Abstract
Background
Although molecular tests are considered the reference standard for coronavirus disease 2019 (COVID-19) diagnostics, serological and immunological tests may be useful in specific settings.
Objectives
This review summarizes the underlying principles and performance of COVID-19 serological and immunological testing.
Sources
Selected peer-reviewed publications on COVID-19 related serology and immunology published between December 2019 and March 2021.
Content
Serological tests are highly specific but heterogeneous in their sensitivity for the diagnosis of COVID-19. For certain indications, including delayed disease presentations, serological tests can have added value. The presence of antibodies against SARS-CoV-2 may indicate a recent or past COVID-19 infection. Lateral flow immunoassay (LFIA) antibody tests have the advantages of being easy and fast to perform, but many have a low sensitivity in acute settings. Enzyme-linked immunosorbent assay (ELISA) and chemiluminescence immunoassays (CLIAs) have higher sensitivities. Besides humoral immunity, cellular immunity is also essential for successful host defences against viruses. Enzyme-linked immunospot (ELISpot) assays can be used to measure T-cell responses against SARS-CoV-2. The presence of cross-reactive SARS-CoV-2-specific T cells in never exposed patients suggests the possibility of cellular immunity induced by other circulating coronaviruses. T-cell responses against SARS-CoV-2 have also been detected in recovered COVID-19 patients with no detectable antibodies.
Implications
Serological and immunological tests are primarily applied for population-based seroprevalence studies to evaluate the effectiveness of COVID-19 control measures and increase our understanding of the immunology behind COVID-19. Combining molecular diagnostics with serological tests may optimize the detection of COVID-19. As not all infected patients will develop antibodies against SARS-CoV-2, assessment of cellular immunity may provide complementary information on whether a patient has been previously infected with COVID-19. More studies are needed to understand the correlations of these serological and immunological parameters with protective immunity, taking into account the different circulating virus variants.
Keywords: Antibodies, COVID-19, Immunity, SARS-CoV-2, Serology, T cell
Introduction
Diagnostics for coronavirus disease 2019 (COVID-19) are mostly performed in cases of a suspected acute respiratory infection or for screening of asymptomatic cases as part of outbreak management. Both aim to detect COVID-19 during the early phase of infection. However, in some cases with negative molecular or antigen tests for COVID-19 but remaining high suspicion, it can be relevant to determine whether a patient has previously been infected with COVID-19. In those cases, serological tests may explain a particular clinical presentation, although it does not assess infectiousness. Moreover, serological tests are important to assess seroprevalence and evaluate the effectiveness of applied containment strategies at the community level. However, humoral immunity is just one part of our immune system. Cellular immunity also plays a potential role in the protection against COVID-19. This review summarizes the basic principles of serological and immunological tests for COVID-19 and provides recommendations for its application.
Humoral immunity
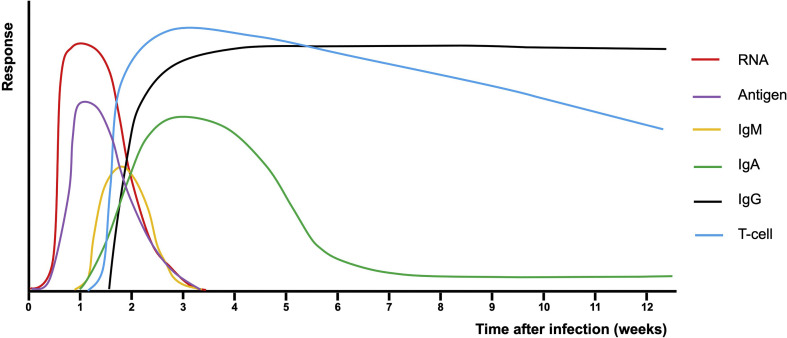
The humoral immunity is characterized by the production of antibodies by B cells as a response to antigens [1]. Immunoglobulin (Ig) M quickly appears but has a short half-life (Fig. 1 ). IgA is most abundant in mucosal surfaces but can also be found in serum, and arises within the first week of symptom onset. IgG is the most abundant antibody type and provides longer-lasting immunity. About 7–14 days after symptom onset, IgG against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is detectable in most patients [2,3]. IgG titres remain stable for at least 4–6 months following diagnosis among COVID-19 polymerase chain reaction (PCR)-confirmed individuals, whereas IgA and IgM titres rapidly decay [[4], [5], [6]]. Antibody titres remain negative in about 5% of symptomatic PCR-positive patients [7], whereas for asymptomatic PCR-positive patients 15% to 40% are seronegative [8,9]. Several studies show that severe cases are associated with higher titres of antibodies and may have a later-onset antibody response in comparison to milder or asymptomatic patients [[9], [10], [11]].
Fig. 1.
Antibody and T-cell responses over time after SARS-CoV-2 infection. Immune responses can be highly heterogenous depending on various factors including patient characteristics and severity of illness. The presented figure is a simplified representation to increase general understanding, but can be variable for different individuals in different settings.
The presence of neutralizing antibodies against SARS-CoV-2 after natural infection indicates protection against reinfection [[12], [13], [14]]. However, the cut-off levels of neutralizing antibodies for protection against reinfection remain to be elucidated. Vaccination also induces neutralizing antibody production, causes protection against COVID-19 infection and reduces the severity of infection. However, the relative independent contributions of humoral and cellular immunity to this protection are difficult to determine [[15], [16], [17]].
The presence of neutralizing antibodies is considered a functional correlate of immunity and provides at least partial resistance to subsequent infections by virus antigen binding to prevent interaction with host cells [1,18]. Although some serological assays showed a high correlation between IgG and neutralizing antibodies [19], other assays have poor correlation [20]. Therefore, comparison with virus-neutralizing tests is important as part of the validation of new serological assays. Most convalescent plasma samples obtained from individuals who recovered from COVID-19 do not contain high levels of neutralizing activity [21]. The antibodies targeting the spike glycoprotein, especially the receptor binding domain (RBD) within the S1 subunit, show the highest neutralizing capacity [1,22]. The degree of cross-reaction to other epidemic and common human coronaviruses largely depends on the target sites to which the antibodies are directed [23]; the S1 subunit is most specific and has the least homology regarding amino acid sequence with other coronaviruses, whereas the S2 subunit is more conservative.
Previously infected individuals develop higher (neutralizing) antibody responses in comparison to infection-naïve individuals after one dose of mRNA vaccine vaccination [24,25], and this single-dose response is comparable or even stronger than in infection-naïve individuals who received two vaccinations [26].
It is of great concern that the presence of neutralizing antibodies against one virus variant after natural infection or vaccination does not automatically mean equally effective protection against other variants [27]. When vaccine-induced neutralizing antibodies are in vitro tested against different SARS-CoV-2 variants, reduced or abolished neutralizing capacity was observed for the K417N, E484K and N501Y virus mutations. This heterogeneity is in line with findings from vaccination studies showing that some vaccines were less effective against infections by these variants compared with wildtype [28].
Serological tests
In contrast to molecular diagnostic tests that detect the presence of SARS-CoV-2 RNA, serological tests detect anti-SARS-CoV-2 antibodies. The most commonly used serological tests include lateral flow immunoassays (LFIAs), enzyme-linked immunosorbent assays (ELISAs) and chemiluminescence immunoassays (CLIAs) (Table 1 ). Depending on the assay used, they may detect IgM, IgA, IgG or total antibodies [29]. In addition, assays vary in the specific antibodies they detect; these include antibodies against the RBD, nucleocapsid (N) protein, spike (S) protein or nucleocapsid and spike (NS) proteins.
Table 1.
Overview principles of serological and immunological tests
| Detection targets | Advantages | Limitations | When to apply | |
|---|---|---|---|---|
| Lateral flow immunoassay (LFIA) | IgM, IgA, IgG or total antibodies |
- Suitable as point-of-care test - Rapid and easy testing |
- Heterogeneous performance with overall limited sensitivity during acute phase of disease - Only qualitative results |
- Population-based epidemiological surveillance - For individual patient care in case of unavailability of molecular diagnostic tests, inconclusive molecular test results, late presentations during disease course or late-onset post-infectious complications - Implications for interpretation after vaccination and correlation with protective immunity remain to be determined |
| Enzyme-linked immunosorbent assay (ELISA) | - Overall higher sensitivity in comparison to LFIA - Suitable for high throughput and automation - Some assays generate quantitative results |
- Not suitable for rapid testing - Need for trained laboratory staff - Batchwise workup in laboratory process |
||
| Chemiluminescence immunoassay (CLIA) | ||||
| Plaque reduction neutralization tests (i.e. conventional virus neutralization test) | Total antibodies (that can inhibit viral replication) | - Presumably high correlation with protective immunity - Gold standard for quantification of neutralizing antibodies |
- Only in biosafety level 3 laboratories possible - Time consuming test |
- To increase scientific understanding regarding immunity - Implications for interpretation after vaccination and correlation with protective immunity remain to be determined |
| Pseudo-neutralizing antibody assays/surrogate virus neutralization test (sVNT) | - High correlation with plaque reduction neutralization tests - Rapid and safe (no need for live biological material) |
- Not considered as gold standard for quantification of neutralizing antibodies | ||
| ELISpot | Antigen-specific T cells (producing a specific cytokine, e.g. IFNγ) | - Quantitative measurements - Commonly used for evaluation of immunity in vaccination trials |
- No information regarding exact cytokine-producing cell types | - To increase scientific understanding regarding immunity - Implications for interpretation after vaccination and correlation with protective immunity remain to be determined |
| Flow cytometry | Different cell types, including T cells | - Identification of specific cell subpopulations and presence of polyfunctional cells | - Test is (relatively) complex | |
LFIA is a rapid immunochromatography-based method, which uses colloidal gold conjugated SARS-CoV-2 antigens [30]. Usually, it requires only a few drops of whole blood from a finger prick placed onto the test strip, whereafter the sample migrates towards fixed bands of bound SARS-CoV-2 antigens [31]. If the sample contains SARS-CoV-2-specific antibodies, these will bind with the antigens, resulting in a visible band. Advantages of LFIAs include their speed (~15 minutes) and ease of use.
ELISA is a plate-based assay of which the microtitre wells are coated with SARS-CoV-2 antigens [32]. After adding the sample, antigen-specific antibodies will bind to these antigens. After washing, a conjugate that binds to the antigen–antibody complex is added. A substrate is added, which will react with the conjugate, resulting in a colour change. The amount of colour change is a quantitative measure of the number of antibodies present in the sample. ELISA is easily adaptable to automation for high throughput.
CLIA utilizes chemiluminescence to quantify the level of antibodies present in the sample [33]. SARS-CoV-2 antigens are conjugated with fluorescein isothiocyanate and bound to magnetic particles. Antibodies in the sample bind to antigens and are then visualized by chemiluminescence using a detection antibody. Advantages of CLIA include the wide dynamic range, high signal intensity, absence of interfering emissions and high stability of reagents.
In contrast to LFIA, which generates only qualitative results, ELISA and CLIA also yield quantitative results. For any serological method, false-positive results due to cross-reactivity are uncommon, with a reported specificity ranging from 96% to 100% [29]. In a meta-analysis, pooled sensitivity of LFIA was 78% (95% confidence interval (CI) 71–83%), of ELISA 86% (95% CI 82–89%) and of CLIA 92% (95% CI 86–95%) [29]. Assays detecting antibodies against the RBD may be more sensitive than assays using other antibodies. LFIAs have the possibility of point-of-care application and do not require highly equipped devices or trained laboratory staff to perform the test. Nevertheless, it will also depend on the a priori probability whether such tests are useful. In high endemic settings and among persons having symptoms longer than 1 week, the test could be useful to decrease time to result and improve hospital logistics, in which positive results confirm the presence of COVID-19 and could accelerate decision-making in emergency rooms and routing to appropriate hospital wards [2].
Although most currently available serology tests assess antibodies against S and N proteins, other antigenic epitopes could also induce strong immune responses. Among 15 different SARS-CoV-2 antigens, nucleocapsid and open reading frame (ORF) 8 and ORF3b induce the strongest specific antibody responses [34]. The combined ORFs had a specificity of 99.5%, suggesting that second-generation diagnostics using novel targets, like non-structural proteins, might improve the performance of serological assays in the future.
Neutralizing antibodies can be detected by plaque reduction neutralization tests [1]. Alternatively, cell-free and protein-based pseudo-neutralizing antibody assays or surrogate virus neutralization tests have been developed, where cells are replaced by receptors, and the virus is replaced by surface proteins [20]. Surrogate virus neutralization tests have the advantage that no biosafety level 3 containment is needed as these do not require live viruses and cells, while having a very high correlation with plaque reduction neutralization tests [35].
Cellular immunity
Cellular immunity is of paramount importance in containing SARS-CoV-2 infection [1]. Lymphopenia is a characteristic feature in moderate and severe COVID-19. It correlates with disease severity and mortality [36], thus raising questions about the adequacy and effectiveness of T-cell responses in severe cases. The cause of lymphopenia could be the recruitment and sequestration of activated lymphocytes in the lungs [37], induction of cell death or immune dysregulation [38,39]. The latter, manifesting either as immunosuppression or excessive immune activation and cytokine release syndrome, is characterized by increased interleukin (IL)-6 production and has been a major concern as it correlates with increased severity and mortality in COVID-19 [38,40,41]. The chronic pro-inflammatory state that accompanies old age and obesity may contribute to the immune imbalance seen in COVID-19, putting these populations at higher risk for severe infection [42].
Robust SARS-CoV-2 T-cell responses were observed in acute COVID-19 as well as in the majority of convalescent individuals [[43], [44], [45], [46]]. Both CD4+ and CD8+ responses were characterized by the secretion of interferon (IFN)γ, IL-2 and tumour necrosis factor α, indicative of T helper (Th) 1 polarization, and weak Th2 and Th17 responses [43,44,46,47]. SARS-CoV-2 S-specific CD4+ T-cell responses were detected in the majority of COVID-19 cases, with a substantial fraction representing T follicular helper (TFH) cells required for effective humoral immunity and affinity-matured B cell memory [41,43,45,48]. Moreover, there is a positive correlation between S-specific T-cell responses and anti-S antibody titres [47]. CD8+ specific responses were also identified both in acute COVID-19 and during convalescence, characterized by the secretion of IFNγ, granzyme B and perforin, and the expression of degranulation marker CD107a [43,46]. After stimulation with SARS-CoV-2 peptides in COVID-19 patients 26 days after symptom onset, specific T-cell responses were elicited against the membrane (M) and N protein, and to a lesser extent, non-structural proteins (e.g. nsp3, nsp4, ORF8) [1,46].
Memory T-cell responses are detectable during early convalescence (1–2 months after symptom onset) in the majority of infected individuals, and they are accentuated in those with more severe disease [[40], [41], [42],44]. In addition, dominant central memory differentiation among CD4+ T cells and effector memory differentiation among CD8+ T cells can be found [49]. Another study identified an early differentiated memory phenotype with stem-like properties (CCR7+ CD127+ CD45RA–/+ TCF1+) among CD8+ T cells [43]. Memory T cells show a preferential specificity for S protein epitopes, but reactivity against M, N and non-structural proteins has also been observed [49]. Robust T-cell immunity is substantial even 6–8 months after SARS-CoV-2 infection, with CD4+ responses being the most frequent [50].
Importantly, cross-reactive SARS-CoV-2-specific T cells are present in 20–50% of unexposed healthy donors, possibly induced by previous exposure to other circulating endemic coronaviruses [43,44,46,49,51]. Whether these T-cell responses could influence clinical outcomes in COVID-19 remain unclear. Responses against S protein primarily aim at the S2 domain, which shows great homology to the S2 domain of endemic coronaviruses [44]. Cross-reactivity has also been observed against M, N and other non-S proteins and was more common among CD4+ T cells [43,46]. T-cell responses against SARS-CoV-2 have also been detected in recovered COVID-19 patients with no detectable antibodies, indicating that, in some cases, cellular immunity could be maintained independently of antibody responses [42,43]. This finding is consistent with previous reports regarding SARS-CoV and MERS [52,53]. Interestingly, using a bioinformatics approach, in silico data showed the presence of T-cell cross-reactivity between SARS-CoV-2 peptides and several allergens that could be beneficial against COVID-19 in atopic individuals [54].
To achieve protective immunity, vaccines against SARS-CoV-2 should elicit effective and lasting T-cell responses in addition to the induction of neutralizing antibodies [39]. In clinical trials, mRNA-1273 (Moderna™) vaccine induced CD4+ Th1-biased and CD8+ T-cell responses, with a lack of Th2 cytokine responses, demonstrating a favourable immunological signature [16]. Similar results have been reported for another mRNA vaccine (i.e. BNT162b2, Pfizer/BioNTech™) and a chimpanzee adenovirus-vectored vaccine (i.e. ChAdOx1, University of Oxford/AstraZeneca™) [15,17]. In the latter, S-specific T-cell responses peaked at day 14 of vaccination and were still detectable on day 56 [17]. Previously infected individuals develop much stronger T-cell responses against spike protein peptides comparison with infection-naïve individuals after one dose of mRNA vaccine vaccination [24].
ELISpot
Enzyme-linked immunospot (ELISpot) is an antigen-specific T-cell functional assay that can measure the proportion of T cells producing a specific cytokine [55] (Table 1). It is a highly sensitive approach and has been used to assess SARS-CoV-2-specific T-cell responses [36,43,47,51,56]. IFNγ-secreting T cells were reactive against M, N and S peptides in 70–100% of convalescent COVID-19 patients depending on the specific antigens and techniques used in the test [47,51,[56], [57], [58]]. Illness severity is correlated with anti-M and anti-S T-cell responses [47,56]. IFNγ-ELISpot assays have also been used for the assessment of cellular responses after SARS-CoV-2 vaccination, with vaccine candidates reporting robust S-specific T-cell responses after vaccination [59]. As ELISpot assay cannot provide further information about the exact cytokine-producing cell types, intracellular cytokine staining with flow cytometry can be used to identify specific cell subpopulations and the presence of polyfunctional cells [60].
Recommendations for clinical practice
Although several aspects of the hosts' immune responses against SARS-CoV-2 remain to be further unravelled, some serological and immunological tests can be used to gain valuable information. Humoral immunity responses are variable and highly dependent on various assay-based and host factors. Hence, the optimization of a standardized approach regarding the correct timing and the appropriate type of serology test that should be performed in different disease phases, severity classes, patient ages and settings remains challenging.
Although the diagnostic utility of serological testing in the acute phase of illness is limited, it can be used for SARS-CoV-2 diagnostics in case of unavailability of molecular diagnostic tests in, e.g., resource-deprived settings. However, in such settings also rapid antigen tests can be considered. Antibody tests may also be utilized when molecular test results are inconclusive or in cases of late-onset post-infectious complications, such as the multisystem inflammatory syndrome in children [61]. In patients who are highly suspected of COVID-19 but in whom the molecular test was negative, serological testing may be helpful to yet establish the diagnosis.
As point-of-care serological tests enable a timely and convenient way of antibody testing, they may be preferred when rapid results are needed or when access to central laboratories requires precarious logistics, like community-based screening, non-referral hospitals, outpatient practice or in home-based settings. Self-sampling approaches might be useful in population-based screening studies, but further validation is needed.
Most importantly, serological tests for SARS-CoV-2 are necessary for population-based epidemiological surveillance. Serological tests are useful for public health policy-making to address the extent of SARS-CoV-2 spread in the community and assess the effectiveness of infection control strategies.
The introduction of community-wide vaccination programmes may complicate the interpretation of serological test results. The majority of currently available vaccines induce anti-S protein or anti-RBD neutralizing antibody response [1]. Consequently, assays targeting only anti-S protein or anti-RBD antibodies will not be able to discriminate between natural and post-vaccination-induced immunity. Nevertheless, the measurable effect of a post-vaccination immune response is of great significance for the affirmation of SARS-CoV-2 immune communities. As of writing this review, community-wide monitoring of antibody levels after vaccination to determine whether sufficient protection levels have been obtained is not recommended according to most guidelines. Nevertheless, it is conceivable to consider measuring vaccine response in patient groups that are at high risk for reduced immune responses, e.g. immunocompromised patients, and to identify those with insufficient protection despite completing the standard vaccination schedule. However, this needs to be further evaluated in clinical studies, and more supporting evidence to reach consensus on cut-off values for presumed protection is mandatory.
The choice of antibody testing strategy and interpretation of the results should be based on the assay performance characteristics and serological tests should only be used in settings where the prevalence is not too low. Otherwise, even a very high specific test can lead to a substantial absolute number of false-positive results when the prevalence is very low. Moreover, when tests have low specificities, combining different tests may be applied to increase overall specificity and maintain high sensitivity rates [62].
Finally, many information gaps need to be clarified regarding long-term antibody kinetics and T-cell responses after natural infections by various SARS-CoV-2 variants and vaccination strategies. Longitudinal studies should be employed in order to delineate the clinical sensitivity and specificity rates of serology and immunology tests in various settings with different prevalence.
Conclusion
Serological tests provide information about previous COVID-19 infections or vaccinations. They are not suitable as stand-alone diagnostics for acute-phase infections. However, in cases of more prolonged existing symptoms and when molecular diagnostic results are unavailable or inconclusive, serological diagnostics could identify additional COVID-19 cases among suspected patients. Awareness of the limitations of serological and immunological tests in a particular setting is required, because of the large heterogeneity in test performance of different assays. Currently, it remains uncertain how serological and immunological parameters are precisely correlated with the extent of protective immunity.
Transparency declaration
R.F.C.: Research Grants from Gilead, Pulmotec, Janssen, Karius, Chimerix, Merck, Viracor, Takeda/Shire and Ansun Pharmaceuticals. Advisory Board/Consultant for ADMA Biologics, Pulmotec, Ablynx, Janssen, Merck, ReViral, Kyorin, Chimerix, Partner Therapeutics and Ansun Pharmaceuticals. All R.F.C. disclosures are not related to the topic discussed. C.S.: Consultancy and research funding, Hycor Biomedical, Bencard Allergie and Thermo Fisher Scientific. Research Funding from Mead Johnson Nutrition (MJN). C.S. is supported by the Universities Giessen and Marburg Lung Center (UGMLC), the German Center for Lung Research (DZL), University Hospital Giessen and Marburg (UKGM) research funding according to article 2, section 3 cooperation agreement, and the Deutsche Forschungsgemeinschaft (DFG)-funded SFB 1021 (C04), KFO 309 (P10) and SK 317/1-1 (project number 428518790) as well as by the Foundation for Pathobiochemistry and Molecular Diagnostics.
Author contributions
D.S.Y.O. and C.S. contributed to the conception and design of the review. D.S.Y.O., P.C.F., V.A.S. and C.D.M. performed the literature search, and contributed to the writing of the first draft of this manuscript. D.S.Y.O. and C.S. supervised the project. D.S.Y.O., P.C.F., V.A.S., R.F.C., C.D.M. and C.S. revised the subsequent manuscript versions critically for important intellectual content, and all authors approved the final manuscript version to be submitted.
Editor: L. Leibovici
References
- 1.Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong D.S.Y., de Man S.J., Lindeboom F.A., Koeleman J.G.M. Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clin Microbiol Infect. 2020;26:1094.e7–1094.e10. doi: 10.1016/j.cmi.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 4.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5:abe5511. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.L'Huillier A.G., Meyer B., Andrey D.O., Arm-Vernez I., Baggio S., Didierlaurent A., et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin Microbiol Infect. 2021;12:910. doi: 10.1016/j.cmi.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oved K., Olmer L., Shemer-Avni Y., Wolf T., Supino-Rosin L., Prajgrod G., et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29:100651. doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang C., Wang Y., Hu M., Wen L., Wen C., Wang Y., et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Transl Immunol. 2020;9 doi: 10.1002/cti2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren L., Zhang L., Chang D., Wang J., Hu Y., Chen H., et al. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun Biol. 2020;3:780–787. doi: 10.1038/s42003-020-01526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumley S.F., O'Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., et al. Antibody status and incidence of SARS-CoV-2 infection in health care Workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffery-Smith A., Iyanger N., Williams S.V., Chow J.Y., Aiano F., Hoschler K., et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021;26:2005. doi: 10.2807/1560-7917.ES.2021.26.5.2100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.-L., et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58:77. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgarth N., Nikolich-Žugich J., Lee F.E.-H., Bhattacharya D. Antibody responses to SARS-CoV-2: let's stick to known knowns. J Immunol. 2020;205:2342–2350. doi: 10.4049/jimmunol.2000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GeurtsvanKessel C.H., Okba N.M.A., Igloi Z., Bogers S., Embregts C.W.E., Laksono B.M., et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17317-y. 3436–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Focosi D., Maggi F., Mazzetti P., Pistello M. Viral infection neutralization tests: a focus on severe acute respiratory syndrome-coronavirus-2 with implications for convalescent plasma therapy. Rev Med Virol. 2020;7821 doi: 10.1002/rmv.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D., Li J. Immunologic testing for SARS-CoV-2 infection from the antigen perspective. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02160-20. e02160–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prendecki M., Clarke C., Brown J., Cox A., Gleeson S., Guckian M., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu Jabal K., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021:1–10. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mekonnen D., Mengist H.M., Derbie A., Nibret E., Munshea A., He H., et al. Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome coronavirus 2 antibody: a systematic review and meta-analysis. Rev Med Virol. 2020;11 doi: 10.1002/rmv.2181. [DOI] [PubMed] [Google Scholar]
- 30.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peeling R.W., Wedderburn C.J., Garcia P.J., Boeras D., Fongwen N., Nkengasong J., et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20:e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. 2020;6:591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cinquanta L., Fontana D.E., Bizzaro N. Chemiluminescent immunoassay technology: what does it change in autoantibody detection? Auto Immun Highlights. 2017;8 doi: 10.1007/s13317-017-0097-2. 9–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachim A., Kavian N., Cohen C.A., Chin A.W.H., Chu D.K.W., Mok C.K.P., et al. ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection. Nat Immunol. 2020;21:1293–1301. doi: 10.1038/s41590-020-0773-7. [DOI] [PubMed] [Google Scholar]
- 35.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I.-C., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 36.Ni L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H., et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–973. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 38.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–993. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiPiazza A.T., Graham B.S., Ruckwardt T.J. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem Biophys Res Commun. 2020;3:363. doi: 10.1016/j.bbrc.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remy K.E., Mazer M., Striker D.A., Ellebedy A.H., Walton A.H., Unsinger J., et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5:337. doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Candia P., Prattichizzo F., Garavelli S., Matarese G. T cells: warriors of SARS-CoV-2 infection. Trends Immunol. 2021;42:18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 45.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sette A., Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol. 2020;20:457–458. doi: 10.1038/s41577-020-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H., et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021:371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 52.Li C.K.-F., Wu H., Yan H., Ma S., Wang L., Zhang M., et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao J., Alshukairi A.N., Baharoon S.A., Ahmed W.A., Bokhari A.A., Nehdi A.M., et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balz K., Chen M., Kaushik A., Cemic F., Heger V., Renz H., et al. Homologies between SARS-CoV-2 and allergen proteins may direct T cell-mediated heterologous immune responses. Sci Rep. 2021;11:4792. doi: 10.1038/s41598-021-84320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slota M., Lim J.-B., Dang Y., Disis M.L. ELISpot for measuring human immune responses to vaccines. Expert Rev Vaccines. 2011;10:299–306. doi: 10.1586/erv.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demaret J., Lefèvre G., Vuotto F., Trauet J., Duhamel A., Labreuche J., et al. Severe SARS-CoV-2 patients develop a higher specific T-cell response. Clin Transl Immunol. 2020;9:e1217. doi: 10.1002/cti2.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thieme C.J., Anft M., Paniskaki K., Blazquez-Navarro A., Doevelaar A., Seibert F.S., et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med. 2020;1:100092. doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds C.J., Swadling L., Gibbons J.M., Pade C., Jensen M.P., Diniz M.O., et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abf3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freer G., Rindi L. Intracellular cytokine detection by fluorescence-activated flow cytometry: basic principles and recent advances. Methods. 2013;61:30–38. doi: 10.1016/j.ymeth.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 61.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ripperger T.J., Uhrlaub J.L., Watanabe M., Wong R., Castaneda Y., Pizzato H.A., et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–933.e4. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]