Abstract
Background
Drug-resistant tuberculosis (DR-TB), including rifampicin-resistant tuberculosis (RR-TB) and multidrug-resistant tuberculosis (MDR-TB, or RR-TB with additional isoniazid resistance), presents challenges to TB control. In Uganda, the GeneXpert test provides point-of-care testing for TB and rifampicin resistance. Patients identified with RR-TB receive culture-based drug susceptibility testing (DST) to identify additional resistance, if any. There are few data on the epidemiological profiles of current DR-TB patients in Uganda. We described patients with RR-TB in Uganda and assessed the trends of RR-TB to inform TB control interventions.
Methods
We identified patients with RR-TB whose samples were referred for culture and DST during 2014–2018 from routinely-generated laboratory surveillance data at the Uganda National Tuberculosis Reference Laboratory. Data on patient demographics and drug sensitivity profile of Mycobacterium tuberculosis isolates were abstracted. Population data were obtained from the Uganda Bureau of Statistics to calculate incidence. Descriptive epidemiology was performed, and logistic regression used to assess trends.
Results
We identified 1474 patients whose mean age was 36 ± 17 years. Overall incidence was 3.8/100,000 population. Males were more affected by RR-TB than females (4.9 vs. 2.7/100,000, p ≤ 0.01). Geographically, Northern Uganda was the most affected region (IR = 6.9/100,000) followed by the Central region (IR = 5.01/100,000). The overall population incidence of RR-TB increased by 20% over the evaluation period (OR = 1.2; 95% CI 1.15–1.23); RR-TB in new TB cases increased by 35% (OR = 1.35; 95% CI 1.3–1.4) and by 7% in previously-treated cases (OR = 1.07; 95% CI 1.0–1.1). Of the 1474 patients with RR-TB, 923 (63%) were culture-positive of whom 670 (72%) had full DST available. Based on the DST results, 522/670 (78%) had MDR-TB.
Conclusion
Between 2014 and 2018, the incidence of RR-TB increased especially among newly-diagnosed TB patients. We recommend intensified efforts and screening for early diagnosis especially among previously treated patients. Mechanisms should be in put to ensure that all patients with RR-TB obtain DST.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-021-00947-2.
Keywords: Tuberculosis, Rifampicin-resistance, Multi-drug resistant, Epidemiology, Uganda
Introduction
Drug-resistant tuberculosis (DR-TB) undermines the global efforts to combat TB. Rifampicin-resistant-TB (RR-TB) defined as a Mycobacterium tuberculosis isolate with resistance to rifampicin detected using genotypic or phenotypic methods, with or without resistance to other first-line anti-TB drugs [1]. When TB is resistant to both rifampicin and isoniazid (the two traditional first-line drugs), it is termed multidrug-resistant TB (MDR-TB). Drug-resistant TB can be present in a primary TB infection or can develop as a complication during the course of patient treatment [2]. In 2018, 3.4% of newly-diagnosed and 18% previously-treated TB patients were estimated to have MDR-TB worldwide [3]. In Uganda, MDR-TB prevalence was estimated at 1% and 12% among new TB cases and previously treated patients respectively in 2018 [4]. Infection with MDR-TB has been associated with poorer treatment outcomes, longer treatment times, and higher treatment costs as compared to infection with drug-sensitive TB [5]. The impact is exacerbated in resource-limited settings due to the presence of other infectious diseases and limited access to well-equipped healthcare facilities [6, 7].
Tuberculosis control depends on the rapid detection and successful treatment of infectious patients, both to save lives and prevent onward transmission, especially for MDR-TB. To improve targeted diagnosis and treatment, in 2010, WHO recommended the GeneXpert MTB/RIF assay, which identifies Mycobacterium tuberculosis mutations associated with rifampicin with high sensitivity [8]. This test has a shorter turn-around time (about 2 h) than the gold standard method of culture (2–6 weeks) [9, 10]. In 2019, 236 of the 1500 TB diagnosis and treatment units in Uganda had GeneXpert machines [11]. At these GeneXpert sites, patients whose samples are identified as rifampicin-resistant are referred to a TB drug-resistance treatment unit.
Because nearly all RR-TB isolates also are resistant to isoniazid [12], RR-TB cases diagnosed by GeneXpert are treated using a standard regimen for MDR-TB [13]. However, RR-TB isolates may also be resistant to other drugs. To further guide management of patients with RR-TB, culture and drug susceptibility testing (DST) is recommended to identify any other anti-TB drug resistance. Patients diagnosed with RR-TB at the GeneXpert sites are referred to one of the 17 TB drug-resistance treatment units in Uganda [14], where the practice is to take a second sputum sample for culture and DST at the National Tuberculosis References Laboratory (NTRL). The DST results are then used to guide patient management [15].
Active surveillance involving active case finding through culture and DST at all levels of the health system is the most effective way to monitor the spread of resistant TB strains. However, it is resource-intensive, and many countries affected by TB are unable to implement this approach. This is why in Uganda, surveillance for drug-resistant TB is largely passive, relying on individuals presenting themselves to health care facilities [16]. For TB patients identified, contact tracing is done to identify and treat those who would have been exposed. In addition, culture and DST capacity for Mycobacterium tuberculosis is limited to a centralized NTRL facility. All specimens requiring these specialized tests must be referred from the GeneXpert health facilities located in rural and urban settings throughout the country to the NTRL [17]. This centralized testing requires rapid and safe transport of specimens from health facilities or lower-level laboratories to the higher-level laboratory, as well as expedient reporting of results back to clinicians [18]. However, there are challenges in specimen referral and result reporting systems which contribute to diagnostic delays in routine practice [19, 20]. Such delays have a potential to translate into under-reporting of drug resistance among TB isolates. In such situations, routinely generated surveillance data are important to monitor the effectiveness of TB control programs [21]. We described the epidemiology of rifampicin resistant TB patients and their drug resistance profiles in Uganda based on routinely generated laboratory surveillance data at the NTRL during 2014–2018.
Methods
Study site and design
This was a retrospective analysis of laboratory data collected at the National TB Reference Laboratory (NTRL) of Uganda from 2014 to 2018. All samples from the entire country are submitted to the NTRL for both culture and DST for Mycobacterium tuberculosis isolates. The NTRL is a one of the WHO approved Supra National Reference Laboratories for Africa and it is accredited by the South African National Accreditation System.
Study population
All records of patients with RR-TB whose samples were submitted to the NTRL for culture and DST during the period 2014–2018 were included in this study. The data were retrieved from the from the Laboratory Information System (LIS) where they are entered at sample submission and testing conclusion. The LIS of the NTRL is used to track patient samples from entry into the laboratory to the point when results are dispatched to the requesting health facilities. At the drug resistance treatment sites, data are entered in the Drug-Resistant Management Information System (DRMIS) for patient management.
Study variables and data abstraction
The primary outcome was the presence of any rifampicin resistance, in the form of mono-resistance, poly-resistance, MDR, or extensive drug resistance (XDR) in a Mycobacterium tuberculosis isolate. For each case-patient with RR-TB, we abstracted data on age, sex, district of residence of the patient, date of enrolment on treatment, culture results, patient categorization a new or previously treated, and the drug susceptibility profile from the LIS. Classification of drug resistance was based on the DST profiles of clinical isolates, as described by the WHO definitions and reporting framework [1, 22, 23] (Table 1). Using unique identifiers, we matched laboratory data in the LIS with information in the DRMIS to obtain the case-patient’s HIV status and history of any TB treatment. Records of patients with incomplete DST, patients without DST results, and those who were susceptible to all drugs were excluded in the classification of drug resistance type. Data on GeneXpert installations across the country over the study period were collected to compare with the numbers diagnosed with RR-TB.
Table 1.
Definitions used for the classification of drug susceptibility profiles of patients with rifampicin-resistant tuberculosis, Uganda, 2014–2018
| Variable | Definition |
|---|---|
| Rifampicin resistance (RR) | Resistance to rifampicin detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs. It includes any resistance to rifampicin, in the form of monoresistance, polyresistance, MDR or XDR |
| Monoresistance | Resistance to one first-line anti-TB drug only (streptomycin, rifampicin, isoniazid, ethambutol, pyrazinamide) |
| Polydrug resistance | Resistance to more than one first-line anti-TB drug (other than both isoniazid and rifampicin) |
| Multidrug resistance (MDR) | Resistance to at least both isoniazid and rifampicin |
| Pre-extensively drug-resistant tuberculosis (Pre-XDR TB) | MDR-TB with resistance to fluoroquinolones (ofloxacin, levofloxacin and moxifloxacin) or a second-line injectable (amikacin, kanamycin, or capreomycin), but not both |
| Extensively drug resistant TB (XDR TB) | Resistance to isoniazid and rifampin plus resistance to any fluoroquinolone (ofloxacin, levofloxacin and moxifloxacin) and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin) |
Data management and analysis
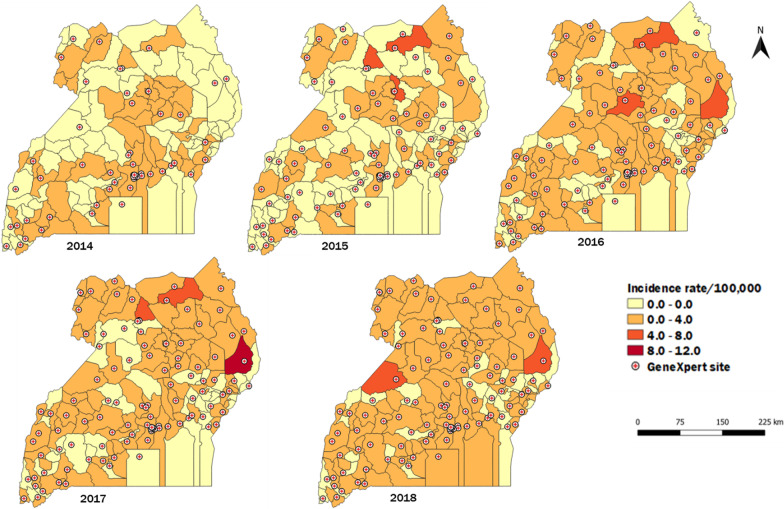
Data were entered into EpiInfo 7.2 for analysis. Descriptive statistics of the sample by person, place, and time were calculated. Line graphs were drawn to demonstrate trends whose significance was tested using logistic regression. Quantum Geographic Information System (QGIS) software was used to show the spatial trends of RR-TB in Uganda. DR-TB incidence rates were calculated using RR-TB cases per district and individual district populations. These were displayed on choropleth maps. District population estimates were calculated based on data from the 2014 National Population and Housing Census (cite), with a national growth rate of 3% to estimate the yearly populations [24]. Data on GeneXpert machine site distribution were superimposed on the incidence rate maps to analyze the relationship between incidence and GeneXpert machine availability.
Results
Characteristics of patients with RR-TB
During the period under assessment, 1474 RR-TB patients, with a median age of 36 (IQR 17) years, were identified. Of these, 943 (64%) were male; 568 (38.5%) were new and 848 (57.5%) were previously-treated patients. In addition, 58 (3.9%) had an unknown / unrecorded history of treatment. Further, approximately half (687; 47%) were HIV-positive and 923 (63%) had positive cultures (Table 2).
Table 2.
Socio-demographic and baseline clinical characteristics of patients with rifampicin resistant tuberculosis, Uganda, 2014–2018
| Variable | All patients N = 1474 | % |
|---|---|---|
| Sex | ||
| Female | 531 | 36 |
| Male | 943 | 64 |
| Age group | ||
| 0–14 | 51 | 3.5 |
| 15–24 | 210 | 14 |
| 25–34 | 449 | 30 |
| 35–44 | 396 | 27 |
| 45–54 | 216 | 15 |
| 55–64 | 98 | 6.7 |
| 65+ | 54 | 3.7 |
| HIV status | ||
| Negative | 681 | 46 |
| Positive | 687 | 47 |
| Unknown | 106 | 7.2 |
| Patient category | ||
| New | 568 | 39 |
| Previously treated | 848 | 58 |
| Unknown | 58 | 3.93 |
| Culture result | ||
| Negative | 531 | 36 |
| Positive | 923 | 63 |
| Others | 20 | 1.4 |
Incidence rate of rifampicin resistant tuberculosis, Uganda, 2014–2018
The overall incidence of RR-TB was 3.8/100,000 population. Males were more affected than females (4.9 vs 2.7/100,000, p ≤ 0.01). Persons aged 35–44 years were most affected (IR = 12/100,000) followed by those between 25 and 34 (IR = 8.5/100,000) while those aged 0–14 years were the least affected (IR = 0.23/100,000).
Spatial distribution of rifampicin resistant tuberculosis patients, Uganda, 2014–2018
District coverage with GeneXpert machines in 1500 TB diagnostic units was 4.8% in 2014, 7.4% (2015), 7.5% (2016), 9% (2017), and 17% (2018). Similarly, there were increases in RR-TB diagnosis over time (Fig. 1). The northern region of Uganda had the highest rates of rifampicin-resistant TB patients (IR = 6.9/100,000), followed by the central region (IR = 5/100,000), western region (IR = 2.7/100,000), and eastern region (IR = 2.4/100,000) (Fig. 1). The districts with the highest incidence rates in the years 2014 to 2018 are shown in Additional file 1: Table S1.
Fig. 1.
Rifampicin-resistant tuberculosis incidence per 100,000 population, Uganda, 2014–2018
Drug-resistance profiles of patients with RR-TB, Uganda, 2014–2018
Of the 923 culture-positive patients, 707 (77%) had their DST done. Among those with DST results, 37 (5%) had incomplete DST (first-line susceptibility testing only) and 670 (95%) had complete DST (both first and second-line). Twenty-eight (3%) of the 670 patient isolates with complete DST were susceptible to all drugs.
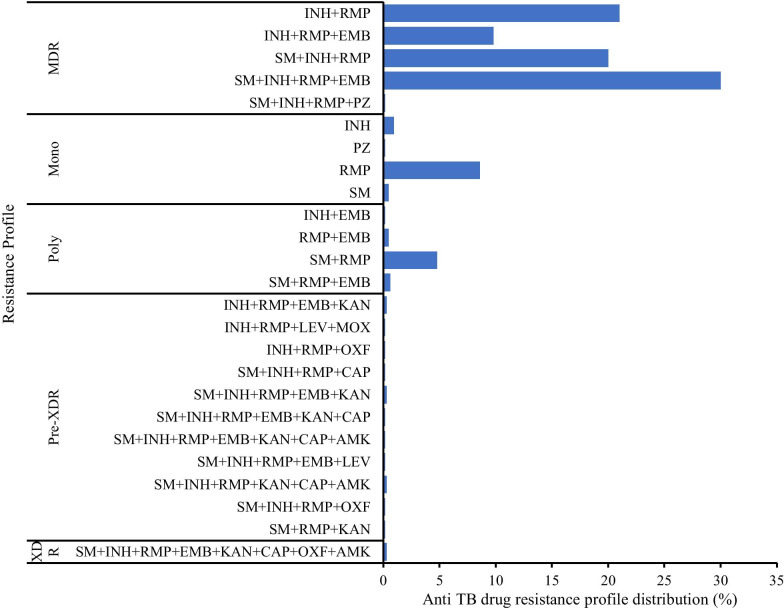
Of the 642 isolates resistant to any TB drug, multidrug resistance was the most common (522; 81%) followed by monoresistance (65; 10%), poly-resistance (39; 6%), pre-XDR (11; 1.7%), and XDR (5; 0.8%) (Fig. 2).
Fig. 2.
Anti-tuberculosis drug resistance profile of patients with rifampicin resistant tuberculosis, N = 642, Uganda 2014–2018. INH, Isoniazid; RMP, Rifampicin; SM, Streptomycin; EMB, Ethambutol; PZ, Pyrazinamide; KAN, Kanamycin; LEV, Levofloxacin; MOX, Moxifloxacin; OFL, Ofloxacin; CAP, Capreomycin; AMK, Amikacin
Trends in incidence of rifampicin resistant tuberculosis, Uganda, 2014–2018
The incidence rate of rifampicin-resistant TB increased over time from 2014 to 2018, with a 20% increase across the study period (OR = 1.2; 95% CI 1.1–1.2). The incidence rates per 100,000 population were 0.49 in 2014, 0.63 in 2015, 0.91 in 2016, and 1.05 in 2018. GeneXpert machine installation around the country also increased over time from 33 in 2014 to 249 machines in 2018.
Trends in incidence of rifampicin-resistant tuberculosis among new and previously-treated patients
The incidence rates of RR-TB among both new and previously-treated cases increased from 2014 to 2018. Patients with previous TB treatment showed a greater incidence compared to the new cases over the entire study period. There was a bigger increase in the incidence among new TB cases (OR = 1.35; 95% CI 1.3–1.4) compared to previously-treated TB cases (OR = 1.07; 95% CI 1.03–1.13) (Fig. 3).
Fig. 3.
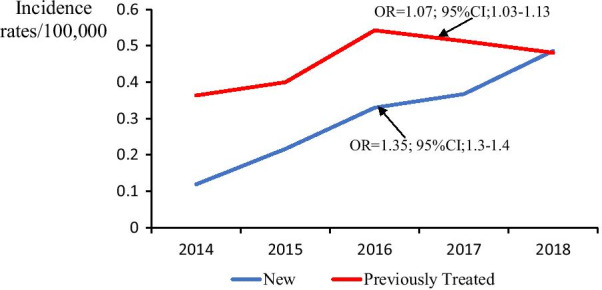
Trends of rifampicin resistant tuberculosis rates among new and previously treated cases, Uganda, 2014–2018
Trends in incidence of different drug resistance types among patients with rifampicin-resistant tuberculosis
The incidence of multidrug resistance was persistently higher than the other types of drug resistance, with an overall incidence of 1.3 per 100,000 population over the 5 years (Additional file 1: Figure S1). Changes in incidence over time were not significant for any of the types of drug resistance over the study period except pre-XDR TB (OR = 1.36; 95% CI 1.09–1.7). Among patients with MDR-TB, there was an overall increase in incidence of 5% while among patients with mono-resistance, there was a 4% increase. In addition, there was an 8% decrease among patients with poly-resistance, and a 1% increase among patients with XDR. The pre-XDR TB resistance patients had the largest increase, at 36% (Additional file 1: Table S2).
Discussion
The study shows an increase in incidence of rifampicin-resistant TB in Uganda during 2014–2018. Although the majority of patients with RR-TB had been previously treated, the increase in incidence was greater among new cases than among previously treated cases. The coverage of GeneXpert machines greatly improved during the study period, and this might have contributed to the improved detection for rifampicin resistance in most parts of Uganda.
This study found an overall incidence of RR-TB of 3.8 per 100,000 population in Uganda. This is fairly comparable with the 2018 WHO estimate of RR-TB incidence for the country of 3.5 per 100,000 [4]. The largest proportion of patients with RR-TB had MDR-TB, with resistance to streptomycin, isoniazid, rifampicin, and ethambutol. The TB National guidelines have evolved from resistance testing largely targeting retreatment cases only in 2011 to resistance testing for all TB patients with RR-TB regardless of prior TB history in 2016 [25, 26]. Our observation that previously-treated TB patients were more likely than new cases to have MDR-TB has been made in multiple other studies [27–29]. Such findings call for attention to treatment completion rates for new cases and extra vigilance for drug resistance monitoring among those with history of previous treatment for TB. In addition, these findings are a good basis for strict implementation of infection control practices to prevent transmission and emergence of new cases, including drug-resistant cases.
Although the incidence of XDR-TB was low and no significant change was observed during the study period, pre-XDR TB showed a significant increase in incidence. This level of second-line resistance is a cause for concern, especially in resource-limited countries such as Uganda. This has the potential of introducing case management repercussions such as adjustment of treatment regimens, need for new therapeutic agents and introduction of new rapid diagnostic tools. Data from previous studies suggest that a similar increase is being observed worldwide [30–34]. Such trends point to the need to expand laboratory capacity for culture and DST for both first and second line anti-TB drugs in TB endemic countries like Uganda. Currently, this capacity is limited to two public health laboratories; decentralization of laboratory services with improved specimen collection and transport for culture and DST TB is needed in the country. Early identification of pre-XDR TB patients would enable closer monitoring to prevent the progression to XDR-TB. The distribution of GeneXpert machines in Uganda has greatly improved which has contributed to the TB case detection. However, even with specimen referral system a17% GeneXpert coverage is still below the desired target for the population in Uganda.
Males and persons aged 25–44 had the highest incidence rates of RR-TB. Male predominance in active TB is widely-reported globally and has been previously reported in Uganda [35, 36]. Persons aged 25–54 have also been identified as the most-affected age group in other countries [37]. Males in this age group have been reported to undertake occupations that are associated with an elevated risk for TB [36, 38]. However, our findings contradict other studies elsewhere which have found more females than males with MDR-TB [39]. Despite such contradictions, we think that targeted screenings for persons aged 25–54 may help reduce the overall burden of MDR-TB, as well as the burden in this age group.
Northern Uganda, with widespread poverty [40] and poor living conditions in overcrowded communities, has been reported previously to have a high prevalence of TB [14, 26]. The northern region, especially the Karamoja region, has been reported to have TB treatment success rates below 72%, mostly attributed to the loss to follow up (LTFU) [14]. Studies have shown that patients who are LTFU are at higher risk of developing MDR-TB, compared to those who are not LTFU [41]. Therefore, programs that can enhance treatment follow-up are suitable for such regions.
The HIV prevalence in the north-central region is also among the highest in the country, at 7.2% compared to the national prevalence of 6.2%, while in the northeast it is 5.3% [42]. HIV co-infection is known to increase the risk for developing drug-resistant TB as mechanisms linking drug-resistant TB to HIV infection have been suggested. Drug malabsorption in HIV-infected patients, especially for rifampin and ethambutol, can also lead to drug resistance as the patient receives a subclinical dose [43–46]. Similarly, Kampala, with an HIV prevalence of 6.9%, had higher incidence rates of drug-resistant TB compared to other districts [42]. Multiple studies have shown that there is an association between drug-resistant TB and HIV [43], although at least one study in Uganda found the opposite [47]. Extra efforts are needed to control tuberculosis transmission in the different parts of the country after having understood each area’s specific risk factors.
Study limitations
Our findings are based on routinely-generated laboratory surveillance data. This has the potential of underestimating the true burden of RR-TB because some patients do not access the healthcare system. Besides, RR-TB is only detected by GeneXpert which has a coverage of about 17% of all the TB diagnostic units in the country. This may have limited our ability to accurately determine the true incidence, and therefore the findings should be interpreted in this context. However, these findings provide a good reflection of the general trends in RR-TB incidence in the country over the study period.
Conclusion
Rifampicin-resistant TB incidence rates have consistently risen throughout the last 5 years in Uganda, but the burden is regionally diverse. There has been a significant increase in new patients diagnosed with RR-TB, with most being among those previously treated. Males and persons in the age-group 24–54 years were more affected by drug-resistant TB than females and the other age-groups. Strengthening prevention and control programs, especially among the most affected sub-populations, is crucial to the goal of minimizing the burden of resistant tuberculosis especially in resource limited settings.
Recommendations
It is important that all TB samples from patients with RR detected by the GeneXpert are referred for culture and DST before treatment initiation and contact tracing is initiated. It may also be efficient to adopt rapid diagnostic tests such as Cepheid Xpert MTB/XDR once they are approved in order to support the decentralization of DST testing. The National TB program should also consider having more machines installed in districts to enhance accessibility as well as instituting mechanisms to improve sample referral in areas without GeneXpert. Lastly, there is need to conduct a national TB drug resistance survey to determine the actual burden and risk factors associated with TB drug resistance in Uganda, and similar settings.
Supplementary Information
Additional file 1: Table S1. Districts with highest RR TB incidence rates per 100,000 population, Uganda, 2014–2018. Figure S1. Trends of anti-tuberculosis drug resistance types among patients with rifampicin resistant tuberculosis, Uganda 2014–2018. Table S2. Odds ratios for changes in incidence by drug resistance types among RR-TB patients in Uganda 2014–2018.
Acknowledgements
We would like to thank the Ministry of Health for giving us the opportunity to access the National Tuberculosis and Leprosy and the National Tuberculosis Reference Laboratory databases. We are highly indebted to Dr. Julie. R. Harris, US Centers for Disease Control and Prevention, for technical guidance during the data analysis, critical review, and revision of the final manuscript. We thank the US-CDC for supporting the Uganda Public Health Fellowship Program activities, the Uganda National Tuberculosis and Leprosy Program for the support rendered by the NTRL data manager Diana Nadunga during data abstraction, and the laboratory team.
Abbreviations
- IR
Incidence Rate
- RR-TB
Rifampicin Resistant TB
- MDR
Multidrug Resistance
- XDR
Extensively Drug Resistance
- TB
Tuberculosis
- QGIS
Quantum Geographic Information System software
- DST
Drug sensitivity testing
- HIV
Human immune deficiency Virus
- INH
Isoniazid
- NTRL
National tuberculosis reference laboratory
- RMP
Rifampicin
- SM
Streptomycin
- EMB
Ethambutol
- PZ
Pyrazinamide
- KAN
Kanamycin
- LEV
Levofloxacin
- MOX
Moxifloxacin
- OFL
Ofloxacin
- CAP
Capreomycin
- AMK
Amikacin
- WHO
World Health Organization
Authors' contributions
GB conceptualized and took lead in writing the manuscript. GB and RKM participated in study designing, manuscript drafting, and data analysis. RKM, SK, NA, BK, LB, ARA, MLJ, and ST participated in manuscript writing and reviewed the manuscript for intellectual content and scientific integrity. All authors read and approved the final manuscript.
Funding
This project was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention Cooperative Agreement Number GH001353-01 through Makerere University School of Public Health to the Uganda Public Health Fellowship Program, MoH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention, the Department of Health and Human Services, Makerere University School of Public Health, or the MoH. The staff of the funding body provided technical guidance in the design of the study, ethical clearance and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
For confidentiality reasons the datasets are not publicly available. However, the data sets can be availed upon reasonable request from the corresponding author and with permission from the Uganda Ministry of Health.
Declarations
Ethics approval and consent to participate
We sought permission to use the routinely generated program data from the Uganda Ministry of Health, the National Tuberculosis and Leprosy Program and the National Tuberculosis Reference Laboratory. In addition, we received a project determination as non-research and clearance from the Centers for Disease Control and Prevention (CDC) to undertake this analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO|TB drug resistance types. WHO.
- 2.Ragonnet R, et al. High rates of multidrug-resistant and rifampicin-resistant tuberculosis among re-treatment cases: where do they come from? BMC Infect Dis. 2017;17(1):36. doi: 10.1186/s12879-016-2171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global tuberculosis report, in World Health Organisation. 2018.
- 4.World Health Organization, WHO_HQ_Reports-G2-PROD-EXT-TBCountryProfile 2018. 2018.
- 5.World Health Organization . Drug-resistant tuberculosis now at record levels. Geneva: World Health Organization; 2010. [Google Scholar]
- 6.Coll-Black S, et al. Reaching the poor: challenges for the TB programmes in the Western Pacific Region. 2004.
- 7.Jassal MS, Bishai WR. Epidemiology and challenges to the elimination of global tuberculosis. Clin Infect Dis. 2010;50(Supplement_3):S156–S164. doi: 10.1086/651486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Rapid implementation of the Xpert MTB/RIF diagnostic test: technical and operational 'How-to'; practical considerations. Geneva: World Health Organization; 2011. [Google Scholar]
- 9.Saeed M, et al. GeneXpert technology: a breakthrough for the diagnosis of tuberculous pericarditis and pleuritis in less than 2 hours. Saudi Med J. 2017;38(7):699–705. doi: 10.15537/smj.2017.7.17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helb D, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48(1):229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Tuberculosis and Leprosy Program, M.o.H.U., National tuberculosis and leprosy program quarterly bulletin. Kampala: Ministry of Health, Republic of Uganda; 2018.
- 12.Hofmann-Thiel S, et al. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent. Uzbekistan Eur Respir J. 2009;33(2):368–374. doi: 10.1183/09031936.00089808. [DOI] [PubMed] [Google Scholar]
- 13.NTLP M. Algorithm for TB screening, diagnosis and Management_final 2017.
- 14.Ministry of Health and National Tuberculosis and Leprosy Programme. NTLP June 2017–July 2018 Report. 2017–2018.
- 15.National Tuberculosis and Leprosy Programme M. Algorithm for TB screening, diagnosis and Management_final 7th August 2017. 2017.
- 16.Parsons LM, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24(2):314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health, National health facility master facility list 2018. 2018, Kampala: MOH.
- 18.Joloba M, et al. Strengthening the tuberculosis specimen referral network in Uganda: the role of public-private partnerships. J Infect Dis. 2016;213(Suppl 2):S41–S46. doi: 10.1093/infdis/jiw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harries AD, et al. Using a bus service for transporting sputum specimens to the Central Reference Laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis. 2004;8(2):204–210. [PubMed] [Google Scholar]
- 20.Yagui M, et al. Timely diagnosis of MDR-TB under program conditions: is rapid drug susceptibility testing sufficient? Int J Tuberc Lung Dis. 2006;10(8):838–843. [PMC free article] [PubMed] [Google Scholar]
- 21.Diriba G, et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect Dis Poverty. 2019;8(1):54. doi: 10.1186/s40249-019-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee R, et al. Extensively drug-resistant tuberculosis in California, 1993–2006. Clin Infect Dis. 2008;47(4):450–457. doi: 10.1086/590009. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Definitions and reporting framework for tuberculosis–2013 revision. 2013, World Health Organization.
- 24.Uganda Bureau of Statistics, The National Population and Housing Census 2014–Main Report, Kampala. 2016.
- 25.Ministry of Health, The National Tuberculosis and Leprosy Programme. Uganda national guidelines for the programmatic management of drug resistant tuberculosis. 2011.
- 26.Ministry of Health Uganda, National Tuberculosis and Leprosy Control Programme. Revised National Strategic Plan 2015/16–2019/20, Kampala, Uganda. Uganda: Ministry of Health; 2017.
- 27.Onyedum CC, Alobu I, Ukwaja KN. Prevalence of drug-resistant tuberculosis in Nigeria: a systematic review and meta-analysis. PLoS ONE. 2017;12(7):e0180996. doi: 10.1371/journal.pone.0180996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasiri MJ, et al. Prevalence of drug-resistant tuberculosis in Iran: systematic review and meta-analysis. Am J Infect Control. 2014;42(11):1212–1218. doi: 10.1016/j.ajic.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Berhan A, Berhan Y, Yizengaw D. A meta-analysis of drug resistant tuberculosis in Sub-Saharan Africa: how strongly associated with previous treatment and HIV co-infection? Ethiop J Health Sci. 2013;23(3):271–282. doi: 10.4314/ejhs.v23i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta C, et al. Drug-resistant tuberculosis in Eastern Europe: challenges and ways forward. Public Health Action. 2015;4:S3–S12. doi: 10.5588/pha.14.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal V, et al. Prevalence of drug-resistant pulmonary tuberculosis in India: systematic review and meta-analysis. BMC Public Health. 2017;17(1):817. doi: 10.1186/s12889-017-4779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasnim T, et al. Pre-extensively drug resistant tuberculosis (Pre-XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) patients in Bangladesh. J Tuberc Res. 2018;6(3):199–206. [Google Scholar]
- 33.Daniel O, et al. Pre-extensive drug resistant tuberculosis (pre-XDR-TB) among MDR-TB patents in Nigeria. Glob Adv Res J Microbiol. 2013;2:22–25. [Google Scholar]
- 34.Adwani S, Desai UD, Joshi JM. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai. JKIMSU. 2016;5:13–19. doi: 10.4103/lungindia.lungindia_182_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boum Y, et al. Male Gender is independently associated with pulmonary tuberculosis among sputum and non-sputum producers people with presumptive tuberculosis in Southwestern Uganda. BMC Infect Dis. 2014;14(1):638. doi: 10.1186/s12879-014-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerra-Silveira F, Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS ONE. 2013;8(4):e62390. doi: 10.1371/journal.pone.0062390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zignol M, et al. Multidrug-resistant tuberculosis in children: evidence from global surveillance. Eur Respir J. 2013;42(3):701–707. doi: 10.1183/09031936.00175812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oni T, et al. Smoking, BCG and employment and the risk of tuberculosis infection in HIV-infected persons in South Africa. PLoS ONE. 2012;7(10):e47072. doi: 10.1371/journal.pone.0047072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Repossi A, et al. Gender and other risk factors for multidrug-resistant (MDR) tuberculosis (TB) among migrants to Milan, Italy. Eur Respir J. 2011;38(Suppl 55):3299. [Google Scholar]
- 40.Uganda Bureau of Statistics (UBOS), Uganda National Household Survey 2016/2017. UBOS: Kampala, Uganda; 2018.
- 41.Caminero J. Multidrug-resistant tuberculosis: epidemiology, risk factors and case finding [State of the art series. Drug-resistant tuberculosis. Edited by CY. Chiang. Number 4 in the series] Int J Tuberc Lung Dis. 2010;14(4):382–390. [PubMed] [Google Scholar]
- 42.Ministry of Health Uganda. Uganda population-based HIV impact assessment (UPHIA): final report, 2016–2017. Kampala: Ministry of Health; 2019.
- 43.Mesfin YM, et al. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2014;9(1):e82235. doi: 10.1371/journal.pone.0082235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dean AS, et al. HIV and multidrug-resistant tuberculosis: overlapping epidemics. Eur Respir J. 2014;44(1):251–254. doi: 10.1183/09031936.00205413. [DOI] [PubMed] [Google Scholar]
- 45.Patel KB, Belmonte R, Crowe HM. Drug malabsorption and resistant tuberculosis in HIV-infected patients. N Engl J Med. 1995;332(5):336–337. doi: 10.1056/NEJM199502023320518. [DOI] [PubMed] [Google Scholar]
- 46.Dye C, et al. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science. 2002;295(5562):2042–2046. doi: 10.1126/science.1063814. [DOI] [PubMed] [Google Scholar]
- 47.Lukoye D, et al. Rates of anti-tuberculosis drug resistance in Kampala-Uganda are low and not associated with HIV infection. PLoS ONE. 2011;6(1):e16130–e16130. doi: 10.1371/journal.pone.0016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Districts with highest RR TB incidence rates per 100,000 population, Uganda, 2014–2018. Figure S1. Trends of anti-tuberculosis drug resistance types among patients with rifampicin resistant tuberculosis, Uganda 2014–2018. Table S2. Odds ratios for changes in incidence by drug resistance types among RR-TB patients in Uganda 2014–2018.
Data Availability Statement
For confidentiality reasons the datasets are not publicly available. However, the data sets can be availed upon reasonable request from the corresponding author and with permission from the Uganda Ministry of Health.