Abstract
Objectives
The worldwide spread of coronavirus disease 2019 (COVID-19) highlights the need for assessment of long-term humoral immunity in convalescent subjects. Our objectives were to evaluate long-term IgG antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and B-cell memory response in COVID-19 convalescent subjects.
Methods
Blood samples were collected from a cohort of subjects recovering from COVID-19 and from healthy subjects who donated blood. SARS-CoV-2 IgG antibodies were quantitatively detected by ELISA using anti-S1 spike IgG. SARS-CoV-2 spike-specific IgG memory B cells were evaluated by reversed B-cell FluroSpot based on human IgG SARS-CoV-2 receptor-binding domain in a randomly selected group of subjects recovering from COVID-19. Statistical analysis was performed with clinical variables and time post COVID-19 infection.
Results
Antibody response was not detected in 26 of 392 COVID-19 convalescent subjects (6.6%). Over a period of 9 months, the level of antibodies decreased by 50% but stabilized at 6 months, and a protective level prevailed for up to 9 months. No differences were found regarding IgG SARS-CoV-2 antibody levels for age, gender, and major blood types over time. Over time, asymptomatic COVID-19 subjects did not differ in antibody level from subjects with mild to severe disease. Repeated paired IgG SARS-CoV-2 antibody level analyses disclosed that, over 6 and 9 months, 15.3% (nine of 59) and 15.8% (three of 19) of subjects became SARS-CoV-2 IgG-seronegative, respectively, all with a low antibody level at 3 months. Rate of antibody decline was not affected by age, gender, or clinical symptomatology. In a subgroup of recovering subjects, memory B-cell response up to 9 months post-COVID-19 infection was undetectable in 31.8% of subjects (14/44), and there was no correlation with age, SARS-CoV-2 antibody level, or time post infection.
Conclusions
The majority of convalescent COVID-19 subjects develop an IgG SARS-CoV-2 antibody response and a protective level prevails over a period of up to 9 months, regardless of age, gender, major blood types or clinical symptomatology.
Keywords: Anti-SARS-CoV-2 IgG, COVID-19, Memory B-Cells, Time-related humoral response, Antibody
Introduction
Coronavirus disease 2019 (COVID-19) is a devastating severe disease responsible for over 140 million infections since its emergence in December 2019, leading to high rates of morbidity and mortality [1]. The basis of protective immunity after infections includes the production of an antigen-specific antibody response, and the generation of a memory adaptive immune response mediated by B and T cells [2,3]. Little is known about the development of long-term humoral immunity and the generation of memory B cells following COVID-19 infection, or about the ease of activating a humoral immune response during recurrent infection. Factors that determine the fate of activated B cells after primary antigen encounter need to be investigated to shed light on the following: (a) whether COVID-19 infection can lead to sustained antibody protection, and whether the absence of specific S1 spike antibodies necessarily mean absence of immune response, (b) what is the longevity of this humoral response, and (c) what is the magnitude of immune protection for individuals in whom the early post-infection humoral immunity has decayed over time. Better understanding of the humoral response and testing the behaviour of memory B cells following COVID-19 infection are crucial for planning future trajectories and will likely enhance our ability to overpower the disease. In the current study we longitudinally portrayed the antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in symptomatic and asymptomatic COVID-19 convalescent subjects, and we assessed memory B-cell response in relation to time post infection.
Methods
Participants
Blood samples were collected from a cohort of subjects recovering from COVID-19 and from healthy subjects who donated blood.
Convalescent subjects were recruited through local networking and local media, and all had a prior diagnosis of COVID-19 disease by positive reverse transcriptase polymerase chain reaction (RT-PCR) detection of SARS-CoV-2 RNA in nasopharyngeal swabs. Antibody response was measured at multiple time points defined as months after positive SARS-CoV-2 RNA nasopharyngeal swab, as follows: 0–1, 1–2, 2–3, 3–4, 4–5, 5–6, 6–7, 7–8, and 8–9 months. In a sub-cohort the same subjects were followed over time and samples were collected respectively.
Ethics statement
This study was approved by Sheba Institutional Review Board SMC-750320), and informed consent was obtained from all enrolled participants. Each patient record was coded for anonymity to ensure confidentiality during statistical analyses.
Detection of SARS-CoV-2 IgG antibodies
Immunoassay for the detection of SARS-CoV-2 IgG antibodies in blood samples was performed using Euroimmun (EI, Lubeck, Germany) anti-SARS-CoV-2 IgG quantitative ELISA kit based on a recombinant S1 subunit of the SARS-CoV-2 spike protein. The test has a sensitivity of 90% and a specificity of 100%. Tests were performed in accordance with the manufacturer's instructions, using an AGILITY® automated ELISA analyser (DYNEX Technologies Inc., Chantilly, CA, USA), and optical density was measured at 450 nm. Results are presented as a range from 0 to 15, and a value > 0.8 was considered positive [4].
Analysis was performed at various time periods following positive RT-PCR detection of SARS-CoV-2 RNA in nasopharyngeal swabs.
Detection of COVID-19 memory B-cells
Cross-sectional analysis of SARS-CoV-2 spike-specific IgG memory B cells was performed in a randomly selected group of subjects recovering from COVID-19. We used reversed antigen human IgG SARS-CoV-2 receptor-binding domain (RBD) ELISpotPLUS (ALP kit, Mabtech, Sweden) according to the manufacturer's instructions. Briefly, peripheral-blood mononuclear cells (PBMCs) were incubated (250 000 cells/well) on an anti-IgG FluoroSpot plate after stimulation with a mixture of Toll-like receptor agonist R848 (resiquimod, 1 mg/mL) and IL2 (10 ng/mL) (B-Cell stimpack, Mabtech, Sweden).
The number of SARS-CoV-2-specific IgG-secreting B cells was measured as spot-forming units (SFUs) using a Mabtech IRIS-TM reader. The results were expressed as the SFUs per 250 000 seeded cells after subtracting the background of unstimulated cells. The positive cut-off value was determined as >10 SFUs (the highest number of SFUs in COVID-19-uninfected healthy subjects, n = 5).
Statistical analysis
Continuous variables were evaluated for a normal distribution and described as medians and interquartile ranges (IQRs). Categorical variables were described as numbers and percentages. To compare the relative change in SARS-CoV-2 IgG antibody measurements between various time points, the two-tailed, non-parametric Mann–Whitney U test and the Kruskal–Wallis test were used. The rate of IgG SARS-CoV-2 antibody decline was calculated as the change in SARS-CoV-2 IgG level divided by the time difference between the repeated tests. Pearson correlation was applied for assessing the time from positive SARS-CoV-2 nasopharyngeal swab and antibody level. The paired T-test was applied (paired observations) to evaluate the statistical significance of SARS-CoV-2 IgG antibody measurements in the same subjects at different time points. A one-way ANCOVA was conducted to compare between groups whilst adjusting for time following positive RT-PCR detection of SARS-CoV-2 RNA in nasopharyngeal swabs. A multivariate linear regression model using the enter approach was applied for simultaneously assessing the effects of independent variables on a quantitative dependent variable; p < 0.05 was considered statistically significant. Statistical analyses were performed using Python, IBM SPSS and R software (versions 3.0, 24, and 3.6.0, respectively).
Results
Participants
We screened for SARS-CoV-2 antibodies 392 COVID-19 convalescent subjects, 284 males (72.4%) and 108 females (27.6%), median (25–75 IQR) age 33.4 (22.2–45.5) years, and 180 healthy blood donors, 82 males (45.5%) and 98 females (54.5%), median (25–75 IQR) age 29.7 (17.2–45.9) years.
Detection of SARS-CoV-2 IgG
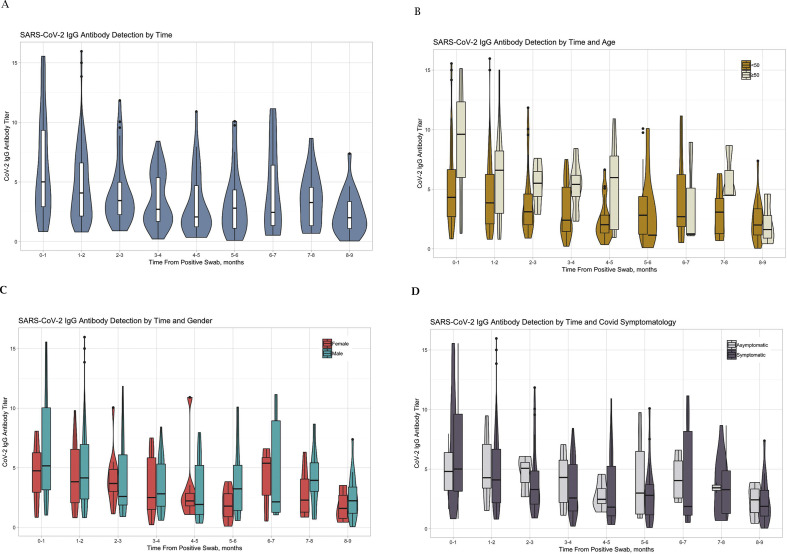
Of the 392 COVID-19 convalescent subjects, 366 (93.4%) were positive for SARS-CoV-2 IgG antibodies. There were 26 COVID-19 convalescent subjects (6.6%) who, although positive for RT-PCR SARS-CoV-2 RNA by nasopharyngeal swab, did not develop a humoral response and had no detectable SARS-CoV-2 IgG antibodies within a median (IQR) 1.63 (2.49) months after the infection. All healthy subjects were negative for SARS-CoV-2 IgG antibodies, with an IgG level median (25–75 IQR) of 0.17 (0.10–0.23). In convalescent subjects, SARS-CoV-2 IgG antibody titer distribution (median and 25–75 IQR) by time from positive nasopharyngeal swab is shown in Fig. 1 A; the median (25–75 IQR) SARS-CoV-2 IgG antibody levels by time from infection are presented in Table 1 . Time from positive SARS-CoV-2 nasopharyngeal swab correlated with SARS-CoV-2 IgG antibody level (Pearson r –0.281, p < 0.001). There was a decline in the level of antibodies by 50% within 6 months; however, the level was still high above the positive cut-off. Thereafter, up to 9 months post-infection, the antibody level stabilized and remained similar to that at the 6-month level.
Fig. 1.
Violin chart presentation (median and 25–75 IQR) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG antibody levels plotted against the time (months) since positive coronavirus disease 2019 (COVID-19) nasopharyngeal swab. (A) All subjects. Population stratified by: (B) age (>50 years, n = 70; ≤50 years, n = 318); (C) gender (females n = 108; males n = 284); and (D) clinical symptomatology (symptomatic, n = 358; asymptomatic, n = 34). The plots show the medians (middle line) and the first and third quartiles (boxes).
Table 1.
Severe acute respiratory disease coronavirus 2 (SARS-CoV-2) IgG antibody level by time from positive coronavirus disease 2019 (COVID-19) RT-PCR of nasopharyngeal swabs
| Time post infection (months) | Number of tested subjects n = 456 | SARS-CoV-2 IgG median (25–75 IQR) | pa |
|---|---|---|---|
| ≤1 | 75 | 5.0 (3.14–9.60) | _ |
| ≤3 | 217 | 4.1 (2.36–6.23) | 0.0032 |
| ≤6 | 109 | 2.4 (1.43–5.21) | 0.00006 |
| ≤9 | 55 | 2.9 (1.26–4.26) | 0.67 |
Compared with previous time measurement.
Demographic and clinical variables related to SARS-CoV-2 IgG antibody level
Assessing the effects of age, gender, blood type and clinical symptomatology on SARS-CoV-2 IgG antibody level demonstrated that in older subjects (≥50 years, n = 70), the level of SARS-CoV-2 IgG antibodies was higher than that in younger subjects (<50 years, n = 318), median (25–75 IQR) 6.2 (2.79–8.73) versus 3.3 (1.80–5.38), p < 0.01; similarly, male subjects (n = 284) had higher antibody levels compared to females (n = 108), 3.9 (1.99–6.64) versus 3.4 (1.78–5.17), p 0.015. After adjustment for the time following positive RT-PCR detection of SARS-CoV-2 RNA by nasopharyngeal swab, no statistical differences related to age and gender were found.
Of 392 COVID-19 convalescent subjects, 358 (91.3%) recovered from mild to severe disease, while 34 (8.7%) were asymptomatic and developed humoral IgG response without any clinical symptomatology. SARS-CoV-2 IgG antibody levels did not differ between asymptomatic subjects and symptomatic subjects: median (25–75 IQR) 3.5 (2.10–5.07) versus 3.7 (1.86–6.26), p 0.57.
Blood type analysis in 306 convalescent COVID-19 subjects disclosed similar SARS-CoV-2 IgG antibodies level, median (25–75 IQR), between blood groups as follows: type A (n = 124), 3.7 (2.10–6.12); type O (n = 107), 3.3 (1.71–5.77); type B (n = 52), 4.2 (2.27–6.33); type AB (n = 23), 5.4 (2.93–7.77), p 0.11.
Time-related changes stratified by age, gender and clinical symptomatology in relation to the level of SARS-CoV-2 IgG antibodies are presented in Fig. 1B–D.
Paired repeated SARS-CoV-2 IgG antibodies measurements
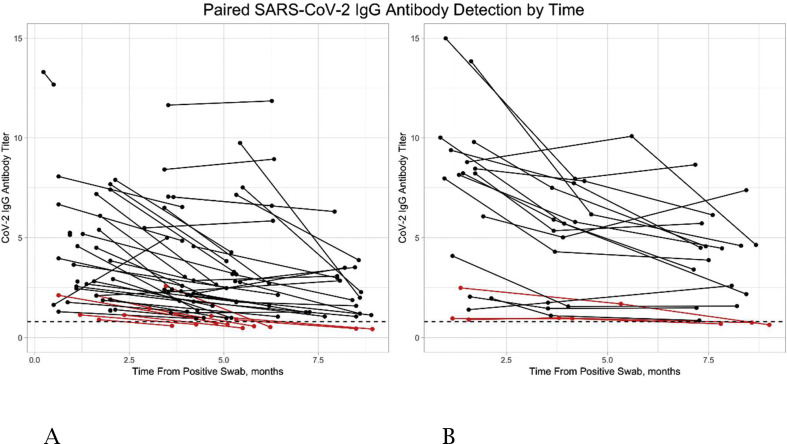
One hundred and seventy-five repeated SARS-CoV-2 IgG antibody measurements were performed in COVID-19 convalescent subjects: median (25–75 IQR) time to the first IgG test 1.7 (1.23–3.5) months, and to the second IgG test 5.1 (3.94–6.43) months. In 59 subjects two serial measurements were performed, and in 19 COVID-19 convalescent subjects three serial measurements were obtained (Fig. 2 A and B, respectively).
Fig. 2.
Paired repeated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG antibody levels over-time. (A) Paired SARS-CoV-2 IgG antibody levels plotted against the time (months) since positive nasopharyngeal swab in each subject. (B) SARS-CoV-2 IgG antibody levels plotted against the time (months) since positive coronavirus disease 2019 (COVID-19) nasopharyngeal swab in each subject. Red dots signify subjects who became seronegative.
In nine of 59 subjects (15.3%) with duplicate tests, SARS-CoV-2 IgG antibodies decreased below the positive cut-off, and they became negative within a median (25–75 IQR) of 5.5 (4.8–6.23) months. All had a low baseline SARS-CoV-2 IgG antibody titer median (25–75 IQR) of 1.1 (1.08–2.11). Similarly, in three of 19 subjects (15.8%) with triplicate repeated tests, SARS-CoV-2 IgG antibodies decreased below the positive cut-off, and their third test became negative within a median (25–75 IQR) of 8.6 (8.18–8.78) months. All had a low baseline titer median (25–75 IQR) of 0.96 (0.93–1.72).
Multivariate linear regression analysis disclosed that the only variable which predicted the decay of IgG SARS-CoV-2 antibodies over time was the previous IgG SARS-CoV-2 antibody level. The rate of IgG SARS-CoV-2 antibody decline over time was not affected by age, gender, or symptomatology.
SARS-CoV-2 spike-specific IgG memory B-cell response
In a randomly selected group of 44 convalescent COVID-19 subjects median (25–75 IQR) age 32.3 (19.2–47.9) years, 31 of whom were males, a B-cell memory response analysed 1–9 months post infection was detected in 68.2% of the subjects. There were no differences between subjects with or without SARS-CoV-2 spike-specific IgG memory B-cell response related to SARS-COV-2 IgG level, time post infection or age.
Discussion
Understanding the pathway of immune regulation following COVID-19 infection has important implications to recovering patients and to healthcare authorities regarding decisions related to safety behaviour in daily life and the need for vaccination. Our findings that 6.6% of COVID-19 convalescent subjects did not develop SARS-CoV-2 IgG antibodies are in accordance with those of previous studies [4,5].
The failure of infected subjects to mount an effective primary immune response can be attributed to false-positive RT-PCR results, but can also suggest that, similarly to many other infections, some patients have immune barriers possibly related to antigen capture that prevent a proper humoral response. Further research is needed to unveil factors that lead to such failure.
Our findings demonstrated long-lasting protective humoral immunity against COVID-19 by S1 IgG antibodies in most COVID-19 convalescent subjects.
We used the Euroimmune ELISA assay to quantify SARS-CoV-2 antibody level as it was reported to correlate highly with the neutralizing antibody assay [6,7]. It is well known that high neutralizing titers are particularly important for protection against COVID-19 [8].
This protective immune response was sustained for up to 9 months post infection. The level of humoral protection was not affected by age, gender, blood type, or clinical symptomatology, as both asymptomatic subjects and those with mild to severe disease developed immune responses with similar levels of SARS-CoV-2 IgG antibodies. Although we observed a time-related antibody decline by 50% over a period of 9 months, the antibody level in most convalescent COVID-19 subjects was still in the protective range and reached a sustained plateau at 6 months that remained stable up to 9 months post infection.
We found no significant differences in SARS-CoV-2 IgG antibodies levels between female and male convalescent subjects over time. This finding agrees with a recent study that reported no gender differences in 331 recovering COVID-19 patients following the early post-infection period [9], although a previous small study of 42 convalescent COVID-19 subjects with only four females reported that the generation of IgG antibodies was higher in females in the early phase of the disease [10]. It has been reported that, regardless of age, females tend to show greater antibody responses, higher basal Ig levels, and higher B-cell numbers than do males [11]. However, these gender-related immune differences were not observed following COVID-19 infection [11].
The absence of clinical symptomatology in COVID-19 infected subjects was reported to be associated with lower virus-specific IgG levels and a higher rate of seroconversion in the early convalescent phase [12]. Our findings, in contrast, demonstrated that asymptomatic COVID-19 convalescent subjects developed similar levels of SARS-CoV-2 IgG to those of symptomatic subjects, and the decline over time was similar in both groups.
A recent study reported that there were no significant differences in anti-spike IgG titres by ABO blood group [13]. Similarly, we did not find any association between the level of SARS-CoV-2 IgG antibodies and blood groups.
Previous studies reported low rates of seroconversion within the early period (2–3 months) post infection, mainly in patients who had low levels of IgG antibodies from the beginning or were immunosuppressed [14,15]. We found a higher percentage (15.3% and 15.8%) of subjects who became SARS-CoV-2 IgG-seronegative within 6–9 months post infection, respectively. All had low SARS-CoV-2 antibody levels during the early 3 months post-infection, suggesting that subjects with a-priori low SARS-CoV-2 IgG antibody titers are at risk of losing their humoral immunity. This antibody decay raises a question regarding the longevity of the humoral response in convalescent subjects and provides important data regarding healthcare decisions related to the magnitude of protective humoral immunity and the need for COVID-19 vaccination, particularly in recovering subjects with SARS-CoV-2 antibody decay or absence of B-cell memory response.
The absence of specific S1 spike antibodies in the serum does not necessarily mean the absence of an immune response. Antibody titers might not be the only determining factor for judging the success of COVID-19 immunization. It is possible that memory B cells can operate and provide a secondary immune response during a future encounter with SARS-CoV-2 infection. Another possibility is that patients who became negative for SARS-CoV-2 IgG antibodies may still have SARS-CoV-2-specific memory T cells [16], [17]. We failed to observe a correlation between circulating IgG titers and numbers of memory B cells. We found SARS-CoV-2 spike-specific IgG memory B-cell response in only 68.2% of subjects, regardless of IgG antibody level or the time post infection. It is evident that COVID-19 convalescent subjects who maintain both high SARS-CoV-2 antibodies and memory B cells are best protected from recurrent infection. Subjects who lose their antibodies but still hold a memory B-cell response are expected to have a milder disease when re-infected, as memory humoral immunity can provide long protection despite effector humoral decay.
The problem remains regarding convalescent subjects who maintain a SARS-CoV-2 IgG antibody response in the absence of B-cell memory response. Is it possible that serum antibodies will persist in the absence of memory B cells and provide life-long protection? Or should these groups-that comprise more than a third of convalescent subjects—need to be vaccinated despite prior COVID-19 infection? Is it possible that following vaccination they will develop antigen-specific memory B cells that will induce a long-lasting immune response?
The limitations of the study are the lack of longitudinal assessments for the majority of the subjects, as only 78/392 (19.9%) had serial samples. The interval between the two and three repeated antibody measurements in the same subjects, although variable, adds valuable data to show a protective level up to 9 months post infection. Another limitation of the study is the missing knowledge regarding the role of memory T cells, especially in patients who did not develop humoral immunity.
Our findings may help in the adoption of COVID-19 vaccination strategies in relation to time post infections, and specifically regarding subjects who over time lose their circulating SARS-CoV-2 antibody response.
Author contributions
AA conceived and supervised the study, contributed to the enrolment of patients and patient data curation, contributed to the analysis and interpretation of data, original draft preparation, writing, reviewing, and editing the final manuscript. MG contributed to sample preparation and the ELISpot experiments, and drafted the manuscript. RF designed and performed the ELISpot experiments. SD-A contributed to the analysis and interpretation of data. PS contributed to sample preparation and performed the IgG assay. MM supervised the study, contributed to the enrolment of patients, patient management, and collection of clinical data, and contributed to patient data curation and writing, reviewing and editing the final manuscript.
Transparency declaration
All authors declare no conflicts of interest. This work was supported by the Laura Schwarz-Kipp Research Fund for Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University and the Nitzan Research Fund, Sheba Medical Centre, Israel.
Acknowledgements
We thank Dr Bartek Makower, Mabtech, Sweden, for his technical support and Ms Shani Tomer, Mrs Yamit Titngi and Mrs Ravit Shanni for their technical help in collecting the data.
Editor: L. Kaiser
References
- 1.[a] Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]; [b] Dying in a leadership vacuum. N Engl J Med. 2020;383:1479–1480. doi: 10.1056/NEJMe2029812. [DOI] [PubMed] [Google Scholar]
- 2.Tangye S.G., Tarlinton D.M. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009;39:2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 3.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Euroimmun . 2020. Anti-SARS-CoV-2 ELISA IgG, package insert. EI_2606G_A_US_C02.docx version: 2020-05-04. [Google Scholar]
- 5.Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P., et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbe. Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S., et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6 doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendrone-Junior A., Dinardo C.L., Ferreira S.C., Nishya A., Salles N.A., de Almeida Neto C., et al. Correlation between SARS-COV-2 antibody screening by immunoassay and neutralizing antibody testing. Transfusion. 2021;61:1181–1190. doi: 10.1111/trf.16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang M.S., Case J.B., Franks C.E., Chen R.E., Anderson N.W., Henderson J.P., et al. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng X., Yin J., Zhang J., Hu Y., Ouyang Y., Qiao S., et al. Longitudinal profiling of antibody response in patients with COVID-19 in a tertiary care hospital in Beijing, China. Front Immunol. 2021;12:614436. doi: 10.3389/fimmu.2021.614436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayak K., Gottimukkala K., Kumar S., Reddy E.S., Edara V.V., Kauffman R., et al. Characterization of neutralizing versus binding antibodies and memory B cells in COVID-19 recovered individuals from India. Virology. 2021;558:13–21. doi: 10.1016/j.virol.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng F., Dai C., Cai P., Wang J., Xu L., Li J., et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sthoeger Z.M., Chiorazzi N., Lahita R.G. Regulation of the immune response by sex hormones. In vitro effects of estradiol and testosterone on pokeweed mitogen-induced human B cell differentiation. J Immunol. 1988;141:91–98. [PubMed] [Google Scholar]
- 13.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 14.Bloch E.M., Patel E.U., Marshall C., Littlefield K., Goel R., Grossman B.J., et al. ABO blood group and SARS-CoV-2 antibody response in a convalescent donor population. Vox Sang. 2021 doi: 10.1111/vox.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.B., Olsson A., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]