Significance
This study demonstrates that damage to lung epithelial architecture induces aberrant cellular signals that promote the development of precancerous airway lesions. These signals are mediated by the uncontrolled activation of the transcriptional effectors YAP and TAZ, which drive the expression of the growth factor NRG1 and the consequent activation of the ERBB receptors. Genetic deletion of YAP/TAZ or pharmacological inhibition of ERBB receptors prevents and treats precancer airway lesion development following epithelial damage, offering important knowledge that may guide therapeutic strategies for intercepting the development of squamous lung cancers.
Keywords: lung precancer, epithelial polarity, YAP/TAZ, lung basal cells
Abstract
Molecular events that drive the development of precancerous lesions in the bronchial epithelium, which are precursors of lung squamous cell carcinoma (LUSC), are poorly understood. We demonstrate that disruption of epithelial cellular polarity, via the conditional deletion of the apical determinant Crumbs3 (Crb3), initiates and sustains precancerous airway pathology. The loss of Crb3 in adult luminal airway epithelium promotes the uncontrolled activation of the transcriptional regulators YAP and TAZ, which stimulate intrinsic signals that promote epithelial cell plasticity and paracrine signals that induce basal-like cell growth. We show that aberrant polarity and YAP/TAZ-regulated gene expression associates with human bronchial precancer pathology and disease progression. Analyses of YAP/TAZ-regulated genes further identified the ERBB receptor ligand Neuregulin-1 (NRG1) as a key transcriptional target and therapeutic targeting of ERBB receptors as a means of preventing and treating precancerous cell growth. Our observations offer important molecular insight into the etiology of LUSC and provides directions for potential interception strategies of lung cancer.
Lung cancer is the leading cause of cancer-related deaths worldwide, with lung squamous cell carcinoma (LUSC) being a major subtype that develops in the airways. Patients diagnosed with LUSC generally exhibit poor survival, in part due to poor detection and treatment strategies. While genomic alterations associated with LUSCs have been identified, the etiology of LUSC is not well understood. LUSC is typically associated with inhaled toxin induced injury, such as that caused by cigarette smoke, and as a result, the epithelial layer of the airways exhibits traits that are normally observed with injury repair. In particular, an expansion of cells expressing markers associated with basal airway stem cells, including cytokeratin-5 (Krt5) and TP63 (p63), is observed (1). The expansion of basal-like cell populations is associated with the step-wise histological progression of LUSC that is characterized by the transition of a normal pseudostratified epithelial morphology to lesions composed of irregular cellular and tissue architecture that transition through stages of hyperplasia, squamous metaplasia, and different severities of bronchial dysplasia (mild, moderate, and severe dysplasia). These precancerous pathologies are susceptible to the development of carcinoma in situ (CIS) that can progress to invasive and metastatic LUSC (2).
The human and murine tracheobronchial epithelium are lined by a pseudostratified cell layer that exhibits defined apical–basal polarity in luminal positioned secretory cells and multiciliated cells. Polarization of the epithelium offers important functions, including barrier and structural support, directional secretion, and motile cilia-directed mucosal flow (3). Polarity-regulating protein complexes have emerged as important signaling centers that direct developmental processes (4). Notable signals include those regulated by the Hippo pathway, a conserved signaling pathway that plays tumor suppressor functions. Hippo pathway signals direct the localization and activity of the transcriptional regulators YAP and TAZ (YAP/TAZ) (5). The dynamics of YAP/TAZ activity mediate a host of essential development and homeostatic processes, including essential roles in lung patterning and morphogenesis and in defining basal airway stem cell identity (6–8). Aberrant YAP/TAZ activity is well documented to associate with a wide range of cancers, including basal and squamous carcinomas (9–11), but how aberrant YAP/TAZ activity is initiated and how this activity is linked to tumorigenic development is poorly understood.
Here, we report that epithelial polarity plays a key role in maintaining the homeostasis of the adult airway. We demonstrate that the conditional deletion of Crb3, the gene encoding the transmembrane Crumbs3 (Crb3) protein that functions as a determinant of the apical domain of epithelial cells (12), leads to the development of precancerous airway lesions. We show that loss of Crb3 drives increased nuclear YAP/TAZ activity that initiates intrinsic luminal and paracrine luminal-to-basal signals that result in a rewiring of the local microenvironment that sustains the expansion of cells expressing basal epithelial markers. By profiling YAP/TAZ-regulated genes in airway epithelial cells, we have identified a YAP/TAZ-directed transcriptional signature that is enriched in human precancerous airway lesions and is associated with high-grade dysplastic lesion progression in human patients. We further identified the ERBB receptor ligand Neuregulin-1 (NRG1) as a key YAP/TAZ-regulated factor that is induced in precancerous lesions of mouse Crb3-deleted airways and human patient airways and that inhibition of ERBB receptors can prevent and treat existing precancerous lesions in the mouse airway. Collectively, our observations indicate that loss of airway epithelial polarity is a driver of precancerous airway disease and offers a direction for the early interception of lung cancer.
Results
Loss of Crb3 in Adult Luminal Airway Epithelial Cells Drives the Aberrant Expansion of Airway Cells Expressing Basal Cell Markers.
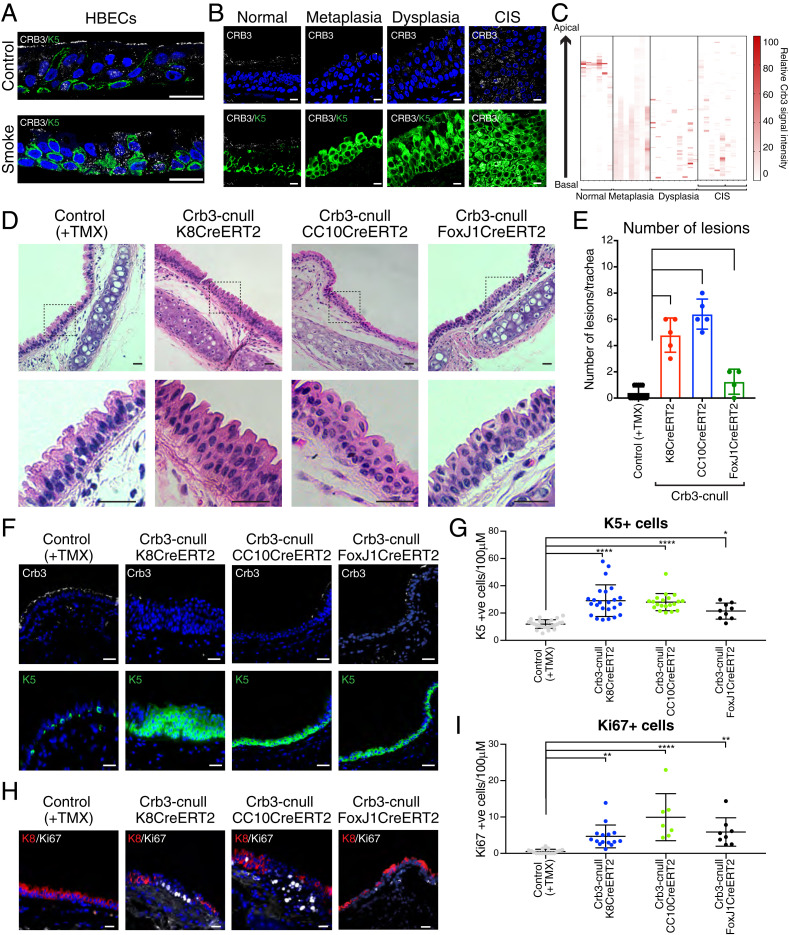
Factors associated with the development of LUSC, such as exposure to cigarette toxins, are known to impair airway epithelial cell adhesion and barrier function (13, 14). Consistent with these prior studies, we observed that differentiated human bronchial epithelial cells (HBECs) (Fig. 1A) and mouse tracheal epithelial cells (mTECs) (SI Appendix, Fig. S1A) exhibit drastic morphological changes following exposure to cigarette smoke that are accompanied by reduced epithelial tight junction barrier function (SI Appendix, Fig. S1 B and C). Mature mammalian airway epithelium is normally arranged as a pseudostratified layer with basal-positioned stem cells and luminal secretory and multiciliated cells that exhibit well-defined apical–basal polarity (SI Appendix, Fig. S1 D–F). Apical–basal polarity defects were evident in smoke exposed cells, which included a loss and disorganization of Crb3 from the apical domain of luminal cells (Fig. 1A and SI Appendix, Fig. S1A).
Fig. 1.
Loss of Crb3 in luminal airway epithelium results in the development of Krt5-positive lesions that resemble precancer pathology. (A) Primary HBECs were differentiated in ALI cultures and then exposed to a single dose of cigarette smoke (6.5% dose as described in Materials and Methods) or air as a control. The cells were fixed 4 h after exposure and then examined by IF microscopy for Crb3 and Krt5 levels and organization. Representative images from three independent samples per condition are shown. (B) CRB3 and KRT5 proteins were examined by IF microscopy in human bronchial brush biopsies with the indicated pathology, and (C) the pixel intensity for CRB3 was quantified across the apical–basal plane of the epithelium. Representative images are shown in B from normal (n = 6), metaplasia (n = 7), dysplasia (n = 8), and CIS (n = 2) pathologies that were quantified. Note that our CIS patient sample size was limited, and we therefore examined multiple different sections with CIS pathology across these patient samples. (D) Hematoxylin and eosin (H&E) staining was performed on trachea tissues isolated from the indicated conditional Crb3-deletion models 21 d post-tamoxifen (TMX) treatment. These models included targeting pan-luminal airway epithelial cells (Krt8CreERT2), secretory luminal airway epithelial cells (CC10CreERT2), and multiciliated luminal epithelial cells (FoxJ1CreERT2). Low magnification images are shown highlighting the positioning of the lesions near the cartilage rings, as well as higher magnification of stratified lesions outlined by the dotted black box. Representative images are shown from a minimum of three tissues across at least four mice. (E) The number of epithelial lesions that showed expansion and stratification of Krt5-positive cells were quantified across individual tracheas isolated from the indicated conditional Crb3-deletion models 21 d post-TMX treatment. Control (n = 14), K8CreERT2 (n = 5), CC10CreERT2 (n = 5), and FoxJ1CreERT2 (n = 4). (F) IF microscopy for Krt5 and Crb3 protein was performed on trachea tissues isolated from the indicated conditional Crb3-deletion models 21 d post-TMX treatment. (G) The number of Krt5-positive cells per 100 μM of tracheal epithelium was quantified within the respective conditional deletion models 21 d post-TMX treatment. Images from Control (n = 26), K8CreERT2 (n = 24), CC10CreERT2 (n = 25), and FoxJ1CreERT2 (n = 9) isolated from a minimum of four mice per condition were quantified. (H) Ki67-positive cells were identified by IF microscopy in the listed models of conditional Crb3 deletion 21 d post-TMX treatment. (l) The number of Ki67-positive cells per 100 μM within the trachea was quantified in the respective conditional deletion models 21-d post-TMX treatment. Images from Control (n = 17), Krt8CreERT2 (n = 15), CC10CreERT2 (n = 7), and FoxJ1CreERT2 (n = 9) isolated from a minimum of four mice per condition were quantified. Shown is the average ± SEM. Significance for all experiments in this figure was determined by the use of a Dunnett’s multiple comparisons test. ****P < 0.0001, **P < 0.01, and *P < 0.05. (Scale bar, 20 μm.)
Observation from our in vitro smoke exposure experiments prompted us to examine Crb3 in endobronchial biopsies obtained from high-risk smokers undergoing lung cancer screening at Roswell Park Comprehensive Cancer Center (RPCC) (15) (details outlined in SI Appendix, Table S1). Immunofluorescence (IF) microscopy analysis of Crb3 in histologically normal airways showed high levels of apical membrane localization in luminal cells and low levels of unorganized Crb3 in basal-positioned cells (Fig. 1B). Regions with metaplasia, dysplasia, and CIS pathology showed lower, more disorganized Crb3 levels throughout the epithelium compared to normal (Fig. 1 B and C). These aberrantly organized precancer lesions consisted primarily of Krt5-positive, basal-like cells, suggesting a role for Crb3 in airway luminal-basal epithelial homeostasis.
To test whether dysregulation of Crb3 affects adult mammalian lung epithelial homeostasis, we crossed a Crb3-floxed mouse (16) with a mouse expressing tamoxifen-inducible Cre from a Krt8 promoter (Krt8-CreERT2) (17), allowing conditional deletion of Crb3 in adult lung luminal epithelial cells. Deletion of Crb3 in Krt8-positive luminal cells resulted in abnormal organization in the tracheal epithelium and the rapid (8 to 21 d post-tamoxifen treatment) development of stratified epithelial lesions that were found adjacent to the cartilage rings (Fig. 1 D and E). The organization of the epithelium of the intrapulmonary airways was largely normal, suggesting that the location of Crb3 deleted cells within the airway impacted the observed pathology. A distinct feature of the mouse tracheal epithelium is the presence of Krt5-positive basal cells, which function as resident stem cells (18). Interestingly, Crb3-deleted tracheal lesions were composed primarily of Krt5-positive cells, which were increased in number and stratified (Fig. 1 F and G and SI Appendix, Fig. S1G). Cells within the Crb3-cnull lesions also expressed Krt14 (SI Appendix, Fig. S1H), a cytokeratin that is induced with airway injury (19, 20) but lacked the expression of mature ciliated or secretory markers (SI Appendix, Fig. S1I).
Many of the cells within Crb3-cnull derived lesions, particularly those positioned more basal in the stratified layer, expressed p63 (SI Appendix, Fig. S1 J and K), a transcription factor that is required for the development of airway basal cells (21). Cells within stratified lesions of Crb3-deleted mice also expressed the proliferation marker Ki67 (Fig. 1 H and I) as well as Krt13 (SI Appendix, Fig. S1L), a marker recently described to associate with “basal hillock” cells that are found in low numbers in normal tracheal epithelium and exhibit a transitional squamous and proliferative state (22, 23). Notably, Crb3 deleted tracheas showed Krt5-positive expanded lesions after aging the mice for extended lengths of time (26 to 52 wk) (SI Appendix, Fig. S1J). We did not observe transition of these lesions into an invasive cancer pathology or any changes in animal survival over this time, indicating that loss of Crb3 is not sufficient to drive tumorigenesis. Rather, these observations suggest that loss of Crb3 drives aberrant injury response mechanisms that fosters the long-term persistence of precancer lesions, similar to what is observed in human precancer airway pathology.
Krt8-expressing luminal cells of the tracheal epithelium are primarily composed of secretory cells that express the secretoglobin CC10 (encoded by Scgb1a1) and multiciliated cells that express the transcription factor FoxJ1 (24). To better understand the observed phenotypes of Crb3 deletion in luminal cells, we generated mice that allowed for the conditional deletion of Crb3 in CC10-expressing epithelial cells (CC10CreERT2) (25) or FoxJ1-expressing epithelial cells (FoxJ1-CreERT2) (26). Deletion of Crb3 in CC10-expressing tracheal cells resulted in airway lesions near the cartilage rings of the trachea similar to the Krt8 model (Fig. 1 D and E), with these lesions composed of expanded and stratified Krt5-positive cells (Fig. 1 F and G and SI Appendix, Fig. S1G). Deletion of Crb3 in FoxJ1-positive multiciliated cells also resulted in abnormal tracheal epithelial morphology with the presence of stratified epithelial lesions, albeit at a reduced number compared to the CC10Cre or Krt8Cre models (Fig. 1 D and E), with these lesions also being composed of expanded Krt5-positive cells (Fig. 1 F and G and SI Appendix, Fig. S1G).
Deletion of Crb3 Drives YAP/TAZ-Mediated Signals that Promote Cellular Plasticity and Paracrine Expansion of Airway Basal Stem Cells.
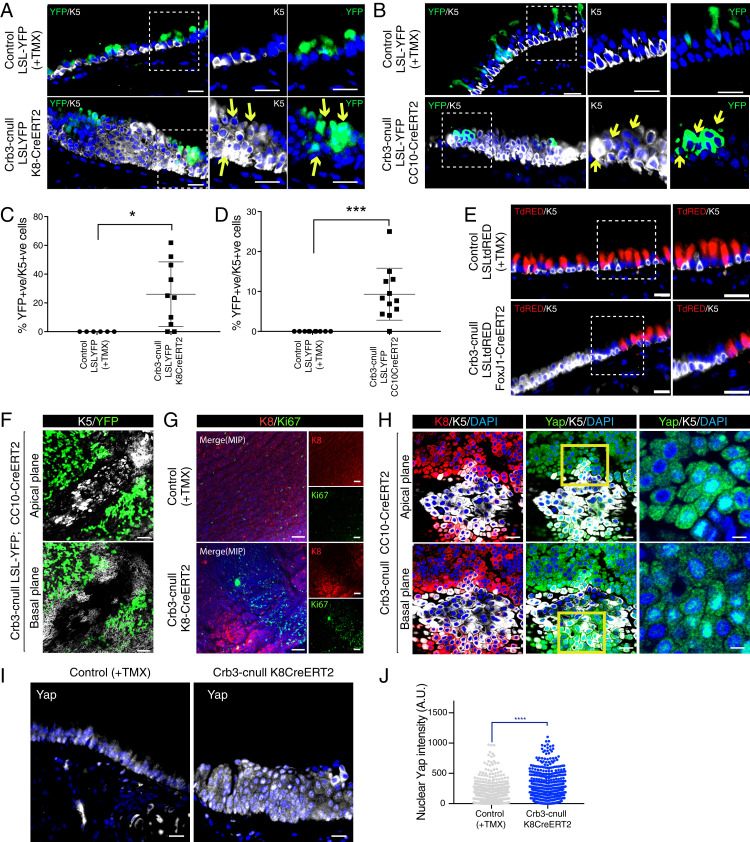
Given that conditional deletion of Crb3 in luminal airway epithelial cells resulted in the expansion of cells expressing basal airway markers, we hypothesized two possible mechanisms: 1) luminal-to-basal plasticity of Crb3-deleted cells, and/or 2) paracrine signals from Crb3-deleted luminal cells stimulate the expansion of neighboring wild-type basal cells. To test these possibilities, we incorporated a Lox-STOP-lox-EYFP–expressing lineage trace into the Krt8-CreERT2 and CC10-CreERT2 models, and a Lox-STOP-lox-TomatoRed into the FoxJ1-CreERT2 model to track cells with active Cre recombinase activity (27). We started by quantifying the Cre efficiency of each model we used in the tracheal epithelium, which revealed that ∼63% of Krt8-expressing cells were labeled with YFP in the Krt8CreERT2 model, with efficient labeling of both CC10-positive and FoxJ1-positive luminal cells but no labeling of any Krt5-positive cells. Similarly, the CC10CreERT2 and FoxJ1CreERT2 models showed no YFP labeling of Krt5-positive cells but did faithfully label ∼92% of CC10-expressing cells or FoxJ1-expressing cells in the respective models (SI Appendix, Fig. S2 A–D).
Combining these lineage-trace models with our Crb3-floxed mice showed YFP expression in ∼10% of Krt5-positive cells from the Krt8CreERT2 model (Fig. 2 A and C) and in ∼25% of Krt5-positive cells from the CC10-CreERT2 deletion model (Fig. 2 B and D). Given the absence of YFP labeling of Krt5-positive cells in controls these observations indicated that loss of Crb3 promotes some luminal cells to adopt a Krt5-positive basal-like state (i.e., luminal-basal plasticity). However, the majority of Krt5-positive cells within lesions in these models were not marked by YFP, which suggested that paracrine signals may also stimulate the expansion of nontargeted basal cells. Supporting paracrine contributions, we did not detect any lineage-labeled Krt5-positive cells in expanded lesions using the FoxJ1-CreERT2 model, indicating that deletion of Crb3 does not induce basal-like plasticity in multiciliated cells, but rather induces paracrine signals that expand Krt5-positive cells (Fig. 2E). Further supporting paracrine contributions, whole mount imaging of Crb3-cnull tracheas allowed us to detect large regions of Krt5-positive cells that were flanked by YFP-labeled (Cre-positive) cells (Fig. 2F). Many of these Krt5-positive cells expressed the proliferation marker Ki67 (Fig. 2G). Cells surrounding the expanded lesions were marked by both Krt5 and Krt8 (i.e., a transitional cellular state) and, similar to the Krt5-positive cells in the center of the lesions, also exhibited elevated levels of nuclear Yap (Fig. 2 H–J), a transcriptional regulator that has been shown to control lung epithelial differentiation and cell plasticity (6, 7).
Fig. 2.
Deletion of Crb3 in luminal airway epithelial cells results in luminal-basal plasticity and paracrine cell expansion. (A–D) Conditional deletion of Crb3 using (A) Krt8CreERT2 or (B) CC10CreERT2 in combination with a Lox-STOP-lox-EYFP (LSL-YFP) marker reveals that a population of cells that exhibit Cre activity/YFP expression and expression of the basal cell marker Krt5 (highlighted by the yellow arrows). Images shown are from tissues isolated 21 d post-TMX treatment. (Scale bars, 20 μm.) Quantitation of cells expressing both YFP and Krt5 showed that (C) the Krt8CreERT2 model led to ∼22% of Cre-active/YFP-expressing cells that exhibited basal-like plasticity following Crb3-deletion, and (D) the CC10CreERT2 model led to ∼10% of Cre-active/YFP-expressing cells that exhibited basal-like plasticity following Crb3 deletion. Images from control (n = 10) and the Crb3-cnull tracheas (n = 12 for Krt8CreERT2, and n = 10 for CC10CreERT2) were analyzed by IF for YFP and Krt5 from a minimum of four mice per condition. Shown is the average percentage of Krt5-positive/YFP-positive cells ± SEM. Significance was determined by the use of a Dunnett’s multiple comparisons test. ***P < 0.001 and *P < 0.05. (E) Conditional deletion of Crb3 using the FoxJ1CreERT2 model, in combination with a Lox-STOP-lox-TomatoRed (LSL-tdRED) marker, revealed that no Cre-active cells express the basal cell marker Krt5. Images shown are from tissues isolated 21 d post-TMX treatment. (Scale bars, 20 μm.) (F) Whole mount IF imaging of isolated tracheas from YFP lineage–traced Crb3-cnull mice shows that Cre-active/YFP-expressing cells flank luminal expanding Krt5-positive cells. (G and H) Whole mount IF imaging of Crb3-cnull tracheas shows the presence of (G) cells expressing the proliferation maker Ki67, and (H) Krt5 or Krt8/Krt5 double-positive cells (zoomed in image) with high levels of nuclear Yap. Images shown are from tracheas isolated 21 d post-TMX treatment. (Scale bars, 20 μm.) (I) Yap protein was examined by IF microscopy in control and Crb3-cnull trachea tissue sections, revealing elevated nuclear Yap throughout stratified epithelial lesions. (J) Quantitation of nuclear Yap intensity demonstrated significantly higher levels of nuclear Yap in Crb3-cnull trachea epithelium. Shown is the average nuclear Yap intensity ± SEM measured from cells across tracheal tissues isolated from three control and Krt8CreERT2 Crb3-cnull mice 21 d post-TMX treatment. Significance was determined by the use of a Dunnett’s multiple comparisons test for all panels. ****P < 0.0001.
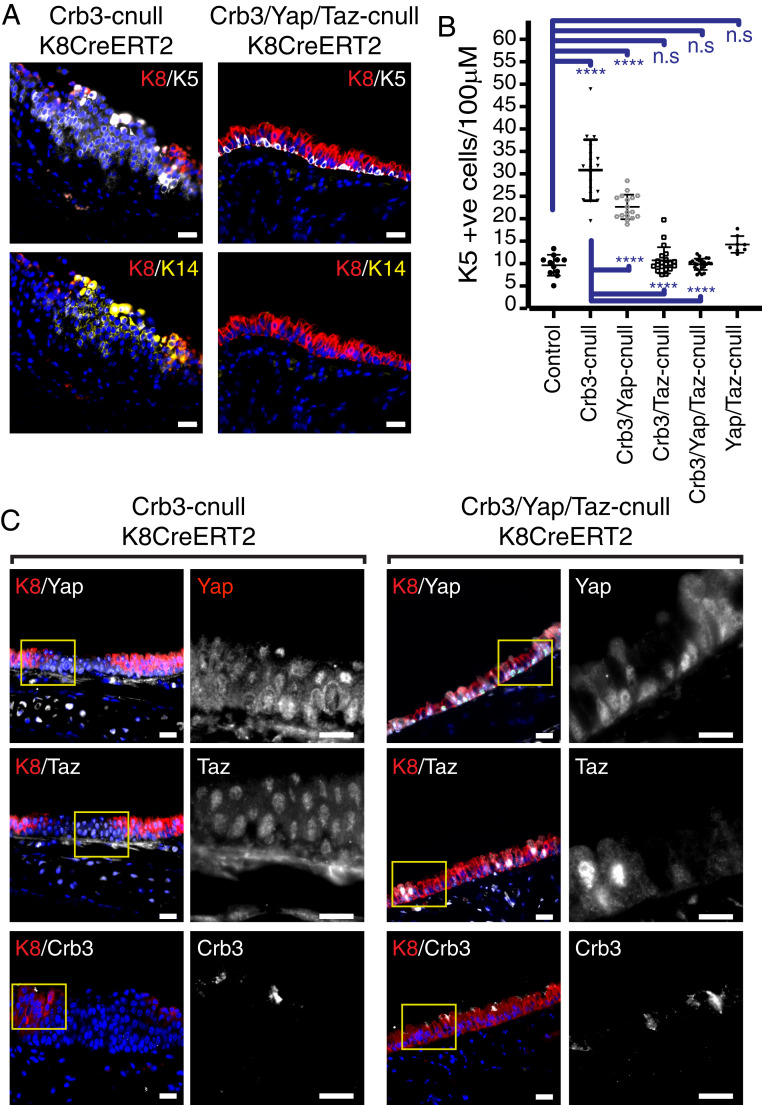
To gain insight into the involvement of Yap in the development of the expanding Krt5-positive lesions, we tested whether codeletion of Yap (Crb3-floxed; Yap-floxed; and Krt8CreERT2) affected the observed phenotypes that arise from Crb3 deletion. Given that Taz can function redundantly in some contexts with Yap (28, 29), we also tested the consequences of Taz codeletion (Crb3-floxed; Taz-floxed; and Krt8CreERT2), as well as Yap/Taz codeletion (Crb3-floxed; Taz-floxed; Yap-floxed; and Krt8CreERT2) together with Crb3. We found that the deletion of Yap or Taz alone partially rescued the Krt5-positive cell expansion resulting from Crb3-deletion (SI Appendix, Fig. S3A) and that codeletion of Yap and Taz together resulted in a rescue of the phenotype, leading to similar numbers of Krt5 positive cells compared to control wild type or Yap/Taz-deleted (Yap-floxed; Taz-floxed; and Krt8CreERT2) mice (Fig. 3 A and B and SI Appendix, Fig. S3A). Immunofluorescence analyses and lineage-trace labeling of these models demonstrated that Crb3/Yap/Taz-codeleted cells were not simply removed from the airways of these mice (Fig. 3C and SI Appendix, Fig. S3B) and that targeted cells did not lead to altered luminal cell fates (SI Appendix, Fig. S3 C and D). Rather, our observations indicated that loss of Yap/Taz in polarity-defective cells had lost the signaling capacity to drive cell expansion.
Fig. 3.
Yap/Taz are required for the expansion of Krt5-positive lesions that result from the conditional luminal deletion of Crb3. (A) Tracheal epithelium isolated from mice with the indicated genotypes were examined by IF microscopy for Krt8, Krt5, and Krt14. The images show that codeletion of Yap and Taz in conjunction with Crb3 prevents the formation of Krt5-positive lesions that are normally observed following the conditional deletion of Crb3. (Scale bar, 20 μm.) (B) The number of Krt5-positive cells per 100 μM of tracheal epithelium was quantified from the indicated mice, demonstrating that Yap/Taz are required for the expansion of Krt5-positive cells following conditional Crb3 deletion. Images from control (n = 11), Crb3-cnull (n = 19), Crb3/Yap-cnull (n = 17), Crb3/Taz-cnull (n = 20), and Crb3/Yap/Taz-cnull (n = 24) were obtained from a minimum of six mice per condition and quantified. Shown is the average ± SEM. Significance was determined by the use of a Tukey’s multiple comparisons. ****P < 0.0001, and n.s = not significant. (C) Crb3-cnull and Yap/Taz/Crb3-cnull airway epithelium was examined for Crb3, Yap, and Taz, which confirmed deletion of the proteins in the respective models. (Scale bar, 20 μm.)
Yap/Taz-Regulated Gene Expression Associates with Human Lung Squamous Precancerous Lesion Severity and Progression.
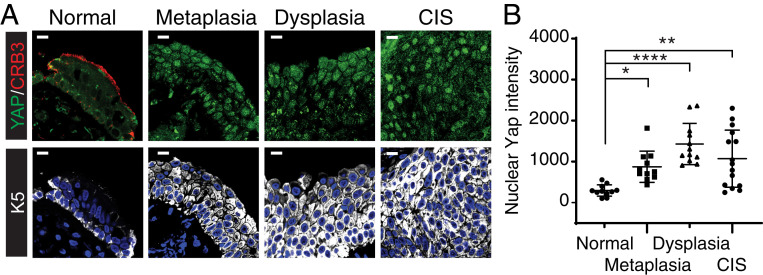
To establish whether aberrant organization of Crb3 associated with dysregulated YAP/TAZ in human precancerous squamous lesions, we immunostained endobronchial biopsies from RPCC for Crb3 and YAP (15) (same tissues as in Fig. 1B, outlined in SI Appendix, Table S1). Along with disrupted Crb3, we observed a significant elevation of nuclear YAP levels in precancerous metaplastic, dysplastic, and CIS lesions when compared to pathologically normal epithelium (Fig. 4 A and B). Analysis of data from The Cancer Genome Atlas (TCGA) indicated frequent amplification of the YAP (YAP1) (∼2% of patient samples) and TAZ (WWTR1) (23% of patient samples) genes in LUSCs tumor, examined across three studies (SI Appendix, Fig. S4), which, together with our observations in mice, supported investigating potential protumorigenic roles for these factors in the development of LUSCs.
Fig. 4.
Elevated nuclear YAP levels are observed in precancerous human airways. (A) Endobronchial patient biopsies exhibiting the noted pathologies were examined by IF microscopy for YAP, KRT5, and CRB3, which revealed a loss of apical CRB3 organization, along with elevated nuclear YAP in KRT5-positive precancerous and carcinoma lesions. Representative images are shown from tissues exhibiting normal (n = 6), metaplasia (n = 7), dysplasia (n = 8), and CIS (n = 2) pathology. (Scale bar, 5 μm.) (B) Quantification of nuclear YAP levels in the noted airway pathologies demonstrated an increase in nuclear YAP levels that is associated with precancerous metaplasia, dysplasia, and CIS pathology. Shown in the average nuclear YAP intensity ± SEM quantified from 11 images across 6 patient tissues with normal pathology, 11 images across 7 patients with metaplastic pathology, 12 images across 8 patients with metaplastic pathology, and 15 images across 2 patients with CIS pathology. Significance was determined with a Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, and ****P < 0.0001.
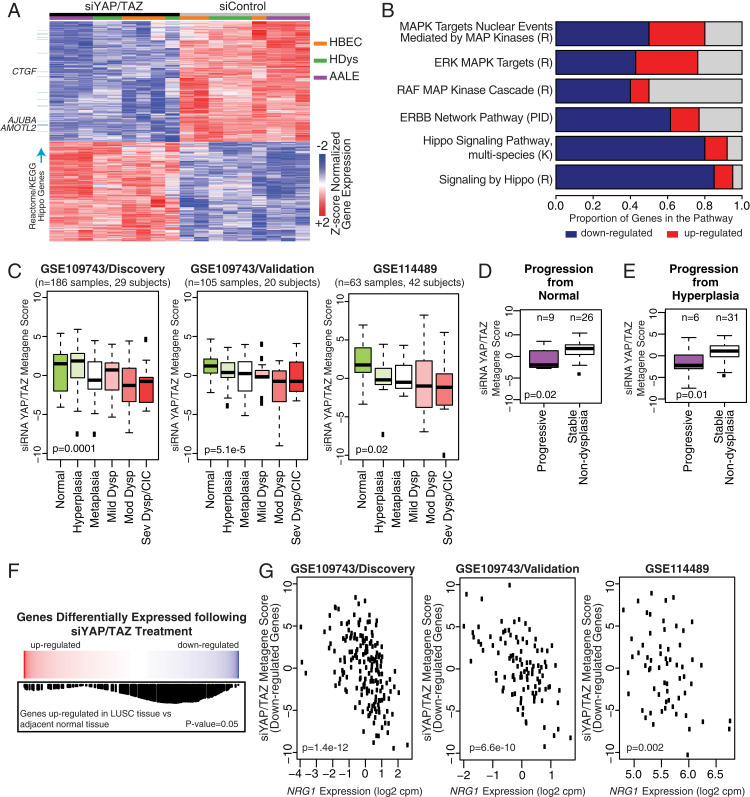
Our experiments in vivo demonstrated high nuclear levels of YAP/TAZ in all expanding basal cells found within Crb3-cnull lesions, including many cells that showed evidence of luminal-to-basal-like plasticity. We therefore reasoned that understanding YAP/TAZ-regulated transcription in basal cells may provide insight into the mechanisms driving precancer growth phenotypes. For this, we used small interfering RNA (siRNA) to deplete YAP/TAZ in proliferating airway cells and performed RNA-seq (alignment statistics are listed in SI Appendix, Table S2). Cells used for this analysis included the following: 1) primary normal HBECs, which grow in vitro in a basal-like state; 2) a transformed normal human bronchial epithelial cell line, AALE, which exhibits hyperplastic phenotypes in vitro (30); and 3) primary human dysplastic bronchial epithelial cells from a moderate dysplasia lesion, isolated from an endobronchial biopsy (human dysplastic epithelial cells [HDys]). Comparison of gene variability across the three different cells indicated overall similarity (Pearson’s correlation coefficient greater than 0.57) but that the HDys and transformed AALEs showed higher similarity with each other (SI Appendix, Fig. S5A). Analysis of differentially expressed genes [false-discovery rate (FDR) < 0.001] resulting from siRNA knockdown of YAP/TAZ revealed 1,334 common genes across the cell lines (Fig. 5A), with reduced expression of 731 genes (i.e., genes normally “activated” by YAP/TAZ) and increased expression of 603 genes (i.e., genes normally “repressed” by YAP/TAZ), resulting from YAP/TAZ knockdown. YAP/TAZ-activated genes were enriched for the Hippo pathway Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome gene expression signatures, as well as for the MAPK, RAF, and ERBB signaling networks (Fig. 5B and Dataset S1).
Fig. 5.
YAP/TAZ-regulated gene expression is associated with progression of precancerous airway pathology. (A) RNA-sequencing was performed on three airway epithelial cell models: primary normal human bronchial epithelial cells (HBECs, orange), primary HDys (green), and transformed AALE (purple) cells, all of which were treated with control siRNA (gray) or siRNA targeting YAP/TAZ (black). A heatmap of the genes significantly altered upon YAP/TAZ depletion is shown (FDR < 0.001; n = 1334 genes, 603 up- and 731 down-regulated). Blue bars on the left denote down-regulated genes associated with “Hippo pathway signaling” in Reactome and KEGG. (B) Bar plot showing enriched pathways within the genes down-regulated with YAP/TAZ depletion (FDR < 0.01). The blue and red bars represent the proportion of genes in the pathway down- and up-regulated following treatment with siRNA targetting YAP and TAZ (siYap/Taz). R, K, and PID denote Reactome, KEGG, and Pathway Interaction Database, respectively. (C) The top 500 most significant genes associated with siRNA YAP/TAZ treatment (n = 180 up-regulated and n = 320 down-regulated) were used to calculate a metagene score representing YAP/TAZ activity across human bronchial precancer datasets (GSE109743, GSE109743, and GSE114489). Negative metagene scores represent a negative correlation between gene signatures, which in these data show that genes repressed with YAP/TAZ knockdown show significant negative correlation with genes associated with histologic severity of the biopsies (P ≤ 0.02) (i.e., YAP/TAZ-activated genes are positively correlated with precancer severity). A similar negative correlation between genes repressed with YAP/TAZ knockdown was observed with (D) normal or (E) hyperplastic lesions that progress to dysplasia compared to lesions that do not progress at future time points (P ≤ 0.02, based on combined data from the discovery and validation cohorts of GSE109743), indicating that increased YAP/TAZ transcriptional activity is positively associated with precancer progression. (F) Genes up-regulated in LUSC tumors compared to adjacent normal tissue are enriched among genes down-regulated with siYAP/TAZ treatment via gene set enrichment analysis (GSEA) (P = 0.05). (Top) Red/blue color bar represents genes ranked by t-statistic for their association with siYAP/TAZ treatment. (Middle and Bottom) The black vertical lines represent the position of the genes in the gene set in the ranked list, and the height (y-axis) corresponds to the magnitude of the running enrichment score from GSEA. (G) Negative correlation is observed between NRG1 expression and the metagene score calculated from the genes down-regulated upon siYAP/TAZ treatment (n = 320 genes) across three distinct precancerous human lung biopsy cohort datasets (discovery and validation cohorts from GSE109743 and GSE114489). The negative correlations are significant (P ≤ 0.002; Pearson correlation), indicating that YAP/TAZ activity positively associates with NRG1 expression in precancerous tissues.
Using the top 500 most differentially expressed genes, we found a significant association between the YAP/TAZ activation signature and genes altered with increased histologic progression in human endobronchial lesion biopsies studied from three distinct patient cohorts (Fig. 5C) (15, 31). Specifically, we calculated YAP/TAZ pathway activity metagene scores for each endobronchial biopsy, profiled via messenger RNA (mRNA) sequencing (GSE109743, both discovery and validation cohorts from RPCC) or Affymetrix Gene 1.0 ST microarray (GSE114489, Colorado Lung Cancer Specialized Programs of Research Excellence (SPORE) bronchoscopy screening study), from high-risk lung cancer screening patients and found that genes reduced in expression, following YAP/TAZ knockdown, were significantly associated with the histologic grade of the biopsy (P = 2.9 × 10−4 in GSE109743/Discovery cohort; P = 5.1 × 10−5 in GSE109743/Validation cohort; and P = 0.05 in GSE114489). In prior work (15), biopsies from GSE109743 were grouped into the four distinct molecular subtypes of disease, and we observed significant YAP/TAZ signature activation in the proliferative molecular subtype enriched for bronchial dysplasia (SI Appendix, Fig. S5B). Information available from these patient cohorts allowed us to investigate potential relationships with biopsy progression/regression, defined by the histology of the biopsy and the worst histology recorded for the same lung anatomic location in the future. Interestingly, we observed YAP/TAZ signature activation among biopsies that progressed from normal or hyperplasia to dysplasia versus biopsies that remained nondysplastic at future time points (P ≤ 0.02, Fig. 5 D and E). Consistent with a role for YAP/TAZ in the onset of LUSC, we also observed that genes “activated” by YAP/TAZ were up-regulated among LUSC tissues compared to adjacent normal tissue in data from TCGA (32) (Fig. 5F). Thus, YAP/TAZ-activated gene expression associates with precancerous human airway disease progression.
Nuclear Yap/Taz Promotes the Expression of Neuregulin-1.
Given that our observations that paracrine signals may influence precancer lesion formation in Crb3-cnull airways in mice, we directed our attention to secreted factors. The top gene encoding a secreted factor that was repressed upon YAP/TAZ knockdown was NRG1 (SI Appendix, Fig. S5C), which encodes for the ERBB receptor ligand NRG1. Given the enrichment in ERBB signaling in the YAP/TAZ gene expression signature, we explored the relationship between YAP/TAZ and NRG1 in the precancerous human patient data, which revealed a strong correlation between YAP/TAZ-“activated” genes (down-regulated with knockdown) and NRG1 expression across the bronchial precancerous lesion datasets (GSE109743, GSE109743, and GSE114489) (Fig. 5G).
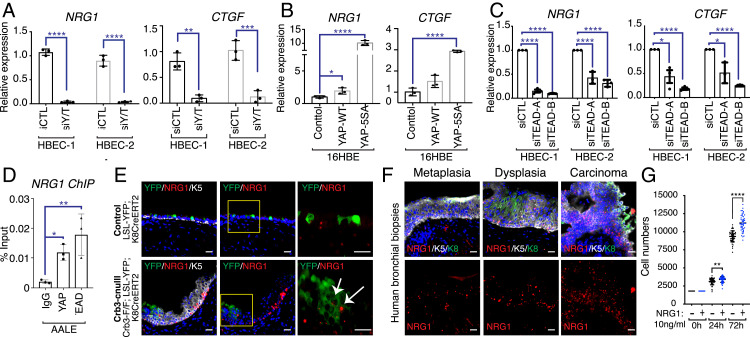
These observations suggested that NRG1 is a transcriptional target of YAP/TAZ in airway epithelial cells and precancerous airway disease. To initially test this, we performed RT-qPCR to examine NRG1 expression following YAP, TAZ, or YAP/TAZ codepletion in two new HBEC patient lines. We found that YAP/TAZ codepletion strongly suppressed NRG1 expression, leading to a similar reduction as the canonical YAP/TAZ target CTGF (Fig. 6A and SI Appendix, Fig. S6A). YAP or TAZ depletion alone did not lead to a similar reduction on NRG1 expression, suggesting redundant control of NRG1 by both factors (SI Appendix, Fig. S6B). We further found that ectopic expression of YAP or a nuclear-localized mutant of YAP (YAP-5SA) (33) led to increased NRG1 expression at even more pronounced levels than CTGF (Fig. 6B). The protumorigenic functions of YAP/TAZ rely on the regulation of the TEAD family of transcription factors (34), prompting us to further test whether NRG1 is TEAD-regulated. Depletion of TEAD1-4 in HBECs, using two different combinations of siRNA, led to marked reduction of NRG1 expression (Fig. 6C and SI Appendix, Fig. S6C). To investigate potential direct regulation, we analyzed chromatin immunoprecipitation (ChIP) sequencing data available for YAP and TEAD4, available from the ENCODE project, searching for binding to regulatory elements associated with the NRG1 gene. This revealed several binding sites for YAP/TAZ and TEADs in the NRG1 gene promoter (SI Appendix, Fig. S6D), which we validated by ChIP quantitative PCR (ChIP-qPCR) in human airway epithelial cells, demonstrating binding for both YAP and TEAD to these NRG1 gene elements (Fig. 6D).
Fig. 6.
YAP/TAZ-TEAD directly regulate NRG1 expression. (A) Two HBEC lines, isolated from different patients (listed as HBEC-1 and HBEC-2), were transfected with control siRNA or siRNA targeting both YAP and TAZ. RNA was then isolated and analyzed by quantitative PCR for NRG1 and CTGF expression. (B) 16HBE cells were infected with lentivirus, transducing the expression of wild-type (WT) YAP or a nuclear-localized active YAP (5SA) and then analyzed by quantitative PCR for NRG1 and CTGF expression. (C) Two patient lines of HBECs were transfected with control siRNA or two different combinations of siRNA targeting all four TEAD family members, and isolated RNA from these cells was analyzed by quantitative PCR for NRG1 and CTGF expression. (D) YAP and TEAD ChIP was performed in human AALE bronchial epithelial cells and examined by quantitative PCR. (E) Airway epithelial tissues from control (LSL-YFP; K8CreERT2) mice and Crb3-cnull (Crb3-F/F; LSL-YFP; and K8CreERT2) mice isolated 21 d post-TMX treatment were examined by RNAscope for NRG1 mRNA and IF microscopy for YFP and Krt5. Elevated NRG1 expression was observed in YFP-marked Crb3-deleted cells (white arrows) and in neighboring nonYFP-marked Krt5-positive cells. (Scale bar, 20 μm.) (F) Human bronchial biopsies with the indicated pathology were examined by RNAscope for NRG1 mRNA levels and IF microscopy for Krt5 protein, revealing high NRG1 expression in precancerous Krt5-positive lesions. (G) Equal numbers of primary HBECs were treated with or without recombinant NRG1, and cell numbers were quantified at the indicated time points. Shown in the average ± SEM. Significance was determined with an unpaired t test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
To test the YAP/TAZ-NRG1 relationship in vivo, we initially turned back to the Crb3-cnull deleted mouse lung tissues and examined them using RNAscope, an RNA in situ hybridization detection method, which revealed increased NRG1 mRNA in Krt5-positive expanded lesions (Fig. 6E). Notably, high levels of NRG1 mRNA were observed in lineage-traced Cre-positive luminal cells in Crb3-cnull lesions, indicating that in addition to high levels of NRG1 in expanding basal cells, aberrant activation of NRG1 also occurs in the polarity-deleted luminal epithelium. Conversely, we observed that NRG1 mRNA levels in wild type airways were very low and restricted to few Krt5-positive basal cells. Consistently, we found that NRG1 mRNA was highly elevated in dysplastic and CIS lesions from human endobronchial biopsies (Fig. 6F). These observations suggested that exogenous NRG1 is capable of stimulating airway epithelial cell growth. To test this idea more directly we treated human primary HBECs with recombinant NRG1 and found significantly increased proliferation of stimulated cells (Fig. 6G). We observed that HBEC treatment with NRG1 stimulated the phosphorylation of the ERBB3 receptor, suggesting NRG1-activated ERBB3 cues contribute to a proliferative phenotype (SI Appendix, Fig. S6E).
Treatment with an ERBB Inhibitor Prevents and Treats Precancerous Lesions in Crb3-Cnull Mice.
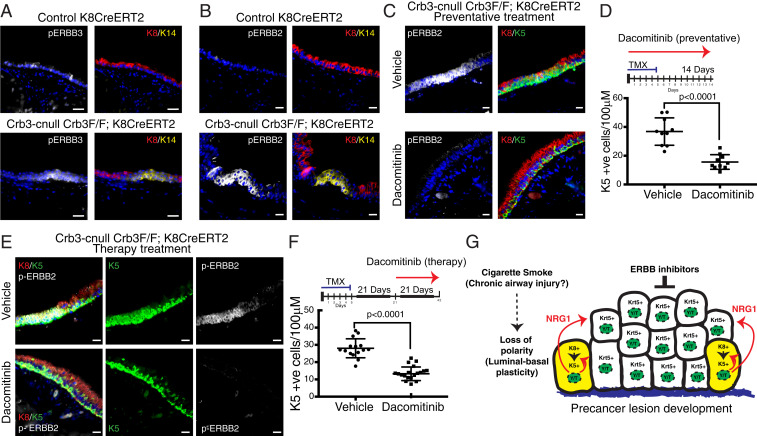
We next examined Crb3-cnull mouse tracheas for potential ERBB receptor activation and found that Krt5-positive/Krt14-positive cells in expanding lesions showed elevated levels of phosphorylated (phospho)-ERBB3 (Fig. 7A) along with increased phosphorylation of ERBB2 (Fig. 7B), suggesting heterodimerization of activated ERBB2 and ERBB3 receptors, similar to what is described in other contexts (35, 36). This observation prompted us to test whether inhibition of ERBB receptors could alleviate the dysplastic phenotypes in Crb3-cnull airways. For this, we tested the effects of the irreversible pan-ERBB receptor inhibitor dacomitinib (also known as PF00299804; trade name, Vizimpro) (37). In vitro, we observed that dacomitinib inhibited NRG1-induced activation of ERBB3 in primary HBECs (SI Appendix, Fig. S6E). In vivo, we found that dacomitinib treatment coincident with tamoxifen-induced Crb3 deletion (preventative therapy strategy) significantly reduced the numbers of lesions with Krt5-positive cells in Crb3-cnull mice from both the CC10CreERT2 and Krt8CreERT2 models, resulting in airways that reflected wild-type tracheobronchial epithelial morphology (Fig. 7 C and D and SI Appendix, Fig. S7 A and B). This phenotype was accompanied by a complete loss of phospho-ERBB2 (Fig. 7C) and phospho-ERBB3 (SI Appendix, Fig. S7C) in the airways of dacomitinib-treated animals. We also found that treatment of established lesions in Crb3-cnull mice with dacomitinib (3 wk following tamoxifen treatment; therapeutic strategy) significantly reduced the number of Krt5-positive cells within Crb3-deleted regions (Fig. 7 E and F). These data therefore indicate that ERBB signaling plays an important role in the expansion of basal-like epithelial cells within precancerous airway lesions of Crb3-cnull airways and suggests that the YAP/TAZ-NRG1-ERBB signaling axis as an attractive target for interception of precancerous lesions.
Fig. 7.
ERBB inhibition prevents and treats Crb3-deleted precancerous lesions. (A) Activated phospho-(Tyr1289)ERBB3 and (B) activated phospho(Tyr1221/1222)-ERBB2 were examined in Crb3-deleted airways by IF microscopy, which showed elevated levels in Krt14-positive Crb3-deleted lesions compared to control airway epithelium. (Scale bar, 20 μm.) (C) Mice were concomitantly treated with TMX to induce Crb3 deletion and Dacomitinib to inhibit ERBB activity (preventative treatment strategy) and examined for the development of Krt5-positive airway lesions and the presence of phospho(Tyr1221/1222)-ERBB2. (Scale bar, 20 μm.) (D) Quantification of Krt5-positive cells per 100 μM of tracheal epithelium showed a significant reduction within Crb3-deleted airways using a preventative strategy (experimental flow is illustrated) of Dacomitinib treatment (n = 10 for vehicle control and n = 10 for Dacomitinib treatment). Shown in the average ± SEM. Significance was determined with an unpaired t test (P values are shown). (E) Developed precancerous lesions in TMX-induced Crb3-cnull mice were treated with Dacomitinib (therapeutic treatment strategy) and examined for the presence of Krt5-positive airway lesions and phospho(Tyr1221/1222)-ERBB2. (Scale bar, 20 μm.) (F) Quantification of a therapeutic Dacomitinib treatment strategy (experimental flow is illustrated) showed a significant reduction in Krt5-positive cells found within treated Crb3-deleted airways (n = 15 for vehicle control and n = 18 for Dacomitinib treatment). Shown in the average ± SEM. Significance was determined with an unpaired t test (P values are shown). (G) An illustrated model of the molecular events resulting from disrupted epithelial polarity that our data suggests contributes to the development of precancerous airway lesions.
Discussion
Our observations have demonstrated that the maintenance of epithelial cell polarity in adult airways is crucial for tissue homeostasis. Using genetic models that allow for the conditional deletion of the Crb3 gene in mice, which encodes a key member of the Crumbs family of apical transmembrane proteins (38), we showed that lost apical–basal polarity drives a network of signals that initiate and sustain precancerous airway lesions. The expanding epithelial lesions that result from disrupted polarity are histologically comparable to lung squamous, precancerous lesions found in the airways of high-risk smokers. Evidence indicates that LUSC originates from the normal airway epithelium that undergoes pathological transitions of epithelial hyperplasia, metaplasia, and dysplasia that transition into CIS (2). The presence and persistence of progressive dysplastic lesions demonstrates an increased risk of LUSC development (39), but little is known about mechanisms that enable the development of early diseased pathologies. Our study demonstrates that loss of airway epithelial polarity is a driver of the precancerous state, and we identify intrinsic and paracrine signals that are induced as a consequence of aberrant polarity that may offer therapeutic opportunity for intercepting the LUSC development (model illustrated in Fig. 7G).
Healthy luminal airway epithelial cells exhibit distinct apical–basal polarity and function to maintain tissue architecture and barrier function. Agents that damage the airway epithelium, such as cigarette smoke, have been linked to precancerous lung pathology (2). Our observations indicate that cigarette smoke exposure rapidly alters the architecture and barrier function of differentiated primary human and mouse airway epithelial cells, with exposure disrupting the luminal-basal epithelial positioning and altering the organization of the key polarity regulator Crb3. We show that Crb3 is aberrantly organized in human precancer pathology and that conditional loss of Crb3 in the luminal airway epithelium drives the expansion of cells that exhibit characteristics of airway basal cells (such as expression of Krt5 and p63), which are resident stem cells that function to regenerate the airway epithelium following injury (18, 40). Our Cre recombinase–induced lineage tracing analyses indicated that loss of Crb3 was sufficient to drive luminal-to-basal plasticity in CC10-expressing secretory epithelium, consistent with these cells having the capacity to transition states under injury conditions (41). However, we also observed that many basal-like cells that were expanded following Crb3 deletion originated from nontargeted cells, suggesting that paracrine signals induced by the knockout cells contributed to lesion development. Similar lineage tracing experiments indicated that FoxJ1-expressing ciliated epithelium did not exhibit luminal-to-basal plasticity but was still capable of driving basal-like cell expansion, further supporting paracrine signaling contributions to basal cell expansion in polarity-defective lesions.
In all the models we studied we observed a consistent pattern of lesion formation near the cartilage rings of the trachea, suggesting that the distinct microenvironment contributes to the observed pathology. In mice, basal cells and transitional “hillock” cells are more enriched near the cartilage, and it is therefore possible that it is these cells respond to the growth stimulating signals produced by polarity-defective cells. In humans, basal cells extend into smaller airways, and thus the microenvironment to support these cells may be present in intrapulmonary regions where LUSC generally arises. Notably, basal cell–enriched lesions in Crb3-deleted airways were observed for long time periods (examined up to a year in our study), suggesting that the precancerous signals persist and are not resolved by normal injury repair mechanisms. Similar long-term persistence of airway precancer lesions is observed in human biopsies from chronic smokers. Interestingly, we did not observe Crb3-deleted lesions progress to an invasive carcinoma state, suggesting that additional signals, potentially those acquired via mutations (42), enable carcinoma onset. Thus, our findings may offer insight into a potential therapeutic window for identifying and targeting precancer lesions before progression to more advanced stages.
Crb3 is a key determinant of Hippo tumor suppressor pathway signaling, a pathway that restricts the activity of the transcriptional regulators YAP and TAZ. Nuclear YAP/TAZ regulate gene expression programs that promote proliferation, control apoptosis, and direct cell fate (5), and YAP/TAZ have emerged as central mediators of lung stem cell biology (6, 7, 43–45). In the airway epithelium, YAP plays a key role in basal stem cell identity, and ectopic nuclear YAP expression has been shown to drive airway basal expansion (6, 7). Crumbs isoforms are known to organize protein complexes that function to promote Hippo pathway signaling in the apical domain of epithelial cells (16, 46–48). These complexes facilitate LATS1/2 association with YAP, thereby restricting YAP from localizing to the nucleus (16, 48). Consistent with these prior studies, loss of Crb3 in adult airway epithelium resulted in uncontrolled nuclear YAP/TAZ localization and activity. Genetic deletion of the Yap and Wwtr1(Taz) genes resolved airways to a normal pathology, demonstrating a critical function for these factors in precancerous lesion development. Interestingly, Yap and Taz exhibited redundancy in this phenotype, as deletion of either gene, together with Crb3, showed less of a rescue effect when compared to double Yap/Taz deletion. Such redundancy has been documented in other contexts, including in early animal development and in lung injury responses (29, 43). Thus, our observations show key roles for Yap and Taz in airway epithelium and highlight Crb3-mediated regulation of these factors as critical for airway epithelial homeostasis.
YAP/TAZ-regulated gene expression is implicated in epithelial regeneration in various organs, including the lung. Thus, airway epithelial polarity-mediated control of Yap/Taz localization likely functions as a mechanism to sense damage and initiate regenerative events. However, chronic damage to the epithelium (as in Crb3 deletion or cigarette smoke exposure) leads to aberrant activation of YAP/TAZ-regulated gene expression that promote an aberrant injury response that drives the precancerous state. Indeed, our profiling of YAP/TAZ-regulated gene expression demonstrated a distinct enrichment in gene expression changes associated with precancerous squamous lesions and LUSC (11). Notably, YAP/TAZ-regulated gene expression is association with bronchial dysplastic progression, suggesting that this gene signature may offer a biomarker for early disease detection and further highlight YAP/TAZ as attractive therapeutic targets for the interception of precancerous airway growth.
Our efforts to identify factors downstream of Crb3-YAP/TAZ signaling that drive paracrine dysplastic cell growth led to the identification of NRG1, a ligand for ERBB receptors. NRG1 has been implicated in squamous tumor growth and differentiation (49), and NRG1 gene fusions are associated with nonsmall cell lung cancers (50, 51). However, roles for NRG1 in precancerous lung biology have not been reported. We demonstrated that YAP/TAZ regulate the expression of NRG1 and that YAP and the TEAD family of transcription factors are recruited to the NRG1 promoter, suggesting direct control. Association of NRG1 expression with YAP/TAZ transcriptional activity showed a striking correlation in precancerous airway lesions isolated from different human patient cohorts. In situ RNA analyses of NRG1 using RNAscope confirmed elevated levels of NRG1 in human and Crb3-deleted precancerous lesions. By combining Cre-mediated lineage tracing with RNAscope, we identified Crb3-deleted cells flanking precancerous lesions that exhibit elevated NRG1 levels, which suggests elevated YAP/TAZ in polarity-defective luminal cells initiate paracrine signals to drive local basal cell growth. Interestingly, activation of ERBB receptors have been shown to induce nuclear YAP/TAZ activity (52–54), suggesting that paracrine activation of ERBB receptors by NRG1 initiate a feed-forward signaling cascade of activated YAP/TAZ > NRG1 > ERBB signaling that propagates in a precancer setting.
NRG1 is known to selectively bind to the ERBB3 and ERBB4 receptors, functioning as a ligand to induce receptor activation that subsequently stimulates homodimerization or heterodimerization with other ERBB receptors. Our experiments demonstrate that NRG1 stimulates the phosphorylation of ERBB3 in HBECs in vitro and that Crb3-cnull lesions exhibit elevated activation of ERBB3 in vivo. We further observed activation of ERBB2 in Crb3-cnull lesions in vivo, suggesting heteromerization of ERBB2/3 receptors. Supporting a direct role for ERBB receptors, we showed that inhibition of ERBB signaling using the irreversible pan-ERBB inhibitor, dacomitinib (37), resulted in a striking resolution of Krt5-positive cell expansion in Crb3-deleted airways. Dacomitinib treatment was capable of both preventing airway lesion development as well as treating predeveloped lesions in Crb3-cnull mice, suggesting ERBB inhibition as an avenue for the treatment of precancerous airways. Collectively, our study identifies epithelial cell polarity as a major factor in mammalian tissue homeostasis and delineates crucial signaling events resulting from epithelial damage that contributes to disease, opening opportunities for clinical therapy.
Materials and Methods
Animals Used in the Study.
Mice used in this study are outlined in SI Appendix, Table S3. All animal experiments were done in accordance with protocols approved by Boston University School of Medicine (Institutional Animal Care and Use Committee protocol AN-15304).
Cell Culture.
Normal HBECs isolated from nondiseased donors [Mattek Corporation, Cryopreserved Normal Human Bronchial Epithelial Cells (NHBE-CRY)], AALE cells (Meyerson Lab) and human bronchial epithelial dysplasia biopsy cells [HDys; primary nonimmortalized bronchial epithelial cells from moderate dysplasia bronchial biopsies obtained from the Colorado Lung SPORE Tissue Bank (55)] were cultured and maintained in Bronchial Epithelial Cell Growth Medium (Cell Applications, 511–500). 16HBE cells (EMD Millipore) were cultured in Dulbecco's modified Eagle medium (DMEM) + 10% fetal bovine serum (FBS) (Gibco, 26140079) and 1% penicillin/streptomycin (Gibco, 15140122). Basal cells isolated from mouse tracheas or from human bronchial brushings were differentiated in air–liquid interface (ALI) conditions (56). ALI-differentiated cells were exposed to gas-phase cigarette smoke using the Vitrocell VC1 in vitro smoke exposure system (VitrocellSystems). Cell cultures were examined 4 h postexposure by immunofluorescence microscopy or transepithelial electrical resistance was measured to determine epithelial permeability.
In Vivo Dacomitinib Administration.
Mice were administered dacomitinib via oral gavage (15 mg/kg) daily. Preventative therapy was composed of 12 daily oral gavages of dacomitinib beginning the same day as tamoxifen treatment. The therapeutic ERBB intervention was composed of 21 daily oral gavages of dacomitinib beginning 21 d after tamoxifen treatment.
Immunohistochemistry.
The tracheas and lungs of the mice were carefully dissected into 4% paraformaldehyde. Murine tissues were processed and embedded in paraffin. Staining was carried out on 5 um sections after standard dewaxing and hydration protocol, followed by heat-mediated antigen retrieval. The primary and secondary antibodies used in this study are listed in SI Appendix, Table S4.
Human Endobronchial Biopsies.
Formalin-fixed paraffin-embedded (FFPE) endobronchial biopsies tissue (n = 14 subjects) utilized to provide representative histological cases were obtained from high-risk subjects undergoing lung cancer screening at ∼1 y intervals by white light and auto-fluorescence bronchoscopy and computed tomography at RPCC. The Institutional Review Boards at Boston University Medical Center and Roswell approved the study and all subjects provided written informed consent.
RNA-Seq Library Preparation, Sequencing, and Data Processing.
Sequencing libraries were prepared from total RNA (extracted with the RNeasy kit, Qiagen) using Illumina TruSeq RNA Sample Preparation Kit v2. Differentially expressed genes associated with siRNA YAP/TAZ treatment were identified using a linear model across the filtered set of genes with independent variables for cell line and treatment. Genes significantly associated with treatment were selected by an FDR < 0.001 and an absolute fold change greater than 1.5 for the treatment term. Rotation gene set testing (57) was used to determine if genes up- and down-regulated with siRNA treatment were enriched (using 9,999 rotations, the mean statistic, and FDR < 0.01).
Krt5 and Nuclear YAP Quantification.
Krt5-positive cells were counted using the Cell Counter plugin for the Fiji software (version 1.52p). To quantify nuclear Yap, raw images were processed using CellProfiler. Nuclei were identified and the signal intensity of Yap within masked nuclei was calculated.
RNA In Situ Using RNAscope.
RNA in situ was carried performed using the RNAscope 2.5 HD Reagent Kit-RED (ACDBio, 322350) according to the manufacturer’s instructions on 5 um human and murine tracheal sections. The probes used are described in SI Appendix, Table S5.
ChIP.
AALE cells were used for ChIP experiments, using methods we have previously described (58, 59). Samples were analyzed by RT-qPCR to determine the percent input using the primers listed in SI Appendix, Table S6.
Real-Time qPCR.
For qPCR, RNA from experimental HBECs and 16HBE cells was extracted with the RNeasy kit (Qiagen) and reverse transcribed using iScript enzymes (BioRad). Gene expression analysis was carried out on the ViiA 7 Real-Time PCR System (Applied Biosystems). TaqMan probes (Life Technologies) used in this study are listed in SI Appendix, Table S7, and SYBR Green primers are listed in SI Appendix, Table S8
Supplementary Material
Acknowledgments
We thank Matthew Meyerson (Dana Farber Cancer Institute) for providing the AALE cell lines used in this study and Dr. Jeffrey Wrana for sharing the Yap-loxP/loxP and Wwtr1-loxP/loxP mice. We also thank Daniel Merrick (University of Colorado, Denver) and the Denver SPORE for facilitating the acquisition of the precancerous primary culture used in this study. We acknowledge support from the Boston University Flow Cytometry core and support by the National Center for Advancing Translational Sciences, NIH, through BU-CTSI Grant Number 1UL1TR001430. X.V. was funded by grant R01HL124392 from the NIH National Heart, Lung, and Blood Institute (NHLBI) and an American Cancer Society–Ellison New England Research Scholar Grant (RSG-17-138-01-CSM); N.M.K. was funded by NIH NHLBIGrant F31HL146163; J.H.-B. was funded by NIH NHLBI Grant F31HL132506; J.G.K. was funded by NIH National Cancer Institute Grant F31CA232683; J.B. was supported by a Janssen Pharmaceuticals Sponsored Research Agreement; and S.M. was supported by T32HL125232.
Footnotes
Competing interest statement: A.S. is an employee of Johnson & Johnson pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2019282118/-/DCSupplemental.
Data Availability
RNA-sequencing data have been deposited in National Center for Biotechnology Information Gene Expression Omnibus (GSE133493).
References
- 1.Rock J. R., Hogan B. L., Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu. Rev. Cell Dev. Biol. 27, 493–512 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Auerbach O., Stout A. P., Hammond E. C., Garfinkel L., Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N. Engl. J. Med. 265, 253–267 (1961). [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Boulan E., Macara I. G., Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halaoui R., McCaffrey L., Rewiring cell polarity signaling in cancer. Oncogene 34, 939–950 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Varelas X., The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Zhao R., et al., Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell 30, 151–165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahoney J. E., Mori M., Szymaniak A. D., Varelas X., Cardoso W. V., The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 30, 137–150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange A. W., et al., Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J. Mol. Cell Biol. 7, 35–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debaugnies M., et al., YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 19, e45809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiemer S. E., Szymaniak A. D., Varelas X., The transcriptional regulators TAZ and YAP direct transforming growth factor β-induced tumorigenic phenotypes in breast cancer cells. J. Biol. Chem. 289, 13461–13474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y.et al.; Cancer Genome Atlas Research Network , Comprehensive molecular characterization of the hippo signaling pathway in cancer. Cell Rep. 25, 1304–1317.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazellieres E., Assemat E., Arsanto J. P., Le Bivic A., Massey-Harroche D., Crumbs proteins in epithelial morphogenesis. Front. Biosci. 14, 2149–2169 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Nishida K., et al., Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L581–L591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghapour M., Raee P., Moghaddam S. J., Hiemstra P. S., Heijink I. H., Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: Role of cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 58, 157–169 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Beane J. E., et al., Molecular subtyping reveals immune alterations associated with progression of bronchial premalignant lesions. Nat. Commun. 10, 1856 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szymaniak A. D., Mahoney J. E., Cardoso W. V., Varelas X., Crumbs3-Mediated polarity directs airway epithelial cell fate through the hippo pathway effector Yap. Dev. Cell 34, 283–296 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Keymeulen A., et al., Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–193 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Rock J. R., et al., Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U.S.A. 106, 12771–12775 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock J. R., Randell S. H., Hogan B. L., Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 3, 545–556 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh M., et al., Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am. J. Respir. Cell Mol. Biol. 45, 403–410 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniely Y., et al., Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. Cell Physiol. 287, C171–C181 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Montoro D. T., et al., A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plasschaert L. W., et al., A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basil M. C., et al., The cellular and physiological basis for lung repair and regeneration: Past, present, and future. Cell Stem Cell 26, 482–502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawlins E. L., et al., The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525–534 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muthusamy N., Vijayakumar A., Cheng G. Jr, Ghashghaei H. T., A knock-in Foxj1(CreERT2:GFP) mouse for recombination in epithelial cells with motile cilia. Genesis 52, 350–358 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivas S., et al., Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J. K., et al., An FAK-YAP-mTOR signaling Axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell 21, 91–106.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishioka N., et al., The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Lundberg A. S., et al., Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene 21, 4577–4586 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Merrick D. T., et al., Altered cell-cycle control, inflammation, and adhesion in high-risk persistent bronchial dysplasia. Cancer Res. 78, 4971–4983 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research Network , Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012). Corrected in: Nature491, 288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao B., et al., Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B., et al., TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fock V., et al., Neuregulin-1-mediated ErbB2-ErbB3 signalling protects human trophoblasts against apoptosis to preserve differentiation. J. Cell Sci. 128, 4306–4316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sliwkowski M. X., et al., Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J. Biol. Chem. 269, 14661–14665 (1994). [PubMed] [Google Scholar]
- 37.Engelman J. A., et al., PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 67, 11924–11932 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Margolis B., The Crumbs3 polarity protein. Cold Spring Harb. Perspect. Biol. 10, a027961 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merrick D. T., et al., Persistence of bronchial dysplasia is associated with development of invasive squamous cell carcinoma. Cancer Prev. Res. (Phila.) 9, 96–104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong K. U., Reynolds S. D., Watkins S., Fuchs E., Stripp B. R., Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 164, 577–588 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tata P. R., et al., Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503, 218–223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell J. D.et al.; Cancer Genome Atlas Research Network , Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 48, 607–616 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaCanna R., et al., Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Invest. 129, 2107–2122 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun T., et al., TAZ is required for lung alveolar epithelial cell differentiation after injury. JCI Insight 5, e128674 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z., et al., MAPK-mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep. 16, 1810–1819 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Chen C. L., et al., The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 107, 15810–15815 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ling C., et al., The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. U.S.A. 107, 10532–10537 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varelas X., et al., The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 19, 831–844 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Hegde G. V., et al., NRG1 is a critical regulator of differentiation in TP63-driven squamous cell carcinoma. eLife 8, e46551 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drilon A., et al., Response to ERBB3-directed targeted therapy in NRG1-rearranged cancers. Cancer Discov. 8, 686–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Cuesta L., et al., CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 4, 415–422 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Haskins J. W., Nguyen D. X., Stern D. F., Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci. Signal. 7, ra116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komuro A., Nagai M., Navin N. E., Sudol M., WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J. Biol. Chem. 278, 33334–33341 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Aharonov A., et al., ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 22, 1346–1356 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Franklin W. A., et al., Expansion of bronchial epithelial cell populations by in vitro culture of explants from dysplastic and histologically normal sites. Am. J. Respir. Cell Mol. Biol. 15, 297–304 (1996). [DOI] [PubMed] [Google Scholar]
- 56.You Y., Richer E. J., Huang T., Brody S. L., Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L1315–L1321 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Wu D., et al., ROAST: Rotation gene set tests for complex microarray experiments. Bioinformatics 26, 2176–2182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiemer S. E., et al., A YAP/TAZ-Regulated molecular signature is associated with oral squamous cell carcinoma. Mol. Cancer Res. 13, 957–968 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beyer T. A., et al., Switch enhancers interpret TGF-β and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep. 5, 1611–1624 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-sequencing data have been deposited in National Center for Biotechnology Information Gene Expression Omnibus (GSE133493).