Abstract
Objective
Higher mortality in COVID-19 in men compared to women is recognized, but sex differences in cardiovascular events are less well established. We aimed to determine the independent contribution of sex to stroke, myocardial infarction and death in the setting of COVID-19 infection.
Methods
We performed a retrospective cohort study of hospitalized COVID-19 patients in a racially/ethnically diverse population. Clinical features, laboratory markers and clinical events were initially abstracted from medical records, with subsequent clinician adjudication.
Results
Of 2060 patients, myocardial injury (32% vs 23%, p = 0.019), acute myocardial infarction (2.7% vs 1.6%, p = 0.114), and ischemic stroke (1.8% vs 0.7%, p = 0.007) were more common in men vs women. In-hospital death occurred in 160 men (15%) vs 117 women (12%, p = 0.091). Men had higher odds of myocardial injury (odds ratio (OR) 2.04 [95% CI 1.43–2.91], p < 0.001), myocardial infarction (1.72 [95% CI 0.93–3.20], p = 0.085) and ischemic stroke (2.76 [95% CI 1.29–5.92], p = 0.009). Despite adjustment for demographics and cardiovascular risk factors, male sex predicted mortality (HR 1.33; 95% CI:1.01–1.74; p = 0.041). While men had significantly higher markers of inflammation, in sex-stratified analyses, increase in interleukin-6, C-reactive protein, ferritin and d-dimer were predictive of mortality and myocardial injury similarly in both sexes.
Conclusions
Adjusted odds of myocardial injury, ischemic stroke and all-cause mortality, but not myocardial infarction, are significantly higher in men compared to women with COVID-19. Higher inflammatory markers are present in men but associated similarly with risk in both men and women. These data suggest that adverse cardiovascular outcomes in men vs. women are independent of cardiovascular comorbidities.
Keywords: COVID-19, Cardiovascular disease, Stroke, Myocardial infarction, Myocardial injury, Death
1. Introduction
COVID-19, caused by coronavirus SARS-CoV2, carries high mortality and morbidity, especially for cardiovascular disease (CVD) [1,2]. Men have consistently higher unadjusted and adjusted mortality from COVID-19 compared to women as reported by various international agencies and prior literature [[3], [4], [5], [6]]. This sex difference in mortality may be related to greater underlying comorbidities in men compared to women, such as hypertension and CVD, which have been linked to increased severity of COVID-19 [5,7,8]. In addition, two factors that may contribute to mortality in COVID-19 include myocardial injury defined by troponin elevation, and increased systemic inflammation, both independently associated with in-hospital mortality [1,9,10]. However, little is known regarding sex differences in myocardial injury, systemic inflammation and CVD outcomes, although plausible explanations for sex differences include variations in immune response and angiotensin converting enzyme (ACE) 2 expression. We hypothesized that adverse cardiovascular (CV) outcomes occur more frequently in men compared to women in the setting of COVID-19, even after adjusting for demographics and CV comorbidities. Further, as recent evidence suggests a link between increased systemic inflammation and worse outcomes, [11] we aimed to perform a sex-stratified evaluation of inflammatory markers and their association with mortality and CV outcomes in hospitalized patients with COVID-19.
2. Methods
2.1. Data collection
Data were obtained from the Johns Hopkins Health System COVID-19 Precision Medicine Analytic Platform Registry (JH-CROWN) on a racially and ethnically diverse patient population. This registry extrapolates data from 5 hospitals using electronic medical records. Consecutive adult patients (>18 years of age) with confirmed COVID-19 by reverse transcriptase polymerase chain reaction test, who were admitted and died, or were discharged between March 1, 2020 and July 4, 2020, were included. Only the index hospitalization for each patient was included. Any patients who were still admitted after the end date were excluded.
Comorbidities and clinical events were obtained using ICD-10 codes or key words (Supplement), and sex and race were self-identified. Myocardial infarction and ischemic and hemorrhagic stroke, were further adjudicated by a neurologist, 2 cardiologists and a research nurse [10,12]. All laboratory data were obtained using the first recorded values during hospitalization. COVID-19 patient management at our institution is based on specific institutional protocols, which include protocols for medical interventions, laboratory measurements and cardiac and venous thromboembolism evaluation and management. The study was approved by the Johns Hopkins University Institutional Review Board. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
2.2. Statistical methods
We compared demographics, comorbidities, laboratory values and clinical events using parametric two-sample Student's t-test for normally distributed continuous variables, nonparametric Mann-Whitney U test for non-normally distributed continuous variables, or Chi-squared test for categorical variables. Unadjusted and adjusted Cox proportional-hazard models were used to estimate hazard ratios (HR) of death. Model 1 included adjustment for age, race and body mass index (BMI) and Model 2 added CV comorbidities (hypertension, diabetes, heart failure, coronary artery disease and history of smoking). Logistic regression was performed for myocardial injury (defined as troponin I ≥ 0.04 ng/mL) with models 1 and 2. For myocardial infarction and ischemic stroke, only model 1 for logistic regression was conducted given a smaller number of events. Inflammatory and cardiovascular biomarkers were divided into quintiles. Given that biomarkers were not available for the entire cohort, analyses were used to identify differences in demographics and comorbidities among patients with/without laboratory values available. Identifiable differences were considered potential confounders and included for adjustment in Cox and logistic regression looking at sex-stratified association of biomarker elevation with all-cause mortality and myocardial injury. For ischemic stroke and myocardial infarction, inflammatory markers were only evaluated without adjustment given fewer events. All proportional hazard assumptions were confirmed.
3. Results
Of 2060 patients, 1088 were men. Medial length of stay for men was 7.0 (3.2–15.1) days, and for women was 6.1 (2.7–13.0) days (p = 0.006). Men had lower BMI (27.5 vs 29.7 kg/m2, p < 0.001) and greater pre-existing coronary artery disease (10% vs 6%, p = 0.002) and smoking history (21% vs 14%, p < 0.001). On admission, men had higher interleukin-6 (72 vs 43 pg/mL, p < 0.001), C-reactive protein (12.2 vs 8.6 mg/dL, p < 0.001), ferritin (786 vs 416 ng/dL, p < 0.001) and fibrinogen (554 vs 510 mg/dL, p = 0.005) levels. Men were more likely to have myocardial injury (32% vs 23%, p = 0.002) and ischemic stroke (1.8% vs 0.7%, p = 0.019). Although there were a greater number of deaths (15% vs 12%, p = 0.076) and myocardial infarction (2.7% vs 1.6%, p = 0.114) in men vs. women, these differences were not statistically significant (Table 1 ).
Table 1.
Comparison of demographics, laboratory markers and clinical outcomes between women and men in COVID-19.⁎
| Variable | Overall (n = 2060) |
Women (n = 972) |
Men (n = 1088) |
p-value |
|---|---|---|---|---|
| Age (years) | 61 (46–74) | 62 (43–77) | 61 (48–72) | 0.340 |
| Race/Ethnicity | 0.075 | |||
| White | 721 (35%) | 353 (36%) | 368 (34%) | |
| Black | 554 (27%) | 235 (24%) | 319 (29%) | |
| Hispanic | 588 (28%) | 288 (30%) | 300 (28%) | |
| Other | 197 (10%) | 96 (10%) | 101 (9%) | |
| Body mass index, kg/m2 | 28.3 (24.4–33.5) | 29.7 (24.9–35.5) | 27.5 (24.2–31.6) | <0.001 |
| Comorbidities | ||||
| Hypertension | 1088 (53%) | 519 (53%) | 577 (53%) | 0.869 |
| Diabetes | 607 (29%) | 290 (30%) | 317 (29%) | 0.728 |
| Coronary artery disease | 170 (92%) | 61 (6%) | 109 (10%) | 0.002 |
| Heart failure | 165 (8%) | 89 (9%) | 76 (7%) | 0.070 |
| History of smoking | 367 (18%) | 139 (14%) | 228 (21%) | <0.001 |
| Medications | ||||
| Aspirin | 213 (10%) | 90 (9%) | 123 (11%) | 0.128 |
| Statin | 321 (16%) | 144 (15%) | 177 (16%) | 0.364 |
| ACEi or ARB | 286 (14%) | 121 (12%) | 165 (15%) | 0.075 |
| Clinical variables | ||||
| Heart rate (beats/min) | 86 (76–98) | 86 (76–98) | 87 (75–99) | 0.956 |
| Systolic blood pressure (mmHg) | 122 (109–138) | 122 (108–137) | 123 (110–138) | 0.191 |
| Diastolic blood pressure (mmHg) | 70 (62–79) | 68 (60–77) | 72 (64–81) | <0.001 |
| Fraction of inspired oxygen (FiO2), % | 75 (50–100) | 60 (45–97) | 80 (55–100) | 0.104 |
| Length of inpatient admission (days) | 6.7 (3.0–13.9) | 6.1 (2.7–13.0) | 7.0 (3.2–15.1) | 0.006 |
| Intensive care unit admission | 1719 (83%) | 799 (82%) | 920 (85%) | 0.151 |
| Intensive care length of stay (days) | 5.1 (2.8–9.6) | 5.1 (2.9–9.6) | 5.1 (2.7–9.6) | 0.715 |
| Laboratory values | ||||
| White blood cell count, K/cu mm | 7.2 (5.2–9.9) | 7.2 (5.2–9.8) | 7.2 (5.3–10.2) | 0.345 |
| Absolute lymphocyte count, K/cu mm | 0.9 (0.5–1.4) | 0.9 (0.5–1.5) | 0.9 (0.5–1.4) | 0.117 |
| Neutrophil/lymphocyte ratio | 5.7 (3.0–12.3) | 5.3 (2.9–11.0) | 5.9 (3.2–13.0) | 0.060 |
| Interleukin-6, pg/mL | 55 (23–129) | 43 (18–101) | 72 (27–158) | <0.001 |
| C-reactive protein, mg/dL | 10.4 (4.1–27.5) | 8.6 (3.0–22.1) | 12.2 (5.1–34.7) | <0.001 |
| D-dimer, mg/dL | 1.0 (0.6–2.1) | 1.0 (0.6–2.2) | 1.0 (0.5–2.1) | 0.397 |
| Ferritin, ng/mL | 621 (269–1171) | 416 (169–873) | 786 (434–1424) | <0.001 |
| Fibrinogen, mg/dL | 532 (417–644) | 510 (407–623) | 554 (437–673) | 0.005 |
| Troponin I, ng/mL | 0.04 (0.02–0.04) | 0.04 (0.02–0.04) | 0.04 (0.02–0.04) | 0.255 |
| Troponin I positive | 275 (28%) | 106 (23%) | 169 (32%) | 0.002 |
| N-terminal pro b-type natriuretic peptide, pg/mL | 231 (59–1238) | 238 (55–1351) | 227 (60–1106) | 0.695 |
| Creatinine, mg/dL | 1.0 (0.8–1.4) | 0.8 (0.7–1.2) | 1.1 (0.9–1.5) | <0.001 |
| Estimated glomerular filtration rate, mL/min/1,73 sqm | 80 (48–107) | 81 (45–109) | 80 (51–104) | 0.516 |
| Clinical outcomes | ||||
| Death | 277 (13%) | 117 (12%) | 160 (15%) | 0.076 |
| Myocardial infarction | 45 (2.2%) | 16 (1.6%) | 29 (2.7%) | 0.114 |
| Stroke, ischemic | 25 (1.2%) | 6 (0.7%) | 19 (1.8%) | 0.019 |
| Stroke, hemorrhagic | 11 (0.5%) | 3 (0.3%) | 8 (0.7%) | 0.185 |
| Deep venous thrombosis or pulmonary embolism | 66 (3%) | 30 (3%) | 36 (3%) | 0.775 |
| Extracorporeal membrane oxygenation | 10 (0.5%) | 2 (0.2%) | 8 (0.8%) | 0.016 |
| Ventricular tachycardia | 19 (1%) | 11 (1%) | 8 (1%) | 0.319 |
| Mechanical ventilation | 330 (16%) | 127 (13%) | 203 (19%) | 0.001 |
| Clinical treatments received | ||||
| Dexamethasone | 133 (7%) | 57 (6%) | 76 (7%) | 0.323 |
| Remdesivir | 216 (11%) | 97 (10%) | 119 (11%) | 0.516 |
| Hydroxychloroquine | 437 (22%) | 191 (20%) | 246 (23%) | 0.120 |
ACEi = angiotensin-converting enzyme inhibitors, ARB = angiotensin II receptor blockers, DVT = deep venous thrombosis, PE = pulmonary embolism.
In adjusted analysis, male sex was associated with greater risk of death (HR 1.33, 95% CI 1.01–1.74; p = 0.041 in Model 2). Men had greater odds of myocardial injury (OR 2.04; 95% CI 1.43–2.91; p < 0.001 in Model 2) and ischemic stroke in an adjusted model including age, BMI and race [OR 3.10 (1.11–8.65; p = 0.031), but not of myocardial infarction (Table 2 ).
Table 2.
Association of sex with myocardial injury, myocardial infarction, ischemic stroke and mortality.
| Unadjusted | Model 1 (adjusted for age, race and BMI) |
Model 2 (adjusted for age, race, body mass index, hypertension, coronary artery disease, heart failure and history of smoking) |
|
|---|---|---|---|
| Myocardial injury | |||
| Odds Ratio (95% Confidence Interval) and p-value | |||
| Male sex | 1.58 (1.19–2.09) p = 0.002 |
2.04 (1.44–2.90) p < 0.001 |
2.04 (1.43–2.91) p < 0.001 |
| Myocardial infarction | |||
| Odds Ratio (95% Confidence Interval) and p-value | |||
| Male sex | 1.64 (0.88–3.03) p = 0.118 |
1.71 (0.89–3.29) p = 0.109 |
XXX |
| Ischemic stroke | |||
| Odds Ratio (95% Confidence Interval) and p-value | |||
| Male sex | 2.72 (1.27–5.82) p = 0.010 |
3.10 (1.11–8.65) p = 0.031 |
XXX |
| Mortality | |||
| Hazard Ratio (95% Confidence Interval) and p-value | |||
| Male sex | 1.07 (0.84–1.35) p = 0.595 |
Male sex | 1.07 (0.84–1.35) p = 0.595 |
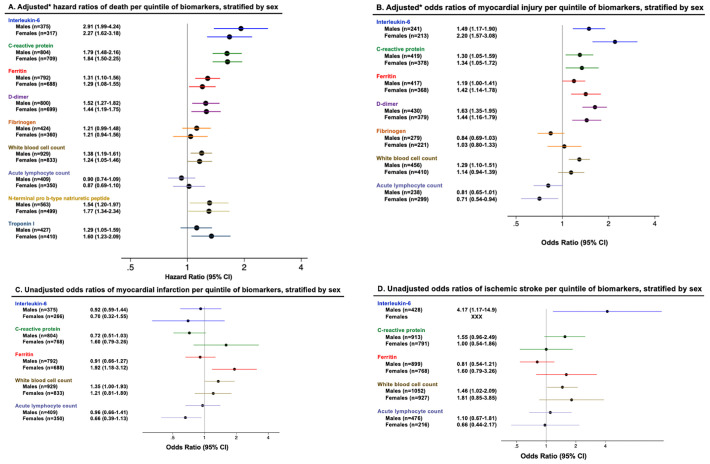
After adjustment, per quintile increase in several inflammatory markers was associated with greater risk of death and odds of myocardial injury in both men and women with similar effect estimates (Fig. 1 ). Interleukin-6 was associated with increased odds of ischemic stroke (Fig. 1).
Fig. 1.
Association of inflammatory markers with mortality, myocardial injury, myocardial infarction and ischemic stroke, stratified by sex.
*Hazard ratios of death and odds ratios of myocardial injury are adjusted for age, race, body mass index, and history of hypertension, diabetes, coronary artery disease, congestive heart failure and smoking.
4. Discussion
We present sex data comparing important cardiovascular indices among men and women from a large cohort of racially/ethnically diverse hospitalized patients with COVID-19. Although men had more underlying CV comorbidities, after adjusting for these, men had a significantly increased risk of death compared to women. Moreover, the risk of both myocardial injury and ischemic stroke was higher in men, even after adjustment for clinical confounders. Finally, we report markedly increased levels of systemic inflammatory markers in men compared to women early during hospital admission for COVID-19, although there was no notable difference in the ability of inflammatory markers to predict adverse outcomes between sexes. To our knowledge, this is one of the first studies to investigate sex differences in COVID-19 in important CV outcomes and the degree of systemic inflammation after adjustment for comorbidities [6,13]. These findings support differences in sex-specific responses, but suggest that these differences are likely independent of common cardiovascular comorbidities, as has previously been advocated but not well studied [8,14,15].
Prior studies have shown that elevated systemic inflammation, as measured by clinical inflammatory biomarkers, is associated with COVID-19 severity [11]. However, there are few data on sex-stratified analyses of systemic inflammation and the extent of association with mortality and cardiovascular outcomes. In addition, although women have been reported to have higher levels of inflammatory markers in the outpatient setting, such as interleukin-6, compared to men, [16] our study in acutely ill patients with COVID-19 showed that men had significantly higher levels of several inflammatory markers including interleukin-6, C-reactive protein and ferritin. Despite high levels of acute inflammation in men, we demonstrate similar increases in the risk of mortality and odds of myocardial injury, myocardial infarction and ischemic stroke with elevations in inflammatory biomarkers between men and women. These findings raise suspicion that perhaps men are more likely to have a hyper-inflammatory response than women, although this alone may not explain the observed sex differences in mortality and cardiovascular outcomes. Recent studies also suggest that hyperactivation of the coagulation system, perhaps as demonstrated by rise in D-dimer, may be associated with myocardial injury and COVID-19 mortality [[17], [18], [19]]. COVID-19-related coagulopathy may result in the occurrence of thrombotic microangiopathy in various organs [20]. While we did not specifically investigate coagulopathy in our study and did not find D-dimer to be differentially elevated among men and women, it is certainly possible that COVID-19 related coagulopathy may be contributing to outcomes such as myocardial infarction, ischemic stroke or death.
Limitations of this work include retrospective study design. Additionally, all laboratory data were measured as clinically indicated, which raises potential selection bias. However, we adjusted for possible confounders to limit selection bias.
5. Conclusions
In conclusion, men are at significantly greater risk than women for myocardial injury, ischemic stroke and all-cause mortality in the setting of hospitalization with COVID-19. This increased risk in men remains despite adjustment for demographics and cardiovascular comorbidities. In addition, increased inflammatory markers are associated with a greater likelihood of developing myocardial injury, ischemic stroke and mortality, although this association is similar between men and women. These data support male sex as an independent risk factor of myocardial injury, ischemic stroke and mortality in acute COVID-19 illness.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Minhas is supported by NHLBI Training Grant T32HL007024. Dr. Hays is supported by the Magic that Matters Fund of Johns Hopkins Medicine and NIH/NHLBI 1R01HL147660.
Competing interests
The authors declare that there is no conflict of interest.
Ethics approval
The study was approved by the Johns Hopkins University Institutional Review Board. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Consent to participate
Consent to participate was waived per the Johns Hopkins University Institutional Review Board.
Availability of data and material
All data relevant to the study are included in the article or uploaded as supplementary information. Any requests for direct access to the data should be made directly to the JH-CROWN Registry.
Code availability
Statistical software used was: StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. Code is available upon request.
Authors' contributions
Allison Hays and Anum Minhas conceived the presented idea. Julie Shade and Natalia Trayanova assisted in data abstraction. Anum Minhas and Julie Shade performed computations. Sung-Min Cho, Allison Hays and Anum Minhas adjudicated clinical events. Erin Michos, Thomas Metkus, Nisha Gilotra and Garima Sharma contributed to revising analytic plans and interpreting the results. Anum Minhas took the lead in writing the manuscript. Allison Hays and all other coauthors edited the manuscript and provided critical feedback. Anum Minhas and Allison Hays are responsible as guarantors of the overall work.
Acknowledgements
The data utilized for this publication were part of the JH-CROWN: The COVID PMAP Registry which is based on the contribution of many patients and clinicians.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2021.05.011.
Appendix A. Supplementary data
Supplementary material
References
- 1.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epidemiology Working Group for NCIP Epidemic Response Chinese center for disease control and prevention, [the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Health 50/50, COVID-19 Sex-disaggregated Data Tracker, (n.d.). https://globalhealth5050.org/covid19/sex-disaggregated-data-tracker/ (accessed July 25, 2020).
- 6.Gomez J.M.D., Du-Fay-de-Lavallaz J.M., Fugar S., Sarau A., Simmons J.A., Clark B., Sanghani R.M., Aggarwal N.T., Williams K.A., Doukky R., Volgman A.S. Sex differences in coronavirus disease 2019 (COVID-19) hospitalization and mortality. J. Women's Health (Larchmt) 2021 doi: 10.1089/jwh.2020.8948. [DOI] [PubMed] [Google Scholar]
- 7.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed M.O., Gale C.P., Kontopantelis E., Doran T., de Belder M., Asaria M., Luscher T., Wu J., Rashid M., Stephenson C., Denwood T., Roebuck C., Deanfield J., Mamas M.A. Sex-differences in mortality rates and underlying conditions for COVID-19 deaths in England and Wales. Mayo Clin. Proc. 2020;S0025619620307618 doi: 10.1016/j.mayocp.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L., Zhong W., Bian Z., Li Z., Zhang K., Liang B., Zhong Y., Hu M., Lin L., Liu J., Lin X., Huang Y., Jiang J., Yang X., Zhang X., Huang Z. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J. Infect. 2020;S0163445320304606 doi: 10.1016/j.jinf.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D. Fourth universal definition of myocardial infarction (2018) J. Am. Coll. Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 11.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., Deng G. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int. J. Infect. Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco R.L., Kasner S.E., Broderick J.P., Caplan L.R., Connors J.J., Culebras A., Elkind M.S.V., George M.G., Hamdan A.D., Higashida R.T., Hoh B.L., Janis L.S., Kase C.S., Kleindorfer D.O., Lee J.-M., Moseley M.E., Peterson E.D., Turan T.N., Valderrama A.L., Vinters H.V. An updated definition of stroke for the 21st Century: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma G., Volgman A.S., Michos E.D. Sex differences in mortality from COVID-19 pandemic. JACC: Case Rep. 2020;2:1407–1410. doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein S.L., Dhakal S., Ursin R.L., Deshpande S., Sandberg K., Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M., Liu S., Yang J.-K. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor M.-F., Motivala S.J., Valladares E.M., Olmstead R., Irwin M.R. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am. J. Phys. Regul. Integr. Comp. Phys. 2007;293:R145–R151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 17.Pisa Covid Study Group, Mengozzi A., Georgiopoulos G., Falcone M., Tiseo G., Pugliese N.R., Dimopoulos M.A., Ghiadoni L., Barbieri G., Forfori F., Carrozzi L., Santini M., Monzani F., De Marco S., Menichetti F., Virdis A., Masi S. The relationship between cardiac injury, inflammation and coagulation in predicting COVID-19 outcome. Sci. Rep. 2021;11:6515. doi: 10.1038/s41598-021-85646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Any requests for direct access to the data should be made directly to the JH-CROWN Registry.