Abstract
Hypothesis:
Cochlear dysfunction is not common in human meningioma of the internal auditory canal.
Background:
Meningiomas arising from the cerebellopontine angle and internal auditory canal typically cause hearing loss. Cochlear dysfunction is known to contribute to sensorineural hearing loss induced by vestibular schwannoma, the most common tumor of the internal auditory canal. Detailed cochlear histopathology in meningioma has not been reported.
Methods:
Retrospective analysis of cochlear histopathology in 5 unoperated and 5 operated meningiomas of the internal auditory canal identified after screening human temporal bone collections from three academic medical centers.
Results:
While some dysfunction of all analyzed cochlear cell types was identified, a predominant or exclusive loss of hair cells was not observed in any meningioma. Only 14.3% of temporal bones showed significantly more hair cell damage on the side of the tumor when compared to the contralateral ear; cochlear neuronal damage was more prevalent in meningiomas. The incidence of hydrops, perilymphatic or endolymphatic precipitate was low.
Conclusions:
Substantial cochlear damage in human meningioma of the internal auditory canal is not common. This may explain the anecdotal hearing improvement observed after surgical resection of meningioma. Our findings underline the importance of developing therapeutic strategies to prevent cochlear neuronal degeneration due to tumors of the internal auditory canal.
INTRODUCTION
Meningioma is the most common intracranial tumor in adults (1), and the second most common tumor of the cerebellopontine angle (2). Though usually benign, if located in the cerebellopontine angle and internal auditory canal, meningioma can lead to degenerative changes in the facial, vestibular and cochlear nerves (1). Vestibular schwannoma (VS) is the most common tumor of the cerebellopontine angle and internal auditory canal, accounting for 85% of tumors in these locations. Both VS and meningioma typically present with hearing loss and tinnitus (3,4). While histopathologic study of human temporal bones reveals that cochlear damage contributes to VS-induced hearing loss (5), it is unclear to what degree cochlear damage also contributes to meningioma-induced hearing loss. To our knowledge, there is only one published case of temporal bone histopathology in a patient affected by meningioma of the cerebellopontine angle (6).
The goal of this study was to analyze a series of human temporal bones with internal auditory canal meningioma and quantify damage to specific cochlear cell types. Our results can inform pre-operative counseling about the likelihood of hearing improvement after surgery for internal auditory canal meningioma.
METHODS
Temporal bone preparation and study
Archival collections of human temporal bones from the US Temporal Bone Registry at Massachusetts Eye and Ear (Harvard Medical School), House Research Institute (University of California, Los Angeles) and the University of Minnesota were screened for cases of meningioma located within the internal auditory canal. This inclusion criterion was chosen because VSs often arise in close proximity to the cochlea, while meningiomas can be found throughout the cranial cavity and in the spinal canal. Such a difference in distance of tumor to the fundus of the internal auditory canal may lead to a bias in the analysis of cochlear dysfunction. Written informed consent from all patients had been obtained prior to death. Out of 26 initially identified temporal bones, 16 were excluded because the tumor was not in the internal auditory canal (5 bones), the cochlear tissue was insufficiently preserved (2 bones), the appropriate sections were missing (1 bone) or patients had comorbidities that made it impossible to separate meningioma-related damage from sequelae of other diseases, including neurofibromatosis type II with bilateral VSs (4 bones), spontaneous VS (2 bones), Menière’s disease (1 bone), and bilateral Mondini dysplasia (1 bone). Of the remaining 10 cases, half had undergone surgical resection. Because surgery could have contributed to cochlear damage, we analyzed the unoperated meningiomas separately (5 bones, age range 34–81 years, median 65 years, 60% women) and in the context of all identified meningiomas (10 bones, age range 34–89 years, median 67 years, 60% women). Patient characteristics are summarized in Table, Supplemental Digital Content 1.
Temporal bones were prepared as detailed previously (7,8). Briefly, bones were extracted and fixed post-mortem with Heidenhain Susa solution or 10% neutral buffered formalin, decalcified with ethylenediaminetetraacetic acid, embedded in celloidin, serially sectioned (20 μm), stained (hematoxylin and eosin on every tenth section), and examined under a light microscope.
Assessment of cochlear pathology
Two independent researchers (LDL and FHL) evaluated cochlear sections ipsilateral and contralateral to the internal auditory canal meningioma for inner hair cell (IHC) and outer hair cell (OHC) loss, atrophy of the stria vascularis, and loss of spiral ganglion neurons (SGNs) in a semiquantitative manner as described for VS specimens by Roosli et al (5). Briefly, cellular degeneration for all four structures was graded as absent cellular loss (−), mild (+, less than one-third missing/atrophied), moderate (++, one-third to two-thirds missing/atrophied) or severe degeneration (+++, more than two-thirds missing/atrophied).
In addition, the predominantly damaged cell type in each temporal bone was further classified using one of five graded designations. “No/minimal damage” denotes that no category had more than one-third of degenerated cells/structures (i.e. − or +). “Predominant HC damage,” “Predominant stria damage,” and “Predominant SGN damage” denote that only one category (specifically, hair cells (HCs), SGNs or stria vascularis) was damaged by more than one-third (i.e. ++ or +++), while all the other cell types had less than one-third of damaged cells (i.e. − or +). The “2+ cell types damaged” category denotes that at least two categories had more than one-third (i.e. ++ or +++) of damaged cells.
The presence of hydrops was determined by a convexity of Reissner’s membrane toward the scala vestibuli in the cochlea and an abnormal distension of the membranous walls of the vestibular organs (saccule, utricle, or ampullae). Furthermore, the presence of endolymphatic and/or perilymphatic precipitate was assessed. The four-point semiquantitative scale (− to +++) was translated to a numerical scale (0 to 3) and the average damage per cell type in the ipsilateral ear was calculated by adding all points per group and dividing by the maximal number of points per group (i.e. 3 x the number of temporal bones per group). The presence of hydrops and endolymphatic and/or perilymphatic precipitate was analyzed on a binary scale (0 or 1) (in the case of a potential artifact, 0.5 points were assigned).
Before starting data collection for this study, inter-observer agreement was established by re-analyzing several VS temporal bones included in the published VS study (5). Moreover, one of the investigators (FHL) participated in both studies.
RESULTS
Mean distance from tumor to fundus of the internal auditory canal was 1 cm for unoperated specimens and 0.98 cm for all temporal bones. When analyzing the predominantly damaged cell type for each temporal bone ipsilateral to meningioma, the most striking finding was that there was not a single case with predominant or exclusive loss of hair cells (including operated meningiomas, i.e. 0/10 temporal bones). As summarized in Table 1, predominant SGN damage was most pronounced, with 40% in the unoperated and 30% in the operated group. Cochlear damage was absent or minimal in 20% of temporal bones with meningiomas. Moderate or severe damage to two or more cochlear cell types was observed in 20% of unoperated meningiomas, while presumed iatrogenic damage doubled this number (40%).
Table 1.
Predominantly damaged cochlear cell type per temporal bone ipsilateral to meningioma.
| No/minimal damage | Predominant HC damage | Predominant Stria damage | Predominant SGN damage | 2+ cell types damaged | |
|---|---|---|---|---|---|
| Meningioma (excl. surg.) | 1 (20.0%) | 0 (0.0%) | 1 (20.0%) | 2 (40.0%) | 1 (20.0%) |
| Meningioma (incl. surg.) | 2 (20.0%) | 0 (0.0%) | 1 (10.0%) | 3 (30.0%) | 4 (40.0%) |
Excl./incl. surg. = meningiomas excluding/including those that underwent surgical resection, HC = hair cell, stria = stria vascularis, SGN = spiral ganglion neuron.
Similar trends were observed when assessing the average damage per cell type per temporal bone ipsilateral to meningioma (Table 2). For this analysis, all points per cell type were summed within a group (i.e. unoperated meningiomas or all meningiomas) and divided by the maximal number of points per group (i.e. 3 x the number of temporal bones). The data show that every structure in the inner ear can be affected. However, cochlear hydrops was absent in all meningiomas (with only one questionable case versus artifact in the surgical group). Similarly, endolymphatic and perilymphatic precipitates were infrequently observed in meningiomas (20% and 20%, respectively, in unoperated cases) (Table 2). In patients where ipsi- and contralateral temporal bones were available, only one out of seven (14.3%) showed significantly more HC damage on the side of the tumor (at least two categories higher; in this case +++ vs. +).
Table 2.
Percent damage to different cell types per temporal bone ipsilateral to meningioma.
| IHCs | OHCs | Stria | SGNs | Hydrops | EL | PL | |
|---|---|---|---|---|---|---|---|
| Meningioma (excl. surg.) | 6/15=40.0% | 6/15=40.0% | 6.5/15=43.3% | 7.5/15=50.0% | 0/5=0% | 1/5=20.0% | 1/5=20.0% |
| Meningioma (incl. surg.) | 12/30=40.0% | 12/30=40.0% | 14/30=46.7% | 19.5/30=65.0% | 0.5/10=5.0% | 1.5/10=15.0% | 2.5/10=25.0% |
Abbreviations as in Table 1. For each cell type (IHCs, OHCs, stria, SGNs), the number of maximal points is 3 per temporal bone (i.e. 15 for 5 unoperated meningiomas, 30 for 10 meningiomas incl. surg.). For hydrops, endolymphatic precipitate (EP) and perilymphatic precipitate (PL), the number of maximal points is 1 per temporal bone.
A typical example of meningioma-related cochlear histopathology is shown in Fig. 1. This patient (MB2) experienced a late-onset bilateral progressive sensorineural hearing loss and died of acute pulmonary edema and atherosclerotic heart disease at the age of 81 years. An accumulation of psammoma bodies in the right internal auditory canal is visible (consistent with a small psammomatous meningioma), along with bilateral moderate patchy atrophy of the stria vascularis in the cochlear apex (16.4% on the right) and bilateral moderate to severe primary neuronal degeneration in the cochlear base. Inner and outer hair cells remained intact.
Figure 1.
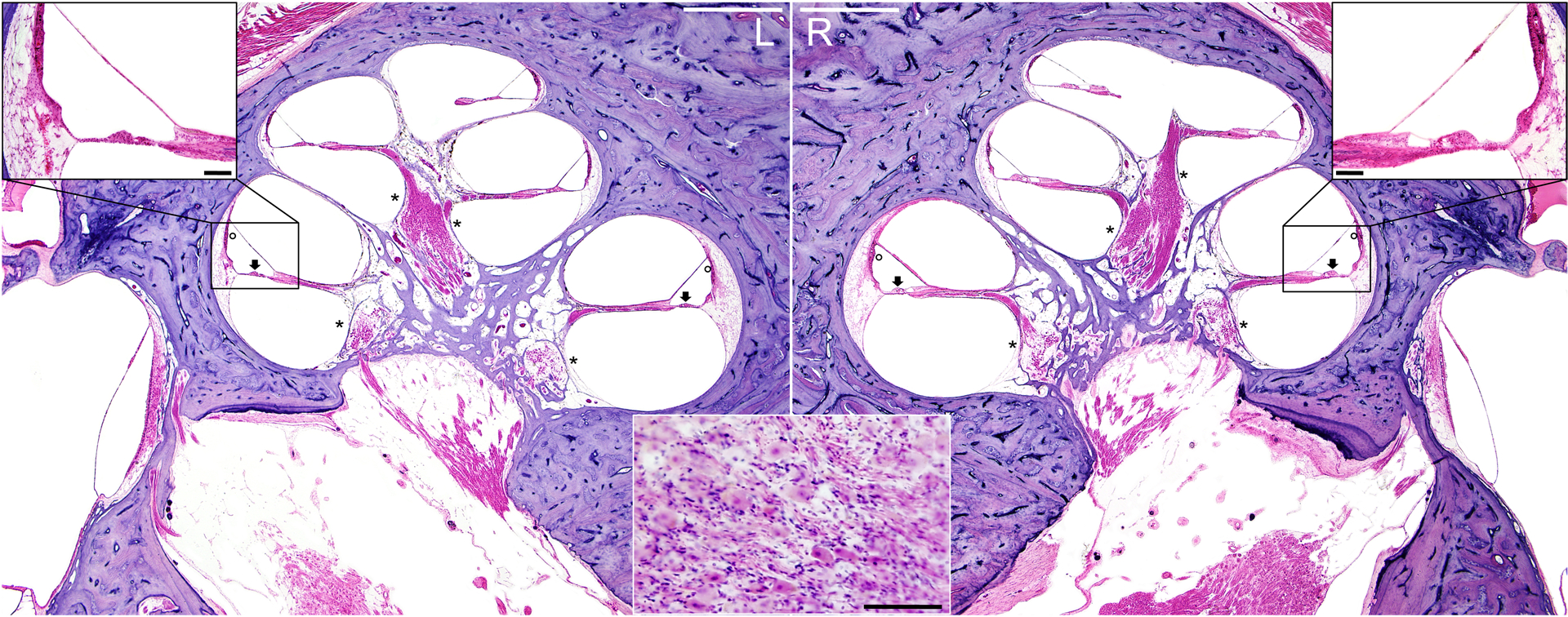
Hematoxylin and eosin stain of mid-modiolar sections of left (L) and right (R) temporal bones of a patient (MB2) with a R-sided meningioma of the internal auditory canal and cerebellopontine angle. Stria (adjacent to circles), spiral ganglion neurons (adjacent to asterisks) and hair cells (adjacent to arrows) have similar appearance between the two sides. Magnified view in the central inset depicts psammoma bodies. Scale bars = 1 mm (white) in overview images, 100 μm (black) in close-ups.
DISCUSSION
To our knowledge, this is the largest histopathologic study of human temporal bones affected with internal auditory canal meningioma. Although audiometric data from patients who donated these temporal bones are not detailed (see Table, Supplemental Digital Content 1), audiometric thresholds in general do not predict cell type-specific damage (8), and our results provide new insight into common clinical observations. For decades, clinicians have observed hearing improvement after surgical removal of meningiomas and published detailed accounts of this phenomenon (9–14). Even among patients with preoperative deafness, presumably caused by infiltration of the auditory nerve and subsequent degeneration of cochlear structures, recovery of hearing has been observed in 1.8% of patients (15). Conversely, as even subtle improvement in hearing thresholds is rare after surgical excision of a VS, the primary goal of VS surgery is to preserve hearing at the preoperative level (5,16–20).
When comparing our results to the published VS study (5) to provide a possible mechanistic insight into this disparity, several limitations of such a comparison have to be pointed out: the small sample size, the historic nature of the VS data and the limited available information regarding tumor size prevent any generalization. Nonetheless, Roosli et al. reported that “tumor size, distance from the cochlea and nerve of origin did not correlate with structural changes” (5). In terms of median age, our study population is comparable to the VS study (5), where median age was 72 years (range 25–100 years). Gender distribution in both cohorts is also relatively characteristic for each tumor, because meningioma is more common in women than men (21) while VS equally affects women and men (18 men and 14 women in the Roosli et al. study) (22). Mean distance from fundus to tumor was 1.76 cm by Roosli et al. (data obtained after contacting the authors). The VS study reported that 18.8% of VSs demonstrated a predominant or exclusive loss of hair cells, while only 3.1% showed more pronounced SGN damage; 14 of these 32 unoperated specimens (43.8%) had moderate or severe damage to two or more cochlear cell types. The average damage per cell type in the ipsilateral ear revealed differences at the level of OHCs (60.4% for VSs). Furthermore, cochlear hydrops was present in 21.9% of VSs with endolymphatic and perilymphatic precipitates observed in 31.3% and 43.8% of cases, respectively. Of the patients where ipsi- and contralateral temporal bones were available, the number with significantly more ipsilateral HC damage rose to 28.1% (9/32) in VSs.
Although precise reasons for the apparent differences between VS- and meningioma-induced cochlear damage remain to be determined, one possible contributor is a difference in tumor-secreted ototoxic and neurotoxic factors. We have previously shown that VSs secrete soluble molecules (23) and extracellular vesicles (24) that can cause direct cochlear damage. Such cochlear damage may exacerbate hearing loss due to tumor-induced compression of the auditory nerve and prevent hearing improvement after surgical tumor resection and decompression of the auditory nerve. Soluble factors secreted by meningioma have not been studied in the context of hearing loss.
Taken together, our findings emphasize the importance of developing therapeutic strategies to prevent cochlear nerve degeneration in meningiomas of the internal auditory canal.
Supplementary Material
Acknowledgment:
This work was supported by the NIDCD grant R01DC015824, Department of Defense grant W81XWH-14-1-0091, the Bertarelli Foundation, Nancy Sayles Day Foundation, and Lauer Tinnitus Research Center, all awarded to K.M.S. The authors declare no conflict of interest. We would like to thank Sonaali Aggarwal, B.A., Garyfallia Pagonis, and Meng Yu Zhu for help with the identification of patients in the Massachusetts Eye and Ear temporal bone collection and with the imaging of stained sections. We are grateful for Dr. Sebahattin Cureoglu’s screening of the University of Minnesota temporal bone collection. We are indebted to Jessica E. Sagers, B.A. and Dr. Christof Roosli for valuable input regarding the manuscript.
Footnotes
List of Supplemental Digital Content:
Supplemental_Digital_Content_1.xlsx
References:
- 1.McNeill KA. Epidemiology of Brain Tumors. Neurologic clinics 2016;34:981–98. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann DR, Grobelny B, Golfinos JG et al. Nonschwannoma tumors of the cerebellopontine angle. Otolaryngologic clinics of North America 2015;48:461–75. [DOI] [PubMed] [Google Scholar]
- 3.von Kirschbaum C, Gurkov R. Audiovestibular Function Deficits in Vestibular Schwannoma. BioMed research international 2016;2016:4980562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerganov V, Bussarsky V, Romansky K et al. Cerebellopontine angle meningiomas. Clinical features and surgical treatment. Journal of neurosurgical sciences 2003;47:129–35; discussion 35. [PubMed] [Google Scholar]
- 5.Roosli C, Linthicum FH Jr., Cureoglu S et al. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2012;33:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvinelli F, Trivelli M, Greco F et al. Acoustic neuromas and meningiomas. Histopathological aspect: a post mortem study on temporal bones. European review for medical and pharmacological sciences 1999;3:221–4. [PubMed] [Google Scholar]
- 7.Schuknecht H Temporal bone removal at autopsy. Preparation and uses. Archives of otolaryngology 1968;87:129–37. [DOI] [PubMed] [Google Scholar]
- 8.Landegger LD, Psaltis D, Stankovic KM. Human audiometric thresholds do not predict specific cellular damage in the inner ear. Hearing research 2016;335:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen CB, Greisen O. Reversible hearing loss in tumours of the cerebello-pontine angle. The Journal of laryngology and otology 1975;89:1161–4. [DOI] [PubMed] [Google Scholar]
- 10.Maurer PK, Okawara SH. Restoration of hearing after removal of cerebellopontine angle meningioma: diagnostic and therapeutic implications. Neurosurgery 1988;22:573–5. [DOI] [PubMed] [Google Scholar]
- 11.Sekhar LN, Jannetta PJ, Burkhart LE et al. Meningiomas involving the clivus: a six-year experience with 41 patients. Neurosurgery 1990;27:764–81; discussion 81. [PubMed] [Google Scholar]
- 12.Goebel JA, Vollmer DG. Hearing improvement after conservative approach for large posterior fossa meningioma. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 1993;109:1025–9. [DOI] [PubMed] [Google Scholar]
- 13.Kileny PR, Edwards BM, Disher MJ et al. Hearing improvement after resection of cerebellopontine angle meningioma: case study of the preoperative role of transient evoked otoacoustic emissions. Journal of the American Academy of Audiology 1998;9:251–6. [PubMed] [Google Scholar]
- 14.Kashio A, Suzuki M. Bilateral hearing loss due to a meningioma located in the left posterior fossa: a case report. Acta oto-laryngologica. Supplementum 2007:168–71. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M, Roser F, Dormiani M et al. Facial and cochlear nerve function after surgery of cerebellopontine angle meningiomas. Neurosurgery 2005;57:77–90; discussion 77–90. [DOI] [PubMed] [Google Scholar]
- 16.Nassif PS, Shelton C, Arriaga M. Hearing preservation following surgical removal of meningiomas affecting the temporal bone. The Laryngoscope 1992;102:1357–62. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M, Roser F, Dormiani M et al. Intraoperative auditory brainstem responses in patients with cerebellopontine angle meningiomas involving the inner auditory canal: analysis of the predictive value of the responses. Journal of neurosurgery 2005;102:637–42. [DOI] [PubMed] [Google Scholar]
- 18.Roser F, Nakamura M, Dormiani M et al. Meningiomas of the cerebellopontine angle with extension into the internal auditory canal. Journal of neurosurgery 2005;102:17–23. [DOI] [PubMed] [Google Scholar]
- 19.Tringali S, Ferber-Viart C, Fuchsmann C et al. Hearing preservation in retrosigmoid approach of small vestibular schwannomas: prognostic value of the degree of internal auditory canal filling. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2010;31:1469–72. [PubMed] [Google Scholar]
- 20.Rabelo de Freitas M, Russo A, Sequino G et al. Analysis of hearing preservation and facial nerve function for patients undergoing vestibular schwannoma surgery: the middle cranial fossa approach versus the retrosigmoid approach--personal experience and literature review. Audiology & neuro-otology 2012;17:71–81. [DOI] [PubMed] [Google Scholar]
- 21.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. Journal of neuro-oncology 2010;99:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Propp JM, McCarthy BJ, Davis FG et al. Descriptive epidemiology of vestibular schwannomas. Neuro-oncology 2006;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dilwali S, Landegger LD, Soares VY et al. Secreted Factors from Human Vestibular Schwannomas Can Cause Cochlear Damage. Scientific reports 2015;5:18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares VY, Atai NA, Fujita T et al. Extracellular vesicles derived from human vestibular schwannomas associated with poor hearing damage cochlear cells. Neuro-oncology 2016;18:1498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


