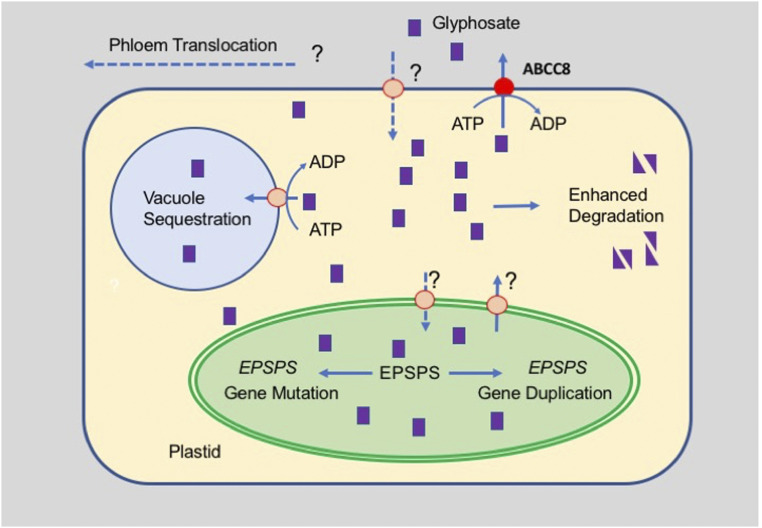
Synthetic herbicides have been used widely for more than 70 y and have substantially contributed to the efficiency of agriculture. Glyphosate [N-(phosphonomethyl)glycine] was marketed in 1974 under the trade name Roundup and has become the most used herbicide worldwide. It is a postemergence, nonselective herbicide of low toxicity to animals and humans. While its metabolism in plants is limited, its breakdown in the soil is relatively fast (1). The slow action of glyphosate is related to the fact that it needs to be transported to meristematic areas to become effective. The observation that plants treated with glyphosate accumulate large amounts of shikimate led to the discovery that glyphosate inhibits the 5-enolpyruvyl-shikimate-3 phosphate synthase (EPSPS), an enzyme of the shikimate pathway, which in plants resides in the plastid (Fig. 1) (2). EPSP is converted to chorismate, which is a central metabolite in the synthesis of the three aromatic amino acids. For more than 20 y after its introduction no notable resistance to glyphosate was observed in weeds. In 1996, a transgenic glyphosate-tolerant soybean (Roundup Ready) was introduced, which carried a bacterial gene coding for a glyphosate-insensitive form of EPSPS. Other Roundup Ready major crop plants soon followed, leading to an enormous increase in the application of the herbicide (1, 3). This has raised increasing concern about ground and surface-water pollution and the appearance of residues in food products (4). As a consequence of the increased selective pressure, an increasing number of weeds in fields around the world developed glyphosate resistance (3, 4). Target (EPSPS)-related insensitivity to glyphosate was traced to either mutations in the enzyme or its overproduction by gene amplification or enhanced expression (Fig. 1). The stepwise evolution of mutations (up to three) increased the severity of weed resistance in the field. Other known mechanisms contributing to glyphosate resistance are vacuolar sequestration, reduced translocation, and degradation by an aldo-keto reductase (Fig. 1) (4). Additional, hitherto putative, mechanisms of resistance against glyphosate are indicated in Fig. 1, i.e., the active export across the plastid envelope or across the plasma membrane. The latter mechanism has now been identified. In their publication in PNAS, Pan et al. show that up-regulation of an ABC transporter of the ABCC subfamily residing in the plasma membrane confers glyphosate resistance to Echinocloa colona populations (5).
Fig. 1.
Mechanisms of resistance to glyphosate. Glyphosate (purple squares) enters the cytoplasm/symplasm by an unknown mechanism. Its cytosolic concentration can be reduced by degradation (aldo-ketoreductase), vacuolar sequestration (ATP-dependent vacuolar import of glyphosate is likely, but the involvement of an ABC transporter is not proven), or by extrusion into the extracellular space mediated by ABCC8. No information is available on the mechanism of the transport of glyphosate across the plastid envelope. The target of glyphosate, EPSPS, resides in the plastid, and glyphosate resistance can result from nuclear EPSPS mutation, duplication, or enhanced expression. Solid lines indicate up-regulation of a process, and dashed lines indicate down-regulation. Modified from ref. 3.
ABC proteins belong to an evolutionarily ancient family present in all living organisms (6, 7). The transport process catalyzed by ABC transporters is directly energized by the hydrolysis of adenosine 5′-triphosphate (ATP) (7, 8). Plants contain more ABC proteins than organisms of any other phylum. Most plants encode for 120 to 140 members of this family, but in some plants their number may exceed 200 (7, 9, 10). This large number may be due to the sessile lifestyle of plants, which cannot escape stresses and hence require an adaptive defense mechanism. A huge number of secondary compounds, which are part of this defense mechanism, are potential substrates for this class of transporters. The ABC protein family can be subdivided into the subclasses ABCA to ABCI. Plants contain members of all classes with the exception of ABCH (7, 9). Most of them act as transporters and are involved in the deposition of surface lipids, in allocation of hormones, and in detoxification processes. In plants, members of the ABCC transporter subfamily have so far only been localized to the vacuolar membrane (7). They are known to transport plant-derived compounds and xenobiotics conjugated to glutathione, glucuronic acid, or glucose. Further substrates for plant ABCCs are chlorophyll degradation metabolites, free phytochelatins, and phytochelatin–metal(oid) complexes and phytate (7, 8). Thus, ABCCs have so far been described as transporters involved in internal detoxification.
In previous field work in Western Australia, the authors had discovered a population of E. colona (Poaceae) that exhibited a pronounced resistance against glyphosate. They could exclude that the resistance was due to a mutation in EPSPS or to impaired translocation of glyphosate (11). In their present work, Pan et al. have now used RNA sequencing followed by an RT-PCR verification to identify proteins possibly involved in the resistance mechanism (5). They identified two genes coding for ABC transporters of the ABCC subfamily, EcABCC8 and EcABCC10, that are consistently up-regulated in glyphosate-resistant E. colona plants as compared to the sensitive plants. Interestingly, higher temperatures caused expression of the two ABCC transporter genes at an even higher level. The amino acid sequences of the two transporters in the resistant and susceptible plants were identical, suggesting that different expression levels of the ABCCs between the resistant and sensitive plants were responsible for their differential sensitivity to glyphosate. For functional characterization of the ABC transporters the authors expressed them heterologously in rice. While rice plants ectopically expressing EcABCC10 were sensitive to glyphosate, the growth of plants containing the EcABCC8 construct was unaffected by the compound at herbicidal concentration. The resistance was specific for glyphosate, since EcABCC8-expressing rice plants (EcABCC8-E rice) did not survive when exposed to the primary glyphosate metabolite aminomethylphosphonic acid (AMPA) or to glufosinate (phosphinothricin, a herbicide inhibiting glutamine synthetase). When the EcABCC8 orthologs of rice, maize, and soybean were overexpressed in the respective plants, all transgenic plants exhibited a reduced sensitivity to glyphosate. The weakest effect was observed in soybean. This may be due to the intrinsic high susceptibility of soybean to glyphosate and/or to the fact that the soybean ABCC8 ortholog has a lower sequence homology to EcABCC8 than the rice and maize orthologs. Rice lines lacking their ABCC8 gene were then produced using CRISPR-Cas9 and were indeed found to be even more susceptible to glyphosate than the wild type.
The analysis of the respective promoters of the EcABC8 genes in the susceptible and resistant E. colona lines revealed only three single-nucleotide polymorphisms at the base level, while the methylation patterns differed in two regions and may thus be responsible for the enhanced expression of the transporter in the resistant plants.
EcABCC8 was found to be predominantly expressed in stem and leaf tissues but also in roots. The patterns of distribution were similar in resistant and sensitive plants; however, the expression was up to 10-fold higher in all tissues of the resistant variety. Cellular localization using a 35S:EcABCC8-GFP construct indicated that this ABC transporter is targeted to the plasma membrane. This finding was surprising since, as mentioned above, plant ABCC-type transporters, in contrast to those of animals and humans, have so far been localized only to the vacuolar membrane. In line with the reported plasma membrane localization, efflux of glyphosate from glyphosate-loaded leaf discs of EcABCC8-E rice was twice as fast as from those of wild-type rice plants, while it was slower than in wild type in leaf discs of the loss-of-function rice plants. Correspondingly, glyphosate contents were lowest in leaf discs of EcABCC8-E rice and highest in those of the loss-of-function plants. Furthermore, while uptake of glyphosate into mesophyll protoplasts from sensitive rice plants was continuous over time, glyphosate levels declined in the ABCC8-E protoplasts after an initial uptake phase, which supports the hypothesis that EcABCC8 exports glyphosate. No such results were found for AMPA, to which the glyphosate-resistant plants are sensitive. Modeling studies using the human HsABCC1 as structural homolog identified a pocket into which glyphosate fits well, while AMPA binding is insufficiently stabilized, explaining why AMPA is not transported by EcABCC8.
Even though no direct transport experiments with isolated plasma membrane vesicles are presented in this work, the authors present convincing data that EcABCC8 and its homologs transport glyphosate, are located at the plasma membrane, and extrude glyphosate from the cell. At physiological pH, glyphosate is an organic anion and hence a potential substrate candidate for ABCCs. It will now be interesting to search for physiological substrates. The plasma membrane localization is intriguing, and at first sight overexpression of EcABCC8 may have resulted in its mistargeting. However, the larger amount of glyphosate retained in leaf discs of the loss-of-function plants compared to the wild type indicates that EcABCC8 localizes to the plasma membrane even when the protein is not up-regulated. Hence, it will be interesting to identify the component(s) that change the targeting of an ABCC from the vacuole to the plasma membrane.
Putatively ATP-dependent vacuolar sequestration of glyphosate in resistant Conyza canadensis (horseweed, Asteraceae) has been demonstrated employing 31P NMR in vivo, and involvement of an ABC transporter was suggested (12). In this case, the putative transporter did not differentiate between glyphosate and AMPA. The same group had previously conducted a transcriptomic analysis of glyphosate-sensitive and -insensitive plants of C. canadensis and found differential expression of various ABC transporters, but no specific evidence for the involvement of any of them in nontarget glyphosate resistance was found (13). It remains to be seen 1) how universally plants use ABCC8 for glyphosate export across the plasma membrane and 2) which transporters are involved in its vacuolar sequestration.
Footnotes
The authors declare no competing interest.
See companion article, “An ABCC-type transporter endowing glyphosate resistance in plants,” 10.1073/pnas.2100136118.
References
- 1.Duke S. O., Powles S. B., Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 64, 319–325 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Steinrücken H. C., Amrhein N., The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem. Biophys. Res. Commun. 94, 1207–1212 (1980). [DOI] [PubMed] [Google Scholar]
- 3.Gaines T. A., et al., Mechanisms of evolved herbicide resistance. J. Biol. Chem. 295, 10307–10330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapp J., Explaining growing glyphosate use: The political economy of herbicide-dependent agriculture. Glob. Environ. Change 67, 102239 (2021). [Google Scholar]
- 5.Pan L., et al., An ABCC-type transporter endowing glyphosate resistance in plants. Proc. Natl. Acad. Sci. U.S.A. 118, e2100136118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins C. F., ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992). [DOI] [PubMed] [Google Scholar]
- 7.Kang J., et al., Plant ABC transporters. Arabidopsis Book 9, e0153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang J. U., et al., Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant 9, 338–355 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Verrier P. J., et al., Plant ABC proteins—A unified nomenclature and updated inventory. Trends Plant Sci. 13, 151–159 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Mishra A. K., Choi J., Rabbee M. F., Baek K. H., In silico genome-wide analysis of the ATP-binding cassette transporter gene family in soybean (Glycine max L.) and their expression profiling. BioMed Res. Int. 2019, 8150523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh S. S., et al., Non-targeted-site glyphosate resistance in Echinocloa colona from Western Australia. Crop Prot. 112, 257–263 (2018). [Google Scholar]
- 12.Ge X., d’Avignon D. A., Ackerman J. J., Sammons R. D., In vivo 31P-nuclear magnetic resonance studies of glyphosate uptake, vacuolar sequestration, and tonoplast pump activity in glyphosate-resistant horseweed. Plant Physiol. 166, 1255–1268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J. S., et al., Functional genomics analysis of horseweed (Conyza canadensis) with special reference to the evolution of non–target-site glyphosate resistance. Weed Sci. 58, 109–117 (2010). [Google Scholar]