Abstract
Objectives
Data on the immune response after two doses of BNT162b2 are so far limited. Previously infected individuals were excluded from pivotal clinical trials and the optimum dose regimen in this population has not been clearly studied. The CRO-VAX HCP study aims to investigate the early antibody response in a population of health-care professionals having received two doses of the BNT162b2 mRNA coronavirus disease 2019 (COVID-19) vaccine.
Methods
The CRO-VAX HCP study is a multicentre, prospective, interventional study conducted in several sites in Belgium. The study included 231 health-care professional volunteers who received the two-dose regimen of the BNT162b2 mRNA COVID-19 vaccine. Of these, 73 were previously infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and 158 were uninfected and seronegative. In the first group, blood samples were collected at baseline and after 2, 4, 7, 10, 14, 21 and 28 days. In the second group, samples were obtained at baseline and after 14 and 28 days. Antibodies against the SARS-CoV-2 nucleocapsid and the receptor binding domain of the S1 subunit of the spike protein were measured in all individuals at different time-points.
Results
In uninfected individuals, 95.5% (95% CI 91.0%–98.2%) developed anti-spike antibodies after 14 days and a 24.9-fold rise (95% CI 21.4%–28.9%) in antibody titre was observed after the second dose. In previously infected individuals, peak antibody response was reached after 7 days (i.e. 6347 U/mL) and the second dose did not lead to significantly higher antibody titres (i.e. 8856–11 911 U/mL). Antibody titres were higher in previously infected individuals.
Conclusions
This study supports the concept that a single dose of BNT162b2 would be sufficient in previously infected individuals.
Keywords: Antibody, BNT162b2, Coronavirus disease 2019, Humoral response, Severe acute respiratory syndrome coronavirus 2, Vaccine
Introduction
The efficacy and safety of the two-dose regimen BNT162b2 mRNA coronavirus disease 2019 (COVID-19) vaccine (Pfizer-BioNTech, Mainz, Germany) has been proved and led in late December to its approval by several regulatory authorities [[1], [2], [3]]. Nevertheless, data on the immune response after two doses of BNT162b2 are so far limited [[4], [5], [6], [7]]. Additionally, individuals who had previous clinical or microbiological diagnosis of COVID-19 were excluded from pivotal clinical trials [2,3,6], precluding the evaluation of the vaccine response in this particular subpopulation.
Materials and methods
The CRO-VAX HCP study is a multicentre, prospective and interventional study designed to assess the antibody response in a population of health-care professionals having received two doses of the BNT162b2 mRNA COVID-19 vaccine. Two-hundred and thirty-one volunteers from three medical centres in Belgium were enrolled. All participants provided informed consent before collection of data and specimen. The study was approved by the ethics committees of the three medical centres (approval number: 2020-006149-21). Participants received the first vaccine dose from 18 January 2021 to 17 February 2021. The second dose was administered 21 days after the first dose. All volunteers underwent a blood draw within 2 days before the first vaccine dose. Volunteers were then included in two follow-up protocols in a 1:2 ratio. In the first group, samples were collected at baseline and after 2, 4, 7, 10, 14, 21 and 28 days, whereas in the second group, samples were obtained at baseline and after 14 and 28 days.
Antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid (anti-NCP; Elecsys Anti-SARS-CoV-2 NCP qualitative ECLIA, Roche Diagnostics, Machelen, Belgium) [9] and the receptor binding domain of the S1 subunit of the spike protein (anti-S; Elecsys anti-SARS-CoV-2 spike quantitative ECLIA, Roche Diagnostics) were measured at each time-point in all serum samples.
Statistical analysis was performed with GraphPad Prism 9.0.1 (GraphPad, San Diego, CA, USA). Antibody titres between groups were tested using a Dunn's multiple comparisons test, with p < 0.05 considered significant.
Results
In our cohort, 73.6% (n = 170) were female (mean age 42.6 years; range 23–66 years) and 26.4% (n = 61) were male (mean age 42.8 years; range 23–64 years). Sixty-five individuals had a previous positive RT-PCR diagnosis (mean days since RT-PCR 99; range 34–337). Among these individuals, 63 had shown symptoms, only two had been asymptomatic and none had required hospitalization. Eight additional participants with positive anti-NCP antibodies at baseline but without evidence of clinical or microbiological diagnosis of COVID-19 in the past were recategorized as previous COVID-19-positive patients (detailed information of the population is presented in the Supplementary material, Table S1).
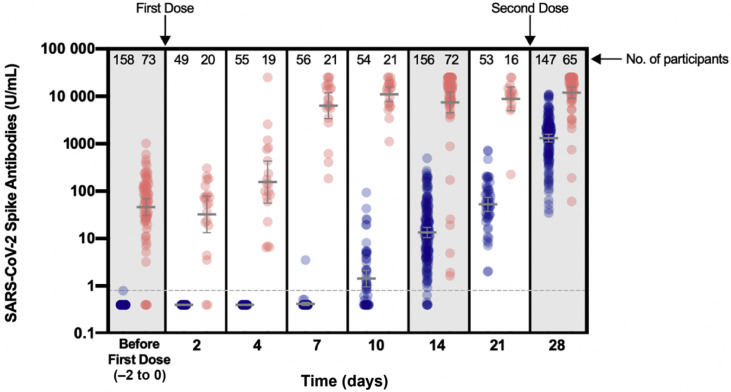
In uninfected, seronegative individuals, the rate of seroconversion after the first dose was 55.6% (95% CI 41.4%–69.1%) and 95.5% (95% CI 91.0%–98.2%) at days 10 and 14, respectively (Fig. 1 ). Among individuals included in the first group, none had positive anti-S antibodies before day 4 and only one participant seroconverted at day 7 (1.8%; 95% CI 0.1%–9.4%). From day 21, all participants had detectable anti-S antibodies (100%; 95% CI 93.3%–100%). At day 28 and following the second vaccine dose, a 24.9-fold (95% CI 21.4–28.9) increase was observed compared with day 21.
Fig. 1.
Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibodies (U/mL) in individuals with previous SARS-CoV-2 infection (red points) and in seronegative persons without declared history of infection (blue points). Blood samples before the first vaccine dose were obtained a maximum of 2 days before. Geometric means with 95% CI are shown, if applicable. The grey dotted line corresponds to the positivity cut-off (0.8 U/mL) of the Elecsys anti-SARS-CoV-2 spike quantitative electrochemiluminescent immunoassay. An automatic dilution of 1/100 at >250 U/mL was performed by the analyser to extend the measurement domain up to 25 000 U/mL. Forty-two samples were rounded to 25 000 U/mL out of 1038 (4%). Results <0.4 U/mL (limit of quantification) were rounded up to 0.4. Up to day 4, blood samples taken 1 day earlier or later compared with the expected blood collection times were allowed. From day 7, 2 days were allowed. Individuals with incomplete samplings were not excluded from the analysis.
In individuals with a previous clinical or microbiological diagnosis of COVID-19, no change in anti-S titres was observed up to day 4. Only five samples, from three participants with a previous molecular diagnosis of SARS-CoV-2 infection but who were seronegative at inclusion, turned seropositive after 4 days. At day 7, a significant 139.9-fold (95% CI 110.8–172.1) increase in anti-S titres was observed. Following the second dose, a 262.4-fold (95% CI 228.1–294.4) increase from baseline was observed. Nevertheless, mean titres at days 14 (7437 U/mL), 21 (8856 U/mL) and 28 (11 911 U/mL) were not significantly different from those at day 7 (6347 U/mL) (p > 0.99). Anti-NCP titres remained unchanged over the 28 days (see Supplementary material, Fig. S1).
Considering each time-point separately, anti-S titres of previously infected individuals were always statistically higher compared with uninfected individuals (see Supplementary material, Table S2). At day 7, anti-S titres from previously infected individuals (6347 U/mL) were not significantly different from titres detected after the second dose of BNT162b2 in previously uninfected individuals (i.e. 1312 U/mL). From 14 days after the first dose of BNT162b2, anti-S titres of uninfected individuals (from 13.5 to 52.7 U/mL) were similar to anti-S titres of individuals with a previous clinical or microbiological diagnosis of COVID-19 at baseline (45.4 U/mL) (p > 0.99). After the second dose, anti-S titres of uninfected individuals (1312 U/mL) were statistically higher compared with baseline levels of previously infected individuals (45.4 U/mL; p < 0.0001).
Discussion
In this study, we report a stronger humoral response in individuals with previous SARS-CoV-2 infection after the first dose of BNT162b2, supporting the concept that this first dose would act as a boost of a previous immunization, as also observed by others [[4], [5], [6], [7]]. This is further supported by the non-significant increase in antibody titres reported after the second dose compared with antibody titres already observed 7 days after the first dose. Evaluation of the pre-vaccinal serological status could therefore be proposed as a strategy to identify individuals who will only require the booster dose [4]. Pan-immunoglobulin assays should be preferred in this context to ensure maximum sensitivity to previous SARS-CoV-2 immunization [8]. Further studies are needed to determine whether a booster dose in previously infected individuals or delayed administration of the second dose in uninfected persons could provide sufficient and effective long-term protection.
Our study has some limitations. The findings should be completed by the assessment of the neutralizing capacity of the anti-S antibodies and by investigation of the cellular immune response. Importantly, while the conclusions of this study are of interest to support the concept of a single booster dose strategy in previously infected individuals, the efficacy of this dose regimen should be confirmed in a sufficiently powered study evaluating clinical outcomes.
This study (EudraCT registration number: 2020-006149-21) has a planned follow up of 2 years. We would therefore be able to determine the long-term kinetics of the humoral response in both uninfected and previously infected participants.
Transparency declaration
The authors declare that they have no conflicts of interest.
Author contributions
JF, JLB, FM, JMD, MC and JD designed the study and performed the data analysis and interpretation; JF, JLB and MC performed the sample analyses and collected the data; JF performed the statistical analysis and wrote the manuscript; and JMD and JD supervised the study.
Acknowledgements
We would like to thank the following persons for their technical support: Clara David, Hélène Haguet and Laure Morimont from Qualiblood; Grégoire Wieërs and Tatiana Roy from Ottignies; Laura Di Chiaro, Marc Elsen, Christine Eucher and Sandrine Van Eeckhoudt from Bouge; Nathalie Ausselet, Christine Laurent, Alexe Momal and Sabrina Onorati from Yvoir. We also would like to thank all the volunteers who agreed to participate. We thank Roche Diagnostics for providing the kits for the evaluation.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manisty C., Otter A.D., Treibel T.A., McKnight A., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prendecki M., Clarke C., Brown J., Cox A., Gleeson S., Guckian M., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saadat S., Tehrani Z.R., Logue J., Newman M., Frieman M.B., Harris A.D., et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favresse J., Eucher C., Elsen M., Gillot C., Van Eeckhoudt S., Dogné J.-M., et al. Persistence of anti-SARS-CoV-2 antibodies depends on the analytical kit: a report for up to 10 months after infection. Microorganisms. 2021;9:556. doi: 10.3390/microorganisms9030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favresse J., Eucher C., Elsen M., Tre-Hardy M., Dogne J.M., Douxfils J. Clinical performance of the Elecsys electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin Chem. 2020;66:1104–1106. doi: 10.1093/clinchem/hvaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.