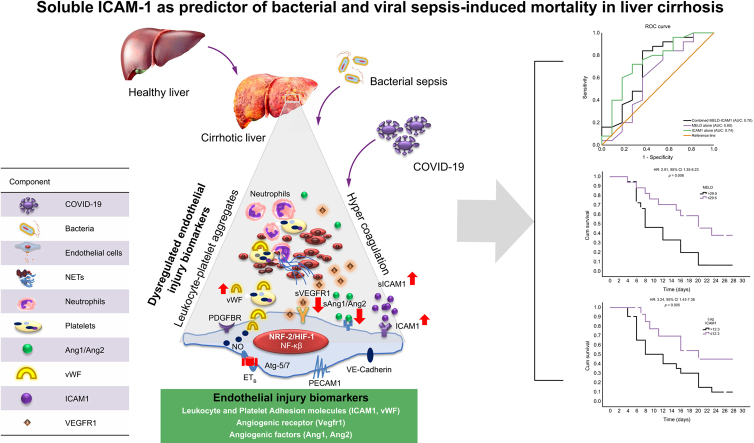
Abstract
Background & Aims
Endothelial injury and dysfunction play a detrimental role in the pathogenesis of infections. Endothelium-related molecules have been reported as potential diagnostic and/or prognostic biomarkers of infection. The prognostic value of these biomarkers in patients with cirrhosis and infections remains elusive.
Methods
In this study, we investigated the performance of key soluble endothelial injury biomarkers, including intercellular adhesion molecule 1 (ICAM1), von Willebrand factor (vWF), vascular endothelial growth factor receptor 1 (VEGFR1), and angiopoietin 1 and 2 (Ang1, 2) as mortality predictors in patients with cirrhosis and severe COVID-19 or bacterial sepsis.
Results
A total of 66 hospitalized patients (admitted to the COVID-19 ward or liver intensive care unit [ICU]) were included. Twenty-two patients had COVID-19 alone, while 20 patients had cirrhosis plus COVID-19. Twenty-four patients had cirrhosis plus bacterial sepsis. Among patients with cirrhosis, the most common aetiology of liver disease was alcohol. ICAM1 was increased (p = 0.003) while VEGFR1 (p <0.0001) and Ang1 (p <0.0001) were reduced in patients with COVID-19 and cirrhosis, compared to patients with COVID-19 alone. Endothelial biomarker levels did not differ significantly between patients with cirrhosis and severe COVID-19 or bacterial sepsis in the ICU. In these patients, ICAM1 levels significantly and independently predicted mortality (hazard ratio 3.24; 95% CI 1.19–8.86) along with model for end-stage liver disease (MELD) score, renal and coagulation failures. The AUC for ICAM1 was 0.74, MELD was 0.60 and combined ICAM1 and MELD was 0.70. ICAM1 also positively correlated with the composite organ failure scores recorded 3–5 days post ICU admission (CLIF-OF and SOFA) in this subgroup of patients.
Conclusion
The study indicates that in patients with cirrhosis, elevated plasma ICAM1 serves as an independent predictor of severe COVID-19- or sepsis-associated 28-day mortality.
Lay summary
Bacterial sepsis and COVID-19 lead to increased mortality in patients with cirrhosis. In this study, we demonstrate that high plasma levels of ICAM1, an endothelial injury biomarker, is one of the important factors predicting mortality in critically ill cirrhotic patients with severe COVID-19 or bacterial sepsis.
Keywords: Biomarkers, COVID-19, Endothelial Injury, Liver Cirrhosis, Sepsis
Abbreviations: ACLF, acute-on-chronic liver failure; Ang1, angiopoietin 1; Ang2, angiopoietin 2; AST, aspartate aminotransferase; CCI, Charlson comorbidity index; HR, hazard ratio; ICAM1, intercellular adhesion molecule 1; ICU, intensive care unit; LDH, lactate dehydrogenase; MELD, model for end-stage liver disease; NLR, neutrophil to lymphocyte ratio; PCT, procalcitonin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, sequential organ failure assessment; vWF, von Willebrand factor; VEGFR1, vascular endothelial growth factor receptor 1
Graphical abstract
Highlights
-
•
Altered plasma levels of endothelial injury biomarkers observed in patients with cirrhosis and COVID-19.
-
•
No difference in the endothelial injury biomarkers among cirrhotic patients with COVID-19 or bacterial sepsis.
-
•
Enhanced ICAM1 levels were an independent predictor of mortality in COVID-19 patients with or without cirrhosis in ICU.
-
•
ICAM1 predicted 28-day mortality better than MELD in cirrhotic patients with severe COVID-19 or sepsis.
-
•
ICAM1 positively correlated with organ failure scores in cirrhotic ICU patients with bacterial infections or COVID-19.
Introduction
The endothelium is a highly specialized and dynamic organ that regulates several processes in both physiology and pathophysiology.1 It is the first organ to discern and mount compensatory responses to any injury signals, thus acting as a shield to protect the underlying tissue. An unhealthy endothelium is associated with profound changes in many physiological systems including expression of adhesion molecules, maintenance of adequate blood vessel tone and haemostasis. Endothelial activation/injury is thought to be a key event in the development and pathogenesis of distinct human vascular diseases, including cirrhosis, hypertension, diabetes, atherosclerosis, sepsis and now even COVID-19.2 Patients with COVID-19 exhibit attributes correlating with endothelial dysfunction involving changes in vascular permeability, inflammation, accumulation and extravasation of leucocytes, activation of procoagulant pathways and disruption of the alveolar-capillary barrier.3,4 A high mortality rate and poor clinical outcomes due to COVID-19 infections in elderly patients and those with comorbid conditions including diabetes, obesity and hypertension has also been postulated to be because of underlying endothelial dysfunction.5 In patients with cirrhosis, COVID-19 leads to a rapid clinical deterioration in otherwise stable patients and increased mortality.6,7 Since biomarkers of endothelial activation and dysfunction are associated with poor outcomes in patients with both COVID-19 and sepsis, we studied these biomarkers in patients with cirrhosis with either COVID-19 or bacterial sepsis. Plasma levels of some of the well-known biomarkers of endothelial injury, including intercellular adhesion molecule 1 (ICAM1), von Willebrand Factor (vWF) antigen, vascular endothelial growth factor receptor 1 (VEGFR1), angiopoietin 1 and 2 (Ang1, 2) were studied for their association with 28-day mortality in patients with COVID-19 with and without cirrhosis, as well as in those with sepsis and cirrhosis.
Patients and methods
Patients
COVID-19 patients (≥18 years) were selected based on a confirmed diagnosis on PCR assays (2 viral genes, envelope protein or E gene and RNA-dependent RNA polymerase or RdRP gene) of nasopharyngeal swab samples. COVID-19 infections were categorised as mild, moderate and severe.8 Patients with cirrhosis plus bacterial sepsis hospitalized in the intensive care unit (ICU) of ILBS were also included in the study. Sepsis was diagnosed based on an increase in the sequential organ failure assessment (SOFA) score of ≥2 points (from baseline SOFA) as a result of documented infection.9 Some samples were also collected from non-hospitalized, mild and asymptomatic COVID-19 patients (namely, hospital staff). Patient groups included in the study are described in Fig. S1. Patients were excluded if they had previously received a liver transplant or if they were transplanted during the hospital stay, as were patients with any malignancy. The ILBS ethics committee approved this study (Ethics protocol no IEC/2020/82/NA01). Biobank samples were collected as per the ILBS biobank guidelines, and samples from in-house hospitalized patients and hospital staff were obtained after proper informed consent from the patient or his/her relative following the national bioethical guidelines. Commercial ELISAs were used to measure the biomarker levels [(ICAM1 and vWF (R & D systems), Ang1, Ang2, VEGFR1 (elabsciences)]. Detailed analyses of patients and other methods are reported in the supplementary information and CTAT table.
Statistical analysis
Categorical variables were expressed as percentages while continuous variables were expressed either as mean (SD) for continuous distribution or as median (minimum-maximum) for variables with skewed distribution. Categorical data were analysed with Chi-square test or Fisher’s exact test wherever appropriate. Continuous variables were compared with independent sample t test or Mann-Whitney U test for 2 groups and by one-way Anova or Kruskal-Wallis test for more than 2 groups depending on the distribution. Differences between endothelial cell markers among various groups, and between survivors and non-survivors, were calculated using the unpaired 2-tailed Mann-Whitney U test. Survivors were those alive or discharged within 28 days. Clinical predictors were entered to the Cox hazard regression model to assess the effects of factors on 28-day in-hospital mortality. We used unadjusted linear regression models and multivariate models adjusted for clinical covariates. The receiver-operating characteristic curves were plotted to explore the AUC. Cut-off points to discriminate the survivors from the non-survivors were calculated by obtaining the best Youden index (sensitivity% + specificity% − 100). The cut-off values were taken where sensitivity and specificity were optimal. AUCs were expressed with their 95% CIs. The study participants were divided into 2 groups according to the optimal cut-off value of the probability of 28-day mortality, and then the 28-day mortality rates of the groups were compared using Kaplan-Meier curve analysis. A log-rank test was conducted to compare the survival curves of the groups. Correlation analysis between the variables was performed using Pearson’s Correlation analysis. All statistical analysis was done using GraphPad Prism (version Prism 8.4.3; GraphPad Software, San Diego, CA, USA) and SPSS Statistics (version 20.0, SPSS Inc., Chicago, IL, USA). Software details are provided in the supplementary CTAT table. A p <0.05 was considered statistically significant.
Results
Baseline characteristics of the COVID-19 patients with and without cirrhosis
A total of 42 hospitalized COVID-19 patients (admitted to dedicated COVID-19 wards or ICUs) were included in the first analysis (Table 1). Out of these, 22 had COVID-19 alone and 20 had COVID-19 plus cirrhosis. We also included asymptomatic non-hospitalized patients (aged 34–61 years old) with mild COVID-19 (n = 6) as a comparator group for endothelial biomarker levels in COVID-19 patients without cirrhosis. In the hospitalized patients, there was no significant difference among the groups with respect to age and sex. In patients with cirrhosis and COVID-19, the most common aetiology of liver disease was alcohol (accounting for 50% of cases) followed by non-alcoholic steatohepatitis. Most patients with cirrhosis (65%) were Child-Pugh class C. Ascites presenting as decompensating event was observed in 70% and hepatic encephalopathy was observed in 40% of COVID-19 patients with cirrhosis. The incidence of acute respiratory distress syndrome was similar in patients from both groups.
Table 1.
Baseline characteristics of the COVID-19 patients with and without cirrhosis.
| Variable | COVID-19 alone (n = 22) | COVID-19 + cirrhosis (n = 20) | p value |
|---|---|---|---|
| Age (years) | 54 (34–65) | 54 (34–78) | n.s. |
| Male, n (%) | 19 (86.3) | 17 (85) | n.s. |
| Female, n (%) | 3 (13.6) | 3 (15) | |
| Neutrophil to lymphocyte ratio | 8.15 (2.5–39.3) | 7 (4.4–18) | n.s. |
| Platelet count (x 109/L) | 210 (57–449) | 71 (22–143) | <0.0001 |
| Bilirubin (mg/dl) | 0.77 (0.33–3.74) | 2.91 (0.63–29.8) | 0.009 |
| AST (U/L) | 54 (27–210) | 55 (23–273) | n.s. |
| ALT (U/L) | 68.5 (18–172) | 63 (21–101) | n.s. |
| Albumin (g/dl) | 3.29 (0.37) | 2.8 (0.57) | 0.001 |
| Serum sodium (mEq/L) | 131.5 (4.03) | 134 (7.24) | n.s. |
| INR | 1.05 (0.96–1.74) | 1.4 (0.98–2.54) | 0.0002 |
| Creatinine (mg/dl) | 0.9 (0.53–1.65) | 1.19 (0.4–3.4) | n.s. |
| ICU, n (%) | 11 (50) | 12 (60) | n.s. |
| Etiology of liver diseases, n (%) | |||
| Alcohol | 0 (0) | 10 (50) | - |
| HBV | 0 (0) | 1 (5) | - |
| HCV | 0 (0) | 0 (0) | - |
| NASH | 0 (0) | 6 (30) | - |
| Others | 0 (0) | 3 (15) | - |
| Ascites, n (%) | 0 (0) | 14 (70) | - |
| Hepatic encephalopathy, n (%) | 0 (0) | 8 (40) | - |
| ARDS, n (%) | 12 (54.5) | 9 (45) | n.s. |
| Liver disease severity n (%) | – | ||
| Child-Pugh A | 0 (0) | 2 (10) | |
| Child-Pugh B | 0 (0) | 5 (25) | |
| Child-Pugh C | 0 (0) | 13 (65) | |
| MELD score | – | 22 (8.59) | -- |
| Comorbidities n (%) | |||
| Hypertension | 9 (40.9) | 4 (20) | n.s. |
| Diabetes mellitus | 11 (50) | 9 (45) | n.s. |
| Coronary artery disease | 5 (22.7) | 3 (15) | n.s. |
| Obesity | 2 (9.09) | 3 (15) | n.s. |
| Liver disease | 0 | 20 (100) | P<0.0001 |
| Charlson comorbidity index | 1 (0–5) | 5 (1–7) | P<0.001 |
For continuous variables, values are given as mean (SD) or median (min-max) and p values have been calculated by Student's t test or Mann-Whitney U test. Categorical variables are given as n (%) and p values for categorical variables have been calculated using Fisher's exact test. ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; ICU, intensive care unit; INR, international normalized ratio; MELD, model for end-stage liver disease; NASH, non-alcoholic steatohepatitis.
As far as comorbidities were concerned, diabetes was the most common in both groups (50% in COVID-19 alone and 45% in COVID-19 plus cirrhosis groups). The Charlson comorbidity index or CCI was significantly different between patients with COVID-19 and those with COVID-19 plus cirrhosis (1 [0–5] vs. 5 [1–7]; p <0.001). Among laboratory variables; neutrophil to lymphocyte ratio (NLR), aspartate aminotransferase (AST), alanine aminotransferase and sodium levels were not significantly different between COVID-19 and COVID-19 plus cirrhosis groups. Platelet count and albumin levels were lower while bilirubin and international normalized ratio (INR) were higher in the COVID-19 plus cirrhosis group. Platelet counts may be low in COVID-19 with cirrhosis due to effect of both liver disease and COVID-19 itself. The number of ICU-admitted patients were similar in both groups.
Endothelial biomarkers in different category of patients
We first compared the endothelial biomarkers in hospitalized and non-hospitalized patients with mild COVID-19. The levels of the endothelial injury factors, ICAM1, vWF, VEGFR1 and Ang2 were markedly increased in the hospitalized COVID-19 patients compared to the asymptomatic and non-hospitalized COVID-19 comparator group (Table S2). Next, we studied these biomarkers in patients with cirrhosis and COVID-19. Patients with COVID-19 plus cirrhosis had significantly higher levels of ICAM1 (p = 0.003), while the levels of VEGFR1 (p <0.0001) and Ang1 (p <0.0001) were substantially reduced compared to those observed in patients with COVID-19 alone (Table 2). The levels of vWF and Ang2 were not significantly different between patients with COVID-19 alone and those with COVID-19 and cirrhosis (Table 2).
Table 2.
Levels of endothelial biomarkers in hospitalized COVID-19 patients with and without liver disease.
| Variables (pg/ml) | Hospitalized patients with COVID-19 alone (n=22) | Hospitalized patients with cirrhosis and COVID-19 (n= 20) | p value |
|---|---|---|---|
| ICAM1 | 67,475 (30,930-261,600) | 149,600 (56,580-353,600) | 0.003∗ |
| vWF | 24,720 (5,011-63,560) | 28,380 (9,037-59,000) | 0.39 |
| Vegfr1 | 3,600 (851.8-13,744) | 903.9 (688-2,267) | <0.0001∗ |
| Ang1 | 4,708 (909.7-13,744) | 1,089 (465.8-4,721) | <0.0001∗ |
| Ang2 | 3,572 (754-13059) | 2,456.5 (628-11,625) | 0.128 |
Data are presented as median (min-max).
Denotes significant p values (Mann-Whitney U test). Significance was taken as p <0.05.
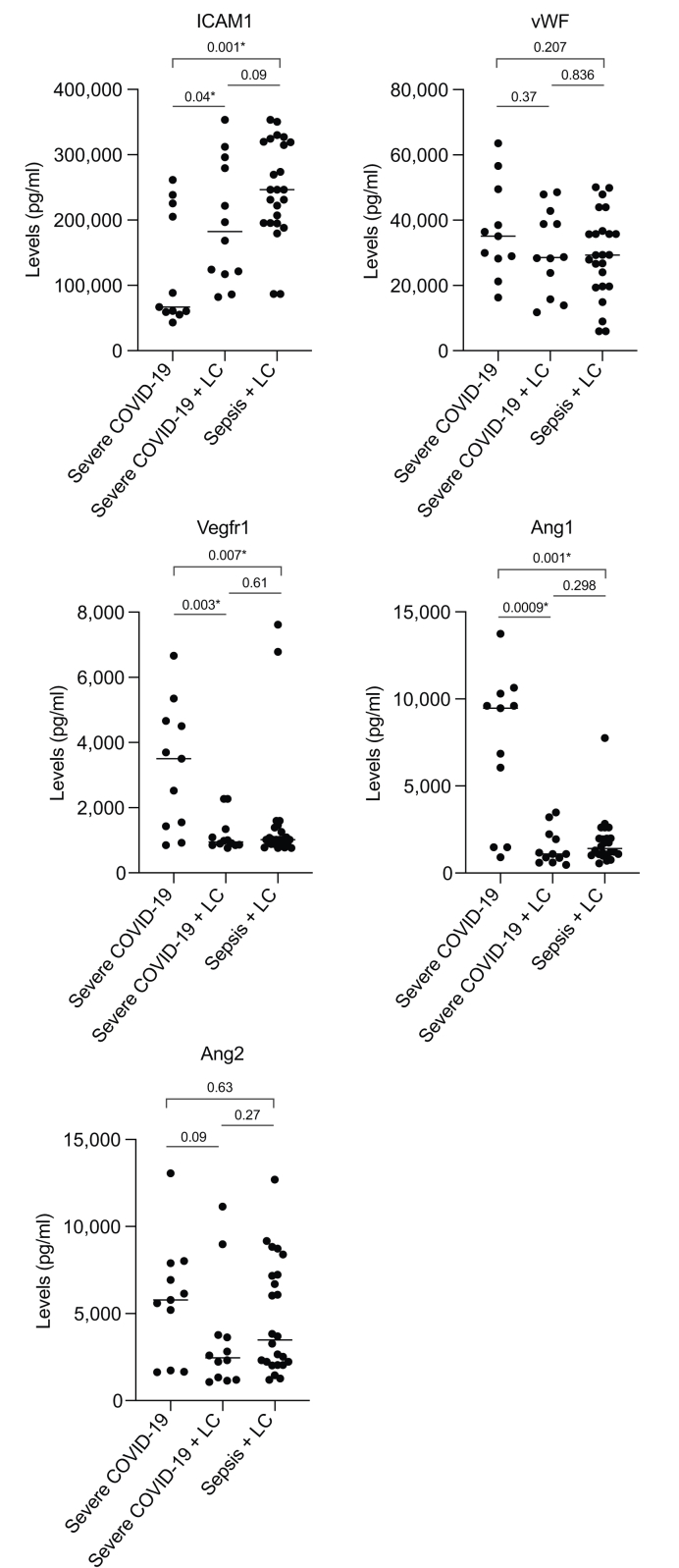
The endothelium is a key component involved in the development of macro- and microcirculatory disturbances and organ dysfunction in severely ill ICU patients with infections; hence, we next studied these biomarkers in a subgroup of ICU patients with severe COVID-19 and those with severe COVID-19 plus cirrhosis. We also included patients admitted to the ICU with cirrhosis and sepsis (Table 3). In addition to usual clinical parameters, factors that are known to be associated with clinical outcomes in severe COVID-19 and bacterial sepsis in ICUs, such as ferritin, lactate dehydrogenase (LDH), procalcitonin (PCT) and D-dimers, were recorded for these patients (Table 3). Among these 3 categories, platelet counts, bilirubin levels, albumin, INR, creatinine, ferritin levels and CCI score were significantly different. Alcohol was the major aetiology and diabetes the major comorbidity in patients with sepsis and cirrhosis, as in patients with COVID-19 and cirrhosis. In the sepsis plus cirrhosis group, ascites was present in 95% and hepatic encephalopathy in 66.6% of patients, which was again similar to the cirrhosis plus COVID-19 group. Like the cirrhosis plus COVID-19 group, most of the patients with sepsis also had Child-Pugh class C cirrhosis but their model for end-stage liver disease (MELD) scores were higher (p = 0.01). Among the 3 categories, the number of patients requiring mechanical ventilation was lowest in the COVID-19 alone group, but similar in patients with cirrhosis and sepsis or severe COVID-19 (Table 3). With respect to SOFA, chronic liver failure-organ failure (CLIF-OF) scores and individual organ failures in patients with cirrhosis at day 3-5 in the ICU, the scores were comparable between patients with COVID-19 infection and those with sepsis. In terms of biomarker levels, patients with cirrhosis and COVID-19 or bacterial sepsis, respectively, showed significantly higher levels of ICAM1 (p = 0.04, 0.001) and reduced levels of VEGFR1 (p = 0.003, 0.007) and Ang1 (p = 0.0009, 0.001) compared to patients with severe COVID-19 alone. Intriguingly, we did not observe any significant differences in the levels of the endothelial biomarkers ICAM1, vWF, VEGFR1, Ang1 and Ang2 between patients with cirrhosis and sepsis and those with cirrhosis and COVID-19 (Fig. 1).
Table 3.
Baseline characteristics of patients in intensive care unit.
| Variable | Severe COVID-19 (n = 11) | Severe COVID-19 + cirrhosis (n = 12) | Sepsis + cirrhosis(n = 24) | p value (all groups) |
|---|---|---|---|---|
| Age (years) | 54 (34-65) | 52.5 (35-72) | 44 (34-78) | |
| Male, n (%) | 10 (90.9) | 10 (83.3) | 21 (87.5) | |
| Female, n (%) | 1 (9.09) | 2 (16.6) | 3 (12.5) | |
| Neutrophil to lymphocyte ratio | 8.15 (2.5-39.3) | 7.8 (4.4-22.23) | 13.5 (1.41-39) | n.s. |
| Platelet count (x109/L) | 210 (57-449) | 59 (36-122) | 114 (14-339) | 0.0002 |
| Bilirubin (mg/dl) | 0.77 (0.33-3.74) | 10.65 (1.07-29.8) | 9.35 (1.30-32) | <0.0001 |
| AST (U/L) | 54 (27-210) | 49.5 (23-273) | 79 (40-553) | n.s. |
| ALT (U/L) | 54.2 (18-89) | 40 (21-101) | 45 (16-208) | n.s. |
| Albumin (g/dl) | 3.29 (0.37) | 2.8 (0.56) | 2.65 (0.6) | 0.001 |
| Serum sodium (mEq/L) | 131.5 (4.03) | 130.5 (7.07) | 128.1 (8.7) | n.s. |
| INR | 1.05 (0.96-1.74) | 1.8 (0.8) | 2.3 (0.7) | <0.0001 |
| Creatinine (mg/dl) | 0.9 (0.53-1.65) | 1.3 (0.2-3.4) | 2.85 (0.5-10.22) | 0.003 |
| D-dimers (pg/ml) | 2,498.3 (1,039.02) | 2,916 (660.6) | 3,098.6 (682.4) | n.s. |
| Ferritin (ng/ml) | 230 (133-1,540) | 884.5 (276-1,670) | 651.4 (103-2,220) | 0.02 |
| LDH (U/L) | 533.3 (260.8) | 605.1 (276.5) | 561.2 (206.6) | n.s. |
| PCT (ng/ml) | 2.0 (0.9) | 2.1 (1.5) | 4.2 (3.1) | n.s. |
| Mechanical ventilation, n (%) | 4 (36.3) | 8 (66.6) | 17 (70.3) | - |
| Etiology of liver diseases, n (%) | ||||
| Alcohol | 0 (0) | 6 (50) | 10 (41.6) | - |
| HBV | 0 (0) | 2 (16.6) | 4 (16.6) | - |
| HCV | 0 (0) | 0 (0) | 3 (12.5) | - |
| NASH | 0 (0) | 3 (25) | 6 (25) | - |
| Other | 0 (0) | 1 (8.3) | 1 (4.1) | - |
| Ascites, n (%) | 0 (0) | 9 (75) | 23 (95.8) | - |
| Hepatic encephalopathy, n (%) | 0 (0) | 8 (66.6) | 16 (66.6) | - |
| ARDS, n (%) | 9 (81.8) | 8 (66.6) | 15 (62.5) | |
| Liver disease severity n (%) | ||||
| Child-Pugh A | 0 (0) | 1 (8.3) | 0 (0) | |
| Child-Pugh B | 0 (0) | 1 (8.3) | 4 (16.6) | |
| Child-Pugh C | 0 (0) | 10 (83.3) | 20 (83.3) | |
| MELD score | - | 23.3 (9.5) | 31.2 (8.8) | 0.01 |
| SOFA (day 3-5) | 11.08 (2.6) | 11.83 (1.8) | n.s. | |
| CLIF-OF (day 3-5) | 11.9 (2.6) | 12.5 (2.3) | n.s. | |
| Organ failures, n (%) | ||||
| Liver | 6 (50) | 14 (58.3) | n.s. | |
| Renal | 7 (58.3) | 18 (75) | n.s. | |
| Respiratory | 10 (83.3) | 19 (79.2) | n.s. | |
| Brain | 6 (50) | 11 (45.8) | n.s. | |
| Circulation | 6 (50) | 18 (75) | n.s. | |
| Coagulation | 7 (58.3) | 13 (54.2) | n.s. | |
| Infections, n (%) | ||||
| Pneumonia | 12 (100) | 18 (75) | ||
| SBP | 0 | 3 (12.5) | ||
| Blood sepsis | 0 | 3 (12.5) | ||
| Comorbidities n (%) | ||||
| Hypertension | 6 (54.5) | 5 (41.6) | 10 (41.6) | |
| Diabetes mellitus | 7 (63.6) | 7 (58.3) | 11 (45.8) | |
| Coronary artery disease | 3 (27.7) | 2 (16.6) | 4 (16.6) | |
| Obesity | 2 (18.1) | 2 (16.6) | 5 (20.8) | |
| Liver disease | 0 (0) | 12 (100) | 24 (100) | - |
| Charlson comorbidity index | 2 (0-5) | 4 (1-7) | 4 (3-6) | 0.0004 |
For continuous variables, values are given as mean (SD) or median (min-max) and p values have been calculated by One-way Annova or Kruskal-Wallis test. Categorical variables are given as n (%). ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CLIF-OF, chronic liver failure-organ failure; ICU, intensive care unit; INR, international normalized ratio; LDH, lactate dehydrogenase; MELD, model for end-stage liver disease; NASH, non-alcoholic steatohepatitis; PCT, procalcitonin; SBP, spontaneous bacterial peritonitis; SOFA, sequential organ failure assessment.
Fig. 1.
Endothelial injury biomarkers in patients in the intensive care unit.
Data points indicate individual measurements and horizontal bars are median values. ∗Denotes significant p values as computed by Mann-Whitney U test and are mentioned in the figure. LC, liver cirrhosis.
Endothelial biomarkers and survival in ICU patients
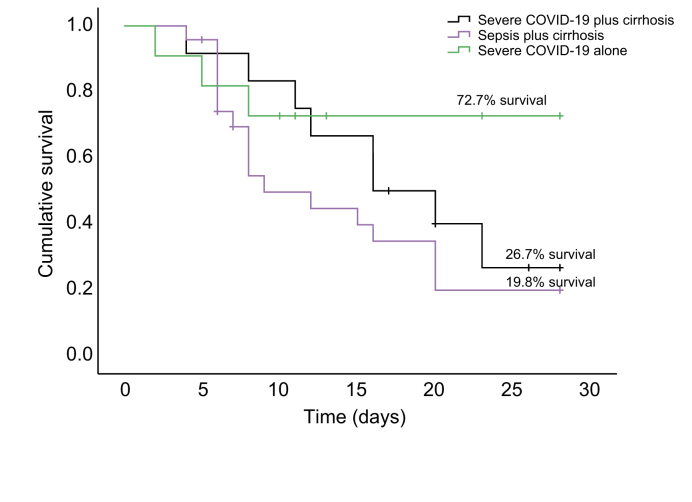
We estimated the 28-day survival in the 3 different categories of severely ill ICU patients. Survival at 28 days was 72.7% for patients with severe COVID-19, while being only 26.7% for those with severe COVID-19 and cirrhosis, and only 19.8% for those with sepsis and cirrhosis (Fig. 2). Log-rank analysis revealed a significant difference between the survival of patients with sepsis and cirrhosis in comparison to patients with severe COVID-19 alone (chi-square = 4.03, df = 1, p = 0.04). Median survival time was 16 days (95% CI 10.33–21.66) in patients with severe COVID-19, compared to 16 days (95% CI 7.51–24.48) in patients with severe COVID-19 and cirrhosis, and 9 days (95% CI 4.70–13.2) in patients with sepsis plus cirrhosis. In patients with cirrhosis and sepsis, mortality was due to severe sepsis or septic shock with progressive liver failure or multi-organ failure. We studied whether endothelial biomarkers predicted survival in these severely ill ICU patients. The levels of ICAM1 (p <0.0001) were significantly elevated while those of VEGFR1 (p = 0.04) and Ang1 (p = 0.02) were decreased in non-survivors compared to survivors (Table S3).
Fig. 2.
A 28-day cumulative probability of survival in severely ill patients in the intensive care unit.
Survival curves were estimated by the Kaplan-Meier method. Mortality as seen by log-rank analysis between severe COVID-19 and severe COVID-19 plus cirrhosis was not significant (p = 0.48) while it was significantly different between severe COVID-19 and sepsis plus cirrhosis (p = 0.03).
ICAM1 as an independent predictor of 28-day mortality in patients with cirrhosis and severe COVID-19 or sepsis
We next studied these biomarkers as mortality predictors along with other clinical biomarkers in the cohort of ICU patients using logistic regression. The association between log-transformed biomarkers and death was analysed. Unadjusted univariate and adjusted hazard ratios (HRs) of all the clinical variables derived from the Cox hazards model in ICU patients are given in Table S4. In the univariate analysis, D-dimers, AST, creatinine and ICAM1 were significant predictors of mortality in the full cohort of ICU patients. AST (HR 1.87; 95% CI 1.08–3.31; p = 0.03) and ICAM1 (HR 2.94; 95% CI 1.3–6.65; p = 0.01) emerged as significant and independent predictors of mortality after multivariate analysis (Table S4). We then performed a separate regression analysis in ICU patients with cirrhosis and either sepsis or COVID-19. NLR, AST, creatinine, MELD, renal failure, coagulation failure, CLIF-OF, SOFA and ICAM1 appeared as mortality predictors in the univariate analysis. On multivariate analysis, ICAM1 (HR 2.92; 95% CI 1.02–8.31; p = 0.04) and MELD score (HR 1.07 95% CI 1.02-1.11; p = 0.006) emerged as independent predictors, when organ failures or their composite scores were not entered into the model. On inclusion of individual organ failures and not composite scores, AST, renal failure, coagulation failure and ICAM1 appeared as independent mortality predictors (Table 4). ICAM1 (HR 3.24; 95% CI 1.19–8.86; p = 0.02) significantly predicted mortality in patients with cirrhosis and either COVID-19 or bacterial sepsis in this model (Table 4). When CLIF-OF and SOFA composite scores were included in the multivariate analysis along with other significant predictors, they only appeared as independent mortality predictors without ICAM1 (Table 4).
Table 4.
ICAM1, MELD and organ failures as independent mortality predictors in patients with cirrhosis and severe COVID-19 or sepsis in the intensive care unit.
| Risk factor | Univariate analysis |
|
|---|---|---|
| HR (95% CI) | p value | |
| Age | 0.98 (0.96-1.01) | 0.44 |
| Sex | 0.72 (0.2-2.43) | 0.59 |
| CCI score | 1.03 (0.78-1.38) | 0.79 |
| D-dimers# | 2.89 (0.40-20.45) | 0.28 |
| Ferritin | 1.08 (0.66-1.76) | 0.75 |
| LDH# | 1.51 (0.78-2.90) | 0.213 |
| NLR | 1.05 (1.00-1.11) | 0.04∗ |
| PCT | 0.94 (0.83-1.07) | 0.41 |
| Albumin | 1.13 (0.59-2.188) | 0.69 |
| Bilirubin | 1.03 (0.99-1.08) | 0.08 |
| Sodium | 1 (0.95-1.04) | 0.97 |
| AST# | 1.86 (1.04-3.31) | 0.03∗ |
| ALT# | 1.17 (0.59-2.30) | 0.64 |
| INR | 1.25 (0.81-1.92) | 0.3 |
| Creatinine | 1.26 (1.03-1.54) | 0.02∗ |
| Child-Pugh | 1.08 (0.91-1.28) | 0.37 |
| MELD | 1.066 (1.01-1.11) | 0.007∗ |
| Liver Failure | 1.68 (0.75-3.78) | 0.2 |
| Renal Failure | 3.41 (1.23-9.47) | 0.018∗ |
| Respiratory Failure | 0.93 (0.31-2.77) | 0.9 |
| Circulatory Failure | 1.06 (0.46-2.43) | 0.87 |
| Coagulation Failure | 3.22 (1.35-7.66) | 0.008∗ |
| CLIF-OF | 1.29 (1.10-1.52) | 0.002∗ |
| SOFA | 1.47 (1.12-1.93) | 0.005∗ |
| ARDS | 1.06 (0.47-2.38) | 0.87 |
| ICAM1# | 2.68 (1-7.21) | 0.04∗ |
| vWF# | 1.02 (0.53-1.96) | 0.94 |
| Vegfr1# | 0.95 (0.45-2.02) | 0.9 |
| Ang1# | 1.49 (0.86-2.59) | 0.14 |
| Ang2# | 0.93 (0.57-1.52) | 0.79 |
| Multivariate analysis (without inclusion of organ failures and composite scores) | ||
| ICAM1# | 2.92 (1.02-8.31) | 0.04 |
| MELD | 1.07 (1.02-1.11) | 0.006 |
| AST# | 2.17 (1.15-4.11) | 0.02 |
| Multivariate analysis (with inclusion of various organ failures and not composite scores) | ||
| ICAM1# | 3.24 (1.19-8.86) | 0.02 |
| Renal failure | 4.57 (1.54-13.6) | 0.006 |
| Coagulation failure | 2.79 (1.08-9.7.15) | 0.03 |
| Multivariate Analysis (with inclusion of composite scores without individual organ failures) | ||
| SOFA | 1.47 (1.12-1.93) | 0.005 |
| CLIF-OF | 1.29 (1.10-1.53) | 0.002 |
Significance was taken as p <0.05. CCI, Charlson comorbidity index; CLIF-OF, chronic liver failure-organ failure; HR, hazard ratio; LDH, lactate dehydrogenase; MELD, model for end-stage liver disease; NLR, neutophil to lymphocyte ratio; PCT, procalcitonin; SOFA, sequential organ failure assessment.
Log values of these parameters were taken.
Denotes significant p values (Cox regression).
ICAM1 is a better mortality predictor than MELD score in patients with cirrhosis and severe COVID-19 or sepsis
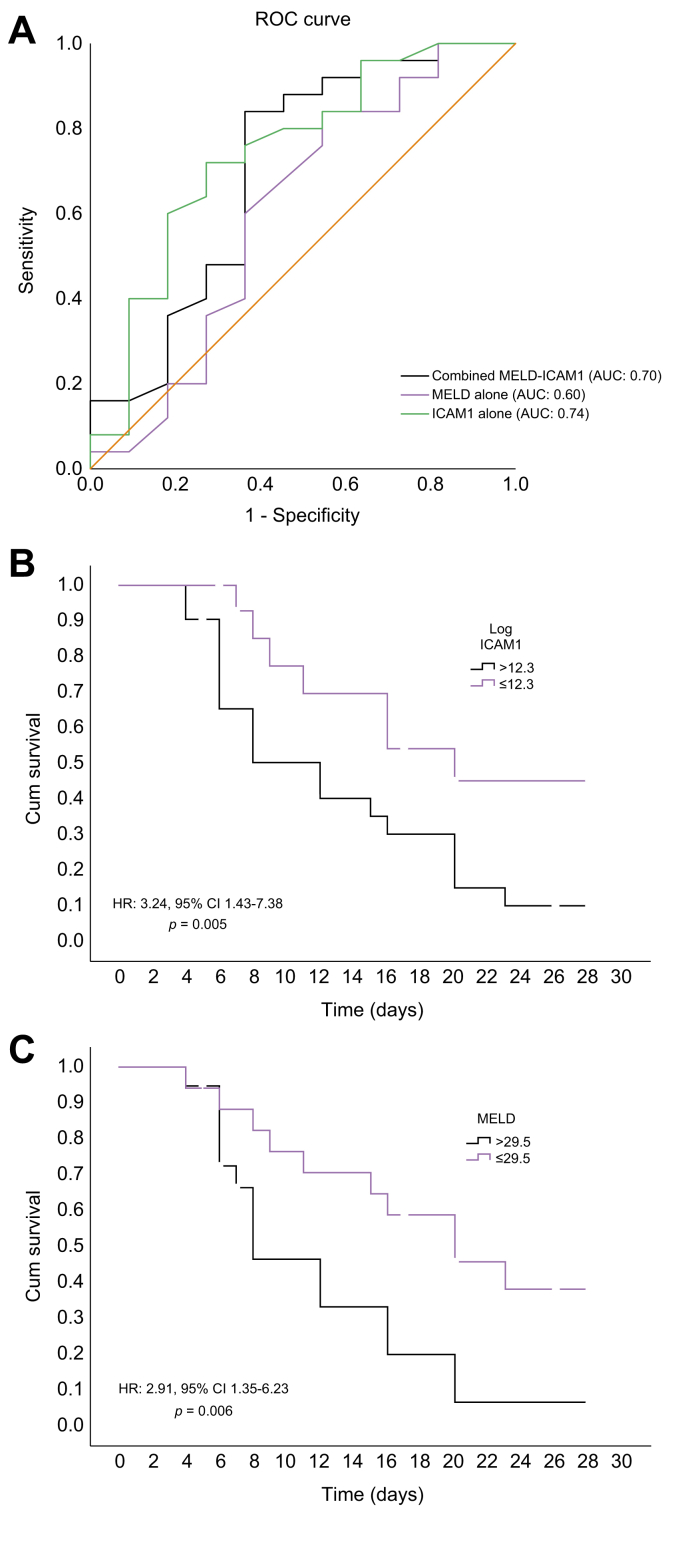
The comparison between discriminatory values of ICAM1 and various scoring systems was performed in patients with cirrhosis and severe COVID-19 or sepsis (Table S5). The AUC was 0.83 (95% CI 0.67–0.97) for the CLIF-SOFA score and 0.82 (95% CI 0.66-0.98) for the SOFA score. The AUC was 0.75 (95% CI 0.56-0.93) for ICAM1 and 0.60 (95% CI 0.38-0.83) for the MELD score (Fig. 3A). We then evaluated the sensitivity and specificity of prediction at cut-off points that provided the best Youden index. The sensitivity and specificity of ICAM1 was comparable to the organ failure scores at the respective cut-off values but higher than the MELD score. The best Youden indices were obtained for the organ failure scores and ICAM1 (Table S5). A log ICAM1 level above 12.3 was used as the cut-off value, with sensitivity of 72%, specificity of 72.7% and Youden index of 44.7% (AUC 0.74; 95% CI 0.56 –0.93, Fig. 3A). With the obtained cut-off values of ICAM1, we next constructed survival curves in patients with cirrhosis using the Cox regression. The log-rank analysis showed that mortality in patients with a logICAM1 value less than or equal to 12.3 was significantly lower at 28 days than patients with logICAM1 levels greater than 12.3 (Chi-Square: 5.10; p = 0.02). The HR at this cut-off value of ICAM1 was 3.24 (95% CI 1.43–7.38; p = 0.005) (Fig. 3B). The optimal cut-off value for MELD was 29.5, with a sensitivity of 60%, specificity of 63.6% and Youden index of 23.6%. Using this MELD score cut-off value, we studied survival curves in patients with cirrhosis. The analysis showed a significant difference in mortality in this study cohort using this cut-off of MELD score (Chi-Square: 6.1; p = 0.013). The HR at this cut-off value of MELD was 2.91 (95% CI 1.35–6.23; p = 0.006) (Fig. 3C). Overall, ICAM1 showed a net benefit of 14% compared to MELD alone in discriminating survivors from non-survivors in this category of patients. Using a combined model of ICAM1 and MELD in liver disease patients with severe COVID-19 or sepsis, the AUC value to predict 28-day mortality was 0.70 (95% CI 0.49 –0.91), demonstrating a net benefit of 10% compared to the use of MELD alone (Fig. 3A).
Fig. 3.
Plasma ICAM1 and MELD scores as mortality predictors in patients with cirrhosis and COVID-19 or sepsis in the intensive care unit.
(A) Receiver-operating characteristics curve of logICAM1, MELD and combined logICAM1-MELD in discriminating survivors from non-survivors. (B) Kaplan-Meier curve of survival with low and high and plasma logICAM1 levels (cut-off value: 12.3), p = 0.005 (log-rank analysis). (C) Kaplan-Meier curve of survival with low and high and MELD score (cut-off value: 29.5), p = 0.006 (log-rank analysis). HR, hazard ratio; MELD, model for end-stage liver disease.
ICAM1 and organ failure scores
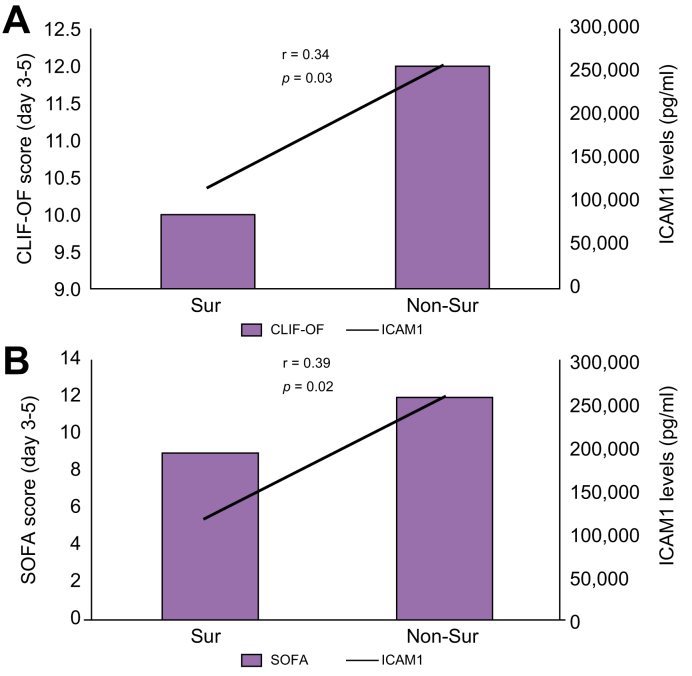
We next analysed the correlation between the plasma ICAM1 levels and some of the known prognostic factors of critical disease including age, CCI score, CLIF-OF and SOFA scores (3-5 days post ICU admission), LDH, NLR, ferritin, PCT and D-dimers in patients with cirrhosis and severe COVID-19 or sepsis. Of all the parameters, ICAM1 levels were positively and significantly correlated with the CLIF-OF (r = 0.34, p = 0.03) and SOFA (r = 0.39, p = 0.02) scores (Fig. 4A&B). CLIF-OF and SOFA scores and ICAM1 levels were significantly higher in non-survivors compared to survivors. The median CLIF-OF scores in survivors vs. non-survivors were 10 (8-13) vs. 12 (10-17) and the median SOFA scores in survivors vs. non-survivors were 9 (6-13) vs. 12 (9-15). The median (min-max) ICAM1 levels (pg/ml) in survivors vs. non-survivors were 117,100 (82,200-231,000) vs. 258,000 (86,970-353,600).
Fig. 4.
Pearson's correlation between CLIF-OF and SOFA score with ICAM1 levels in patients with cirrhosis and COVID-19 or sepsis in the intensive care unit.
A combined graph showing either CLIF-OF or SOFA scores and ICAM1 levels in survivors (sur) and non-survivors (non-sur). For ICAM1 and CLIF-OF, p = 0.03 and for ICAM1 and SOFA, p = 0.02 (Pearson's correlation analysis). r' is Pearson's correlation co-efficient. CLIF-OF, chronic liver failure-organ failure; SOFA, sequential organ failure assessment.
Discussion
The study fortifies the findings of previous studies showing elevated plasma levels of biomarkers of endothelial activation including ICAM1, vWF, VEGFR1 and Ang2 in hospitalized COVID-19 patients, indicating endothelial activation as an important hallmark of COVID-19 infection. It has been proven that thrombomodulin and vWF predict survival in critically ill COVID-19 ICU patients, indicating a central role of endothelial activation in the progression of the disease.10 Ang2/Ang1 ratios have also been shown to be a relevant predictive factor for ICU admission in COVID-19 patients.11 In our study, we observed significantly enhanced levels of ICAM1 and decreased levels of VEGFR1 and Ang1 among survivors and non-survivors in the ICU. Our findings corroborate previous findings that increased expression of endothelial cell adhesion molecules is related to COVID-19 disease severity.12,13
Intriguingly, we found that the soluble ICAM1 levels were further elevated while VEGFR1 and Ang1 were decreased in age- and sex-matched patients with COVID-19 and cirrhosis compared to those with COVID-19 alone. Since we have not performed a longitudinal analysis of ICAM1 in patients before and after the diagnosis of COVID-19, we cannot say whether these levels have increased due to COVID-19. Indeed, ICAM1 and vascular cell adhesion molecule 1 are also highly expressed on inflamed liver sinusoidal endothelial cells. Circulating ICAM1 levels and even other biomarkers of endothelial injury (such as Ang2) have been reported to be significantly elevated in patients with cirrhosis compared to normal healthy individuals.[14], [15], [16], [17] Thus, patients with cirrhosis might present with pre-existing altered levels of some of these biomarkers due to an ongoing endothelial injury and activation, In our cohort, liver disease patients with COVID-19 had a higher CCI in comparison to those with COVID-19 alone. The presence of comorbidities has been appreciably correlated with endothelial injury in previous reports.1
Among all the clinical variables in our study, high AST, creatinine, D-dimers and soluble ICAM1 plasma levels emerged as important predictors of 28-day mortality in ICU patients, with AST and ICAM1 appearing as independent predictors after multivariate analysis. Elevated D-dimers have been reported to be one of the strong predictors of severe prognosis in patients with COVID-19.18 AST and ICAM1 levels also predicted mortality independently in ICU patients with cirrhosis and either COVID-19 or sepsis. In terms of receiver-operating characteristic analysis, we observed that ICAM1 alone or a combined ICAM1 and MELD model was 10% better at predicting short-term mortality compared to MELD score alone in patients with cirrhosis and severe COVID-19 or bacterial sepsis, underscoring the relevance of endothelial injury as one of the most important prognostic factors in patients with cirrhosis and bacterial or viral infections. COVID-19 has been reported to increase mortality rates in patients with cirrhosis, even in patients with low MELD scores. Also, in patients with acute-on-chronic liver failure (ACLF), with sepsis and ≥1 organ failure(s), it has recently been reported that MELD-Na does not correctly capture 90-day mortality risk.19 Our findings reinforce these findings that prognosis and survival cannot only be explained by differences in the severity of liver disease.6
Although not the sole component of host response to infections, the endothelium is a key perpetuator of inflammation and coagulation that leads to microvascular occlusion, hypoxia, and organ dysfunction. ICAM1 is mainly involved in leukocyte recruitment to the underlying tissues. Enhanced expression of ICAM1 on activated endothelial cells has been reported to contribute to increased neutrophil adhesion and transmigration into the tissues in inflammation.20 The activation of neutrophils has been linked to platelet activation and intravascular coagulation, thus causing impaired organ perfusion. High soluble ICAM1 levels have been well correlated with the development of multi-organ dysfunction in patients with severe sepsis.21 We also observed a significant positive correlation between high ICAM1 levels and organ failure severity (both SOFA score and CLIF-OF score). Compared to other liver disease severity markers such as APACHE II and Child-Pugh scores, studies have shown that the organ failure scores perform far better in terms of their ability to predict survival in critically ill patients with cirrhosis in ICUs.22 In fact, when we entered the CLIF-OF or SOFA scores in our regression models, ICAM1 did not appear as an independent predictor given its close correlation with organ failure scores. When we used the individual organ failures in our statistical analysis, along with ICAM1, renal and coagulation failures appeared as independent mortality predictors in our study cohort, which was in line with a recently published study.23
Another interesting observation from our study was that in patients with cirrhosis, most of the endothelial dysfunction biomarkers were not different in either sepsis or severe COVID-19, implying that both COVID-19 infection and bacterial sepsis involve similar mechanisms of vascular injury and organ dysfunction. Loss of endothelial barrier function and increased permeability is already well-established in sepsis. Serum concentrations of several endothelial injury biomarkers correlate with sepsis severity and patient mortality.24 In fact, patients with cirrhosis and severe COVID-19 or sepsis were also very similar in their baseline clinical profile, suggestive of similar ongoing host responses to these infections. Several studies have now emphasized that critically ill patients with sepsis or severe COVID-19 have similar immune profiles and that beyond hyperinflammation, immunosuppression is responsible for mortality in these patients.25 Severe depletion of immune effector cells and lymphopenia has been unequivocally reported to be a strong predictor of prognosis in patients with sepsis or COVID-19.25,26 Aberrations in the innate and adaptive components of the immune system also characterize patients with decompensated cirrhosis, whereby cirrhosis-associated immune dysfunction leads to sepsis and mortality.27 Although increased expression of endothelial ICAM1 is correlated with inflammation and neutrophil transmigration in tissues, it is known that as the inflammatory responses progress, very high levels of soluble isoforms of the adhesion molecules are shed from endothelial cells to dampen inflammation (and specifically to reduce leucocyte–endothelial interactions) and protect the host from excessive collateral damage.28 An elegant study has depicted that soluble ICAM1 shed into the vascular compartment may lead to a self-limiting inflammatory response and hence immunosuppression, while the presence of ICAM1 in the distal airway compartment could propagate existing inflammation caused by enhanced production of cytokines by lung macrophages.29 Determining how soluble levels of ICAM1 cause immunosuppression during severe infections is crucial and warrants further investigations. One of the important limitations of our study is the small sample size. Also, we have not assessed serial levels of these markers, particularly in resolved phase of the disease. However, to our knowledge, this is the first study that has demonstrated the role of a dysfunctional endothelium in COVID-19- or sepsis-associated mortality in patients with cirrhosis. Since the study has been performed in the Asia Pacific region, where we follow the APASL definition for ACLF,30 none of the decompensated cirrhotic patients in our cohort could be characterized as ACLF according to this criteria. However, given the fact that organ failures are significant predictors of mortality in patients with ACLF, it would be worthwhile to study the association of ICAM1 levels and other vascular injury biomarkers with mortality in patients with ACLF, as specified by the EASL as well as APASL guidelines.19,22,30
To summarize, increased plasma levels of ICAM1, an endothelial injury marker, represent a significant predictor of COVID-19- or sepsis-associated mortality in severely ill patients with cirrhosis. Elevated levels of plasma ICAM1, due to pre-existing vascular/endothelial injury in these patients, may comprise one of the key factors leading to a dampened immune system, thus making them most susceptible to COVID-19- or sepsis-associated organ failures and deaths. The use of ICAM1 and other biomarkers of endothelial injury should be validated in a larger population of patients with cirrhosis and viral or bacterial sepsis; if validated, this could be an important prognostic marker for mortality. In cirrhotic patients with high ICAM1 levels, a timely therapy that mitigates endothelial injury and boosts immune competence could affect outcomes.
Financial support
The study was supported partly by research funds from the Institute of liver and biliary Sciences (ILBS), New Delhi and partly by Department of Science and Technology (DST), Government of India.
Authors’ contributions
SK designed the study along with KK, DMT and SKS; SK performed all the experiments, along with SH and wrote the manuscript; SH collected all samples and performed ELISA analysis; KK, GK and DMT performed statistical analysis along with SK; AT guided for understanding all the clinical variables of the COVID-19 patients and helped in selection of such patients; PK was involved in collection of culture-positive samples of patients with sepsis; AN, CB and MB aided in collection of clinical data from patients with COVID-19; RM was involved in selection of cirrhotic patients with sepsis and also analysis of data from ICU patients; EG provided the RT-PCR data of all COVID-19 patients; SKS provided all clinical inputs for the study and finalized the manuscript draft; All authors approved the final draft.
Data availability statement
The data generated during and/or analysed during the current study are available with the corresponding authors on reasonable request.
Conflict of interests
The authors declare no conflict of interests.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
We would like to thank all the supporting hospital staff who have helped us with the collection of blood samples.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100303.
Contributor Information
Savneet Kaur, Email: savykaur@gmail.com.
Shiv K. Sarin, Email: shivsarin@gmail.com.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Galley H.F., Webster N.R. Physiology of the endothelium. Br J Anaesth. 2004;93:105–113. doi: 10.1093/bja/aeh163. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur S., Tripathi D.M., Yadav A. The enigma of endothelium in COVID-19. Front Physiol. 2020;11:989. doi: 10.3389/fphys.2020.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iavarone M., D'Ambrosio R., Soria A., Triolo M., Pugliese N., Del Poggio P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marjot T., Moon A.M., Cook J.A., Abd-Elsalam S., Aloman C., Armstrong M.J. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.09.024. S0168-8278:33667-33669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Government of India. Ministry of family health and welfare . 13th June, 2020. Guidelines on clinical management of COVID–19. [Google Scholar]
- 9.Lambden S., Laterre P.F., Levy M.M., Francois B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23:374. doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smadja D.M., Guerin C.L., Chocron R., Yatim N., Boussier J., Gendron N. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23:611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong M., Jiang Y., Xia D., Xiong Y., Zheng Q., Chen F. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J Infect Dis. 2020;222:894–898. doi: 10.1093/infdis/jiaa349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagashima S., Mendes M.C., Camargo Martins A.P., Borges N.H., Godoy T.M., Miggiolaro A.F.R.D.S. Endothelial dysfunction and thrombosis in patients with COVID-19—brief report. Arteriosclerosis, Thromb Vasc Biol. 2020;40:2404–2407. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalor T., Adams D. The liver: a model of organ-specific lymphocyte recruitment. Expert Rev Mol Med. 2002;4:1–15. doi: 10.1017/S1462399402004155. [DOI] [PubMed] [Google Scholar]
- 15.Mandi Y., Nagy I. Circulating ICAM1 in alcoholic liver cirrhosis. Int Arch Allergy Immunol. 1995;106:302–304. doi: 10.1159/000236859. [DOI] [PubMed] [Google Scholar]
- 16.Kasztelan-Szczerbinska B., Surdacka A., Slomka M., Rolinski J., Celinski K., Cichoz-Lach H. Angiogenesis-related biomarkers in patients with alcoholic liver disease: their association with liver disease complications and outcome. Mediat Inflamm. 2014;2014:673032. doi: 10.1155/2014/673032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Bartolomé Á., López-Rodríguez R., Borque M.J., González-Moreno L., Real-Martínez Y., García-Buey L. Angiopoietin-2/angiopoietin-1 as non-invasive biomarker of cirrhosis in chronic hepatitis C. World J Gastroenterol. 2016;22:9744. doi: 10.3748/wjg.v22.i44.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastug A., Bodur H., Erdogan S., Gokcinar D., Kazancioglu S., Kosovali B.D. Clinical and laboratory features of COVID-19: predictors of severe prognosis. Int Immunopharmacol. 2020;88:106950. doi: 10.1016/j.intimp.2020.106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernaez R., Liu Y., Kramer J.R., Rana A., El-Serag H.B., Kanwal F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J Hepatol. 2020;73:1425–1433. doi: 10.1016/j.jhep.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Barthel L. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amalakuhan B., Habib S.A., Mangat M., Reyes L.F., Rodriguez A.H., Hinojosa C.A. Endothelial adhesion molecules and multiple organ failure in patients with severe sepsis. Cytokine. 2016;88:267–273. doi: 10.1016/j.cyto.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong V., Karvellas C.J. Acute-on-chronic liver failure: objective admission and support criteria in the intensive care unit. JHEP Rep. 2019;1:44–52. doi: 10.1016/j.jhepr.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Méndez-Guerrero O., Calle-Rodas D.A., Cervantes-Alvarez E., Alatorre-Arenas E., Pérez-Escobar J., Navarro-Alvarez N. Renal and brain failure predict mortality of patients with acute-on-chronic liver failure admitted to the intensive care unit. Ann Hepatol. 2020;22:100270. doi: 10.1016/j.aohep.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Dolmatova E.V., Wang K., Mandavilli R., Griendling K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 2020;117:60–73. doi: 10.1093/cvr/cvaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remy K.E., Mazer M., Striker D.A., Ellebedy A.H., Walton A.H., Unsinger J. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI insight. 2020;5 doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziadi A., Hachimi A., Admou B., Hazime R., Brahim I., Douirek F. Lymphopenia in critically ill COVID-19 patients: a predictor factor of severity and mortality. Int J Lab Hematol. 2021;43:e38–e40. doi: 10.1111/ijlh.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Wang H.W., Babic A.M., Mitchell H.A., Liu K., Wagner D.D. Elevated soluble ICAM1 levels induce immune deficiency and increase adiposity in mice. FASEB J. 2005;19:1018–1020. doi: 10.1096/fj.04-3094fje. [DOI] [PubMed] [Google Scholar]
- 29.Schmal H., Czermak B.J., Lentsch A.B., Bless N.M., Beck-Schimmer B., Friedl H.P. Soluble ICAM1 activates lung macrophages and enhances lung injury. J Immunol. 1998;161:3685–3693. [PubMed] [Google Scholar]
- 30.Sarin S.K., Choudhury A., Sharma M.K., Maiwall R., Al Mahtab M., Rahman S. APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during and/or analysed during the current study are available with the corresponding authors on reasonable request.