Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is involved in a global outbreak affecting millions of people who manifest a variety of symptoms. Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 is increasingly associated with cardiovascular complications requiring hospitalizations; however, the mechanisms underlying these complications remain unknown. Nitric oxide (NO) and hydrogen sulfide (H2S) are gasotransmitters that regulate key cardiovascular functions.
Methods
Blood samples were obtained from 68 COVID-19 patients and 33 controls and NO and H2S metabolites were assessed. H2S and NO levels were compared between cases and controls in the entire study population and subgroups based on race. The availability of gasotransmitters was examined based on severity and outcome of COVID-19 infection. The performance of H2S and NO levels in predicting COVID-19 infection was also analyzed. Multivariable regression analysis was performed to identify the effects of traditional determinants of gasotransmitters on NO and H2S levels in the patients with COVID-19 infection.
Results
Significantly reduced NO and H2S levels were observed in both Caucasian and African American COVID-19 patients compared to healthy controls. COVID-19 patients who died had significantly higher NO and H2S levels compared to COVID-19 patients who survived. Receiver-operating characteristic analysis of NO and H2S metabolites in the study population showed free sulfide levels to be highly predictive of COVID-19 infection based on reduced availability. Traditional determinants of gasotransmitters, namely age, race, sex, diabetes, and hypertension had no effect on NO and H2S levels in COVID-19 patients.
Conclusion
These observations provide the first insight into the role of NO and H2S in COVID-19 infection, where their low availability may be a result of reduced synthesis secondary to endotheliitis, or increased consumption from scavenging of reactive oxygen species.
Keywords: SARS-CoV-2, COVID-19, Nitric oxide, Hydrogen sulfide, Gasotransmitters, Cardiovascular disease
Highlights
-
•
NO and H2S availability is decreased in COVID-19 patients compared to healthy controls.
-
•
Decreased NO and H2S availability in COVID-19 patients is independent of race, other demographics and comorbidities.
-
•
Decreased NO levels in COVID-19 patients parallel an increase in nitrotyrosine, an oxidative stress marker.
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected over 77.8 million people in over 220 countries during the recent worldwide pandemic, approximately 18.5 millions of whom are in the United States. Although COVID-19 causes significant morbidity and mortality when it manifests as ‘viral pneumonia,’ available evidence suggests that COVID-19 is associated with cardiovascular complications. These are rapidly emerging as a key threat, leading to increasing hospitalizations accompanied by a host of complications, including myocarditis, thrombo-embolism, acute coronary syndrome, and resultant cardiac arrhythmias, together referred to as Acute COVID-19 Cardiovascular Syndrome (ACovCS)[1,2]. The complications of COVID-19 are significantly exacerbated due to preexisting comorbidities, including pulmonary and cardiovascular disease. Studies of the SARS and SARS-CoV-2 viruses reveal a potential role for cytokine storm, altered blood pressure regulation, and thrombosis in the pathogenesis of COVID-19 [[3], [4], [5]]. Moreover, COVID-19 has been shown to directly target endothelial cells and cause endotheliitis, thus affecting downstream functions that may contribute to cardiovascular complications[6]. However, the link between cardiovascular complications and COVID-19, along with the underlying molecular mechanisms, remains poorly understood.
Nitric oxide (NO) and hydrogen sulfide (H2S) are ubiquitous signaling molecules popularly referred to as gasotransmitters that play protective roles in limiting the severity of cardiovascular disease[7,8]. NO acts as a vasodilator and an antiviral agent in patients with SARS and can inhibit in vitro replication of SARS-CoV-2 [9, 10]. While several recent reviews also suggest an association between H2S and SARS-CoV-1/2, they provide little evidence of any of such relationship[[11], [12], [13], [14]]. Consistent with these suppositions is the possibility that endothelial dysfunction concomitant with COVID-19 infection is likely to result in reduced NO and H2S metabolite availability. However, no studies have been reported to date evaluating specific levels of gasotransmitters in relation to COVID-19. In this study, we assessed the relationship between NO and H2S metabolite availability in patients with COVID-19 and further evaluated them as prognostic biomarkers in severely ill COVID-19 patients.
2. Methods
2.1. Study design
This was a case-control study approved by the Institutional Review Board (IRB) of Louisiana State University Health Sciences Center at Shreveport (LSUHSC-S) (STUDY00001501). Consecutive patients admitted with COVID-19 viral pneumonia to Ochsner-LSU hospital in Shreveport were approached for inclusion in the study. Patients who tested positive for COVID-19 by rapid testing or by PCR within 14 days were included. Pregnant women, prisoners, and patients younger than 18 years of age or older than 89 years of age were excluded from the study. Among those who met the inclusion criteria, a total of 73 patients were consented; two patients withdrew their consent, we could not obtain blood samples from two other patients, and one sample was inadequate for performing analysis. Volunteers were invited to enroll in the study using flyers and by word of mouth. Blood samples from healthy race- and sex-matched volunteers with no prior history of COVID-19 infection were also obtained in the cardiology clinic at Ochsner-LSU Hospital in Shreveport after the volunteers provided an informed consent.
2.2. Human blood collection
After obtaining an informed consent, blood samples were collected from human healthy subjects and COVID-19 patients into 6 mL BD vacutainer tubes with lithium heparin. Samples were transported to the lab within 15 min on ice and were centrifuged at 1500 RCF for 4 min at 4 °C; plasma was collected and snap frozen for further analyses. Medical record data pertaining to baseline characteristics and comorbidities of healthy subjects and COVID-19 patients were collected and compared (Table 1, Table 2).
Table 1.
Demographics of COVID-19 cases and healthy controls included in the study.
| Mean Age (range) | Total (N) | Caucasians (C) | African Americans (AA) | Other Race | Males | Females | |
|---|---|---|---|---|---|---|---|
| Controls | 43.09 (22–68) | 33 | 19 (58%) | 13 (39%) | 1 (3%) | 18 (54%) | 15 (45%) |
| Covid-19 Patients | 58.19 (27–85) | 68 | 21 (31%) | 45 (66%) | 2 (3%) | 35(51.5%) | 33 (48.5%) |
Table 2.
Patient and disease characteristics of COVID-19 cases included in the study.
| Patient Characteristics | Total Number (% of Total Number) | African Americans (AA) | Caucasians (C) |
|---|---|---|---|
| Comorbidities | |||
| DM | 29/68 (42.6%) | 21/45 (46.7%) | 6/21 (28.5%) |
| Hypertension | 51/68 (75%) | 39/45 (86.6%) | 11/21 (52.4%) |
| BMI>30 | 41/68 (60%) | 27/45 (60%) | 12/21 (57.1%) |
| COPD | 6/68 (8.8%) | 5/45 (11.1%) | 1/21 (4.7%) |
| CVD | 18/68 (26.4%) | 12/45 (26.7%) | 6/21 (28.6%) |
| Severity | |||
| Mild-Moderate | 46/68 (67.6%) | 33/45 (73.3%) | 12/21 (57.1%) |
| Severe | 22/68 (32.3%) | 12/45 (26.7%) | 9/21 (42.8%) |
DM = Diabetes Mellitus; BMI= Body Mass Index; COPD = chronic obstructive pulmonary disease; CVD = cardiovascular disease. Two patients in the study were Hispanic.
2.3. NO metabolite measurements
NO metabolites (NOx) were measured using an ozone-based chemiluminescence assay (Sievers Nitric Oxide Analyzer 280i, Weddington, NC) as described previously[15]. Plasma samples were collected in NO stabilization buffer (1.25 mol/L potassium ferricyanide, 56.9 mmol/L N-ethylmaleimide, 6% Nonidet P-40 substitute in PBS), or free nitrite and S-nitrosothiol (SNO) preservation buffers (Zysense, Weddington, NC), respectively. Aliquots of samples were injected into the analyzer and tested for total NO and for individual NO metabolites.
2.4. Measurement of biological pools of H2S
Plasma samples were analyzed for free sulfide, acid-labile sulfide (ALS), bound sulfane sulfur (BSS), and total sulfide levels using the monobromobimane (MBB) method reported previously[16]. Free sulfide was measured using 30 μL of plasma with MBB; for detection of ALS and BSS, 50 μL of plasma was processed separately in two 4 mL BD vacutainer tubes with 100 mM phosphate buffer (pH 2.6, 0.1 mM DTPA) for the ALS reaction, and 100 mM phosphate buffer (pH 2.6, 0.1 mM DTPA) containing 1 mM TCEP for the total sulfide reaction. Following a 30-minute incubation on a nutator mixer, to trap the evolved sulfide gas and incubation with 100 mM Tris-HCl buffer (pH 9.5, 0.1 mM DTPA) for 30 minutes on a nutator mixer, the trapped sulfide was then measured using the MBB method and calculations performed to determine total sulfide and its pools as previously described [16].
2.5. Measurement of nitrotyrosine
Quantitative determination of nitrotyrosine in the plasma of control subjects and COVID-19 patients was performed by a competitive ELISA kit (Cell biolabs, Inc.) as per manufacturer’s instructions.
2.6. Statistical analyses
Levels of NO and sulfide metabolites were assessed by group means and standard deviations with subsequent pairwise comparison using analysis of variance (ANOVA). Receiver-operating characteristic analysis (ROC) was conducted to assess the predictive accuracy in correlating NO and sulfide levels with COVID-19 infection. Cutoff values for positive classification were included in the curve, with a nonparametric distribution assumption and a confidence level of 95%. These statistical analyses were performed using GraphPad Prism 5.0. We also conducted multivariable regression analyses to estimate the effect of various predictor variables on NO and H2S in separate models with 95% confidence intervals. A descriptive analysis of study variables was performed using SPSS Version 26.0 (IBM Corp., Armonk, NY). A Chi-square test of independence was used to determine associations between categorical variables. For continuous variables, means of two independent groups were compared using the independent samples Student’s t-test. For all analyses, a p-value of <0.05 was considered statistically significant. We assumed equal variance for the independent samples Student’s t-test result when Levene’s test had a p-value <0.05. Otherwise, we used the results from equal variance not assumed.
3. Results
3.1. NO metabolites are reduced with COVID-19 infection
A total of 68 COVID-19 cases and 33 controls were included in the study. Plasma NO availability was measured and compared between control subjects and COVID-19 patients (Fig. 1). We found a significant reduction in the total NO levels in the plasma of COVID-19 patients compared to that of healthy controls (Fig. 1A; 418.84 ± 153.03 nM vs 286.69 ± 140.39 nM, p<0.0001). In addition, to observe the effect of COVID-19 infection on individual NO metabolites, we measured free nitrite (Fig. 1B) and bound SNO fractions (Fig. 1C) using commercially available stabilization buffers. Free nitrite (292.63 ± 141.67 nM vs 179.945 ± 164.0 nM, p = 0.0017) and SNO fractions (243.19 ± 91.60 nM vs 152.89 ± 85.39 nM, p<0.0001) were significantly reduced in the plasma of COVID-19 patients compared to that of the controls (Fig. 1 B and C).
Fig. 1.
Plasma NO availability in COVID-19 patients. Results show significantly reduced total NO (A), free nitrite (B), and s-nitrosothiol (C) metabolites in COVID-19 patients (n = 68) compared to control subjects (n = 33).
3.2. Sulfide pools are reduced with COVID-19 infection
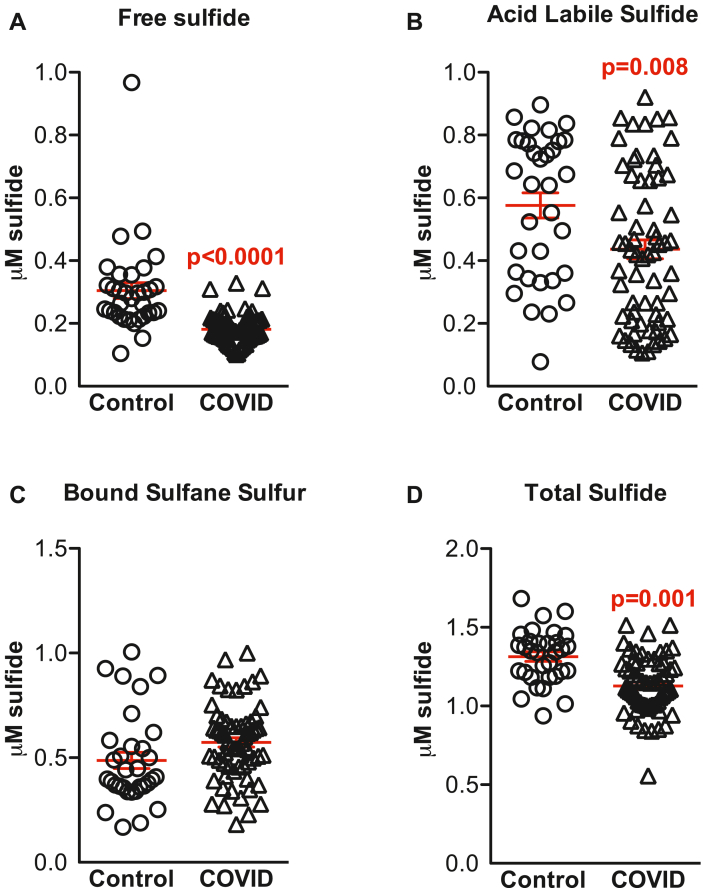
We next examined the impact of COVID-19 infection on sulfide metabolites. Fig. 2 illustrates free, acid labile, bound sulfide, and total sulfide pools that were quantified in plasma samples from healthy controls and COVID-19 patients. Sulfide levels, including free (0.31 ± 0.14 μM vs 0.18 ± 0.05 μM, p<0.0001; Fig. 2A), ALS (0.59 ± 0.23 μM vs 0.45 ± 0.24 μM, p = 0.008; Fig. 2B) and total (1.37 ± 0.31 μM vs 1.15 ± 0.21 μM, p = 0.001; Fig. 2D), were significantly reduced in COVID-19 patients compared to the healthy controls. No significant differences were observed in BSS (0.53 ± 0.32 μM vs 0.59 ± 0.19 μM; Fig. 2C).
Fig. 2.
Plasma sulfide pools in COVID-19 patients. Scatter bar graphs showing plasma free sulfide (A), acid labile sulfide (B), bound sulfane sulfur (C) and total sulfides (D) in Control and COVID-19 subjects. Results show significantly reduced sulfide metabolites with the exception of bound sulfane sulfur in COVID-19 patients (n = 68) compared to Controls (n = 33).
3.3. Race-based comparison of NO metabolites in COVID-19 patients
The association of plasma NO levels were compared between COVID-19 patients and control subjects based on race. Analysis by race revealed a significant reduction in plasma total (451.8 ± 158 nM vs 286.35 ± 120.55 nM; p = 0.0005), free nitrite (301.16 ± 128.37 nM vs 229.55 ± 79.09 nM; p = 0.03), and SNO (259.56 ± 115.10 nM vs 131.80 ± 83.98 nM; p<0.0001) metabolites in Caucasian COVID-19 patients compared to race matched controls (Fig. 3A–C), whereas NO metabolites in African Americans (AA) showed a significant reduction in total NO (384.8 ± 157 nM vs 287.6 ± 150.3 nM; p = 0.0494) and SNO levels (222.62 ± 44.57 nM vs 164.7 ± 85.06 nM; p = 0.013) in COVID-19 patients compared to AA controls (Fig. 3D and F). Although a trend towards decreased free nitrite (281.58 ± 171.04 nM vs 224.1 ± 85.37 nM; p = 0.275) was seen in AA COVID-19 patients compared to AA controls, no statistical significance was observed (Fig. 3E). Moreover, no race-based differences were observed when NO levels were compared between control and/or COVID-19 groups in Caucasians vs AA.
Fig. 3.
NO availability by race. Total NO, free nitrite, and s-nitrosothiol metabolites are significantly reduced in Caucasian (A–C) COVID patients (n = 21) compared to controls (n = 19). There was a trend towards lower free nitrite levels and significantly reduced total NO and s-nitrosothiol metabolites in African American (D–F) COVID-19 patients (n = 44) compared to control subjects (n = 13).
3.4. Race-based comparison of sulfide metabolites in COVID-19 patients
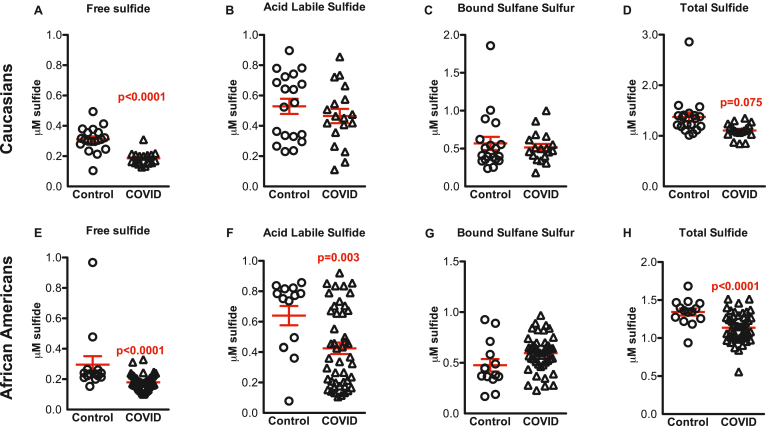
We next compared subjects based on race for sulfide metabolites (Fig. 4A–D). A significant reduction was seen in free sulfide pools (0.31 ± 0.08 μM vs 0.19 ± 0.06 μM, p<0.0001) and total sulfide levels (1.37 ± 0.40 μM vs 1.19 ± 0.24 μM, p = 0.075) in Caucasian COVID-19 patients compared to healthy subjects (Fig. 4A and D). The reduced levels of ALS and BSS in Caucasian COVID-19 patients were not statistically significant. In the AA population, a significant decrease was seen in free (0.25 ± 0.08 μM vs 0.18 ± 0.05 μM, p<0.0001), acid labile (0.67 ± 0.23 μM vs 0. 43 ± 0.250 μM, p = 0.003), and total sulfide levels (1.37 ± 0.13 μM vs 1.13 ± 0.19 μM, p<0.0001), while no significant changes were seen in the levels of BSS (Fig. 4G). When sulfide levels were compared between Caucasian and AA controls, there was a significant reduction in free sulfide levels (0.31 ± 0.08 μM vs 0.25 ± 0.08 μM; p = 0.04) in AA subjects. No significance was seen in other pools of sulfide in comparisons between these races in either the control or COVID-19 groups.
Fig. 4.
Sulfide pools by race. Free sulfide, acid labile sulfide, bound sulfane sulfur and total sulfides in Caucasian (A–C) and African American (D–F) COVID-19 subjects compared to control subjects respectively. Scatter bar graphs show a significantly reduced total and free sulfide levels but not bound sulfane sulfur and acid labile sulfide levels in Caucasian COVID-19 patients (n = 18) compared to controls (n = 19); and significantly reduced total, free and acid labile sulfide levels but comparable bound sulfane sulfur levels in African American COVID-19 patients (n = 46) compared to controls (n = 13).
3.5. Nitrotyrosine levels are elevated in COVID-19 patients
To determine NO-derived oxidants, we measured systemic levels of nitrotyrosine in the plasma from healthy controls and COVID-19 patients (Fig. 5). Nitrotyrosine levels were significantly higher among patients with COVID-19 compared to healthy controls (107.049 ± 7.907 nM vs 44.7606 ± 12.85 nM; P<0.0001; Fig. 5A). Analysis by race showed a significant increase in nitrotyrosine levels both in Caucasian COVID-19 patients (108.2 ± 13.62 nM vs 48.54 ± 16.92 nM; p = 0.01; Fig. 5B), and in African Americans COVID-19 patients (106.2 ± 10.01 nM vs 40.69 ± 22.01 nM; p = 0.006; Fig. 5C) compared to respective race matched controls.
Fig. 5.
Nitrotyrosine levels in controls vs COVID-19 patients. Nitrotyrosine levels are significantly increased in COVID-19 patients (n = 68) compared to healthy controls (n = 33) in the overall study population (A); There was a similar increase in nitrotyrosine levels in the Caucasian (n = 21 vs 19) (B) and African American (n = 44 vs 13) COVID patients compared to race matched controls.
3.6. A case-study of a single COVID-19 patient – association between CRP and gasotransmitters
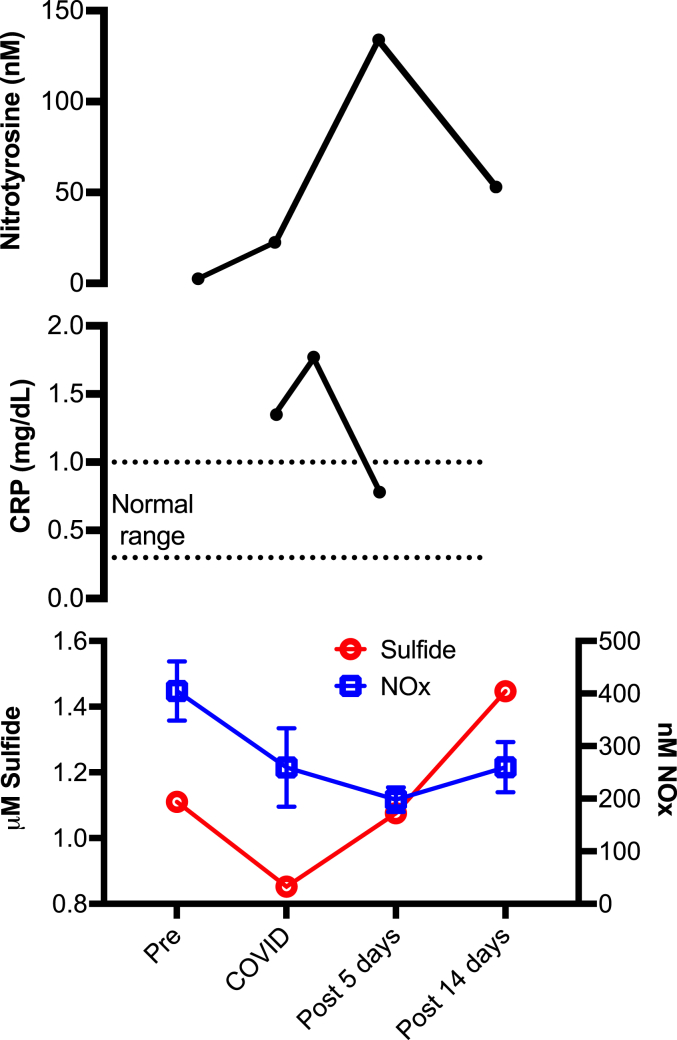
C-reactive protein (CRP) levels have been shown to be an early prognosticator in COVID-19 pneumonia and can indicate disease severity, whereas the gasotransmitters NO and H2S are known for their anti-inflammatory properties[17]. We measured nitrotyrosine, CRP as well as NO and H2S levels in a single subject who was initially a control subject, but 9 days later contracted a COVID-19 infection (Fig. 6). The subject’s CRP levels, which were significantly elevated with COVID-19 infection (1.35 mg/dL, normal range 0.3–1.0 mg/dL), were further elevated (1.77 mg/dL) within 3 days of infection, and then returned to the normal range (0.78 mg/dL) following antiviral therapy with remdesivir for 5 days (Fig. 6, middle panel). Total NO and sulfide levels were significantly reduced during COVID-19 infection (280 nM and 0.8523 μM, respectively) in this individual from pre-infection baseline (400 nM and 1.11039 μM, respectively) (Fig. 6, bottom panel). However, both the NO and sulfide levels were elevated following remdesivir antiviral therapy coinciding with decreased COVID-19 symptoms (5 days post-treatment: 200 nM and 1.07555 μM; 14 days post-treatment: 280 nM and 1.44706 μM). The subject’s level of nitrotyrosine, an oxidant marker, was significantly increased with COVID-19 infection and at day-5 post COVID-19 infection (22.52 nm and 133.99 nM respectively) compared to the baseline (2.56 nM) (Fig. 6, top panel), in close alignment with the increasing CRP levels and decreasing NO and H2S levels. Nitrotyrosine level then steeply decreased at day-14 post COVID-19 infection (52.97 nM) with corresponding increase in NO and H2S levels.
Fig. 6.
Single case of COVID-19 infection and its association with CRP, NO and sulfide levels. Nitrotyrosine levels of the subject pre-COVID, during and post-COVID at 5 days and 14 days (top panel); CRP levels during and post-COVID, 3 days and 5 days (middle panel); NO and sulfide levels before, during and post-COVID at 5 days and 14 days (bottom panel).
3.7. Nitric oxide as an indicator of COVID-19 infection
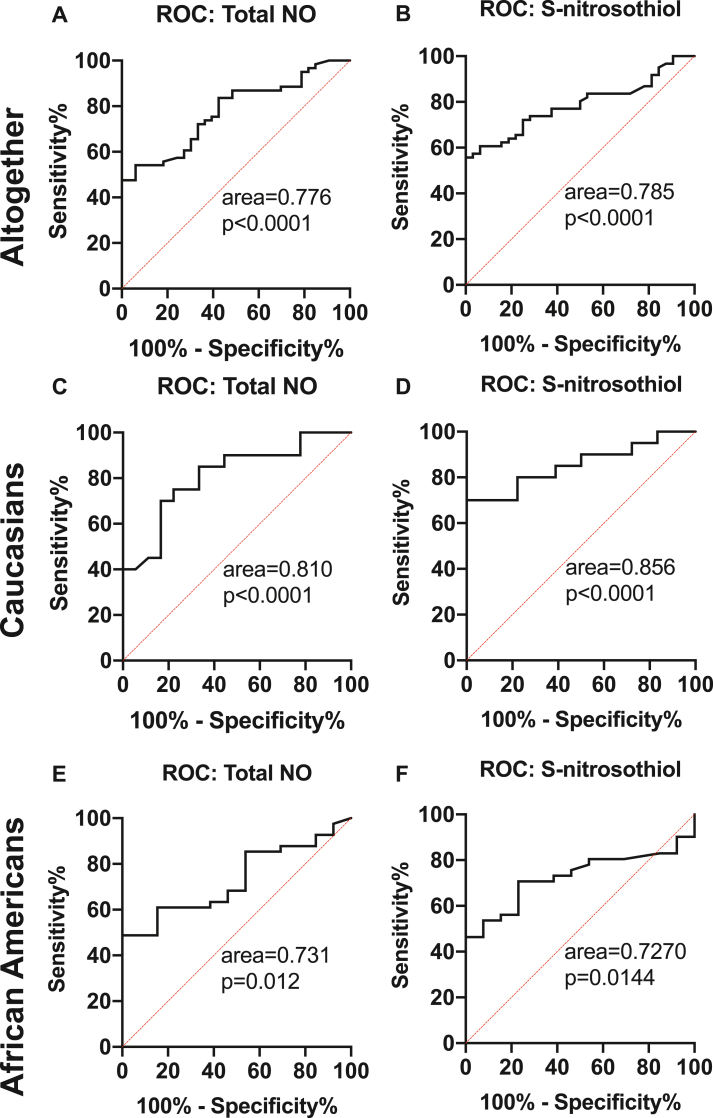
We performed receiver-operating characteristic analysis (ROC) (Fig. 7, Suppl Fig 1) to determine the accuracy of reduced NO levels as an indicator of COVID-19. Analysis of plasma NO and its metabolites between COVID-19 patients and controls revealed areas under the curve (AUC) of 0.776 (p<0.0001), 0.640 (p = 0.02), and 0.785 (p<0.0001) for total NO, free nitrite, and SNO, respectively (Fig. 7A and B; Suppl. Fig.1A). Plasma NO metabolites were then analyzed based on race in Caucasian and AA subjects, and found to be a stronger indicator of COVID-19 infection in Caucasian patients (AUC of 0.810, p<0.0001; 0.703, p = 0.03; and 0.856, p<0.0001 (Fig. 7D and E; Suppl. Fig.1B) for total NO, free nitrite, and SNO, respectively) compared to AA (AUC 0.731, p = 0.012 and 0.727, p = 0.014 total and SNO, respectively (Fig. 7G, I)). However, free nitrite levels in AA subjects did not show any significant predictability for COVID-19 infection (AUC of 0.547, p = 0.625, Suppl. Fig. 1C).
Fig. 7.
Receiver-operating characteristic analysis (ROC) of NO metabolites in controls vs COVID. ROC curves with area under the curve of Total NO, free nitrite and S-nitrosothiol – altogether (A–C); Caucasians (D–F) and African American (G–I) populations respectively.
3.8. Sulfide pools as indicators of COVID-19 infection
We next performed ROC with sulfide and its metabolites by analyzing the AUC in healthy controls and COVID-19 patients. Free sulfide with an AUC of 0.8697 (95% CI - 0.7878- 0.9517, p < 0.0001) was a strong predictor of COVID-19 in the overall study population (Fig. 8A). A free sulfide of 0.30 μM or below had a sensitivity of 96% and a specificity of 33% of predicting COVID-19 infection and a level of 0.24 μM or below had a sensitivity of 91% with a specificity of 67%. Total sulfide was also fairly able to predict COVID-19 infection with an AUC of 0.753(p<0.0001, Fig. 8B). We further analyzed the accuracy of reduced sulfide levels as a predictor of COVID-19 based on race in Caucasian and AA subjects. We found that free sulfide was a powerful predictor of COVID-19 infection in Caucasians with an AUC of 0.915 (p<0.0001, Fig. 8C). A free sulfide level of 0.30 μM or less was 94% sensitive and 58% specific in predicting COVID-19 infection in Caucasians. Total sulfide with an AUC of 0.8041 (p = 0.0016) in Caucasians was a fair predictor of COVID-19 infection in this population. Total and free sulfide with an AUC of 0.7873 (p<0.0001) and 0.8276 (p<0.0001) respectively were good predictors of COVID-19 infection in AA patients (Fig. 8E and F). With a free sulfide level of 0.30 μM or below, the sulfide levels were able to predict COVID-19 with 95% sensitivity and 14% specificity in AA.
Fig. 8.
Receiver-operating characteristic analysis (ROC) of Sulfide in controls vs COVID. ROC curves with area under the curve of Free and total sulfides of COVID-19 subjects altogether (A, B); Caucasian population (C, D); and African American populations (E, F) respectively.
3.9. Correlation and regression analyses between COVID-19 severity and gasotransmitters
Independent sample Student’s t-tests were performed to study the association between biomarkers (cardiac injury, thrombosis, and inflammatory) and NO and H2S levels in the COVID-19 cases (Table 3). LDH levels ≤260 U/L compared to LDH >260 U/L showed significant differences in NO levels (210.50 ± 64.41 nM vs 323.16 ± 167.63 nM; p = 0.004) (Table 3). In addition, there was a trend towards a difference in NO levels in patients with low and high levels of lactate (235.65 ± 64.79 nM vs 311.26 ± 177.96 nM, p = 0.052) and procalcitonin (251.34 ± 102.73 nM vs 334.70 ± 165.37 nM, p = 0.053). Based on the level of respiratory support, COVID-19 patients were categorized as mild-to-moderately ill or severely ill. Compared to patients with mild-to-moderately severe COVID-19 illness, patients with severe illness had slightly elevated NO (274.02 ± 133.67 nM (n = 46) versus 314.68 ± 154.25 nM (n = 22), p = 0.299). Similarly, patients who died had significantly higher levels of NO compared to levels in patients who survived (263.65 ± 124.34 nM (n = 60) versus 464.43 ± 137.41 nM (n = 8), p<0.0001).
Table 3.
NO and H2S levels based on biomarkers and disease severity.
| NO Levels (nM) |
p-Value | H2S Levels (μM) |
p-Value | |||
|---|---|---|---|---|---|---|
| Mean (n) | ±SD | Mean (n) | ±SD | |||
| Troponin | 0.14 | 0.64 | ||||
| Troponin ≤0.04 ng/ml | 273.87(30) | 137.36 | 1.17 (31) | 0.21 | ||
| Troponin >0.04 ng/ml | 347.54 (13) | 176.18 | 1.14 (15) | 0.25 | ||
| D-Dimer | 0.08 | 0.85 | ||||
| D-Dimer ≤1000 ng/ml | 240.00 (14) | 111.99 | 1.14 (15) | 0.15 | ||
| D-Dimer >1000 ng/ml | 326.45 (31) | 163.98 | 1.13 (36) | 0.24 | ||
| Ferritin | 0.43 | 0.94 | ||||
| Ferritin ≤600 ng/ml | 266.12 (17) | 141.11 | 1.14(18) | 0.13 | ||
| Ferritin >600 ng/ml | 300.94 (35) | 153.24 | 1.14(40) | 0.23 | ||
| CRP | 0.56 | 0.76 | ||||
| CRP ≤ 1.8 mg/dl | 325.33 (9) | 138.17 | 1.15 (9) | 0.21 | ||
| CRP > 1.8 mg/dl | 294.62 (37) | 141.57 | 1.18 (43) | 0.21 | ||
| LDH | 0.004 | 0.22 | ||||
| LDH ≤ 260 U/L | 210.50 (10) | 64.41 | 1.08 (11) | 0.17 | ||
| LDH > 260 U/L | 323.16 (31) | 167.63 | 1.17 (35) | 0.22 | ||
| BNP | 0.21 | 0.11 | ||||
| BNP ≤ 1000 pg/ml | 255.00 (15) | 131.61 | 1.09 (16) | 0.12 | ||
| BNP > 1000 pg/ml | 322.53 (17) | 163.45 | 1.19 (20) | 0.21 | ||
| RDW | 0.16 | 0.92 | ||||
| RDW ≤ 14.5% | 265.86 (36) | 109.38 | 1.14 (37) | 0.23 | ||
| RDW > 14.5% | 323.17 (24) | 174.51 | 1.15 (30) | 0.18 | ||
| Lactate | 0.05 | 0.10 | ||||
| Lactate ≤ 1.25 mmol/L | 235.65 (17) | 64.79 | 1.08 (17) | 0.22 | ||
| Lactate> 1.25 mmol/L | 311.26 (27) | 177.96 | 1.19 (31) | 0.21 | ||
| Procalcitonin | 0.05 | 0.14 | ||||
| Procalcitonin ≤0.5 ng/ml | 251.34 (29) | 102.73 | 1.12 (31) | 0.23 | ||
| Procalcitonin >0.5 ng/ml | 334.70 (20) | 163.57 | 1.21 (23) | 0.19 | ||
| Severity of COVID | 0.29 | 0.009 | ||||
| Mild - Moderate | 274.02 (46) | 133.67 | 1.10 (46) | 0.17 | ||
| Severe | 314.68 (22) | 154.25 | 1.23 (22) | 0.24 | ||
| Outcome | <0.001 | 0.013 | ||||
| Alive | 263.65 (60) | 124.34 | 1.11 (60) | 0.17 | ||
| Expired | 464.43 (8) | 137.41 | 1.40 (8) | 0.25 | ||
CRP - C-Reactive Protein; LDH - Lactate Dehydrogenase; BNP - Brain Natriuretic Peptide; RDW - Red cell Distribution Width.
We further analyzed the relationship between biomarkers and H2S levels in the COVID-19 patients and found a significant increase in H2S levels (1.10 ± 0.17 μM versus 1.23 ± 0.24 μM, p = 0.009) in patients with severe COVID-19 illness compared to those with mild-to-moderately severe COVID-19 illness. Levels of H2S significantly increased in expired patients compared to levels in those who survived (1.11 ± 0.17 μM versus 1.40 ± 0.25 μM, p<0.013). To assess if the higher NO and H2S levels in sicker COVID-19 patients and COVID-19 patients who expired reflected a higher demand due to advanced oxidant stress, we compared nitrotyrosine levels in mild to moderately ill COVID-19 patients and patients who survived the COVID-19 infection to severely ill COVID-19 patients and COVID-19 patients who succumbed to their illness. Severely ill COVID-19 patients had significantly higher nitrotyrosine levels compared to mild to moderately ill patients (128.76 ± 55.55 nM versus 93.51 ± 60.95 nM, p = 0.04). Similarly, patients who died from COVID-19 infection had a trend towards a higher nitrotyrosine level compared to patients who survived (139.45 ± 59.26 nM versus 99.96 ± 60.40 nM, p = 0.11). The patients who had higher levels of cardiac, inflammatory, and thrombosis biomarkers had higher NO and H2S levels although most were non-significant (Table 3).
We performed multivariable regression analysis to identify any association between comorbidities and total NO and sulfide levels (Table 4). It is worth noting that we did not find any further association between NO and H2S levels and cardiovascular risk factors, including age, race, sex, diabetes, and hypertension (Table 4).
Table 4.
Multivariable regression analysis of association of demographics and comorbidities with total NO and sulfide levels.
| Multivariable Regression analysis in COVID positive cases- Total Nitric Oxide | ||
|---|---|---|
| Risk Factor | Coefficient±SD | p-Value |
| Age | 0.35 ± 1.45 | 0.81 |
| Race | -2.31 ± 38.76 | 0.95 |
| Gender | 51.41 ± 40.3 | 0.21 |
| Diabetes Mellitus | -32.29 ± 44.62 | 0.47 |
| Hypertension | 27.03 ± 49.1 | 0.58 |
| Multivariable Regression analysis in COVID positive cases- Total Sulfide | ||
| Risk Factor | Coefficient±SD | p-Value |
| Age | 0.0 | 0.8 |
| Race | -0.02 ± 0.06 | 0.68 |
| Gender | -0.04 ± 0.06 | 0.49 |
| Diabetes Mellitus | 0.04 ± 0.06 | 0.5 |
| Hypertension | -0.07 ± 0.07 | 0.31 |
4. Discussion
The gasotransmitters NO and H2S have overlapping pathophysiological roles with significant influence in regulating cardio– and vaso– protective functions and possessing anti-inflammatory, anti-thrombotic, and antiviral properties[7,[18], [19], [20], [21]]. While researchers have pondered the possible use of NO and H2S in the treatment of COVID-19, studies exploring the availability of these two gasotransmitters in COVID-19 patients are limited[11,13,14,[22], [23], [24]]. For the first time, our study analyzed and compared both NO and sulfide metabolites in healthy subjects and COVID-19 patients and observed a significant and parallel reduction in both NO and sulfide metabolites in the COVID-19 patients compared to controls (Fig. 1, Fig. 2).
NO plays a key protective role in limiting the severity of cardiovascular disease (CVD), and as a selective pulmonary vasodilator, improves pulmonary function in subjects with acute and chronic pulmonary hypertension [24]. Previously, NO has been negatively associated with viral replication in severe acute respiratory syndrome (SARS/SARS-CoV) [10,25,26]. In vitro studies with SARS-CoV suggested that NO has anti-viral properties as shown by its specific inhibition of the viral replication cycle [25,26]. Chen et al. demonstrated the favorable effect of inhaled NO on arterial oxygenation in patients with acute respiratory distress syndrome [10]. Similar to SARS-CoV-1, SARS-CoV-2 infects the upper respiratory tract, but with increased complications mediated through vascular inflammation and injury. It has been predicted that COVID-19 mortality could be associated with decreased endothelial NO production and availability [27]. Based on earlier reports from studies of SARS-CoV-1, the inhibitory effect of NO on SARS-CoV-2 has been evaluated recently in vitro and found to promote significant reduction in SARS-CoV-2 protease activity [9]. Although there are now quite a few clinical trials using NO therapy to alleviate viral pneumonia and the bronchopulmonary effects of SARS-CoV-2 [28], interestingly, there have been no reports suggesting a decrease in NO availability in COVID-19 patients. Recently, a study by Alamdari et al. showed a significant increase in NO levels in 25 COVID-19 patients in ICU compared to non-infected controls [28] but did not include data from mildly ill COVID-19 patients. In contrast, our study found significantly lower NO metabolites in patients with COVID-19 infections of different severities compared to controls.
H2S is another gasotransmitter with antiviral properties that is cardioprotective, anti-inflammatory, and antioxidant [8,29,30]. Considering its varied functions, it has been contemplated as a possible therapy in COVID-19 infection [11,22,31]. We have previously reported H2S availability as a predictive biomarker for cardiovascular disease in a race- and sex-based manner [16]. A recent study has suggested a correlation between the severity of SARS‐CoV‐2 infection, cytokine production, and H2S plasma levels [13]. H2S levels were significantly reduced in deceased patients compared to those who survived following COVID-19 infection, suggesting a possible role of H2S in the outcome of pneumonia caused by SARS‐CoV‐213. However, that study was limited to COVID-19 patients with viral pneumonia and did not include non-infected controls. In a biological system, H2S can be present in various forms including free, acid labile, and bound sulfane sulfur that regulate and contribute to the total amount of bioavailable sulfide [16]. For the first time, we demonstrate that all of these sulfide biochemical forms are significantly reduced in COVID-19 patients compared to healthy controls (Fig. 2). The interaction between H2S and NO can be complex and could range from synergism, based on evidence from the cardiovascular disease models [32,33] to antagonistic regulation of each other found in inflammatory cells [34], especially in pulmonary infections [35]. Our finding that both H2S and NO are reduced in COVID-19 infection simultaneously hints at a more synergistic role for these two gasotransmitters in this context.
There are known variations in NO and H2S levels based on race in vascular disease patients [16,36]. ROC analyses with NO showed a significantly predictable relationship between COVID-19 and NO levels, including total NO, free nitrite, and SNO metabolites in all of the COVID-19 subjects, irrespective of race (Fig. 6A–C). Interestingly, sulfide metabolites, especially total sulfide and free sulfide, were more predictive of COVID-19 infection than NO metabolites. ROC analysis of free sulfide showed that a free sulfide level of 0.30 μM was 96% sensitive and 33% specific in predicting COVID-19 infection in the general population; >94% sensitive and 58% specific in the Caucasian population; and 95% sensitive and 14% specific in the AA population. Assuming a roughly 10% prevalence of COVID-19 infection in the United States, free sulfide levels of 0.30 μM predicted COVID-19 infection with a positive predictive value (PPV) of 14% but a negative predictive value (NPV) of 99% in the general population and a PPV of 20% and a NPV of 99% in Caucasians, suggesting that higher free sulfide levels can rule out COVID-19 infection with certainty. The majority of the control population in this study was healthy and did not have significant comorbidities, while 25% of COVID-19 cases had CVD. Previously, we have shown that while the levels of other sulfide metabolites in the plasma are decreased with cardiovascular disease, free sulfide levels are elevated in these patients [37,38] Therefore, the finding that free sulfide levels are significantly reduced in and are the best predictors of COVID-19 infection in the COVID-19 cases with 25% CVD prevalence assumes prominence.
Elevated levels of multiple biomarkers including lactate dehydrogenase (LDH) and procalcitonin were associated with poor outcomes in COVID-19 infection [39]. We therefore analyzed the effects of various inflammatory and cardiovascular biomarkers on NO and H2S in COVID-19 patients (Table 3). We saw a significant association between LDH and NO levels in COVID-19 infected subjects (Table 3). Surprisingly, patients with LDH levels >260 U/L had higher total NO levels compared to patients with LDH levels ≤260 U/L. NO also showed a significant association with mortality, with increased NO levels in expired COVID-19 subjects compared to patients who survived. This is in agreement with the findings in the study by Alamdari et al. [28] Similarly, COVID-19 patients who were severely ill or expired had a significantly higher plasma H2S levels compared to patients who were mild-to-moderately ill or survived. Although the gasotransmitter levels were significantly reduced in COVID-19 patients compared to controls, it is unclear why sicker COVID-19 patients had relatively elevated levels compared to less sick patients. One possible explanation is that the elevated NO and H2S levels in sicker COVID-19 patients is a last-ditch compensatory response to the severely noxious effects of the COVID-19 infection. Another reason could be a hypothetical inability to utilize or underutilization of NO and H2S to reduce oxidative stress leading to poor outcomes. NO-derived oxidant generation can also reduce NO availability, thereby reducing its levels. Peroxynitrite is one such oxidant that promotes nitration of protein tyrosine residues such as nitrotyrosine [40]. We observed a significant increase in nitrotyrosine levels in the plasma of COVID-19 patients (Fig. 5) in conjunction with reduced NO levels. In addition, severely ill COVID-19 patients had significantly higher nitrotyrosine levels compared to mild-moderately ill COVID-19 patients and COVID-19 patients who died had a trend towards higher nitrotyrosine levels compared to COVID-19 patients who survived, lending credibility to this hypothesis. Finally, changes in circulating NO levels could reflect alterations in nitric oxide synthase (NOS). Decreased NO availability generally can be attributed to reduced eNOS; whereas, iNOS is correlated with high NO production [41,42]. A direct association of eNOS and iNOS, including eNOS polymorphisms have been proposed to critically regulate defense against SARS-CoV-2 and COVID-19 severity [42]. While iNOS is likely activated by the inflammation and cytokine storm caused by COVID-19, our finding that total NO levels in patients with COVID-19 is low could suggest an overwhelming effect of COVID-19 on endothelial NOS, resulting in high oxidant stress which in turn could possibly result in NOS uncoupling. The variations in the level of iNOS between moderately and severely ill COVID-19 patients could also explain the differences in NO levels in these patient groups and the findings in our study compared to the study by Alamdari et al. However, future studies focusing on the enzyme expression and activity, and their polymorphisms, including their substrate availability in COVID-19 patients are warranted.
Comorbidities in COVID-19 patients may be associated with increased hospitalizations, complications, and mortality [[43], [44], [45]]. Therefore, we used multivariable regression analyses to find the association between the gasotransmitters NO and H2S and other risk factors (Table 3) in COVID-19 positive cases. Remarkably, there were no further differences in either NO or sulfide metabolites with patient demographics or cardiovascular comorbidities known to affect their levels, including age, race, sex, diabetes, and hypertension (Table 4), suggesting that the effect of COVID-19 on these gasotransmitters was overwhelming, leaving no room for variations.
5. Conclusion and future directions
In summary, our findings reveal that the availability NO and sulfide metabolites is significantly reduced in individuals with COVID-19 infection but is not affected by comorbidities. In addition, reduced free sulfide levels have a high sensitivity in predicting COVID-19 infection in the study population regardless of race. Based on a case study within the cohort, inflammatory and oxidative stress markers CRP and nitrotyrosine, were inversely related to and NO/H2S availability with the onset of COVID-19 infection, which should be studied in a wider population. Overall, our study further substantiates the need for NO as a therapeutic modality for COVID-19, consistent with ongoing clinical trials. Additionally, our study also suggests exogenous H2S therapy as a pharmacological strategy at least in mild to moderate COVID-19 disease, to restore its availability and counteract the severe consequences of COVID‐19 infection. Finally, based on this association of decreasing NO and H2S availability with COVID-19 infection, it is worth exploring these gasotransmitters as potential protective factors and novel therapeutic alternatives.
Clinical implications
-
•
NO and H2S are prognostic biomarkers in COVID-19 infection.
-
•
Supplementation with H2S-based drugs as a therapeutic approach may have potential protective effects in COVID-19 infection.
Declaration of competing interest
P.Dominic, Gopi K Kolluru, A Wayne Orr and C.Kevil have a pending provisional patent application for the use of hydrogen sulfide and nitric oxide compounds in the treatment of Covid-19. Other authors do not have any conflict of interest to disclose.
Acknowledgements
We thank Georgia Morgan for her assistance editing this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101982.
Contributor Information
Paari Dominic, Email: pdomi2@lsuhsc.edu.
Gopi K. Kolluru, Email: gkollu@lsuhsc.edu.
Funding support
This work was supported by LSUHSC Shreveport Research Council COVID-19 Intramural Grant awarded to P. Dominic. and G.K. Kolluru; by HL098435, HL133497, and HL141155 to A.W. Orr, an Institutional Development Award from the National Institutes of General Medical Sciences of the National Institutes of Health (NIH) under grant number P20GM121307, and a R01HL149264 grant to C.G. Kevil.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., Rajagopal S., Pai A.R., Kutty S. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front. Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potus F., Mai V., Lebret M., Malenfant S., Breton-Gagnon E., Lajoie A.C., Boucherat O., Bonnet S., Provencher S. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;319:L277–L288. doi: 10.1152/ajplung.00195.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolluru G.K., Shen X., Yuan S., Kevil C.G. Gasotransmitter heterocellular signaling. Antioxidants Redox Signal. 2017;26:936–960. doi: 10.1089/ars.2016.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajendran S., Shen X., Glawe J., Kolluru G.K., Kevil C.G. Nitric oxide and hydrogen sulfide regulation of ischemic vascular growth and remodeling. Comp. Physiol. 2019;9:1213–1247. doi: 10.1002/cphy.c180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akaberi D., Krambrich J., Ling J., Luni C., Hedenstierna G., Jarhult J.D., Lennerstrand J., Lundkvist A. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020;37:101734. doi: 10.1016/j.redox.2020.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L., Liu P., Gao H., Sun B., Chao D., Wang F., Zhu Y., Hedenstierna G., Wang C.G. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citi V., Martelli A., Brancaleone V., Brogi S., Gojon G., Montanaro R., Morales G., Testai L., Calderone V. Anti-inflammatory and antiviral roles of hydrogen sulfide: rationale for considering H2 S donors in COVID-19 therapy. Br. J. Pharmacol. 2020;177:4931–4941. doi: 10.1111/bph.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dattilo M. The role of host defences in Covid 19 and treatments thereof. Mol Med. 2020;26:90. doi: 10.1186/s10020-020-00216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renieris G., Katrini K., Damoulari C., Akinosoglou K., Psarrakis C., Kyriakopoulou M., Dimopoulos G., Lada M., Koufargyris P., Giamarellos-Bourboulis E.J. Serum hydrogen sulfide and outcome association in pneumonia by the SARS-CoV-2 coronavirus. Shock. 2020;54:633–637. doi: 10.1097/SHK.0000000000001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang G. H2S as a potential defense against COVID-19? Am. J. Physiol. Cell Physiol. 2020;319:C244–C249. doi: 10.1152/ajpcell.00187.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolluru G.K., Yuan S., Shen X., Kevil C.G. H2S regulation of nitric oxide metabolism. Methods Enzymol. 2015;554:271–297. doi: 10.1016/bs.mie.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajpal S., Katikaneni P., Deshotels M., Pardue S., Glawe J., Shen X., Akkus N., Modi K., Bhandari R., Dominic P., Reddy P., Kolluru G.K., Kevil C.G. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biol. 2018;15:480–489. doi: 10.1016/j.redox.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akaike T., Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazhanov N., Ansar M., Ivanciuc T., Garofalo R.P., Casola A. Hydrogen sulfide: a novel player in airway development, pathophysiology of respiratory diseases, and antiviral defenses. Am. J. Respir. Cell Mol. Biol. 2017;57:403–410. doi: 10.1165/rcmb.2017-0114TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swiatkowska M., Cierniewska-Cieslak A., Pawlowska Z., Cierniewski C.S. Dual regulatory effects of nitric oxide on plasminogen activator inhibitor type 1 expression in endothelial cells. Eur. J. Biochem. 2000;267:1001–1007. doi: 10.1046/j.1432-1327.2000.01086.x. [DOI] [PubMed] [Google Scholar]
- 21.Emerson M. Hydrogen sulfide and platelets: a possible role in thrombosis. Handb. Exp. Pharmacol. 2015;230:153–162. doi: 10.1007/978-3-319-18144-8_7. [DOI] [PubMed] [Google Scholar]
- 22.Evgen'ev M.B., Frenkel A. Possible application of H2S-producing compounds in therapy of coronavirus (COVID-19) infection and pneumonia. Cell Stress Chaperones. 2020;25:713–715. doi: 10.1007/s12192-020-01120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alamdari D.H., Moghaddam A.B., Amini S., Keramati M.R., Zarmehri A.M., Alamdari A.H., Damsaz M., Banpour H., Yarahmadi A., Koliakos G. Application of methylene blue -vitamin C -N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur. J. Pharmacol. 2020;885:173494. doi: 10.1016/j.ejphar.2020.173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sim J.Y. Nitric oxide and pulmonary hypertension. Korean J Anesthesiol. 2010;58:4–14. doi: 10.4097/kjae.2010.58.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akerstrom S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akerstrom S., Mousavi-Jazi M., Klingstrom J., Leijon M., Lundkvist A., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozdemir B., Yazici A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Med. Hypotheses. 2020;144:109970. doi: 10.1016/j.mehy.2020.109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez R.A., Berra L., Gladwin M.T. Home nitric oxide therapy for COVID-19. Am. J. Respir. Crit. Care Med. 2020;202:16–20. doi: 10.1164/rccm.202005-1906ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 30.Kolluru G.K., Shen X., Kevil C.G. Reactive sulfur species: a new redox player in cardiovascular pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2020;40:874–884. doi: 10.1161/ATVBAHA.120.314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang G. H2S as a potential defense against COVID-19? Am. J. Physiol. Cell Physiol. 2020;319:C244–C249. doi: 10.1152/ajpcell.00187.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolluru G.K., Shen X., Kevil C.G. A tale of two gases: NO and H2S, foes or friends for life? Redox Biol. 2013;1:313–318. doi: 10.1016/j.redox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo Faro M.L., Fox B., Whatmore J.L., Winyard P.G., Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47. doi: 10.1016/j.niox.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Oh G.S., Pae H.O., Lee B.S., Kim B.N., Kim J.M., Kim H.R., Jeon S.B., Jeon W.K., Chae H.J., Chung H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Saini V., Chinta K.C., Reddy V.P., Glasgow J.N., Stein A., Lamprecht D.A., Rahman M.A., Mackenzie J.S., Truebody B.E., Adamson J.H., Kunota T.T.R., Bailey S.M., Moellering D.R., Lancaster J.R., Jr., Steyn A.J.C. Hydrogen sulfide stimulates Mycobacterium tuberculosis respiration, growth and pathogenesis. Nat. Commun. 2020;11:557. doi: 10.1038/s41467-019-14132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams S.F., Nicholas S.B., Vaziri N.D., Norris K.C. African Americans, hypertension and the renin angiotensin system. World J. Cardiol. 2014;6:878–889. doi: 10.4330/wjc.v6.i9.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peter E.A., Shen X., Shah S.H., Pardue S., Glawe J.D., Zhang W.W., Reddy P., Akkus N.I., Varma J., Kevil C.G. Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watts M., Kolluru G.K., Dherange P., Pardue S., Si M., Shen X., Trosclair K., Glawe J., Al-Yafeai Z., Iqbal M., Pearson B.H., Hamilton K.A., Orr A.W., Glasscock E., Kevil C.G., Dominic P. Decreased bioavailability of hydrogen sulfide links vascular endothelium and atrial remodeling in atrial fibrillation. Redox Biology. 2021;38:101817. doi: 10.1016/j.redox.2020.101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., Gabrilove J.L., Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2020 doi: 10.1136/bmjebm-2020-111536. bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shishehbor M.H. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 41.Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., Ghiladi R.A., Wang J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021;163 doi: 10.1016/j.freeradbiomed.2020.12.008. 153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan S.P., Seet R.C.S., Kennedy B.K. Does eNOS derived nitric oxide protect the young from severe COVID-19 complications? Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101201. 101201-101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh A.K., Gillies C.L., Singh R., Singh A., Chudasama Y., Coles B., Seidu S., Zaccardi F., Davies M.J., Khunti K. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes. Metabol. 2020 doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.