Abstract
The permeability of cultured endothelial monolayers is higher than the permeability of endothelium in vivo. Co-culture with astrocytes can induce a tight, blood-brain-barrier phenotype in aortic endothelium in vitro. We hypothesised that dendritic cells, which reside in the intima of non-cerebral arteries and have features in common with astrocytes, may also reduce the permeability of cultured aortic endothelium. The permeability of porcine aortic endothelial monolayers was reduced by non-contact co-culture with dendritic cells (but not with the peripheral blood monocytes from which they were derived) and by dendritic cell conditioned medium, indicating a soluble mediator. The reduction in permeability was similar to that obtained by co-culture with astrocytes; however, dendritic cells did not up-regulate P-glycoprotein and there was no synergy with the effect of chronic shear stress on permeability, contrary to observations with astrocytes. Endothelial permeability was reduced by sphingosine-1-phosphate, which mediates the barrier-tightening effect of platelets, but inhibitors of sphingosine-1-phosphate receptors did not block the effect of dendritic cells. Rates of endothelial mitosis and apoptosis were also unaffected by co-culture. Hence dendritic cells reduce permeability by different mechanisms from those mediating barrier-tightening effects of astrocytes and platelets, although factors mediating the permeability-lowering effects of chronic shear stress may be involved. We speculate that dendritic cells influence endothelial permeability in vivo.
Keywords: porcine aorta, sphingosine-1-phosphate, shear stress
Introduction
Vascular endothelium provides a significant barrier to the transport of water and solutes between blood and tissue. Elevated endothelial permeability is implicated in the pathogenesis of a number of diseases and in particular appears to play a key role in the development of atherosclerosis35. Transendothelial transport is often studied in vitro but the permeability of endothelium under these conditions is substantially higher than in vivo2. One factor which may contribute to this discrepancy is that endothelial cells are usually cultured in the absence of supporting cell types. It is well established that co-culture with glial cells such as astrocytes can lower the permeability of cerebral endothelial monolayers and induce other properties in them that are characteristic of the blood-brain barrier (BBB)1,14,25. In vivo, astrocytes underlie the cerebral endothelium and project processes that closely appose it. The effect of astrocytes is useful for studying BBB permeability in vitro but, perhaps more importantly, also has implications for the control and breakdown of the BBB in vivo.
We draw attention to the demonstration by Stanness et al.31 that co-culture with astrocytes induces BBB features, including lowered permeability, in aortic endothelium. (Interestingly, the effect was not observed with venous endothelium.) It is plausible, therefore, that companion cells may control barrier function in non-cerebral vasculature. We hypothesised that the permeability of arterial endothelium is controlled by vascular-associated dendritic cells (VDC). These cells are abundantly present in the sub-endothelial space of normal, non-atherosclerotic aortic intima4,15,19, and they project processes that closely appose the endothelium4. Dendritic cells can be derived from glial cells32 and share structural homology with them - for instance, they express the astrocyte marker S1003. VDC are not found in the intima of veins19.
Recent evidence suggests that aortic VDC derive from peripheral blood monocytes15. Here we demonstrate that dendritic cells differentiated from peripheral blood monocytes lower the permeability of porcine aortic endothelial cells (PAEC) in vitro. We further show that the effects of dendritic cells on PAEC and the pathways involved in tightening the barrier are different from those occurring with astrocytes and platelets.
Materials and Methods
Cell isolation and culture
Pig aortic endothelial cells (PAEC) were isolated as previously described5,33. Briefly, thoracic aortas of Landrace Cross pigs (age 4-6 months, weight approx. 80 kg), were obtained after slaughter (Fresh Tissue Supplies, East Sussex) and stored for 24 h in Hank’s balanced salt solution containing penicillin (200 U.ml−1), streptomycin (200 μg.ml−1), amphotericin (5 μg.ml−1) and gentamycin (100 μg.ml−1). They were trimmed of fat and connective tissue, and intercostal arteries were ligated. The proximal end of the vessel was cannulated and the vessel flushed with phosphate buffered saline (PBS). The distal end of the vessel was clamped and the aorta filled with 0.2 mg.ml−1 type II collagenase. After incubation at 37°C for 10 minutes, aortas were massaged gently to loosen endothelial cells and the collagenase solution was collected and centrifuged at 1,000 rpm for 10 minutes. Cells were re-suspended in Dulbecco’s modified eagles medium (DMEM) supplemented with fetal calf serum (10% v/v), newborn calf serum (10% v/v), l-glutamine (5 mM) and endothelial cell growth factor (5 μg.ml−1), and plated into flasks coated with 1% gelatin. Medium was replaced every 2-3 days. Dil-labeled acetyl-LDL is taken up by 99.8% of the cells, indicating essentially pure endothelial monolayers, when using these culture methods; <1 in 104 cells stains with anti-smooth muscle actin33.
Astrocytes of the DI TNC1 cell line, derived from neonatal Sprague-Dawley rats26, were seeded into flasks and cultured in astrocyte complete growth medium (ATCC) supplemented with foetal bovine serum (FBS; 10% v/v) and penicillin-streptomycin (100 U.ml−1) until 80-90% confluent, when they were sub-cultured by exposure to trypsin-EDTA (0.1%:0.02%). At passage 6-9 they were seeded in stages into 12-well plates at 1.5 x 104 cells.cm−2.
Dendritic cells were derived from peripheral blood mononuclear cells (PBMC) isolated from porcine blood (Fresh Tissue Supplies, East Sussex) by density gradient centrifugation. The blood, anti-coagulated with heparin (14.3 USP units/ml), was diluted 2-fold in Earle’s balanced salt solution (EBSS) and layered onto Ficoll-Paque PLUS (GE Healthcare) at room temperature. PBMC were collected following centrifugation at 400 g for 40 minutes and washed twice in excess EBSS, with centrifugation at 100 g for 10 minutes between each wash. They were re-suspended in RPMI-1640 supplemented with 10% FBS, 5 mM L-glutamine and 100 U.ml−1 penicillin-streptomycin, and seeded onto 12-well Transwell plates coated with 50 μg.ml−1 fibronectin. Non-adherent cells were removed after 3 h by washing with medium. Porcine interleukin-4 (IL-4; 10 ng.ml−1) and granulocyte-macrophage colony stimulating factor (GM-CSF; 20 ng.ml−1) obtained from ProSpec (Rehovot, Israel) were added to the medium to promote differentiation into monocyte-derived dendritic cells (MoDC); half the medium was replaced every 2-3 days. Cytokines were omitted in control (monocyte) cultures.
All cells were cultured at 37°C and 5% CO2 in a humidified incubator.
Detection of monocyte and dendritic cell markers
Monocytes were seeded onto Transwell inserts (polyester, 0.4 μm pore size, 12 mm diameter) and cultured in serum-supplemented RPMI with or without IL-4 and GM-CSF. After 6-7 days, the cells were fixed on the insert in ice-cold methanol for 15 minutes and permeabilised with 0.1% Triton X-100 for 5 minutes before blocking for 1 h with 10% goat serum. They were then incubated overnight at 4°C with either anti-S100 (B32.1; Abcam) or anti-CD163 (JPZ01; R&D systems) primary antibodies (omitted in controls), and then for 1 h with either FITC-labelled goat anti-mouse antibody or TRITC-labelled rabbit anti-goat diluted antibody respectively (Abcam). Nuclei were stained by incubation with DRAQ5 (Cell Signaling Technology). All staining steps described here and in subsequent sections were carried out at room temperature unless otherwise stated and were preceded by several washes with PBS.
Transwell insert membranes were mounted in Vectashield® (Vector Labs) and imaged with a Leica SP5 confocal microscope; excitation wavelengths and emission bandwidths were 488 and 510-530 nm for FITC, 561 and 570-590 nm for TRITC, and 633 and 670-700 nm for DRAQ5. Mean pixel intensities were calculated for regions of interest in images obtained at constant laser power and detector gain, and were corrected by subtraction of the mean pixel intensity from cells treated with only the secondary antibody.
Co-culture of endothelial cells with dendritic cells, monocytes or astrocytes
Transwell filter inserts pre-coated with 50 μg.ml−1 fibronectin were placed in wells that already contained MoDC, PBMC or astrocytes adhered to the well base. The filters were seeded in stages over 30 minutes with PAEC (105 cells.cm−2) at passage 2. For co-cultures with MoDC and PBMC, the PAEC were cultured in serum-supplemented RPMI-1640, which additionally contained cytokines for MoDC. Half the lower-compartment medium and all the upper-compartment medium was replaced every 2-3 days. For astrocytes, the PAEC were cultured in serum-supplemented ATCC and medium was replaced in both the lower and upper compartment every 2-3 days. The ratio of MoDC or PBMC to endothelial cells was typically 1:10, whilst astrocytes were confluent.
In some experiments PAEC seeded onto Transwells (as above, without co-culture) were treated with MoDC-conditioned medium. Two wells of a 12-well plate seeded with MoDC were used to provide conditioned media for one Transwell lower compartment. Half of the MoDC-conditioned medium was removed every 24 h and transferred to the PAEC. This volume was then replaced with fresh cytokine-supplemented media. Control wells were treated with cytokine-supplemented RPMI-1640 every 24 h.
In some experiments MoDC-PAEC co-cultures were exposed to chronic shear stress (CSS) from 24 h after seeding the PAEC, as previously described33. Six-well plates containing the Transwell filters were placed on the platform of an orbital shaker (POS-300, Grant Instruments) housed in the incubator. The orbit of the platform was circular with a radius of 5 mm and a rotation rate set to 150 rpm; this movement induced a swirling motion of the medium over the cells. The endothelial monolayers were exposed to shear stress (mean level of ~2 dyne.cm−2 - see reference 33) in this way for 5 days before permeability was measured. (MoDC on the bottom of the well were at a greater depth from the free surface and were partially shielded from the effects of the swirl by the Transwell filter; nevertheless, we cannot exclude the possibility that they also experienced a significant shear).
Measurement of endothelial monolayer permeability
Rhodamine-labelled albumin was used as a tracer. It was labelled and purified as previously described33. Sulforhodamine B acid chloride was dissolved in acetone and added to 20 times its own weight of fatty acid-free bovine serum albumin (fraction V) at 2% wt/vol in 0.33 mol/L carbonate buffer, pH 9, at 4°C. The conjugate was purified of free dye by gel filtration (Sephadex G-25, GE Healthcare), frozen drop-wise in liquid nitrogen, freeze-dried and stored at −20°C. Prior to use the conjugate was reconstituted in buffer and purified of any remaining free dye by mixing for 1 h with neutralised activated charcoal (0.35g/g of protein). Charcoal was removed by centrifugation (twice at 3,000 rpm for 30 minutes) followed by filtration through a 0.2μm filter.
The fraction of free dye remaining was assessed by ultrafiltration of the conjugate through a centrifugal filter with a 10 kDa molecular weight cut-off at 3,000 rpm for 30 minutes, and the endotoxin content of the conjugate was determined using the limulus amebocyte lysate assay, as previously described33. More than 99.9% of the dye was bound to albumin and the endotoxin content of the conjugate was <0.015 EU/ml.
The permeability of PAEC monolayers was assessed as previously described33. Trace quantities (1 mg.ml−1) of rhodamine-labelled albumin were added to the upper compartment of the Transwell. Samples were taken from the bottom compartment after 60 minutes and tracer fluorescence in them was measured using a fluorimeter (Jenway; Model 6285) with excitation and emission wavelengths of 570 and 600 nm respectively. The concentration of rhodamine albumin in each sample was determined from a standard curve.
The permeability (P) of the monolayer is defined as the rate of transport of solute (Js) across unit area of membrane (S) per unit concentration difference across the membrane (ΔC):
Js was determined from the concentration of rhodamine albumin in the lower compartment (CLC; μg.ml−1), the volume of the lower compartment (VLC; ml) and the time after addition of tracer to the upper compartment (t; seconds):
Permeability was derived from Js using the area of the Transwell filter (S) and the concentration of rhodamine-labelled albumin in the upper compartment (CUC);
The permeability recorded from each treatment group was corrected for the permeability of the cell-free filter by subtracting the resistance of the filter from the resistance of the monolayers in the same plate. (Resistances were calculated as reciprocals of permeability). Since monolayer permeability varies somewhat between PAEC isolations, despite pooling cells from several aortas, comparisons between experimental conditions were always made using the same batches of cells33. There was similarly a variation in the effect of MoDC between batches, and the same precaution was taken in the use of these cells.
Detection of P-glycoprotein expression in endothelial cells
PAEC monolayers on Transwell inserts were fixed and blocked as described for MoDC cells, incubated overnight at 4°C with anti-P-glycoprotein (Pgp) antibody (C219; Abcam) and then for 1 h with FITC-labelled goat anti-mouse antibody. Primary antibodies were omitted in controls. Nuclei were stained and Transwell inserts were imaged and analysed as above.
Detection of mitotic or apoptotic endothelial cells
Mitosis rates were assessed by incorporation of 5-bromo-2’deoxyuridine (BrdU) into DNA. PAEC were incubated with BrdU labeling reagent (GE Healthcare) for 1 h at 37°C and then fixed for 30 minutes in acid-alcohol fixative (5% glacial acetic acid, 5% de-ionised water, 90% ethanol), rehydrated in PBS and blocked for 15 minutes with 10% goat serum. They were then incubated with an anti-BrdU antibody containing a nuclease (GE Healthcare) followed by an FITC-labelled goat anti-mouse secondary antibody. Nuclei were stained using DRAQ5 and Transwell inserts were imaged as above. The ratio of BrdU-positive nuclei to DRAQ5 stained nuclei was determined in at least 10 randomly-selected fields per sample, each containing approximately 100 cells.
Expression of cleaved caspase-3 was used as an indicator of rates of endothelial cell apoptosis. PAEC were fixed and blocked as described for MoDC and incubated overnight at 4°C with anti-cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology) and then for 1 h with Alexa-488 goat anti-rabbit antibody. They were counterstained overnight at 4°C with an anti-CD31 antibody (clone LC1.4) and then for 1 h with an Alexa-568 goat-anti-mouse secondary antibody, and Transwell inserts were imaged as described above. The percentage of cleaved caspase-3 positive cells was determined in at least 10 randomly-selected fields per sample, each containing approximately 100 cells.
Statistics
Significance was assessed by Student’s unpaired t-test using a criterion of p<0.05.
Results
Monocytes cultured in the presence of IL-4 and GM-CSF differentiate into dendritic cells
The intensity of immunostaining for S100 was negligible in PBMC incubated in medium alone but was clearly detected after incubation in medium with cytokines (Fig 1; mean pixel intensity ± SEM of 60±6 vs. 10±2; P=0.03), indicating that exposure to IL-4 and GM-CSF causes differentiation of PBMC into MoDCs. The intensity of immunostaining for the monocyte/macrophage marker CD163 was halved in PBMC exposed to cytokine-supplemented medium compared to medium alone (Fig 1; mean pixel intensity of 34±3 vs. 59±3; P=0.03). 30 cells were analysed in each experimental group (n=3 independent experiments).
Figure 1. Monocyte-derived dendritic cells are S100 positive and lose expression of CD163.
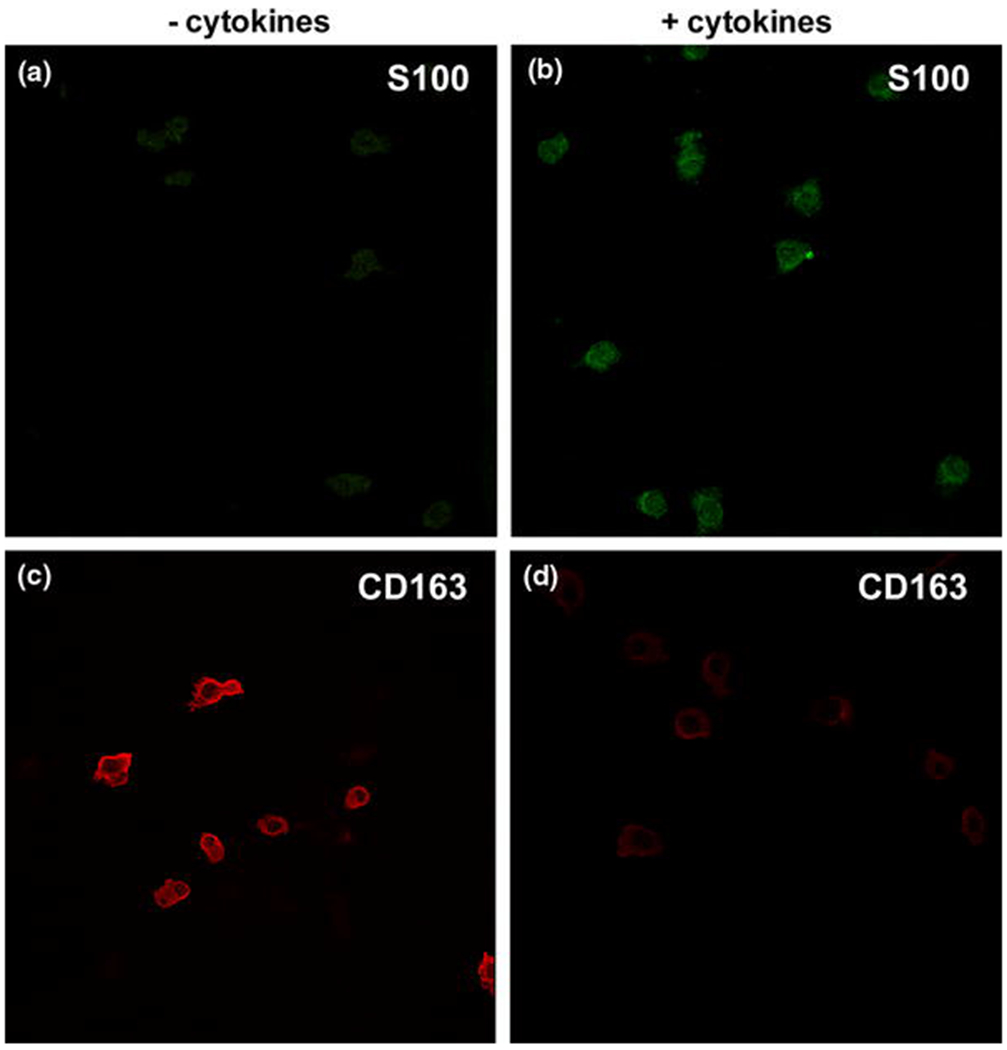
Immunostaining for the dendritic cell marker S100 was significantly increased in cells exposed to IL-4 and GM-CSF (B) compared to exposure to RPMI alone (A; P=0.0303). Exposure to these cytokines also caused decreased immunostaining for the monocyte/macrophage marker CD163 (D) compared to cells cultured in RPMI alone (C; P = 0.026). All images were taken using the same microscope settings. Representative images from 3 independent experiments.
Astrocytes, dendritic cells and DC-conditioned medium but not monocytes reduce permeability
Non-contact co-culture of PAEC for 5 days with rat astrocytes produced a significant decrease in permeability to rhodamine-labelled albumin, compared to PAEC alone (Fig 2; 43% reduction; P<0.0001, n=4 independent experiments). The permeability of PAEC monolayers was also decreased by non-contact co-culture with MoDC for 5 days, compared to PAEC alone (Fig 2; 31% reduction; P=0.0002; n=7). It was also decreased by a 5-day exposure to MoDC-conditioned medium, compared to monolayers treated with unconditioned cytokine-supplemented medium (Fig 2; 40% reduction; P<0.0001; n=3). (There was a tendency for endothelial permeability to increase following exposure to unconditioned cytokine-supplemented RPMI, but this did not reach statistical significance (P=0.163; n=3)). Endothelial permeability was not affected by co-culture with monocytes (Fig 2; P=0.7552; n=7).
Figure 2. Effect of co-culture and conditioned medium on endothelial monolayer permeability.
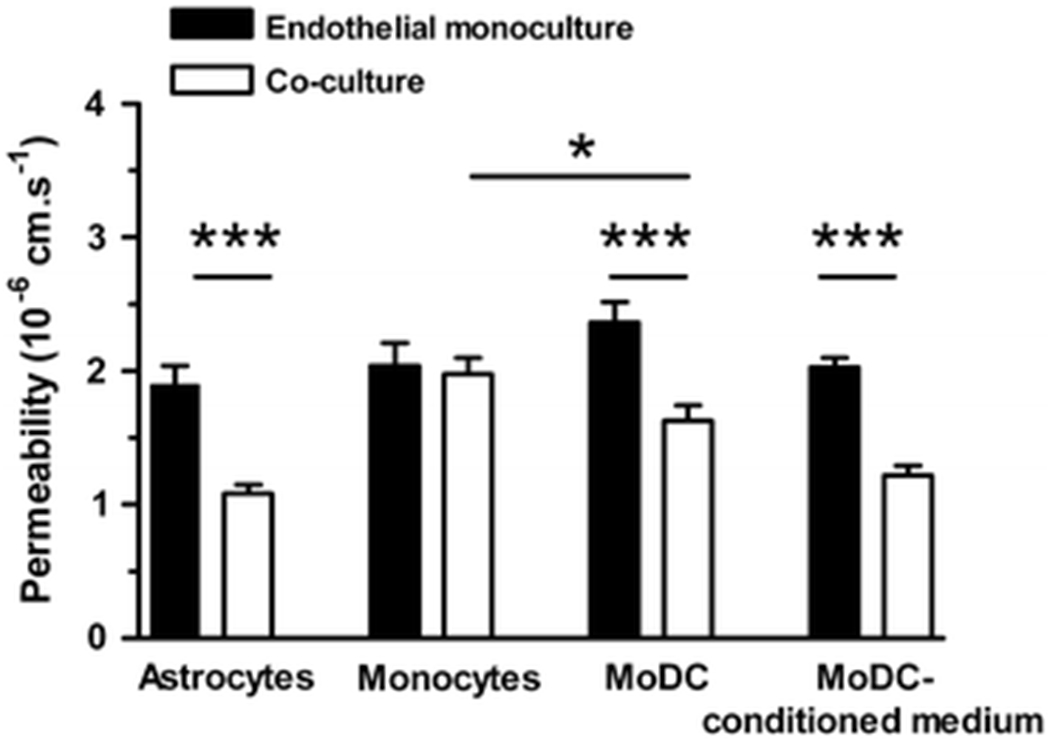
Non-contact co-culture of confluent endothelial monolayers with rat astrocytes for 5 days reduced monolayer permeability to albumin compared to the permeability of monolayers cultured alone (P<0.0001, n=4). Non-contact co-culture with monocyte-derived dendritic cells (MoDC) for 5 days also reduced permeability (P=0.0002, n=7) but non-contact co-culture with undifferentiated monocytes was without effect (P = 0.7552; n=7). Exposure of monolayers to MoDC-conditioned medium for 5 days reduced permeability compared to exposure to cytokine-supplemented medium (P<0.0001, n=3). *P <0.05, **P<0.01, ***P<0.001.
P-glycoprotein expression by endothelial cells is increased by co-culture with astrocytes but not dendritic cells
Non-contact co-culture of PAEC with rat astrocytes significantly increased the intensity of immunostaining for Pgp in the endothelial cells, compared to endothelial cells cultured alone (Fig 3; P=0.001, n=3). Non-contact co-culture with MoDC for 5 days did not elicit a change in the intensity of immunostaining for Pgp (Fig 3; P=0.254, n=3 independent experiments). The intensities obtained for PAEC monocultures were not significantly different from those obtained in the absence of primary antibody (data not shown; n=3), suggesting negligible basal expression of Pgp.
Figure 3. P-glycoprotein expression is up-regulated in endothelium co-cultured with astrocytes but not with monocyte-derived dendritic cells.
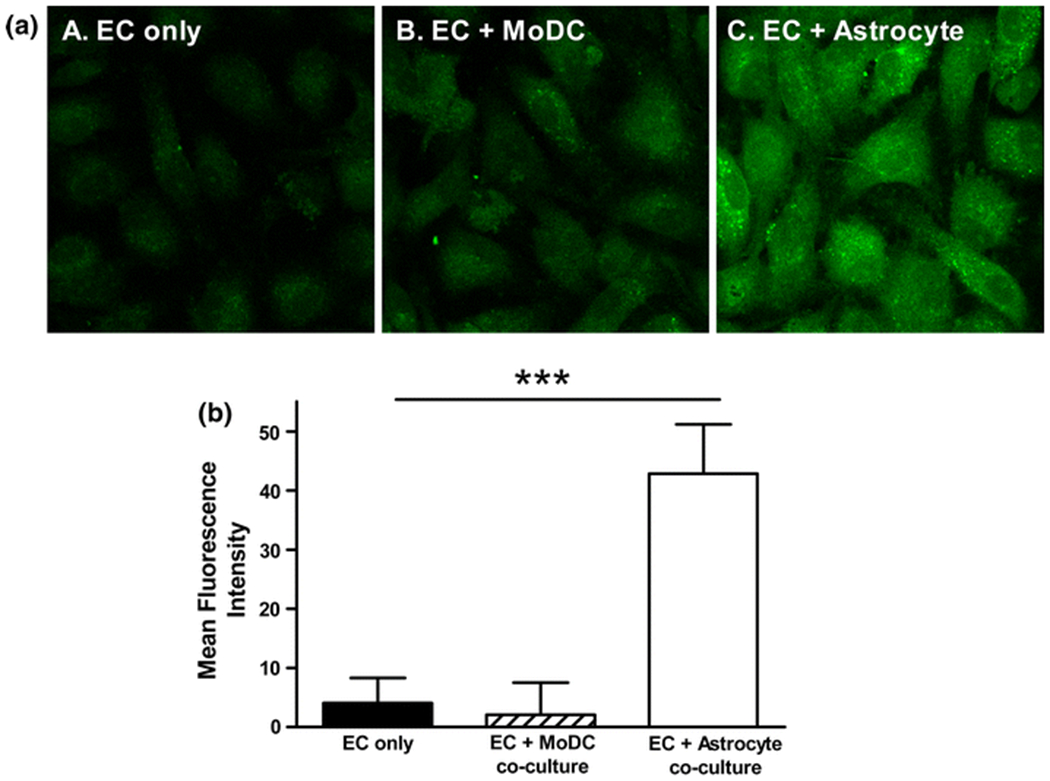
The intensity of immunostaining for the blood-brain barrier protein, PgP was significantly increased in endothelial monolayers co-cultured with astrocytes for 5 days (A; panel C) compared to monolayers cultured alone (A; panel A), represented graphically in B; P=0.0010; n=3 independent experiments). P-glycoprotein expression was not significantly altered in monolayers co-cultured with MoDC for 5 days (A; panel B) compared to monolayers cultured alone (A; panel A), represented graphically in B; P=0.254; n=3 independent experiments). Values obtained without the primary antibody have been subtracted in B. *P <0.05, **P<0.01, ***P<0.001.
Sphingosine-1-phosphate reduces permeability but S1P receptor antagonists do not block the permeability-reducing effect of dendritic cells
Pre-treatment of PAEC monolayers with sphingosine-1-phosphate (S1P; Cayman Chemicals, MI, USA) for 1 h before the addition of tracer produced a dose-dependent reduction in permeability that was abolished by the presence of the S1P-1 and −3 receptor dual antagonist, VPC-23019 (1 μM; Avanti Polar Lipids, AL, USA), (Fig 4; P<0.05; n=4 independent experiments). Pre-treatment with VPC-23019 did not significantly alter monolayer permeability in the absence of S1P (Fig 4; P=0.58; n=3). Non-contact co-culture of PAEC with MoDC caused a reduction in permeability, as before, which was not significantly different in the presence of VPC-23019 (Fig 4; P=0.246; n=3 independent experiments). In some experiments, the pre-treatment with VPC-23019 was extended to 4 h but this also failed to alter the permeability of control monolayers or co-cultures (data not shown; P=0.411; n=2 independent experiments). Similarly, pre-treatment for 1 or 4 h with the S1P-1 receptor antagonist W146 (1 μM; Avanti Polar Lipids; AL, USA) did not alter the reduction in PAEC permeability caused by MoDC (data not shown; 1 h P=0.411 and 4 h P=0.625; n=3 independent experiments).
Figure 4. Sphingosine-1-phosphate reduces permeability but S1P receptor inhibition does not alter the MoDC-driven reduction in permeability.
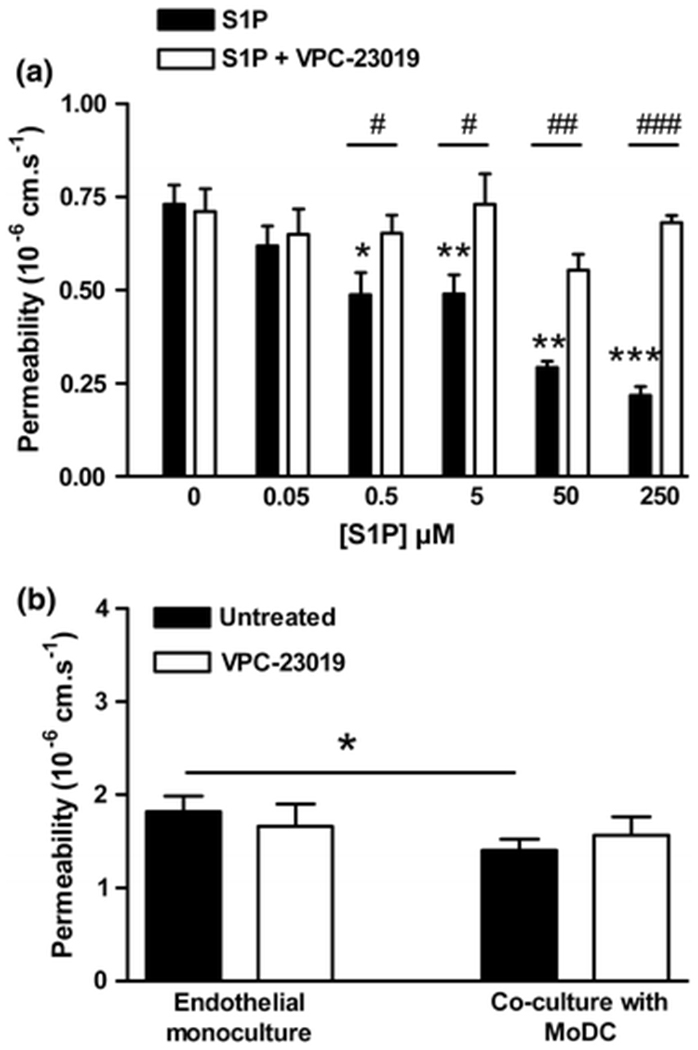
(A) Pre-treatment with S1P for 1 h produced a dose-dependent reduction in endothelial monolayer permeability to albumin that was abrogated by the S1P-1 and -3 receptor inhibitor VPC-23019 (1 μM) (n=4 independent experiments). (B) Non-contact co-culture of monolayers with MoDC for 5 days significantly reduced endothelial permeability (P=0.0254; n=3 independent experiments). Pre-treatment with VPC-23019 (1 μM) for 1 h before the addition of tracer did not significantly alter permeability in the presence or absence of MoDC (P=0.246). *P <0.05, **P<0.01, ***P<0.001.
Rates of proliferation and apoptosis are not altered in confluent endothelial cells co-cultured with dendritic cells
The percentage of replicating cells in PAEC monolayers co-cultured with MoDC for 5 days was not significantly different from that in PAEC monocultures (0.63%±0.1% vs. 0.55%±0.1%; P=0.613; Fig 5a; n=3 independent samples). Similarly the rate of apoptosis in PAECs was not altered by co-culture with MoDC for 5 days (2.73%±0.67% vs. 2.69%±0.58%; P=0.968; Fig 5b; n=3 independent samples). Inspection of confocal images of the CD31 counter-staining in these experiments (3 wells per condition from 3 isolations) showed that the PAEC monolayers were confluent whether cultured on their own (Fig 5c; image I) or in the presence of MoDC (Fig 5c; image II).
Figure 5. Co-culture with MoDCs does not alter the rate of mitosis or apoptosis in endothelial monolayers.
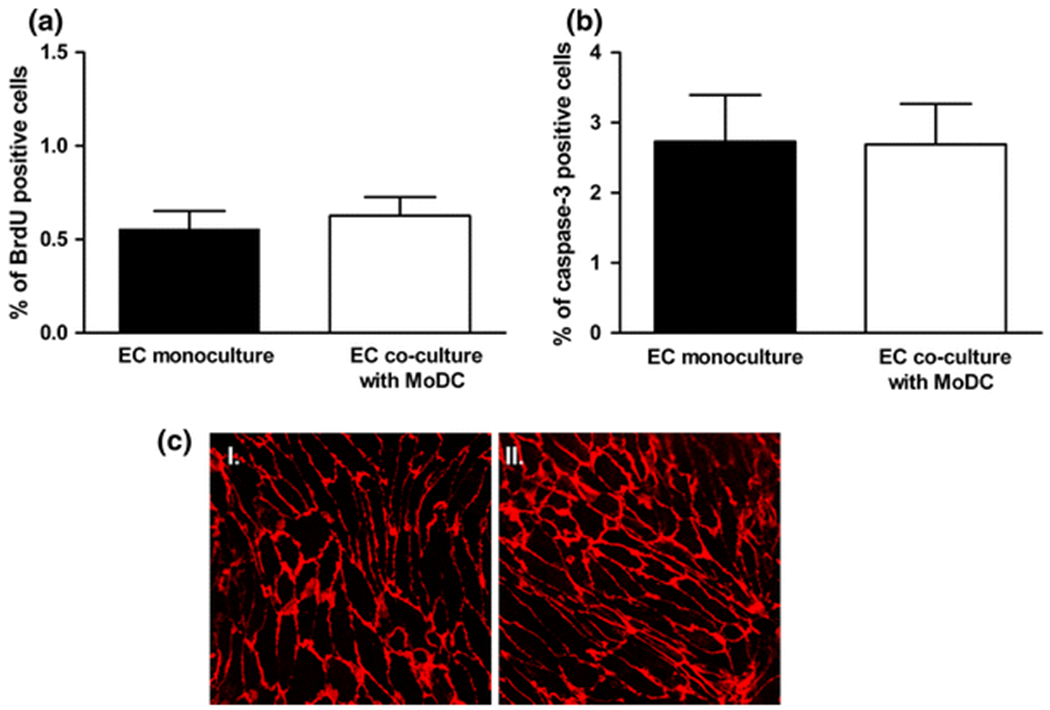
(A) Co-culture of endothelial monolayers with MoDC for 5 days did not alter the rate of mitosis when compared to endothelial monocultures as assessed by incorporation of BrdU into cellular DNA (P = 0.613; n = 3 independent experiments). (B) Co-culture of endothelial monolayers with MoDC for 5 days did not alter the rate of apoptosis when compared to endothelial monocultures as assessed by the expression of cleaved-caspase 3 (P = 0.968; n = 3 independent experiments). (C) CD31 staining shows confluent monolayers and no obvious difference in endothelial morphology between monocultures (image I) and co-cultures (image II).
Chronic shear stress does not enhance the barrier-tightening effect of dendritic cells
Exposure of PAEC to CSS by swirling the cell culture plates for 5 days significantly reduced their permeability compared to static controls (P=0.001; n=3), as previously reported33. Swirling of plates containing MoDC seeded on fibronectin caused the MoDC to detach, resulting in a loss of their permeability-reducing effect (data not shown). This was circumvented by seeding the MoDC onto a gel prepared from bovine collagen solution (PureCol; Advanced Biomatrix). Static co-culture of PAEC with MoDC on collagen produced a significant reduction in permeability (P<0.0001; Fig 6), as with MoDC cultured on fibronectin. The reduction in permeability elicited by MoDC when the PAEC were exposed to CSS was significant (P=0.0019). However, the permeability of PAEC monolayers exposed to MoDC and CSS was not different from that of monolayers exposed to MoDC under static conditions (P=0.876; Fig 6; n=3 independent experiments).
Figure 6. Chronic application of shear stress decreases endothelial monolayer permeability but does not enhance the effect of MoDCs.
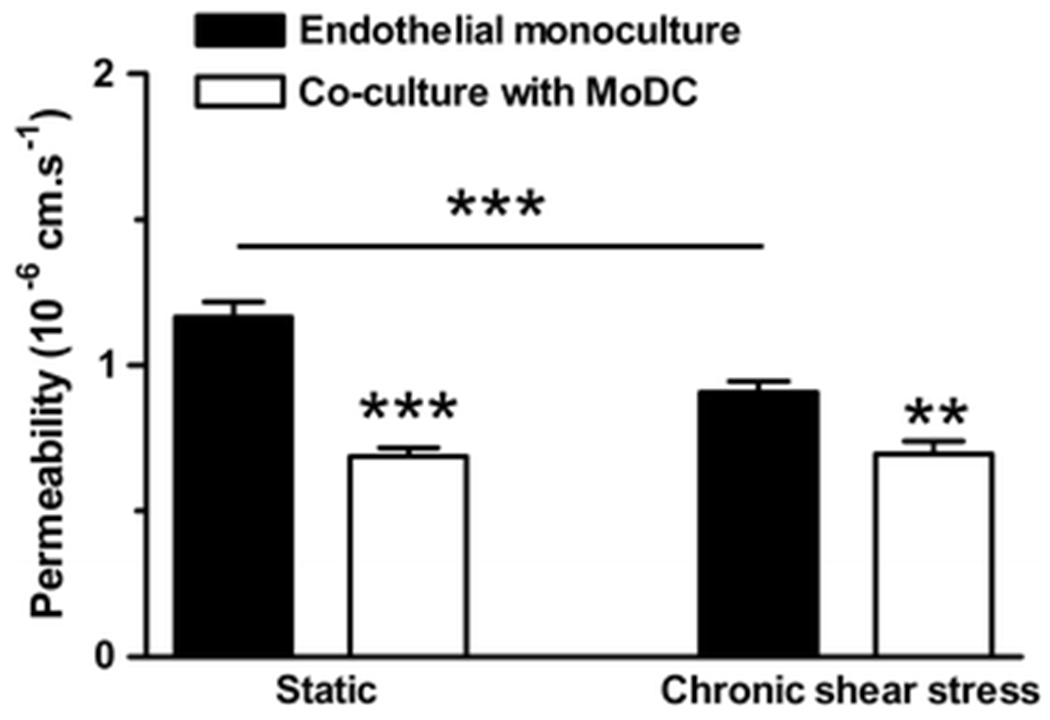
Application of shear stress for 5 days (chronic shear stress) significantly reduced monolayer permeability compared to static controls (P=0.001). Co-culture of endothelial monolayers with MoDC significantly reduced permeability under static conditions (P<0.0001) and with CSS (P=0.0019), but permeability did not differ significantly between static and CSS co-cultures (P = 0.876; n=3 independent experiments). *P <0.05, **P<0.01, ***P<0.001.
Discussion
Intimal cells with a stellate morphology were first observed in the normal human aorta by Krushinsky et al17. Bobryshev and Lord identified a fraction of them as dendritic cells3. Wick et al. confirmed the presence of dendritic cells in human vessels and also found them in normal rabbit aorta19. More recently, they have been detected in the arterial wall of wild-type mice15. The latter cells, which have a dendritic morphology and express both CD68 (a myeloid cell marker) and CD11c (a pan-dendritic cell marker), were most frequently found in the lesser curvature of the aortic arch, a site susceptible to atherosclerosis in apoE −/− mice. Pulse labelling with BrdU demonstrated that the intimal cells in these regions derive predominantly from recruitment of blood monocytes15.
Using established methods27, we differentiated porcine peripheral blood monocytes (PBMC) into dendritic cells in vitro. Dendritic cells derived from porcine PBMC using these methods lose expression of monocyte markers such as CD14 but express CD11b/c, CD68 and many other surface markers found on dendritic cells22. In the present study, we found that the monocyte-derived dendritic cells (MoDC) expressed S100, unlike the monocytes themselves, and they lost expression of the monocyte/macrophage marker CD163, as expected24. They were cultured in wells containing PAEC on Transwell filter inserts in order to establish their influence on the permeability of the endothelial monolayer to rhodamine-labelled albumin, a stable, relatively inert macromolecular tracer with similar physical, chemical, and biological properties to the unlabelled protein34. As a positive control, we confirmed that astrocytes decreased permeability in this non-contact co-culture system. The dendritic cells also did so, and by a similar fraction (about one third), whereas undifferentiated PBMC (and any contaminating cells in this preparation) did not.
There are a number of similarities between astrocytes and dendritic cells. Indeed S100 is an astrocyte marker and dendritic cells can be differentiated from glial cells32, as noted above. Nevertheless, although the two cell types had similar effects on endothelial permeability, this was not because MoDC were inducing a BBB phenotype: monolayers co-cultured with astrocytes expressed permeability glycoprotein (Pgp, CD243), an ATP-binding cassette transporter characteristic of the BBB9, but monolayers cultured with MoDC cells did not.
Further differences between the effects of astrocytes and dendritic cells are evident from their interactions with effects of mechanical forces. Astrocytes act synergistically with chronically-applied fluid dynamic shear stress to reduce permeability in vitro30. In the present study, we obtained a reduction in PAEC permeability with chronically-applied shear stress but did not find synergy with the influence of MoDC: the permeability of PAEC co-cultured with MoDC was the same regardless of whether the cells were sheared or not. The lack of synergy suggests that there are common components to the pathways mediating influences of MoDC and shear stress. We have recently shown that CSS reduces the permeability of PAEC monolayers via a pathway involving phosphatidylinositol-3-OH kinase, nitric oxide and soluble guanylyl cyclase33; however, preliminary data from the present study (not shown) indicate that the influence of MoDC is not altered by inhibiting nitric oxide synthesis.
We also investigated whether MoDC reduced mitosis and apoptosis rates in endothelial monolayers. Mitosis leads to foci of enhanced endothelial permeability in vivo that may account for a significant fraction of total macromolecule entry into the aortic wall8 and we have recently shown that endothelial mitosis rates are higher in vitro than in vivo33. Apoptosis also appears to enhance permeability in vitro7. Reductions in mitosis or apoptosis would be expected to lower baseline permeability to macromolecules. However, no effect of co-culture with MoDC on these rates was observed. Furthermore, CD31 staining did not reveal any obvious effect on endothelial cell morphology and the cells were confluent in the presence or absence of MoDC.
MoDC-conditioned medium was also effective in reducing the permeability of PAEC monolayers, implying the presence of a long-acting soluble mediator. Platelets and platelet-conditioned medium reduce the permeability of endothelium12, and this effect is mediated at least in part by sphingosine-1-phophate (S1P)10,28. We showed here for the first time that S1P substantially reduces the permeability of aortic endothelium; to our knowledge these are the lowest aortic monolayer permeabilities to albumin yet reported. (Since S1P can increase apoptosis11,20, its effect on the transport of larger molecules may be different). The effect of S1P was mediated by the S1P-1 and/or S1P-3 receptor. However, the effect of MoDC on permeability was not attenuated by an antagonist of these receptors, suggesting the involvement of a different mediator. When identified, the mediator might have therapeutic value, for example in reducing low density lipoprotein entry into the arterial intima, if the permeability-reducing effect that we demonstrated in vitro also occurs in vivo and if it applies to the uptake of LDL. (Transendothelial albumin transport may occur through smaller pores, and have a lower convective component, than LDL transport). The magnitude of the effect was of the same order as the difference in albumin permeability seen between atheroprone and atheroprotected sites in vivo29, and it has recently been shown that S1P analogues reduce aortic atherosclerosis in mice16.
Although MoDC reduced the permeability of cultured endothelium, they did not bring it to the level (c. 10−8 - 10−7 cm.s−1) observed in the aorta in vivo6,29. Soluble mediators released by VDC could have greater effects on endothelium in vivo because the cells are closer to the endothelium and because the mediator is not diluted by a large volume of fluid, as in vitro. VDC may additionally influence endothelium in vivo through the direct cell-cell contacts that have been demonstrated in ultrastructural studies4. Influences other than VDC may also contribute to the gap between in vivo and conventional in vitro permeabilities. The S1P released by platelets is one such factor.
The primary role of dendritic cells is immunosurveillance; they present novel antigens to lymphoid tissue. Indeed, Millonig et al.19 coined the term “Vascular Associated Lymphoid Tissue” to describe VDC. Cybulsky and co-workers23 recently identified a novel role by showing that the majority of foam cells in experimental murine atherosclerosis are derived from VDC. Consistent with this, previous studies have demonstrated that the number of VDC is greater in atherosclerosis-prone regions than in resistant regions and that it increases in naturally-occurring or experimentally-induced disease3,4,19. The results of our study demonstrate another novel role of dendritic cells - as modulators of endothelial permeability. We speculate that the same effect occurs in vivo and that variation in VDC density may help account for spatial variation in arterial permeability. However, a permeability-reducing effect, if it also applies to the larger lipoproteins implicated in the initiation of atherogenesis, would suggest an anti-atherogenic role, leading to the expectation of greater numbers of VDC in regions protected from atherosclerosis. This apparent discrepancy requires further investigation. One possibility is that soluble factors and direct cell-cell contacts mediate different types of effect of VDC on endothelium; only the former were investigated here. Another is that there are sub-types of VDC (perhaps reflecting different levels of maturation) that have different functions and different distributions within the vasculature.
Finally we note that pericytes, like astrocytes, dendritic cells and perhaps smooth muscle cells18, are implicated in the control of endothelial permeability13,21. In the last few decades, the endothelium has emerged as the controller of vascular homeostasis, with influences on circulating cells and on other cells of the vessel wall. The effects reported in this paper, and the results noted above, suggest that endothelial properties (including but not restricted to permeability) may in turn be under the control of other cells of the vessel wall.
Acknowledgments
This work was supported, in part, by the National Institutes of Health [EY018373 to D.R.O]. We thank Professor W. Daniel Stamer (University of Arizona) for kindly providing the S1P receptor antagonists.
Footnotes
Conflict of Interest
None
References
- 1.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neuro 7:41–53, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Albelda SM, Sampson PM, Haselton FR, McNiff JM, Mueller SN, Williams SK, Fishman AP, Levine EM: Permeability characteristics of cultured endothelial cell monolayers. J. Appl. Physiol 64:308–322, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Bobryshev YV, Lord RSA. S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovas. Res 29:689–696, 1995. [PubMed] [Google Scholar]
- 4.Bobryshev YV, Lord RSA. Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of vascular dendritic cells in athero-resistant and athero-prone areas of the normal aorta. Arch. Histol. Cytol 58:307–322, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Bogle RG, Baydoun AR, Pearson JD, Mann GE. Regulation of L-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J. Physiol 490:229–241, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratzler RL, Chisolm GM, Colton CK, Smith KA, Zilversmit DB, Lees RS. The distribution of labeled albumin across the rabbit thoracic aorta in vivo. Circ. Res 40:182–190, 1977. [DOI] [PubMed] [Google Scholar]
- 7.Cancel LM, Tarbell JM. The role of apoptosis in LDL transport through cultured endothelial cell monolayers. Atherosclerosis. 208:335–341, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien S, Lin SJ, Weinbaum S, Lee MM, Jan KM. The role of arterial endothelial cell mitosis in macromolecular permeability. Adv. Exp. Med. Biol 242:59–73, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem 38:1277–1287, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest 108:689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gencer EB, Ural AU, Avcu F, Baran Y. A novel mechanism of dasatinib-induced apoptosis in chronic myeloid leukemia; ceramide synthase and ceramide clearance genes. Ann. Hematol 90:1265–1275, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Gimbrone MA Jr., Aster RH, Cotran RS, Corkery J, Jandl JH, Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature 222(5188):33–36, 1969. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki T, Kanda T, Mizusawa H. Effects of pericytes and various cytokines on integrity of endothelial monolayer originated from blood-nerve barrier: an in vitro study. J. Med. Dent. Sci 46:31–40, 1999. [PubMed] [Google Scholar]
- 14.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325(6101):253–257, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med 203:2073–2083, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keul P, Tölle M, Lucke S, von Wnuck Lipinski K, Heusch G, Schuchardt M, van der Giet M, Levkau B: The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol 27:607–613, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Krushinsky AV, Orekhov AN, Smirnov VN. Stellate cells in the intima of human aorta. Application of alkaline dissociation method in the analysis of the vessel wall cellular content. Acta. Anat. (Basel) 117:266–269, 1983. [PubMed] [Google Scholar]
- 18.Kurzen H, Manns S, Dandekar G, Schmidt T, Prätzel S, Kräling BM. Tightening of endothelial cell contacts: a physiologic response to cocultures with smooth-muscle-like 10T1/2 cells. J. Invest. Dermatol 119:143–153, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler. Thromb. Vasc. Biol 21:503–508, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Müller HC, Hocke AC, Hellwig K, Gutbier B, Peters H, Schönrock SM, Tschernig T, Schmiedl A, Hippenstiel S, N’Guessan PD, Rosseau S, Suttorp N, Witzenrath M. The Sphingosine-1 Phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulm. Pharmacol. Ther 24:377–385, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, Tanaka K, Niwa M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int 54:253–259, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Paillot R, Laval F, Audonnet J-C, Andreoni C, Juillard V. Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunology 102:396–404, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res 106:383–390, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Piriou-Guzylack L, Salmon H. Membrane markers of the immune cells in swine: an update. Vet. Res 39:54, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia 35:145–155, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Radany EH, Brenner M, Besnard F, Bigornia V, Bishop JM, Deschepper CF. Directed establishment of rat brain cell lines with the phenotypic characteristics of type 1 astrocytes. Proc. Natl. Acad. Sci. USA 89:6467–6471, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson SP, Stagg AJ, eds. Dendritic Cell Protocols. Totowa: Humana Press, 2001. [Google Scholar]
- 28.Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F, Garcia JG. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am. J. Physiol. Lung. Cell. Mol. Physiol 285:L258–L267, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Sebkhi A, Weinberg PD. Effect of age on the pattern of short-term albumin uptake by the rabbit aortic wall near intercostal branch ostia. Arterioscler. Thromb. Vasc. Biol 16:317–327, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 1147:39–50, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanness KA, Westrum LE, Fornaciari E, Mascagni P, Nelson JA, Stenglein SG, Myers T, Janigro D. Morphological and functional characterisation of an in vitro blood-brain barrier model. Brain Research 771:329–342, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Xu LY, Yang JS, Xiao BG. TGF-beta1-conditioned glial cell-derived dendritic cells inhibit expansion of MBP-reactive T cells in vitro. Neuroreport 13:35–39, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Warboys CM, Berson RE, Mann GE, Pearson JD, Weinberg PD. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am. J. Physiol. Heart. Circ. Physiol 298:H1850–H1856, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg PD Application of fluorescence densitometry to the study of net albumin uptake by the rabbit aortic wall up- and downstream of intercostal ostia. Atherosclerosis 74:139–148, 1988. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg PD Rate-limiting steps in the development of atherosclerosis: the response-to-influx theory. J. Vasc. Res 41:1–17, 2004. [DOI] [PubMed] [Google Scholar]


