Abstract
Background:
Diabetes mellitus (DM) is an endocrine disorder characterized by hyperglycemia, polyuria, polydipsia, and glucosuria. γ-aminobutyric acid (GABA) is an inhibitory neurotransmitter in the central nervous system (CNS) of humans and other mammals. GABA acts on two different receptors, which are GABA-A and GABA-B. Pancreatic β-cells synthesize GABA from glutamic acid by glutamic acid decarboxylase (GAD).
Aim:
The objective of this study was to explore the potential role of pancreatic GABA on glycemic indices in DM.
Methods:
Evidence from experimental, preclinical, and clinical studies are evaluated for bidirectional relationships between pancreatic GABA and blood glucose disorders. A multiplicity of search strategies took on and assumed included electronic database searches of Medline and Pubmed using MeSH terms, keywords and title words during the search.
Results:
The pancreatic GABA signaling system has a role in the regulation of pancreatic hormone secretions, inhibition of immune response, improve β-cells survival, and change α cell into β-cell. Moreover, a GABA agonist improves the antidiabetic effects of metformin. In addition, benzodiazepine receptor agonists improve pancreatic β-cell functions through GABA dependent pathway or through modulation of pancreatic adenosine and glucagon-like peptide (GLP-1).
Conclusions:
Pancreatic GABA improves islet cell function, glucose homeostasis, and autoimmunity in DM. Orally administered GABA is safe for humans, and acts on peripheral GABA receptors and represents a new therapeutic modality for both T1DM and T2DM. Besides, GABA-A receptor agonist like benzodiazepines improves pancreatic β-cell function and insulin sensitivity through activation of GABA-A receptors.
Keywords: Benzodiazepines, diabetes mellitus, gamma-aminobutyric acid
Introduction
Diabetes mellitus (DM) is an endocrine disorder characterized by hyperglycemia, polyuria, polydipsia, and glucosuria. By the year 2040, there will be about 640 million living people affected. In type 1 DM (T1DM), 70% of pancreatic β-cells are destroyed while in type 2 DM (T2DM) 40–60% of pancreatic β-cells are destroyed at the time of diagnosis.[1]
The remaining pancreatic β-cells underwent a burden to increase insulin levels to overcome the deficiency status, which progressively leads to induction of exhaustion and acceleration of β-cell apoptosis. Therefore, residual pancreatic β-cell is a novel pathway in the treatment of DM.[2]
γ-aminobutyric acid (GABA) is an inhibitory neurotransmitter in the central nervous system (CNS) of humans and other mammals, Figure 1. It synthesized by glutamate decarboxylase (GAD) from glutamate, which stored in the presynaptic vesicles. The release of GABA into the synaptic cleft is occurring through the activation of presynaptic voltage-gated calcium channels leading to hyperpolarization and inhibition of postsynaptic potential. The effect of GABA is terminated and ceased by plasma membrane cotransporters at glial cells and neuronal presynaptic terminal. GABA acts on two different receptors, which are GABA-A and GABA-B.[3]
Figure 1.
Chemical structure of GABA
Elements of the different neurotransmitter signaling machinery are found within human pancreatic islets and one of them is the GABA signaling system. This system has been shown to modulate exocytosis, insulin and glucagon secretion, and regulate β cell replication. In addition, the GABA receptors in β-cells in intact human pancreatic islets and their functional properties have been recently characterized in detail.[4] Therefore, the objective of this study was to explore the potential role of pancreatic GABA on glycemic indices in DM.
Method and Search Strategy
In general, the endeavor of this study article was to present a narrative review regarding the potential effect of pancreatic GABA on pancreatic β-cell functions, glucose homeostasis, insulin release, and risk of DM. Evidence from experimental, preclinical, and clinical studies are evaluated for bidirectional relationships between pancreatic GABA and blood glucose disorders are discussed and opportunities for elaborating these models briefly alluded. Given the nature of the subject area, it remains clear that this literature search cannot be regarded as a systemic review.
A multiplicity of search strategies took on and assumed which included electronic database searches of Medline and Pubmed using MeSH terms, keywords and title words during the search. The terms used for these searches were as follows: [γ-aminobutyric acid OR GABA] AND [cognitive function OR vigilance OR depression OR schizophrenia OR addiction OR diabetes mellitus OR blood glucose OR hyperglycemia]. [GABA OR GABA antagonists OR GABA agonists] AND [sleep disorders OR diabetes mellitus OR hyperglycemia OR hypoglycemia OR insulin resistance]. Reference lists of identified and notorious articles were reviewed. In addition, only English articles were considered and case reports were not concerned in the review. The key features of recognized applicable search studies were considered and the conclusions summarized in a narrative review.
Pancreatic GABA
Regardless of CNS distribution, GABA is also distributed in different tissues, including; adrenal medulla, uterus, placenta, and ovaries with a high comparable concentration in the pancreatic β-cells. As well, GABA together with GAD and GABA transporters are highly expressed in different islets of Langerhans suggesting the potential role of GABA in pancreatic biology.[5]
Pancreatic β-cells synthesize GABA from glutamic acid by GAD and store it in the synaptic like microvesicles with insulin granules till the time of secretion. The potential role of GABA in pancreatic islet is regulation of hormone secretions, inhibition of immune response, improve β-cells survival, and change α cell into β-cell. Notably, auto-antibody against GAD is linked with the development of T1DM in which β-cell mass is reduced and eventually disappears thus; pancreatic GABAergic neurotransmission will be blunted.[4] Similarly, GABA-A receptors are activated by glucagon-like peptide (GLP-1) and exendin-1 thus; these metabolic hormones may exert their effect on pancreatic β-cell via GABA-A receptors.[6] GLP-1 activates pancreatic GAD leads to elevation of β-cells GABA concentrations which per se increase insulin synthesis and secretions.[7]
In addition, GABA of the enteric nervous system activates the release of GLP-1 from L-cell via activation of GABA-A and GABA-C receptors in myenteric neuronal plexus.[8]
Purwana et al., a study illustrated that pancreatic GABA has a role in the regulation of insulin and glucagon secretions, protection of β-cells, β-cells regeneration, and neogenesis.[9] Moreover, GABA inhibits and attenuates immune activations and inflammation in DM that results in the regulation of glucose homeostasis and the reduction of diabetic complications.[10]
Recently, Liu et al., study confirmed that GABA loaded chitosan-nanoparticles improve glycemic indices in streptozocin-induced DM in mice through the protection of pancreatic β-cells.[11]
The effect of pancreatic GABA is mediated by either GABA-A receptor which increases intracellular Ca+2 via voltage-gated Ca+2 channel activation and release of Ca+2 from endoplasmic reticulum or through GABA-B receptor which activates intracellular Ca+2 only. Therefore, GABA mediated Ca+2 concentrations are totally inhibited by GABA-A receptor antagonist and partially by GABA-B receptor antagonist.[12]
Furthermore, the GABA-B receptor plays a role in regulation of blood glucose since low blood glucose produces no effect on GABA-B receptor, while high blood glucose activates it, leading to inhibition of insulin release.[13]
Different studies illustrated the potential responsibility of the GABA-B receptor in regulation of insulin secretions. GABA-B receptor agonists like baclofen prevent and attenuates the onset of T1DM through activation of pancreatic β-cells proliferation and survival.[14] Whereas GABA-B receptor-deficient mice illustrated a good glucose tolerance, improved glucose-stimulated insulin secretion and high pancreatic β-cells insulin content. Therefore, GABA-B receptor showed inhibitory or excitatory effect on pancreatic β-cells.[15] Recently, Wang et al., discussed extensively and showed that GABA is a fundamental in the regulation of pancreatic β-cells through suppression and stimulation of glucagon and insulin secretions, respectively. Also, GABA has a specific ability in restoration of β-cells following streptozocin-induced β-cells destruction via enhancement of β-cells mitotic activity and through antiapoptotic activity. Therefore, GABA agonists add on antidiabetic pharmacotherapy may be a future option in treating both types of DM.[16]
The signaling mechanisms of GABA action on the pancreatic islet are related to different pathways. In response to increasing glucose levels, insulin released from β-cells activates the insulin receptor present on α-cells. Subsequent activation of Akt leads to phosphorylation of β-subunits of GABA-A receptor which causes rapid translocation of the receptor to the plasma membrane. Although GABA is constantly released, the efficacy of the receptor-mediated membrane hyperpolarization is enhanced, due to increased GABA-A receptor numbers at the cell surface. In turn, membrane hyperpolarization inhibits α-cell exocytosis and glucagon release. Therefore, impaired α-cell GABA-A receptor pathway might be an underlying mechanism for unsuppressed glucagon secretion, despite hyperglycemia, in diabetic subjects.[10,17]
Signaling differs sharply in β-cells, where GABA induces membrane depolarization. In isolated rodent and human islets, GABA was shown to stimulate Akt activation, promoting β-cell proliferation and insulin release. GABA activates GABA-A receptor on β-cells leading to depolarization through the opening of voltage-gated calcium channel (VGCC) which activates PI3k/Akt pathway and insulin secretion.[18] Therefore, GABA exerts differential effect on the different pancreatic islet cells, through GABA-A receptors; it causes α-cell hyperpolarization and β-cell depolarization causing inhibition of glucagon and stimulation of insulin secretions, respectively Figure 2.[19]
Figure 2.
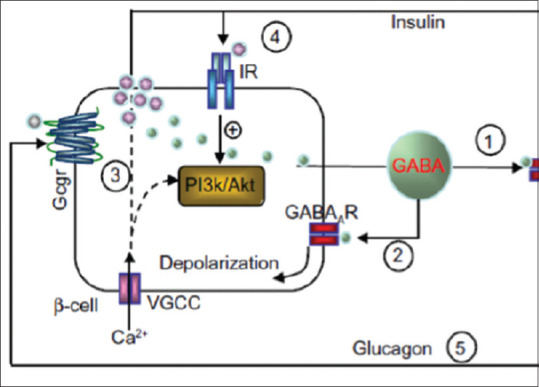
Differential effect of GABA on the pancreatic α and β cells. VGCC: Voltage-gated calcium channel; Gcgr: Glucagon receptor; PI3K: Phosphatidylinositol 3 kinases; Akt: Protein kinase B
Pancreatic GABA and the immune response
Regarding the anti-inflammatory and immune-modulating effect of GABA, activation of the GABA-A receptor leads to noteworthy inhibition of T and B immune cells with inhibition of lymphocyte proliferation and attenuate the progression of T1DM through reduction of T cell activation against antigens of pancreatic β-cells.[20] This immunosuppressive effect of GABA is mainly mediated by GABA-A receptor which upon its activation causes significant inhibition of inflammatory cytokines synthesis and secretion from activated macrophage while GABA-Breceptor activation showed an insignificant effect on immune cells.[21] Moreover, the GABA effect on GABA-A receptors attenuates the toxic effect of immunosuppressive agents and improves the immunotherapy during islet transplantation.[22]
Similarly, GABA through both of its receptors preserves β-cells mass and prevents the progression of DM due to immune-regulatory and anti-inflammatory effects. Indeed, GABA possesses the trophic effect on β-cells through activation of PI3K/Akt pathway.[23]
Certainly, GAD enzyme involved in the synthesis of GABA is an antigenic target for T cells during pathophysiology of TIDM. Destruction of GAD results in reduction of β-cells GABA through induction of inflammatory reactions and β-cell apoptosis. Therefore, vaccination to protect β-cells GAD may be promising future immunotherapy in the prevention of T1DM.[24]
Moreover, the effects of GABA on pancreatic islet cells may be mediated by alpha-Klotho which an antiaging agent expressed in many tissues in humans, including islet β- cells, kidneys, brain, blood vessels, and skin.[25] Klotho consists of a single-pass membrane protein, with a very short intracytoplasmic segment. Circulating Klotho acts as an endocrine hormone that protects β-cells in both T1DM and T2DM models. In T1DM, it possibly acts in part through the blockade of NF-?B activation. Klotho is depleted in the islets of diabetic patients and depressed in the serum of both T1DM and T2DM patients. Klotho-deficient mice exhibit rapid aging, multiorgan disease, and atrophy of the islets of Langerhans.[26] Moreover, GABA therapy increases circulating levels and expression of Klotho in a murine model of T1DM promotes survival and proliferation of pancreatic β-cells. This reveals a mechanism by which GABA protects against diabetes, and has potential clinical applications.[20]
Therefore, GABA therapy enhances pancreatic β-cells Klotho levels which protect against development of T1DM and T2DM through stimulatory and anti-apoptotic effects on the pancreatic β-cells. Similarly, GABA-dependent Klotho effect improves insulin secretion and regeneration of pancreatic β-cells. Thus, therapy with GABA, other GABAergic drugs, or Klotho protein injections, may be beneficial in the prevention or treatment of T1DM and T2DM, as both GABA-A and GABA-B receptor agonists could be effective therapy in the management of DM through augmentation of serum Klotho levels.[27]
Effects of pancreatic GABA/GABA agonist on blood glucose
Different preclinical studies illustrated that the oral administration of GABA improves insulin and C-peptide levels in healthy volunteers. Also, intravenous administration of GABA reduces blood glucose in diabetic patients, but not in normal subjects'. Similarly, baclofen therapy improves postprandial glucose level and insulin response without significant effect on the basal glucose levels.[28,29]
GABA of pancreatic islets is released by exocytosis together with insulin from the dense-core vesicle. Interstitial GABA activates extra-synaptic channels on pancreatic β-cells during insulin secretion. It has been shown that GABA-A channels are down-regulated in patients with T2DM. Phenobarbital improves blood glucose and insulin secretion through activation of pancreatic β-cells extra-synaptic channels.[30] Oda et al. confirmed that phenobarbital leads to the hypoglycemic effect due to suppression of phosphoenolpyruvate carboxykinase enzyme (PEPCK) which is involved in hepatic gluconeogenesis.[31] Besides, activation of pancreatic GABA by phenobarbital leading to crucial down-regulation of insulin receptors through inhibition of protein kinase B in a rat model.[32]
Recently, GABA-B agonist and GABA-A antagonists increase blood glucose by an unknown mechanism but a dose-dependent effect of baclofen failed to illustrate this effect.[29]
GABA-A agonist alprazolam leads to blunting the counter-regulatory response during exercise in patients with T1DM through inhibition of sympathetic activation and neuroendocrine response which eventually inhibit hepatic gluconeogenesis and glucagon secretion. These events contribute to glucose normalization and activation of insulin secretion.[33] Furthermore, diazepam ads on metformin therapy lead to significant anxiolytic, antistress, and antidiabetic effects compared with metformin monotherapy. This combination reduced hepatic insulin resistance and improves insulin sensitivity through reduction of oxidative stress, modulation of stress-induced hypothalamic-pituitary-adrenal axis disorders since; diazepam reduces corticosterone plasma levels in patients with T2DM.[34] Thus, both diazepam and metformin improve mitochondrial integrity in patients with T2DM.[35]
In recent times, Untereiner et al., in their study confirmed that the administration of GABA leads to increase in β-cell mass and reverses the development of T1DM without significant effect on glucose impairment induced by high-fat diet.[36] In addition, Sohrabipour et al., in a study showed that GABA administration reduces insulin resistance via up-regulation of GLUT4 with significant amelioration of blood glucose in high fat and streptozocin-induced diabetes mellitus through reduction of glucagon receptor and inhibition of hepatic gluconeogenesis.[28]
Similarly, a combination of GABA with sitagliptin improves glucose tolerance through the reduction of glucagon, increasing insulin, augmentation of β-cell mass, and inhibition of apoptosis.[37]
In spite of the significant effect of GABA on blood glucose, Nzor et al., the study showed an insignificant effect of bromazepam and diazepam on blood glucose.[38] Furthermore, midazolam attenuates hyperglycemia-induced endothelial injury by inhibition of vascular endothelial growth factor (VEGF) in diabetic mice since; midazolam improves endothelial function indirectly through activation of endothelial GABA which counteracts oxidative stress-induced endothelial injury.[39,40]
Moreover, Li et al. found that artemisinin represses Arx which is a regulatory transcription factor for the conversion of α cell to functional β-like cells. In addition, artemisinin improves GABA-A signaling through up-regulation of gephyrin protein, which is mammalian target of artemisinin. Gephyrin is a key regulator of γ-aminobutyric acid (GABA) synthesis and GABAA receptor trafficking. Thus, artemisinin activates the regeneration of pancreatic β cell mass from α cell though pancreatic GABA-A signaling.[41]
The number of positive allosteric modulators (PAMs) that enhance GABA's actions on neuronal GABAA receptors can increase significantly β-cell replication, which is enhanced by exogenous GABA application. The combination of a PAM and low levels of exogenous GABA further increased human islet cell replication. These findings suggest that PAMs may potentiate the actions of GABA secreted by islet β-cells on GABAA receptors and provide a new class of drugs for diabetes treatment. This may explain a past clinical observation of a GABAA PAM reducing HbA1c levels in diabetic patients.[24]
Thus, activation of GABA-A signaling by different drugs regardless of its class will improve pancreatic β cell mass and functions.
Pleiotropic effects of benzodiazepines
Sympathetic activation is associated with insulin resistance and hyperglycemia therefore, suvorexant which is an orexin receptor antagonist improves blood glucose in patients with T2DM. In addition, distorted sleep in patients with T2DM is linked with high nocturnal catecholamine causing early morning hyperglycemia.[42] Benzodiazepine improves sleep quality with a noteworthy reduction of sympathetic overactivity in patients with T2DM. Thus, benzodiazepine reduces insulin resistance and improves insulin sensitivity independent on benzodiazepine receptor activations.[43]
It has been shown that peripheral benzodiazepine receptor (PBR) which consists of 18kDa protein is not linked to GABA/chloride activation. PBR is found in the contact site between the outer and inner mitochondrial membranes. PBR is highly expressed in pancreatic islet which is involved in the induction of apoptosis through increasing mitochondrial permeability transition pore (MPTP) accordingly; PBR is involved in β-cell death during inflammatory changes. Conveniently, PBR is not mediating the effect of benzodiazepine agonist agents.[44]
Previously, Park et al., study illustrated that peripheral but not central benzodiazepine agonist leads to induction of apoptosis in insulinoma. Therefore, the consequence of GABA activation is differing according to the type of benzodiazepine receptors.[45]
On the other hand, adenosine, which is a key molecule, regulates different aspects of tissue metabolism through activation of G-protein-coupled adenosine receptors. Adenosine improves blood glucose in T1DM and T2DM through regulation of insulin secretion and regulation of β-cell homeostasis. It has been shown that adenosine stimulates pancreatic β-cell proliferation and regeneration with considerable inhibition of inflammatory and immunological-induced β-cell destruction.[46] In general, adenosine activates insulin secretion through the A2A receptor but inhibits insulin secretion via A2B receptor. Also, A2A receptor promotes β- cell proliferation via mTOR pathway. Thus, reduction in the activity of A1 receptor leads to elevation of glucagon and subsequent hyperglycemia.[47] Regardless of GABA activations, Narimatsu et al. confirmed that benzodiazepine acts as an adenosine reuptake inhibitor concerned with anticonvulsant effect of benzodiazepine.[48] Thus, benzodiazepine agonists improve pancreatic β-cell functions through potentiating of adenosine pathway.
Conclusions
The presence of a GABAergic system within the pancreas as a potential target for treating DM becomes more known recently. In α-cells, GABA induces membrane hyperpolarization and inhibits glucagon secretion, and this involves an insulin-mediated GABA-A-trafficking mechanism. In β-cells, GABA induces membrane depolarization and enhances insulin secretion. GABA also has beneficial effects on β-cell survival and regeneration, which results in enlarged β-cell mass. Furthermore, GABA inhibits systemic inflammatory and cytokine production, so GABA therapy in regulating islet cell function, glucose homeostasis, and autoimmunity is promising in the management of DM. Orally administered GABA is safe for humans, and acts on peripheral GABA receptors but does not affect CNS functions, since it does not cross the blood-brain barrier (BBB). Thus it represents a new therapeutic modality for both T1DM and T2DM. Furthermore, GABA-A receptor agonist like benzodiazepines improves pancreatic β-cell function and insulin sensitivity through activation of GABA-A receptors or indirectly through amelioration of diabetes-induced sympathetic over-activity and other pleiotropic effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors express deep thanks for all staff members, Department of Clinical Pharmacology and Medicine.
References
- 1.Al-Kuraishy HM, Al-Gareeb AI. Erectile dysfunction and low sex drive in men with type 2 DM: The potential role of diabetic pharmacotherapy. J Clin Diagn Res. 2016;10:FC21–6. doi: 10.7860/JCDR/2016/19971.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Kuraishy HM, Al-Gareeb AI, Waheed HJ, Al-Maiahy TJ. Differential effect of metformin and/or glyburide on apelin serum levels in patients with type 2 diabetes mellitus: Concepts and clinical practice. J Adv Pharm Technol Res. 2018;9:80–6. doi: 10.4103/japtr.JAPTR_273_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talebi N, Nematbakhsh M, Monajemi R, Mazaheri S, Talebi A, Vafapour M. The protective effect of γ-aminobutyric acid on kidney injury induced by renal ischemia-reperfusion in ovariectomized estradiol-treated rats. Int J Prev Med. 2016;7:6. doi: 10.4103/2008-7802.173796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korol SV, Jin Z, Jin Y, Bhandage AK, Tengholm A, Gandasi NR, et al. Functional characterization of native, high-affinity GABAA receptors in human pancreatic β cells. EBioMedicine. 2018;30:273–82. doi: 10.1016/j.ebiom.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pourmasoumi M, Vosoughi N, Derakhshandeh-Rishehri SM, Assarroudi M, Heidari-Beni M. Association of Omega-3 fatty acid and epileptic seizure in epileptic patients: A systematic review. Int J Prev Med. 2018;9:36. doi: 10.4103/ijpvm.IJPVM_281_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korol SV, Jin Z, Babateen O, Birnir B. GLP-1 and exendin-4 transiently enhance GABAA receptor–mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. Diabetes. 2015;64:79–89. doi: 10.2337/db14-0668. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Mao R, Van De Casteele M, Pipeleers D, Ling Z. Glucagon-like peptide-1 stimulates GABA formation by pancreatic β-cells at the level of glutamate decarboxylase. Am J Physiol Endocrinol Metab. 2007;292:E1201–6. doi: 10.1152/ajpendo.00459.2006. [DOI] [PubMed] [Google Scholar]
- 8.Gameiro A, Reimann F, Habib AM, O'malley D, Williams L, Simpson AK, et al. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J Physiol. 2005;569:761–72. doi: 10.1113/jphysiol.2005.098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purwana I, Zheng J, Li X, Deurloo M, Son DO, Zhang Z, et al. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes. 2014;63:4197–205. doi: 10.2337/db14-0153. [DOI] [PubMed] [Google Scholar]
- 10.Mendu SK, Bhandage A, Jin Z, Birnir B. Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes. PLoS One. 2012;7:e42959. doi: 10.1371/journal.pone.0042959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Weng W, Wang S, Long R, Li H, Li H, et al. Effect of γ-aminobutyric acid–chitosan nanoparticles on glucose homeostasis in mice. ACS Omega. 2018;3:2492–7. doi: 10.1021/acsomega.7b01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwirtlich M, Emri Z, Antal K, Máté Z, Katarova Z, Szabó G. GABAA and GABAB receptors of distinct properties affect oppositely the proliferation of mouse embryonic stem cells through synergistic elevation of intracellular Ca2+ FASEB J. 2010;24:1218–28. doi: 10.1096/fj.09-143586. [DOI] [PubMed] [Google Scholar]
- 13.Brice NL, Varadi A, Ashcroft SJ, Molnar E. Metabotropic glutamate and GABAB receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2002;45:242–52. doi: 10.1007/s00125-001-0750-0. [DOI] [PubMed] [Google Scholar]
- 14.Ligon B, Yang J, Morin SB, Ruberti MF, Steer ML. Regulation of pancreatic islet cell survival and replication by γ-aminobutyric acid. Diabetologia. 2007;50:764–73. doi: 10.1007/s00125-007-0601-8. [DOI] [PubMed] [Google Scholar]
- 15.Bonaventura MM, Catalano PN, Chamson-Reig A, Arany E, Hill Dj, Bettler B, et al. GABAB receptors and glucose homeostasis: Evaluation in GABAB receptor knock-out mice. Am J Physiol Endocrinol Metab. 2008;294:E157–67. doi: 10.1152/ajpendo.00615.2006. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Ren L, Wan Y, Prud'homme GJ. GABAergic regulation of pancreatic islet cells: Physiology and antidiabetic effects. J Cell Physiol 2019. :28214. doi: 10.1002/jcp.28214. doi: 10.1002/jcp. [DOI] [PubMed] [Google Scholar]
- 17.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Gromada J, Chabosseau P, Rutter GA. The α-cell in diabetes mellitus. Nat Rev Endocrinol. 2018;14:694–704. doi: 10.1038/s41574-018-0097-y. [DOI] [PubMed] [Google Scholar]
- 19.Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci. 2011;108:11692–7. doi: 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y, Wang Q, Prud'homme GJ. GABAergic system in the endocrine pancreas: A new target for diabetes treatment. Diabetes Metab Syndr Obes. 2015;8:79–87. doi: 10.2147/DMSO.S50642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prud'homme GJ, Glinka Y, Kurt M, Liu W, Wang Q. The anti-aging protein Klotho is induced by GABA therapy and exerts protective and stimulatory effects on pancreatic beta cells. Biochem Biophys Res Commun. 2017;493:1542–7. doi: 10.1016/j.bbrc.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Donate-Correa J, Martín-Núñez E, Delgado NP, de Fuentes MM, Arduan AO, Mora-Fernández C, et al. Implications of Fibroblast growth factor/Klotho system in glucose metabolism and diabetes. Cytokine Growth Factor Rev. 2016;28:71–7. doi: 10.1016/j.cytogfr.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Jin T, Wang Q. 337-LB: Combined oral administration of GABA and GLP-1 promotes human ß-Cell proliferation and reduces apoptosis. Diabetes. 2019;68(Suppl 1) doi: 10.2337/db19-337-LB. [Google Scholar]
- 24.Tian J, Dang H, Middleton B, Kaufman DL. Clinically applicable GABA receptor positive allosteric modulators promote β-cell replication. Sci Rep. 2017;7:374. doi: 10.1038/s41598-017-00515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjurstöm H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, et al. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18:833–45. doi: 10.3727/096368909X471198. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghi A, Hami J, Razavi S, Esfandiary E, Hejazi Z. The effect of diabetes mellitus on apoptosis in hippocampus: Cellular and molecular aspects. Int J Prev Med. 2016;7:57. doi: 10.4103/2008-7802.178531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohrabipour S, Sharifi MR, Talebi A, Sharifi M, Soltani N. GABA dramatically improves glucose tolerance in streptozotocin-induced diabetic rats fed with high-fat diet. Eur J Pharmacol. 2018;826:75–84. doi: 10.1016/j.ejphar.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 29.Bharadwaj G, Satyanarayana V, Shabeer D, Vardhan GV. A study of the effect of Baclofen on blood glucose level in alcohol dependence syndrome (ADS) patients at a tertiary care hospital. Indian J Pharm Pharmacol. 2017;4:55–61. [Google Scholar]
- 30.Taneera J, Jin Z, Jin Y, Muhammed SJ, Zhang E, Lang S, et al. γ-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia. 2012;55:1985–94. doi: 10.1007/s00125-012-2548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oda H, Okuda Y, Yoshida Y, Kimura N, Kakinuma A. Phenobarbital reduces blood glucose and gluconeogenesis through down-regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression in rats. Biochem Biophys Res Commun. 2015;466:306–11. doi: 10.1016/j.bbrc.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Yasujima T, Saito K, Moore R, Negishi M. Phenobarbital and insulin reciprocate activation of the nuclear receptor constitutive androstane receptor through the insulin receptor. J Pharmacol Exp Ther. 2016;357:367–74. doi: 10.1124/jpet.116.232140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedrington MS, Mikeladze M, Tate DB, Younk LM, Davis I, Davis SN. Effects of γ-aminobutyric acid a receptor activation on counterregulatory responses to subsequent exercise in individuals with type 1 diabetes. Diabetes. 2016;65:2754–9. doi: 10.2337/db16-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garabadu D, Krishnamurthy S. Diazepam potentiates the antidiabetic, antistress and anxiolytic activities of metformin in type-2 diabetes mellitus with cooccurring stress in experimental animals. Biomed Res Int. 2014;2014:693074. doi: 10.1155/2014/693074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91:409–14. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Untereiner A, Abdo S, Bhattacharjee A, Gohil H, Pourasgari F, Ibeh N, et al. GABA promotes β-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB J. 2018;30:fj–201801397R. doi: 10.1096/fj.201801397R. [DOI] [PubMed] [Google Scholar]
- 37.Liu W, Son DO, Lau HK, Zhou Y, Prud'homme GJ, Jin T, et al. Combined oral administration of GABA and DPP-4 inhibitor prevents beta cell damage and promotes beta cell regeneration in mice. Front Pharmacol. 2017;20:8–362. doi: 10.3389/fphar.2017.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nzor JN, Uwakwe AA, Onuoha SC. Impact of benzodiazepines administration on selected biochemical parameters of albino Wistar rats (Rattus rattus) Egypt Pharm J. 2018;17:40–7. [Google Scholar]
- 39.Lee YJ, Kim M, Lee JY, Jung SH, Jeon HY, Lee SA, et al. The benzodiazepine anesthetic midazolam prevents hyperglycemia-induced microvascular leakage in the retinas of diabetic mice. FASEB J. 2018;32:6089–99. doi: 10.1096/fj.201800014RR. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Z, Shi Z, Xie C, Gong W, Hu Z, Peng Y. A novel mechanism of Gamma-aminobutyric acid (GABA) protecting human umbilical vein endothelial cells (HUVECs) against H2O2-induced oxidative injury. Comp Biochem Physiol C: Toxicol Pharmacol. 2019;217:68–75. doi: 10.1016/j.cbpc.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, et al. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell. 2017;168:86–100. doi: 10.1016/j.cell.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toi N, Inaba M, Kurajoh M, Morioka T, Hayashi N, Hirota T, et al. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J Clin Transl Endocrinol. 2019;15:37–44. doi: 10.1016/j.jcte.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khandelwal D, Dutta D, Chittawar S, Kalra S. Sleep disorders in type 2 diabetes. Indian J Endocrinol Metab. 2017;21:758–61. doi: 10.4103/ijem.IJEM_156_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marselli L, Trincavelli L, Santangelo C, Lupi R, Del Guerra S, Boggi U, et al. The role of peripheral benzodiazepine receptors on the function and survival of isolated human pancreatic islets. Eur J Endocrinol. 2004;151:207–14. doi: 10.1530/eje.0.1510207. [DOI] [PubMed] [Google Scholar]
- 45.Park SY, Cho N, Chang I, Chung JH, Min YK, Lee MK, et al. Effect of PK11195, a peripheral benzodiazepine receptor agonist, on insulinoma cell death and insulin secretion. Apoptosis. 2005;10:537–44. doi: 10.1007/s10495-005-1884-1. [DOI] [PubMed] [Google Scholar]
- 46.Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G. Adenosine signalling in diabetes mellitus—pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11:228–41. doi: 10.1038/nrendo.2015.10. [DOI] [PubMed] [Google Scholar]
- 47.Sällström J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A1-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol. 2007;190:253–9. doi: 10.1111/j.1748-1716.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- 48.Narimatsu E, Niiya T, Kawamata M, Namiki A. The mechanisms of depression by benzodiazepines, barbiturates and propofol of excitatory synaptic transmissions mediated by adenosine neuromodulation. Masui. 2006;55:684–91. [PubMed] [Google Scholar]


