMG) electrodes (0.2 mm flexible stainless steel Teflon-insulated wires) were implanted bilaterally into the m. gastrocnemius lateralis (Gast, ankle extensor) and m. tibialis anterior (Tib, ankle flexor), as described previously (Gerasimenko et al. 2009). Anesthesia was discontinued after the surgical procedures. The experiments were started 2–3 h thereafter. During the experiments, the animals were spontaneously breathing room air.
During the experiment, the rectal temperature and mean blood pressure of the animals were continuously monitored. The rectal temperature was maintained at 37 ± 0.5°C with the help of heat irradiation. The blood pressure was kept at > 80 mmHg. If needed, injection of prednisolone (3 mg/kg, i.m.) was done to stabilize the arterial pressure and to reduce brain swelling after decerebration.
Experimental design
The experimental design (Fig. 1A) was in many respects similar to that used in the previous studies (Musienko et al. 2012, 2014). The head, the vertebral column, and the pelvis of the decerebrate cat were fixed in a rigid frame. The forelimbs had no support, whereas the hindlimbs were positioned on the treadmill with two separate belts (left and right) moving at the same speed (0.5 m/s) and below referred to as the “treadmill belt”. The distance between the treadmill belt and the fixed pelvis was 21–25 cm (depending on the animal size), which determined a hemi-flexed limb configuration in the middle of stance typical for walking. The treadmill belt was moving backward in relation to the animal.
Figure 1. Experimental design for recording of the same neurons during MLR- and ES-evoked locomotion.
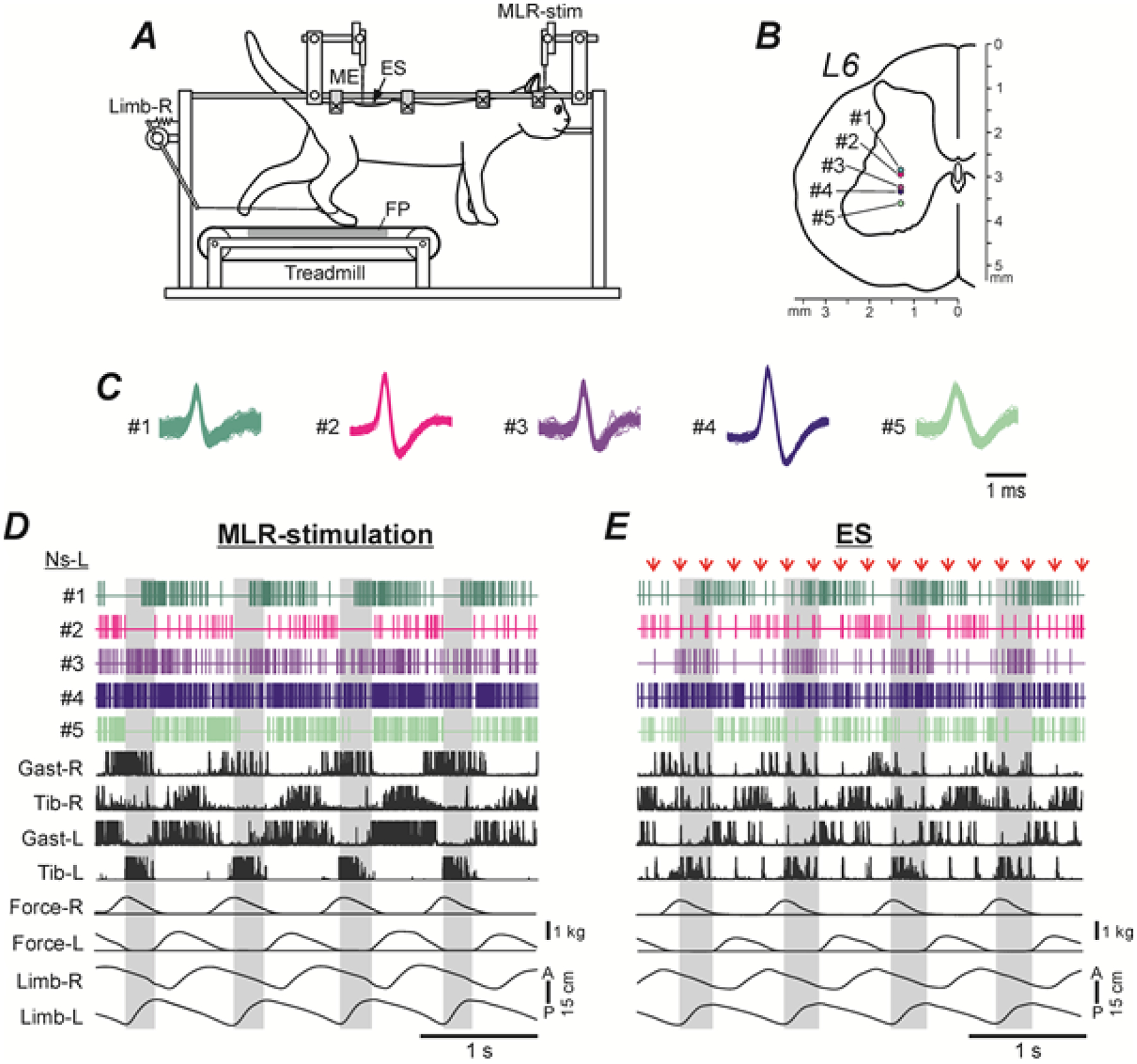
A, The head, vertebral column and pelvis of a decerebrate cat were fixed in a rigid frame (points of fixation are indicated by X). The hindlimbs were positioned on the treadmill. Walking of the hindlimbs was evoked by stimulation of the mesencephalic locomotor region (MLR-stim) or the epidural stimulation of the spinal cord (ES). Anterior/posterior movements of each limb were recorded by mechanical sensors (only the right sensor, Limb-R, is shown). The contact force under each limb was measured by a force plate (FP). Neuronal activity was recorded by a multichannel electrode array (ME). B-D, Location of five spinal neurons (#1–5) recorded simultaneously by the multichannel electrode array on the left side of L6 (B), waveforms of their spikes extracted from the mass activity by spike sorting procedure (C), as well as their activity during MLR- and ES-evoked locomotion (D and E, respectively). Neuronal activity was recorded along with movements of the right and left limbs (Limb-R and Limb-L), contact forces (Force-R and Force-L), and EMGs of the left and right gastrocnemius and tibialis muscles (Gast-L, Gast-R and Tib-L, Tib-R, respectively). The EMGs were rectified. In E, the EMG signals contained large responses to individual epidural stimuli (indicated by red arrows).
Swing phases of the left hindlimb are highlighted.
In each cat, locomotion was evoked by electrical stimulation of the mesencephalic locomotor region (MLR, Shik et al. 1966; Shik & Orlovsky, 1976; Jordan, 1986; Garcia-Rill & Skinner, 1987a,b) and by electrical epidural stimulation (ES) of the spinal cord (Iwahara et al. 1992; Musienko et al. 2007, 2012; Gerasimenko et al. 2009; Merkulyeva et al. 2018). The stimulation started 2–3 s after onset of the treadmill belt motion. For MLR stimulation, a bipolar electrode (two 150 μm wires isolated except for the tips and separated by 0.5 mm) was inserted into the brainstem area (Horsley-Clarke coordinates P2, R/L4, H0) by means of a micromanipulator (MLR-stim in Fig. 1A). We used the following parameters of stimulation: frequency, 30 pulses per second (Hz); pulse duration, 0.5–1 ms; current, 50–200 μA. For ES, a ball electrode (d=0.5 mm) was positioned on the dura mater in the middle of the dorsal surface of the spinal cord at the L5 level (ES in Fig. 1A). ES stimulation at this site produced well-coordinated forward locomotion (Merkulyeva et al. 2018). We used the following parameters of stimulation: frequency, 5 Hz; pulse duration, 0.2–0.5 ms; current, 100–300 μA.
The side views of the walking cat were recorded by two video cameras (50 frames/s; Sony HDR-CX160E). In addition¸ we recorded the anterior-posterior locomotor limb movements (by means of two mechanical sensors, one of which, Limb-R, is shown in Fig. 1A) and the vertical forces developed by each of the limbs (by means of two force plates positioned under the left and right parts of the moving belt, FP in Fig. 1A).
Recording of neurons
For the first time, the activity of a limited population (n = 30) of individual spinal neurons was recorded during real MLR-evoked locomotion by using single extracellular microelectrodes (Orlovsky & Feldman, 1972). In the present study, spinal neurons were recorded extracellularly from the spinal segment L4 and L6 by means of commercially available multichannel electrode arrays (ME in Fig. 1A). Each array consisted of a shaft with 32 Pt/Ir/Au electrode sites (A1×32-10mm-50-177; Neuronexus, Ann Arbor, MI). Each site surface area was 177 μm2. Electrode sites were distributed 50 μm apart vertically. Thus the recording length of the array was approximately 1.5 mm, which allowed for simultaneous recordings of many individual neurons from different areas of the gray matter. The array was mounted on an electrode holder driven by a manual micromanipulator. To increase the stability of the recording that is to prevent displacement of the nervous tissue in relation to the recording electrodes caused by movements (locomotor, breathing, etc.), the space between the dura mater and the walls of the spinal canal was filled with tissue adhesive glue Indermil X Fine (Henkel Ireland Operations and Research Ltd, Dublin, Ireland) in the area of recording.
We attempted to explore systematically the whole cross-section of the gray matter except for the areas of motor nuclei. Recording of putative spinal interneurons at the same position of the array in the gray matter was performed during 7–10 s before the treadmill was switched on (to reveal the spontaneous activity of neurons), as well as during forward locomotion evoked by MLR and by ES stimulation. Under each condition, the neuronal activity was recorded in ~20–60 locomotor cycles. In addition, to estimate the possible contribution of sensory feedback from the limb to the locomotor modulation of a neuron, the majority of neurons were recorded during passive movements of the ipsilateral and passive movements of the contralateral limb performed manually along the locomotor trajectory. For this purpose, in the resting animal, one of the hindlimbs was suspended while the other one (held by the metatarsus) was moved forward from the posterior extreme position (similar to that observed at the end of stance during locomotion) to the anterior extreme position (similar to that observed at the end of swing during locomotion) where the limb was landed (imitation of swing). Then the limb was moved backward together with the moving treadmill belt (imitation of stance). At each position of the electrode array, neurons were recorded during 5–10 cycles of passive movements performed by each of the hindlimbs.
To determine the location of individual neurons (recorded at a definite position of the array by a particular electrode site) on the spinal cord cross-section, the lateral and vertical co-ordinates of the array tip were marked.
The signals from the electrode array (neuronal activity), EMG electrodes, mechanical sensors and force plates were amplified and digitized with a sampling frequency of 30 kHz (neurons), 5 kHz (EMGs), and 1 kHz (sensors). These signals, together with the signals indicating the switching-on and switching-off the treadmill, video recording, and MLR or ES stimulation were recorded on a computer disk using the data acquisition system (RZ5 BioAmp Processor, Medusa preamplifiers, Tucker-Davis Technologies, Alachua, Florida, USA). For synchronization of the video recording with other recorded signals, synchro pulses were used that were recorded by the acquisition system, and produced light flashes recorded by video cameras.
Data analysis
The multiunit spike trains recorded by each electrode were separated into unitary waveforms representing the activity of individual neurons using the spike-sorting procedure in an analysis software (Spike2, Cambridge Electronic Design, Cambridge, UK). The spikes with the same waveform were supposedly generated by the same spinal neuron during MLR- and ES-evoked locomotion (Fig. 1C). Only neurons with a stable spike shape were used for the analysis. Many neurons were recorded simultaneously by several neighboring sites, which increased the confidence of the spike sorting. An example of activity of five neurons simultaneously recorded during MLR- and ES-evoked locomotion, waveforms of their spikes, as well as their positions on the cross-section of the gray matter, is shown in Fig. 1, D, E, C and B, respectively.
The activity of the neurons was typically modulated in the rhythm of stepping movements (Fig. 1D,E). To characterize this modulation, a phase histogram of neuronal activity in a step cycle was created. Because of some variability in the duration and structure of step cycles within a test and between the tests (MLR- and ES-evoked locomotion), we used dual-referent phase analysis (Berkowitz and Stein, 1994), that is, we normalized the swing and stance phase separately. The swing was normalized to the swing proportion in the locomotor cycle averaged across all episodes of MLR- and ES-evoked locomotion in all cats. The stance was normalized correspondingly. Normalized swing and stance constituted 38% and 62% of the locomotor cycle, respectively. Such normalization ensured that neuronal activity during a definite phase (swing or stance) in one test was compared to the activity during the same phase in the other tests or when these characteristics were compared in different steps within the same test.
The spike time sequence of an individual neuron was converted to the instantaneous rate versus time and then to the instantaneous rate versus phase (380 points for the swing, 720 points for the stance). The dependence of the instantaneous rate on the phase was averaged over all steps of a given test. Then the histogram was smoothed (sliding averaging, window width, 30 bins) to remove high-frequency noise. Examples of the rasters and the resulting histograms (termed “the entire activity phase histograms”) are shown in Fig. 4A–L.
Figure 4. Comparison of modulation patterns of individual neurons during MLR- and ES-evoked locomotion.
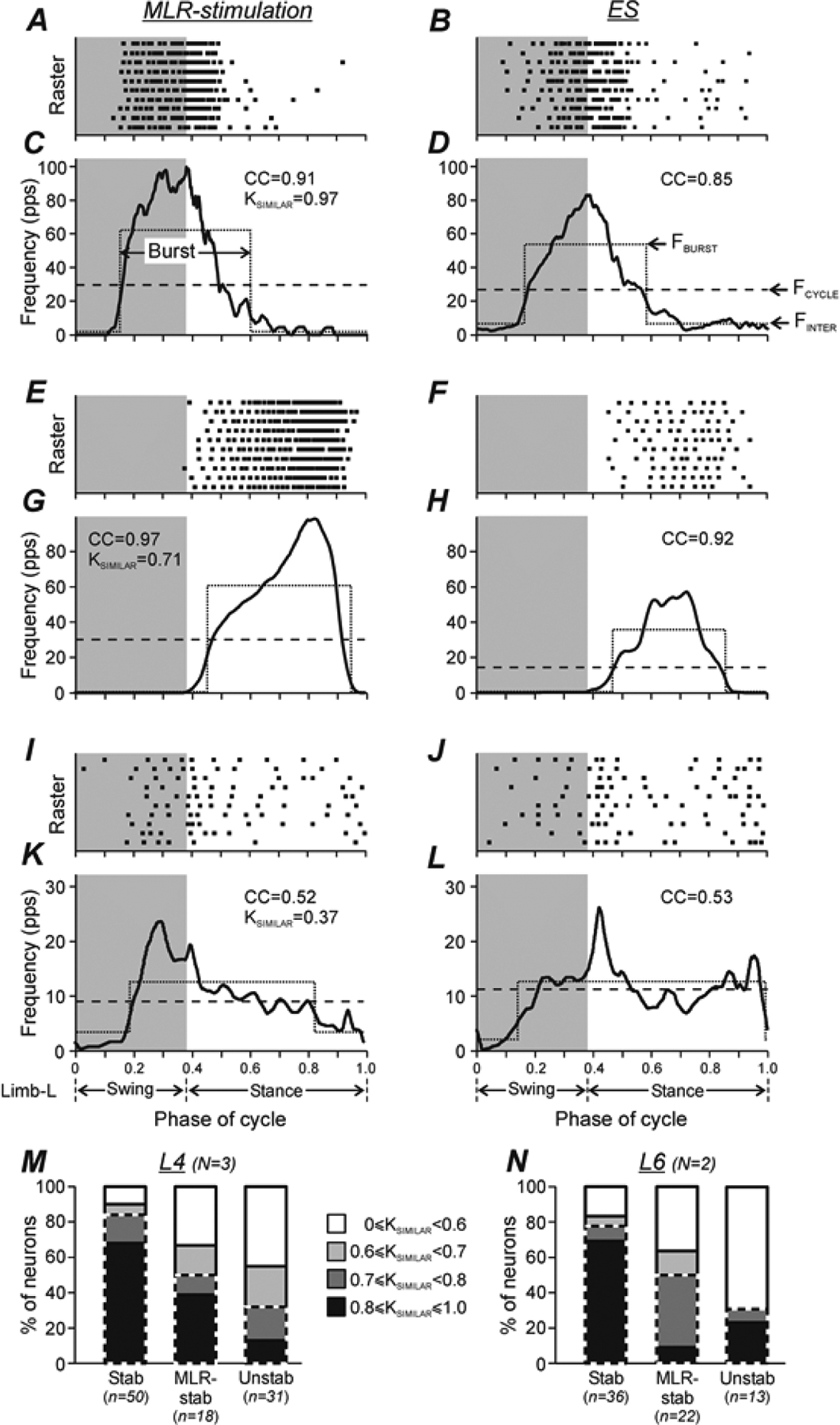
A-L, Examples of modulation patterns of three neurons (A-D, E-H, and I-L, respectively) during MLR-stimulation (A,C,E,G,I,K) and ES (B,D,F,H,J,L). For each condition (MLR-stimulation and ES), the raster (for clarity, only 10 out of 20–60 recorded cycles are shown in each neuron) and the histogram (thick line) of the activity of the neuron in the cycle of the ipsilateral hindlimb are shown. The swing phase is highlighted. The dotted lines show the best two-level rectangular approximations of the histograms, with the burst period (upper level indicating also the mean burst frequency, FBURST in D) and interburst period (lower level indicating also the mean interburst frequency, FINTER in D). The dashed lines show the mean cycle frequency (FCYCLE in D). The average correlation coefficient (CC) between the profiles of activity in individual locomotor cycles and in the entire activity histogram, as well as the coefficient of similarity (KSIMILAR), that is the correlation coefficient between the activity phase histograms obtained under two conditions, are indicated. Two neurons shown in A-H were classified as Stable neurons, and the neuron shown in I-L – as Unstable one. M,N, Comparison of the relative number of neurons with different values of the coefficient of similarity in populations Stable (Stab), MLR-stable (MLR-stab), and Unstable (Unstab) neurons recorded in L4 (M) and L6 (N). Parts of the bars outlined by dashed line indicate relative number of neurons with KSIMILAR ≥ 0.7. Number of animals and number of Stable, MLR-stable, Unstable neurons in M: N = 3 and n = 50, 18, 31, respectively. Number of animals and number of Stable, MLR-stable, Unstable neurons in N: N = 2 and n = 36, 22, 13, respectively.
To evaluate the depth of step-related modulation of neuronal activity, we used the best two-level rectangular fit for the entire activity histogram (Zelenin et al. 2011); the upper level was defined as a burst and the lower level as an inter-burst period (see Fig. 4) (Zelenin et al. 2011). For each neuron, the mean cycle frequency, the mean burst frequency, and the mean interburst frequency (Fig. 4D) were calculated for the entire activity histogram. In addition, the mean frequency of spontaneous activity (recorded before the treadmill was switched on) was calculated.
The activity of neurons was considered modulated if, first, the mean burst frequency was significantly different from the mean interburst frequency (Student’s t test, p < 0.05), and, second, the pattern of modulation in the majority of the locomotor cycles was consistent (the Pearson’s correlation coefficient between the profiles of the activity in individual locomotor cycles and in the entire activity histogram was higher than 0.3 in more than 60% of the locomotor cycles). The coefficient of frequency modulation was defined as KMOD = (1 − FINTER / FBURST) × 100%, where FINTER and FBURST are mean interburst and burst frequencies, respectively.
To evaluate the degree of similarity between the modulation patterns of the same neuron during MLR- and ES-evoked locomotion, we calculated the coefficient of similarity (KSIMILAR), that is, the Pearson’s correlation coefficient between the entire activity phase histograms obtained in these tests. Such analysis reveals co-variations of the two functions, i.e., parallel changes of the instantaneous discharge frequency within the cycle, while dismissing differences in mean frequencies and depths of modulation. Examples of neuronal discharges recorded under the two conditions and then compared using this method are shown in Fig. 4.
To evaluate the variability of the activity phase of individual neurons in the locomotor cycle, the instantaneous frequency of the neuron versus the normalized phase of the locomotor cycle (see above) was used. The best two-level rectangular fit for instantaneous frequency of the neuron within each individual step cycle was used to determine its burst onset and burst offset phases in this cycle. Then the average and SD for the burst onset phase and for the burst offset phase were calculated across all step cycles using circular statistics methods (Batschelet, 1981).
Statistical analyses
All quantitative data in this study are presented as mean ± SD. Paired Student’s t test (two-tailed) was used for pairwise comparisons. Welch’s t test (two-tailed) was used to characterize the statistical significance when comparing different means. To evaluate the statistical significance of difference in percentages of different types of modulated neurons recorded in L4 and in L6, we used Pearson’s χ2 test. The significance level for all tests was set at p = 0.05.
Histological procedures
At the termination of the experiments, the cats were deeply anesthetized with isoflurane (5%), and then perfused transcardially with isotonic saline followed by 4% paraformaldehyde solution, which caused euthanasia. The L4 and L6 spinal segments were removed from the spine and stored in 20% and 30% sucrose until they sank. Then, regions of segments containing the recording sites were cut on a freezing microtome into 50 μm frontal sections. The sections were collected in 0.1 M PBS (pH 7.4) and then stained with cresyl violet. The positions of the array in the spinal cord were verified by observation of the array tracks. The positions of the recording sites were estimated in relation to the array track position.
Results
Comparison of MLR- and ES-evoked locomotor movements
Examples of the forward locomotion evoked by MLR stimulation and ES in the same cat are shown in Fig. 1, D and E, respectively. In general, the two locomotor patterns were similar. In both cases, the stepping limb movements were rather uniform. The right and left hindlimbs were stepping in antiphase. The EMG patterns were also similar in these two cases: the ankle extensor (Gast) was active during the stance phase of the limb and the flexor (Tib) was active during the swing phase. The EMG signals during ES-evoked locomotion contained large short-latency responses to stimulating pulses coming at a frequency of 5 Hz (Fig. 1E). These general features of the two locomotor patterns were similar in all studied cats; they were also similar to those described in earlier studies (Iwahara et al. 1992; Musienko et al. 2012).
However, despite the similarity of the basic features of locomotor patterns evoked by MLR stimulation and ES, we found some differences. Figure 2, A and B, shows a side view of the left foot trajectory during one cycle of locomotion performed by the same cat during MLR stimulation and ES, respectively. One can see that during MLR stimulation, the limb landed at a more rostral position in relation to the trunk as compared with that during ES (compare the distances between the paw and the projection of the hip on the support surface in A and B). To estimate quantitatively the paw excursion in relation to the trunk, we connected the hip with the tip of the paw at the extreme anterior and posterior limb positions in stance by lines and calculated angles between the pelvis and these lines (angles αA and αP in Fig. 2, A and B, respectively). To evaluate the magnitude of limb oscillations, the angle between the extreme anterior and posterior limb positions (angle Δα=αP–αA in Fig. 2A) was calculated. Since the step magnitudes varied among the individual cats, in each of five cats, the steps recorded in sequential MLR-evoked and ES-evoked locomotor episodes (n=28 and 28, respectively) were used. Then, we averaged a particular parameter across 140 steps obtained in all cats during MLR stimulation and during ES. The mean (± SD) value of αA was significantly smaller during MLR stimulation than that during ES (Fig. 2C): 73 ± 11° vs 85 ± 8°, respectively (Welch’s t test, p = 6 × 10−21). Thus, during MLR stimulation, the limb landed at a more rostral position in relation to the trunk, compared with that during ES. By contrast, the mean (± SD) values of αP during MLR stimulation and ES were similar (Fig. 2C). Correspondingly, the magnitude of limb oscillations [the mean (± SD) value of angle Δα in Fig. 2C] was significantly larger during MLR stimulation: 54 ± 12° vs 43 ± 7° during ES (Welch’s t test, p = 2 × 10−19). We also found small but significant differences in cycle structure and cycle duration during MLR stimulation and ES. As shown in Fig. 2D, during MLR stimulation, the cycle duration was longer [mean (±SD), 0.86 ± 0.11 s (N = 5, n = 106) vs 0.80 ± 0.11 s (N = 5, n = 113), Welch’s t test, p = 0.0004], as compared to ES and the swing proportion was smaller [mean (± SD), 0.37 ± 0.05 (N = 5, n = 106) vs 0.40 ± 0.04 (N = 5, n = 113) fraction of cycle, Welch’s t test, p = 6 × 10−6)]. Finally, the mean (± SD) value of the peak contact force produced by the limb during steps in MLR-evoked locomotor episodes was significantly higher than that during sequential ES-evoked episodes [1.03 ± 0.43 kg (N = 5, n = 60) vs 0.80 ± 0.46 kg (N = 5, n = 60 locomotor cycles), Welch’s t test, p = 3 × 10−4)].
Figure 2. Comparison of locomotor movements evoked by MLR-stimulation and by ES of the L5.
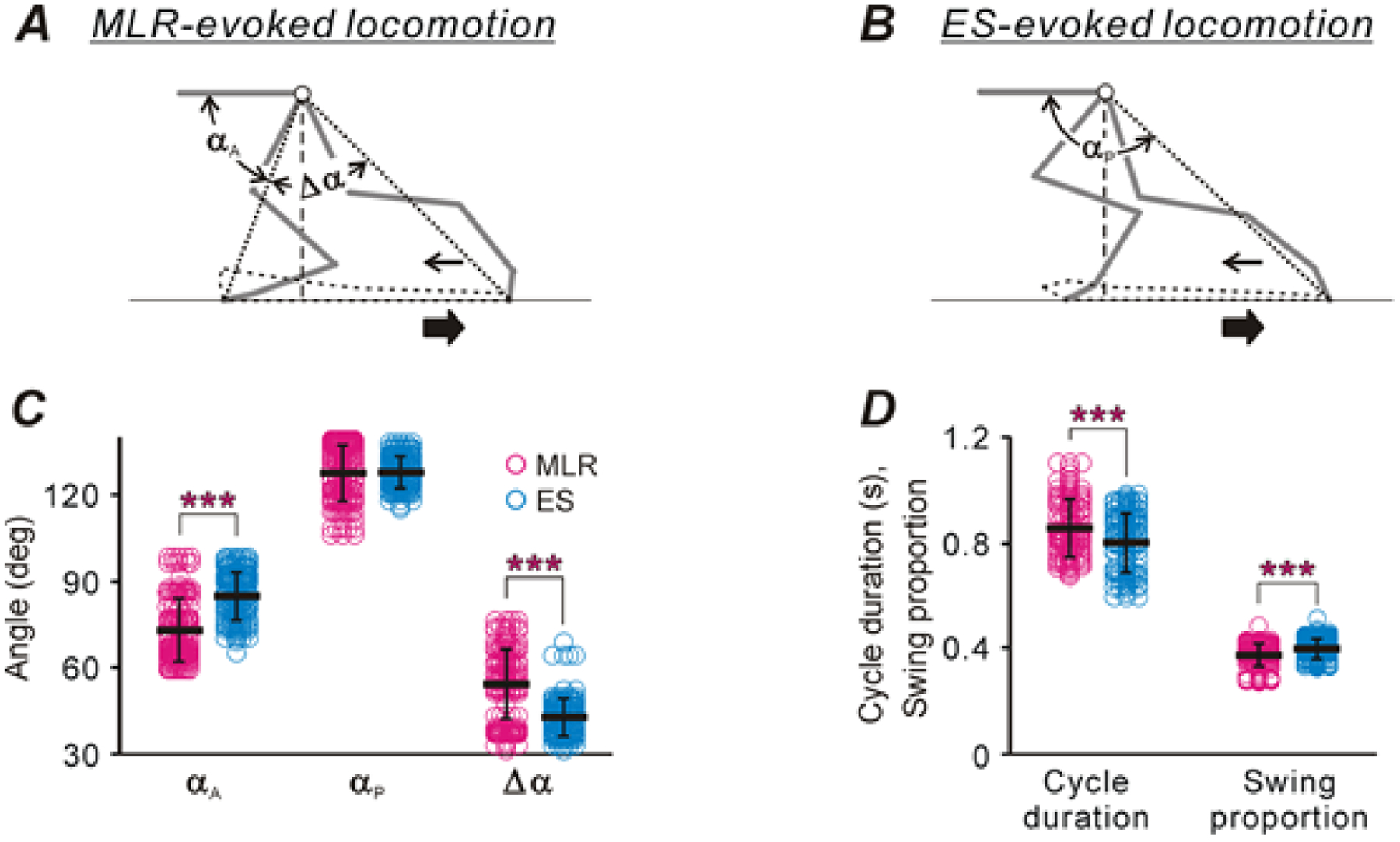
A,B, Extreme left hindlimb positions during one step cycle evoked by MLR-stimulation (A) and by ES (B). Angles αA and αP characterize the extreme anterior and posterior paw positions in relation to the trunk, respectively. Angle Δα characterizes the magnitude of limb movements. Thick and thin arrows indicate directions of the treadmill belt movement and the foot movement during swing, respectively. Note, that during stepping evoked by MLR stimulation the limb is landed at more rostral position in relation to the trunk as compared with that observed during stepping evoked by ES (compare distance between the anterior extreme paw position and the projection of the hip on the treadmill surface in A and B, respectively). C,D, Comparison of characteristics of stepping evoked by MLR stimulation and ES (mean ± SD are indicated by black lines across corresponding dot clouds showing the values of the parameter in individual cycles. In C, the number of animals, episodes and cycles analyzed during MLR-evoked as well as during ES-evoked locomotion: N=5, nep=17 and ncyc=140, respectively. In D, N=5, nep=107, ncyc=2711 were analyzed during MLR-evoked locomotion, and N=5, nep=113, ncyc=1797 were analyzed during ES-evoked locomotion. Indication of significance level: * p < 0.05, *** p < 0.001).
Characterization of neuronal database
Altogether, the activity of 242 individual spinal neurons was extracted from the multiunit spike trains recorded by the electrode array in the five cats, including 127 neurons recorded in the spinal segment L4 and 115 neurons in the spinal segment L6 (Table 1). Out of all the recorded neurons, a few were “inactive” (i.e., they had a mean cycle frequency of less than 1 Hz, and thus, their contribution to the control of locomotion was very weak) or active but non-modulated (see criteria for modulation in Materials and Methods) during both or one of the locomotor tests (Table 1). The majority of neurons (~78% from L4 and ~67% from L6) were active and modulated during both MLR- and ES-evoked locomotion. Only these neurons were used for further analysis.
Table 1.
Neurons recorded in individual cats.
| Active | Inactive | T | Number of locomotor episodes/cycles | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modulated | Non-modulated | |||||||||||||
| S | M | E | U | T | M | E | M+E | M | E | M+E | MLR | ES | ||
| L4 | ||||||||||||||
| Cat #1 | 31 | 15 | 0 | 23 | 69 | 3 | 8 | 7 | 1 | 1 | 1 | 90 | 33/669 | 33/754 |
| Cat #2 | 13 | 2 | 0 | 7 | 22 | 2 | 1 | 0 | 0 | 2 | 1 | 28 | 3/66 | 3/83 |
| Cat #3 | 6 | 1 | 0 | 1 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 9 | 34/881 | 34/560 |
| Total (n) | 50 | 18 | 0 | 31 | 99 | 6 | 9 | 7 | 1 | 3 | 2 | 127 | 70/1616 | 70/1397 |
| Total (%) | 51 | 18 | 0 | 31 | 78 | 5 | 7 | 5 | 1 | 2 | 2 | 100 | ||
| L6 | ||||||||||||||
| Cat #4 | 21 | 13 | 6 | 8 | 48 | 3 | 11 | 12 | 3 | 2 | 3 | 82 | 15/331 | 15/750 |
| Cat #5 | 15 | 9 | 0 | 5 | 29 | 1 | 2 | 0 | 0 | 0 | 1 | 33 | 21/764 | 28/1047 |
| Total (n) | 36 | 22 | 6 | 13 | 77 | 4 | 13 | 12 | 3 | 2 | 4 | 115 | 36/1095 | 43/1797 |
| Total (%) | 47 | 28 | 8 | 17 | 67 | 4 | 11 | 10 | 2 | 2 | 4 | 100 | ||
Abbreviations: Modulated, modulated neurons (S, Stable neurons; M, MLR-stable neurons; E, ES-stable neurons; U, Unstable neurons). Non-modulated and Inactive, respectively, non-modulated and inactive neurons during MLR-evoked (M), ES-evoked (E) and during both MLR- and ES-evoked locomotion (M+E). T, total number of neurons. MLR, ES, MLR- and ES-evoked locomotor episodes, respectively.
We supposed that spinal neurons of the locomotor rhythm generator, as well as neurons strongly affected by the generator, should have “stable” modulation. That is, they should have a small dispersion of the burst phase in the sequential locomotor cycles and the profile of their activity in individual locomotor cycles should be consistent. We suggested the following criteria for the stability of modulation: (i) the SD of burst onset and/or burst offset should not be more than 0.1 part of the locomotor cycle (0.1 was close to the median value for all observed SDs; moreover, 0.1 comprised less than 30% of the swing phase and less than 20% of the stance phase; therefore an onset/offset phase with SD < 0.1 could be considered as rather precisely bound to a particular phase of the locomotor cycle). We accepted the stability of only one edge of the burst since a subset of the recorded neurons had a ramp-up or ramp-down burst shape with stable burst offset and burst onset phase, respectively. (ii) The average correlation coefficient between the profiles of the activity in individual locomotor cycles and in the entire activity histogram should not be less than 0.6. According to these criteria, all modulated neurons were divided into four types: neurons with stable modulation during both MLR stimulation and ES (“Stable neurons”), neurons exhibiting stable modulation during MLR stimulation only (“MLR-stable neurons”), neurons exhibiting stable modulation during ES only (“ES-stable neurons”), and neurons which had unstable modulation during both MLR stimulation and ES (“Unstable neurons”). Among the five individual neurons shown in Fig. 1B,D,E, neurons #1 and # 5 are Stable neurons while neurons #2 and #3 are MLR-stable and ES-stable, respectively. Neuron #4 is an Unstable neuron.
Figure 3A–D shows the location of individual modulated (A,C) as well as non-modulated and inactive neurons (B,D) recorded in L4 (A,B) and in L6 (C,D) on the corresponding cross-sections of the spinal cord. One can see that the overwhelming majority of these neurons were recorded in the intermediate and ventral parts of the gray matter outside the area of the motor nuclei (Vanderhorst and Holstege, 1997), and thus, were considered as putative interneurons. Stable, MLR-stable, and Unstable neurons were found in both L4 (A) and L6 (C) spinal segments. Modulated neurons of different types (A,C), inactive and non-modulated neurons (B,D), were intermixed. The distribution of modulated neurons across the gray matter revealed in the present study was similar to the distribution of a limited population (n=30) of modulated neurons recorded in the decerebrate cat during real forward locomotion evoked by MLR-stimulation (Orlovsky & Feldman, 1972), as well as to the distribution of modulated neurons observed after deafferentation (Orlovsky & Feldman, 1972) and during fictive locomotion (Baev et al. 1979).
Figure 3. Neurons recorded in L4 and L6 spinal segments during both locomotion evoked by MLR stimulation and by ES.
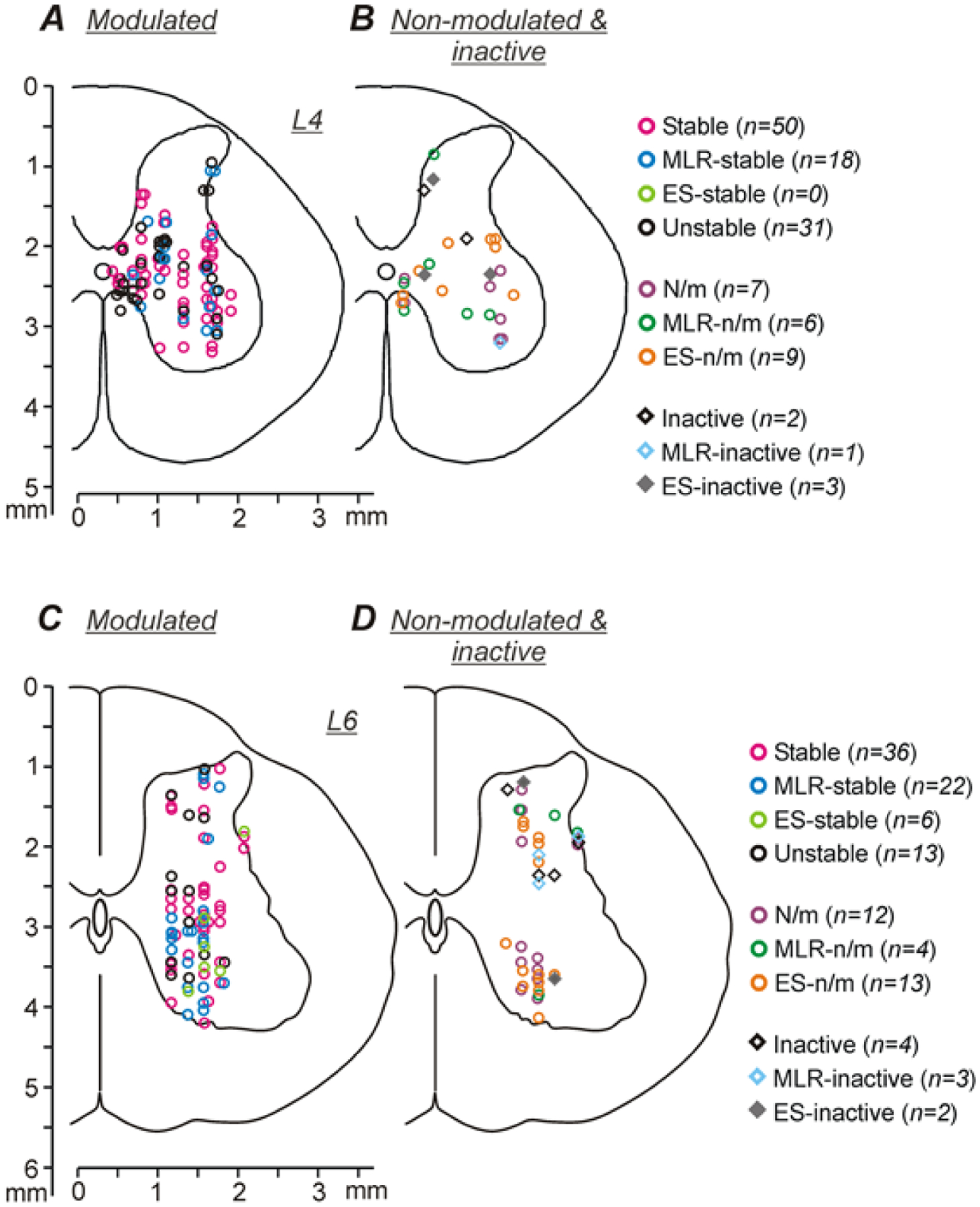
A-D, Position of different types of neurons modulated under both conditions (A,C), as well as non-modulated, inactive and modulated under only one condition neurons (B,D) on the cross-section of the spinal cord, recorded in L4 (A,B) and L6 (C,D). Designations: Stable, neurons with stable modulation under both conditions; MLR-stable, neurons with stable modulation during MLR-stimulation and unstable modulation during ES; ES-stable, neurons with stable modulation during ES and unstable modulation during MLR-stimulation; Unstable, neurons with unstable modulation under both conditions; N/m, non-modulated neurons; MLR-n/m, non-modulated during MLR stimulation and modulated during ES; ES-n/m, non-modulated during ES and modulated during MLR stimulation; Inactive, Neurons with the mean cycle frequency less than 1 Hz under both conditions; MLR-inactive, inactive during MLR stimulation only; ES-inactive, inactive during ES only.
Stable, MLR-stable, and Unstable neurons were found in all cats (Table 1). By contrast, a few ES-stable neurons were found only in L6 and only in one cat. In both L4 and L6, Stable neurons constituted about half of all modulated neurons (50 out of 99 and 36 out of 77, respectively). In the population of modulated neurons recorded in L4, the relative number of Unstable neurons was significantly higher than that in L6 (31 out of 99 in L4 vs 13 out of 77 in L6, χ2 test, p = 0.03). By contrast, the proportions of MLR-stable neurons in the populations of modulated neurons recorded in L4 and in L6 did not differ significantly (18 out of 99 and 22 out of 77, respectively, χ2 test, p = 0.10).
Activity of the same individual spinal neurons during MLR- and ES-evoked locomotion
Comparison of activity patterns.
To evaluate the degree of similarity between modulation patterns of the same neuron during MLR- and ES-evoked locomotion, we calculated the coefficient of similarity (KSIMILAR) (see Materials and Methods). Figure 4A–L shows the rasters and histograms of activity during MLR- and ES-evoked locomotion for three individual neurons with their coefficients of similarity. Two of them (shown in A-D and E-H, respectively) were Stable neurons and one (shown in K-L) was an Unstable neuron. One of the Stable neurons had almost the same pattern of modulation during MLR- and ES-evoked locomotion (compare C and D) and its coefficient of similarity was very high (KSIMILAR=0.97). Another one had less similar (but still rather similar) patterns of modulation under the two conditions (compare G and H), and correspondingly, a slightly lower coefficient of similarity (KSIMILAR=0.71). Finally, the Unstable neuron had substantially different patterns of modulation during MLR- and ES-evoked locomotion (compare K and L) and, correspondingly, a low value of coefficient of similarity (KSIMILAR=0.37).
Figure 4M,N shows the proportion of neurons with different values of coefficient of similarity in each of the three types of modulated neurons recorded in L4 (M) and in L6 (N). One can see that in both L4 and L6 segments, the majority (about 80%) of Stable neurons had rather high values of coefficient of similarity (KSIMILAR ≥ 0.7) and about 70% of Stable neurons had KSIMILAR ≥ 0.8. In both segments, neurons with KSIMILAR ≥ 0.7 constituted about 50% and 30% of MLR-stable and Unstable neurons, respectively. In both L4 and L6 segments, the relative number of neurons with KSIMILAR ≥ 0.7 (part of the bar outlined by the dashed line) was significantly higher in the population of Stable neurons compared to that in the populations of MLR-stable and Unstable neurons [42 out of 50 Stable neurons vs 9 out of 18 MLR-stable ones (χ2 test, p = 0.004), and 42 out of 50 Stable neurons vs 10 out of 31 Unstable ones (p = 2 × 10−5) in L4; 30 out of 36 Stable neurons vs 11 out of 22 MLR-stable ones (p = 0.007) and 30 out of 36 Stable neurons vs 4 out of 13 Unstable ones (p = 4 × 10−4)] in L6.
We suggest that Stable neurons with similar patterns of modulation during MLR- and ES-evoked locomotion (KSIMILAR ≥ 0.7) are the elements of the spinal locomotor network that operates similarly during stimulation of MLR and ES. We termed this population as “S-neurons”. For further analysis three major populations of modulated neurons (S-neurons, MLR-stable neurons, and Unstable neurons) were used. A few Stable but dissimilar (KSIMILAR < 0.7), as well as ES-stable neurons, were neglected.
Comparison of activity phases.
Figure 5A–L shows the distribution of the activity phases of individual S-neurons (A,D,G,J), MLR-stable (B,E,H,K) and Unstable (C,F,I,L) neurons recorded in L4 (A-F) and L6 (G-L) over the MLR-evoked (A-C,G-I) and ES-evoked (D-F,J-L) locomotor cycles. Each point on these graphs represents an individual neuron. Its abscissa and ordinate indicate the mean phase of the burst onset and the burst offset in the locomotor cycle, respectively.
Figure 5. Phases of activity in the locomotor cycle of individual S-neurons, MLR-stable, and Unstable neurons during MLR- and ES-evoked locomotion.
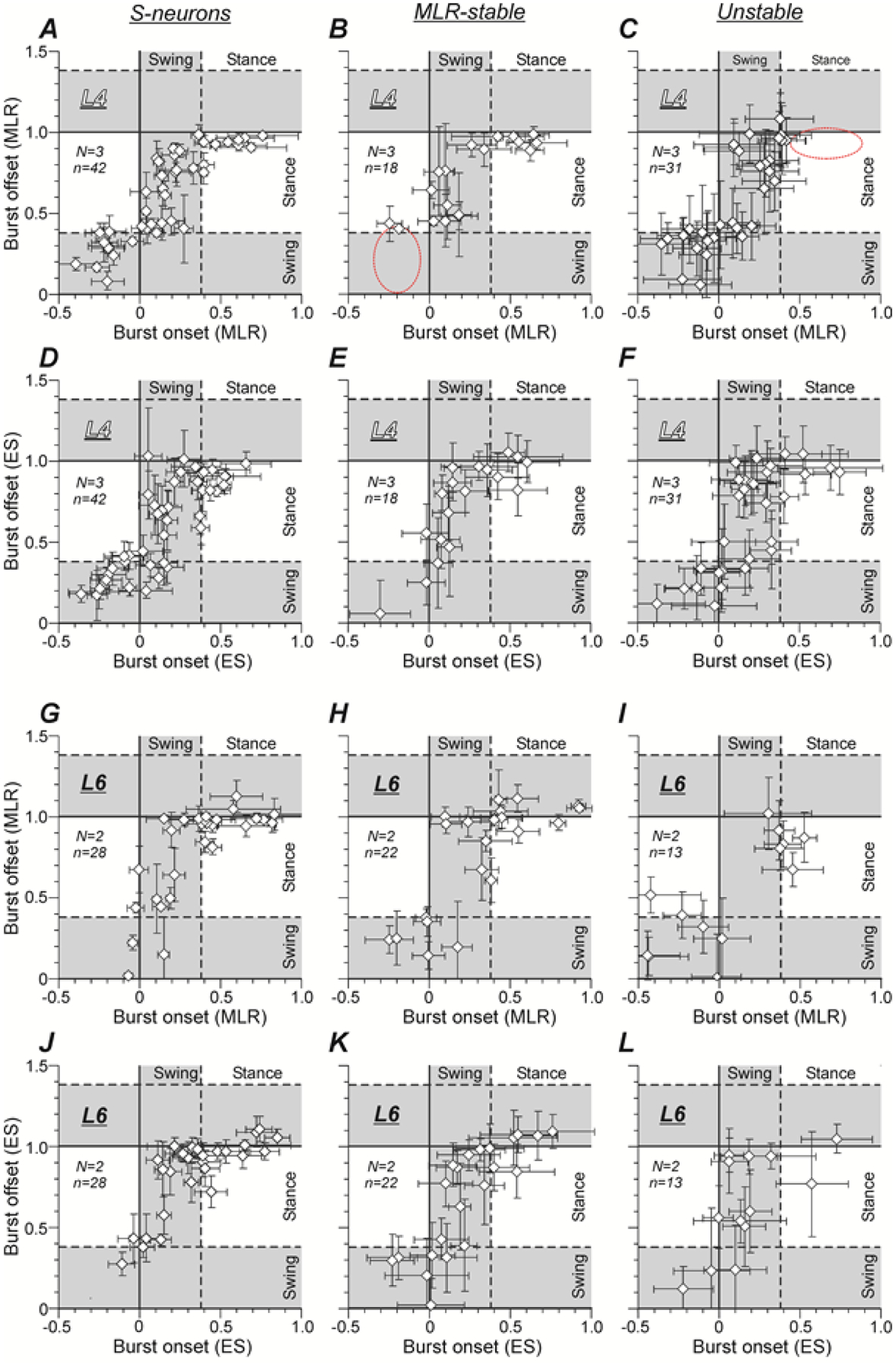
A-L, Phase distribution of bursts of individual S-neurons (A,D,G,J), MLR-stable (B,E,H,K) and Unstable (C,F,I,L) neurons recorded in L4 (A-F) and L6 (G-L) in the cycle of MLR-evoked (A-C,G-I) and ES-evoked (D-F,J-L) locomotion. The x and y values of each point show the mean ± SD phases of the burst onset and the burst offset in the locomotor cycle, respectively. A dashed red ellipse deliniates a part of the locomotor cycle in which a subpopulation of S-neurons recorded in L4 had bursts of activity, but in a given population such subpopulation was absent. Number of animals and number of S-neurons, MLR-stable, Unstable neurons in A-F: N = 3 and n = 42, 18, 31, respectively. Number of animals and number of S-neurons, MLR-stable, Unstable neurons in G-L: N = 2 and n = 30, 22, 13, respectively.
In general, the patterns of the activity phase distribution of the three populations of the spinal neurons [S-neurons (A), MLR-stable neurons (B) and Unstable neurons (C)] recorded in L4 during MLR stimulation were similar, although some small differences could be seen. For example, in the population of MLR-stable neurons, the sub-population of neurons with the burst onsets in the second half of the stance and the burst offsets in the swing (the area delineated by dashed line in B), which was present in the populations of S-neurons and Unstable neurons, was absent (compare B with A and C). In the population of Unstable neurons, the sub-population of neurons with the burst onsets within the first half of the stance and the burst offsets at the end of the stance (the area delineated by dashed line in C), which was present in the populations of MLR-stable and S-neurons, was absent (compare C with A and B). In each of the three populations of neurons recorded in L4, the pattern of the phase distribution during MLR stimulation was rather similar to that observed during ES (compare A and D, B and E, C and F, respectively).
The patterns of the activity phase distribution of the three populations of the spinal neurons [S-neurons (G), MLR-stable neurons (H) and Unstable neurons (I)] recorded in L6 during MLR stimulation had some differences compared with the patterns of the corresponding populations recorded in L4 (compare G and A, H and B, I and C, respectively). In particular, the majority of S-neurons and MLR-stable neurons generated the main part of the burst during stance.
Similar to the neurons recorded in L4, the pattern of the activity phase distribution of S-neurons during MLR stimulation was similar to that observed during ES (compare G and J, respectively). However, it was less similar in MLR-stable neurons (compare H and K, respectively) and substantially different in Unstable neurons (compare I and L, respectively).
In both L4 and L6 segments, the neurons generating the main part of the burst during swing and those generating the main part of the burst in stance were intermixed in the gray matter (not illustrated).
To estimate the difference in the phases of activity of individual neurons during MLR and ES stimulation, we compared the phases of their burst onset and burst offset under these two conditions. In Figure 6, A–C and G–I, the phase of the burst onset during MLR-stimulation was plotted against the burst onset phase during ES for individual neurons of the three populations: S-neurons (A,G), MLR-stable (B,H), and Unstable neurons (C,I) recorded in the L4 (A-C) and L6 (G-I) segments. Similar plots are presented for the phases of the burst offsets of the same neurons recorded in L4 and L6 under the two conditions (Fig. 6, D–F and J–L, respectively). One can see that the overwhelming majority of points in A and D (78% and 71%, respectively), as well as in G and J (75% and 86%, respectively), are located within a strip delimited by dotted lines. Thus, the majority of S-neurons located in both L4 and L6 exhibited the shift of the burst onset and the burst offset that was less than or equal to 0.1 part of the locomotor cycle. In both L4 and L6 segments, the relative number of such neurons was smaller in the populations of MLR-stable neurons (66% and 41%, 56% and 59% in B and H, E and K, respectively) and it was the smallest in the populations of Unstable neurons (58% and 16%, 52% and 38% in C and I, F and L, respectively).
Figure 6. Comparison of burst onsets and burst offsets of individual S-neurons, MLR-stable, and Unstable neurons in MLR-evoked and ES-evoked locomotor cycles.
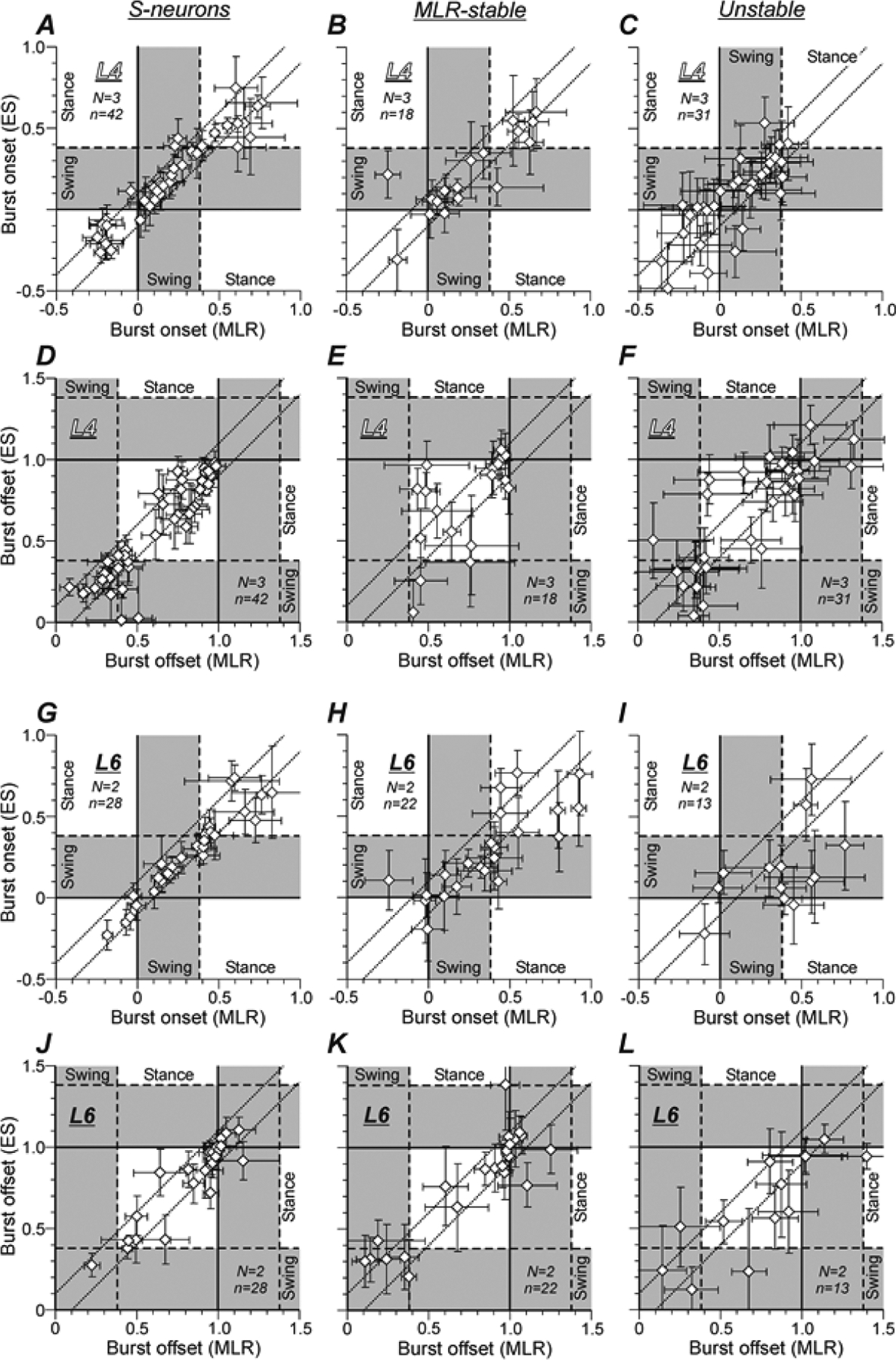
In scatter plots A-L, the x and y values of each point show the mean ± SD phase of the burst onset (A-C,G-I) or the mean ± SD phase of the burst offset (D-F,J-L) in the MLR- and ES-evoked locomotor cycle, respectively. The data are presented separately for S-neurons (A,D,G,J), MLR-stable (B,E,H,K), and Unstable (C,F,I,L) neurons recorded in L4 (A-F) and in L6 (G-L). Dotted lines delineate the neurons with a shift of the burst onset or the burst offset less or equal to 0.1 part of the locomotor cycle. Number of animals and number of S-neurons, MLR-stable, Unstable neurons in A-F: N = 3 and n = 42, 18, 31, respectively. Number of animals and number of S-neurons, MLR-stable, Unstable neurons in G-L: N = 2 and n = 30, 22, 13, respectively.
One can also note that while burst onsets were distributed rather evenly across the locomotor cycle (Fig. 6A–C,G–I), burst offsets (Fig. 6D–F,J–L) had some tendency of clustering into two groups located around the end of the swing – beginning of the stance and around the end of the stance – beginning of the swing, respectively. Both groups could be seen in the burst offset distribution of S-neurons and Unstable neurons from L4 (Fig. 6, D and F, respectively) and MLR-stable neurons from L6 (Fig. 6K), while in the burst offset distribution of MLR-stable neurons from L4 and S-neurons from L6, only the latter group was seen (Fig. 6, E and J, respectively).
Figure 7 compares the relative numbers of S-neurons, MLR-stable and Unstable neurons with less than or equal to 0.1 part of the cycle shift of the burst onset and/or the burst offset recorded in L4 (A) and in L6 (B). One can see that almost all S-neurons in both segments had at least one edge of the burst (onset or offset) with a phase shift that is less than or equal to 0.1 part of the cycle and more than 50% had such phase shift of both edges of the burst. In both segments, the proportion of such neurons was lower in the populations of MLR-stable and Unstable neurons. In L6, it was lower than in L4. One can also see also that in all three populations recorded in L6, the relative number of neurons with stable phase of burst offset was substantially higher than the relative number of neurons with stable phase of burst onset (B). By contrast, in the corresponding populations recorded in L4 (A), slightly more neurons with stable phase of burst onset than those with stable phase of burst offset were observed.
Figure 7. Comparison of burst phases of individual S-neurons, MLR-stable, and Unstable neurons in MLR-evoked and ES-evoked locomotor cycles.
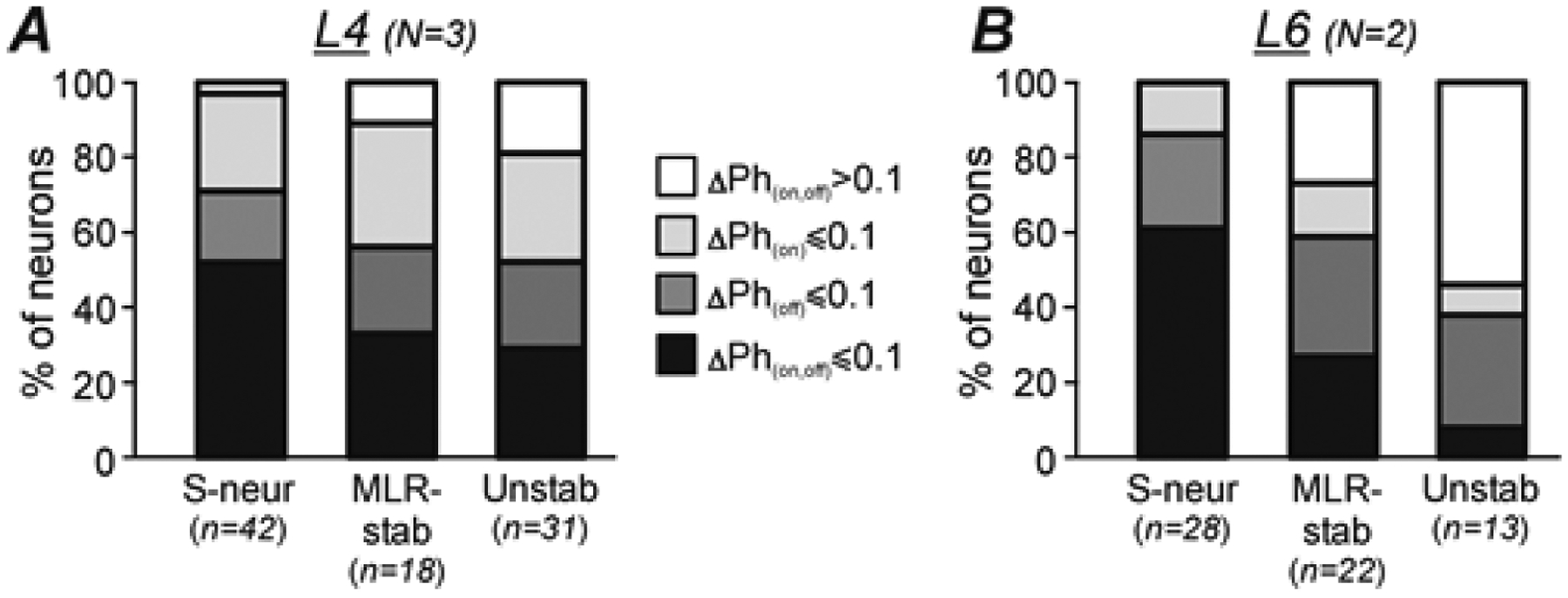
A,B, Relative number of S-neurons, MLR-stable (MLR-stab), and Unstable (Unstab) neurons with less or equal to 0.1 part of the cycle shift of both the burst onset and the burst offset (ΔPh(on,off) ≤ 0.1, black parts of bars), of the burst onset only (ΔPh(on) ≤ 0.1, light gray parts of bars), of the burst offset only (ΔPh(off) ≤ 0.1, dark gray parts of bars), and those with more than to 0.1 part of the cycle shift of both the burst onset and the burst offset (ΔPh(on,off) > 0.1, white parts of bars) recorded in L4 (A) and in L6 (B). Number of animals and number of S-neurons, MLR-stable, Unstable neurons in A: N = 3 and n = 42, 18, 31, respectively. Number of animals and number of S-neurons, MLR-stable, Unstable neurons in B: N = 2 and n = 30, 22, 13, respectively.
Comparison of activity levels.
In Figure 8, different characteristics of activity [the mean values of cycle frequency (FCYCLE, A,D,G), burst frequency (FBURST, B,E,H)) and interburst frequency (FINTER, C,F,I)] of individual S-neurons (A-C), MLR-stable (D-F), and Unstable (G-I) neurons recorded in L4 (dark green, yellow, and gray diamonds, respectively) and in L6 (light green, orange, and black diamonds, respectively) during MLR-stimulation were plotted against the corresponding characteristics during ES. The overwhelming majority of S-neurons recorded in L4 had higher mean cycle frequency and mean burst frequency during MLR-stimulation compared to that during ES (the overwhelming majority of the dark green diamonds are below the line x = y in A and B, respectively). By contrast, the majority of MLR-stable neurons recorded in both L4 and L6 segments had higher value of mean interburst frequency during ES as compared to that during MLR stimulation (the majority of both orange and yellow diamonds are above the line x = y in F).
Figure 8. Comparison of different characteristics of activity of individual S-neurons, MLR-stable, and Unstable neurons recorded in L4 and in L6 during MLR- and ES-evoked locomotion.
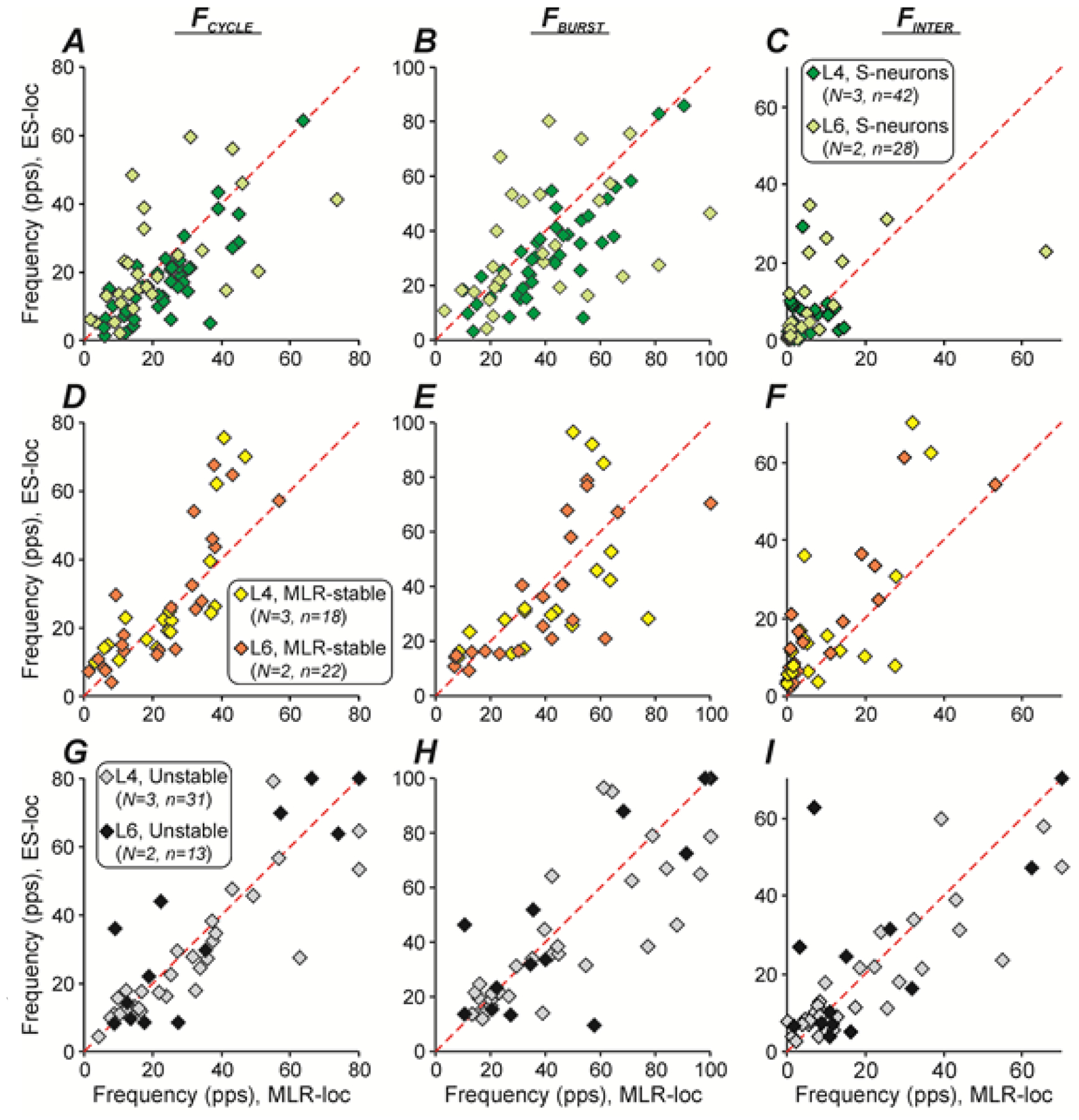
A-I, Comparison of mean cycle frequencies (FCYCLE, A,D,G), mean burst (FBURST, B,E,H) and interburst (FINTER, C,F,I) frequencies of individual S-neurons (A-C), MLR-stable (D-F) and Unstable (G-I) neurons during MLR- and ES-evoked locomotion. The x and y values of each point show the frequency of an individual neuron during MLR- and ES-evoked locomotion, respectively. Number of animals, number of S-neurons MLR-stable, Unstable neurons from L4 and L6: N = 3, n = 42, 18, 31 and N = 2, n = 30, 22, 13, respectively.
Figure 9 compares the mean ± SD values of different characteristics of activity for S-neurons, MLR-stable, and Unstable neurons recorded in the L4 and L6 spinal segments (indicated by lines across corresponding clouds of diamonds that show data points obtained in individual neurons). One can see that the average values of spontaneous activity of S-neurons and MLR-stable neurons located in L4 were substantially higher than those observed in the corresponding populations located in L6 [(7.3 ± 9.1 Hz (N = 5, n = 42) vs 3.9 ± 6.0 Hz (N = 5, n = 28) and 13.7 ± 13.3 Hz (N = 5, n = 18) vs 4.5 ± 6.5 Hz (N = 5, n = 22), respectively; Fig. 9A)]. For the MLR-stable neurons, the difference was statistically significant (Welch’s t test, p = 0.01). Thus, the level of excitability of S-neurons and MLR-stable neurons located in L4 was higher than those located in L6. By contrast, the mean values of the spontaneous activity of Unstable neurons recorded in L4 and those recorded in L6 were similar (Fig. 9A).
Figure 9. Comparison of different characteristics of population activity of S-neurons, MLR-stable, and Unstable neurons recorded in L4 and in L6 during MLR- and ES-evoked locomotion.
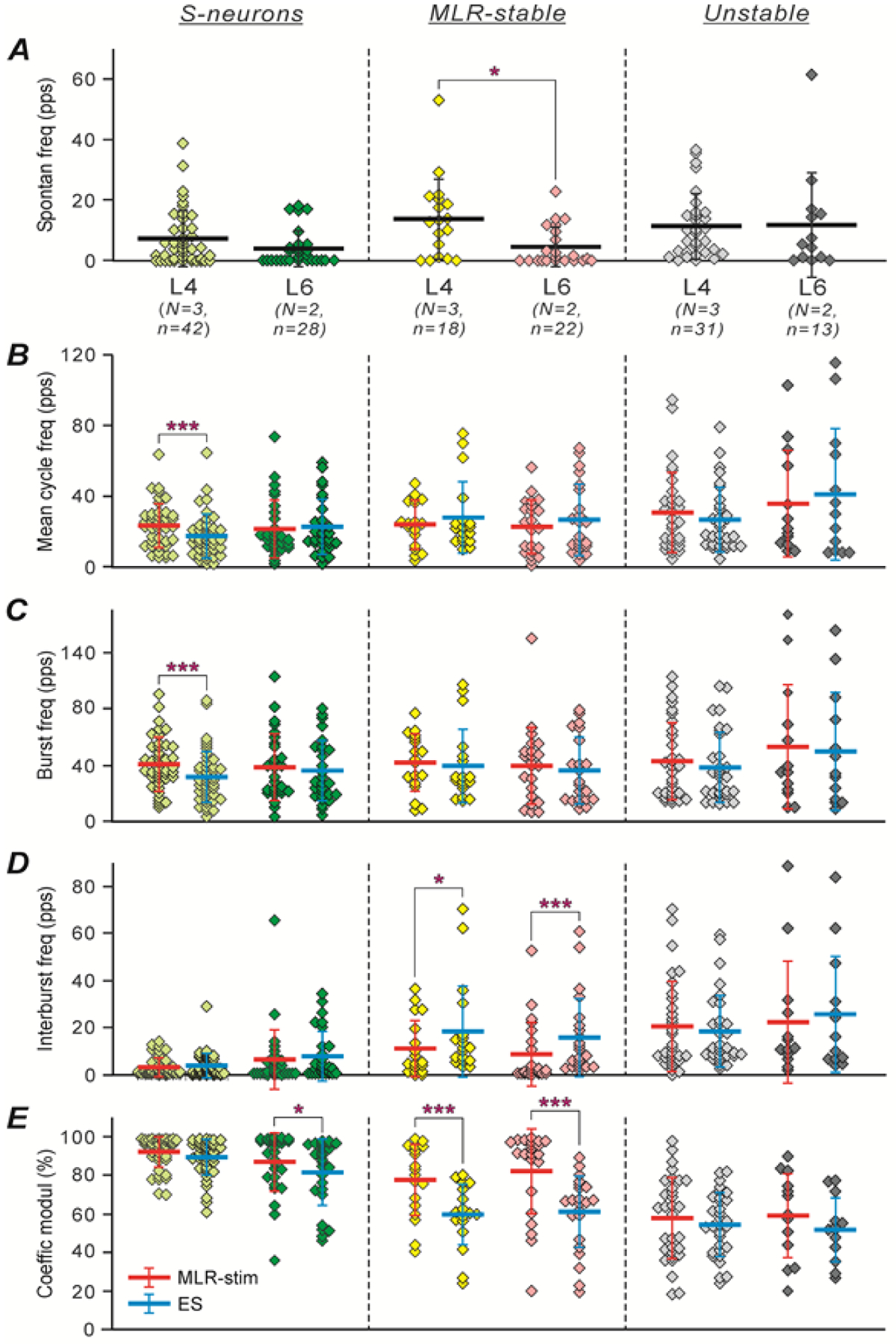
A-E, Mean ± SD values of spontaneous frequency (A), cycle frequency (B), burst (C) and interburst (D) frequency, coefficient of modulation (E) for populations of S-neurons, MLR-stable and Unstable neurons recorded in L4 and in L6 during MLR-evoked (indicated by red lines across corresponding clouds of diamonds showing the mean values of the parameter for individual neurons) and ES-evoked (indicated by blue lines) locomotion. Number of animals, number of S-neurons MLR-stable, Unstable neurons from L4 and L6: N = 3, n = 42, 18, 31 and N = 2, n = 30, 22, 13, respectively. Indication of significance level: * p < 0.05, *** p < 0.001.
Figure 9B–E compares the different characteristics of activity of S-neurons, MLR-stable and Unstable neurons recorded in L4 and L6 segments during locomotion evoked by MLR-stimulation and ES. One can see that the values of the mean cycle frequency shown in B (indicated by red and blue thick horizontal lines for conditions of MLR-stimulation and ES, respectively) were rather similar under the two conditions in L4 and L6 populations of S-neurons, MLR-stable, and Unstable neurons. The only significant though small difference was observed for the mean cycle frequency of S-neurons located in L4 (17.6 ± 12.2 Hz during ES vs 23.5 ± 12.6 Hz during MLR stimulation; N = 5, n = 42; paired Student’s t test, p = 10−5). The populations of S-neurons, MLR-stable, and Unstable neurons had rather similar mean cycle frequencies. Taking into account that the spontaneous activity of S-neurons and MLR-stable neurons in L4 was substantially higher than that in L6 (Fig. 9A), one can suggest that both MLR-stimulation and ES of L5 produced weaker activation of populations of these neurons located in L4 and stronger activation of those located in L6. One can also see that the mean value of the spontaneous activity of S-neurons located in L4 was significantly higher than the value of their mean interburst frequency during both MLR-stimulation and ES [7.3 ± 9.1 Hz vs 3.4 ± 4.0 Hz and 4.0 ± 5.1 Hz (N = 5, n = 42), paired Student’s t test, p = 0.02 and p = 0.01, respectively; compare the thick horizontal lines across the light green clouds in A and D)], suggesting that they receive inhibitory input in this part of the locomotor cycle. By contrast, S-neurons located in L6, as well as MLR-stable neurons and Unstable neurons located in both L4 and L6, had either similar or higher level of activity in the interburst period during both MLR- and ES-evoked locomotion as compared with the level of their spontaneous activity (compare the thick horizontal lines across the dark green, yellow, pink, gray, and black clouds in D and in A). One can propose that because of the stronger activation of these neurons by MLR-stimulation and by ES, the inhibitory input to these neurons during locomotion was masked.
Similar to values of the mean cycle frequency, the values of the mean burst frequency (shown in Fig. 9C) were also comparable in all populations of S-neurons, MLR-stable, and Unstable neurons, as well as in each population under the two conditions. The only significant difference in the mean burst frequency was observed in the population of S-neurons located in L4 during ES as compared with that during MLR stimulation [31.6 ± 18.4 Hz during ES vs 41.1 ± 18.3 Hz during MLR stimulation, (N = 5, n = 42), paired Student’s t test, p = 10−6; compare the red and blue thick horizontal lines across the light green clouds in Fig. 9C]. By contrast, S-neurons, MLR-stable, and Unstable neurons had different values of mean interburst frequency (Fig. 9D). This value was more than threefold higher in both L4 and L6 populations of Unstable neurons as compared with that observed in corresponding populations of S-neurons both during MLR-stimulation [20.5 ± 19.1 Hz (N = 5, n = 31) vs 3.4 ± 4.0 Hz (N = 5, n = 42), Welch’s t test, p = 3 × 10−5, in L4, and 22.7 ± 25.5 Hz (N = 5, n = 13) vs 6.4 ± 13.0 Hz (N = 5, n = 28), Welch’s t test, p = 0.04, in L6] and during ES [18.4 ± 15.4 Hz (N = 5, n = 31) vs 4.0 ± 5.14 Hz (N = 5, n = 42), Welch’s t test, p = 2 × 10−5, in L4, and 25.5 ± 25.0 Hz (N = 5, n = 13) vs 8.2 ± 10.4 Hz (N = 5, n = 28), Welch’s t test, p = 0.03, in L6]. One can also see that during MLR stimulation and ES, the mean interburst frequency was similar in the L4 and L6 populations of both S-neurons and Unstable neurons (compare the red and blue thick horizontal lines, respectively, across the light green, dark green, gray, and black clouds in Fig. 9D). By contrast, the value of the mean interburst frequency in both L4 and L6 populations of MLR-stable neurons was almost twofold higher during ES as compared with that during MLR-stimulation: 18.5 ± 19.4 Hz vs 11.2 ± 12.0 Hz in L4 population (N = 5, n = 18; paired Student’s t test, p = 0.04), and 16.1 ± 16.6 Hz vs 8.8 ± 13.4 Hz in L6 population (N = 5, n = 22; paired Student’s t test, p = 2 × 10−4); compare the blue and red thick horizontal lines across the yellow and pink clouds, respectively, in Fig. 9D. Most likely, this increase in interburst frequency was caused by responses of MLR-stable neurons to individual epidural stimuli during the interburst period, which also led to the loss of stability of modulation of these neurons during ES.
Figure 9E compares the mean values of the coefficient of modulation in L4 and L6 populations of S-neurons, MLR-stable, and Unstable neurons during MLR-stimulation and ES. One can see that both populations of S-neurons had rather similar and high coefficient of modulation under both conditions (compare the red and blue thick horizontal lines across the light and dark green clouds), although in the L6 population, a small but significant decrease in the coefficient value was observed during ES as compared to that during MLR-stimulation (81.9 ± 17.1 Hz vs 87.1 ± 15.3 Hz, respectively; N = 5, n = 28; paired Student’s t test, p = 0.01). Similar to S-neurons, in L4 and L6 populations of Unstable neurons, the mean value of the coefficient of modulation was almost the same under the two conditions (compare the red and blue thick horizontal lines across the gray and black clouds). However, it was significantly lower than that in corresponding populations of S-neurons during both MLR-stimulation [57.7 ± 21.5 Hz (N = 5, n = 31) vs 92.1 ± 8.3 Hz (N = 5, n = 42), Welch’s t test, p = 4 × 10−10, respectively, in L4; 58.8 ± 22.3 Hz (N = 5, n = 13) vs 87.1 ± 15.3 Hz (N = 5, n = 28), respectively, Welch’s t test, p = 7 × 10−4, in L6] and during ES [54.6 ± 16.2 Hz (N = 5, n = 31) vs 89.2 ± 9.3 Hz (N = 5, n = 42), respectively, Welch’s t test, p = 8 × 10−14, in L4; 51.9 ± 16.4 Hz (N = 5, n = 13) vs 81.9 ± 17.1 Hz (N = 5, n = 28), respectively, Welch’s t test, p = 2 × 10−5, in L6]. Finally, in the L4 and L6 populations of MLR-stable neurons, the coefficient of modulation during ES was significantly lower than that during MLR-stimulation (59.5 ± 16.0 Hz and 60.7 ± 19.2 Hz vs 77.7 ± 18.5 Hz and 82.3 ± 22.1 Hz, respectively; N = 5, n = 18 and 22, respectively; paired Student’s t test, p = 10−5 and p = 4 × 10−9, respectively). One can see that during MLR-stimulation, the values of the coefficient of modulation in L4 and L6 populations of MLR-stable neurons were close to those in the corresponding populations of S-neurons (compare the thick red horizontal lines across the yellow and pink clouds with those across the light green and dark green ones, respectively). By contrast, during ES, their values were close to those in the corresponding populations of Unstable neurons (compare the thick blue horizontal lines across the yellow and pink clouds with those across the gray and black ones, respectively).
Contribution of sensory feedback to locomotor modulation of individual neurons
To reveal movement-related sensory inputs from the ipsilateral and from the contralateral hindlimb to individual neurons, as well as to estimate their possible contribution to locomotor modulation of these neurons, the majority of S-neurons (n=55), MLR-stable (n=23), and Unstable (n=31) neurons were recorded during both passive movements of the ipsilateral hindlimb and during passive movements of the contralateral hindlimb performed along the forward walking trajectory.
Figure 10A shows a relative number of neurons with different responses to passive limb movements in each of the three populations. One can see that the majority of S-neurons, MLR-stable, and Unstable neurons (77%, 87%, and 90%, respectively) were modulated by movements of at least one of the hindlimbs. About half of the neurons in each of the three populations were modulated by movements of the ipsilateral, and the contralateral limb (Ipsi- & Co- in A), about 20–30% by movements of the ipsilateral limb only (Ipsi- in A), and about 5–10% by movements of the contralateral limb only (Co- in A).
Figure 10. Effects of passive movements of the ipsilateral and of the contralateral hindlimb along the locomotor trajectory on activity of S-neurons, MLR-stable, and Unstable neurons.
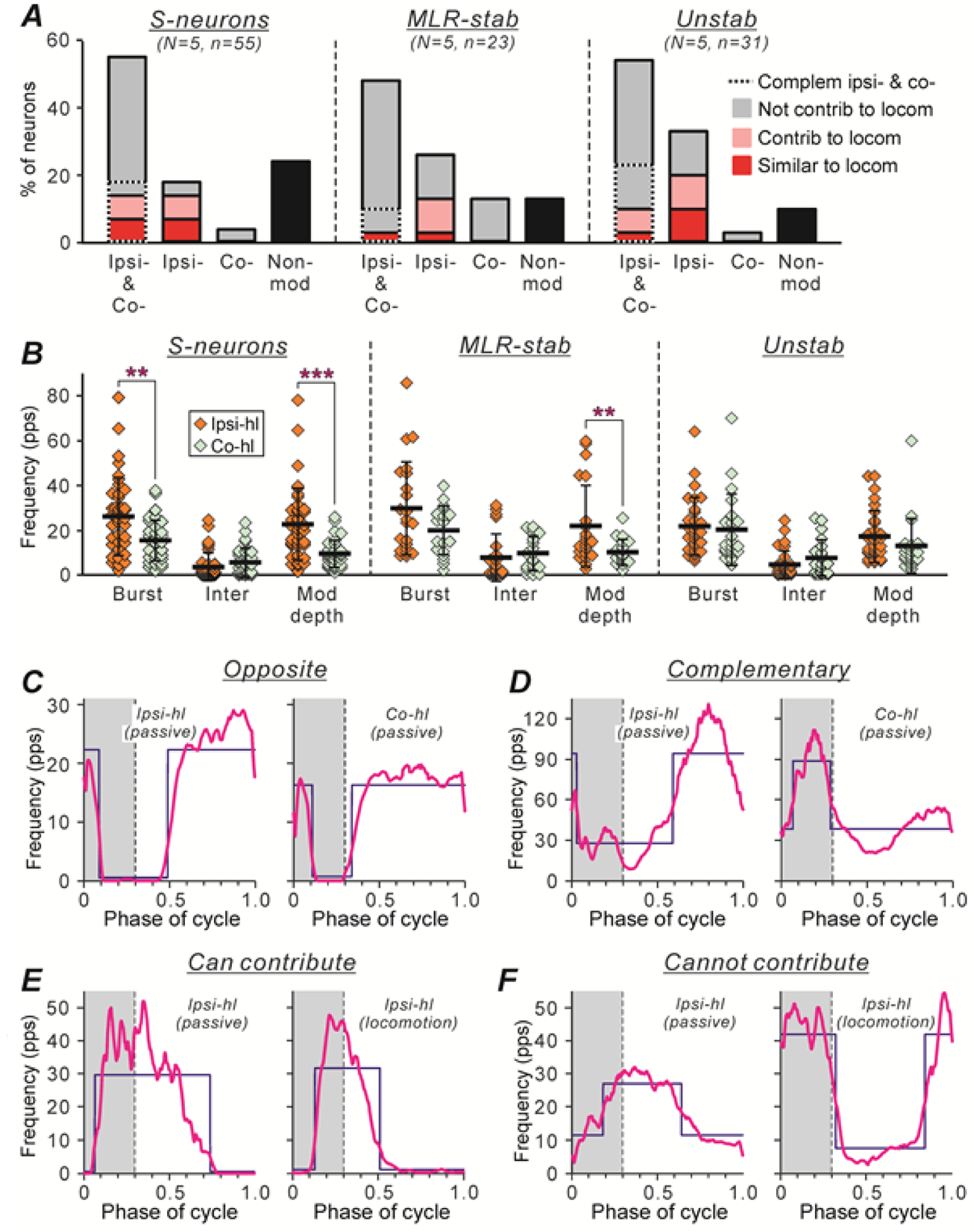
A, Relative numbers of neurons modulated by passive movements of either hindlimb (Ipsi- & Co- bars), neurons modulated by movements of only ipsilateral or only contralateral limb (Ipsi- and Co- bars, respectively), and neurons non-modulated by passive limb movements (Non-mod bars), in populations of S-neurons, MLR-stable, and Unstable neurons. Parts of the Ipsi- & Co- bars with dotted and solid outlines show the proportions of neurons with complimentary and opposite inputs from ipsilateral and contralateral hindlimb (see Results for explanation), respectively. Red, pink, and gray parts of the bars indicate the proportion of neurons with the phase of modulation caused by sensory feedback similar to, strongly overlapping with, and different from that observed during locomotion (Similar to loc, Contrib to loc, and Cannot contrib to loc, respectively). Number of S-neurons, MLR-stable, and Unstable neurons recorded in 5 cats: n=55, 23, and 31, respectively. B, Mean ± SD values of the burst (Burst) and interburst (Inter) frequency, and the depth of modulation in S-neurons, MLR-stable, and Unstable neurons caused by passive movements of the ipsilateral limb (Ipsi-hl, N = 5, n = 39, 22, 31, respectively) and of the contralateral hindlimb (Co-hl, N = 5, n = 31, 19, 21, respectively) are indicated by black lines across corresponding clouds of diamonds showing the mean frequencies for individual neurons. Indication of significance level: ** 0.001 < p < 0.01, *** p < 0.001. C,D, Examples of two neurons with opposite (C) and complementary (D) sensory feedback from ipsilateral and contralateral hindlimbs. E,F, Examples of two neurons with sensory feedback from ipsilateral limb only, which can contribute (E) and cannot contribute (F) to locomotor modulation of the neuron. For each neuron, the histogram (thick line) of the activity of the neuron in the cycle of the ipsilateral hindlimb (Ipsi-hl) passive movements (left panels in C-F), and in the cycle of the contralateral hindlimb (Co-hl) passive movements (right panels in C,D) or in the cycle of the ipsilateral hindlimb locomotor movements (right panels in E,F) are shown. The swing phase is highlighted. The blue lines show the best two-level rectangular approximations of the histograms (as in Fig. 4A–L).
Figure 10B compares the mean ± SD values of the burst (Burst) and interburst (Inter) frequency, as well as the depth of modulation (Mod depth) of S-neurons, MLR-stable, and Unstable neurons, caused by passive movements of the ipsilateral hindlimb with those caused by movements of the contralateral limb (indicated by black lines across the orange and light green clouds, respectively). One can see that in populations of S-neurons and MLR-stable neurons, the mean burst frequency during movements of the ipsilateral limb was substantially higher compared to that during movements of the contralateral one (compare the thick horizontal lines across the orange and light green Burst clouds). For the population of S-neurons, this difference was statistically significant [26.24 ± 17.18 Hz (N = 5, n = 39) vs 15,29 ± 9.44 Hz (N = 5, n = 31), Welch’s t test, p = 0.001]. By contrast, the values of the mean interburst frequency during movements of the ipsilateral and the contralateral hindlimb were rather similar (compare the thick horizontal lines across the orange and light green Inter clouds). Correspondingly, in populations of S-neurons and MLR-stable neurons, the mean value of the depth of the modulation caused by movements of the ipsilateral limb was about twofold higher than that caused by movements of the contralateral limb [respectively, 22.56 ± 16.14 Hz (N = 5, n = 39) and 22.03 ± 17.90 Hz (N = 5, n = 22) vs 9.57 ± 6.09 Hz (N = 5, n = 31) and 10.20 ± 5.72 Hz (N = 5, n = 19); Welch’s t test, p = 3 × 10−5 and p = 0.007, respectively; compare the thick horizontal lines across the orange and light green Mod depth clouds). These results suggest that the populations of S-neurons and MLR-stable neurons received stronger sensory input from the ipsilateral limb than from the contralateral one.
For neurons that were modulated by passive movements of the limbs, we tried to assess a possible contribution of this sensory-produced modulation to their locomotor modulation. For this purpose, for each neuron that was modulated by passive movements performed by either hindlimb, first, we compared the phase of its modulation in the cycle of the ipsilateral limb movement with that observed in the cycle of the contralateral limb movement (Fig. 10C,D). We found that in each of the three populations, more than half of the neurons modulated by sensory inputs from any hindlimb (parts of Ipsi- & Co- bars with solid outline in Fig. 10A) had the same phase of modulation in the cycle of the ipsilateral and in the cycle of the contralateral limb movement (as a neuron in Fig. 10C). Thus, sensory inputs from the limbs cannot explain the locomotor modulation of these neurons since during walking, the hindlimbs moved in antiphase. The rest of the neurons had either opposite phases of modulation in the cycles of ipsilateral and contralateral limb movement (e.g., in the middle of the stance during ipsi-limb movement and in the swing during contra-limb movement, as a neuron in Fig. 10D, or at the end of the stance during ipsi-limb movement and at the beginning of stance during contra-limb movement, etc.). Thus, such neurons received complementary sensory inputs from the two limbs (Complem ipsi- & co-, parts of Ipsi- & Co- bars outlined by the dashed line in Fig. 10A), which could potentially contribute to locomotor modulation of these neurons.
Second, to find out if it was the case, we compared the phase of the modulation of these neurons in the cycle of the ipsilateral limb during MLR-evoked locomotion with that observed during passive movements of the ipsilateral limb. We found that in less than half of the neurons with complementary sensory inputs, the phase of modulation was similar under the two conditions (Similar to locom, red parts of the Ipsi- & Co- bars in Fig. 10A), suggesting that in these neurons, locomotor modulation can be explained by sensory feedback from the hindlimbs. In a part of S-neurons and Unstable neurons with complementary sensory inputs, we also found neurons that had largely overlapping phases of modulation under the two conditions (as a neuron shown in Fig. 10E), suggesting that sensory feedback from the hindlimbs can potentially contribute to locomotor modulation of these neurons (Contrib to locom, pink parts of the Ipsi- & Co- bars in Fig. 10A). However, the majority of MLR-stable and Unstable neurons, as well as a part of S-neurons with complementary sensory inputs had the opposite phase of modulation under the two conditions (as a neuron shown in Fig. 10F), suggesting that sensory feedback from the hindlimbs cannot contribute to locomotor modulation of these neurons (Not contrib to locom, gray parts of the Ipsi- & Co- bars outlined by the dashed line in Fig. 10A). Neurons with similar, largely overlapping, and opposite phases of modulation during locomotion and during passive limb movements were found also within neurons modulated by passive movements of ipsilateral limb only (red, pink and gray parts of Ipsi- bars in Fig. 10A). Within neurons modulated by passive movements of the contralateral limb only, we did not find neurons whose modulation phase in the cycle of passive movements of the contralateral limb was opposite to that observed in the locomotor cycle of the ipsilateral limb, suggesting that sensory feedback from the hindlimbs can neither explain nor contribute to locomotor modulation of these neurons.
To conclude, we found that only in a small proportion of S-neurons, MLR-stable, and Unstable neurons, sensory feedback from the hindlimbs can explain (in 14%, 6%, and 13% of the neurons, respectively) or contribute (in 14%, 10%, and 15% of the neurons, respectively) to locomotor modulation. However, it is necessary to note that passive limb movement along the locomotor trajectory only partly mimicked the sensory feedback during real locomotion. One can expect that many limb afferents were activated similarly during passive and during locomotor limb movement (e.g., joint afferents, mechanoreceptors of the foot sole), while other afferents most likely had weaker activity (e.g., muscle spindle afferents that have central control mediated by gamma-motoneurons, Murphy et al. 1984) or were silent (e.g., Ib afferents). Thus, one cannot exclude that the proportion of neurons modulated during locomotion exclusively by sensory feedback was underestimated.
Discussion
In the present study, we compared the activity of the same individual spinal neurons during real (with normal sensory feedback from limbs) forward locomotion evoked in decerebrate cats by MLR stimulation and ES of the spinal cord. We found that the overwhelming majority of neurons active during locomotion had modulation of their activity in the locomotor rhythm, suggesting that the network generating forward locomotion during MLR stimulation and ES are formed by the same neurons. The majority of these neurons were located in the intermediate and ventral parts of the gray matter outside the motor nuclei (Vanderhorst & Holstege, 1997), suggesting that they are putative interneurons.
Among the modulated neurons, we revealed and characterized three populations of neurons (S-neurons, MLR-stable, and Unstable neurons), which differed in the stability of their modulation within a locomotor trial (dispersion of their activity phase in sequential cycles), in the degree of similarity of the phase and shape of activity in MLR- and ES-evoked locomotor cycles, as well as in the strength of the activity modulation (reflected in the coefficient of modulation value).
S-neurons had stable modulation (very small dispersion of their activity phase in sequential cycles), similar phase and shape of activity in the cycles of MLR- and ES-evoked locomotion. They also had high coefficient of modulation under both conditions. Thus, a part of the locomotor network formed by S-neurons operated similarly during MLR stimulation and ES. We suggest that this part of the network is critically important for generation of basic features of the forward locomotor pattern common for MLR- and ES-evoked locomotion (Iwahara et al. 1992; Musienko et al. 2012).
In contrast to S-neurons, Unstable neurons had unstable modulation (large dispersion of their activity phase in sequential cycles) and the majority of them had dissimilar phase or shape of activity in the cycles of MLR-evoked and ES-evoked locomotion. Although the level of activity of Unstable neurons during locomotion (the mean cycle frequency) was similar to that observed in S-neurons, their coefficient of modulation was significantly (almost twofold) lower. Thus, it is unlikely that Unstable neurons are essential for generation of the locomotor pattern. Since they were strongly activated by both MLR stimulation and ES, one of the possible functions of Unstable neurons could be maintenance of the high excitability level of locomotor networks necessary for generation of stepping. Alternatively, they could belong to postural networks, which are activated simultaneously with locomotor networks by both MLR stimulation and ES (Musienko et al. 2014).
In contrast to S-neurons and Unstable neurons, which had similar stability of modulation and coefficient of modulation during MLR- and ES-evoked locomotion, MLR-stable neurons behaved differently under the two conditions. During MLR-evoked locomotion, they had stable modulation and high coefficient of modulation (like S-neurons). However, during ES-evoked locomotion, their modulation was unstable and their coefficient of modulation was similar to that in Unstable neurons, although in contrast to Unstable neurons, about half of MLR-stable neurons had similar phase of modulation and shape of activity in MLR- and ES-evoked locomotor cycles. Thus, ES distorted operation of a part of the locomotor network formed by MLR-stable neurons.
Recently, we demonstrated that kinematics of forward locomotor movements evoked by ES depend on the site of the spinal cord stimulation (Merkulyeva et al. 2018). In the present study, we found that stepping movements evoked by ES of L5 and MLR-stimulation differed to some extent. In particular, the vigor of locomotion was lower, and the anterior-posterior movements of the limbs had smaller amplitude during ES as compared to those during MLR-stimulation. We suggest that some of these differences in kinematics are caused by a change in operation of a part of the locomotor network formed by MLR-stable neurons during ES as compared to that during MLR-stimulation.
We found that although the majority of neurons were modulated by passive movements of the ipsilateral and/or contralateral hindlimb performed along the locomotor trajectory, the locomotion-related modulation of the majority of S-neurons, MLR-stable, and Unstable neurons was not determined by the sensory feedback from the limbs. We suggest that S-neurons and MLR-stable neurons belong to locomotor CPG while Unstable neurons are modulated by strongly fluctuating polysynaptic input from CPG. The obtained result suggests that sensory feedback from the limbs to the majority of the spinal neurons observed at rest state of the cat was strongly modified when the locomotor network was activated. This result is in line with earlier studies that demonstrated phase-dependent gating, as well as disappearance or appearance of sensory input from specific limb afferents to spinal neurons during locomotion (Duysens & Pearson, 1980; Conway et al. 1987; Shefchyk et al. 1990; Gossard et al. 1994; Quevedo et al. 1998; McCrea, 1998; Angel et al. 2005). One should note, however, that during real locomotion, sensory feedback from the limb controls a critical point in the step cycle, that is, onset of the swing (Grillner, 1975; Grillner & Rossignol, 1978; Orlovsky et al. 1999; Rossignol et al. 2006). Thus neurons transmitting sensory feedback to CPG also represent important elements of the locomotor network controlling real stepping.
It was demonstrated that although the network with rhythmogenic potential is distributed in the lumbosacral enlargement (Deliagina et al. 1983; Cazalets & Bertrand, 2000; Lev-Tov et al. 2000), a part of this network located in the rostral segments has a higher level of excitability and plays a leading role in entraining the rhythm in more caudal segments (Deliagina et al. 1983; Cazalets et al. 1995; Kjaerulff & Kiehn, 1996; Gerasimenko et al. 2019). In the present study, for the first time, we compared the locomotion-related activity of neurons located in L4 (which is critical for the expression of locomotion in cats, Barthelemy et al. 2007; Langlet et al. 2005) with that recorded in L6. In both segments, we revealed S-neurons, MLR-stable, and Unstable neurons and found that during MLR-evoked, as well as during ES-evoked locomotion, different parameters of activity were similar in the sub-populations of MLR-stable and Unstable neurons located in L4 and in L6, suggesting that they are similarly involved in the control of locomotion. By contrast, activity of subpopulation of S-neurons located in L4 was significantly lower during ES compared with that during stimulation of MLR. The decrease in the activity of this subpopulation can contribute to a change in vigor and kinematics of locomotor movements observed during ES as compared with those during MLR-stimulation.
In addition, we found a rostro-caudal difference of excitability in S-neurons and MLR-stable neurons expressed in the level of spontaneous activity of subpopulations located in the L4 and L6 segments. This result represents some indirect evidence in favor of our hypothesis that the majority of S-neurons and MLR-stable neurons belong to the locomotor CPG.
We also found some differences in the activity phase distribution of neurons located in L4 and L6. In particular, S-neurons located in L4 contained both swing- and stance-related neurons but those located in L6 were mainly stance-related. Neurons recorded in the present study had a broad distribution of activity phases over the locomotor cycle with a predominance of neurons with the main part of their bursts in the stance. This result is similar to that obtained earlier in spinal cats during air stepping (AuYong et al. 2011). We also found that if burst onsets were rather evenly distributed across the locomotor cycle, the bursts of the majority of the neurons were terminated either around the end of the stance - beginning of the swing or around the end of the swing - beginning of the stance. A similar burst offset distribution with two peaks was observed in deafferented cats (Orlovsky & Feldman, 1972).
There are two main conceptual models of organization of locomotor CPG. In the framework of the two-layer hypothesis (McCrea & Rybak, 2008; Rybak et al. 2015), one can suggest that S-neurons belong both to the rhythm generating and pattern formation layers while MLR-stable neurons belong to the pattern formation layer. In the framework of the one-layer hypothesis (Grillner, 2006), one can suggest that S-neurons belong to unit CPGs while MLR-stable neurons are responsible for the coordination of the unit CPGs activity, leading to formation of the locomotor pattern.
In both L4 and L6 segments, we found neurons which were active but not modulated during locomotion. It is possible that these neurons mediate the effect of MLR stimulation and/or ES on spinal locomotor networks, and thus, contribute to their activation and/or maintenance of their excitability level required for generation of locomotion.
To conclude, in the present study, for the first time, the operations of spinal locomotor networks during real locomotion evoked by ES of the spinal cord and by stimulation of the MLR of the brainstem have been compared. A new method of analysis of rhythm-related neuronal activity based on stability and similarity of modulation pattern, which can be used for analysis of neuronal activity related to other rhythmical movements, has been introduced. By using this method, we revealed three components of active during locomotion spinal networks with presumably different functional roles. Results of the present study advance our understanding of the neuronal mechanisms of the ES therapeutic effects in subjects with intact spinal cord suffering from impairment of locomotion (e.g., caused by Parkinson’s disease; Zong et al. 2019) and can potentially be used for the development of more effective strategies for the recuperation of impaired locomotor function in such subjects. One of the important questions is whether the effects of ES on the spinal networks in spinal cord-injured subjects and in subjects with intact spinal cord are similar. Results of the present study represent the basis for addressing this important question in future investigations.
Key points.
Epidural electrical stimulation of the spinal cord (ES) restores/improves locomotion in patients. ES-evoked locomotor movements differ to some extent from the normal ones. Operation of the locomotor network during ES is unknown.
We compared activity of individual spinal neurons during locomotion initiated by signals from the brainstem and by ES.
We demonstrated that the spinal network generating locomotion under each of the two conditions is formed by the same neurons.
A part of this network operates similarly under the two conditions, suggesting that it is essential for generation of locomotion under both conditions.
Another part of this network operates differently under the two conditions, suggesting that it is responsible for differences in the movement kinematics observed under the two conditions.
Funding
This work was supported by grants from Russian Science Foundation (No14-15-00788) and by Russian Foundation for Basic Research (No16-04-01791) to PEM; by grant from NIH (R01 NS-100928) to TGD and PEM; by grants from Swedish Research Council to TGD (2017-02944) and to PVZ (no. 21076).
Biography

Pavel Musienko received his PhD from the Pavlov Institute of Physiology (St. Petersburg, Russia) in 2005. In 2005–2007, Dr. Musienko was a postdoctoral scholar at the Karolinska Institute (Stockholm, Sweden). Dr. Musienko is currently a professor in neuroscience and the Head of Laboratory of Neuroprosthetics at St. Petersburg State University. His long-term research goals are to examine neural mechanisms underlying pathophysiology of spinal cord injuries and to develop neurorehabilitation interventions to restore the motor function after neuromotor disorders.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Angel MJ, Jankowska E & McCrea DA (2005). Candidate interneurons mediating group I disynaptic EPSPs in extensor motoneurons during fictive locomotion in the cat. J Physiol 563, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AuYong N, Olliver-Lanvin K & Lemay A (2011). Preferred locomotor phase of activity of lumbar interneurons during air-stepping in subchronic spinal cats. J Neurophysiol 105, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baev KV, Degtyarenko AM, Zavadskaya TV & Kostyuk PG (1979). Activity of lumbar interneurons during fictitious locomotion in thalamic cats. Neirofiziologiya 11, 329–338. [PubMed] [Google Scholar]
- Baev KV, Beresovskii VK, Kebkalo TG & Savoskina LA (1988). Afferent and efferent connections of brainstem locomotor regions study by means of horseradish peroxidase transport technique. Neuroscience 26, 871–892. [DOI] [PubMed] [Google Scholar]
- Barthélemy D, Leblond H & Rossignol S (2007). Characteristics of mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. J Neurophysiol 97, 1986–2000. [DOI] [PubMed] [Google Scholar]
- Barschelet E (1981). Circular statistics in biology. New York, USA: Academic Press. [Google Scholar]
- Berkowitz A & Stein PS (1994). Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: phase analyses. J Neurosci 14, 5105–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G & Kiehn O (2018). Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli P, Pivetta C, Esposito MS & Arber S (2017). Locomotor speed control circuits in the caudal brainstem. Nature 551, 455–460. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G & Micera S (2013). A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33, 19326–19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR & Bertrand S (2000). Coupling between lumbar and sacral motor networks in the neonatal rat spinal cord. Eur J Neurosci 12, 2993–3002. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Borde M & Clarac F (1995). Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15, 4943–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H & Kiehn O (1987). Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68, 643–656. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV & Edgerton VR (2009). Transformation of nonfunctional spinal circuits into functional states after loss of brain input. Nature Neurosci 12, 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN & Pavlova GA (1983). The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res 53, 81–90. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Gerasimenko Y & Pinter MM (1998). Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci 860, 360–376. [DOI] [PubMed] [Google Scholar]
- Duysens J & Pearson KG (1980). Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187, 321–332 [DOI] [PubMed] [Google Scholar]
- Ferreira-Pinto MJ, Ruder L, Capelli P & Arber S (2018). Connecting circuits for supraspinal control of locomotion. Neuron 100, 361–374. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E & Skinner RD (1987a). The mesencephalic locomotor region. i. Activation of a medullary projection site. Brain Res 411, 1–12. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E & Skinner RD (1987b). The mesencephalic locomotor region. ii. Projections to reticulospinal neurons. Brain Res 411, 13–20. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA & Lavrov IA (2003). Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci Behav Physiol 33, 247–254. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Bogacheva IN, Shcherbakova NA, Kucher VI & Musienko PE (2005). Formation of locomotors patterns in decerebrate cats in conditions of epidural stimulation of the spinal cord. Neurosci Behav Physiol 35, 291–298. [PubMed] [Google Scholar]
- Gerasimenko Y, Musienko P, Bogacheva I, Moshonkina T, Savochin A, Lavrov I, Roy RR & Edgerton VR (2009). Propriospinal bypass of the serotonergic system that can facilitate stepping. J Neurosci 29, 5681–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Preston C, Zhong H, Roy RR, Edgerton VR & Shah PK (2019). Rostral lumbar segments are the key controllers of hindlimb locomotor rhythmicity in the adult spinal rat. J Neurophysiol 122, 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani CA & Smith JL (1987). Stepping behaviors in chronic spinal cats with one hindlimb deafferented. J Neurosci 7, 2537–2546. [PMC free article] [PubMed] [Google Scholar]
- Goldberger ME (1977). Locomotor recovery after unilateral hindlimb deafferentation in cats. Brain Res 123, 59–74. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I & Hultborn H (1994). Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98, 213–228. [DOI] [PubMed] [Google Scholar]
- Grillner S (1975). Locomotion in vertebrates - central mechanisms and reflex interaction. Physiol Rev 55, 247–304. [DOI] [PubMed] [Google Scholar]
- Grillner S (2006). Biological patterm generation: cellular and computational logic of networks in motion. Neuron 52, 751–766. [DOI] [PubMed] [Google Scholar]
- Grillner S & Rossignol S (1978). On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res 146, 269–277. [DOI] [PubMed] [Google Scholar]
- Grillner S & Zangger P (1979). On the central generation of locomotion in the low spinal cat. Exp Brain Res 34, 241–261. [DOI] [PubMed] [Google Scholar]
- Grillner S & Zangger P (1984). The effect of dorsal root transection on the efferent motor pattern in the cat’s hindlimb during locomotion. Acta Physiol Scand 120, 393–405. [DOI] [PubMed] [Google Scholar]
- Grillner S & El Manira A (2015). The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol 31, 244–249. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E & Skinner RD (1992). Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull 28, 99–105. [DOI] [PubMed] [Google Scholar]
- Jordan LM (1986). Initiation of locomotion from the mammalian brainstem. In: Neurobiology of vertebrate locomotion, ed. Grillner S, Stein PSG, Stuart DG & Forssberg H, pp. 21–37. Macmillan, London. [Google Scholar]
- Kiehn O (2006). Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29, 279–306. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O & Kiehn O (1996). Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neirosci 16, 5777–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet C, Leblond H & Rossignol S (2005). Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J Neurophysiol 93, 2474–2488. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Delvolve I & Kremer E (2000). Sacrocaudal afferents induce rhythmic efferent bursting in isolated spinal cords of neonatal rats J Neurophysiol 83, 888–894. [DOI] [PubMed] [Google Scholar]
- McCrea DA (1998). Neuronal basis of afferent-evoked enhancement of locomotor activity. Ann NY Acad Sci 860, 216–225. [DOI] [PubMed] [Google Scholar]
- McCrea DA & Rybak IA (2008). Organization of mammalian locomotor rhythm and pattern generator. Bain Res Rev 57, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkulyeva N, Veshchitskii A, Gorsky O, Pavlova N, Zelenin PV, Gerasimenko Y, Deliagina TG & Musienko P (2018). Distribution of spinal neuronal networks controlling forward and backward locomotion. J Neurosci 38, 4695–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F & Dimitrijevic MR (2004). Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42, 401–416. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Stein RB & Taylor J (1984). Phasic and tonic modulation of impulse rates in gamma-motoneurons during locomotion in premammillary cats. J Neurophysiol 52, 228–243. [DOI] [PubMed] [Google Scholar]
- Musienko PE, Bogacheva IN & Gerasimenko YP (2007). Significance of peripheral feedback in the generation of stepping movements during epidural stimulation of the spinal cord. Neurosci Behav Physiol 37, 181–190. [DOI] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Lyalka VF, Gerasimenko YP, Orlovsky GN & Deliagina TG (2012). Spinal and supraspinal control of the direction of stepping during locomotion. J Neurosci 32, 17442–17453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Deliagina TG, Gerasimenko YP, Orlovsky GN & Zelenin PV (2014). Limb and trunk mechanisms for balance control during locomotion in quadrupeds. J Neurosci 34, 5704–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN (1969). Spontaneous and induced locomotion of the thalamic cat. Biophysics 14, 1154–1162. [Google Scholar]
- Orlovsky GN (1970a). Connections of the reticulo-spinal neurons with the ‘locomotor regions’ of the brain stem. Biophysics 15, 178–186. [Google Scholar]
- Orlovsky GN (1970b). Activity of the reticulo-spinal neurons during locomotion. Biophysics 15, 761–771. [Google Scholar]
- Orlovsky GN & Feldman AG (1972). Classification of lumbosacral neurons by their discharge pattern during evoked locomotion. Neirofiziologiya 4, 311–317. [Google Scholar]
- Orlovsky GN, Deliagina TG & Grillner S (1999). Neuronal control of locomotion. From mollusc to man. Oxford UP, Oxford, UK. [Google Scholar]
- Quevedo J Fedirchuk B, Gasgnach S & McCrea D (1998). Group I disynaptic excitation in flexor and bifunctional motoneurons during locomotion. In: Neuronal Mechanisms for Generating Locomotor Activity, ed. Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H & Kudo N, vol. 860, pp. 499–501. Ann. NY Acad. Sci., New York. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R & Gossard J-P (2006). Dynamic sensorimotor interactions in locomotion. Physiol Rev 86, 89–154. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Dougherty KJ & Shevtsova NA (2015). Organization of the Mammalian Locomotor CPG: Review of Computational Model and Circuit Architectures Based on Genetically Identified Spinal Interneurons(1,2,3). eNeuro 2, doi: 10.1523/ENEURO.0069-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefchyk S, McCrea DA, Kriellaars D, Fortier P & Jordan L. (1990). Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Exp Brain Res 80, 290–295. [DOI] [PubMed] [Google Scholar]
- Shik ML & Orlovsky GN (1976). Neurophysiology of locomotor automatism. Physiol Rev 56, 465–501. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV & Orlovsky GN (1966). Control of walking and running by means of electrical stimulation of the mid-brain. Biophysics 11, 756–765. [PubMed] [Google Scholar]
- Vanderhorst VG & Holstege G (1997). Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382, 46–76. [PubMed] [Google Scholar]
- Waller WH (1940). Progression movements elicited by subthalamic stimulation. J Neurophys 3, 300–307. [Google Scholar]
- Zelenin PV, Deliagina TG, Orlovsky GN, Karayannidou A, Stout EE, Sirota MG & Beloozerova IN (2011). Activity of motor cortex neurons during backward locomotion. J Neurophysiol 105, 2698–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV, Musienko PE, Gorskii OV, Lyalka VF, Gerasimenko YP, Orlovsky GN & Deliagina TG (2015). Activity of individual spinal neurons during locomotion initiated from brainstem and from spinal cord. Soc Neurosci Abstr, 798.11. [Google Scholar]
- Zhong H, Zhu C, Minegishi Y, Richter F, Zdunowski S, Roy RR, Vissel B, Gad P, Gerasimenko Y, Chesselet MF, Edgerton VR (2019). Epidural Spinal Cord Stimulation Improves Motor Function in Rats With Chemically Induced Parkinsonism. Neurorehabil Neural Repair 33, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]


