Abstract
Background
Patients with coronavirus disease-2019 (COVID-19) with preexisting diabetes and cardiovascular metabolic diseases have higher fatality rate. The circulation of new variants with emerging clinical characteristics requires more studies focusing the impact of preexisting health conditions on outcome of COVID-19 accurately.
Aims
Main aim of this study was to investigate the impact of diabetes and cardiovascular disease (CVD) on disease prognosis and severe health outcomes among patients with COVID-19.
Methods
A retrospective study was performed on 799 patients with COVID-19 during December 10, 2020, to February 10, 2020 in Bangladesh. Logistic regression analysis was performed for age, sex, diabetes, CVD and symptoms on fatality. Kaplan-Meier survival analysis was conducted to predict the survival rate.
Results
Fatality was detected in 40% (318 of 799) patients with COVID-19. Among 318 fatalities, 90.6% were detected in patients with CVD and 74.5% in patients with diabetes. Case fatality rate was highest in patients with COVID-19, CVD and diabetes (94, 184 of 195). Fever (91%) and dry cough (71%) were the most frequent symptoms. CVD (42.2%), diabetes (32.7%) and obesity (18%) were prevalent. The highest odds of risk was detected in patients with COVID-19, CVD and diabetes (OR: 6.98, 95% CI, 4.21 to 7.34). Female patients had the highest survival rate.
Conclusions
In this study, 318 fatality was seen in 799 patients with COVID-19. The highest odds of fatality risk was detected in patients with COVID-19, CVD and diabetes. The risk increased many folds when CVD and diabetes coexisted in patients.
Keywords: COVID-19, Diabetes, Cardiovascular disease, Poor prognosis, Fatality
1. Introduction
An infectious virus namely, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has set out the pandemic called coronavirus disease-2019 (COVID-19) that has transmitted promptly from Wuhan, China from late December 2020 throughout 211 countries and territories around the world [[1], [2], [3]]. On March 11, 2020, the World Health Organization announced COVID-19 a pandemic due to the rapid transmission. The COVID-19 pandemic has become one of the leading health burdens with soaring number of cases and fatalities [[1], [2], [3], [4]]. As of March 26, 2021, about 126 million cases and 2.7 million fatalities of COVID-19 have been officially reported [3,4]. A high prevalence of underlying health conditions including hypertension, diabetes, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), and obesity are present in poor prognosis and fatalities among patients with COVID-19 [5,6]. The ICU admission rate and case-fatality rate are high in older patients with preexisting comorbidities. Among comorbidities, diabetes and CVD are most significantly associated with poor prognosis of patients with COVID-19 [5]. The existing clinical records indicate that type 2 diabetes mellitus is prevalent followed by type 1 diabetes [[5], [6], [7], [8]]. Recently, published work found that COVID-19 has the potential to trigger the onset of different types of diabetes. The prevalence of diabetes in patients with COVID-19 were 9.8% in Wuhan, 9.7% in China, 8.9% in Italy and 32% in the UK [5,6]. Clinical data from previous studies showed that patients with COVID-19 with diabetes had higher odds of hospitalization and fatality in China, the UK and Italy [6]. Further, prognosis of severe health conditions and fatalities in patients with COVID-19 are not only induced by the common clinical symptoms of respiratory failure but also by the cardiovascular diseases by SARS-CoV-2 infection [9]. Prevalence of CVD including arrhythmias, acute myocardial injury, coronary heart disease, cardiomyopathy, and hypertension in patients with COVID-19 varied from 33% to 7% in previous studies [9]. Both diabetes and CVD are associated with the severe outcomes and fatalities in patients with COVID-19 [[10], [11], [12], [13], [14]]. However, specific information on the exact mechanism of effects of COVID-19 on patients with CVD and diabetes are still lacking [15]. Only limited number of studies have been conducted to determine the association of CVD, diabetes and COVID-19 on the disease prognosis among patients with COVID-19. Preliminary study on the effects of CVD and diabetes on prognosis of COVID-19 and vice versa is lacking in Bangladesh and South Asia. Therefore, we investigated the impact of diabetes and CVD on disease prognosis among patients with COVID-19 in Bangladesh.
2. Materials and methods
2.1. Study design and population
A retrospective, observational, multi-center, study was performed on data from 799 participants from eight different hospitals and clinics from eight divisions in Bangladesh. Patients with COVID-19 positive results by RT-PCR diagnosis following the guideline of the World Health Organization were retrospectively analyzed [16]. The study was conducted from December 10, 2020, to February 10, 2020. Records of the patients of hospitalization or discharge were collected directly from them and authorities, while death reports were collected from the authorities and relatives of the patients. Appropriate ethical approval was taken from the Biosafety, Biosecurity & Ethical Committee at Jahangirnagar University under the clearance ref no. BBEC, JU/M 2021/COVID-19/(2)1. Consent was taken from patients or relatives of the patients and the hospitals/clinics.
2.2. Data collection
Nasal or pharyngeal swab specimens were used for the test. The World Health Organization guidance were followed for testing of samples [16]. Positive result was defined by a positive laboratory test for SARS-CoV-2 by real-time reverse-transcriptase– PCR (RT-PCR) assay or high throughput sequencing [16]. Sociodemographic characteristics including age, sex, monthly income, origin, residing place, underlying health conditions (defined by the International Classification of Diseases, 10th Revision, Clinical Modification), medical history, complication, treatment (antiviral, antibiotic, steroid therapies, immune therapy, plasma therapy, respiratory support by mechanical ventilation and ICU support) and outcome were incorporated in this study [13]. Preexisting health conditions included diabetes mellitus, a history of hypertension, hyperlipidemia, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD) and malignancy.
2.3. Outcome
The primary outcome was fatality associated with COVID-19 infection. Secondary outcomes included admission to ICU, requirement of mechanical ventilation and hospitalization. Recovered patients criteria contained resolution of clinical symptoms, inflammation, normal body temperature, normal chest radiography, and minimum of two successive negative results shown by real time RT-PCR tests of COVID-19. Acute respiratory distress syndrome malignant arrhythmia, and acute myocardial injury were defined according to the previously published works [11,13].
2.4. Statistical analysis
Data representing categorical variables are shown as percentage, frequency and rate. Central tendency of continuous variables were represented as means and standard deviations. The primary analysis included determining the relationship of preexisting diabetes, cardiovascular disease and comorbidities with the outcomes. Independent sample t-tests were performed with 95% confidence intervals. Logistic-regression analysis was conducted using multivariate approach to determine the impacts of age, sex, origin, coexisting conditions (diabetes mellitus, CVD, COPD, hypertension, and comorbidities) on the probability of hospitalization, ICU admission and fatality in patients with COVID-19. Adjusted odds ratios were calculated with 95% confidence intervals. Charlson Comorbidity Index (CCI) were computed for age and the preexisting comorbidities. The Kaplan-Meier survival analysis was conducted by considering sex, presence or absence of comorbidities in patients with COVID-19. All statistical analyses were performed by using International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS) version 26.0 (Chicago, IL, USA) and Microsoft Excel 2019.
3. Results
3.1. Sociodemographic characteristics of the patients
Our study population included 799 patients with COVID-19 from Bangladesh during the second wave. About 65.8% (526 of 799, p = .004) patients were male. Mean (SD) age of the patients were 49 ± 7.8 years, while 54.5% of the patients were aged above 40 years (Table 1 ). Most of the patients (75%) were from district or divisional regions in Bangladesh. Patients in cities had better access to health facilities than villages. About 100% of the patients were from Bangladeshi origin. Occupation and monthly income revealed the capability of the patients to undergo effective treatment after being infected with COVID-19 (Table 1).
Table 1.
Socio-demographic characteristics of COVID-19 positive participants.
| Variables | Male (%) | Female (%) | Total (%) | P value |
|---|---|---|---|---|
| Study Population | 526/799 (65.8) | 273/799 (34.2) | 799/799 (100.0) | .004 |
| Age (years) | ||||
| 0–9 | 3/3 (100.0) | 0/3 (0.0) | 3/799 (0.4) | .041 |
| 10–19 | 16/29 (55.2) | 13/29 (44.8) | 29/799 (3.6) | .024 |
| 20–29 | 123/190 (64.7) | 67/190 (35.3) | 190/799 (23.8) | .001 |
| 30–39 | 92/142 (64.8) | 50/142 (35.2) | 142/799 (17.8) | .048 |
| 40–49 | 101/170 (59.4) | 69/170 (40.6) | 170/799 (21.3) | .021 |
| 50–59 | 123/169 (72.8) | 46/169 (27.2) | 169/799 (21.2) | .004 |
| 60–69 | 55/79 (69.6) | 24/79 (30.4) | 79/799 (9.9) | .05 |
| Above 70 | 13/17 (76.5) | 4/17 (23.5) | 17/799 (2.1) | .049 |
| Monthly income (thousand tk) | ||||
| Less than 10 | 117/281 (41.6) | 164/281 (58.4) | 281/799 (35.2) | .047 |
| 10–29 | 160/216 (74.1) | 56/216 (25.9) | 216/799 (27.0) | .864 |
| 30–49 | 129/163 (79.1) | 34/163 (20.9) | 163/799 (20.4) | .043 |
| 50–79 | 75/88 (85.2) | 13/88 (14.8) | 88/799 (11.0) | .737 |
| More than 80 | 45/51 (88.2) | 6/51 (11.8) | 51/799 (6.4) | .073 |
| Residence | ||||
| Village | 129/200 (64.5) | 71/200 (35.5) | 200/799 (25.0) | .062 |
| District town | 210/299 (70.2) | 89/299 (29.8) | 299/799 (37.4) | .057 |
| Divisional city | 187/300 (62.3) | 113/300 (37.7) | 300/799 (37.5) | .004 |
| Occupation | ||||
| Physician | 23/36 (63.9) | 13/36 (36.1) | 36/799 (4.5) | .079 |
| Teacher | 33/55 (60.0) | 22/55 (40.0) | 55/799 (6.9) | .043 |
| Researcher | 7/12 (58.3) | 5/12 (41.7) | 12/799 (1.5) | .061 |
| Farmer | 17/17 (100.0) | 0/17 (0.0) | 17/799 (2.1) | .832 |
| Nurse | 1/12 (8.3) | 11/12 (91.7) | 12/799 (1.5) | .317 |
| Student | 91/137 (66.4) | 46/137 (33.6) | 137/799 (17.1) | .037 |
| Journalist | 6/8 (75.0) | 2/8 (25.0) | 8/799 (1.0) | .317 |
| Political person | 7/9 (77.8) | 2/9 (22.2) | 9/799 (1.1) | .329 |
| Lawyer | 9/11 (81.8) | 2/11 (18.2) | 11/799 (1.4) | .614 |
| Police | 22/22 (100.0) | 0/22 (0.0) | 22/799 (2.8) | .421 |
| Banker | 14/21 (66.7) | 7/21 (33.3) | 21/799 (2.6) | .322 |
| Administrative Officer | 30/36 (83.3) | 6/36 (16.7) | 36/799 (4.5) | .043 |
| Private employee | 81/102 (79.4) | 21/102 (20.6) | 102/799 (12.8) | .032 |
| Rickshaw/Van/Car driver | 14/14 (100.0) | 0/14 (0.0) | 14/799 (1.8) | .425 |
| Businessman | 104/105 (99.0) | 1/105 (1.0) | 105/799 (13.1) | .001 |
| Others | 67/202 (33.2) | 135/202 (66.8) | 202/799 (25.3) | .014 |
P value < .05 were statistically significant.
3.2. Clinical characteristics and underlying health conditions of the patients
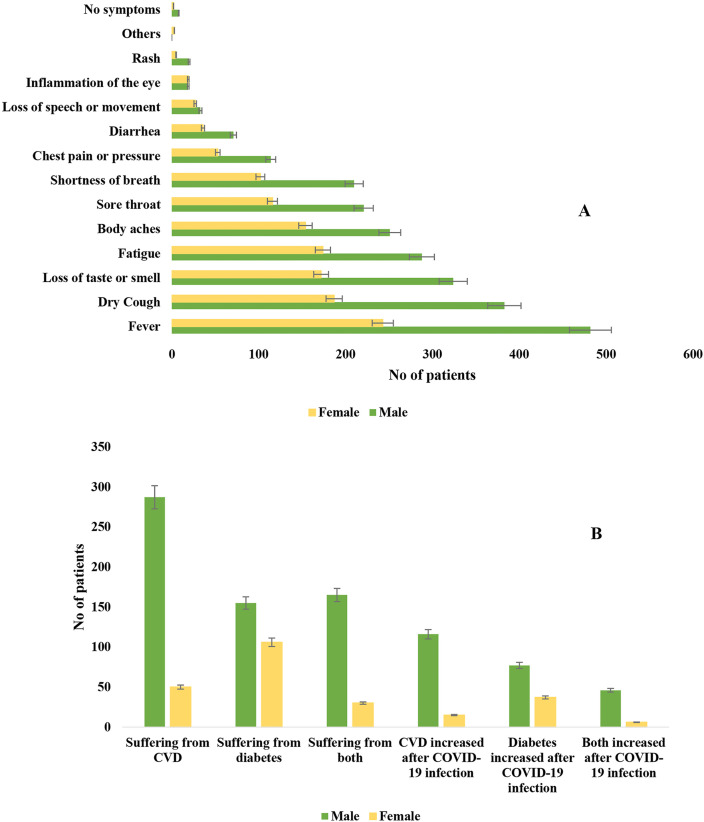
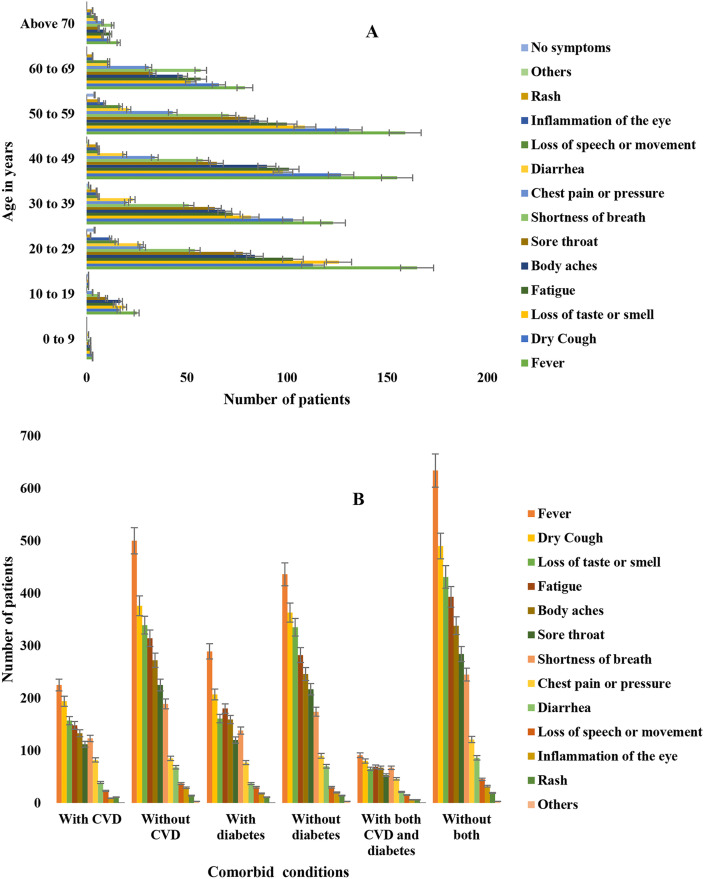
Among 799 patients with COVID-19, fever (91%) was the most frequent clinical symptom followed by dry cough (71%), loss of taste or smell (62%) and fatigue (58%) in both male and female (Fig. 1 ). Number of symptoms and the frequency increased with increasing age of the patients. Magnitude of clinical complications were intensified in patients aged above 27 years (Fig. 2 ). Fever and dry cough was the most frequent symptoms in patients with CVD, diabetes and COVID-19 (Fig. 2). The distribution of CVD (85%, 287 of 337) and diabetes (59%, 155 of 261) was highest among male patients (Fig. 1). Among the preexisting comorbidities, CVD (42.2%, 337 of 799) was most prevalent followed by diabetes (32.7%, 261 of 799; Type 2 diabetes mellitus was 22.7% and Type 1 was 10%), obesity (18%), COPD (13%), renal problem (9.7%), endocrine diseases (6.2%), cancer (3.7%) and other comorbidities, respectively (Table 2 ). Fatality was detected in 40% (318/799) of the patients. About 90.6% (288/318) fatalities were detected in patients with CVD and COVID-19, 74.5% (237/318) in patients with diabetes and COVID-19, 58% (184/318) in patients with both CVD, diabetes and COVID-19. Case fatality rate was the highest in patients with COVID-19, CVD and diabetes (94.3, 184/195) followed by, COVID-19 and diabetes (90.8, 237/261) and COVID-19 and CVD (85.5, 288/337), respectively (Table 2).
Fig. 1.
Trends of the distribution of A. symptoms in male and female, B. comorbidities in male and female patients with COVID-19 in Bangladesh.
Fig. 2.
Distribution of clinical manifestations in A. different age groups, B. comorbid groups among study population.
Table 2.
Frequency distribution of cardiovascular disease and diabetes in different sex and age groups in patients with COVID-19.
| Variables | Age groups in years (%) |
P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | Above 70 | ||
| Sex | |||||||||
| Male | 3/526 (0.6) | 16/526 (3.0) | 123/526 (23.4) | 92/526 (17.5) | 101/526 (19.2) | 123/526 (23.4) | 55/526 (10.5) | 13/526 (2.5) | .013 |
| Female | 0/273 (0) | 13/273 (4.8) | 67/273 (24.5) | 50/273 (18.3) | 69/273 (25.3) | 46/273 (16.8) | 24/273 (8.8) | 4/273 (1.5) | .024 |
| Suffering from cardiovascular diseases (CVD) | |||||||||
| Yes | 0/337 (0) | 0/337 (0) | 7/337 (2.1) | 28/337 (8.3) | 90/337 (26.7) | 135/337 (40) | 63/337 (18.7) | 14/337 (4.2) | .001 |
| No | 2/462 (0.4) | 24/462 (5.2) | 152/462 (32.9) | 100/462 (21.7) | 88/462 (19.0) | 61/462 (13.2) | 29/462 (6.3) | 6/462 (1.3) | .043 |
| Suffering from diabetes | |||||||||
| Yes | 0/261 (0) | 1/261 (0.4) | 5/261 (1.9) | 20/261 (7.7) | 74/261 (28.4) | 92/261 (35.2) | 56/261 (21.5) | 13/261 (5.0) | .029 |
| No | 3/538 (0.6) | 28/538 (5.2) | 185/538 (34.4) | 122/538 (22.7) | 96/538 (17.8) | 77/538 (14.3) | 23/538 (4.3) | 4/538 (0.7) | .047 |
| Suffering from both diabetes and CVD | |||||||||
| Yes | 0/195 (0) | 0/195 (0) | 0/195 (0) | 2/195 (1) | 37/195 (18.9) | 82/195 (42.1) | 60/195 (30.7) | 14/195 (7.3) | .004 |
| No | 3/604 (0.5) | 25/604 (4.1) | 163/604 (27) | 121/604 (20) | 130/604 (21.5) | 110/604 (18.3) | 43/604 (7.1) | 9/604 (1.5) | .067 |
| Complication related with CVD increased after COVID-19 infection | |||||||||
| Yes | 0/131 (0) | 0/131 (0) | 3/131 (2.3) | 11/131 (8.4) | 33/131 (25.2) | 52/131 (39.7) | 27/131 (20.6) | 5/131 (3.8) | .050 |
| No | 3/668 (0.4) | 29/668 (4.3) | 187/668 (28.0) | 131/668 (19.6) | 137/668 (20.5) | 117/668 (17.5) | 52/668 (7.8) | 12/668 (1.8) | .034 |
| Complication related with diabetes increased after COVID-19 infection | |||||||||
| Yes | 0/114 (0) | 1/114 (0.9) | 3/114 (2.6) | 4/114 (3.5) | 28/114 (24.6) | 42/114 (36.8) | 30/114 (26.3) | 6/114 (5.3) | .731 |
| No | 3/685 (0.4) | 28/685 (4.1) | 187/685 (27.3) | 138/685 (20.1) | 142/685 (20.7) | 127/685 (18.5) | 49/685 (7.2) | 11/685 (1.6) | .468 |
| Symptoms of CVD and diabetes worsen after COVID-19 infection | |||||||||
| Yes | 0/52 (0) | 0/52 (0) | 0/52 (0) | 1/52 (1.9) | 6/52 (11.5) | 24/52 (46.2) | 18/52 (34.6) | 3/52 (5.8) | .389 |
| No | 3/747 (0.4) | 29/747 (3.9) | 190/747 (25.4) | 141/747 (18.9) | 164/747 (22.0) | 145/747 (19.4) | 61/747 (8.2) | 14/747 (1.9) | .347 |
| Fatality in patients with CVD and COVID-19 | |||||||||
| Yes | 1/288 (0.3) | 3/288 (1.0) | 53/288 (18.4) | 47/288 (16.3) | 71/288 (24.7) | 75/288 (26.0) | 31/288 (10.8) | 7/288 (2.4) | .046 |
| No | 2/511 (0.4) | 26/511 (5.1) | 137/511 (26.8) | 95/511 (18.6) | 99/511 (19.4) | 94/511 (18.4) | 48/511 (9.4) | 10/511 (2.0) | .017 |
| Fatality in patients with diabetes and COVID-19 | |||||||||
| Yes | 1/237 (0.4) | 3/237 (1.3) | 46/237 (19.4) | 39/237 (16.5) | 55/237 (23.2) | 62/237 (26.2) | 24/237 (10.1) | 7/237 (3.0) | .056 |
| No | 2/562 (0.4) | 26/562 (4.6) | 144/562 (25.6) | 103/562 (18.3) | 115/562 (20.5) | 107/562 (19.0) | 55/562 (9.8) | 10/562 (1.8) | .049 |
| Fatality in patients with diabetes, CVD and COVID-19 | |||||||||
| Yes | 1/184 (0.5) | 1/184 (0.5) | 38/184 (20.7) | 22/184 (12.0) | 48/184 (26.1) | 48/184 (26.1) | 21/184 (11.4) | 5/184 (2.7) | .049 |
| No | 2/615 (0.3) | 28/615 (4.6) | 152/615 (24.7) | 120/615 (19.5) | 122/615 (19.8) | 121/615 (19.7) | 58/615 (9.4) | 12/615 (2.0) | .028 |
| Treatment problems during COVID-19 | |||||||||
| Yes | 0/124 (0) | 1/124 (0.8) | 2/124 (1.6) | 11/124 (8.9) | 30/124 (24.2) | 47/124 (37.9) | 29/124 (23.4) | 4/124 (3.2) | .769 |
| No | 3/675 (0.4) | 28/675 (4.1) | 188/675 (27.9) | 131/675 (19.4) | 140/675 (20.7) | 122/675 (18.1) | 50/675 (7.4) | 13/675 (1.9) | .083 |
P value < .05 were statistically significant.
3.3. Multivariate logistic regression analysis
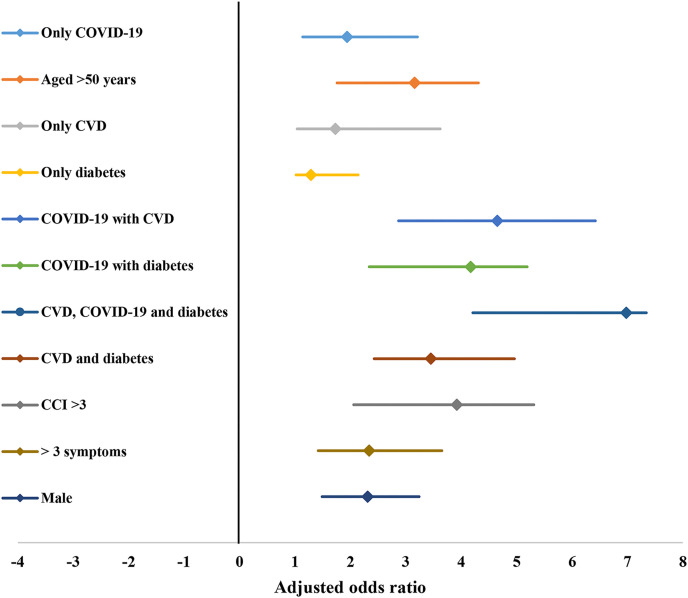
A multivariate logistic-regression model was designed and incorporated for the collected data. Independent predictors of fatality among patients with COVID-19 and respective odds ratios with 95% confidence intervals are represented in forest plot (Fig. 3 ). Among the higher odds of death, age greater than 50 years, CVD (including cardiac arrhythmia, coronary artery disease, congestive heart failure), diabetes, Charlson Comorbidity Index (CCI) > 3, coexistence of CVD, diabetes and COVID-19, male sex were the major predictors. The highest odds ratio was detected in patients with COVID-19, CVD and diabetes (OR: 6.98, 95% CI, 4.21 to 7.34), followed by COVID-19 and CVD (OR: 4.65, 95% CI, 2.87 to 6.42) and COVID-19 and diabetes (OR: 4.17, 95% CI, 2.34 to 5.19), respectively (Fig. 3). Presence of CVD, diabetes, and COVID-19 were independent predictors of higher odds of fatality among patients.
Fig. 3.
Forest plot with odds ratio of independent predictors of poor prognosis and fatality in patients with COVID-19.
3.4. Survival rate analysis
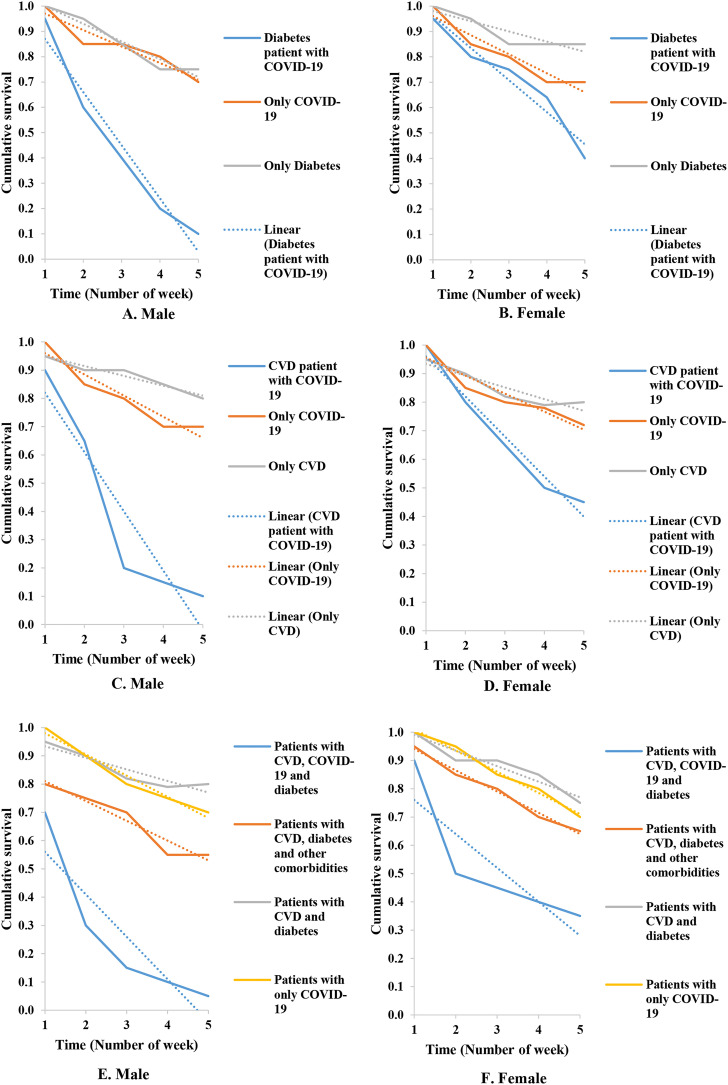
Kaplan-Meier model equation was incorporated to conduct survival analysis of the study population. The cumulative survival rate of the patients were plotted against the time periods of their survival or recovery. The findings are given in Fig. 4 A-F. The cumulative survival rate of the patients with diabetes and CVD decreased drastically from the end of third week. The cumulative survival rate decreased to 0.1 in fourth week from 0.7 in first week in male patients with diabetes, CVD and COVID-19. Male patients with coexisting diabetes or CVD had lower rate of survival compared to female (Fig. 4A–F). The Kaplan-Meier model was conducted for the data of patients aged >50 years with the highest risk of poor prognosis and fatality in this study.
Fig. 4.
A-F. Kaplan-Meier survival analysis for patients with diabetes in A. Male, B. Female, patients with cardiovascular disease in C. Male, D. Female, patients with both cardiovascular disease and diabetes in E. Male, F. Female.
4. Discussions
The rapidity and severity of the COVID-19 pandemic have become a continuous health burden from 2020 globally [17,18]. In this study we analyzed predictors associated with poor prognosis and fatality in patients with COVID-19. Our findings reflected the previous reports of independent and combined impacts of older age, sex, diabetes, coronary heart disease, COPD and other comorbidities on developing severity and fatality among patients with COVID-19 [5,8,9,19,20]. Young adults aged below 40 years and female had lower odds of developing severity and fatality related with COVID-19. Previous studies have also detected that young adults and female have stronger innate and adaptive immunity against viral infections as a result, they have less frequency of comorbidities like diabetes and CVD [13,19,20]. In our study, we detected that male patients aged above 50 years with preexisting diabetes or CVD, diabetes and CVD with COVID-19 had higher odds of poor prognosis and in-hospital fatality. In similarity with previous studies, we detected fever and dry cough as the most prevalent symptoms [18,20]. We found that CVD (42.2%) and diabetes (32.7%; Type 2 diabetes mellitus, 22.7% and Type 1, 10%) were most frequent. Case fatality (40) rate was very high in the study population than previous studies. Cardiovascular disease was involved in 91% of the fatalities, followed by diabetes (74.5%). In similar with previous studies, we detected that the highest case fatality rate (94.3) was present among patients with COVID-19, CVD and diabetes [5,8,11,13]. The highest odds of fatality was also found in patients with COVID-19, CVD and diabetes (OR: 6.98, 95% CI, 4.21 to 7.34). These findings reflected the previous original studies and meta-analysis in different countries across the globe [5,8,[11], [12], [13],[22], [23], [24]]. In previous studies the prevalence of diabetes in patients with COVID-19 varied from 10% to 100% with the odds of risk 0.13 to 2.36 [6,10,11]. Further, previous works reported that the prevalence of cardiovascular disease varied from 2% to 40% with odds of risk from 1.09 to 5.09 in patients with COVID-19 [[13], [14], [15]]. Of note, our findings on the prevalence and odds ratio on diabetes and CVD in patients with COVID-19 reflected the previous studies [6,10,11,[13], [14], [15]]. Among the patients with diabetes, median glycaemia during hospital stay was 7·8 mmol/L (IQR 6·2–8·4) which was also similar with previous studies [6]. Further, among CVD complications, hypertension was most prevalent followed by coronary artery disease, cardiac arrhythmia and congestive heart failure, respectively in patients with COVID-19. Majority of the in-hospital patients with diabetes were prescribed antivirals and antibiotics with insulin with no significant change in glycaemia. In severe cardiac arrests and myocardial infarction majority of the patients with COVID-19 couldn't underwent operation due to older age and presence of diabetes. In severe cases of COVID-19 both diabetes and CVD situations worsen leading to fatalities in patients. These findings in our study about the association of underlying health conditions reflected the previous findings on poor prognosis and fatality in patients with COVID-19 [6,10,11,[13], [14], [15],[21], [22], [23]].
As COVID-19 has transmitted globally, there have been concerns that individuals with underlying diabetes and cardiovascular disease are the main risk group of poor prognosis in many countries [[19], [20], [21], [22], [23]]. Recently, studies have suggested that COVID-19 may also stimulate Type 1 and Type 2 diabetes, as well as a proposal for stimulating new type of diabetes by COVID-19 has been hypothesized [15]. Further, concerns have been raised about the role of medical therapy used in cardiovascular disease in poor prognosis in patients with COVID-19 [13,[25], [26], [27]]. Previous case series studies have confirmed higher incidence of cardiac arrest, myocardial infarction, cardiac arrhythmias, and cardiomyopathy as outcome in patients with COVID-19 [13,14,[21], [22], [23]]. Previous studies suggested that acute viral infections in the respiratory system may trigger the activation of pro-inflammatory and coagulation pathways, resulting in endothelial cell dysfunction [13]. Angiotensin-converting enzyme 2 (ACE2) receptor is the primary site of binding for SARS-CoV-2 in human cells [15]. Membrane bound ACE2 receptors are expressed by different cells in human including pancreatic beta cells, pulmonary alveolar, heart, intestine, kidney and many more [28,29]. Though the exact mechanism is uncertain, it is proposed that presence of ACE2 receptors in pancreases and heart increase the risk of infection by SARS-CoV-2 during severe illness [[25], [26], [27],29].
However, previously studies have demonstrated the association of diabetes and CVD in poor prognosis of patients with COVID-19 in developed countries, studies in poor developing countries where the incidence of CVD and diabetes are high are lacking [6,13,[19], [20], [21], [22], [23]]. In this study, we analyzed CVD and diabetes both as independent and combined predictors of fatal outcomes among patients with COVID-19. In future, studies with larger sample and clinical as well as diagnostic data should be conducted following this study. This study will add knowledge in understanding the burden of preexisting health conditions in poor prognosis of COVID-19. Further, the findings will complement the future studies to find the mechanism and impact of SARS-CoV-2 in patients with CVD and diabetes. Policy makers and public health agencies can extract the available knowledge from this study to take appropriate measures for aged people with comorbidities in reducing the health burden and fatality associated with COVID-19.
The main limitation of the study was the size of the population. Larger sample size would give more statistical power of the analysis. Another limitation included lack of data on hemoglobin A1c (HbA1c) and duration of diabetes of the patients. For some patients we couldn't collect data on the clinical manifestations and in some cases the data were self-reported. However, this study was conducted using appropriate statistical analysis and the sample size was ensured to be large enough to conduct the study.
Ethical approval
Appropriate ethical clearance was taken from the Bio-safety, Bio-security and Ethical Committee at Jahangirnagar University and the reference number is BBEC, JU/M 2021/COVID-19/(2)1.
Author contributions
Nadim Sharif: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead). Shamsun Nahar Ahmed: Data curation (equal); Methodology (equal). Rubayet Rayhan Opu: Data curation (equal); Investigation (equal). Mahmuda Rahman Tani: Data curation (equal). Dolly Dewan:Data curation (equal). Muktasid Ud Daullah:Data curation (equal). Rakibul Islam Shanto:Data curation (equal). Anowar Khasru Parvez: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead). Ali Azam Talukder: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead). Shuvra Kanti Dey: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead).
Declaration of competing interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. J Am Med Assoc. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.Healthmap Novel coronavirus (COVID-19) https://www.healthmap.org/covid-19/ accessed.
- 4.WHO Coronavirus disease (COVID-19) Dashboard. https://covid19.who.int// URL. accessed.
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Cui Y., Shen M., Zhang J., Liu B., Dai M. Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–812. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuschieri S., Grech S. COVID-19 and diabetes: the why, the what and the how. J Diabet Complicat. 2020;34(9):107637. doi: 10.1016/j.jdiacomp.2020.107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382(25) doi: 10.1056/nejmoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Ceriello A., Schnell O. COVID-19: considerations of diabetes and cardiovascular disease management. J Diabetes Sci Technol. 2020;14(4):723–724. doi: 10.1177/2F1932296820930025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratoryinfection accessed.
- 17.Sharif N., Dey S.K. Impact of population density and weather on COVID-19 pandemic and SARS-CoV-2 mutation frequency in Bangladesh. Epidemiol Infect. 2021;149 doi: 10.1017/S0950268821000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharif N., Sarkar M.K., Ahmed S.N., Ferdous R.N., Nobel N.U., Parvez A.K. Environmental correlation and epidemiologic analysis of COVID-19 pandemic in ten regions in five continents. Heliyon. 2021 doi: 10.1016/j.heliyon.2021.e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with covid-19: evidence from meta-analysis. Aging. 2020;12(7):6049–6057. doi: 10.18632/2Faging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imam Z., Odish F., Gill I., O'Connor D., Armstrong J., Vanood A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye C., Zhang S., Zhang X., Cai H., Gu J., Lian J. Impact of comorbidities on patients with COVID-19: a large retrospective study in Zhejiang, China. J Med Virol. 2020;92(11):2821–2829. doi: 10.1002/jmv.26183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 26.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? J Am Med Assoc. 2020;323(18):1769–1770. doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 27.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/2FS2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41(19):1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18(9):2128–2130. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]