Abstract
Cyclic adenosine monophosphate (cAMP) contributes to maintenance of a quiescent (relaxed) state in the myometrium (i.e. uterine smooth muscle) during pregnancy, which most commonly has been attributed to activation of protein kinase A (PKA). PKA-mediated phosphorylation of cytosolic contractile apparatus components in myometrial smooth muscle cells (mSMCs) are known to promote relaxation. Additionally, PKA also regulates nuclear transcription factor (TF) activity to control expression of genes important to the labour process; these are mostly involved in actin-myosin interactions, cell-to-cell connectivity and inflammation, all of which influence mSMC transition from a quiescent to a contractile (pro-labour) phenotype. This review focuses on the evidence that cAMP modulates the activity of TFs linked to pro-labour gene expression, predominantly cAMP response element (CRE) binding TFs, nuclear factor κB (NF-κB), activator protein 1 (AP-1) family and progesterone receptors (PRs). This review also considers the more recently described exchange protein directly activated by cAMP (EPAC) that may oppose the pro-quiescent effects of PKA, as well as explores findings from other cell types that have the potential to be of novel relevance to cAMP action on TF function in the myometrium.
Keywords: bZIP transcription factors, cAMP, muscle contraction, pregnancy, signal transducers and activators of transcription, uterus
Introduction
In the uterus, smooth muscle cells of the myometrium (mSMCs) are maintained in a quiescent state throughout pregnancy to facilitate fetal growth and maturation. Labour typically starts at term (defined as ≥37 weeks of gestation), when the myometrium undergoes transition from quiescent to contractile state. This involves up-regulated expression/activity of contraction-associated proteins (CAPs), which include connexin-43, oxytocin receptor (OXTR) and sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2; changes in concentrations of prostaglandins and cytokines/chemokines also occur to indicate that labour is a pro-inflammatory process [1–3]. Sterile inflammation is suggested to increase within the myometrium, as pregnancy advances towards labour, to promote uterine contractions by up-regulating the expression of CAPs [4–6]. Spontaneous preterm (<37 weeks of gestation) labour (sPTL), a leading cause of mortality and morbidity of children under 5 years old [7,8], is linked to premature up-regulation of labour-related protein expression/activity; stimuli proposed to promote this include extrinsic inflammation (from infection), thrombin and mechanical stretch [9]. Our knowledge of the cellular processes involved in the spontaneous onset of labour are limited, especially with regards to the intracellular signalling events responsible for mSMC transition from a quiescent to contractile phenotype, which has hindered the development of effective therapies for preventing sPTL.
Transcription factor (TF) activity is expected to form an important connection between changes to in utero signals and mSMC phenotype adaptation. Among the most well-studied TFs for pregnancy/labour are nuclear factor κB (NF-κB), activator protein 1 (AP-1) and progesterone receptors (PRs; isoforms A and B). Regulation of these TFs in mSMCs is typically associated with pro-inflammatory mediator activation of mitogen-activated protein kinase (MAPK) signalling cascades. However, TF activity is also influenced by cyclic adenosine monophosphate (cAMP), which is a ubiquitous second messenger better known for promoting myometrial relaxation via its effects on contractile apparatus proteins by acting primarily through protein kinase A (PKA) [10–12]. The actions of cAMP can also be mediated via exchange protein directly activated by cAMP (EPAC; isoforms 1 and 2), which interacts with Ras-like small GTPase (Rap) proteins [13,14] to activate MAPKs [15] but its role in mSMCs is less understood. In this review, we will summarise established observations of labour-associated changes in abundance of cAMP signalling components in the myometrium, as well as discuss how they may impact on TFs known to bind to cAMP response element (CRE) sequences at gene promoters, along with the aforementioned labour-related TFs, to modulate contractility and inflammation.
Dynamics of myometrial cAMP signalling
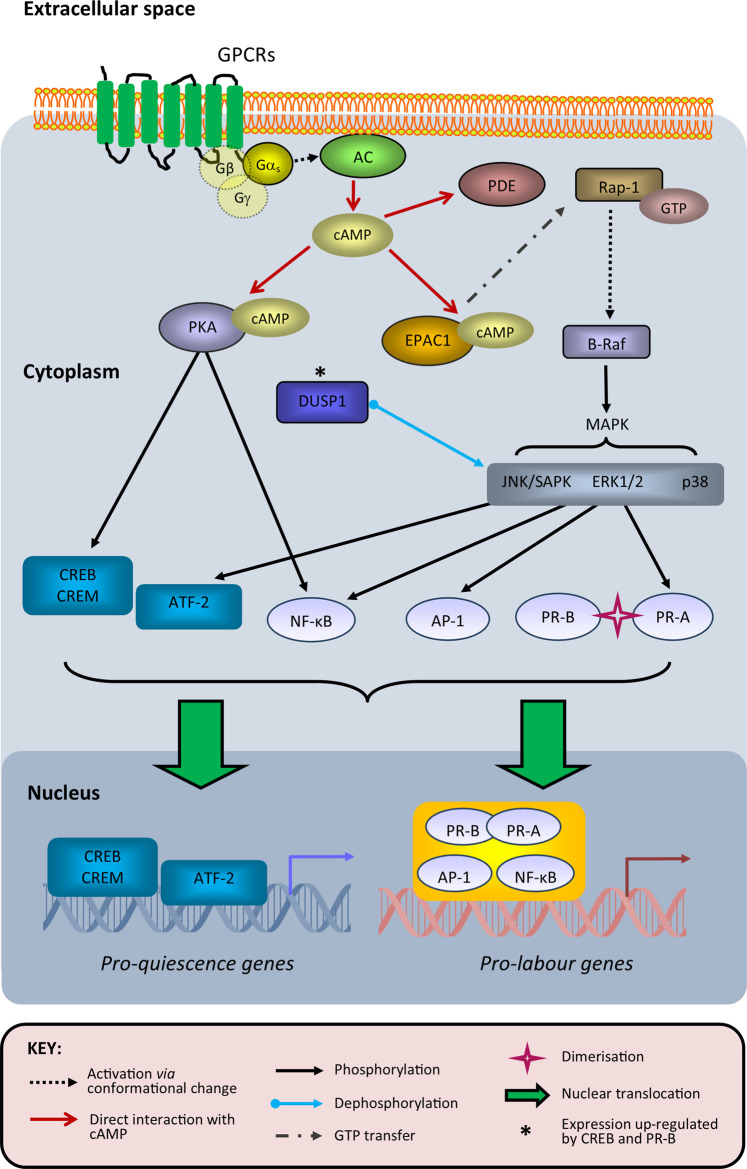
The cAMP pathway is fundamental to all mammalian cells and primarily driven by G-protein coupled receptor (GPCR) activation, specifically through those coupled to either Gαs (cAMP-activating) or Gαi (cAMP-inhibiting) transducer proteins [16,17]. For mSMCs, the most recognised GPCRs involved in cAMP signalling during pregnancy/labour are β-adrenoreceptors [18] and prostaglandin E2 (PGE2) receptors [19]. The generic signal transduction steps that link GPCR–ligand interaction to cellular response is well described for many cell types [20,21] and their components that are relevant to our discussion of mSMCs are depicted in Figure 1. The potential importance of adenylate cyclase (AC; activated by Gαs to synthesise cAMP) and phosphodiesterase (PDE; hydrolyses cAMP to eliminate its activity) isoforms in shaping PKA-driven responses, as well as their likelihood of acting as drug targets, in the context of labour have been reviewed previously [22,23].
Figure 1. Overview of cAMP-regulated transcription factor activity in myometrial smooth muscle cells during pregnancy and labour.
Upon agonist binding to G-protein coupled receptors (GPCRs) that promote cyclic adenosine monophosphate (cAMP) signalling, Gαs dissociates from its trimeric G-protein complex to activate adenylate cyclase (AC) and thus increase cAMP synthesis. Elevation of cAMP concentrations increase the probability of cAMP to bind both regulatory subunits of each tetrameric protein kinase A (PKA) complex, which causes the dissociation and activation of both its catalytic subunits that subsequently phosphorylate proteins with exposed serine/threonine-containing motifs compatible to their active sites. These include transcription factors that bind to the cAMP response element (CRE) sequence within compatible gene promoters, such as CRE-binding protein (CREB) and CRE modulator (CREM); cAMP-dependent transcription factor 2 (ATF-2) can bind to CRE sites but is not a known PKA substrate. CREB and CREM activities have been proposed to promote expression of pro-quiescence genes, and this is potentially influenced by CREB heterodimerisation with ATF-2. PKA (via CREB) and PR-B activity can enhance expression of dual specificity phosphatase 1 (DUSP1), which dephosphorylates mitogen-activated protein kinases (MAPKs) to reduce phosphorylation of their downstream targets; these include progesterone receptor A (PR-A), which is phosphorylated at its Ser344/345 residue by c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) to promote its heterodimerisation and subsequent transrepression of PR-B. In addition to PKA, exchange protein directly activated by cAMP 1 (EPAC1) can also bind cAMP, which leads to activation of guanosine triphosphate (GTP)-bound Ras-like small GTPase 1 (Rap1); this promotes the phosphorylation activity of B-Raf to increase downstream MAPK signalling via extracellular signal-regulated kinase 1/2 (ERK1/2), JNK, SAPK and/or p38. Subsequently, activator protein 1 (AP-1) and nuclear factor κB (NF-κB) transcription factors enhance the expression of pro-labour genes, which can involve interaction with PR heterodimers. PKA and EPAC1 activities are reduced by phosphodiesterase (PDE)-mediated degradation of cAMP.
Known significant pregnancy and labour-associated changes in myometrial mRNA/protein abundance for cAMP signalling components are summarised in Table 1. With regards to their functional output, basal PKA and GPCR/Gαs-dependent AC activities, when specifically detected in membrane fractions prepared from myometrial tissues, are increased by pregnancy and subsequently decreased at labour [24–26], and activity ratios of cAMP-specific PDE4 isoforms are altered by pregnancy to sustain high cAMP concentrations in myometrial tissues [27,28]. Data from in vitro experiments with mSMCs have suggested that up-regulation of PDE4B may partly be mediated by increased pro-inflammatory interleukin (IL)-1β stimulation [29]. Together, existing expression and activity data supports the concept that pregnancy favours a high cAMP content within the myometrium to maintain quiescence by promoting PKA activity, which declines at labour. This is potentially paralleled by an increase in EPAC1 levels, which has been observed in mSMCs in vitro to be capable of enhancing oxytocin receptor (OXTR) expression [30] and thus promote a pro-contractile phenotype. Labour-related changes in expression/activity of TFs that can be influenced by PKA/EPAC have also been observed in human myometrium-derived samples and these will be discussed in the following sections.
Table 1. Changes in myometrial tissue expression of cAMP signalling components associated with human pregnancy and term gestation labour.
| Component of cAMP signalling | Pregnancya | Labourb | ||
|---|---|---|---|---|
| mRNA | protein | mRNA | protein | |
| Gαs | [-] [26] | [-] [25]; ⇑ [25,143] | [-] [30] | [-] [30]; ⇓ [25] |
| Adenylate cyclases (ACs) | n/a | ⇓ AC1, AC3, AC8, AC9 [144]; ⇑ AC2, AC4, AC5, AC7 [144] | n/a | n/a |
| A-kinase anchoring proteins (AKAPs) | n/a | [-] AKAP95, [-] AKAP79 [24] |
↓ AKAP79 [30] | [-] AKAP79 [30]; [-] AKAP95, [-] AKAP79 [24]; ↓ AKAP79 [87] |
| Protein kinase A (PKA) – regulatory (R) subunits | ↑ RIIα [24] | [-] RIα, ↑ RIIα [24]; ↑ RIIα [24] |
↓ RIIα [24,30] | [-] RIα [24], ↓ RIIα [24,30]; ↓ RIIα/β [24,87] |
| Protein kinase A (PKA) – catalytic (C) subunits | n/a | [-] Cα, [-] Cβ [24]; ↑ Cα/β [24] |
n/a | [-] Cα, [-] Cβ [24]; ↓ Cα/β [24,87] |
| Exchange protein directly activated by cAMP 1 (EPAC1) | n/a | n/a | ↑ [30] | ↑ [30] |
| cAMP-phosphodiesterases (PDEs) | [-] PDE4A, PDE4C, PDE4D [145]; ↑ PDE4B2 [145] |
⇑ PDE4B, [=] PDE4D [27] |
[-] PDE4B [30] | ↓ PDE4B [30] |
Associated references in brackets, where qualitative (⇓/⇑/[=]) or quantitative (↑/↓/[-]) findings presented were based on comparisons between apregnant (term gestation) vs non-pregnant and bterm labouring vs term non-labouring women (decrease (⇓↓)/increase (⇑↑)/no change ([=][-]) of former compared with latter for each pair of comparisons) for myometrial tissues obtained from the lower uterine segment at Caesarean section; n/a indicates the comparison has not been assessed; changes at protein level that were specifically observed in membrane fractions are shown in bold font.
CRE-binding TFs
TFs that bind to the CRE nucleotide sequence (5′-TGACGTCA-3′) include CRE-binding protein (CREB), CRE modulator (CREM) and cAMP-dependent transcription factor (ATF), which are all members of the basic leucine zipper (bZIP) superfamily [31]. CREB is activated by PKA phosphorylation at its Ser133 residue to promote its nuclear translocation and dimerisation [32]. Active CREB dimers can interact with coregulators such as CREB-binding protein (CBP) or p300, which augment the ability of CREB to assemble transcriptional complexes at its target gene promoters by modifying chromatin structure via histone acetylation [33]. CREB protein abundance in human myometrium has been observed to decrease during pregnancy with no further change at labour [34]. Additionally, CBP abundance in myometrial tissues is increased during pregnancy and decreased at labour, whereas p300 is unchanged during both events [35]. Other CREB-binding partners, such as nuclear receptor sub-family 6, group A, member 1 (NR6A1) [36] and CREB-regulated transcription co-activator 1 (CRTC1) [37], have yet to be studied using mSMCs.
CREM is phosphorylated by PKA, which occurs specifically at the Ser117 residue of its CREMτ isoform [38]. In human myometrium, alternative splicing of CREM produces transcription activator (CREMτ2α) or repressor (CREMα) isoforms, which are decreased and increased, respectively, in their protein abundance both by pregnancy and, more so for CREMα, at labour [34,39]. This myometrial CREM isoform switch has been attributed to the activity of serine/arginine-rich splicing factor 5 (SRSF5; also known as SRp40), which decreases in its protein abundance during pregnancy and more so at labour; repression of its activity increases the presence of CREMα [40]. Microarray-based transcriptome analysis of mSMC cultures have shown that CREMτ2α and CREMα overexpression results in differential expression of 220 and 118 genes, respectively, only 16 of which were common between these two CREM isoforms; the same study also showed 958 genes were differentially expressed after CREB overexpression [39].
ATF-2 is the only member of the ATF family that has been detected at protein level in human myometrial tissues, in both its full-length form and a short ‘ATF2-sm’ variant [34] both of which were decreased in their protein abundance by pregnancy and, more so for full-length ATF-2, at labour. The same study also showed ATF2-sm expression was higher in upper (than lower) segment myometrium in pregnant (non-labouring and labouring) women, but they were of near-equal abundance in these two uterine regions in non-pregnant women. ATF-2 interaction with co-activators can be enhanced by CBP [41]. Phosphorylation of ATF-2 is more associated with stress-activated protein kinases (SAPKs) and c-Jun N-terminal kinases (JNKs) than PKA [42]. ATF-2 can bind to CRE as a homodimer but can also form heterodimers with Jun proteins; the latter bind with less affinity to CRE and cannot bind the AP-1 response element [43]. ATF-2 can also form heterodimers with CREB, thus forming an intersection between MAPKs and PKA for their regulation of CRE-containing gene promoters. The CRE-binding activity of ATF-2 in mSMCs has been demonstrated in vitro but endogenous labour-related gene targets for ATF-2 in the myometrium have yet to be determined [44].
Combined effects of cAMP and progesterone on gene expression
Progesterone is well recognised for its pro-quiescent effects; classic labour-related genes that encode cyclooxygenase-2 (COX-2; PTGS2), connexin-43 (GJA1) and OXTR have been shown to be transcriptionally repressed by progesterone acting via PR-B during pregnancy [45,46]. With the onset of human labour, it has been proposed that pro-quiescent effects of PR-B are reduced by increased expression/activity of the transrepressive PR-A isoform, which forms heterodimers with PR-B to sequester it from promoter sequences to remove its suppressive effect on the expression of labour-related genes [47,48]. Other mechanisms for nuclear PR action mostly involve interactions with transcriptional coregulator proteins [49]; those proposed to contribute to progesterone-regulated gene expression in the myometrium for labour include GATA zinc finger domain-containing 2B (GATAD2B) [50], non-POU-domain-containing octamer binding protein (p54nrb) [51] and polypyrimidine tract-binding protein-associated-splicing factor (PSF) [52,53]. Regulation of mSMC progesterone content by 20α-hydroxysteroid dehydrogenase (20α-HSD) activity has also been considered as an important aspect of myometrial progesterone responsiveness at labour [46,54].
PR-A is 164 amino acids shorter than PR-B at its N-terminus [55], which results in the lack of an activation function (AF) domain (specifically AF3) that is present in PR-B and the first 140 residues of its N-terminus acts as a repressor region instead [56]. The canonical progesterone response element (PRE) sequence is 5′-ACAnnnTGT-3′ but PR can interact with promoter sequences that do not contain PREs [57,58]. Transrepression by PR-A requires sumoylation at its N-terminus [59]. In human mSMCs, pro-inflammatory stimulants lipopolysaccharide (LPS) and IL-1β can increase PR-A protein stability [60], which has been attributed to SAPK/JNK-mediated phosphorylation at its Ser344/345 residue [61,62]. Consequently, mSMC inflammation as a result of increased cytokines expression, which has been observed in myometrium biopsies from labouring (compared to non-labouring) women [63], is expected to reduce the pro-quiescent effect of PR-B at labour.
In relation to cAMP signalling, forskolin (AC agonist) can enhance the ability of progesterone to suppress IL-1β-induced PTGS2 expression in human mSMC cultures [64]. This apparent synergistic effect against inflammation-driven transcriptional activity is likely to be mediated via modulation of MAPKs, whereby cAMP and progesterone together (via CREB [65] and PR-B [66], respectively) increase dual specificity phosphatase 1 (DUSP1) expression that represses SAPK/JNK activity [67,68] to reduce PR-A Ser344/345 phosphorylation [62]. PR-A and PR-B abundance has been observed to exist at a 1 : 1 ratio in myometrium biopsies from non-labouring pregnant women at term gestation [69,70]; when this is modelled in vitro using the human myometrium hTERT-HMA/B cell line [48], treatment with a combination of forskolin and progesterone can maintain a transcriptome profile that resembles the in vivo non-labouring state more closely than treatment with either agent alone [71].
The endometrium is another uterine tissue where the effects of simultaneously up-regulating cAMP and progesterone signalling have been studied, mostly in the context of their ability to promote decidualisation when modelled in cell cultures using 8-bromo-cAMP (cAMP analogue) and medroxyprogesterone acetate (progestin). Here, this treatment combination increases Forkhead box protein O1 (FOXO1) TF expression and activity, which is needed to up-regulate IL-8 [72]; RNA-seq data suggests signal transducer and activator of transcription (STAT) TF activities are also increased at the same time, which is eventually replaced by NF-κB as expected in vivo prior to the end of the menstrual cycle [73]. Thus, cAMP and progesterone signalling together have been observed to promote opposing effects on inflammation in endometrial cells and mSMCs, at least where there are also differences in in vitro protocols and stage of reproduction.
NF-κB
The NF-κB family of TFs consists of RelA (p65), RelB, c-Rel, p100/p52 and p105/p50 proteins, which form different combinations of dimers that bind to consensus sequence 5′-GGGRNYYYCC-3′ to control the expression of inflammation-related genes [74–76]. Various pregnancy/labour-related stresses (e.g. uterine stretch and pro-inflammatory cytokines) activate NF-κB in mSMCs [77,78]. The p65 : p50 complex, which is the most commonly explored NF-κB dimer for mSMCs, is inactive in the cytoplasm while bound to inhibitor of NF-κB (IκB). Pro-inflammatory stimuli, such as LPS, IL-1β and tumour necrosis factor α (TNFα), activate IκB kinases (IKKs) to promote IκB phosphorylation, which leads to its ubiquitination and subsequent proteolysis. This removal of IκB binding from p65 and p50 exposes their nuclear localisation sequence, which results in their translocation into the nucleus to bind promoter sequences of genes that include CXCL8, CCL2, PTGS2 and OXTR.
A study that examined NF-κB protein abundance in myometrium biopsies from non-pregnant and pregnant (non-labouring and labouring) women showed levels of c-Rel, p105, p50 and p100 were reduced by pregnancy, while p65 (in lower, but not upper, uterine segment myometrium) was only reduced at labour [79]. Additionally, NF-κB dimer composition was found to change during pregnancy, from being predominantly p50 homodimers in non-pregnant women to being mostly p65 : p50 heterodimers in pregnant women; data from electromobility shift assays suggested that p65 : p50 DNA binding increases at labour despite a decrease in total p65 protein levels. The relevance of PKA to NF-κB activity was demonstrated in the same study; p65 was bound to PKA (catalytic subunits) and phosphorylated at its Ser536 residue in non-pregnant and pregnant women, most likely to maintain p65 in an inactive state prior to labour.
In other cell types, PKA is capable of phosphorylating p65 at its Ser276 residue [80], which can either activate NF-κB via enhanced CBP/p300 recruitment [81] or suppress its nuclear translocation [82]. In myometrium, it has been suggested that complex formation with PKA and IκBα together prevents p65 Ser276 phosphorylation in both non-pregnant and pregnant (non-labouring) women [79]. An alternative mechanism for cAMP-enhanced NF-κB activity has been proposed for human foetal lung epithelial cells, where miRNAs (specifically miR-199a and miR-214) that suppress NF-κB and COX-2 expression can be decreased in abundance by treatment with cAMP analogue, dibutyryl cAMP [83]; this was attributed to CREB-mediated reduction of zinc finger E-box-binding homeobox 1 (ZEB1) TF expression. Interestingly, progesterone is known to have the opposite effect on ZEB1 in mSMCs, which suppresses COX-2 expression (using the same miRNAs) during pregnancy [45].
The consequences of interactions between PKA and NF-κB on labour-related gene expression in the myometrium have not been directly assessed. Prolonged in vitro treatment of human mSMCs with forskolin has, however, been observed to increase COX-2 expression and PGE2 secretion by promoting MAPK activity [84]; although the functional impact of PGE2 can be either pro-relaxatory or pro-contractile depending on the expression levels of its receptor isoforms present [19,85,86]. The combination of forskolin and progesterone during IL-1β stimulation can also reduce p65 binding to the PTGS2 promoter [64]. Overall, a labour-associated decrease in catalytic and regulatory PKA subunits in myometrium at term gestation [24,87] is expected to enhance pro-inflammatory genes expression driven by NF-κB.
Both enhancement and suppression of NF-κB by cAMP have been demonstrated more directly in other cell types and mostly attributed to PKA. Treating a human monocyte cell line with dibutyryl cAMP can increase gene promoter activity mediated by NF-κB, which is an observation used to explain how cholera toxin causes inflammation [88]; whereas nuclear translocation of NF-κB in brain (hippocampus and cortex) tissues of mice after in vivo LPS injection can be decreased by PDE4 inhibitor, rolipram [89]. In airway SMCs, the anti-inflammatory effects of PKA have been associated with enhanced cell proliferation [90,91]. Whereas for mSMCs, it has not been determined whether their proliferative state, which increases during hyperplasia at early pregnancy before switching to a hypertrophic state at late pregnancy to expand uterine size [92], influences the ability of PKA to regulate inflammation in the same way.
From the perspective of NF-κB regulating cAMP action, the GNAS (Gαs-encoding gene) promoter sequence is rich in GC nucleotides, which makes it ideal for binding TFs like CREB and NF-κB [93]. Indeed, TNFα-stimulated p65 activity can reduce GNAS promoter activity in mSMCs [94] by reducing CBP recruitment [95]. NF-κB is also activated by IL-1β and other pregnancy-related stress mediators; their combined ability to activate NF-κB has been proposed to progressively increase during advancing gestation [60], which eventually reaches a level that reduces cAMP-regulated gene expression (by reducing PKA activity) to promote labour [62]. Currently, it is not certain what the in utero order of events are at labour between the activities of cAMP/PKA and NF-κB in mSMCs.
AP-1
Active AP-1 dimers are formed from Jun (c-Jun, JunB and JunD) and Fos (c-Fos, FosB, Fra-1 and Fra-2) proteins, which can also dimerise with members of the ATF and Jun dimerisation protein (JDP) families, to function as bZIP TFs [96]; the consensus AP-1 binding sequence is 5′-TGA(C/G)TCA-3′ [97]. AP-1 activation requires its phosphorylation by MAPKs [98] that include members of the JNK family, which can be regulated by cAMP via EPAC (discussed below) as demonstrated in vascular SMCs [99]. Expression of c-Fos and c-Jun can be increased by CREB, which involves its phosphorylation by extracellular signal-regulated kinase (ERK)1/2 when demonstrated in a mouse-derived monocyte/macrophage cell line stimulated with IL-33 [100]. Comparisons for protein abundance of each Jun and Fos protein in the myometrium between non-labouring and labouring women have been undertaken for whole tissue extracts [101] and nuclear/cytosolic fractions [102], which demonstrated that they are differentially altered by labour (i.e. not all AP-1 monomers were increased or decreased to the same extents as each other). AP-1 can interact with PR to modulate GJA1 transcription in mSMCs [54]. Here, the activity of AP-1 has been proposed to be repressed by PR-B and activated by PR-A, which suggests that a labour-related increase in PR-A expression [47,48] can enhance AP-1 activity to increase connexin-43 abundance. The ability of PKA to regulate PR-A activity (discussed above) presents a potential layer of complexity to their relationship with AP-1 in mSMCs. Promoter sequences of some labour-related genes contain binding sites for both AP-1 and NF-κB [103–105], and these TFs can also interact with each other to promote labour-related changes [106]; how this impacts on their interaction with cAMP signalling in mSMCs is not yet known.
EPAC and its MAPK-related role
EPAC1 is expressed in a wide range of tissues and believed to be ubiquitous [13]. EPAC2 has so far been observed as more restricted in its expression profile, whereby it has only been detected in the brain, as well as neuroendocrine and endocrine systems [107]. Their roles are likely to differ depending on cell type and micro/nano-domain localisation [108]. For example, mouse genetic knockout models have demonstrated that EPAC1 is mostly anti-inflammatory, while EPAC2 is pro-inflammatory, specifically in lung tissues [109]. PKA and EPAC have similar affinities for cAMP according to enzymology experiments [14,110], although recent cell-based data obtained using fluorescent cAMP biosensors question the physiological relevance of these findings [111,112]. Nevertheless, the assembly of different cAMP effector combinations in discrete micro/nano-domain regions of the intracellular environment is expected to allow compartmentalised control of PKA and EPAC activities [113–115]. There is certainly a deficit in our knowledge for this aspect of cAMP signalling in mSMCs, which needs to be explored to at least the same extent as it has been in other organ systems [116–118] for us to fully assess their roles in regulating uterine contractility and inflammation during pregnancy/labour.
Cellular stresses during pregnancy in mSMCs can activate MAPK signalling and be inhibited by PKA activation. However, as mentioned above, in vitro forskolin stimulation to enhance cAMP accumulation for a prolonged period of time can activate MAPKs [84]. The latter is potentially attributable to EPAC, which activates Rap proteins upon cAMP binding to enhance activities of MAPKs (via phosphorylation by B-Raf) that include ERK1/2, MAPK/ERK kinase (MEK)1/2 and p38 [119,120]. EPAC1 abundance in myometrium biopsies from term pregnant women has been observed to increase during the early phase of labour [30]. Although in vitro RNA interference-based silencing of EPAC1 expression in human mSMCs has demonstrated that this cAMP effector does not contribute to COX-2 up-regulation promoted by forskolin [84]; similar experiments have instead demonstrated that EPAC1 can increase OXTR expression when PKA is suppressed [30]. So far, these are the only studies that have examined the role of EPAC1 in mSMCs and none are available for EPAC2. EPAC1/2 roles in endometrial stromal cells have also only recently been of interest, where they have been proposed to enhance PKA-driven prolactin (PRL) gene promoter activity during decidualisation [121], and mediate cAMP-dependent (but PKA-independent) ERK1/2 phosphorylation promoted by human chorionic gonadotropin [122].
Future use of highly specific EPAC activators, such as 8-pCPT-2′-O-Me-cAMP [119], and inhibitors will be useful in evaluating the importance of EPAC in human mSMCs at labour; this approach has provided valuable information from studies on EPAC action in cardiovascular and respiratory SMCs with regards to their response to stress stimuli [123,124]. For example, 8-pCPT-2′-O-Me-cAMP treatment of wild-type mice-derived aortic SMCs can decrease cofilin phosphorylation to reduce stress fibre formation, which decreases lamellipodia formation needed for vascular remodelling, and this does not occur in aortic SMCs from EPAC1 knockout mice [125]. Treatment of immortalised human bronchial SMCs with either 6-Bnz-cAMP, a PKA-specific activator, or 8-pCPT-2′-O-Me-cAMP can inhibit IL-8 secretion in response to cigarette smoke extract (CSE), but PKA and EPAC distinctly prevent CSE-mediated decrease in IκBα protein abundance and increase in ERK1/2 phosphorylation, respectively [126]. A novel EPAC1-specific inhibitor, AM-001, has recently been shown in vivo to prevent murine cardiac fibrosis, immune cell infiltration and myocyte enhancer factor 2 (MEF2) TF activation in response to prolonged β-adrenoreceptor activation [127].
Cross-talk between cAMP and other messengers
Each TF and their complexes often act as hubs for multiple signalling pathways that are simultaneously activated by numerous combinations of cell surface receptors, which are predominantly GPCRs and receptor tyrosine kinases [128–130]. In addition to cAMP and MAPKs, Ca2+ signalling is important to mSMC function and can regulate TFs [131]; oxytocin and PGF2α receptor activation increases myometrial Ca2+ mobilisation to promote contractility. Along with MAPKs, Ca2+ can also influence cAMP accumulation by regulating PDE activity [132]. In contrast, cyclic guanosine monophosphate (cGMP) has been proposed to have a lesser impact on mSMCs at labour due to lack of effect on their contractions [133]. Nitric oxide can promote myometrial relaxation but a nitrosoproteome analysis of uterine biopsies from pregnant women did not identify cAMP-related TFs of interest as labour-associated nitrosylation targets [134].
CREB Ser133 phosphorylation can be increased by Ca2+-calmodulin-dependent protein kinase IV (CaMKIV), along with MAPKs [135] and protein kinase G (PKG) [136], as demonstrated in other cell types; it can also be decreased by Ca2+-activated calcineurin. NF-κB nuclear translocation can be increased by inhibiting large-conductance Ca2+-activated K+ (BK) channels in mSMCs, which has been proposed to involve IKK activity [137], whereas CaMKIV-driven p65 phosphorylation can promote its transactivation and interaction with CBP in HeLa cells [138]. Members of the AP-1 family can form complexes with those of the nuclear factor of activated T-cells (NFAT) family of calcineurin-dependent TFs, which can also be phosphorylated by MAPKs [139] and multiple NFAT isoforms are expressed in human myometrium during pregnancy [140].
Together, observations like these exemplify the complexity that needs to be considered when working towards identifying upstream factors of TFs that are specifically responsible for promoting myometrial contractions to initiate labour. Various stimuli may promote labour [141], but their relative importance and order of interactions remain to be determined. Integrative analyses of the aforementioned and future data for myometrial cAMP actions with those for other intracellular messengers are needed to build a complete picture of mSMC signalling networks [142]. From this approach, the sequence of cellular events critical to the transition from end of pregnancy to the beginning of labour are more likely to be identified.
Summary
To date, studies related to cAMP in the myometrium have assessed its regulation of cytosolic contractile apparatus function more than its effects on nuclear TF activity. Data for the latter mostly demonstrate how cAMP, either alone or in partnership with progesterone, can modulate labour-related gene expression to complement PKA pro-relaxation effects at the contractile apparatus and/or regulate inflammation. Instead of examining aspects of myometrial cAMP signalling for each TF separately, future work should focus on how the connections between them are altered to result in myometrial activation for initiating labour. Combining this with in-depth consideration of cAMP spatio-temporal organisation, the role of EPAC and cross-talk with other intracellular messengers, will help us to identify new ways to clinically modulate its activity to reduce the occurrence of pregnancy/labour-related complications.
Perspectives
Modulation of gene expression by TF activity is commonly investigated to gain insight into how the myometrium (uterine smooth muscle) transitions from a quiescent to contractile phenotype for the labour process to be initiated. TFs regulated by cAMP action have been shown to influence the expression of several classic labour-related proteins (mostly involved in contraction and inflammation) and thus likely to influence the success or failure of uterine contractions that lead to childbirth.
Cytosolic actions of cAMP are best known for promoting myometrial relaxation by acting on the contractile apparatus via PKA, but cAMP can also control nuclear DNA-binding activities of myometrial TFs at labour-related gene promoters. The expression/activities of several proteins involved in cAMP synthesis/degradation and subsequent PKA activation are up-regulated by pregnancy and down-regulated at labour.
Future efforts in identifying ways to harness the uterine pro-quiescent effects of cAMP will need to focus on the importance of spatio-temporal factors of cAMP signalling, as well as the activities of cAMP-binding proteins other than PKA, in mSMCs. A better understanding of how cAMP controls myometrial contractions will aid the development of therapies that reduce maternal and neonatal risk from obstetric complications, which includes preterm birth.
Acknowledgements
The authors are thankful to Emily Whettlock and Zhengyuan Huang (PhD students; Imperial College London) for proofreading the manuscript and feedback during figure preparation, respectively.
Abbreviations
- 20α-HSD
20α-hydroxysteroid dehydrogenase
- AC
adenylate cyclase
- AF
activation function
- AP-1
activator protein 1
- ATF
cAMP-dependent transcription factor
- BK
large-conductance Ca2+-activated K+
- bZIP
basic leucine zipper
- CaMK
calmodulin-dependent protein kinase
- cAMP
cyclic adenosine monophosphate
- CBP
CREB-binding protein
- cGMP
cyclic guanosine monophosphate
- COX-2
cyclooxygenase-2
- CRE
cAMP response element
- CREB
CRE-binding protein
- CREM
CRE modulator
- CRTC1
CREB-regulated transcription co-activator 1
- CSE
cigarette smoke extract
- DUSP1
dual specificity phosphatase 1
- EPAC
exchange protein activated by cAMP
- ERK
extracellular signal-regulated kinase
- FOXO1
Forkhead box protein O1
- GATAD2B
GATA zinc finger domain-containing 2B
- GPCR
G-protein coupled receptor
- GTP
guanosine triphosphate
- IKK
IκB kinase
- IL
interleukin
- IκB
inhibitor of NF-κB
- JDP
Jun dimerisation protein
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MEF2
myocyte enhancer factor 2
- MEK
MAPK/ERK kinase
- mSMC
myometrial smooth muscle cell
- NFAT
nuclear factor of activated T-cells
- NF-κB
nuclear factor κB
- NR6A1
nuclear receptor sub-family 6, group A, member 1
- p54nrb
non-POU-domain-containing octamer binding protein
- PDE
phosphodiesterase
- PGE2
prostaglandin E2
- PKA
protein kinase A
- PKG
protein kinase G
- PR
progesterone receptor
- PSF
polypyrimidine tract-binding protein-associated-splicing factor
- Rap
Ras-like small GTPase
- SAPK
stress-activated protein kinase
- sPTL
spontaneous preterm labour
- SRSF5
serine/arginine-rich splicing factor 5
- STAT
signal transducer and activator of transcription
- TF
transcription factor
- TNFɑ
tumour necrosis factor α
- ZEB1
zinc finger E-box-binding homeobox1
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
PhD studentship for JKHL is funded by Borne (registered charity number 1167073) with assistance from the Robert McAlpine Foundation (registered charity number 226646). The academic research activities of PFL and MRJ are funded by Borne; RMT is funded by HEFCE (King's College London), with current myometrium-related research funded by Tommy's baby charity (registered charity number 1060508) and Borne.
Open Access
Open access for this article was enabled by the participation of Imperial College London in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contributions
J.K.H.L. wrote the first draft of the manuscript. J.K.H.L., P.F.L., R.M.T. and M.R.J. contributed to all subsequent editing and approved the final submitted draft.
References
- 1.Hadley, E.E., Richardson, L.S., Torloni, M.R. and Menon, R. (2018) Gestational tissue inflammatory biomarkers at term labor: asystematic review of literature. Am. J. Reprod. Immunol. 79, e12776 10.1111/aji.12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tribe, R.M., Moriarty, P. and Poston, L. (2000) Calcium homeostatic pathways change with gestation in human myometrium. Biol. Reprod. 63, 748–755 10.1095/biolreprod63.3.748 [DOI] [PubMed] [Google Scholar]
- 3.Taggart, M.J. and Tribe, R.M. (2007) Cellular ionic mechanisms controlling uterine smooth muscle contraction: effects of gestational state. In New Frontiers in Smooth Muscle Biology and Physiology (Savinneau, J., ed.), pp. 523–549, Research Signpost, India [Google Scholar]
- 4.Romero, R., Miranda, J., Chaiworapongsa, T., Korzeniewski, S.J., Chaemsaithong, P., Gotsch, F.et al. (2014) Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 72, 458–474 10.1111/aji.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero, R., Xu, Y., Plazyo, O., Chaemsaithong, P., Chaiworapongsa, T., Unkel, R.et al. (2018) A role for the inflammasome in spontaneous labor at term. Am. J. Reprod. Immunol. 79, 20 10.1111/aji.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Lopez, N., Romero, R., Panaitescu, B., Leng, Y.Z., Xu, Y., Tarca, A.L.et al. (2018) Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am. J. Reprod. Immunol. 80, e13049 10.1111/aji.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawanpaiboon, S., Vogel, J.P., Moller, A.B., Lumbiganon, P., Petzold, M., Hogan, D.et al. (2019) Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health 7, E37–E46 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubens, C.E., Sadovsky, Y., Muglia, L., Gravett, M.G., Lackritz, E. and Gravett, C. (2014) Prevention of preterm birth: harnessing science to address the global epidemic. Sci. Transl. Med. 6, 12 10.1126/scitranslmed.3009871 [DOI] [PubMed] [Google Scholar]
- 9.Romero, R., Dey, S.K. and Fisher, S.J. (2014) Preterm labor: one syndrome, many causes. Science 345, 760–765 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyson, E.K., Macintyre, D.A., Smith, R., Chan, E.C. and Read, M. (2008) Evidence that a protein kinase A substrate, small heat-shock protein 20, modulates myometrial relaxation in human pregnancy. Endocrinology 149, 6157–6165 10.1210/en.2008-0593 [DOI] [PubMed] [Google Scholar]
- 11.Meera, P., Anwer, K., Monga, M., Oberti, C., Stefani, E., Toro, L.et al. (1995) Relaxin stimulates myometrial calcium-activated potassium channel activity via protein-kinase-A. Am. J. Physiol.-Cell Physiol. 269, C312–C3C7 10.1152/ajpcell.1995.269.2.C312 [DOI] [PubMed] [Google Scholar]
- 12.Guerra, D.D., Bok, R. and Hurt, K.J. (2020) Cyclic nucleotides and myometrial contractility. Curr. Opin. Physiol. 13, 102–107 10.1016/j.cophys.2019.10.014 [DOI] [Google Scholar]
- 13.Kawasaki, H., Springett, G.M., Mochizuki, N., Toki, S., Nakaya, M., Matsuda, M.et al. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 10.1126/science.282.5397.2275 [DOI] [PubMed] [Google Scholar]
- 14.de Rooij, J., Zwartkruis, F.J., Verheijen, M.H., Cool, R.H., Nijman, S.M., Wittinghofer, A.et al. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 10.1038/24884 [DOI] [PubMed] [Google Scholar]
- 15.Breckler, M., Berthouze, M., Laurent, A.C., Crozatier, B., Morel, E. and Lezoualc'h, F. (2011) Rap-linked cAMP signaling Epac proteins: compartmentation, functioning and disease implications. Cell Signal. 23, 1257–1266 10.1016/j.cellsig.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 16.Johnson, M. (2006) Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J. Allergy Clin. Immunol. 117, 18–24 10.1016/j.jaci.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 17.Simonds, W.F. (1999) G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 20, 66–73 10.1016/S0165-6147(99)01307-3 [DOI] [PubMed] [Google Scholar]
- 18.Rouget, C., Breuiller-Fouche, M., Mercier, F.J., Leroy, M.J., Loustalot, C., Naline, E.et al. (2004) The human near-term myometrial beta(3)-adrenoceptor but not the beta(2)-adrenoceptor is resistant to desensitisation after sustained agonist stimulation. Br. J. Pharmacol. 141, 831–841 10.1038/sj.bjp.0705616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slater, D.M., Astle, S., Woodcock, N., Chivers, J.E., de Wit, N.C.J., Thornton, S.et al. (2006) Anti-inflammatory and relaxatory effects of prostaglandin E-2 in myometrial smooth muscle. Mol. Hum. Reprod. 12, 89–97 10.1093/molehr/gal005 [DOI] [PubMed] [Google Scholar]
- 20.Musheshe, N., Schmidt, M. and Zaccolo, M. (2018) cAMP: from long-range second messenger to nanodomain signalling. Trends Pharmacol. Sci. 39, 209–222 10.1016/j.tips.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 21.McCormick, K. and Baillie, G.S. (2014) Compartmentalisation of second messenger signalling pathways. Curr. Opin. Genet. Dev. 27, 20–25 10.1016/j.gde.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 22.Yuan, W. and Lopez Bernal, A. (2007) Cyclic AMP signalling pathways in the regulation of uterine relaxation. BMC Pregnancy Childbirth 7, S10 10.1186/1471-2393-7-S1-S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehats, C., Oger, S. and Leroy, M.J. (2004) Cyclic nucleotide phosphodiesterase-4 inhibitors: a promising therapeutic approach to premature birth? Eur. J. Obstet. Gynecol. Reprod. Biol. 117, S15–SS7 10.1016/j.ejogrb.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 24.MacDougall, M.W.J., Europe-Finner, G.N. and Robson, S.C. (2003) Human myometrial quiescence and activation during gestation and parturition involve dramatic changes in expression and activity of particulate type II (RII alpha) protein kinase A holoenzyme. J. Clin. Endocrinol. Metab. 88, 2194–2205 10.1210/jc.2002-021862 [DOI] [PubMed] [Google Scholar]
- 25.Europe-Finner, G.N., Phaneuf, S., Tolkovsky, A.M., Watson, S.P. and Bernal, A.L. (1994) Down-regulation Of G-alpha(S) in human myometrium in term and preterm labor - a mechanism for parturition. J. Clin. Endocrinol. Metab. 79, 1835–1839 10.1210/jcem.79.6.7989491 [DOI] [PubMed] [Google Scholar]
- 26.Gsell, S., Eschenhagen, T., Kaspareit, G., Nose, M., Scholz, H., Behrens, O.et al. (2000) Apparent up-regulation of stimulatory G-protein alpha subunits in the pregnant human myometrium is mimicked by elevated smoothelin expression. FASEB J. 14, 17–26 10.1096/fasebj.14.1.17 [DOI] [PubMed] [Google Scholar]
- 27.Mehats, C., Tanguy, G., Paris, B., Robert, B., Pernin, N., Ferre, F.et al. (2000) Pregnancy induces a modulation of the cAMP phosphodiesterase 4-conformers ratio in human myometrium: consequences for the utero-relaxant effect of PDE4-selective inhibitors. J. Pharmacol. Exp. Ther. 292, 817–823 PMID: [PubMed] [Google Scholar]
- 28.Verli, J., Klukovits, A., Kormanyos, Z., Hajagos-Toth, J., Ducza, E., Seres, A.B.et al. (2013) Uterus-relaxing effect of beta(2)-agonists in combination with phosphodiesterase inhibitors: studies on pregnant rat in vivo and on pregnant human myometrium in vitro. J. Obstet. Gynaecol. Res. 39, 31–39 10.1111/j.1447-0756.2012.01929.x [DOI] [PubMed] [Google Scholar]
- 29.Oger, S., Mehats, C., Dallot, E., Ferre, F. and Leroy, M.J. (2002) Interleukin-1 beta induces phosphodiesterase 4B2 expression in human myometrial cells through a prostaglandin E-2- and cyclic adenosine 3 ‘,5 ‘-monophosphate-dependent pathway. J. Clin. Endocrinol. Metab. 87, 5524–5531 10.1210/jc.2002-020575 [DOI] [PubMed] [Google Scholar]
- 30.Yulia, A., Singh, N., Lei, K.Y., Sooranna, S.R. and Johnson, M.R. (2016) Cyclic AMP effectors regulate myometrial oxytocin receptor expression. Endocrinology 157, 4411–4422 10.1210/en.2016-1514 [DOI] [PubMed] [Google Scholar]
- 31.Zhang, X.M., Odom, D.T., Koo, S.H., Conkright, M.D., Canettieri, G., Best, J.et al. (2005) Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl Acad. Sci. U.S.A. 102, 4459–4464 10.1073/pnas.0501076102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montminy, M. (1997) Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 66, 807–822 10.1146/annurev.biochem.66.1.807 [DOI] [PubMed] [Google Scholar]
- 33.Ogryzko, V.V., Schiltz, R.L., Russanova, V., Howard, B.H. and Nakatani, Y. (1996) The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87, 953–959 10.1016/S0092-8674(00)82001-2 [DOI] [PubMed] [Google Scholar]
- 34.Bailey, J., Sparey, C., Phillips, R.J., Gilmore, K., Robson, S.C., Dunlop, W.et al. (2000) Expression of the cyclic AMP-dependent transcription factors, CREB, CREM and ATF2, in the human myometrium during pregnancy and labour. Mol. Hum. Reprod. 6, 648–660 10.1093/molehr/6.7.648 [DOI] [PubMed] [Google Scholar]
- 35.Long, A.A., Chapman, N.R., Innes, B., Europe-Finner, G.N. and Robson, S.C. (2005) Expression and interaction of the transcriptional coregulators, CBP/p300, in the human myometrium during pregnancy and labor. J. Soc. Gynecol. Investig. 12, 92–97 10.1016/j.jsgi.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 36.Wang, Y., Zhang, Y., Dai, X., Liu, Z., Yin, P., Wang, N.et al. (2015) NR6A1 couples with cAMP response element binding protein and regulates vascular smooth muscle cell migration. Int. J. Biochem. Cell Biol. 69, 225–232 10.1016/j.biocel.2015.10.026 [DOI] [PubMed] [Google Scholar]
- 37.Schumacher, Y., Aparicio, T., Ourabah, S., Baraille, F., Martin, A., Wind, P.et al. (2016) Dysregulated CRTC1 activity is a novel component of PGE2 signaling that contributes to colon cancer growth. Oncogene 35, 2602–2614 10.1038/onc.2015.283 [DOI] [PubMed] [Google Scholar]
- 38.de Groot, R.P., den Hertog, J., Vandenheede, J.R., Goris, J. and Sassone-Corsi, P. (1993) Multiple and cooperative phosphorylation events regulate the CREM activator function. EMBO J. 12, 3903–3911 10.1002/j.1460-2075.1993.tb06068.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey, J., Tyson-Capper, A.J., Gilmore, K., Robson, S.C. and Europe-Finner, G.N. (2005) Identification of human myometrial target genes of the cAMP pathway: the role of cAMP-response element binding (CREB) and modulator (CREM alpha and CREM tau(2)alpha) proteins. J. Mol. Endocrinol. 34, 1–17 10.1677/jme.1.01594 [DOI] [PubMed] [Google Scholar]
- 40.Tyson-Capper, A.J., Bailey, J., Krainer, A.R., Robson, S.C. and Europe-Finner, G.N. (2005) The switch in alternative splicing of cyclic AMP-response element modulator protein CREM tau(2)alpha (activator) to CREM alpha (repressor) in human myometrial cells is mediated by SRp40. J. Biol. Chem. 280, 34521–34529 10.1074/jbc.M505344200 [DOI] [PubMed] [Google Scholar]
- 41.Sano, Y., Tokitou, F., Dai, P., Maekawa, T., Yamamoto, T. and Ishii, S. (1998) CBP alleviates the intramolecular inhibition of ATF-2 function. J. Biol. Chem. 273, 29098–29105 10.1074/jbc.273.44.29098 [DOI] [PubMed] [Google Scholar]
- 42.Huebner, K., Prochazka, J., Monteiro, A.C., Mahadevan, V. and Schneider-Stock, R. (2019) The activating transcription factor 2: an influencer of cancer progression. Mutagenesis 34, 375–389 10.1093/mutage/gez041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hai, T. and Curran, T. (1991) Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA-binding specificity. Proc. Natl Acad. Sci. U.S.A. 88, 3720–3724 10.1073/pnas.88.9.3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey, J., Phillips, R.J., Pollard, A.J., Gilmore, K., Robson, S.C. and Europe-Finner, G.N. (2002) Characterization and functional analysis of cAMP response element modulator protein and activating transcription factor 2 (ATF2) isoforms in the human myometrium during pregnancy and labor: identification of a novel ATF2 species with potent transactivation properties. J. Clin. Endocrinol. Metab. 87, 1717–1728 10.1210/jcem.87.4.8360 [DOI] [PubMed] [Google Scholar]
- 45.Williams, K.C., Renthal, N.E., Gerard, R.D. and Mendelson, C.R. (2012) The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol. Endocrinol. 26, 1857–1867 10.1210/me.2012-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renthal, N.E., Chen, C.C., Williams, K.C., Gerard, R.D., Prange-Kiel, J. and Mendelson, C.R. (2010) miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc. Natl Acad. Sci. U.S.A. 107, 20828–20833 10.1073/pnas.1008301107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesiano, S., Chen, E.C., Fitter, J.T., Kwek, K., Yeo, G. and Smith, R. (2002) Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J. Clin. Endocrinol. Metab. 87, 2924–2930 10.1210/jcem.87.6.8609 [DOI] [PubMed] [Google Scholar]
- 48.Tan, H.Q., Yi, L.J., Rote, N.S., Hurd, W.W. and Mesiano, S. (2012) Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J. Clin. Endocrinol. Metab. 97, E719–EE30 10.1210/jc.2011-3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimm, S.L., Hartig, S.M. and Edwards, D.P. (2016) Progesterone receptor signaling mechanisms. J. Mol. Biol. 428, 3831–3849 10.1016/j.jmb.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 50.Chen, C.C., Montalbano, A.P., Hussain, I., Lee, W.R. and Mendelson, C.R. (2017) The transcriptional repressor GATAD2B mediates progesterone receptor suppression of myometrial contractile gene expression. J. Biol. Chem. 292, 12560–12576 10.1074/jbc.M117.791350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong, X.S., Yu, C., Shynlova, O., Challis, J.R.G., Rennie, P.S. and Lye, S.J. (2009) P54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1). Mol. Endocrinol. 23, 1147–1160 10.1210/me.2008-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong, X.S., Shylnova, O., Challis, J.R.G. and Lye, S.J. (2005) Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J. Biol. Chem. 280, 13329–13340 10.1074/jbc.M409187200 [DOI] [PubMed] [Google Scholar]
- 53.Xie, N., Liu, L.L., Li, Y.Q., Yu, C., Lam, S., Shynlova, O.et al. (2012) Expression and function of myometrial PSF suggest a role in progesterone withdrawal and the initiation of labor. Mol. Endocrinol. 26, 1370–1379 10.1210/me.2012-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nadeem, L., Shynlova, O., Matysiak-Zablocki, E., Mesiano, S., Dong, X.S. and Lye, S. (2016) Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 7, 11565 10.1038/ncomms11565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kastner, P., Krust, A., Turcotte, B., Stropp, U., Tora, L., Gronemeyer, H.et al. (1990) Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9, 1603–1614 10.1002/j.1460-2075.1990.tb08280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giangrande, P.H., Pollio, G. and McDonnell, D.P. (1997) Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J. Biol. Chem. 272, 32889–32900 10.1074/jbc.272.52.32889 [DOI] [PubMed] [Google Scholar]
- 57.Jacobsen, B.M. and Horwitz, K.B. (2012) Progesterone receptors, their isoforms and progesterone regulated transcription. Mol. Cell. Endocrinol. 357, 18–29 10.1016/j.mce.2011.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dinh, D.T., Breen, J., Akison, L.K., DeMayo, F.J., Brown, H.M., Robker, R.L.et al. (2019) Tissue-specific progesterone receptor-chromatin binding and the regulation of progesterone-dependent gene expression. Sci. Rep. 9, 14 10.1038/s41598-018-36846-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdel-Hafiz, H., Takimoto, G.S., Tung, L. and Horwitz, K.B. (2002) The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J. Biol. Chem. 277, 33950–33956 10.1074/jbc.M204573200 [DOI] [PubMed] [Google Scholar]
- 60.Peters, G.A., Yi, L.J., Skomorovska-Prokvolit, Y., Patel, B., Amini, P., Tan, H.Q.et al. (2017) Inflammatory stimuli increase progesterone receptor-A stability and transrepressive activity in myometrial cells. Endocrinology 158, 158–169 10.1210/en.2016-1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amini, P., Michniuk, D., Kuo, K., Yi, L.J., Skomorovska-Prokvolit, Y., Peters, G.A.et al. (2016) Human parturition involves phosphorylation of progesterone receptor-A at serine-345 in myometrial cells. Endocrinology 157, 4434–4445 10.1210/en.2016-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amini, P., Wilson, R., Wang, J.Y., Tan, H.Q., Yi, L.J., Koeblitz, W.K.et al. (2019) Progesterone and cAMP synergize to inhibit responsiveness of myometrial cells to pro-inflammatory/pro-labor stimuli. Mol. Cell. Endocrinol. 479, 1–11 10.1016/j.mce.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 63.Osman, I., Young, A., Ledingham, M.A., Thomson, A.J., Jordan, F., Greer, I.A.et al. (2003) Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9, 41–45 10.1093/molehr/gag001 [DOI] [PubMed] [Google Scholar]
- 64.Chen, L., Lei, K., Malawana, J., Yulia, A., Sooranna, S.R., Bennett, P.R.et al. (2014) Cyclic AMP enhances progesterone action in human myometrial cells. Mol. Cell. Endocrinol. 382, 334–343 10.1016/j.mce.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 65.Che, W.C., Manetsch, M., Quante, T., Rahman, M.M., Patel, B.S., Ge, Q.et al. (2012) Sphingosine 1-phosphate induces MKP-1 expression via p38 MAPK- and CREB-mediated pathways in airway smooth muscle cells. Bioch. Biophys. Acta-Mol. Cell Res. 1823, 1658–1665 10.1016/j.bbamcr.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 66.Chen, C.C., Hardy, D.B. and Mendelson, C.R. (2011) Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1). J. Biol. Chem. 286, 43091–43102 10.1074/jbc.M111.295865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu, Y.S., Gorospe, M., Yang, C.L. and Holbrook, N.J. (1995) Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress - inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J. Biol. Chem. 270, 8377–8380 10.1074/jbc.270.15.8377 [DOI] [PubMed] [Google Scholar]
- 68.Franklin, C.C. and Kraft, A.S. (1997) Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J. Biol. Chem. 272, 16917–16923 10.1074/jbc.272.27.16917 [DOI] [PubMed] [Google Scholar]
- 69.Merlino, A.A., Welsh, T.N., Tan, H.Q., Yi, L.J., Cannon, V., Mercer, B.M.et al. (2007) Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J. Clin. Endocrinol. Metab. 92, 1927–1933 10.1210/jc.2007-0077 [DOI] [PubMed] [Google Scholar]
- 70.Lai, P.F., Georgiou, E.X., Tribe, R.M. and Johnson, M.R. (2020) The impact of progesterone and RU-486 on classic pro-labour proteins & contractility in human myometrial tissues during 24-hour exposure to tension & interleukin-1 beta. Mol. Cell. Endocrinol. 500, 16 10.1016/j.mce.2019.110633 [DOI] [PubMed] [Google Scholar]
- 71.Stanfield, Z., Amini, P., Wang, J.Y., Yi, L.J., Tan, H.Q., Chance, M.R.et al. (2019) Interplay of transcriptional signaling by progesterone, cyclic AMP, and inflammation in myometrial cells: implications for the control of human parturition. Mol. Hum. Reprod. 25, 408–422 10.1093/molehr/gaz028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brighton, P.J., Maruyama, Y., Fishwick, K., Vrljicak, P., Tewary, S., Fujihara, R.et al. (2017) Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 6, e31274 10.7554/eLife.31274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rytkönen, K.T., Erkenbrack, E.M., Poutanen, M., Elo, L.L., Pavlicev, M. and Wagner, G.P. (2019) Decidualization of human endometrial stromal fibroblasts is a multiphasic process involving distinct transcriptional programs. Reprod. Sci. 26, 323–336 10.1177/1933719118802056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayden, M.S. and Ghosh, S. (2008) Shared principles in NF-kappaB signaling. Cell 132, 344–362 10.1016/j.cell.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 75.Lawrence, T. (2009) The nuclear factor NF-kappa B pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, 10 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martone, R., Euskirchen, G., Bertone, P., Hartman, S., Royce, T.E., Luscombe, N.M.et al. (2003) Distribution of NF-kappa B-binding sites across human chromosome 22. Proc. Natl Acad. Sci. U.S.A. 100, 12247–12252 10.1073/pnas.2135255100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hua, R., Pease, J.E., Sooranna, S.R., Viney, J.M., Nelson, S.M., Myatt, L.et al. (2012) Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-κB activation. Endocrinology 153, 481–491 10.1210/en.2011-1506 [DOI] [PubMed] [Google Scholar]
- 78.Khanjani, S., Kandola, M.K., Lindstrom, T.M., Sooranna, S.R., Melchionda, M., Lee, Y.S.et al. (2011) NF-kappa B regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J. Cell. Mol. Med. 15, 809–824 10.1111/j.1582-4934.2010.01069.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chapman, N.R., Europe-Finner, G.N. and Robson, S.C. (2004) Expression and deoxyribonucleic acid-binding activity of the nuclear factor kappa B family in the human myometrium during pregnancy and labor. J. Clin. Endocrinol. Metab. 89, 5683–5693 10.1210/jc.2004-0873 [DOI] [PubMed] [Google Scholar]
- 80.Christian, F., Smith, E.L. and Carmody, R.J. (2016) The regulation of NF-kappa B subunits by phosphorylation. Cells 5, 19 10.3390/cells5010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhong, H.H., Voll, R.E. and Ghosh, S. (1998) Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1, 661–671 10.1016/S1097-2765(00)80066-0 [DOI] [PubMed] [Google Scholar]
- 82.King, C.C., Sastri, M., Chang, P., Pennypacker, J. and Taylor, S.S. (2011) The rate of NF-kappa B nuclear translocation is regulated by PKA and A kinase interacting protein 1. PLoS One 6, 15, e18713 10.1371/journal.pone.0018713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mishra, R., Benlhabib, H., Guo, W., Lerma Cervantes, C.B. and Mendelson, C.R. (2018) Developmental decline in the microRNA 199a (miR-199a)/miR-214 cluster in human fetal lung promotes type II cell differentiation by upregulating key transcription factors. Mol. Cell. Biol. 38, e00037-18 10.1128/MCB.00037-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen, L., Sooranna, S.R., Lei, K.Y., Kandola, M., Bennett, P.R., Liang, Z.Q.et al. (2012) Cyclic AMP increases COX-2 expression via mitogen-activated kinase in human myometrial cells. J. Cell. Mol. Med. 16, 1447–1460 10.1111/j.1582-4934.2011.01413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kandola, M.K., Sykes, L., Lee, Y.S., Johnson, M.R., Hanyaloglu, A.C. and Bennett, P.R. (2014) EP2 receptor activates dual G protein signaling pathways that mediate contrasting proinflammatory and relaxatory responses in term pregnant human myometrium. Endocrinology 155, 605–617 10.1210/en.2013-1761 [DOI] [PubMed] [Google Scholar]
- 86.Mosher, A.A., Rainey, K.J., Giembycz, M.A., Wood, S. and Slater, D.M. (2012) Prostaglandin E-2 represses interleukin 1 beta-induced inflammatory mediator output from pregnant human myometrial cells through the EP2 and EP4 receptors. Biol. Reprod. 87, 10 10.1095/biolreprod.112.100099 [DOI] [PubMed] [Google Scholar]
- 87.Ku, C.Y., Word, R.A. and Sanborn, B.M. (2005) Differential expression of protein kinase A, AKAP79, and PP2B in pregnant human myometrial membranes prior to and during labor. J. Soc. Gynecol. Investig. 12, 421–427 10.1016/j.jsgi.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 88.Terrinoni, M., Holmgren, J., Lebens, M. and Larena, M. (2019) Requirement for cyclic AMP/protein kinase A-dependent canonical NF kappa B signaling in the adjuvant action of cholera toxin and its non-toxic derivative mmCT. Front. Immunol. 10, 13 10.3389/fimmu.2019.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zou, Z.-Q., Chen, J.-J., Feng, H.-F., Cheng, Y.-F., Wang, H.-T., Zhou, Z.-Z.et al. (2017) Novel phosphodiesterase 4 inhibitor FCPR03 alleviates lipopolysaccharide-induced neuroinflammation by regulation of the cAMP/PKA/CREB signaling pathway and NF-κB inhibition. J. Pharmacol. Exp. Ther. 362, 67–77 10.1124/jpet.116.239608 [DOI] [PubMed] [Google Scholar]
- 90.Kaur, M., Holden, N.S., Wilson, S.M., Sukkar, M.B., Chung, K.F., Barnes, P.J.et al. (2008) Effect of beta(2)-adrenoceptor agonists and other cAMP-elevating agents on inflammatory gene expression in human ASM cells: a role for protein kinase A. Am. J. Physiol.-Lung Cell. Mol. Physiol. 295, L505–LL14 10.1152/ajplung.00046.2008 [DOI] [PubMed] [Google Scholar]
- 91.Misior, A.M., Yan, H.D., Pascual, R.M., Deshpande, D.A., Panettieri, R.A. and Penn, R.B. (2008) Mitogenic effects of cytokines on smooth muscle are critically dependent on protein kinase A and are unmasked by steroids and cyclooxygenase inhibitors. Mol. Pharmacol. 73, 566–574 10.1124/mol.107.040519 [DOI] [PubMed] [Google Scholar]
- 92.Buhimschi, C.S., Buhimschi, I.A., Malinow, A.M. and Weiner, C.P. (2003) Myometrial thickness during human labor and immediately post partum. Am. J. Obstet. Gynecol. 188, 553–559 10.1067/mob.2003.77 [DOI] [PubMed] [Google Scholar]
- 93.Kozasa, T., Itoh, H., Tsukamoto, T. and Kaziro, Y. (1988) Isolation and characterization of the human Gs-alpha gene. Proc. Natl Acad. Sci. U.S.A. 85, 2081–2085 10.1073/pnas.85.7.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chapman, N.R., Smyrnias, I., Anumba, D.O., Europe-Finner, G.N. and Robson, S.C. (2005) Expression of the GTP-binding protein (Galphas) is repressed by the nuclear factor kappaB RelA subunit in human myometrium. Endocrinology 146, 4994–5002 10.1210/en.2005-0533 [DOI] [PubMed] [Google Scholar]
- 95.Webster, S.J., Waite, S.L., Cookson, V.J., Warren, A., Khan, R., Gandhi, S.V.et al. (2013) Regulation of GTP-binding protein (G alpha(s)) expression in human myometrial cells: a role for tumor necrosis factor in modulating G alpha(s) promoter acetylation by transcriptional complexes. J. Biol. Chem. 288, 6704–6716 10.1074/jbc.M112.440602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hess, J., Angel, P. and Schorpp-Kistner, M. (2004) AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 117, 5965–5973 10.1242/jcs.01589 [DOI] [PubMed] [Google Scholar]
- 97.Zhou, H.M., Zarubin, T., Ji, Z.L., Min, Z., Zhu, W., Downey, J.S.et al. (2005) Frequency and distribution of AP-1 sites in the human genome. DNA Res. 12, 139–150 10.1093/dnares/12.2.139 [DOI] [PubMed] [Google Scholar]
- 98.Karin, M. (1995) The regulation of AP-1 activity by mitogen-activated protein-kinases. J. Biol. Chem. 270, 16483–16486 10.1074/jbc.270.28.16483 [DOI] [PubMed] [Google Scholar]
- 99.Eid, A.H., Chotani, M.A., Mitra, S., Miller, T.J. and Flavahan, N.A. (2008) Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle alpha(2C)-adrenoceptors. Am. J. Physiol.-Heart Circ. Physiol. 295, H266–HH72 10.1152/ajpheart.00084.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ariyoshi, W., Okinaga, T., Chaweewannakorn, W., Akifusa, S. and Nisihara, T. (2017) Mechanisms involved in enhancement of matrix metalloproteinase-9 expression in macrophages by interleukin-33. J. Cell. Physiol. 232, 3481–3495 10.1002/jcp.25809 [DOI] [PubMed] [Google Scholar]
- 101.Lim, R. and Lappas, M. (2014) Differential expression of AP-1 proteins in human myometrium after spontaneous term labour onset. Eur. J. Obstet. Gynecol. Reprod. Biol. 177, 100–105 10.1016/j.ejogrb.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 102.Nadeem, L., Farine, T., Dorogin, A., Matysiak-Zablocki, E., Shynlova, O. and Lye, S. (2018) Differential expression of myometrial AP-1 proteins during gestation and labour. J. Cell. Mol. Med. 22, 452–471 10.1111/jcmm.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khanjani, S., Terzidou, V., Johnson, M.R. and Bennett, P.R. (2012) NF kappa B and AP-1 drive human myometrial IL8 expression. Mediat. Inflamm. 2012, 504952 10.1155/2012/504952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terzidou, V., Lee, Y., Lindstrom, T., Johnson, M., Thornton, S. and Bennett, P.R. (2006) Regulation of the human oxytocin receptor by nuclear factor-kappa B and CCAAT/enhancer-binding protein-beta. J. Clin. Endocrinol. Metab. 91, 2317–2326 10.1210/jc.2005-2649 [DOI] [PubMed] [Google Scholar]
- 105.Zhu, T.X., Chen, J.F., Zhao, Y.H., Zhang, J.J., Peng, Q.Z., Huang, J.R.et al. (2019) Neuromedin B mediates IL-6 and COX-2 expression through NF-kappa B/P65 and AP-1/C-JUN activation in human primary myometrial cells. Biosci. Rep. 39, 13 10.1042/BSR20192139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng, Q., Liu, Y., Dong, M., Xu, F., Huang, J., Chen, J.et al. (2018) Interaction between NF-kappaB and AP-1 and their intracellular localization at labor in human late pregnant myometrial cells in vivo and in vitro. Medicine (Baltimore) 97, e12494 10.1097/MD.0000000000012494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ozaki, N., Shibasaki, T., Kashima, Y., Miki, T., Takahashi, K., Ueno, H.et al. (2000) cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat. Cell Biol. 2, 805–811 10.1038/35041046 [DOI] [PubMed] [Google Scholar]
- 108.Parnell, E., Smith, B.O. and Yarwood, S.J. (2015) The cAMP sensors, EPAC1 and EPAC2, display distinct subcellular distributions despite sharing a common nuclear pore localisation signal. Cell Signal. 27, 989–996 10.1016/j.cellsig.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oldenburger, A., Timens, W., Bos, S., Smit, M., Smrcka, A.V., Laurent, A.C.et al. (2014) Epac1 and Epac2 are differentially involved in inflammatory and remodeling processes induced by cigarette smoke. FASEB J. 28, 4617–4628 10.1096/fj.13-248930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dao, K.K., Teigen, K., Kopperud, R., Hodneland, E., Schwede, F., Christensen, A.E.et al. (2006) Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J. Biol. Chem. 281, 21500–21511 10.1074/jbc.M603116200 [DOI] [PubMed] [Google Scholar]
- 111.Koschinski, A. and Zaccolo, M. (2017) Activation of PKA in cell requires higher concentration of cAMP than in vitro: implications for compartmentalization of cAMP signalling. Sci. Rep. 7, 12 10.1038/s41598-017-13021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iancu, R.V., Ramamurthy, G., Warrier, S., Nikolaev, V.O., Lohse, M.J., Jones, S.W.et al. (2008) Cytoplasmic cAMP concentrations in intact cardiac myocytes. Am. J. Physiol.-Cell Physiol. 295, C414–CC22 10.1152/ajpcell.00038.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kobrinsky, E., Duong, S.Q., Sheydina, A. and Soldatov, N.M. (2011) Microdomain organization and frequency-dependence of CREB-dependent transcriptional signaling in heart cells. FASEB J. 25, 1544–1555 10.1096/fj.10-176198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bogard, A.S., Xu, C.F. and Ostrom, R.S. (2011) Human bronchial smooth muscle cells express adenylyl cyclase isoforms 2, 4, and 6 in distinct membrane microdomains. J. Pharmacol. Exp. Ther. 337, 209–217 10.1124/jpet.110.177923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ponsioen, B., Zhao, J., Riedl, J., Zwartkruis, F., van der Krogt, G., Zaccolo, M.et al. (2004) Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 5, 1176–1180 10.1038/sj.embor.7400290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bobin, P., Belacel-Ouari, M., Bedioune, I., Zhang, L., Leroy, J., Leblais, V.et al. (2016) Cyclic nucleotide phosphodiesterases in heart and vessels: a therapeutic perspective. Arch. Cardiovasc. Dis. 109, 431–443 10.1016/j.acvd.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 117.Ghigo, A. and Mika, D. (2019) cAMP/PKA signaling compartmentalization in cardiomyocytes: lessons from FRET-based biosensors. J. Mol. Cell. Cardiol. 131, 112–121 10.1016/j.yjmcc.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 118.Smith, S.A., Newby,, A.C. and Bond,, M. (2019) Ending restenosis: inhibition of vascular smooth muscle cell proliferation by cAMP. Cells 8, 1447 10.3390/cells8111447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Enserink, J.M., Christensen, A.E., de Rooij, J., van Triest, M., Schwede, F., Genieser, H.G.et al. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 10.1038/ncb874 [DOI] [PubMed] [Google Scholar]
- 120.Goldsmith, Z.G. and Dhanasekaran, D.N. (2007) G protein regulation of MAPK networks. Oncogene 26, 3122–3142 10.1038/sj.onc.1210407 [DOI] [PubMed] [Google Scholar]
- 121.Kusama, K., Tamura, K., Bai, H., Sakurai, T., Nishi, H., Isaka, K.et al. (2018) Exchange protein directly activated by cAMP (EPAC) promotes transcriptional activation of the decidual prolactin gene via CCAAT/enhancer-binding protein in human endometrial stromal cells. Reprod. Fertil. Dev. 30, 1454 10.1071/RD17483 [DOI] [PubMed] [Google Scholar]
- 122.Tapia-Pizarro, A., Archiles, S., Argandona, F., Valencia, C., Zavaleta, K., Johnson, M.C.et al. (2017) hCG activates Epac-Erk1/2 signaling regulating progesterone receptor expression and function in human endometrial stromal cells. Mol. Hum. Reprod. 23, 393–405 10.1093/molehr/gax015 [DOI] [PubMed] [Google Scholar]
- 123.Roberts, O.L. and Dart, C. (2014) cAMP signalling in the vasculature: the role of Epac (exchange protein directly activated by cAMP). Biochem. Soc. Trans. 42, 89–97 10.1042/BST20130253 [DOI] [PubMed] [Google Scholar]
- 124.Grandoch, M., Roscioni, S.S. and Schmidt, M. (2010) The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br. J. Pharmacol. 159, 265–284 10.1111/j.1476-5381.2009.00458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kato, Y., Yokoyama, U., Yanai, C., Ishige, R., Kurotaki, D., Umemura, M.et al. (2015) Epac1 deficiency attenuated vascular smooth muscle cell migration and neointimal formation. Arterioscler. Thromb. Vasc. Biol. 35, 2617–2625 10.1161/ATVBAHA.115.306534 [DOI] [PubMed] [Google Scholar]
- 126.Oldenburger, A., Roscioni, S.S., Jansen, E., Menzen, M.H., Halayko, A.J., Timens, W.et al. (2012) Anti-inflammatory role of the cAMP effectors Epac and PKA: implications in chronic obstructive pulmonary disease. PLoS One 7, e31574 10.1371/journal.pone.0031574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laudette, M., Coluccia, A., Sainte-Marie, Y., Solari, A., Fazal, L., Sicard, P.et al. (2019) Identification of a pharmacological inhibitor of Epac1 that protects the heart against acute and chronic models of cardiac stress. Cardiovasc. Res. 115, 1766–1777 10.1093/cvr/cvz076 [DOI] [PubMed] [Google Scholar]
- 128.Di Liberto, V., Mudò, G. and Belluardo, N. (2019) Crosstalk between receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCR) in the brain: focus on heteroreceptor complexes and related functional neurotrophic effects. Neuropharmacology 152, 67–77 10.1016/j.neuropharm.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 129.Shaw, A. and Xu, Q. (2003) Biomechanical stress-induced signaling in smooth muscle cells: an update. Curr. Vasc. Pharmacol. 1, 41–58 10.2174/1570161033386745 [DOI] [PubMed] [Google Scholar]
- 130.Abdel-Latif, A.A. (2001) Cross talk between cyclic nucleotides and polyphosphoinositide hydrolysis, protein kinases, and contraction in smooth muscle. Exp. Biol. Med. 226, 153–163 10.1177/153537020122600302 [DOI] [PubMed] [Google Scholar]
- 131.Wray, S. and Prendergast, C. (2019) The myometrium: from excitation to contractions and labour. Adv. Exp. Med. Biol. 1124, 233–263 10.1007/978-981-13-5895-1_10 [DOI] [PubMed] [Google Scholar]
- 132.Houslay, M. (1997) Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem. Sci. 22, 217–224 10.1016/S0968-0004(97)01050-5 [DOI] [PubMed] [Google Scholar]
- 133.Buxton, I.L.O., Milton, D., Barnett, S.D. and Tichenor, S.D. (2010) Agonist-specific compartmentation of cGMP action in myometrium. J. Pharmacol. Exp. Ther. 335, 256–263 10.1124/jpet.110.171934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ulrich, C., Quilici, D.R., Schlauch, K.A. and Buxton, I.L.O. (2013) The human uterine smooth muscle S-nitrosoproteome fingerprint in pregnancy, labor, and preterm labor. Am. J. Physiol.-Cell Physiol. 305, C803–CC16 10.1152/ajpcell.00198.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu, G.-Y., Deisseroth, K. and Tsien, R.W. (2001) Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc. Natl Acad. Sci. U.S.A. 98, 2808–2813 10.1073/pnas.051634198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pilz, R.B. and Casteel, D.E. (2003) Regulation of gene expression by cyclic GMP. Circ. Res. 93, 1034–1046 10.1161/01.RES.0000103311.52853.48 [DOI] [PubMed] [Google Scholar]
- 137.Li, Y., Lorca, R.A., Ma, X., Rhodes, A. and England, S.K. (2014) BK channels regulate myometrial contraction by modulating nuclear translocation of NF-κB. Endocrinology 155, 3112–3122 10.1210/en.2014-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jang, M.K., Goo, Y.H., Sohn, Y.C., Kim, Y.S., Lee, S.-K., Kang, H.et al. (2001) Ca2+/calmodulin-dependent protein kinase IV stimulates nuclear factor-κB transactivation via phosphorylation of the p65 subunit. J. Biol. Chem. 276, 20005–20010 10.1074/jbc.M010211200 [DOI] [PubMed] [Google Scholar]
- 139.Macián, F., López-Rodríguez, C. and Rao, A. (2001) Partners in transcription: NFAT and AP-1. Oncogene 20, 2476–2489 10.1038/sj.onc.1204386 [DOI] [PubMed] [Google Scholar]
- 140.Chin-Smith, E.C., Willey, F.R., Slater, D.M., Taggart, M.J. and Tribe, R.M. (2015) Nuclear factor of activated T-cell isoform expression and regulation in human myometrium. Reprod. Biol. Endocrinol. 13, 9 10.1186/s12958-015-0005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Butler, T.A., Paul, J.W. and Smith, R. (2020) Non-conventional signalling in human myometrium by conventional pathways: looking back for a synergistic future. Curr. Opin. Physiol. 13, 145–154 10.1016/j.cophys.2019.11.010 [DOI] [Google Scholar]
- 142.Stanfield, Z., Lai, P.F., Lei, K., Johnson, M.R., Blanks, A.M., Romero, R.et al. (2019) Myometrial transcriptional signatures of human parturition. Front. Genet. 10, 185 10.3389/fgene.2019.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Europe-Finner, G.N., Phaneuf, S., Watson, S.P. and Bernal, A.L. (1993) Identification and expression of G-proteins in human myometrium - up-regulation of G-alpha-S in pregnancy. Endocrinology 132, 2484–2490 10.1210/endo.132.6.8504751 [DOI] [PubMed] [Google Scholar]
- 144.Price, S.A., Pochun, I., Phaneuf, S. and Bernal, A.L. (2000) Adenylyl cyclase isoforms in pregnant and non-pregnant human myometrium. J. Endocrinol. 164, 21–30 10.1677/joe.0.1640021 [DOI] [PubMed] [Google Scholar]
- 145.Leroy, M.J., Mehats, C., Duc-Goiran, P., Tanguy, G., Robert, B., Dallot, E.et al. (1999) Effect of pregnancy on PDE4, cAMP-specific phosphodiesterase messenger ribonucleic acid expression in human myometrium. Cell Signal. 11, 31–37 10.1016/S0898-6568(98)00028-X [DOI] [PubMed] [Google Scholar]