Abstract
Background & aims
Patients affected by COVID-19 may develop disease related malnutrition (DRM) due to the catabolic situation, symptoms that interfere with intake and prolonged hospital stay. This study aims to know the percentage of patients admitted for COVID-19 who required artificial nutrition (AN), their clinical characteristics, as well as the prevalence of DRM and the risk of sarcopenia at hospital discharge and after 6 months.
Material and methods
Observational, prospective study, with successive inclusion of adult patients admitted for COVID-19 in whom institutional nutritional support (NS) care protocol was applied. Those who received AN underwent a nutritional screening by Short Nutritional Assessment Questionnaire (SNAQ) and an assessment by Subjective Global Assessment (SGA) at hospital discharge, as well as a screening for sarcopenia (SARC-F test) and SNAQ re-test 15 days and 6 months after by a phone call. Symptoms related to food intake, anthropometric and analytical data were also collected.
Results
We evaluated 936 patients with a mean age of 63.7 ± 15.3 years; predominantly male (59.7%), overweight 41%, obesity 40.4%; hypertension 52.9%; diabetes mellitus 26.6% and cancer 10.4%. The stay hospital length was 17.3 ± 13.8 days and 13.6% patients died during hospitalization. The modality of nutritional support was: 86.1% dietary adaptation + oral nutritional supplements (ONS); 12.4% enteral nutrition (EN) by nasogastric (NG) tube; 0.9% parenteral nutrition (PN) and 0.6% EN plus PN. Focusing on patients who received AN, follow-up post discharge was possible in 62 out of 87 who survived. Of these, at the time of hospital discharge, 96.7% presented nutritional risk by SNAQ and 100% malnutrition by SGA (20% B; 80% C). During admission, 82.3% presented intense anorexia and the mean weight loss was 10.9 ± 6 Kg (p < 0.001). Fifteen days after being discharged, 12.9% still had anorexia, while hyperphagia appeared in 85.5% of the patients and risk of sarcopenia by SARC-F was present in 87.1% of them. Six months after discharge, 6.8% still had anorexia and 3.4% hyperphagia, with a global weight gain of 4.03 ± 6.2 Kg (p=<0.0001). Risk of malnutrition was present in only 1.7% of the patients, although risk of sarcopenia persisted in 49.2%.
Conclusion
All patients admitted by COVID-19 for whom EN or PN were indicated following an institutional protocol still presented malnutrition at hospital discharge, and almost all showed risk of sarcopenia, that persisted in almost half of them at 6 months. These findings suggest that nutritional and functional problems persist in these patients after discharge, indicating that they require prolonged nutritional support and monitoring.
Keywords: COVID-19, Nutritional support, Disease related malnutrition, Artificial nutrition, Sarcopenia
1. Introduction
The novel coronavirus disease 2019 (COVID-19) is caused by infection from the newly emerged coronavirus SARS-CoV-2 [1,2] and the COVID-19 pandemic has posed an unprecedented challenge for healthcare systems around the world. The disease mainly affects the respiratory tract, but in severe cases it can cause multiorgan dysfunction and may be fatal [[3], [4], [5]]. Elderly individuals, along with those with pre-existing co-morbid conditions, such as hypertension, cancer, cardiovascular diseases, obesity, diabetes mellitus, and acute kidney injury, have worse outcomes and higher mortality [2,4,6,7].
Patients with COVID-19 may develop, according to the classification of the ESPEN guidelines, acute DRM for different reasons, but mainly because of increased energy requirements related to severe inflammation with difficulty to achieve them due to hyporexia and coexistence of eating difficulties [8]. In particular, COVID-19 infection may be accompanied by gastrointestinal disturbances like nausea, vomiting, and diarrhea that affect both, food intake and absorption [5].
In the context of COVID infection, patients may present loss of muscle mass and functionality due to the catabolic state caused by the viral infection, aggravated by a situation of immobilization and prolonged hospital stay, especially in those patients requiring intensive care unit (ICU) admission.
In addition, it should be borne in mind that many of these patients already have a high risk of malnutrition due to ongoing chronic diseases or aging that is associated to adverse changes in body composition, such as the gradual loss of skeletal muscle mass and impaired muscle function (sarcopenia) [9,10].
Recently, a multicenter, randomized, controlled trial in medical inpatients at nutritional risk (EFFORT trial), observed that nutritional support during the hospital stay improved important clinical outcomes, including survival [11]. For all the above, an adequate assessment of nutritional status in COVID-19 patients, as well as the prescription of a nutritional treatment adapted to their needs, is essential to achieve a better nutrition status, which will certainly improve clinical outcomes.
Only few studies, performed in Chinese population, have evaluated nutritional status in COVID-19 patients, finding a risk of malnutrition in approximately 80% of them during hospitalization [12,13]. To the best of our knowledge, there are no studies reporting nutritional status at discharge, and no studies evaluating nutritional support in this type of subjects.
Our study aims to assess the nutritional status of patients admitted for COVID-19 and to evaluate those who have required AN (EN or/and PN), their clinical characteristics, as well as the prevalence of DRM and risk of sarcopenia after hospital discharge.
2. Methods
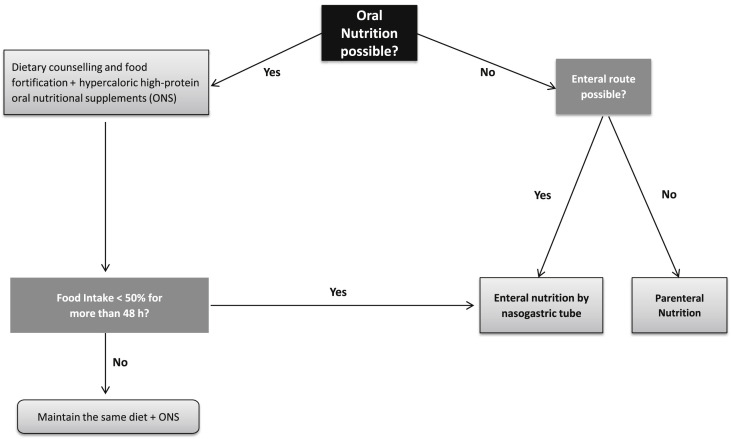
We performed a prospective observational study of adult patients with SARS-CoV-2 infection who were admitted to conventional hospitalization units and/or ICU from April to June 2020 at Germans Trias i Pujol Hospital in Badalona (Barcelona), Spain. All COVID-19 patients admitted in our institution were considered to be at high nutritional risk, given the acute inflammatory situation with high nutritional requirements of this type of patients. Therefore, all of them received nutritional support following an institutional protocol based on ESPEN recommendations. The energy and protein targets were: 25–30 kcal per kg body weight/day and 1.5 g protein per kg body weight/day. The first step was dietary counselling and food fortification plus hypercaloric high-protein ONS. EN by NG was initiated, if possible, when food intake was less than 50% during more than 48 h despite dietary counselling and ONS (Fig. 1 ). When EN was not possible or when nutritional requirements could not be met with EN, PN was started. Clinical and laboratory data were collected from the hospital's electronic medical records. At hospital discharge, surviving patients who received AN were asked for symptoms related to intake and a nutritional evaluation by SNAQ and SGA was performed [14,15]. Dysphagia was assessed by Eating Assessment Tool-10 (EAT-10) test [16]. When risk of dysphagia or malnutrition were detected, nutritional counseling was provided. A sarcopenia screening was also performed by SARC-F test [17]. At 15 days and 6 months after discharge, patients were re-evaluated by a phone call and asked about symptoms related to intake including an EAT-10 test. At 6 months, a SNAQ and SARC-F test were also administered.
Fig. 1.
Nutritional management of COVID-19 patients at Germans Trias i Pujol Hospital.
The study was approved by the local Ethics Committee, and the investigation conformed to the principles outlined in the Declaration of Helsinki.
2.1. Statistical analysis
All statistical analyses were performed in line with the statistical program SPSS V22. We performed descriptive statistics using frequencies to summarize categorical variables (percentage) and expressing quantitative variables as mean ± standard deviation (SD). Normal distribution of quantitative data was assessed. The univariate tests used were Chi-square for categorical variables and Student's test for quantitative variables. A p value of <0.05 was set as statistically significant.
3. Results
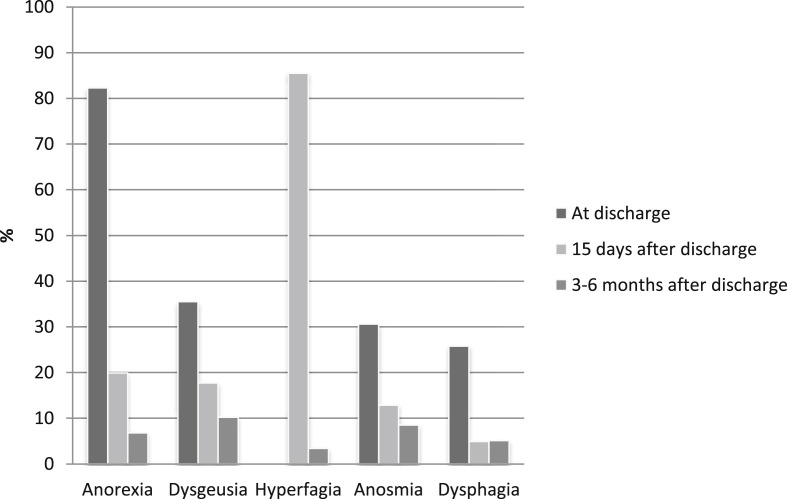
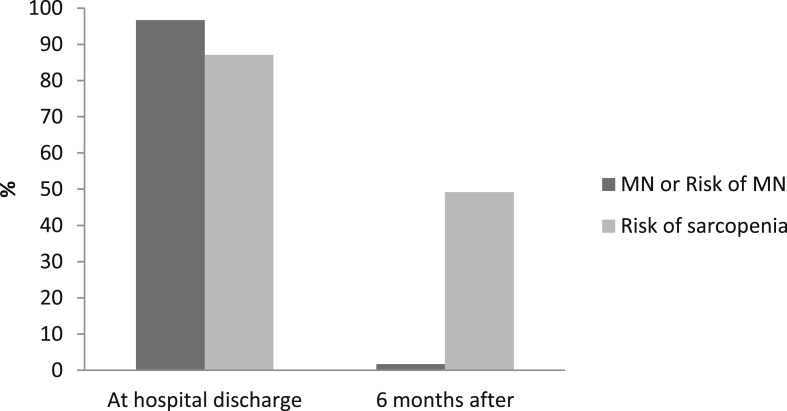
Nine hundred and thirty-two patients consecutively admitted to our institution were included in the analysis. Briefly, patients had a mean age of 63.7 ± 15.2 years and were predominantly overweight (41%) or obese (40.4%). According to clinical judgement and standard care defined in the institutional protocol, the type of nutritional support required during hospitalization was in 86.1% dietary adaptation + ONS and in 13.9% (130 patients) AN. Table 1 shows baseline demographic and clinical data for the total cohort studied and those in which AN was required. Patients requiring AN were younger (61 ± 10.2 vs. 63.7 ± 15.3 years; p = 0.003), predominantly male (76.4 vs. 59.7%; p < 0,001), obesity was present in 44.6% of them, had longer length of hospital stay (41.1 ± 17.9 vs. 17.3 ± 13.8 days; p=<0,001), and higher mortality (33.1% vs. 13.6%; p=<0,001). Moreover, they had lower albumin levels (31.4 ± 5 vs 36 ± 4.8; p < 0.001), lower lymphocyte count (0.6 [0.4–0.8] vs. 0.9 [0.6–1.2] x109/L) and higher inflammatory markers at hospital admission. Results are shown in Table 2 . Focusing on patients who received AN, follow-up post discharge at 2 weeks and 6 months was possible in 62 out of 87 who survived. Those subjects presented a BMI at admission of 30.9 ± 6.4 kg/m2 and 87% (54 patients) required ICU admission with a length of stay of 20.7 ± 11.9 days. Tube feeding was required in 92.1% during 20.5 ± 13.3 days. . During hospitalization, a mean weight loss of 10,9 ± 6 Kg was observed, and albumin levels decreased significantly (31.5 ± 4.8 vs. 26.8 ± 4.2, p < 0.001). At the time of hospital discharge, 96.7% presented nutritional risk by SNAQ and 100% malnutrition by SGA (20% B; 80% C) and risk of sarcopenia evaluated by SARC-F was detected in 87.1% of subjects. Figure 2 shows the prevalence of symptoms related to food intake. Anorexia was reported in 82.3% and dysphagia by EAT-10 was detected in 25.8% of patients during hospitalization. Fifteen days after discharge anorexia persisted in 12.9% and dysphagia in 4.9% of the patients, highlighting the appearance of hyperphagia in 85.5% of them. After 6 months of discharge, 10.2% still presented disorexic symptoms, with 6.8% still having anorexia and 3.4% hyperphagia. Only 3 patients of the 62 evaluated (4.9%) persisted with dysphagia at 6 months. None of them presented risk of malnutrition at the end of follow-up, but all them were at risk of sarcopenia. No complications related to dysphagia were observed. For these patients requiring AN, a global weight gain of 4.03 ± 6.2 Kg (p=<0.0001) was observed at 6 months of discharge. Risk of malnutrition was present in only 1.7% of the patients, although risk of sarcopenia persisted in 49.2%. Figure 3 shows the evolution of the risk of malnutrition and sarcopenia after hospital discharge.
Table 1.
Baseline demographic and clinical data.
| Variable | All patients (n = 936) | Patients with AN (n = 130) | Patients without AN (n = 806) | p |
|---|---|---|---|---|
| Age (years) | 63.7 ± 15.3 | 61 ± 10.2 | 64.2 ± 15.9 | 0.003a |
| Sex (% male) | 59.7 | 76.4 | 57 | <0.001a |
| BMI (kg/m2) | 29.8 ± 5.8 | 30.2 ± 6.4 | 29.4 ± 5.8 | NS |
| Overweight (%) | 41 | 43.8 | 40.3 | NS |
| Obesity (%) | 40.4 | 44.6 | 39.4 | NS |
| Hypertension (%) | 52.9 | 59.2 | 52 | NS |
| Diabetes mellitus (%) | 26.6 | 26.9 | 26.6 | NS |
| Cancer patient (%) | 10.4 | 4.6 | 11.3 | 0.02a |
| Length of stay (days) | 17.3 ± 13.8 | 41.1 ± 17.9 | 14.9 ± 10.7 | <0.001a |
| Exitus (%) | 13.6 | 33.1 | 10.4 | <0.001a |
| Type of nutritional support (%) | ||||
|
86.1 | – | 100 | |
|
12.4 | 91.3 | ||
|
0.6 | 0.8 | ||
|
0.9 | 7.9 | ||
Data are presented as mean ± SD.
P values represent significant comparisons among patients with and without Artificial Nutrition (AN).
Table 2.
Laboratory measures.
| Variable | Patients without AN (n = 806) | Patients with AN (n = 130) | p |
|---|---|---|---|
| Albumin (g/L) | 36.1 ± 4,8 | 31.4 ± 5.1 | <0.001a |
| Lymplocyte count (x 109/L) | 0.9 (0.6–1.2) | 0.6 (0.4–0.8) | <0.001a |
| C-reactive protein (mg/L) | 90.7 ± 79 | 165.7 ± 113.5 | <0.001a |
| D-dimer (ng/mL) | 742.5 (429.7–1361.7) | 1191 (580–2424) | 0.002a |
| Interleukin-6 (pg/mL) | 37.7 (17.2–78.7) | 116.7 (43.8–239.5) | <0.001a |
| Ferritin (ng/mL) | 494 (255–944) | 1292 (670–2107) | <0.001a |
Data are presented as mean ± SD or median (P25–P75).
P values represent significant comparisons among patients with and without Artificial Nutrition (AN).
Fig. 2.
Symptoms related to food intake.
Fig. 3.
Risk of malnutrition and sarcopenia during follow-up.
4. Discussion
This observational prospective study shows that all patients admitted with COVID-19 who received AN presented malnutrition at hospital discharge and almost all showed risk of sarcopenia, that persisted in half of them at 6 months. Moreover, disorexic symptoms were still present at 6 months in about 10% of cases. In our cohort of COVID patients at Germans Trias hospital, a university tertiary center covering about 800,000 people in Northern Barcelona area, it is remarkable that more than 80% of admitted patients had either obesity or overweight.
Until the present we have not found other studies that evaluate the need of artificial nutritional in COVID-19 patients, which in our series is 13.9%. Although all subjects underwent daily sessions of prone position, we observed that tube feeding could be implemented and tolerated in these COVID-19 patients. This finding confirms and supports ESPEN guidelines recommendations that prone position does not represent a limitation for EN in COVID-19 patients [8]. The overall prognosis of patients with AN was worse than those without this type of support, probably in relation to the severity of the disease, as practically all of the patients with AN required ICU admission. This is further supported by the fact that not just clinical characteristics but also biological factors such as lymphocytes count and proinflammatory markers were worse in this subset of patients.
DRM was present in all patients that required AN at hospital discharge. Lower prevalence of malnutrition in COVID-19 patients has been recently described [12]. Nevertheless, in this latter series, patients with milder forms of the disease were included and nutritional assessment was performed during hospitalization and not at discharge, which could explain the differences with the out study.
Anorexia was very prevalent in our patients with AN during admission. Other authors reported less prevalence of this symptom probably because they included mainly mild-to-moderate COVID-19 patients, without need of intensive care [18]. Nevertheless, it was surprising that most of our patients experienced hyperphagia after discharge. No data reporting this finding have been published yet. The profound anorectic feeling accompanying COVID-19 infection and inflammatory disease is quite impressive. It develops very rapidly and also disappears at about two weeks after discharge in most, although not in all patients. The intensity of this symptom and its rapid disappearance in the recovery phase clearly suggests that not just the infective disease state plays a role. It has been described that angiotensin converting enzyme 2 (ACE2), the only confirmed cellular receptor for SARS-CoV-2, it is expressed in the human hypothalamus and a case report confirmed viral invasion and replication in the hypothalamus in a post-mortem brain of a patient affected with COVID-19 [19,20]. One hypothesis could be that some component of the viral particles bind to specific areas of the hypothalamus leading to the profound effect upon orexigenic state. The clearance of the viral particles from the hypothalamus would explain the reactive hyperphagic and increased hunger observed in the majority of patients. The negative effect conferred by the profound anorectic state of these patients together with the catabolic state induced by COVID-19 inflammatory disease contributes to the high prevalence of DRM observed in these patients. Moreover, this disorectic situation doesn't disappear completely at 6 months, as about 10% of cases in our series still presented symptoms at 6 months after discharge. Therefore, there is a need to develop tailored anabolic nutrition strategies for those patients entering the recovery phase. Furthermore, according to our data it is evident that there is a need of an earlier detection and initiation of intensive nutritional support during the first days after admission.
On the other hand, risk of sarcopenia was highly prevalent at discharge and persisted in almost half of the patients at 6 months, although risk of malnutrition was only present in few of them at that time. In our cohort, in whom the vast majority of subjects had either overweight or obesity, the serious weight loss of more than 10 kg together with the high prevalence of sarcopenia risk could indicate that weight loss is focused on muscle mass loss. Furthermore, hyperphagic rebound situation would lead consequently to an increase in adipose reservoir. The net consequence, for these obese/overweight convalescent patients, could be an increment in the previously perturbed body composition and the possibility of increasing sarcopenic obesity at mid-term, which is a very concerning consequence of post-COVID health status. Emerging evidence indicates that low muscle mass and its quality have a strong negative prognostic impact in obese individuals and may lead to frailty, disability, and increased morbidity and mortality, unless the situation is reverted with appropriate nutritional and functional intervention [21].
There is evidence that people with severe COVID-19 need a specific program of exercise therapy to prevent or reverse muscle disability [22,23]. Patients who develop sarcopenia as a consequence of a stressful event often require even lifetime exercise and nutrition therapy. More research regarding the incidence and impact of sarcopenia in post-COVID-19 cases is required and also more attention to rehabilitation programs during the recovery phase is necessary [24,25]. All these considerations indicate that integrated follow-up programs for post-COVID clinics should include dietary counseling and functional rehabilitation. In concordance with other studies, and as previously highlighted, we found a high prevalence of obesity in our SARS-Cov-2 hospitalized patients (40,4%) [26,27]. Nevertheless, this data does not represent the prevalence of obesity in the general Spanish population, which overall is of 17.4%, which indicates that obese individuals who suffer COVID-19 are more prone to a higher risk of hospitalization, as also reported by other authors [28,29].
Despite overweight, all our patients requiring AN were undernourished, which confirms that BMI is not a good indicator of nutritional status [30]. Since malnutrition is defined not only by low BMI but also by inability to preserve a healthy body composition, obese subjects should be screened and investigated by systematic measurement of body composition.
We observed a high prevalence of dysphagia at discharge in our study population, as also reported by other authors in COVID-19 patients [31] and similar to swallowing disorders described after prolonged ICU stay for other diseases [32,33]. One of the reasons could be a direct infection of the digestive epithelial cells by the virus [34]. The presence of severe post-extubating dysphagia has been associated with severe outcomes including pneumonia, reintubation and hospital mortality [35]. Taking this into account, it seems necessary that all patients hospitalized for SARS-CoV-2 must be screened for dysphagia, and if detected, they must undergo a swallowing evaluation, in order to prevent nutritional complications and bronchial aspiration.
There were several limitations in our study. Firstly, there was some lack of data about nutritional status at admission. Chronic comorbidities were present in a non-negligible percentage of patients of the study, which could confer itself an important component of malnutrition risk and sarcopenia prior to SARS-CoV-2 infection. Secondly, we lack of body composition measurements. It has been described that a low lean body mass is an independent risk factor for mortality at 6 months in critically ill patients with mechanical ventilation. Thus, it is important also for clinical care in COVID-19 patients to systematically evaluate body composition, which will allow a better nutritional support for a given patient. Furthermore, considering the high prevalence of obesity in COVID-19 patients in our series, it is important to monitor fat mass during the recovery period, and also identify sarcopenic obesity for prevention of worsening of the metabolic condition in those surviving COVID-19 diseases.
In conclusion, all patients admitted by COVID-19 who received AN presented malnutrition at hospital discharge and almost all of them showed a high risk of sarcopenia, that persisted in half of the cases after 6 months of discharge. This finding indicates that nutritional support must be more intensive and ideally associated to exercise rehabilitation therapy to prevent or reverse sarcopenia. Nutritional intervention should be considered as part of the comprehensive care of all patients with SARS-CoV-2 infection who require hospital admission, to improve hospital outcomes and the medium-term health status of survivors.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 Apr 7;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28 doi: 10.1056/NEJMoa2002032. https://www.nejm.org/doi/10.1056/NEJMoa2002032 [Internet] [cited 2020 Aug 13]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouadma L., Lescure F.-X., Lucet J.-C., Yazdanpanah Y., Timsit J.-F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46(4):579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020 Feb 19:368. doi: 10.1136/bmj.m606. https://www.bmj.com/content/368/bmj.m606 [cited 2020 Aug 13] [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020 Jun 1;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes F., Schuetz P., Bounoure L., Austin P., Ballesteros-Pomar M., Cederholm T., et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr Edinb Scotl. 2018;37(1):336–353. doi: 10.1016/j.clnu.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Volkert D., Beck A.M., Cederholm T., Cruz-Jentoft A., Goisser S., Hooper L., et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr Edinb Scotl. 2019;38(1):10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Schuetz P., Fehr R., Baechli V., Geiser M., Deiss M., Gomes F., et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019 Jun 8;393(10188):2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 12.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020 Jun;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G., Zhang S., Mao Z., Wang W., Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020 Jun;74(6):876–883. doi: 10.1038/s41430-020-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detsky A.S., McLaughlin J.R., Baker J.P., Johnston N., Whittaker S., Mendelson R.A., et al. What is subjective global assessment of nutritional status? JPEN - J Parenter Enter Nutr. 1987 Feb;11(1):8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 15.Screening. Fight malnutrition. https://www.fightmalnutrition.eu/toolkits/hospital-screening [internet][cited 2020 Oct 9]. Available from:
- 16.Burgos R., Sarto B., Segurola H., Romagosa A., Puiggrós C., Vázquez C., et al. Traducción y Validación de la Versión en Español de la Escala EAT-10. Nutr Hosp. 2012 Nov 1;(6):2048–2054. doi: 10.3305/nh.2012.27.6.6100. [DOI] [PubMed] [Google Scholar]
- 17.Parra-Rodríguez L., Szlejf C., García-González A.I., Malmstrom T.K., Cruz-Arenas E., Rosas-Carrasco O. Cross-cultural adaptation and validation of the Spanish-language version of the SARC-F to assess sarcopenia in Mexican community-dwelling older adults. J Am Med Dir Assoc. 2016 01;17(12):1142–1146. doi: 10.1016/j.jamda.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020 Aug 1;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020 Oct 1;183(1):16–27.e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nampoothiri S., Sauve F., Ternier G., Fernandois D., Coelho C., Imbernon M., et al. bioRxiv; 2020 Jun 19. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. 2020.06.08.139329. [Google Scholar]
- 21.Barazzoni R., Bischoff S.C., Boirie Y., Busetto L., Cederholm T., Dicker D., et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr Edinb Scotl. 2018 Dec;37(6 Pt A):1787–1793. doi: 10.1016/j.clnu.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Li J. Effect and enlightenment of rehabilitation medicine in COVID-19 management. Eur J Phys Rehabil Med. 2020 Apr 24:56. [Google Scholar]
- 23.Morley J.E., Kalantar-Zadeh K., Anker S.D. COVID-19: a major cause of cachexia and sarcopenia? J Cach Sarcop Muscle. 2020;11(4):863–865. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brugliera L., Spina A., Castellazzi P., Cimino P., Tettamanti A., Houdayer E., et al. Rehabilitation of COVID-19 patients. J Rehabil Med. 2020 15;52(4) doi: 10.2340/16501977-2678. [DOI] [PubMed] [Google Scholar]
- 25.Kirwan R., McCullough D., Butler T., Perez de Heredia F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. GeroScience. 2020 Oct 1 doi: 10.1007/s11357-020-00272-3. [Internet] [cited 2020 Oct 13]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in patients younger than 60 Years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020 Jul 28;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killerby M.E. Characteristics associated with hospitalization among patients with COVID-19 — metropolitan Atlanta, Georgia, march–April 2020. MMWR Morb Mortal Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6925e1. https://www.cdc.gov/mmwr/volumes/69/wr/mm6925e1.htm [Internet] [cited 2020 Oct 9] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22:369. doi: 10.1136/bmj.m1966. https://www.bmj.com/content/369/bmj.m1966 [Internet] [cited 2020 Oct 9] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H., et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11) doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Ambrosi J., Silva C., Galofré J., Escalada F., Santos S., Millán D., et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes. 2011 May 17;36:286–294. doi: 10.1038/ijo.2011.100. [DOI] [PubMed] [Google Scholar]
- 31.Özçelik Korkmaz M., Eğilmez O.K., Özçelik M.A., Güven M. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur Arch Oto-Rhino-Laryngol. 2020 Oct 3 doi: 10.1007/s00405-020-06396-8. [Internet] [cited 2020 Oct 9]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson S.J., Tsai A.A., Scala C.M., Sowa D.C., Sheean P.M., Braunschweig C.L. Adequacy of oral intake in critically ill patients 1 week after extubation. J Am Diet Assoc. 2010 Mar;110(3):427–433. doi: 10.1016/j.jada.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Skoretz S.A., Flowers H.L., Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010 Mar;137(3):665–673. doi: 10.1378/chest.09-1823. [DOI] [PubMed] [Google Scholar]
- 34.Eder P., Łodyga M., Dobrowolska A., Rydzewska G., Kamhieh-Milz J. Addressing multiple gastroenterological aspects of coronavirus disease 2019. Pol Arch Intern Med. 2020 29;130(5):420–430. doi: 10.20452/pamw.15332. [DOI] [PubMed] [Google Scholar]
- 35.Macht M., Wimbish T., Clark B.J., Benson A.B., Burnham E.L., Williams A., et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care Lond Engl. 2011;15(5) doi: 10.1186/cc10472. [DOI] [PMC free article] [PubMed] [Google Scholar]