Abstract
Background
COVID-19 related in-hospital venous thromboembolism (VTE) incidence is high but data reported vary significantly. Some studies show that up to half of the events are diagnosed early after admission.
Objectives
To study symptomatic VTE incidence in acute COVID-19 hospitalized patients and to describe timing of VTE diagnosis.
Methods
Multicenter cohort of 5966 patients hospitalized with acute COVID-19. Multicenter Registry of 844 hospitalized patients with acute COVID-19 and associated acute VTE.
Results
By the time of cohort data collection, 68 patients (1.14%) were still hospitalized, 19.8% had died, and 5.4% required ICU. During a median follow-up of 6 days (IQR, 4–12), 183 patients (3.07%; 95% CI, 2.64–3.55) presented a symptomatic VTE event. The cumulative incidences of VTE at 7, 14 and 21 days in wards [2.3% (95% CI, 1.9–2.7), 3.6% (95% CI, 3.0–4.3), and 4.3% (95% CI, 3.5–5.1)] were similar to the ones reported in ICU [2.2% (95% CI, 1.0–4.4), 2.9% (95% CI, 1.5–5.3), and 4.1% (95% CI, 2.2–6.8)], but at 30 and 60 days were higher in ICU [6.9% (95% CI, 4.2–10.5), and 12.8% (95% CI, 8.1–18.5)] than in wards. Eighty-eight VTE events (48%) were diagnosed early, within 48 h of admission. VTE was not associated with death (HR, 0.79; 95% CI, 0.55–1.12).
Conclusions
Incidence of symptomatic VTE in our COVID-19 cohort is consistent with that of other real-life studies recently published. Early VTE events are, along with COVID-19, the reason for admission rather than an in-hospital complication.
Keywords: COVID19, Incidence, Multicenter study, Pulmonary embolism, Venous thromboembolism, Thromboprophylaxis
1. Introduction
The SARS-CoV-2 pandemic, which has affected more than 102 million people worldwide, has taken at least two million lives since its appearance [1]. The disease produced by SARS-CoV-2, 2019-nCoV (COVID-19), has been associated to an increased risk of venous thromboembolic disease [[2], [3], [4], [5]].
In addition to classic thrombotic risk factors, new mechanisms that predispose COVID-19 patients to a higher incidence of venous thromboembolism (VTE) have been described. Severe COVID-19 clinical presentation, characterized by acute respiratory distress syndrome, septic shock, and coagulopathy, could be explained by the fact that SARS-CoV-2, through the angiotensin-converting enzyme 2, infects pneumocytes as well as vascular endothelial cells, being the latter its main target [6,7]. Microvasculature damage has also been observed, with vascular wall thickening and microthrombi formation [8]. Likewise, impaired fibrinolytic function during lung inflammation results in fibrin accumulation in the alveolar spaces. These findings may explain the high D-dimer levels observed in severe COVID-19 patients, which strongly correlate with VTE and mortality [[9], [10], [11]].
Current consensus postulates that all patients admitted with COVID-19 should receive prophylactic treatment with low molecular weight heparin (LMWH) unless contraindicated [12]. Several questions like dose escalation usefulness and whether prophylaxis should be extended remain unsolved. LMWH may reduce mortality, especially in those with elevated D-dimer, not only through prevention of VTE but also by preventing microthrombi formation [13,14].
Despite the large number of studies on VTE incidence in hospitalized patients with COVID-19, published results are very inconsistent. Recent real-life studies [15] have shown lower incidences than initial studies, many of which included fewer patients, a higher proportion of ICU patients and asymptomatic VTE events [16].
On the other hand, little has been said about the timing of in-hospital VTE disease in COVID-19 patients. One Italian study observed that at least half of the thromboembolic events were diagnosed within the first 24 h of admission and, therefore, not preventable by in-hospital thromboprophylaxis [5].
The main objective of this real-life study was to determine the cumulative incidence of VTE in acute COVID-19 patients admitted to hospital ward and ICU. The secondary objective was to describe baseline characteristics and outcomes among acute COVID-19 hospitalized patients who presented VTE.
2. Materials and methods
2.1. Patients and study design
Hospitalized adult patients from twenty hospitals within Madrid region, in Spain, with acute COVID-19 and either a probable (high clinical suspicion) or confirmed VTE diagnosis from March 1 to May 30, 2020, were retrospectively evaluated. In-hospital VTE disease included events presented on admission and those occurring during admission [17]. Patients were identified through each center diagnostic coding system and by the imaging tests carried out during the study period. Patient data obtained from electronic medical histories were recorded and uploaded to a secure web platform (TROMBOCOVID) in the electronic data capture software REDCap, supported by Asociación Madrileña de Hematología y Hemoterapia (AMHH). An incidence study was performed in a subgroup of patients from four hospitals (incidence study cohort) that were able to obtain data from all patients admitted for acute COVID19 during this period.
COVID-19 diagnosis was made according to World Health Organization interim guidance and confirmed by real-time one-step reverse transcriptase PCR [18]. Likewise, COVID-19 probable cases were those patients with acute respiratory infection and radiological criteria consistent with COVID-19 but negative or inconclusive PCR [19]. Acute COVID-19 was defined as the presence of COVID-19 signs and symptoms up to 4 weeks [20]. Pneumonia severity was estimated using CURB-65 score [21].
VTE events were diagnosed based on clinical suspicion following standard clinical practice. None of the participating centers followed a screening approach for VTE. Deep venous thrombosis (DVT) was confirmed by a positive venous compression ultrasound, and pulmonary thromboembolism (PE), by high probability ventilation-perfusion scintigraphy or helical chest computed tomography with positive contrast (CTPA). In addition, probable PE cases were those patients with high clinical suspicion [sudden hypoxemia with peripheral capillary oxygen saturation (SpO2) <90% not justified by the radiological lesion, tachycardia >100 bpm, systolic arterial tension <100 mmHg or signs of right ventricular pressure overload, pulmonary hypertension and DVT signs]. PE severity was assessed using the simplified Pulmonary Embolism Severity Index (s-PESI) [22]. Thromboprophylaxis was performed with LMWH. Patients aged <18 years and patients with prior VTE in the last 3 months before the onset of COVID-19 were excluded.
We collected demographic data, comorbidities, VTE risk factors, pharmacological thromboprophylaxis and outcomes. We calculated the time from the first symptoms of COVID-19 infection and hospital admission to the thrombotic event. Laboratory parameters on admission and at VTE onset were also recorded. Variables are described in Table 1 .
Table 1.
Characteristics and use of thromboprophylaxis in hospitalized patients with acute VTE and COVID-19 infection.
| Total patients n = 844 |
Ward patients n = 601 (70.5%) |
ICU patients n = 243 (29.5%) |
|
|---|---|---|---|
| Age- years, median (IQR) | 66 (57–76) | 70 (59–79) | 61 (52–68) |
| Gender- n (%) | |||
| Male | 537 (63.9) | 348 (59.8) | 180 (74.1) |
| Female | 304 (36.1) | 234 (40.2) | 63 (25.9) |
| BMI (kg/m2), median (IQR) | 27.7 (24.9–31.3) | 27.5 (25–31.2) | 27.7 (24.6–33) |
| Comorbidities- n (%) | |||
| Arterial hypertension | 401 (47.5) | 302 (51.9) | 92 (37.9) |
| Dyslipidemia | 307 (36.4) | 220 (37.8) | 82 (33.7) |
| Smoking | 73 (8.6) | 51 (8.8) | 22 (9.1) |
| Diabetes | 165 (19.5) | 120 (20.6) | 43 (17.7) |
| Obesity | 154 (18.2) | 96 (16.5) | 54 (22.2) |
| Chronic lung disease | 119 (14.1) | 84 (14.4) | 33 (13.6) |
| Cardiac disease | 95 (11.3) | 77 (13.2) | 16 (6.6) |
| Renal disease | 34 (4) | 31 (5.3) | 3 (1.2) |
| Risk factors for VTE, n (%) | |||
| Immobilization | 52 (6.2) | 43 (7.4) | 9 (3.7) |
| Active cancer | 50 (5.9) | 44 (7.6) | 4 (1.6) |
| History of VTE | 51 (6) | 41 (7) | 9 (3.7) |
| Trombophilia | 14 (1.7) | 6 (1) | 8 (3.3) |
| Recent surgery | 8 (0.9) | 6 (1) | 2 (0.8) |
| Time from hospital admission to VTE onset- days, median (IQR) | 8 (1–16) | 6 (0−13) | 5(1–9.75) |
| Time from first COVID-19 infection symptoms to VTE onset- days, median (IQR) | 17 (11–25) | 16 (9–23) | 14 (8–18) |
| CURB-65- n (%) score for pneumonia severity, n (%) | |||
| Low risk (0–1 point) | 421 (58.8) | 310 (62.6) | 102 (49) |
| Moderate risk (2 points) | 168 (23.5) | 106 (21.4) | 58 (76.9) |
| Hight risk (≥3 points) | 127 (17.8) | 79 (15.9) | 48 (23.1) |
| VTE type, n (%) | |||
| Confirmed PE | 654 (77.5) | 490 (81.5) | 164 (67.5) |
| Probable PE | 46 (5.5) | 17 (2.9) | 29 (11.9) |
| Confirmed PE and DVT | 36 (4.3) | 25 (4.1) | 11 (4.5) |
| DVT | 76 (9) | 56 (9.3) | 20 (8.3) |
| Other thrombosisa | 32 (3.7) | 13 (2.2) | 19 (7.8) |
| s-PESI, n (%) | |||
| Low risk (0 point) | 214 (29.9) | 169 (33.1) | 42 (21.4) |
| High risk (≥1 point) | 522 (70.1) | 341 (66.9) | 154 (78.6) |
| VTE prophylaxis, n (%) | 524 (64.6) | 314 (56.1) | 197 (83.1) |
| Thromboprophylaxis doses, n (%) | |||
| Standard LMWH dose | 354 (68.3) | 224 (71) | 124 (63.9) |
| Intermediate LMWH dose | 55 (10.6) | 26 (8.4) | 28 (14.4) |
| Therapeutic LMWH dose | 21 (4.1) | 13 (4.2) | 7 (3.6) |
Other thrombosis: atypical location, catheter-related and other thrombosis. BMI = body mass index; COVID-19 = coronavirus disease 2019; DVT = Deep venous thrombosis; ICU = intensive care unit; IQR = interquartile range; LMWH = Low-molecular-weight heparin; PE: pulmonary thromboembolism; s-PESI = simplified Pulmonary Embolism Severity Index; VTE = venous thromboembolism.
Early VTE was defined as any VTE event diagnosed within the first 48 h of hospitalization for acute COVID-19. Late VTE was defined as any VTE event diagnosed after 48 h of hospitalization. Based on radiological images, incident PEs from incidence study cohort were categorized as peripheral, if segmental and/or subsegmental arteries were only involved, and central, if lobar and/or pulmonary arteries were involved.
This multicentric observational study followed Helsinki's Declaration ethical principles and was previously approved by University Hospital Fundación Jiménez Díaz Ethical committee on June 09, 2020, and then by all participating centers' ethical committee. Due to our study's retrospective nature, the ethical committee waived the requirement for informed consent.
2.2. Statistical and incidence analysis
VTE incidence analysis was carried out in the Incidence study cohort, a sample of patients from four University Hospitals out of the twenty that participated in the registry. Patient data were collected from an administrative database of discharge diagnoses called Minimum Data Set (MDS), which is mandatory in Spanish National Health Service hospitals. Patients with a primary discharge diagnosis of confirmed or probable COVID-19 admitted between March 1 and May 10 were included. We analyzed duplicated patients and eliminated admissions not related to acute COVID-19. Between the same dates, we also retrieved patients who had among their diagnoses at discharge, COVID-19 and PE and/or DVT in any location and/or superficial thrombophlebitis. After reviewing the electronic medical records and confirming the diagnoses, patients who presented the thrombotic event before June 1 were included, so that all patients had at least 21 days of follow-up. Since MDS does not retrieve clinical variables, we extracted the date of VTE diagnosis from the medical history. Information on whether PE was central or peripheral according to the angio-CT report was collected only from the Incidence study cohort but not from the registry.
Descriptive analyses were reported as relative frequencies for discrete variables. Continuous variables were reported as mean ± standard deviation (SD) or median and interquartile range for normal and non-normal distributed variables, respectively. Statistical comparisons were calculated using chi-square test, Fisher exact-test, t-test, or Mann-Whitney U test. Clinical variables associated with outcome were analyzed using univariate logistic regression models. All statistical tests were two sided, and p < 0.05 was considered as statistically significant.
The VTE incidence in ICU and hospital ward was calculated as the proportion of patients with VTE, with 95% confidence intervals [95%CI] calculated using Poisson distribution. The VTE cumulative incidence was estimated at 7, 14, 21, 30 and 60 days using a competing risk approach considering death as a competing risk. Patients were followed up since date of admission to date of VTE and censored at date of death or date of discharge. When we estimated ICU's VTE incidence, the date of ICU admission was considered the beginning of the follow-up. When we estimated incidence in ward patients, ICU patients were censored at date of ICU admission. The association between VTE and mortality was analyzed by calculating a time-varying hazard ratio in Cox proportional hazards model. Analyses were performed in STATA v14.
3. Results
3.1. Registry results
Eight hundred and forty-four hospitalized patients from 20 hospitals of Madrid region with acute COVID-19 diagnosis and either a probable (5.7%) or confirmed acute VTE (94.3%) were included in TROMBOCOVID registry. Two hundred and twelve patients (25.1%) had a probable COVID-19. Seven hundred and thirty-six (87%) presented acute PE (with or without coexisting DVT), while 76 (9%) had isolated DVT. Clinical characteristics and outcomes are summarized in Table 1.
Median time from hospital admission to VTE was 8 days (IQR 1–16) and median time from first COVID-19 symptoms to VTE diagnosis was 17 days (IQR 11–25). Two hundred and forty-three patients (29.5%) were admitted in ICU. Most ICU patients (72.1%) presented the VTE episode before ICU admission and 68 (27.9%), during ICU admission. In total, 524 patients (64.6%) received LMWH before VTE diagnosis, 56.1% in ward and 83.1%, in ICU. Most patients (68.3%) received LMWH at standard prophylactic dose. Intermediate and therapeutic doses of prophylactic LMWH were used more in ICU patients (18%) than in ward patients (12.6%). Patients who did not receive thromboprophylaxis had a significantly lower time from hospital admission to VTE than those who did receive it (median 0 days, IQR 0–10 vs. 11 days, IQR 6–18; p < 0.001).
We compared early with late VTE diagnosed patients (Table 2 ). Early VTE accounted for 29.4% of the registry. Compared to late VTE, early VTE patients were older (median age 70 vs. 65 years; p < 0.001), had a lower proportion of males (56% vs. 68.2%; p = 0.001) and a higher incidence of a previous VTE (8.9% vs.4.4%, p = 0.016). Late VTE patients were admitted more often to ICU (38.8% vs.10.4%; p < 0.001). There were no differences in severity between both groups in terms of CURB-65 and s-PESI scores. Most patients who developed a late VTE were on LMWH thromboprophylaxis (82.8%).
Table 2.
Clinical and laboratory characteristics of patients with early and late VTE in the Registry.
| Early VTE* patients n = 225 (29.4%) |
Late VTE** patients n = 541 (70.6%) |
P value | |
|---|---|---|---|
| Age (years), median (IQR) | 70 (59–80.5) | 65 (56–74) | 0.001 |
| Gender, n (%) | |||
| Male | 126 (56) | 369 (68.2) | 0.001 |
| Time from SARS-CoV-2 infection symptoms to admission (days), median (IQR) | 8 (4–16) | 7 (4–10) | 0.052 |
| Time from admission to VTE onset, days median (IQR) | 0 (0–1) | 13 (7–19) | <0.001 |
| Arterial hypertension, n (%) | 105 (46.7) | 258 (47.7) | 0.800 |
| Diabetes, n (%) | 41(18.2) | 109 (20.1) | 0.540 |
| Dyslipidemia, n (%) | 81 (36) | 206 (38.1) | 0.590 |
| Obesity, n (%) | 31 (13.8) | 109 (20.1) | 0.038 |
| Current smoker, n (%) | 15 (6.7) | 52 (9.6) | 0.190 |
| Previous VTE, n (%) | 20 (8.9) | 24 (4.4) | 0.016 |
| Thromboprophylaxis, n (%) | 62 (28.1) | 439 (82.8) | <0.001 |
| CURB-65 score, n (%) | |||
| Low risk (0–1 point) | 114 (58.2) | 268 (57.5) | 0.140 |
| Moderate risk (2 points) | 40 (20.4) | 122 (26.2) | |
| Hight risk (≥3 points) | 42 (21.4) | 76 (16.3) | |
| VTE type, n (%) | |||
| Confirmed PE | 187 (83.1) | 415 (76.7) | 0.049 |
| Probable PE | 7 (3.1) | 40 (7.4) | 0.024 |
| Confirmed PE and DVT | 15 (6.7) | 17 (3.1) | 0.026 |
| DVT | 14 (6.2) | 43 (7.9) | 0.407 |
| Other thrombosis* | 2 (0.9) | 26 (4.9) | 0.002 |
| s-PESI, n (%) | |||
| Low risk (0 point) | 65 (32) | 126 (27.5) | 0.230 |
| High risk (≥1 point) | 138 (68) | 333 (72.5) | |
| ICU admission, n (%) | 23 (10.4) | 206 (38.8) | <0.001 |
| Exitus, n (%) | 35 (15.6) | 109 (20.3) | 0.140 |
| CRP (mg/Dl), median (IQR) | |||
| On admission | 13 (5–30) | 19 (10–45) | 0.004 |
| On VTE onset | 15 (5–45) | 7 (1–24) | <0.001 |
| Fibrinogen (mg/dL), median (IQR) | |||
| On admission | 516 (440–682) | 650 (500–803) | <0.001 |
| On VTE onset | 548 (454–630) | 487 (345–643) | 0.003 |
| Lymphocyte count (×103/μL), median, (IQR) | |||
| On admission | 1.2 (0.8–1.73) | 0.8 (0.6–1.14) | <0.001 |
| On VTE onset | 1.2 (0.8–1.75) | 1.05 (0.68–1.50) | 0.040 |
| Platelet count (×103/μL), median (IQR) | |||
| On admission | 246 (193–322) | 217 (164–288) | 0.001 |
| On VTE onset | 248 (195–322) | 273 (200–373) | 0.043 |
| Ferritin (ng/mL), median (IQR) | |||
| On admission | 508 (243–906) | 1167 (548–1807) | <0.001 |
| On VTE onset | 492 (236–889) | 942 (540–1487) | <0.001 |
| IL-6 (pg/dL), median (IQR) | |||
| On admission | 17 (7–71) | 61 (15–164) | 0.007 |
| On VTE onset | 15 (6–67) | 106 (15–292) | <0.001 |
| D-dimer (μg/L), median (IQR) | |||
| On admission | 15,000 (4000–35,000) | 2000 (750–4000) | <0.001 |
| On VTE onset | 15,000 (4000–35,000) | 7500 (2000–15,000) | <0.001 |
*Early VTE: ≤48 h from admission; ** Late VTE: >48 h from admission; *** Other thrombosis: atypical location, catheter-related and other thrombosis. BMI = body mass index; CRP = c-reactive protein; DVT = Deep venous thrombosis; ICU = intensive care unit; IL-6 = interleukin-6; IQR = interquartile range; LMWH = low-molecular-weight heparin; PE = pulmonary thromboembolism; s-PESI = simplified Pulmonary Embolism Severity Index; VTE = venous thromboembolism.
On admission and at thrombosis diagnosis, early VTE group presented higher D-Dimer and fibrinogen levels. However, ferritin and IL-6 levels at any time were higher in the late VTE group (Table 2).
Missing values early VTE (admission, VTE onset): CRP (45, 60), D-Dimer (9, 8), Ferritin (35, 42), Fibrinogen (52, 26), IL-6 (57, 65), Lymphocyte (3,2), Platelet (3,0). Missing values late VTE (admission, VTE onset): CRP (23,33), D-Dimer (47, 32), Ferritin (234, 225), Fibrinogen (135, 84), IL-6 (389, 404), Lymphocyte (4, 6), Platelet (4, 8).
3.2. Incidence results
Between March 1 and May 10, 2020, 5966 patients were admitted for acute COVID-19 to the four hospitals in Madrid region who participated in the incidence study (incidence study cohort). Patient characteristics are shown in Table 3 . Three hundred twenty-two patients (5.4%) required ICU at some point during admission. In contrast to ward patients, ICU patients were younger (64 years vs. 72 years; p < 0.001) and there was a greater proportion of males (68.6% vs. 54%; p < 0.001).
Table 3.
Basic characteristics and outcomes from the incidence study cohort.
| All patients n = 5966 |
Ward patients n = 5644 (94.6%) |
ICU patients n = 322 (5.4%) |
P value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 71 (56–82) | 72 (56–83) | 64 (54–70) | <0.001 |
| Male gender, n (%) | 3270 (54.8) | 3049 (54) | 221 (68.6) | <0.001 |
| Follow-up (days), median (IQR) | 6 (4–12) | 6 (3−11) | 26.5 (13–42.5) | <0.001 |
| Time from admission to VTE diagnosis, median (IQR) | 2 (0−10) | 1 (0–7) | 16 (7–32) | <0.001 |
| Patients at risk of VTE | All patients n = 5966 |
Ward patients n = 5826 |
ICU patients n = 316 |
P value |
|---|---|---|---|---|
| VTE, n (%) | 183 (3.07) | 158 (2.71) | 25 (7.91) | <0.001 |
| VTE type, n (%) | ||||
| PE, confirmed | 146 (79.8) | 125 (79.1) | 21(84) | |
| PE, probable | 12 (6.5) | 12 (7.6) | 0 | <0.001 |
| PE and DVT | 4 (2.2) | 4 (2.5) | 0 | |
| DVT | 21 (11.5) | 17 (10.8) | 4 (16) | |
| PE type, n (%) | ||||
| Central | 71 (47.3) | 63 (49) | 8 (32) | 0.424 |
| Peripheral | 79 (52.7) | 66 (51) | 13 (68) | |
| Exitus, n (%) | 1186 (19.9) | 1055 (18.7) | 131 (40.7) | <0.001 |
ICU = intensive care unit; IQR = interquartile range; PE = pulmonary embolism; VTE = venous thromboembolism.
By the end of data collection (May 30, 2020), 4717 patients (79.1%) had been discharged, 1181 (19.8%) had died, and 68 (1.14%) were still hospitalized. Patients still hospitalized were followed for at least 21 days.
During a median follow-up of 6 days (IQR, 4–12), 183 patients (3.07%; 95% CI, 2.64–3.55) presented a symptomatic VTE event. After excluding 140 patients admitted directly to the ICU, ward patients at risk were 5826. Similarly, six ICU patients presented VTE before ICU admission so the ICU patients at risk were 316. The proportion of patients with VTE was higher in ICU than in ward (7.91%; 95% CI, 5.12–11.68 vs. 2.71%; 95% CI, 2.31–3.17; p < 0.001) (Table 3).
In ward, the proportion of patients eventually transferred to the ICU was similar among patients with VTE (3.8%, 6 of 158) and without VTE (3.1%, 176 of 5668) (p = 0.638).
As described above with the patients recorded in our registry, we compared early and late VTE patients in the incidence study cohort. Patients with early VTE represented 48.1% of the cohort. Characteristics and outcomes of these two groups are summarized in Table 4 .
Table 4.
Characteristics and outcome of early VTE and late VTE in the incidence study cohort.
| All VTE patients n = 183 |
Early VTE* patients n = 88 (48.1%) | Late VTE** patients n = 95 (51.9%) |
P value | |
|---|---|---|---|---|
| Age (years), median (IQR). | 68 (58–78) | 74 (54–82) | 65 (58–74) | 0.078 |
| Male gender, n (%) | 112 (61.2) | 42 (47.7) | 70 (73.7) | <0.001 |
| VTE type, n (%) | ||||
| PE | 158 (86.4) | 74 (84) | 84 (88.4) | |
| DVT | 21 (11.4) | 11 (12.5) | 10 (10.5) | 0.598 |
| PE and DVT | 4 (2.2) | 3 (3.4) | 1 (1) | |
| PE type, n (%) | ||||
| Central | 73 (46.2) | 43 (57.3) | 30 (37) | 0.011 |
| Peripheral | 85 (53.8) | 32 (42.7) | 51 (63) | |
| ICU admission, n (%) | 31 (16.9) | 4 (4.5) | 27 (28.4) | <0.001 |
| Exitus, n (%) | 33 (18) | 14 (15.9) | 19 (20) | 0.472 |
*Early VTE: ≤48 h from admission; **Late VTE: >48 h from admission. DVT = deep vein thrombosis; ICU = intensive care unit; IQR = interquartile range; PE = pulmonary thromboembolism; VTE = venous thromboembolism.
Although the proportion of PE was similar in both groups, a greater proportion of central PE was observed in early VTE patients than in late VTE patients (57.3% vs. 37%; p = 0.011). Conversely, late VTE patients presented peripheral PE more often than early VTE patients (63% vs. 42.7%; p = 0.011).
In the competing risk model, the cumulative incidences of VTE at ward at 7, 14, 21, 30 and 60 days were 2.3% (95% CI, 1.9–2.7), 3.6% (95% CI, 3.0–4.3), 4.3% (95% CI, 3.5–5.1), 5.4% (95% CI, 4.2–6.8), and 5.4% (95% CI, 4.2–6.8), respectively. The cumulative incidence of VTE at ICU at 7, 14, 21, 30 and 60 days were 2.2% (95% CI, 1.0–4.4), 2.9% (95% CI, 1.5–5.3), 4.1% (95% CI, 2.2–6.8), 6.9% (95% CI, 4.2–10.5), and 12.8% (95% CI, 8.1–18.5), respectively. Fig. 1 shows ward and ICU cumulative incidence graphs.
Fig. 1.
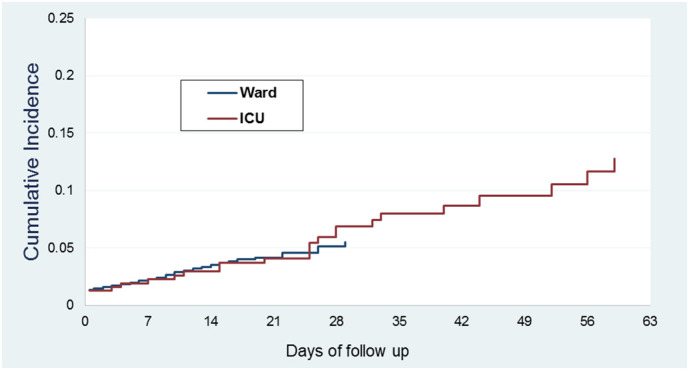
Cumulative incidence of symptomatic VTE in ward and ICU patients.
When analyzed as a time-varying variable, VTE was not associated with death (HR, 0.79; 95% CI, 0.55–1.12; p = 0.182) (Fig. 2 ). (Table 5 in Supplementary material).
Fig. 2.
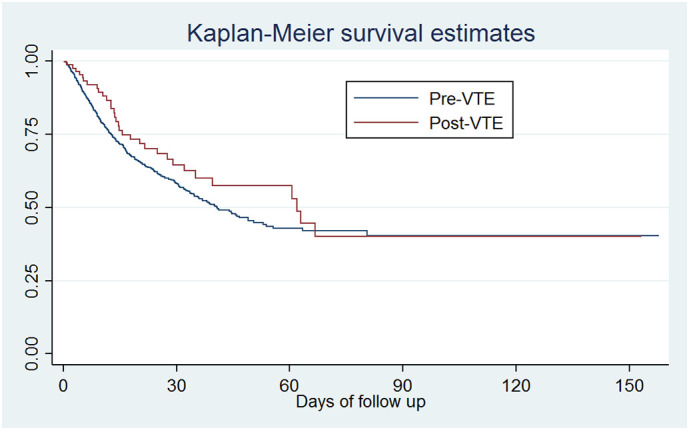
Hospital survival by VTE.
Table 5.
Hospital survival by VTE.
| Time | N | Events | Survival function | CI95% | ||
|---|---|---|---|---|---|---|
| Pre VTE time | 7 | 2886 | 666 | 86.0% | 85.0% | 87.0% |
| 14 | 1148 | 309 | 72.9% | 71.2% | 74.5% | |
| 21 | 538 | 95 | 64.7% | 62.5% | 66.8% | |
| 30 | 264 | 39 | 58.1% | 55.3% | 60.9% | |
| Post VTE time | 7 | 74 | 7 | 92.1% | 84.1% | 96.1% |
| 14 | 54 | 9 | 79.3% | 68.2% | 86.8% | |
| 21 | 45 | 5 | 71.8% | 59.9% | 80.7% | |
| 30 | 34 | 4 | 64.6% | 51.9% | 74.8% | |
4. Discussion
4.1. Descriptive analysis
The TROMBOCOVID registry included data from 844 patients hospitalized for acute COVID-19 between March 1 and May 30, 2020 in Madrid region, Spain, and who were diagnosed with a VTE event during admission.
Most of the clinical characteristics of our patients and VTE risk factors, such as active cancer (5.9%) and past VTE history (6.0%), were similar to those previously described [23,24]. Strikingly, dyslipidemia (36%) was the second most recorded comorbidity in our series, a data not reflected in other studies. As published in other series, the most frequent thrombotic event was PE (87%); however, in our study, 5.5% of PEs, mostly in critical patients, were considered probable PE based on high clinical suspicion, due to the difficulty in performing angio-CT in these patients.
Nevertheless, there are notable differences between our results and those obtained from 455 patients from RIETE (Registro Informatizado de la Enfermedad Tromboembólica) registry, many of whom were admitted in Madrid hospitals [23]. Firstly, only 6% of our patients had been immobilized compared to 78% in the RIETE study. Immobilization is not clearly defined in our study; thus, it is possible that this 6% reflects the patient situation before admission. In RIETE study, immobilization is defined as “bedding of ≥4 days -except for going to the bathroom- in the 2 months prior to the diagnosis of VTE” so most of the patients hospitalized for COVID-19 would meet this criterion. Secondly, even though 29% of patients in our registry were admitted to ICU at some point during their hospitalization, only 8% of events (68) were diagnosed during their stay in ICU, a percentage much lower than the 32% reported in RIETE study, which could explain the higher percentage of male patients in their registry (71%) compared with ours (64%). Third, the percentage of patients who received prophylactic LMWH before the VTE event was lower in our registry than in RIETE study, both in ward (56.1% vs. 84%) and in ICU (83.1% vs. 96%). We found that most of our patients without thromboprophylaxis belonged to the early VTE group, therefore, there was little time to initiate thromboprophylaxis before VTE event. Finally, prophylactic LMWH was used at higher doses in RIETE study. Intermediate and therapeutic doses were used in 28% of patients in ward and 40% in ICU according to RIETE study, but only in 12.4% in ward and 18% in ICU in our study.
The early VTE group represents one third of the registry. As detailed in Results, patients in the early VTE group resemble non-COVID patients with conventional VTE disease. It is very likely that VTE, together with COVID-19, is the reason for admission more than a secondary event due to hospitalization. Conversely, late VTE is undoubtedly an in-hospital event that occurs more frequently in patients with typical characteristics of severe COVID-19 who are admitted to ICU. Surprisingly, peripheral PE was more frequent than central PE among late VTE events in the incidence study cohort. Although at first sight it seems that thromboprophylaxis cannot prevent late peripheral PE, we could hypothesize that thromboprophylaxis succeeds in preventing central PE secondary to hospitalization for severe COVID-19 which usually has more serious clinical consequences. A 2019 study with real life data, found that patients with peripheral PE presented, on admission, less dyspnea, syncope, hypoxemia, hypotension and tachycardia than patients with central PE [25]. Another prospective study confirmed that central PE confers a higher risk of mortality from any cause than peripheral PE [26]. In patients with COVID-19 according to our data there is no difference in s-PESI score at the diagnosis of PE between patients with early and late PE. So, we could assume that there is no difference in s-PESI score between patients with peripheral and central PE. Moreover, the mortality rate is similar in both groups, even though patients with peripheral PE are admitted to ICU more often. These data together suggest that the s-PESI score is not useful for assessing PE severity in this setting, and that peripheral PE in COVID-19 patients, rather than an embolic phenomenon, could be the radiological expression of in-situ thromboses observed in these patients' autopsies [8].
4.2. Incidence analysis
Our cohort is composed by 5966 patients. It represents 0.64% of the population that belongs to the health service area of the four hospitals that make up our incidence study group. This percentage is consistent with the official data reported in Madrid region about COVID-19 hospitalized population until May 10, 2020 [27]. The percentage of deaths among hospitalized patients (19.9%) is also consistent with official results, while the ICU admission rate (5.4%) was somewhat lower than the official percentage (8%).
Our study's incidence results are clearly lower than those reported on the first studies, where sample sizes were less than 200, most patients were in ICU and asymptomatic VTE was included. A meta-analysis of 47 studies published before July 2020, which included 18,093 patients, found that the pooled incidence for VTE using random effects model was 17.0% (95% CI, 13.4–20.9) [28]. In the subgroup meta-analyses, the incidence of VTE was higher when assessed according to screening (33.1% vs. 9.8% clinical diagnosis), among patients in ICU (27.9% vs. 7.1% in ward) and in prospective studies (25.5% vs. 12.4% in retrospective studies).
The role of routine screening for VTE is a matter of discussion. Many clinicians believe that the clinical and analytical characteristics of COVID-19 pneumonia often mask those of PE; so many PEs may go undetected if the indication for objective testing, as CTPA, is based on clinical suspicion. Despite this, there are unresolved issues regarding VTE screening. First, it is unknown the better time to perform the routine imaging studies to detect asymptomatic VTE. Besides, a prospective observational study found that a systematic screening for DVT in patients hospitalized in ICU is not associated with a higher diagnosis of VTE [29]. Therefore, the guidelines of American College of Chest Physicians [30] and the guidelines of International Society on Thrombosis and Haemostasis [31] do not recommend a routine sreenning ultrasound for the detection of DVT in COVID-19 patients. Instead, it is advised that clinicians should have a low threshold for performing ultrasound in patients with a reasonable degree of clinical suspicion for VTE.
Our results are consistent with those of a systematic review presented at the 62nd ASH annual meeting in December 2020, which included more than 12,000 patients from 52 studies published before July 13, 2020 [15]. This review found that VTE reported incidence in hospitalized COVID-19 patients depends on the size of the studies. Those that enrolled fewer than 100 patients showed an overall VTE incidence of 10.6%, whereas the incidence was 8.4% and 3.1% in those between 100 and 1000 patients and more than 1000 patients, respectively. The authors suggest that this trend towards higher incidences among the smaller studies may suggest a publication bias and overestimation in smaller reports.
To date, it is not clear that VTE incidence in hospitalized COVID-19 patients is higher than in patients hospitalized for other pathologies. Historical studies have reported VTE incidences associated to other diseases much lower than those associated to COVID-19, especially for ward patients. Thus, a 2007 meta-analysis [32] described a VTE rate of 0.58% in medical patients on thromboprophylaxis during an average follow-up of 45 days. Another study from 2019 showed an accumulated incidence of VTE in ICU patients on prophylactic LMWH at 7, 14 and 21 days of 4.45%, 7.14% and 7.35% respectively [33].
In contrast to the results reported from a cohort of 198 patients from The Netherlands [2], where VTE cumulative incidences at 7, 14, and 21 days were significantly higher in the ICU than in the ward, we found that symptomatic VTE cumulative incidences in both the ICU and the ward overlapped during the first 21 days of admission, followed by an increase in the ICU at 30 and 60 days. This could reflect the great difficulty in diagnosing PE in the critical early days of ICU patients. According to our results, VTE during hospitalization for COVID-19 did not appear to be associated with either mortality or transfer to ICU.
On the other hand, considering the high percentage (48%) of VTE events diagnosed within first 48 h of admission in the incidence study cohort, and although well-designed prospective studies are strongly needed, it is likely that COVID-19 is a risk factor for VTE disease that contributes, along with other well-known risk factors, to increase the incidence of VTE also in the outpatient setting.
In this setting, another question still to be solved is whether early ambulatory thromboprophylaxis in patients with symptomatic COVID-19 could improve disease prognosis. Several ongoing trials, one with prophylactic enoxaparin versus placebo (NCT04492254), one with rivaroxaban 10 mg once daily versus placebo (NCT04508023) and one with therapeutic apixaban versus placebo-controlled aspirin (NCT04498273), aim to answer this question [34]. To date, no clear recommendations have been established in this context by the guidelines, thus decisions on early ambulatory thromboprophylaxis need to be individualized.
The strengths of our study include: 1) the large sample size used in both the descriptive and the incidence analysis; 2) consecutive patient inclusion; 3) the use of computerized data sources such as the clinical history and the MDS lists which, despite some limitations, are possibly one of the most valuable tools for carrying out studies of VTE incidence [17]; 4) a follow-up period of at least 21 days, longer than usual in other studies, and 5) no loss to follow-up. We believe that the results of our study reflect faithfully the health situation that Madrid region experienced during the first wave of the COVID-19 pandemic.
However, this study has also several limitations. First, its retrospective and observational design. Second, it should be noted that, despite highly clinical suspicion, SARS-CoV-2 infection could not be confirmed by PCR in the entire group due to several reasons, such as sensitivity of initial tests, time of sampling in the course of the infection, and restrictions to repeat the test. Third, it is highly probable that not all symptomatic VTE events were detected due to resources available, patient's critical situation, and differences in clinical suspicion. As discussed above, many PEs may go undetected if the indication for objective testing, as CTPA, is based on clinical suspicion. Accordingly, the lack of routine screening for VTE could lead to selection bias and underestimation of events. Conversely, the events considered as probable may not have been such, although they are few cases (5.7% in the registry, 6.5% in the incidence study cohort). Fourth, it is possible that some VTE events have not been included due to coding problems, either because the VTE event was not reflected among the diagnoses in the discharge report, or because of omission or misinterpretation by the coders. Fifth, the ward or ICU admission criteria may have been different between hospitals and varied over time depending on the resources available. Given the shortage of ICU beds, it is very likely that many critical patients remained in ward. Sixth, due to the nature of the study, an angio-CTs centralized review was not carried out, so the diagnosis of peripheral or central PE has been subject to variability among observers and there could be a risk of misclassification.
In conclusion, the observed rates of symptomatic VTE in our cohort are consistent with other real-life studies recently published. According to our data from a cohort of 5966 patients, global VTE rate was 3.1%. Nearly half (48%) of VTE events were diagnosed within the first 48 h of admission. These early VTE events were, along with COVID-19, the reason for hospital admission rather than a complication secondary to hospitalization.
CRediT authorship contribution statement
KA and IM-A designed the study, collected and interpreted the data and, drafted the manuscript; CD collected data and analyzed statistics; IG enrolled patients in the study and drafted the manuscript; MR, NC and EG enrolled patients in the study; DV-R enrolled patients in the study and critically revised the manuscript; EP-F undertook the statistical analyses; SM-H critically revised the manuscript; PL-S designed the study, collected and interpreted the data and drafted the manuscript. All authors contributed to the acquisition of data, revised the manuscript, and approved the final version.
Declaration of competing interest
The authors declare that they have no known competing financialinterestsor personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to thank all the patients included in this study and also all healthcare staff and the entire leadership team of the participating hospitals. To the Asociación Madrileña de Hematología y Hemoterapia (AMHH) for their support to carry out this study. The authors gratefully acknowledge Ángel Cedillo for the creation of the secure web platform (TROMBOCOVID) in the electronic data capture software REDCap; Mayca Morales (Admission Department, University Hospital Fundación Alcorcón, Madrid, Spain); Adrián García (Admission Department, University Hospital del Sureste, Madrid, Spain) and José Miguel Arce (Clinical Documentation Department, University Hospital Fundación Jiménez Díaz, Madrid, Spain) for providing us with Minimum Data Set.
References
- 1.Weekly epidemiological update - 19 January 2021, (n.d.). https://www.who.int/publications/m/item/weekly-epidemiological-update---19-january-2021 (accessed February 7, 2021).
- 2.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., Bouman C.C.S., Beenen L.F.M., Kootte R.S., Heijmans J., Smits L.P., Bonta P.I., van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.a.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Gandet F. Fagot, Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.-M., Meziani F. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis), High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.-D., Sacco C., Bertuzzi A., Sandri M.T., Barco S. Humanitas COVID-19 Task Force, venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.F., Lo A.W.I. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2) J. Pathol. 2004;203:740–743. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo W., Yu H., Gou J., Li X., Sun Y., Li J., Liu L. 2020. Clinical Pathology of Critical Patient with Novel Coronavirus Pneumonia (COVID-19): Pulmonary Fibrosis and Vascular Changes including Microthrombosis Formation. [DOI] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakka M., Connors J.M., Hékimian G., Martin-Toutain I., Crichi B., Colmegna I., Bonnefont-Rousselot D., Farge D., Frere C. Association between D-dimer levels and mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and pooled analysis. J. Med. Vasc. 2020;45:268–274. doi: 10.1016/j.jdmv.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett C.D., Moore H.B., Yaffe M.B., Moore E.E. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: a comment. J. Thromb. Haemost. 2020;18:2060–2063. doi: 10.1111/jth.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ASH Guidelines on Use of Anticoagulation in Patients with COVID-19 - Hematology.org, (n.d.). https://www.hematology.org:443/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19 (accessed December 23, 2020).
- 14.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galllastegui Crestani N., Zhou J.Y., von Drygalski A., Morris T.A. Incidence of venous thromboembolic events in hospitalized COVID-19 patients: a systematic review. Blood. 2020;136:42–43. doi: 10.1182/blood-2020-143021. [DOI] [Google Scholar]
- 16.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupo Multidisciplinar para el Estudio de la Enfermedad Tromboembólica en España . 2006. Estudio sobre la enfermedad tromboembólica venosa en España, Imago Concept Image DEV, Las Matas (Madrid) [Google Scholar]
- 18.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D., Sidorov I.A., Sola I., Ziebuhr J. Severe acute respiratory syndrome-related coronavirus : the species and its viruses – a statement of the Coronavirus study group. Microbiology. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 19.W.H. Organization WHO COVID-19 case definition. 2020. https://apps.who.int/iris/handle/10665/333912
- 20.COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19, (n.d.) 35. [PubMed]
- 21.Lim W., van der Eerden M.M., Laing R., Boersma W., Karalus N., Town G., Lewis S., Macfarlane J. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez D., Aujesky D., Moores L., Gómez V., Lobo J.L., Uresandi F., Otero R., Monreal M., Muriel A., Yusen R.D. RIETE investigators, simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch. Intern. Med. 2010;170:1383–1389. doi: 10.1001/archinternmed.2010.199. [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Capitán C., Barba R., Díaz-Pedroche M.D.C., Sigüenza P., Demelo-Rodriguez P., Siniscalchi C., Pedrajas J.M., Farfán-Sedano A.I., Olivera P.E., Gómez-Cuervo C., Llamas P., Villares P., Sanchez O., López-Reyes R., Catella J., Bikdeli B., Weinberg I., Tafur A.J., Jiménez D., Monreal M. Presenting characteristics, treatment patterns, and outcomes among patients with venous thromboembolism during hospitalization for COVID-19. Semin. Thromb. Hemost. 2020 doi: 10.1055/s-0040-1718402. [DOI] [PubMed] [Google Scholar]
- 24.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium, Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.C. Fernández-Capitán, A.R. Cobo, D. Jiménez, O. Madridano, M. Ciammaichella, E. Usandizaga, R. Otero, P.D. Micco, F. Moustafa, M. Monreal, Symptomatic subsegmental versus more central pulmonary embolism: clinical outcomes during anticoagulation, Res. Pract. Thromb. Haemost.. n/a (n.d.). doi: 10.1002/rth2.12446. [DOI] [PMC free article] [PubMed]
- 26.Vedovati M.C., Becattini C., Agnelli G., Kamphuisen P.W., Masotti L., Pruszczyk P., Casazza F., Salvi A., Grifoni S., Carugati A., Konstantinides S., Schreuder M., Golebiowski M., Duranti M. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417–1424. doi: 10.1378/chest.11-2739. [DOI] [PubMed] [Google Scholar]
- 27.2019-Nuevo Coronavirus, Comunidad de Madrid. 2020. https://www.comunidad.madrid/servicios/salud/2019-nuevo-coronavirus
- 28.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., Le Mao R., Rodríguez C., Hunt B.J., Monreal M. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2020 doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapébie F.-X., Minville V., Ribes A., Combis B., Thery A., Geeraerts T., Silva S., Bura-Rivière A., Vardon-Bounes F. Systematic screening for deep vein thrombosis in critically ill inpatients with COVID-19: impact on the incidence of venous thromboembolism. Front. Med. 2021;7:624808. doi: 10.3389/fmed.2020.624808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., Holley A.B., Jimenez D., Le Gal G., Rali P., Wells P. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., Levi M., Samama C.M., Thachil J., Giannis D., Douketis J.D., the Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis, Scientific and Standardization Committee communication Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dentali F., Douketis J.D., Gianni M., Lim W., Crowther M.A. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann. Intern. Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C., Zhang Z., Mi J., Wang X., Zou Y., Chen X., Nie Z., Luo X., Gan R. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Search of: interventional studies | Covid19 - results by topic - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/results/browse?type=Intr&cond=Covid19&brwse=intr_cat_AnCoag (accessed January 8, 2021).


