Abstract
Background
Nurses who work in hospitals experience a high level of burnout and the relationship between immune variables and burnout syndrome has yet to be elucidated. The aim of the present study was to investigate the effects of job burnout on immune function in female oncology nurses in a tertiary oncology hospital in Guangxi, China. The aspects of the human immune system evaluated were humoral and cellular immunity and complement components 3 (C3) and 4 (C4).
Material/Methods
We administered the Maslach Burnout Inventory-General Survey (MBI-GS), which includes scales for emotional exhaustion, depersonalization (DP), and personal accomplishment (PA), to measure variables related to immune function in 105 female nurses in a tertiary oncology hospital in Guangxi, China. Levels of humoral immunity and C3 and C4 were detected with immune turbidimetry. Cellular immunity was assessed with indirect immunofluorescence.
Results
A Spearman rank correlation analysis revealed that levels of C3, C4, and CD4- and CD8-positive T cells were significantly associated with burnout symptoms (P<0.05, P<0.01, and P<0.05, respectively). Furthermore, there was a correlation between demographic data and humoral and cellular immunity (both P<0.05). Multivariable linear regression analysis showed that C4 levels were closely related to DP (P<0.05) and that CD4 and CD8 levels were closely related to PA (P<0.01).
Conclusions
These results suggest that DP and PA have an impact on immune function, and that timely psychological and behavioral interventions can be used to reduce the degree of job burnout among nurses and regulate their immunity, thus enabling them to better serve patients.
Keywords: Burnout, Professional; Immunity; Nurses; Oncology Service, Hospital
Background
Maslach and Jackson described burnout as a combination of emotional exhaustion (EE), depersonalization (DP), and poor personal accomplishment (PA) [1]. Job burnout among nurses is an individual response to emotion and stress related to the inability of nurses to deal effectively with long-term occupational demands. As the largest developing country, China is short of medical resources, with hospital nurses on 8-hour shifts and medical staff working intensively. Studies have shown that a shift system [2] and high-intensity work adversely affect the proportion and activity of lymphocytes in health care staff [3]. A meta-analysis of psychological stress and the immune system over the past 30 years also showed that psychological stress had a clear effect on the human immune system [4].
Applying the Job Demands-Resource Model to nurses around the world shows that they have a high incidence of job burnout because of the interaction between high demand for individuals in their profession and limited resources [5,6]. The incidence of burnout is even higher in oncology nurses. A German study showed that the degree of job burnout was more obvious in nurses in the COVID-19 ward than in those in the general ward [7]. A Chinese study, however, showed that nurses in the Oncology Department had a higher level of job burnout than in the COVID-19 ward, even during the epidemic [8]. Książek et al studied job burnout in nurses in Oncology and General Surgery departments in Poland. The results showed significantly more intense job burnout in the nurses in the Oncology Department than in the General Surgery Department [9]. Therefore, special attention should be paid to job burnout in and health and well-being of medical staff in the Oncology Department [10]. Khamisa et al showed that burnout symptoms are related to chronic psychological stress [11], which can affect the mental and physical health of nurses [12]. Given these data, an exploration of the state of burnout and its association with immune variables among oncology nurses would be very valuable.
Humoral and cellular immunity are specific types of immunity in the third line of defense for the human body, and the complement system is considered a non-specific humoral immune mechanism against pathogens. Complement components 3 (C3) and 4 (C4), the 2 most important subtypes of the complement system [13], play important roles in human immune defenses and can exert a variety of biological effects. In recent years, an increasing number of studies have focused on the effect of chronic psychological stress on immune function [14,15]. Although altered immune function is known to be associated with burnout [16,17], the specific roles of humoral and cellular immunity and complement function in nurses with job burnout tendencies have yet to be clarified. Therefore, the present study aimed to investigate the effects of job burnout on immune function in female oncology nurses in a tertiary oncology hospital in Guangxi, China.
Material and Methods
Ethics Approval
The researchers met with the participants to explain the purpose and importance of the study and to answer their questions. The participants then anonymously volunteered for the study and signed the informed consent. An application to perform the study was submitted to the Guangxi Medical University Affiliated Cancer Hospital and approved by a committee (grant no. LW2020048).
Participants
For the present prospective, cross-sectional study, 105 female nurses were recruited from the Guangxi Medical University Affiliated Cancer Hospital. The participants had not worked a night shift in the past 3 days, had no symptoms of cold or fever, and had no history of disease known to affect the immune system. None of the nurses dropped out during the course of the study.
Questionnaire
After the participants were given detailed information about confidentiality and provided informed consent, the researchers explained the meaning of each item on the Maslach Burnout Inventory-General Survey (MBI-GS) to them. When the participants clearly understood the purpose and significance of the study, they completed the self-reported MBI-GS. All 105 questionnaires distributed were returned and eligible for analysis, for a completion rate of 100%.
As described by Kalimo et al [18], higher EE and DP scores indicate stronger tendencies toward these symptoms. Lower PA scores indicate less satisfaction and greater burnout. According to the MBI-GS manual, DP and PA are divided into 3 categories, based on individual scores for each dimension: mild burnout (0–1.49), moderate burnout (1.50–3.49), and severe burnout (3.50–6). The coefficient of reliability for this questionnaire is 0.841.
Blood Collection Procedure
Participants were instructed not to eat or drink anything other than water on the morning that their blood samples were drawn. They also were told not to engage in any strenuous exercise 2 h before or to smoke 30 min before the sampling. Blood was collected between 7 a.m. and 9 a.m. in 10-mL heparinized vacuum tubes by 4 trained researchers at an experimental laboratory.
Humoral Immunity and C3 and C4
Levels of humoral immunity and C3 and C4 were assessed using a specific detection kit (MG05, KANGBAOLAI, China) and with immune turbidimetry [19] (ADVIA 2400, Siemens, Germany). The samples were centrifuged continuously for 10 min at a rate of 3000 revolutions per min to ensure that they were not hemolytic and did not contain fat. During computerized testing, a fixed amount of quality-control serum was added to maintain quality control and the testing was completed strictly according to a predefined protocol. The immunoglobulin in the serum reacted with the anti-human immunoglobulin antiserum to form an antigen-antibody complex and produce turbidity. Its absorbance was detected at a specific wavelength and the change was proportional to the immunoglobulin in the serum. The normal reference ranges for immunoglobulin (Ig) G, IgM, IgA, C3, and C4 are 7.0 to 16 g, 35 to 250 mg, 70 to 400 mg, 0.79 to 1.52 g, and 0.16 to 0.38 g, respectively.
Cellular Immunity
Cellular immunity was assessed using ABC hemolysin and a mouse anti-human monoclonal antibody specific to CD45/CD4/CD8/CD3 and CD45/CD (16–56)/CD19/CD3 (no. 6607013, Beckman, America) and with flow cytometry [20] (Navios EX, Beckman, America). Early in the morning, 2 mL of elbow venous blood was collected from the participants on an empty stomach and placed in an anticoagulation tube containing disodium ethylenediaminetetraacetate (EDTA-Na2). One hundred microliters of the whole blood were mixed with fluorescein-labeled mouse anti-human monoclonal antibody and 20 μL of homotype control IgG. The reaction was kept away from light at room temperature for 30 minutes and computerized detection was completed within 1 h after hemolysis. The lymphocyte group then was identified and 10 000 cells were captured from it. Lymphocyte surface markers were analyzed for 2 parameters and the percentages of cells positive for CD3 alone, CD3 and CD4, CD3 and CD8, CD3 and CD19, and of natural killer (NK) cells were calculated. At the end of the study, participants received a letter with their results and a summary report. A meeting was organized to communicate the overall results to them and to further explain the findings at an individual level.
Statistical Analysis
Data analysis was performed using SPSS software, version 25.0. The normality of data distribution was tested with a Kolmogorov-Smirnov test; if the distribution was abnormal, the data were transformed logarithmically. Spearman rank correlation and multivariable linear regression analyses were used to analyze the relationship between demographic characteristics, immune variables, and job burnout. Because the data regarding EE, DP, PA, C4, and levels of CD4 and CD8 positivity showed abnormal distributions before and after logarithmic transformation, nonparametric tests were carried out to examine the differences in immune variables among meaningful burnout symptom groups in the regression equation. The significance level for all tests was set at P<0.05.
Results
Table 1 shows the demographic data for the nurses. The scores for the EE, DP, and PA scales were analyzed. The median scores were as follows: EE, 2.59±1.03; DP, 2.04±0.94; and PA, 3.28±0.77. The MBI-GS scores are shown in Table 2.
Table 1.
Demographic data for 105 female oncology nurses in a tertiary oncology center in Guangxi, China.
| Demographic data | Number (n) | Constituent ratio (%) |
|---|---|---|
| Age group | ||
| ≤30 years old | 57 | 54.3 |
| >30 years old | 48 | 45.7 |
| Marital status | ||
| Married | 60 | 57.1 |
| Unmarried | 45 | 42.9 |
| Fixed department | ||
| Yes | 82 | 78.1 |
| No | 23 | 21.9 |
| Length of service | ||
| ≤10 years | 73 | 69.5 |
| >10 years | 32 | 30.5 |
| Head nurse or above | ||
| Yes | 11 | 10.5 |
| No | 94 | 89.5 |
| Number of responsible patients per day | ||
| ≤10 patients | 43 | 41.0 |
| >10 patients | 62 | 59.0 |
Table 2.
Maslach Burnout Inventory-General Survey results for 105 female oncology nurses at a tertiary oncology center in Guangxi, China.
| Burnout index | Score | Number (n) | Constituent ratio (%) |
|---|---|---|---|
| EE | 0–1.49 | 10 | 9.5 |
| 1.5–3.49 | 82 | 78.1 | |
| 3.5–6 | 13 | 12.4 | |
| DP | 0–1.49 | 25 | 23.8 |
| 1.5–3.49 | 73 | 69.5 | |
| 3.5–6 | 7 | 6.7 | |
| PA | 0–1.49 | 2 | 1.9 |
| 1.5–3.49 | 55 | 52.4 | |
| 3.5–6 | 48 | 45.7 |
Maslach Burnout Inventory-General Survey (MBI-GS).
The scores of each dimension: (1) mild burnout (scores 0–1.49); (2) moderate burnout (scores 1.50–3.49); (3) severe burnout (scores 3.50–6).
Correlation of Demographic Data and Burnout Index with Immune Variables
Table 3 shows that DP scores were positively correlated with C3 and C4 (P<0.05 and P<0.01, respectively) and that PA scores were negatively correlated with CD4 and CD8 positivity (P<0.05). The demographic data were incorporated into a multivariable linear regression model for correction. The results of the correlations between DP and C4 and between PA and the CD4- and CD8-positive cells then were entered into the regression equation (Table 4). In the nurses whose DP symptoms were more serious, C4 levels increased (P<0.05). In contrast, the more serious the PA symptoms were, the lower the ratio of CD4- and CD8-positive cells and the greater the adverse effect on cellular immune function in the nurses (P<0.01).
Table 3.
Correlation between immune variables and demographic data and burnout index.
| Variables | Humoral immunity (g/L) | Complement (g/L) | Cellular immunity (/mm3) of [% to lymphocytes] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgA | C3 | C4 | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | NK | CD19+ | |
| EE | −0.022 | −0.071 | 0.030 | −0.014 | 0.071 | −0.083 | −0.001 | −0.031 | 0.046 | 0.122 | 0.051 |
| DP | −0.003 | −0.098 | 0.001 | 0.206a | 0.345b | −0.083 | −0.033 | −0.047 | 0.048 | 0.102 | −0.101 |
| PA | 0.063 | 0.027 | −0.089 | −0.097 | −0.138 | 0.016 | −0.147 | 0.182 | −0.242a | −0.071 | 0.042 |
| Age group | 0.032 | 0.098 | 0.243a | 0.274b | 0.133 | −0.084 | 0.032 | −0.034 | 0.057 | 0.005 | −0.172 |
| Marital status | −0.178 | 0.004 | −0.192a | −0.265b | −0.196 | 0.189 | −0.080 | 0.165 | −0.178 | 0.009 | 0.022 |
| Fixed department | 0.073 | 0.015 | −0.203a | −0.318b | −0.335b | 0.158 | −0.014 | 0.138 | −0.107 | −0.035 | 0.038 |
| Length of service | −0.086 | −0.010 | 0.158 | 0.186 | 0.049 | −0.197a | −0.044 | −0.066 | −0.021 | −0.037 | −0.094 |
| Head nurse or above | 0.198a | −0.081 | −0.225a | −0.134 | −0.178 | 0.029 | 0.017 | −0.129 | 0.100 | 0.108 | 0.122 |
| Number of responsible patients per day | 0.095 | −0.100 | 0.158 | 0.234a | −0.022 | 0.003 | 0.094 | −0.055 | 0.092 | −0.058 | 0.198a |
| M±S | 14.725 ±2.357 | 1.501 ±0.592 | 2.423 ±0.780 | 1.011 ±0.162 | 0.205 ±0.065 | 68.442 ±6.879 | 36.008 ±0.832 | 26.077 ±0.558 | 1.450 ±0.414 | 12.043 ±5.603 | 11.050 ±3.630 |
Emotional exhaustion (EE), depersonalization (DP), personal accomplishment (PA),complement component 4 (C4),complement component 3 (C3).
Statistical significance of the Spearman correlation coefficient:
p<0.05,
p<0.001.
Table 4.
Multivariable linear regression analysis of the burnout index for immune variables.
| Criterion variables | Entered variables | b | β | t | P | F | R2 |
|---|---|---|---|---|---|---|---|
| C4 (g/L) | DP (score) | 0.027 | 0.216 | 2.223 | 0.028 | 7.745 | 0.132 |
| CD4+/CD8+ [% to lymphocytes] | PA (score) | −0.198 | −0.256 | −2.693 | 0.008 | 7.252 | 0.066 |
Depersonalization (DP), personal accomplishment (PA),complement component 4 (C4).
Statistical significance of the multiple linear regression analysis coefficients: p<0.05.
Kruskal-Wallis Test of Burnout Index for C4 and the CD4/CD8 Ratio
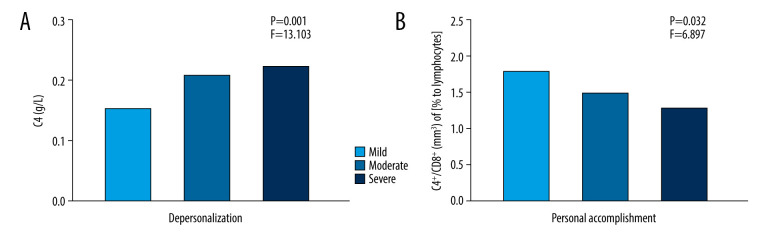
Figure 1 shows the differences in C4 levels among the 3 DP groups and the differences in the levels of CD4 and CD8 positivity among the 3 PA groups (P<0.01). The C4 levels were significantly higher in the severe DP group than in the mild-to-moderate DP group and the levels of CD4 and CD8 positivity were higher in the mild PA group than in the moderate and severe PA groups (P<0.05).
Figure 1.
Differences in complement component 4 (C4) levels among the 3 depersonalization (DP) groups and in levels of CD4 and CD8 positivity among the 3 personal accomplishment (PA) groups. * Serum measurements of C4 and feelings of DP and the CD4/CD8 lymphocyte ratio and feelings of PA, assessed by the Maslach Burnout Inventory-General Survey. (A) C4 levels were significantly increased in the severe DP group compared with the mild and moderate DP groups. (B) The ratio of CD4- to CD8-positive cells was significantly lower in the severe PA group compared with the mild and moderate PA groups.
Discussion
Our study revealed that levels of C3 and C4, and of CD4 and CD8 positivity were significantly associated with burnout symptoms. Furthermore, there was a correlation between demographic data and humoral and cellular immunity. Multivariable linear regression analysis showed that C4 levels were closely related to DP and that levels of CD4 and CD8 positivity were closely related to PA.
The Agency for Healthcare Research and Quality estimated that burnout affects 10% to 70% of nurses and 30% to 50% of physicians, nurse practitioners, and physician assistants [21]. Nurses comprise one of the largest groups of healthcare workers and have a high incidence of job burnout in the face of high-intensity, stressful work. Comparative studies have shown that the rate of job burnout among nurses in an Oncology Department is higher than that among nurses in a General Surgery Department [8,22]. Job burnout is psychological stress that can affect immune function [23]. Exploring part of the immune spectrum among nurses experiencing burnout could reveal information about key physiological changes and provide guidance about how to prevent job burnout.
The Spearman rank correlation analysis performed in the present study showed that C3 and C4 levels were significantly positively correlated with DP but not with EE and PA. That was consistent with the finding on the nonparametric test that C4 levels were higher among people with a greater tendency toward DP. Job burnout, as a kind of chronic stress, reportedly can promote an increase in inflammatory factors in the body [24]. Complement is a key factor in innate and acquired immunity and plays an important role in induction and contraction of T cells and in the inflammatory response [25,26]. When an inflammatory response occurs, complement-adaptive activation plays a dual role in the inflammatory response and protection from it [27]. Therefore, our results suggest that high levels of DP can cause an inflammatory response, thus stimulating an adaptive increase in C4 levels. Conversely, according to the job burnout theory proposed by Jackson et al, EE is similar to the traditional stress variable, whereas DP seems to be a kind of stress response related to working conditions that require a high level of emotional reserves [28]. In a study of doctors and nurses in the Oncology Department, the frequency of job burnout was very high [29]. Comparative studies have shown that nurses, particularly those working in oncology, have a higher risk of burnout than other medical staff [22]. Nurses working in hospitals that specialize in oncology often are faced with patients who have cancer and are in pain and for whom effective treatment is lacking. Thus, these nurses must be able to resist pressure and have greater emotional reserves than their counterparts in other departments, which results in the production of DP. Moreover, the correlation analysis showed that having the rank of Head Nurse or above was positively correlated with the proportion of NK cells, which is consistent with the results from Nagai et al [2]. As a result of that shift in the cells, the subjective experience of fatigue is more intense and their activity is decreased, which seems to reflect the degree of fatigue felt by the medical staff. A report by Okamoto et al [3] also supported this view; however, in China, Head Nurses and nurses above that rank do not work in shifts, their circadian rhythms are normal, and their degree of fatigue is lower than that of other nurses.
In the present study, when PA increased, levels of CD4- and CD8-positivity decreased significantly. A Spearman rank correlation analysis also showed a significant negative correlation between levels of CD4- and CD8-positive cells and PA, which suggested that the more significant PA is, the lower the ratio of CD4- and CD8-positive cells that can influence cellular immunity. Many studies have shown that psychosocial stress (eg, job burnout) is related to immune system disorders that affect immune factors and immune cells, such as a decrease in NK cell activity, an increase in the ratio of CD4- and CD8-positive cells, and inflammatory markers [30]. The nurses in the present study had symptoms of severe job burnout, especially related to PA: 98.1% of respondents reported at least moderate levels of PA. Compared with nurses in other general departments, nurses in oncology departments serve patients with cancer, for whom the effects of treatment and outcomes are not as good as those seen with other disease and conditions; that clinical working environment is an important factor affecting the degree of job burnout [31]. Therefore, nurses have lower awareness of PA and greater psychological pressure regarding the imbalance of efforts. A previous meta-analysis found that an effort/reward imbalance in the work environment is associated with lower immunity [32]. Studies of nurses also have shown that an effort/reward imbalance at work is a strong risk factor for job burnout [33], thus increasing the severity of EE and DP and reducing PA [34]. Other findings from experimental and semi-experimental studies have shown that this effort/reward imbalance is strongly correlated with markers of reduced immune ability [35] and a significant decrease in the ratio of CD4- and CD8-positive cells [36].
Our results indicated that C4 levels increased when nurses in the Oncology Department reported severe DP, whereas levels of CD4- and CD8-positive cells increased in nurses with low PA levels, suggesting that DP and PA are not only psychosomatic diseases but also occupational health problems that are related to some indices that reflect immune function. Nurses with job burnout have DP symptoms, which reduce job effort and adversely affect the care of patients. A literature search showed that improving the working environment, reducing the occurrence of violence in it [37], and enhancing social support can help relieve work-related stress for nurses [38]. Previous studies have shown that psychological [39] and behavioral interventions can enhance brain adaptation to immune function, thus enhancing immunity [40]. This provides a management basis for hospital nursing managers, suggesting that in the early stage of burnout, appropriate psychological motivation and behavioral interventions can be implemented to reduce job burnout, improve levels of PA among nurses, and enhance their immunity, thus enabling them to better serve patients. Therefore, the impact of job burnout and immune function needs to be further studied.
While the present study exploring the relationship between job burnout and immune function in Chinese oncology nurses was innovative, it had some limitations. First, the participants were nurses who met the requirements for enrollment and volunteered to participate in the study and the sample size was not predetermined. Second, the study sample was small and no data from male nurses were analyzed; their job burnout status and biochemical markers need to be studied. Third, the sample was recruited from a single oncology hospital in China and the results should be interpreted cautiously. Finally, the present study measured job burnout and immune function at a single point in time; therefore, it is not possible to speculate about the potential mechanism for the causal relationship between the parameters that were evaluated. Longitudinal research should be carried out to obtain a deeper understanding of this relationship.
Conclusions
In summary, DP and PA have an impact on immune function in nurses in an Oncology Department and timely psychological and behavioral interventions can be used to reduce the degree of job burnout among them and to regulate their immunity, thus enabling them to better serve patients.
Footnotes
Conflict of Interest
None.
Source of support: This work was supported by Guangxi University of Science and Technology (grant no. GuikeAB18126042)
References
- 1.Maslach C, Schaufeli WB, Leiter MP. Job burnout. Annu Rev Psychol. 2001;52:397–422. doi: 10.1146/annurev.psych.52.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Nagai M, Morikawa Y, Kitaoka K, et al. Effects of fatigue on immune function in nurses performing shift work. J Occup Health. 2011;53(5):312–19. doi: 10.1539/joh.10-0072-oa. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto H, Tsunoda T, Teruya K, et al. An occupational health study of emergency physicians in Japan: Health assessment by immune variables (CD4, CD8, CD56, and NK cell activity) at the beginning of work. J Occup Health. 2008;50(2):136–46. doi: 10.1539/joh.l6084. [DOI] [PubMed] [Google Scholar]
- 4.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Sabei SD, Labrague LJ, Miner Ross A, et al. Nursing work environment, turnover intention, job burnout, and quality of care: The moderating role of job satisfaction. J Nurs Scholarsh. 2020;52(1):95–104. doi: 10.1111/jnu.12528. [DOI] [PubMed] [Google Scholar]
- 6.Cañadas-De la Fuente GA, Vargas C, San Luis C, et al. Risk factors and prevalence of burnout syndrome in the nursing profession. Int J Nurs Stud. 2015;52(1):240–49. doi: 10.1016/j.ijnurstu.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Zerbini G, Ebigbo A, Reicherts P, et al. Psychosocial burden of healthcare professionals in times of COVID-19 – a survey conducted at the University Hospital Augsburg. Ger Med Sci. 2020;18:Doc05. doi: 10.3205/000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Wang J, Luo C, et al. A comparison of burnout frequency among oncology physicians and nurses working on the frontline and usual wards during the COVID-19 epidemic in Wuhan, China. J Pain Symptom Manage. 2020;60(1):e60–65. doi: 10.1016/j.jpainsymman.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Książek I, Stefaniak TJ, Stadnyk M, et al. Burnout syndrome in surgical oncology and general surgery nurses: A cross-sectional study. Eur J Oncol Nurs. 2011;15(4):347–50. doi: 10.1016/j.ejon.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Caruso A, Vigna C, Bigazzi V, et al. Burnout among physicians and nurses working in oncology. Med Lav. 2012;103(2):96–105. [PubMed] [Google Scholar]
- 11.Khamisa N, Peltzer K, Ilic D, et al. Work related stress, burnout, job satisfaction and general health of nurses: A follow-up study. Int J Nurs Pract. 2016;22(6):538–45. doi: 10.1111/ijn.12455. [DOI] [PubMed] [Google Scholar]
- 12.Khamisa N, Peltzer K, Oldenburg B. Burnout in relation to specific contributing factors and health outcomes among nurses: A systematic review. Int J Environ Res Public Health. 2013;10(6):2214–40. doi: 10.3390/ijerph10062214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reis ES, Mastellos DC, Hajishengallis G, et al. New insights into the immune functions of complement. Nat Rev Immunol. 2019;19(8):503–16. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnevali L, Montano N, Statello R, et al. Rodent models of depression-cardiovascular comorbidity: Bridging the known to the new. Neurosci Biobehav Rev. 2017;76(Pt A):144–53. doi: 10.1016/j.neubiorev.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Sharif K, Watad A, Coplan L, et al. The role of stress in the mosaic of autoimmunity: An overlooked association. Autoimmun Rev. 2018;17(10):967–83. doi: 10.1016/j.autrev.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Mommersteeg PM, Heijnen CJ, Kavelaars A, et al. Immune and endocrine function in burnout syndrome. Psychosom Med. 2006;68(6):879–86. doi: 10.1097/01.psy.0000239247.47581.0c. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura H, Nagase H, Yoshida M, et al. Natural killer (NK) cell activity and NK cell subsets in workers with a tendency of burnout. J Psychosom Res. 1999;46(6):569–78. doi: 10.1016/s0022-3999(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 18.Kalimo R, Pahkin K, Mutanen P, et al. Staying well or burning out at work: Work characteristics and personal resources as long-term predictors. Work Stress. 2003;17(2):109–22. [Google Scholar]
- 19.Wouters D, Wiessenberg HD, Hart M, et al. Complexes between C1q and C3 or C4: novel and specific markers for classical complement pathway activation. J Immunol Methods. 2005;298(1–2):35–45. doi: 10.1016/j.jim.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Cossarizza A, Chang HD, Radbruch A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur J Immunol. 2019;49(10):1457–973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridgeman PJ, Bridgeman MB, Barone J. Burnout syndrome among healthcare professionals. Am J Health Syst Pharm. 2018;75(3):147–52. doi: 10.2146/ajhp170460. [DOI] [PubMed] [Google Scholar]
- 22.Bernardi M, Catania G, Marceca F. [The world of nursing burnout. A literature review]. Prof Inferm. 2005;58(2):75–79. [in Italian] [PubMed] [Google Scholar]
- 23.Jonsdottir IH, Sjörs Dahlman A. Mechanisms in endocrinology: Endocrine and immunological aspects of burnout: A narrative review. Eur J Endocrinol. 2019;180(3):R147–58. doi: 10.1530/EJE-18-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammadah M, Sullivan S, Pearce B, et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun. 2018;68:90–97. doi: 10.1016/j.bbi.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeley S, Kemper C, Le Friec G. The “ins and outs” of complement-driven immune responses. Immunol Rev. 2016;274(1):16–32. doi: 10.1111/imr.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liszewski MK, Elvington M, Kulkarni HS, et al. Complement’s hidden arsenal: New insights and novel functions inside the cell. Mol Immunol. 2017;84:2–9. doi: 10.1016/j.molimm.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Gil EM, Cadenas-Sanchez C, Santabárbara J, et al. Inflammation in metabolically healthy and metabolically abnormal adolescents: The HELENA study. Nutr Metab Cardiovasc Dis. 2018;28(1):77–83. doi: 10.1016/j.numecd.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Jackson SE, Schwab RL, Schuler RS. Toward an understanding of the burnout phenomenon. J Appl Psychol. 1986;71(4):630–40. [PubMed] [Google Scholar]
- 29.Colombat P, Lejeune J, Altmeyer A, et al. [A better management for a better care]. Bull Cancer. 2019;106(1):55–63. doi: 10.1016/j.bulcan.2018.10.012. [in French] [DOI] [PubMed] [Google Scholar]
- 30.Bargellini A, Barbieri A, Rovesti S, et al. Relation between immune variables and burnout in a sample of physicians. Occup Environ Med. 2000;57(7):453–57. doi: 10.1136/oem.57.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostacoli L, Cavallo M, Zuffranieri M, et al. Comparison of experienced burnout symptoms in specialist oncology nurses working in hospital oncology units or in hospices. Palliat Support Care. 2010;8(4):427–32. doi: 10.1017/S1478951510000295. [DOI] [PubMed] [Google Scholar]
- 32.Eddy P, Heckenberg R, Wertheim EH, et al. A systematic review and meta-analysis of the effort-reward imbalance model of workplace stress with indicators of immune function. J Psychosom Res. 2016;91:1–8. doi: 10.1016/j.jpsychores.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Schulz M, Damkröger A, Heins C, et al. Effort-reward imbalance and burnout among German nurses in medical compared with psychiatric hospital settings. J Psychiatr Ment Health Nurs. 2009;16(3):225–33. doi: 10.1111/j.1365-2850.2008.01355.x. [DOI] [PubMed] [Google Scholar]
- 34.Padilla Fortunatti C, Palmeiro-Silva YK. Effort-reward imbalance and burnout among ICU nursing staff: A cross-sectional study. Nurs Res. 2017;66(5):410–16. doi: 10.1097/NNR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 35.Siegrist J. [Effort-reward imbalance at work and depression: Current research evidence]. Nervenarzt. 2013;84(1):33–37. doi: 10.1007/s00115-012-3667-6. [in German] [DOI] [PubMed] [Google Scholar]
- 36.Nakata A. Psychosocial job stress and immunity: A systematic review. Methods Mol Biol. 2012;934:39–75. doi: 10.1007/978-1-62703-071-7_3. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Wang J, Liu J, et al. The impact of work environment on workplace violence, burnout and work attitudes for hospital nurses: A structural equation modelling analysis. J Nurs Manag. 2020;28(3):495–503. doi: 10.1111/jonm.12947. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Y, Fan G, Feng C, et al. [Effect of occupational stressors on coping resources of nurses in Nanchang]. Wei Sheng Yan Jiu. 2010;39(3):339–41. [in Chinese] [PubMed] [Google Scholar]
- 39.Black DS, Slavich GM. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann NY Acad Sci. 2016;1373(1):13–24. doi: 10.1111/nyas.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Lei B, Yuan Y, et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature. 2020;581(7807):204–8. doi: 10.1038/s41586-020-2235-7. [DOI] [PubMed] [Google Scholar]