Abstract
INTRODUCTION
Cigarette smoking has been hypothesized to be a risk factor for meningioma. However, the results of studies exploring the relationship between smoking exposure and the occurrence of meningioma are inconsistent.
METHODS
A search of PubMed, Medline, Embase, and Science Direct (up to June 2020) databases was performed. Two authors independently extracted the data. The Newcastle–Ottawa Scale was employed for judging the quality of articles. A random-effects model was utilized for meta-analysis. Association analysis between smoking and meningioma was based on the adjusted RR and the 95% CI, as reported by eligible studies. Subgroup and sensitivity analyses were performed and publication bias was assessed. Subgroup analysis was conducted by geographical region, study design, sex, study quality, and adjustments of RR score. Begg’s and Egger’s tests were employed for detecting publication bias.
RESULTS
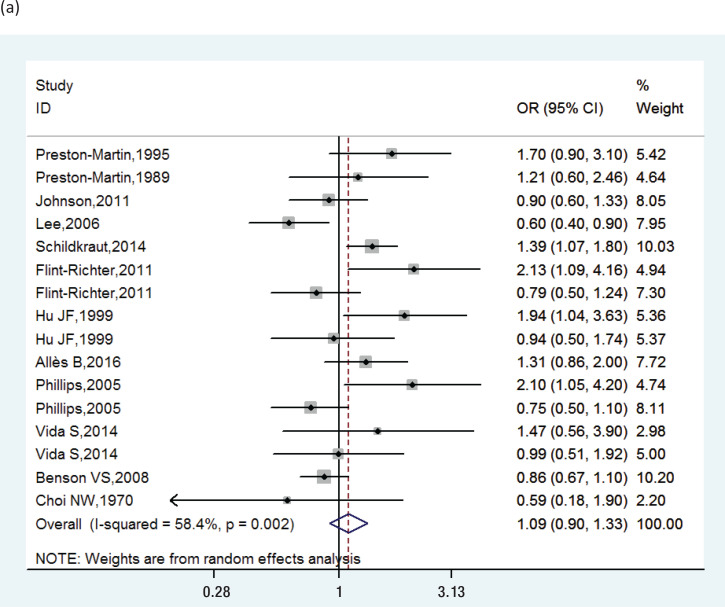
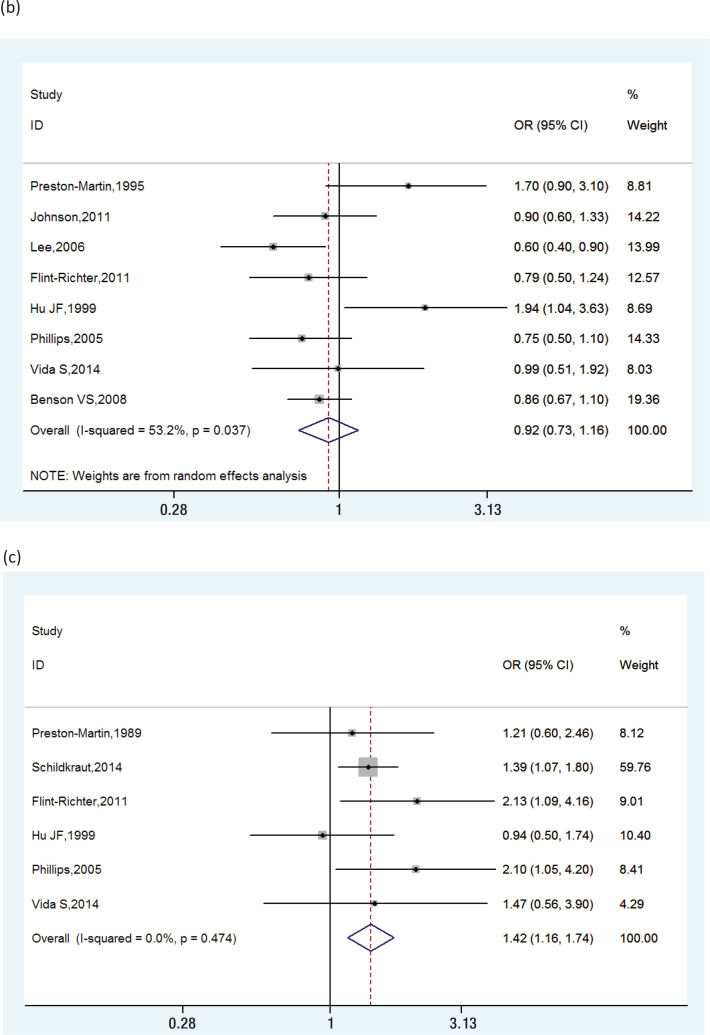
Twelve articles, including 2 cohort studies and 10 case–control studies, and a total of 1210167 participants were identified. The pooled relative risk (RR) with 95% confidence interval (95% CI) implied that smoking was not associated with increased risk of meningioma in men and women combined (RR=1.09; 95% CI: 0.90–1.33). From the sex-stratified subgroup analysis, the risk of meningioma was significant in men (RR=1.42; 95% CI: 1.16–1.74). Risk of meningioma in women did not remain significant (RR=0.92; 95% CI: 0.73–1.16). There was a high heterogeneity in the results (I2=58.4%, p=0.002). Sensitivity analyses showed stable results and there was no evidence of publication bias.
CONCLUSIONS
Cigarette smoking is not associated with a significantly increased risk of meningioma in the whole population, but there is a positive association in men but not in women.
Keywords: meningioma, smoking, meta-analysis
INTRODUCTION
Smoking is a risk factor for many diseases1. There are more than 7000 chemicals in tobacco smoke, hundreds of which are harmful. Smoking is popular all over the world. It is estimated that approximately 6 million people die each year from smoking and environmental tobacco exposure2. Some studies have found that smoking increases the risk of several types of cancer, including lung, oral, throat, esophageal, gastric, colon, and rectal cancer3. In addition, components such as n-nitroso compounds in cigarette smoke can cross the blood–brain barrier4. Animal experiments have proved that smoking is associated with meningioma5-7. Meningioma accounts for approximately 25% of all primary adult intracranial tumors, and it is more common in women. It is more common in middle-aged and elderly patients8. Many epidemiological studies have investigated the possible link between the occurrence of meningioma and smoking, but the results were inconsistent. Three studies found a positive association between active smoking (in men9-11) and meningioma. However, others studies did not find a positive association between active smoking (in men12-14, women9,10,14-17, or both18,19) and meningioma. Recently, a meta-analysis by Fan et al.20 concluded that smoking was not associated with a significantly increased risk of meningioma. We performed the present study to further investigate a possible association between active smoking and the risk of developing meningioma by sex-stratified analysis.
METHODS
Search strategy and selection criteria
According to the meta-analysis of observational studies in epidemiology (MOOSE) guidelines21, two authors searched the relevant publications in PubMed, Medline, Embase, and Science Direct. We restricted our literature search to human studies that were published in English and tried identifying non-published studies. Searching covered single words or combinations, including ‘meningioma’ or ‘meningeal neoplasms’ or ‘meningeal tumor’ with smoking (‘tobacco,’ ‘smoke,’ ‘cigarette’, ‘smoker’). To find more articles, a manual retrieval of relevant articles and references was performed. The inclusion criteria were: 1) the study assessed the relationship between smoking and meningioma; 2) a case-control study or cohort study; 3) the study reported relative risk (RR) or odds ratio (OR) and 95% confidence interval (CI), or the original data allowed this to be calculated; and 4) data of smoking status include smoking (including ever and current) versus never smoking. If the subject inhaled directly cigarettes that was regarded as active smoking. Active smokers were defined as active smoking of at least 100 cigarettes or for six months or more. Otherwise, subjects were classified as never active smokers. Criteria for exclusion were: 1) animal experiments or mechanistic research; 2) the study investigated passive smoking or environment smoking; and 3) the publication was in the form of a letter, conference paper, review, or case report.
Data extraction and quality assessment
Two authors undertook independent evaluations of titles and abstracts of cited articles. The following data were extracted: first author’s name, publication year, study design, number of participants, country, assessment of outcome, estimated effect size (RR), corresponding 95% CI, and adjusted factors. Quality assessment was conducted using the Newcastle– Ottawa Quality Assessment Scale (NOS); studies with NOS score ≥7 were considered of high quality, and studies with NOS score ≥5 were considered of moderate quality22.
Statistical analysis
Association analysis between smoking and meningioma was based on the adjusted RR and the 95% CI, as reported by eligible studies. The Q test and I2 statistic were used to assess heterogeneity among selected studies23. Considering the large variation in terms of study design and study population characteristics of all the included studies, it is more prudent to always use random-effects model regardless of the I2 value.
Subgroup analyses were conducted according to the following characteristics: geographical region (US/Europe or Asia), study design (case–control or cohort), sex (men or women), study quality (high or moderate), and adjustments of RR score (Yes or No).
Begg’s and Egger’s tests were employed for detecting publication bias. For evaluating the stabilities of the meta-estimates, sensitivity analysis was adopted by removing one article at a time. STATA version 13.0 was utilized for performing all data analyses.
RESULTS
Study selection
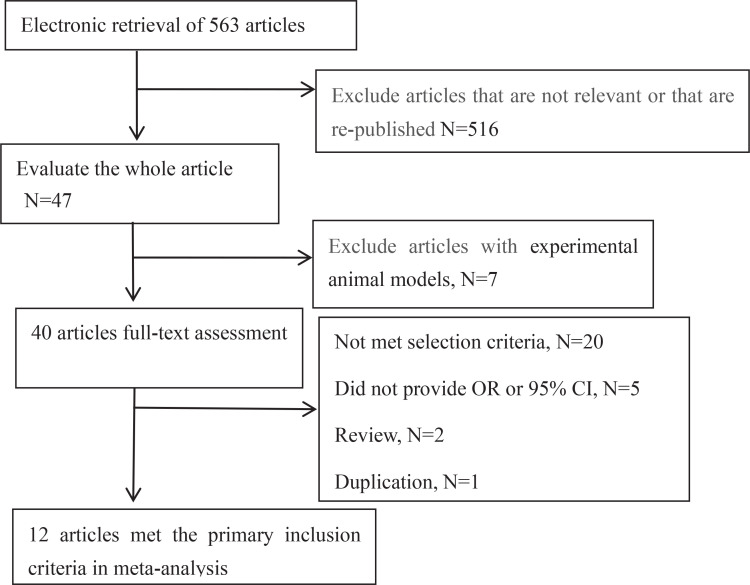
In Figure 1, the details of the whole process and 12 eligible papers for meta-analysis are presented. The selected papers included 10 case–control studies9-15,18,19,24 and 2 cohort studies16,17. The selected studies were conducted in the US9,11,13,15,17,24, Canada14,18, Israel10, China12, UK16, and France19. Of the 12 selected articles, six reported smoking in men9-14 and eight in woman9,10,12,14-17,24. Studies on the different sexes were mostly conducted independently. According to the nine-point NOS, six studies9,10,12,14,16,17 were of high quality and six studies11,13,15,18,19,24 were of moderate quality. The details of each study are provided in Table 1.
Figure 1.
Flowchart presenting the steps of literature search and selection
Table 1.
Main characteristics of included studies on the active smoking and risk of meningioma
| No. | Author, Year | Case Men/Women | Control Men/Women | Study design | Country | Sex | RR | 95% CI | Adjustment | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Phillips et al.9 2005 | 57/143 | 114/286 | Case-control | US | Men | 2.10 | 1.05–4.20 | Education | High |
| Women | 0.75 | 0.50–1.10 | ||||||||
| 2 | Flint-Richter et al.10 2011 | 71/171 | 84/196 | Case-control | Israel | Men | 2.13 | 1.09–4.16 | Radiation | High |
| Women | 0.79 | 0.50–1.24 | ||||||||
| 3 | Schildkraut et al.11 2014 | 456/0 | 452/0 | Case-control | US | Men | 1.39 | 1.07–1.80 | NA | Moderate |
| 4 | Hu et al.12 1999 | 70/113 | 140/226 | Case-control | China | Men | 0.94 | 0.50–1.74 | Income, education, occupational exposure to chemicals, consumption of fruit and vegetables | High |
| Women | 1.94 | 1.04–3.63 | ||||||||
| 5 | Preston-Martin et al.13 1989 | 70/0 | 70/0 | Case-control | US | Men | 1.21 | 0.60–2.46 | NA | Moderate |
| 6 | Vida et al.14 2014 | 26/67 | 317/331 | Case-control | Canada | Men | 1.47 | 0.56–3.90 | Age, sex, education, region | High |
| Women | 0.99 | 0.51–1.92 | ||||||||
| 7 | Preston-Martin et al.15 1995 | 0/81 | 0/155 | Case-control | US | Women | 1.70 | 0.90–3.10 | Age, menstruating, ERT use, OC, radiography | Moderate |
| 8 | Benson et al.16 2008 | 0/372 | 0/1177087 | Cohort | UK | Women | 0.86 | 0.67–1.10 | Height, BMI, strenuous exercise, socioeconomic level, alcohol intake, parity, age at first birth, OC | High |
| 9 | Johnson et al.17 2011 | 0/125 | 0/27791 | Cohort | US | Women | 0.90 | 0.60–1.33 | Education, residence, alcohol use, physical activity index | High |
| 10 | Choi et al.18 1970 | 23 | 23 | Case-control | Canada | Both | 0.59 | 0.18–1.90 | NA | Moderate |
| 11 | Allès et al.19 2016 | 193 | 392 | Case-control | France | Both | 1.31 | 0.86–2.00 | NA | Moderate |
| 12 | Lee et al.24 2006 | 0/217 | 0/248 | Case-control | US | Women | 0.60 | 0.40–0.90 | NA | Moderate |
OC: oral contraceptive. ERT: estrogen replacement therapy. NA: not applicable. BMI: body mass index.
Cigarette smoking and risk of meningioma
The pooled RRs of cigarette smoking with meningioma are shown in Figure 2. We found significant heterogeneity (I2=58.4%), and a random-effects model was used to calculate the pooled RR. The combined RR was 1.09 (95% CI: 0.90–1.33).
Figure 2.
Forest plots showing individual and pooled RRs with 95% CI of the risk between active smoking and meningioma: (a) in men and women combined, (b) in women, (c) in men
Subgroup and sensitivity analysis
Subgroup analysis on the basis of study design, geographical regions, publication year, and adjustments of RR score showed that the results remained similar. In women, smoking was not a significant risk for meningioma (RR=0.92; 95% CI: 0.73–1.16) among 8 studies (I2=53.2%, p=0.037). However, in men, smoking was a significant risk for meningioma (RR=1.42; 95% CI: 1.16–1.74), within six studies (I2=0.0%, p= 0.474). Sensitivity analysis confirmed that the results were stable by the removal of one study at a time. Table 2 and Supplementary file Figure 1 show the data from our subgroup and sensitivity analyses.
Table 2.
Subgroup and sensitivity analysis of all the included studies
| Subgroup | Number of reports | RR (95% CI) | p* | I 2 (%) |
|---|---|---|---|---|
| Geographical region | ||||
| US/Europe | 10 | 1.03 (0.91–1.16) | 0.124 | 44.8 |
| Asia | 2 | 1.20 (0.91–1.60) | 0.044 | 53.7 |
| Study design | ||||
| Case–control | 10 | 1.13 (0.99–1.29) | 0.002 | 60.2 |
| Cohort | 2 | 0.87 (0.71–1.07) | 0.849 | 0.00 |
| Sex | ||||
| Men | 6 | 1.42 (1.16–1.74) | 0.474 | 0.00 |
| Women | 8 | 0.92 (0.73–1.16) | 0.037 | 53.2 |
| Study quality | ||||
| High | 6 | 1.09 (0.93–1.29) | 0.024 | 52.9 |
| Moderate | 6 | 1.02 (0.87–1.18) | 0.004 | 71.4 |
| Adjustments of RR score | ||||
| Yes | 7 | 1.01 (0.88–1.17) | 0.011 | 56.3 |
| No | 5 | 1.12 (0.93–1.35) | 0.009 | 70.3 |
For heterogeneity test.
Publication bias
A funnel plot was employed to evaluate publication bias. There was no obvious publication bias. Begg’s and Egger’s tests yielded no statistical significance (p=0.192 and p=0.360, respectively, Supplementary file Figure 2).
DISCUSSION
Meningioma is the most common subtype of brain tumor in adults, with an incidence rate of 3.5 per 100000 person-years25. The 5-year survival rate is 72% for women and 66% for men26. These tumors are most common in women, with a women-to-men ratio of about 2:1, and most commonly occur between adolescence and menopause. Meningiomas are tumors that originate in the arachnoid layer of the meninges. Although generally benign in histological appearance and behavior, 5–10% of these tumors are malignant. At present, the cause of meningioma is still largely unclear, but several studies have shown that the triggers for their development include radiation, brain injuries, smoking, and female hormones9,27. Cigarette smoke is a complex mixture of chemicals and is the single most important cause of cancer in humans. It has been shown to induce tumors in many organs and tissues.
This is the largest meta-analysis to examine the relationship between cigarette smoking and meningioma risk. A total of 1210167 participants were included. According to our study, active smoking may increase the risk of meningioma in men (RR=1.42; 95% CI: 1.16–1.74), but not significantly in the whole population (RR= 1.09; 95% CI: 0.90–1.33). Our results are similar to those of another meta-analysis20 of smoking and risk of meningioma, which obtained an OR of 0.95 (95% CI: 0.87–1.07). The study by Fan et al.20 included 9 papers and passive smoking, whereas 12 papers were included in the present study. Five studies11,14,15,17,19 which were not included in the Fan et al.20 study were included in our meta-analysis as they met our inclusion criteria. Another meta-analysis obtained an OR for smoking of 0.82 (95% CI: 0.68–0.98, n=6) for women and 1.39 (95% CI: 1.08–1.79, n=5) for men28. Our results show that smoking is not a significant risk for women (RR=0.92; 95% CI: 0.73–1.16, n=8), but it plays a bigger role in men (RR=1.42; 95% CI: 1.16–1.74, n=6). There are three possible reasons to explain this difference. Firstly, our study included a larger sample size and passive smoking was excluded. Secondly, the exposure intensity is different, and active smoking is much stronger. Lastly, the type of tobacco is different between men and women, and men smokers smoke more than women smokers29.
Limitations
There are still some limitations in this study. First, there was a lack of accurate assessment of exposure to cigarette smoking. Despite a feasibility of crude classifications, this was inevitable. Second, the studies used questionnaires to evaluate smoking, but self-reported methods could easily result in reporting bias. Researchers should use biomarkers or specific substrates in the body to determine exposure doses more accurately. Third, we did not study the relationship between different levels of tobacco exposure and the risk for meningioma because there was insufficient information about the dose–response relationship.
CONCLUSIONS
This meta-analysis indicates that cigarette smoking does not increase the risk of developing meningioma, in the whole population. However, sex-stratified subgroup analysis indicates a positive association in men but not in women.
Supplementary Material
CONFLICTS OF INTEREST
The authors have each completed and submitted an ICMJE form for disclosure of potential conflicts of interest. The authors declare that they have no competing interests, financial or otherwise, related to the current work. All the authors report that they received funding by Youth Doctor Fund Projects of Qiqihar Medical University (QMSI2019B-07). H. Chao, Y. Cheng, H.F. Xue, H.J. Li and Meng E report that they also received funding by the University Basic Scientific Research Operating Expenses in Heilongjiang Province (2019-KYYWF-1277/ Heilongjiang Provincial Department of Education).
FUNDING
This work was supported by Youth Doctor Fund Projects of Qiqihar Medical University (QMSI2019B-07) and the University Basic Scientific Research Operating Expenses in Heilongjiang Province (2019-KYYWF-1277).
ETHICAL APPROVAL AND INFORMED CONSENT
Not required, as this is a review of existing literature.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed.
REFERENCES
- 1.Wipfli H, Samet JM. Global economic and health benefits of tobacco control: part 1. Clin Pharmacol Ther. 2009;86:263–271. doi: 10.1038/clpt.2009.93. [DOI] [PubMed] [Google Scholar]
- 2.Giraldi G, Ruggiero GF, Marsella LT, d'Alessandro EDL. Environmental tobacco smoke: health policy and focus on Italian legislation. Clin Ter. 2013;164:e429–e435. doi: 10.7417/CT.2013.1623. [DOI] [PubMed] [Google Scholar]
- 3.The Health Consequences of Smoking: A Report of the Surgeon General. US Centers for Disease Control and Prevention; 2004. Accessed October 27, 2020. https://pubmed.ncbi.nlm.nih.gov/20669512/ [PubMed] [Google Scholar]
- 4.Il’yasova D, McCarthy BJ, Erdal S, et al. Human exposure to selected animal neurocarcinogens: a biomarker-based assessment and implications for brain tumor epidemiology. J Toxicol Environ Health B Crit Rev. 2009;12(3):175–187. doi: 10.1080/10937400902894152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachance DH, Yang P, Johnson DR, et al. Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. Am J Epidemiol. 2011;174(5):574–581. doi: 10.1093/aje/kwr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoemaker MJ, Robertson L, Wigertz A, et al. Interaction between 5 genetic variants and allergy in glioma risk. Am J Epidemiol. 2010;171(11):1165–1173. doi: 10.1093/aje/kwq075. [DOI] [PubMed] [Google Scholar]
- 7.Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41(8):905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088–1095. doi: 10.1227/01.NEU.0000188281.91351.B9. [DOI] [PubMed] [Google Scholar]
- 9.Phillips LE, Longstreth WT, Jr, Koepsell T, Custer BS, Kukull WA, van Belle G. Active and passive cigarette smoking and risk of intracranial meningioma. Neuroepidemiology. 2005;24(3):117–122. doi: 10.1159/000082998. [DOI] [PubMed] [Google Scholar]
- 10.Flint-Richter P, Mandelzweig L, Oberman B, Sadetzki S. Possible interaction between ionizing radiation, smoking, and gender in the causation of meningioma. Neuro Oncol. 2011;13(3):345–352. doi: 10.1093/neuonc/noq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schildkraut JM, Calvocoressi L, Wang F, et al. Endogenous and exogenous hormone exposure and the risk of meningioma in men. J Neurosurg. 2014;120(4):820–826. doi: 10.3171/2013.12.JNS131170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Little J, Xu T, et al. Risk factors for meningioma in adults: a case–control study in northeast China. Int J Cancer. 1999;83(3):299–304. doi: 10.1002/(sici)1097-0215(19991029)83:3<299::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Preston-Martin S, Mack W, Henderson BE. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49(21):6137–6143. Accessed October 27, 2020. https://cancerres.aacrjournals.org/content/49/21/6137.long. [PubMed] [Google Scholar]
- 14.Vida S, Richardson L, Cardis E, et al. Brain tumours and cigarette smoking: analysis of the INTERPHONE Canada case-control study. Environ Health. 2014;13:2–9. doi: 10.1186/1476-069x-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston-Martin S, Monroe K, Lee PJ, et al. Spinal meningiomas in women in Los Angeles County: investigation of an etiological hypothesis. Cancer Epidemiol Biomarkers Prev. 1995;4(4):333–339. Accessed October 27, 2020. https://cebp.aacrjournals.org/content/4/4/333.long. [PubMed] [Google Scholar]
- 16.Benson VS, Pirie K, Green J, Casabonne D, Beral V. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99(1):185–190. doi: 10.1038/sj.bjc.6604445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson DR, Olson JE, Vierkant RA, et al. Risk factors for meningioma in postmenopausal women: results from the Iowa Women’s Health Study. Neuro Oncol. 2011;13(9):1011–1019. doi: 10.1093/neuonc/nor081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi NW, Schuman LM, Gullen WH. Epidemiology of primary central nervous system neoplasms. II. Case-control study. Am J Epidemiol. 1970;91(5):467–485. doi: 10.1093/oxfordjournals.aje.a121158. [DOI] [PubMed] [Google Scholar]
- 19.Allès B, Pouchieu C, Gruber A, et al. Dietary and Alcohol Intake and Central Nervous System Tumors in Adults: Results of the CERENAT Multicenter Case-Control Study. Neuroepidemiology. 2016;47(3-4):145–154. doi: 10.1159/000450580. [DOI] [PubMed] [Google Scholar]
- 20.Fan Z, Ji T, Wan S, et al. Smoking and risk of meningioma: A meta-analysis. Cancer Epidemiol. 2013;37(1):39–45. doi: 10.1016/j.canep.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Lee E, Grutsch J, Persky V, Glick R, Mendes J, Davis F. Association of meningioma with reproductive factors. Int J Cancer. 2006;119(5):1152–1157. doi: 10.1002/ijc.21950. [DOI] [PubMed] [Google Scholar]
- 25.Central Brain Tumor Registry of the United States . Statistical report: primary brain tumors in the United States, 1997-2001. Central Brain Tumor Registry of the United States; 2004. [Google Scholar]
- 26.McCarthy BJ, Davis FG, Freels S, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88(5):831–839. doi: 10.3171/jns.1998.88.5.0831. [DOI] [PubMed] [Google Scholar]
- 27.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 28.Claus EB, Walsh KM, Calvocoressi L, et al. Cigarette Smoking and Risk of Meningioma: The Effect of Gender. Cancer Epidemiol Biomarkers Prev. 2012;21(6):943–950. doi: 10.1158/1055-9965.EPI-11-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eissenberg T, Adams C, Riggins 3rd EC, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999;1(4):317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.