Abstract
Background:
Although antibiotics are well-known for their anti-bacterial effects, their inaugurated immunomodulatory roles in chronic inflammatory diseases have not elucidated yet. Anecdotal reports support the beneficial effects of parenteral penicillin in arthritis suggesting an immunomodulatory other than antibacterial effects for penicillin. The present study was designed to address the possible effects of penicillin G sodium (PCN-G) on different T-helper cells differentiation.
Materials and Methods:
In this experimental study, peripheral blood mononuclear cells (PBMCs) of 10 healthy donors were isolated using Ficoll density gradient. The stimulated PBMCs by anti-CD3, anti-CD28, and anti-CD69 were cultured in the presence of 120 μg/ml of PCN-G. Foxp3, T-bet, RORγT, GATA3 as well as interferon-gamma (IFN-γ) and interleukin (IL)-17A mRNA in stimulated cells were measured by the real-time polymerase chain reaction. Mann–Whitney U-test was used for determining differences between the medium of gene expression levels of stimulated cell population and unstimulated cells by PCN. Correlations between the related genes were determined using the Spearman test.
Results:
Based on the results, T-bet gene expression levels were similar in stimulated cells by PCN G after 24 and 48 h while significant reduction was observed after 72 incubation with PCN G (difference = 3; 0.09–0.34; P = 0.031). Meanwhile, treated cells with PCN G expressed decreased levels of IFN-γ (difference = 8.0; 0.49–1.07; P = 0.001) and IL-17A (difference = 2.2; 0.05–0.75; P ≤ 0.05) genes comparing to unstimulated cell by PCN-G. GATA3 genes expression levels downregulated by PCN G after 72 h of incubation by PBMCs (difference = 1.1; 0.77–0.88; P = 0.035).
Conclusion:
Our results confirmed the immunomodulatory role of PCN G by affecting the expression of different cytokines genes in PBMCs.
Keywords: Autoimmune disease, forkhead box P3 protein, GATA3 protein, inflammation, interferon-gamma, interleukin-17A, lymphocyte, penicillin G benzathine, RORgammaT, T-box transcription factor TBX21 (T-bet)
INTRODUCTION
Accumulating evidence shows some antibiotics exert their beneficial effects not only by direct killing or inhibiting the growth of pathogenic bacterial, but indirectly are able to induce immunomodulatory responses.[1,2] Macrolides, as a common family of antibiotics in clinic, have already approved their immunomodulatory effects.[3] The dominant proinflammatory cytokines, including interleukin 8 (IL-8) and tumor necrosis factor (TNF-α), are downregulated by macrolides. They are also able to inhibit leukocyte chemotaxis by suppressing the synthesis of endogenous chemotactic factors. Moreover, beta-lactam antibiotics, a class of broad-spectrum antibiotics, comprise all antibiotic agents containing a beta-lactam ring in their molecular structure. This family includes penicillin derivatives penams, cephalosporins, monobactams, and penicillins.[4] Penicillin acts by inhibiting bacterial transpeptidase, an essential enzyme for bacterial cell wall biosynthesis.[5,6]
Based on new findings, immunomodulatory functions of antibiotics might affect inflammatory responses in autoimmune diseases and chronic inflammatory conditions. Tetracycline has recently been shown to exert a number of pleiotropic anti-inflammatory and immunomodulatory activities. The tetracycline class of drugs appears to have anti-inflammatory along with cartilage-protective effects in rheumatoid arthritis (RA), most probably by inhibiting matrix metalloproteinase (MMP).[7] In addition, tetracycline therapy in immunoglobulin-mediated autoimmune or allergic diseases is emerging.[8] Minocycline from tetracycline family also inhibited microglia/macrophage activation in animal encephalomyelitis models.[3,9,10,11] Moreover, doxycycline prevents isotype switching in human B cells.[12] Doxycycline and minocycline block inducible nitric oxide synthase (iNOS) and nitric oxide (NO) in rheumatoid arthritis.[13,14,15,16] In the animal model, lipopolysaccharide (LPS)-induced pro-inflammatory cytokines (IL-8, TNF-α, and IL-6) modulated by low dose of doxycycline.[17] While the role of macrolides in modulating immune responses have elucidated, our knowledge of beta lactam antibiotics behavior in inducing inflammatory responses are not clear still. Our recent observations and pilot study showed marked anti-inflammatory activities of penicillin derivatives on several rheumatic conditions. In the present study, we aim to investigate PCN effects on the differentiation of human T-cell population. Essential transcription factors for developing different T-cell population including T-bet, GATA3 protein (GATA3), RORgammaT (RORγt), forkhead box P3 protein (FOXP3) as well as interferon-gamma (IFN-γ) and interleukin 17A (IL-17A) gene expression human T-cells treated by PCN were investigated.
MATERIALS AND METHODS
Human subjects
In the experimental study, 10 healthy male, aged 20–25 (22.5 ± 4.29), were selected of students and staff of the medical faculty, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. All the participants were in full health condition confirmed by the university clinic, and informed consent was obtained. The participants did not show present or past infection diseases at least 1 month before sampling. The sera of donors also was screened for anti-HIV-1, anti-HbsAg, and anti-HCV by commercial enzyme-linked immunosorbent assay kits (Diapro, Diaplus, Italy). None of the donors had received antiviral, anti-bacterial, or anti-inflammatory treatment 1 month before entry into the study to avoid the possible effect of therapy on the expression of genes expression. The people who had a history of autoimmune diseases, organ transplantation, chemotherapy, and metabolic disorders were excluded from the study. Fifteen milliliters fresh heparinzd blood specimen obtained of each healthy donor volunteer and peripheral blood mononuclear cells (PBMCs) enriched by using Ficoll (Biosera, Germany) density gradient and cultured in 96 cell culture plates. For each donor 9 × 107 cells were cultured into 45 wells (in each well 2 × 105 cells). The wells randomly selected by random number table and divided to five groups. In each group, 3 wells were considered as unstimulated (control), 3 wells stimulated by ani-CD3, 3 wells stimulated by anti-CD3/CD28, 3 wells stimulated by Penicillin G sulfate, and 3 wells stimulated simultaneously by anti-CD3/CD28/PCN-G. For reverse transcription polymerase chain reaction (PCR) assessment, each 3 wells harvested and treated for analysis. Therefore, totally with considering 10 donors and three times (24, 48 and 96 h) assessments, 150 samples were assessed by real-time PCR. The Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran approved our study methodology (Ethic code: IR.SSU.MEDICINE.REC.1394.153). All patients who enrolled in the study signed a written informed consent for the study.
Cell culture
To evaluate GATA3, IFN-γ, T-bet, IL-17, RORγt, and Foxp3 genes expression, 15 ml of heparinized peripheral blood was taken from the donors. PBMCs from healthy individuals donors (n = 10) were obtained and layered over a Ficoll-Hypaque gradient (Biosera, Germany). Cells were washed and suspended in RPMI1640 medium, supplemented with 10% fetal bovine serum (Invitrogen, Denmark). PBMCs were cultured in 96-well cell culture microplate at 200 μl per well with 2 × 105 cells. The cells were stimulated by anti-CD3 and anti-CD28 (eBioscience, CA). Penicillin G sulfate was added to the cells in the concentration of 120 μg/ml. The Cultures were performed in triplicate and maintained at 37°C/5% CO2. After 24, 48, and 72 h of incubation, IFN-γ, IL-17, Foxp3, T-bet, and GATA3 mRNA levels were measured by quantitative real time PCR.
Quantitative real-time polymerase chain reaction
To investigate mRNA levels of GATA3, IFN-γ, T-bet, IL-17, RORγt, and Foxp3, quantitative PCR (qPCR) method was used. The PBMCs were stimulated as mentioned in the earlier section and total RNA extracted by Trizol (Roche Diagnostics GmbH, Mannheim, Germany) method. The quantitative and quality of the RNA were carried out by NANODROP 2000c (Thermo Fisher Scientific Inc., USA). Prime-ScriptTM, RT reagent kit (Takara Biotechnology, Otsu-Shiga, Japan) was purchased and complementary DNA (cDNA) was synthesized according to the manufacturer's instructions of the kit. Quantitative real-time PCR was performed on the Mic qPCR Cycler (bio molecular systems, Australia). Primers were designed by web-based “primer 3” software (http://bioinfo.ut.ee/primer3-0.4.0/). PCR assays were carried out in 20 μL reaction volumes containing 100 ng of cDNA, 250 nM of forward and reverse Primers and used QuantiTect SYBR Green PCR Kit (QIAGEN, Germany). qPCR was performed using Mic qPCR Cycler (bio molecular systems, Australia). Real-time PCR reactions were run in triplicates for each gene of interest, and data were analyzed by REST 2009 (v2.0.13) software. The synthesized primers characteristics presented in Table 1. Beta actin as a housekeeping gene was used as an endogenous control to normalize the samples. Expressed target genes normalized to beta actin and delta-delta Ct method was used to measure the expression changes in target genes.
Table 1.
Primer sequences used for the real-time polymerase chain reaction
| Gene | Primer sequence 5’ to 3’ | Temperature | Amplification length (bp) |
|---|---|---|---|
| Foxp3 | Forward: GAAACAGCACATTCCCAGAGTTC | 56.3 | 100 |
| Reverse: ATGGCCCAGCGGATGAG | 58 | ||
| T-bet | Forward: TGTTGTGGTCCAAGTTTAATCAGCA | 57.1 | 158 |
| Reverse: CCCGGCCACAGTAAATGACAG | 58.3 | ||
| RORγt | Forward: TGCAAAGAAGACCCACACCTCACA | 60.7 | 197 |
| Reverse: ATCGGTTTCGGCTGGTGCGG | 63.2 | ||
| Gata3 | Forward: AGATGGCACGGGACACTACCT | 60.3 | 92 |
| Reverse: ACAGTTCAGCCATCACTTGGA | 56.5 | ||
| IFN-tim | Forward: CTAATTATTCGGTAACTGACTTGA | 50.4 | 75 |
| Reverse: ACAGTTCAGCCATCACTTGGA | 56.5 | ||
| IL-17A | Forward: GGAAGAAACAACGATGAC | 48.4 | 88 |
| Reverse: GATTCCTGCCTTCACTAT | 48.6 | ||
| Β-actin | Forward: CAA GAG ATG GCC ACG GCT GCT | 62.5 | 275 |
| Reverse: TCC TTC TGC ATC CTG TCG GCA | 61 |
Gata3=Gata3 protein; RORγt=RORgammaT; FOXP3=Forkhead box P3; IFN-γ=Interferon-gamma; IL-17A=Interleukin 17A; T-bet: T-box transcription factor TBX21
Data analysis
The obtained results were analyzed by the SPSS version. 16.0 software (SPSS Inc., Chicago, IL, USA). Mann–Whitney U-test was used for determining the differences between the medium of gene expression levels of stimulated cell population and unstimulated cells by PCN. P ≤ 0.05 was considered statistically significant. Correlations between the related genes (T-bet, IFN-γ, RORγt, and IL-17A) were determined using the Spearman test.
RESULTS
GATA3 gene expression
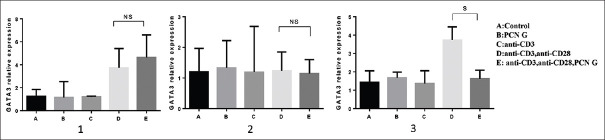
GATA3 has conventionally regarded as a transcription factor that drives the differentiation of TH2 cells. Obtained data of unstimulated PBMCs and stimulated the cells by CD3/CD28/PCN-G after 24 H showed elevated GATA3 expression [Table 2]. However, further evaluation of stimulated cells in 48 and 72 h did not support the elevation trend PCN [Figure 1]. Real-time PCR results are presented in supplementary documents.
Table 2.
Cytokine mRNA expression in stimulated and unstimulated peripheral blood mononuclear cells
| Cytokine | Stimulation time (h) | Unstimulated PBMCs | Stimulated PBMCs by anti-CD3/CD28 | Stimulated PBMCs anti-CD3/CD28/PCN-G | P |
|---|---|---|---|---|---|
| Gata3 | 24 | 1.7 (0.41-3.20) | 4.1 (1.8-11.2) | 4.3 (2.1-10.24) | 0.002** |
| 48 | 1.4 (0.31-0.41) | 1.3 (0.24-4.11) | 1.1 (0.24-3.76) | 0.235 | |
| 72 | 1.5 (0.24-4.56) | 3.5 (1.2-6.11) | 1.4 (0.43-5.23) | 0.243 | |
| FoxP3 | 24 | 0.4 (0.11-.2.45) | 1.2 (0.35-4.89) | 1.5 (0.34-5.34) | 0.001* |
| 48 | 1.2 (0.32-3.67) | 2.4 (0.23-6.12) | 1.85 (0.34-5.48) | 0.081 | |
| 72 | 1.6 (0.26-5.34) | 3.4 (0.35-7.45) | 4.1 (0.42-8.28) | 0.032* | |
| IFN-γ | 24 | 1.4 (0.16-3.55) | 14.5 (1.56-15.78) | 7.3 (0.98-16.55) | 0.021* |
| 48 | 1.8 (0.62-4.67) | 15.8 (1.44-13.98) | 6.4 (1.16-12.55) | 0.045* | |
| 72 | 1.2 (0.28-5.84) | 15.5 (1.67-13.45) | 7.5 (2.16-12.38) | 0.001** | |
| T.bet | 24 | 1.7 (0.26-3.45) | 2.2 (1.22-5.66) | 2.3 (0.98-5.57) | 0.081 |
| 48 | 1.8 (0.66-4.77) | 2.1 (0.23-5.12) | 1.4 (0.16-4.55) | 0.343 | |
| 72 | 1.8 (0.28-4.84) | 4.1 (0.25-4.12) | 1.1 (0.16-3.78) | 0.125 | |
| IL-17 | 24 | 0.9 (0.12-3.15) | 3.3 (0.21-4.11) | 1.2 (0.18-3.23) | 0.236 |
| 48 | 0.88 (0.12-2.67) | 4.9 (0.21-4.23) | 2.4 (0.16-4.69) | 0.045 | |
| 72 | 1.6 (0.29-5.14) | 4.3 (0.21-5.13) | 2.1 (0.16-4.38) | 0.123 | |
| RORγt | 24 | 0.8 (0.19-3.33) | 2.2 (0.23-5.12) | 1.3 (0.18-4.15) | 0.122 |
| 48 | 1.2 (0.32-4.23 | 3.1 (0.34-4.39) | 0.9 (0.23-3.45) | 0.213 | |
| 72 | 0.4 (0.18-4.14) | 1.3 (0.15-4.19) | 0.95 (0.16-3.38) | 0.052* |
*, **Represent P<0.05 and 0.01, respectively. All values are mean and range. Gata3=Gata3 protein; RORγt=RORgammaT; FOXP3=Forkhead box P3; IFN-γ=Interferon-gamma; IL-17A=Interleukin 17A; T-bet: T-box transcription factor TBX21
Figure 1.
GATA3 expression levels in stimulated and unstimulated PBMCs. Analysis of GATA3 mRNA in stimulated PBMCs with anti-CD3 (1 μg/ml), anti-CD28 (2 μg/ml) and PCN G (120 μg/ml) did not show significant differences after 24 h and 48 h (graph 1 and 2); however, expression of the gene was down regulated after 72 h stimulation of the cells in the presence of PCN G (P = 0.035). The Bars represent mean ± standard deviation values. NS = Nonsignificant; S = Significant; PBMC = Peripheral blood mononuclear cell
Forkhead box P3 protein gene expression
QPCR analysis of Foxp3 gene expression in stimulated PBMCs did not show significant changes in the cells after 24 and 48 h in the presence of PCN G [Figure 2, P = 0.45]. After 72 h of stimulation PBMCs by PCN G produced elevated levels of Foxp3; however, statistical analysis did not show significant differences (P > 0.05).
Figure 2.
Foxp3 mRNA levels in PBMCs stimulated by 120μg/ml PCN, 1 μg/ml of anti-CD3 and 2 μg/ml of anti-CD28 are depicted in Graph 1, 2, and 3 after 24, 48, and 72, respectively. Although PCN G augmented expression of Foxp3 gene comparing to control group statistical analysis did not show significant differences (P > 0.05). Bars represent mean ± standard deviation values. NS = Nonsignificant; PBMC = Peripheral blood mononuclear cell
Interferon-gamma gene expression
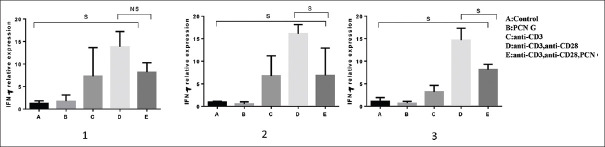
IFN-γ as a pluripotent cytokine plays a vital role in developing TH1 cells and leading cell-mediated immunity. IFN-γ mRNA measurements by QPCR results of the study showed that PCN G effectively down regulated of the gene after 72 h [Figure 3].
Figure 3.
Decreased IFN-γ gene expression in stimulated PBMCs 48 h and 72 h (graph 2 and 3) of cultures with 120 μg/ml of PCN G, 1 μg/ml of anti-CD3 and 2 μg/ml of anti-CD28 (P = 0.02). The Bars represent mean ± standard deviation values. NS = Nonsignificant; S = Significant; IFN-γ = Interferon-gamma; PBMCs = Peripheral blood mononuclear cells
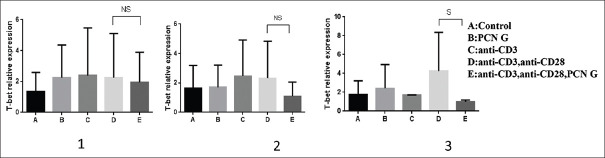
T-bet gene expression
T-bet, as effective transcription factor, promotes the development of TH1 cells. This transcription factor also is essential for the expression of IFN-γ in human T-lymphocytes. Our results showed that penicillin is able to modulate T-bet in activated T-cells by CD3 and CD28 pathways. After 72 h of coculturing PBMCs with PCN and anti-CD3 and anti-CD28, levels of T-bet mRNA decreased significantly comparing with stimulated T-cells by anti-CD3 and anti-CD8 [Figure 4].
Figure 4.
T-bet gene mRNA levels down regulated significantly in stimulated PBMCs after 72 h (graph 3) of incubation with 120 μg/ml of PCN G, 1 μg/ml of anti-CD3 and 2 μg/ml anti-CD28 (P = 0.03). Bars represent mean ± standard deviation values. NS = Nonsignificant; S = Significant; PBMCs = Peripheral blood mononuclear cells
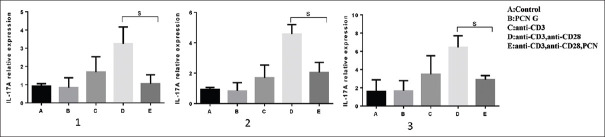
Interleukin-17A gene expression
IL-17A is considered as an inflammatory cytokine that produce mainly by TH17 cells. Neutrophils related inflammation is related to some extent to this cytokine's function. The results of the present revealed that PCN G is able to reduce the expression of IL-17 when it administrated by anti-CD3 and anti-CD28 [Figure 5].
Figure 5.
PCN G affected IL-17 gene expression levels in stimulated PBMCs negatively. To induce activation state in T-cells, 1 μg/ml of anti-CD3 and 2 μg/ml of anti-CD28 were used. Statistical analysis approved that PCN G significantly has down regulated IL-17 gene (Graph 1, 2, 3) (P < 0.05). The bars represent mean ± standard deviation values, S = Significant; PBMCs = Peripheral blood mononuclear cells; IL = Interleukin
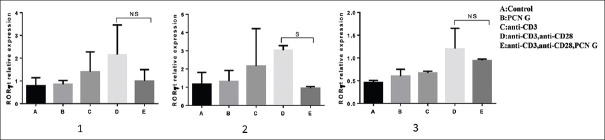
RORγt gene expression
TH17 cells development is related to the expression of RORγt. The present study investigated IL-17A and RORγt in stimulated T-cells simultaneously. Our results showed that after 48 h of stimulation T-cell by anti-CD3, anti-CD28 and PCN G, median of RORγt gene expression significantly downregulated comparing to activated T-cell by anti-CD3 and anti-CD28 [Figure 6] (P = 0.03).
Figure 6.
RORγt gene expression levels in stimulated T-cells by 1 μg/ml of anti-CD3, 2 μg/ml of anti-CD28, and 120 μ/ml of PCN G after 24, 48 and 72 h of incubation. Statistical analysis approved that PCN G significantly has down regulated RORγt expression after 48 h of stimulation (Graph 2) (P = 0.01). The bars represent mean ± standard deivation values. NS = Nonsignificant; S = Significant
DISCUSSION
We had shown previously that the prevention of articular symptoms in acute rheumatic fever during monthly penicillin injections could be due to anti-inflammatory, rather than antibiotic effects of penicillin.[18] To address the possible anti-inflammatory properties of PCN-G, the current study is designed to assess the PBMCs of healthy donors' reactions to the stimulation by PCN-G in an ex-vivo condition. A limitation of the study was investigation of PBMCs of healthy donors without inflammatory diseases in in-vitro condition. Several factors, including enzymes, neutralizing proteins, genetic factors of host are working in in-vivo conditions, which can affect the treatment. Moreover, PBMCs in many inflammatory situations and infectious diseases are not the only involved cellular players. Despite the mentioned limitation to get better knowledge of PCN-G functions, in the first step we designed the study by regular high dose of PCN-G which already has been used in human for treatment in different pathologic conditions. It has been approved that besides antimicrobial activity, antibiotics have immunomodulatory properties. Some distinct antibiotics are able to downregulate pro-inflammatory cytokines including IL-1 β, IL-6, IL-8, TNF-α, IFN-γ.[8] On the other hand, certain antibiotics ameliorate acute inflammation by inducing IL-10.[17] In addition to the effects of certain antibiotics (moxifloxacin and ciprofloxacin) on development of Th1 and Th2 cells,[8] nuclear transcription factors such as NF-κB, and activator protein-1 (AP-1) are also modulated by distinct antibiotics.[19] Clarithromycin also decreases the expression of IL-13, with anti-inflammatory effects, in goblet cell in human bronchial epithelial cells.[20,21] Based on current evidence, there are growing attention on immunomodulatory role of antibiotics in recent years.
According to our results, PCN-G has upregulated GATA3 gene expression 24 h after stimulation. Physiological effects of GATA3 leading TH2 responses causing anti-inflammatory responses. GATA3 has conventionally been regarded as a transcription factor that drives the differentiation of TH2 cells.[22] Sacha et al. showed that clarithromycin has ameliorating role in severe asthma because of inhibiting IL-13 effects on respiratory tracts epithelial cells.[23] It would be assumed that a part of immunomodulatory role of PCN-G is dependent to upregulation TH2 responses.
Furthermore, macrolides including clarithromycin suppress LPS-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors.[24] Our results also showed that PCN G is able to diminish IFN-γ expression after 72 h stimulation [Figure 4]. Studies on infected mice by Listeria monocytogenes showed, rifampicin, cephradine, amikacin, and ticarcillin have also prevented IFN-γ production while penicillin G and ampicillin did not affect T cell IFN-γ responses.[23] We also investigated vital transcription factor for IFN-γ production, T-bet, to see possible mechanism for downregulation of IFN-γ in T cells treated by PCN G. Based on our results, PCN G has downregulated T-bet gene expression after 48 and 72 h. On the other hand, neomycin, chloramphenicol, gentamycin, rifampicin and oxytetracycline in mice model did not alter the IFN-γ levels in blood. However, only in high doses of chloramphenicol (2500 μg/mouse) a reduction of IFN-γ was appeared.[23,25] According to the present study and other surveys, some kind of antibiotics may have the capacity to affect TH1 and dependent mediators which might lead to suppress the inflammation.
In the present study we also measured IL-17A gene expression and its major transcription factor, RORγt, in stimulated PBMCs with PCN G. Real-time PCR results showed that PCN significantly has downregulated IL-17A expression in T cells while RORγt levels did not show significant changes. RORγt is a key transcription factor playing an important role in TH17 cell differentiation. These cells are characterized to produce IL-17A, IL-17F, IL-21 and IL-22. TH17 cells and their effector cytokines mediate host-defensive mechanisms to various infections, especially extracellular bacterial infections. According to our results, PCN-G potentially is able to ameliorate inflammation responses in ex-vivo conditions.
On the other hand, Regulatory CD4+ T cells (Treg cells) are the key cells to suppress inflammation responses dependent to TH1 and TH17 cells. Development of the Treg cells is critically dependent on X-linked transcription factor Foxp3 (forkhead box P3). Our results did not show significant effects of PCN G on Foxp3 mRNA expression. Acquired results in arthritis related to chronic Borrelia burgdoferi infection showed that following antibiotic therapy the number of Treg has increased in the peripheral and synovial fluid of the patients.[26,27] However in experimental autoimmune encephalomyelitis and adjuvant arthritis rat models treated by cefuroxime or penicillin, numerous genes associated with TH2 and Treg differentiation were down-regulated.[28] The results of the study did not support anti-inflammatory PCN-G properties related to Treg cells.
CONCLUSION
Our results confirmed the immunomodulatory role of PCN G by affecting the expression of different cytokines genes in PBMCs.
Financial support and sponsorship
This study supported financially by a grant numbered IR.SSU.MEDICINE.REC.1394.153 of Shahid Sadoughi University of Medical Sciences Yazd, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Here we should appreciate of immunology department staff Shahid Sadoughi University of Medical Sciences Yazd, Iran for their technical assistance and the blood donors who accepted to take part in the study. Authors also do appreciate the kind contribution of Masoud Tajamolian, in the preparation of primers utilized in this study.
REFERENCES
- 1.Tauber SC, Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008;1:68–79. [PubMed] [Google Scholar]
- 2.Swathirajan CR, Rameshkumar MR, Solomon SS, Pradeep A, Chithra DA, Balakrishnan R, et al. Bacterial etiology and antibiotic resistance profile of bloodstream infections in human immunodeficiency virus patients from Southern India. J Res Med Sci. 2019;24:82. doi: 10.4103/jrms.JRMS_55_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R, Tintinger G, Cockeran R, Potjo M, Feldman C. Beneficial and harmful interactions of antibiotics with microbial pathogens and the host innate immune system. Pharmaceuticals (Basel) 2010;3:1694–710. doi: 10.3390/ph3051694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper RD. The enzymes involved in biosynthesis of penicillin and cephalosporin; their structure and function. Bioorg Med Chem. 1993;1:1–7. doi: 10.1016/s0968-0896(00)82098-2. [DOI] [PubMed] [Google Scholar]
- 5.June CM, Vaughan RM, Ulberg LS, Bonomo RA, Witucki LA, Leonard DA. A fluorescent carbapenem for structure function studies of penicillin-binding proteins, β-lactamases, and β-lactam sensors. Anal Biochem. 2014;463:70–4. doi: 10.1016/j.ab.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishino F, Mitsui K, Tamaki S, Matsuhashi M. Dual enzyme activities of cell wall peptidoglycan synthesis, peptidoglycan transglycosylase and penicillin-sensitive transpeptidase, in purified preparations of Escherichia coli penicillin-binding protein 1A. Biochem Biophys Res Commun. 1980;97:287–93. doi: 10.1016/s0006-291x(80)80166-5. [DOI] [PubMed] [Google Scholar]
- 7.Duivenvoorden WC, Hirte HW, Singh G. Use of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cells. Invasion Metastasis. 1997;17:312–22. [PubMed] [Google Scholar]
- 8.Williams AC, Galley HF, Watt AM, Webster NR. Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother. 2005;56:502–6. doi: 10.1093/jac/dki251. [DOI] [PubMed] [Google Scholar]
- 9.Hou Y, Heon Ryu C, Jun JA, Kim SM, Jeong CH, Jeun SS. Interferon β-secreting mesenchymal stem cells combined with minocycline attenuate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;274:20–7. doi: 10.1016/j.jneuroim.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Niimi N, Kohyama K, Matsumoto Y. Minocycline suppresses experimental autoimmune encephalomyelitis by increasing tissue inhibitors of metalloproteinases. Neuropathology. 2013;33:612–20. doi: 10.1111/neup.12039. [DOI] [PubMed] [Google Scholar]
- 11.Nessler S, Dodel R, Bittner A, Reuss S, Du Y, Hemmer B, et al. Effect of minocycline in experimental autoimmune encephalomyelitis. Ann Neurol. 2002;52:689–90. doi: 10.1002/ana.10353. [DOI] [PubMed] [Google Scholar]
- 12.McEvoy T. Minocycline: Rheumatoid arthritis. Hosp Pharm. 2016;51:535–8. doi: 10.1310/hpj5107-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin AR, Patel RN, Thakker GD, Lowenstein CJ, Attur MG, Abramson SB. Post-transcriptional regulation of inducible nitric oxide synthase mRNA in murine macrophages by doxycycline and chemically modified tetracyclines. FEBS Lett. 1997;410:259–64. doi: 10.1016/s0014-5793(97)00605-4. [DOI] [PubMed] [Google Scholar]
- 14.Huang TY, Chu HC, Lin YL, Lin CK, Hsieh TY, Chang WK, et al. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicol Appl Pharmacol. 2009;237:69–82. doi: 10.1016/j.taap.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Sadowski T, Steinmeyer J. Minocycline inhibits the production of inducible nitric oxide synthase in articular chondrocytes. J Rheumatol. 2001;28:336–40. [PubMed] [Google Scholar]
- 16.Defaux A, Zurich MG, Honegger P, Monnet-Tschudi F. Minocycline promotes remyelination in aggregating rat brain cell cultures after interferon-γ plus lipopolysaccharide-induced demyelination. Neuroscience. 2011;187:84–92. doi: 10.1016/j.neuroscience.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 17.Di Caprio R, Lembo S, Di Costanzo L, Balato A, Monfrecola G. Anti-inflammatory properties of low and high doxycycline doses: An in vitro study. Mediators Inflamm. 2015;2015:329418. doi: 10.1155/2015/329418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owlia M, Mirzaei M. Acute rheumatic fever: Over-estimation or mis-conception? Int J Cardiol. 2013;168:5107–8. doi: 10.1016/j.ijcard.2013.07.245. [DOI] [PubMed] [Google Scholar]
- 19.Aghai ZH, Kode A, Saslow JG, Nakhla T, Farhath S, Stahl GE, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62:483–8. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 20.Nagashima A, Shinkai M, Shinoda M, Shimokawaji T, Kimura Y, Mishina K, et al. Clarithromycin suppresses chloride channel accessory 1 and inhibits interleukin-13-induced goblet cell hyperplasia in human bronchial epithelial cells. Antimicrob Agents Chemother. 2016;60:6585–90. doi: 10.1128/AAC.01327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe T, Kanoh S, Tsushima K, Yamazaki Y, Kubo K, Rubin BK. Clarithromycin inhibits interleukin-13-induced goblet cell hyperplasia in human airway cells. Am J Respir Cell Mol Biol. 2011;45:1075–83. doi: 10.1165/rcmb.2010-0327OC. [DOI] [PubMed] [Google Scholar]
- 22.Allen JE, Wynn TA. Evolution of Th2 immunity: A rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacha PT, Zaremba ML, Jakoniuk P. The effect of selected antibacterial antibiotics on production of interferon gamma (IFN-G) by mouse T lymphocytes stimulated by Listeria monocytogenes. Med Dosw Mikrobiol. 1999;51:413–9. [PubMed] [Google Scholar]
- 24.Kikuchi T, Hagiwara K, Honda Y, Gomi K, Kobayashi T, Takahashi H, et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother. 2002;49:745–55. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 25.Rollag H, Jr, degré M. Effect of antibiotics on interferon production in mice. Acta Pathol Microbiol Scand B. 1976;84B:369–72. doi: 10.1111/j.1699-0463.1976.tb01954.x. [DOI] [PubMed] [Google Scholar]
- 26.Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, et al. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum. 2010;62:2127–37. doi: 10.1002/art.27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strle K, Sulka KB, Pianta A, Crowley JT, Arvikar SL, Anselmo A, et al. T-Helper 17 Cell Cytokine responses in Lyme disease correlate with Borrelia burgdorferi antibodies during early infection and with autoantibodies late in the illness in patients with antibiotic-refractory Lyme arthritis. Clin Infect Dis. 2017;64:930–8. doi: 10.1093/cid/cix002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mor F, Cohen IR. Beta-lactam antibiotics modulate T-cell functions and gene expression via covalent binding to cellular albumin. Proc Natl Acad Sci U S A. 2013;110:2981–6. doi: 10.1073/pnas.1215722110. [DOI] [PMC free article] [PubMed] [Google Scholar]