Abstract
Objective:
To examine the relationship of the Childhood Cancer Symptom Cluster - Leukemia (CCSC-L) with health-related quality of life (HRQOL).
Sample and Setting:
327 children receiving treatment for acute lymphoblastic leukemia (ALL) from four pediatric oncology programs across the United States.
Methods and Variables:
Participants completed fatigue, sleep disturbance, pain, nausea and depression symptom questionnaires at four time points; these symptoms comprised the CCSC-L. HRQOL was measured at the start post-induction therapy then at the start of maintenance therapy. Relationships between the CCSC-L and HRQOL scores were examined with longitudinal parallel-process modeling.
Results:
The mean HRQOL significantly increased over time (p < .001). The CCSC-L had a significant negative association with HRQOL scores at the start of post-induction therapy (β = −0.53, p < .005) and the start of maintenance therapy (β = −0.33, p < .015). Participants with more severe symptoms in the CCSC-L over time had significantly lower HRQOL at the start of maintenance therapy (β = −0.42, p < .005).
Implications for Nursing:
Nurses are pivotal in providing management strategies to minimize symptom severity that may improve HRQOL.
Keywords: pediatric oncology, symptom cluster, health-related quality of life
Approximately 80% of children with cancer endure at least one symptom during treatment but more commonly these children experience multiple symptoms throughout treatment (Buckner et al., 2014; Hockenberry et al., 2017). Symptoms of fatigue, nausea, pain, sleep disturbances, and depression commonly occur among children undergoing cancer treatment (Daniel, Li, Kloss, Reilly, & Barakat, 2016; Kestler & LoBiondo-Wood, 2012; Rodgers, Hooke Ward, & Linder, 2016). A recent study evaluating the trajectory of symptoms among children with acute lymphoblastic leukemia (ALL) confirmed that sleep disturbance and nausea persist during post-induction chemotherapy treatment while fatigue, pain, and depression decreased but never completely resolved during this time (Hockenberry et al., 2017). ALL is the most common type of childhood cancer that requires approximately three years of chemotherapy treatment (Scheurer, Lupo, & Bondy, 2016). ALL treatment is divided into three phases: induction therapy that is started urgently after the cancer diagnosis and lasts approximately one month; post-induction therapy (also referred to as consolidation or intensification therapy) that begins after induction therapy and includes at least 8 months of intensified treatment; and maintenance therapy that starts after post-induction therapy and consists of less intensive treatment for approximately two years (Margolin, Rabin, Steuber, & Poplack, 2016). Post-induction therapy is an important time as children are receiving intensive treatment with several courses of chemotherapy that are associated with numerous symptoms (Hockenberry et al., 2014; Margolin et al., 2016). Children describe symptoms as the worst part of cancer treatment that causes distress and increases suffering (Woodgate, 2008; Ameringer, Elswick, Shockey, & Dillon, 2013).
It is no surprise that children with ALL have low health-related quality of life (HRQOL) during their cancer treatment (Mitchell et al., 2016; van Litsenburg et al., 2014; Sung et al., 2011). Despite the prevalence of symptoms and low HRQOL, only a few studies have evaluated the relationship between symptoms and HRQOL in children undergoing treatment for ALL. Three cross-sectional studies revealed an association of a single symptom, fatigue, with poor HRQOL in children who were receiving treatment for any type of cancer (Al-Gamal & Long, 2016; Pan, Wu, & Wen, 2017; Nunes et al., 2017). Another cross-sectional study of 61 children receiving myelosuppressive chemotherapy found poor HRQOL scores among children with higher symptom distress scores (Baggott et al., 2011). Finally, lower HRQOL scores were noted among children with high symptoms related to their oral mucositis compared to children with low oral mucositis symptoms (Cheng, Lee, Li, Yuen, & Epstein, 2012). These cross-sectional studies provide a snapshot of the relationship between symptoms and HRQOL; however, symptoms are dynamic and descriptions of the relationship of multiple symptoms and HRQOL are missing from the literature.
Symptoms rarely occur alone and there is now an increased priority to identify co-occurring symptoms in children with cancer, referred to as symptom clusters. Baggott and colleagues (2012) identified three clusters (chemotherapy sequelae cluster, mood disturbance cluster, and neuropsychological discomfort cluster) among 131 children receiving cancer therapy. Likewise, Atay and colleagues (2012) noted distinct symptom clusters among 54 adolescents at one, two and three months post cancer diagnosis. Hockenberry and colleagues (2011) noted two clusters: (1) fatigue and depression, and (2) nausea, sleep disturbance, and performance status among 67 children receiving cisplatin, doxorubicin, or ifosfamide for their cancer treatment.
Buckner and colleagues (2014) were among the first to use an advanced modeling technique, latent profile analysis, to characterize similar levels of symptom severity with functional outcomes in 200 children who were receiving or had completed therapy for a variety of cancer diagnoses. They identified that children with high symptom severity including anxiety, depression, fatigue, and pain reported the lowest functional outcomes including peer relationships, upper extremity physical functioning, and mobility. Although HRQOL is likely associated with symptom clusters, no study has evaluated these relationships in children receiving leukemia therapy.
Our group recently identified a symptom cluster of fatigue, sleep disturbance, pain, nausea, and depression, referred to as the Childhood Cancer Symptom Cluster - Leukemia (CCSC-L), among 327 children receiving treatment for ALL (Hooke et al., 2018). Furthermore, the CCSC-L was noted to act as a mediator between physical activity and cognition/memory (Hooke et al., 2018). The purpose of this paper is to extend this analysis by examining the relationship between CCSC-L and HRQOL.
Methods
A repeated-measures design was used for this prospective study. This work was part of a larger study funded by the National Institutes of Health to characterize and explore associations of the phenotypic and genotypic characteristics in children experiencing symptoms related to leukemia treatment. The focus of this analysis is to identify the longitudinal association of the CCSC-L and HRQOL among children receiving ALL therapy. Participants completed symptom questionnaires at four time points during their post-induction treatment (i.e., T1: start of post-induction therapy, T2: 4 months post-induction therapy, T3: 8 months post-induction therapy, and T4: the start of maintenance therapy). HRQOL was measured at the start of post-induction then again at the start of maintenance therapy; the time interval between these two measures was approximately 12 months but varied depending on individual treatment delays. The conceptual model was developed by the authors and illustrated in Figure 1. The figure represents data collected during ALL treatment and the potential relationship of HRQOL with both the initial level of symptoms (cluster intercept) and the rate of change in symptoms over time (cluster slope).
Figure 1.
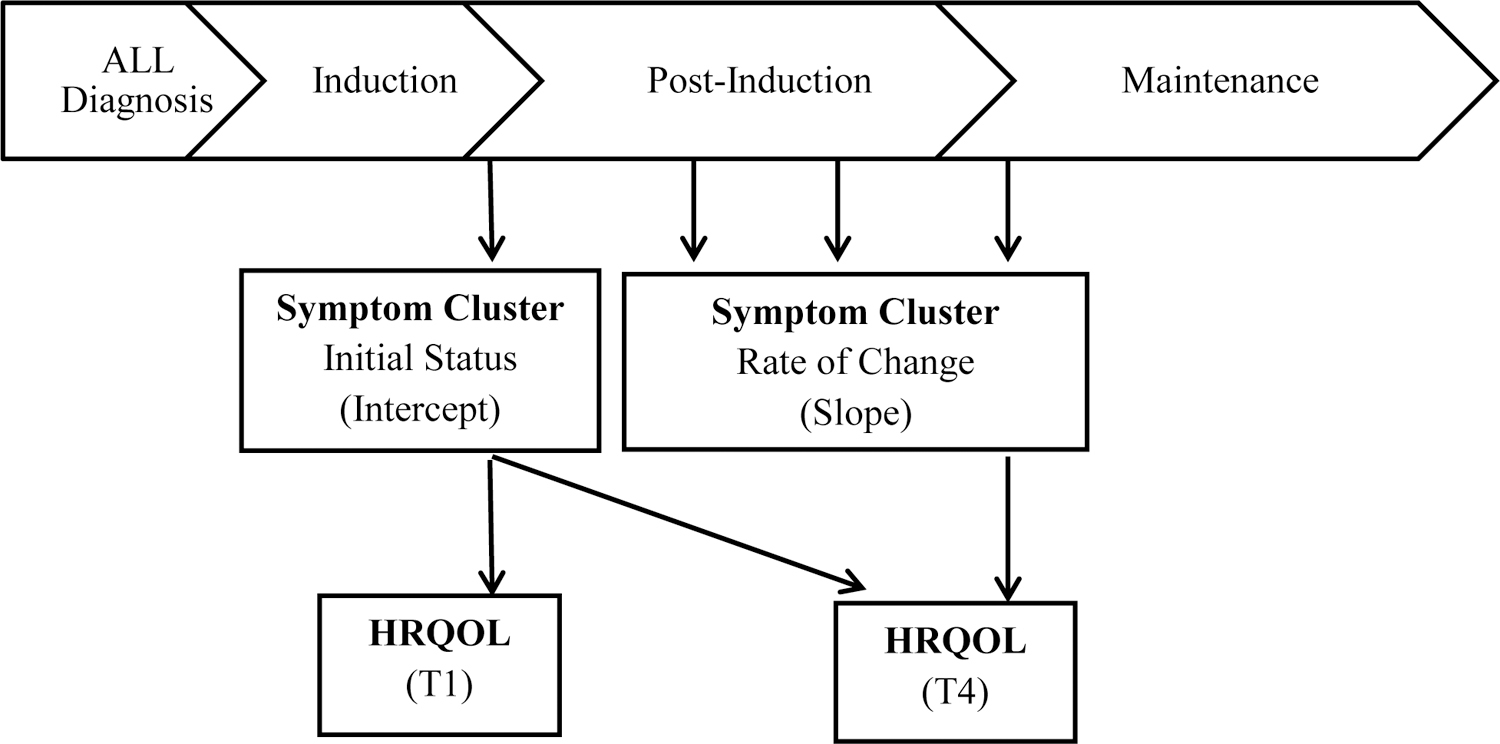
Conceptual Model
Setting and Sample
Participants were recruited from four pediatric oncology programs across the United States including one site in the Southwest, one site in the northern section of the Midwest, one site in the southern section of the Midwest, and one site on the East Coast. Potential participants were invited to participate in the study if the patient was 3–18 years of age, receiving first-time treatment according to an acute lymphoblastic leukemia (ALL) protocol, and fluent in English or Spanish. Exclusion criteria consisted of patients with relapsed ALL or any cognitive disability that was established before the cancer diagnosis.
All participants were treated on a lymphoblastic leukemia protocol and received similar therapy. Post-induction therapy involved several courses of treatment that included asparaginase, methotrexate, vincristine, doxorubicin, corticosteroid, cytarabine, and mercaptopurine. Intrathecal methotrexate was given on Day 1 of each 12-week cycle.
Measures
Severity of each of the five symptoms within the CCSC-L were measured individually with self-reported questionnaires for children 7 years of age and older or parent-proxy questionnaires for children 3 to 6 years old. Each questionnaire is listed in Table 1 along with the established reliability and validity. To be consistent with the scoring direction of other symptom measures, scores on the sleep wake scale were reversed and the scale was renamed as sleep disturbance so that higher scores for each symptom represented higher severity of the symptom.
Table 1.
Study Instruments
| Variable | Questionnaire: patient age in years | Reliability | Scoring |
|---|---|---|---|
| Fatigue | Parent Fatigue Scale: 3–6 Child Fatigue Scale: 7–12 Adolescent Fatigue Scale: 13–18 |
Internal consistency for all scales = 0.67–0.95 (Mandrell et al., 2011;Hinds et al., 2010; Hockenberry et al., 2003) | 5-point Likert scale Total score: T-score ranging from 20–80 |
| Sleep disturbance | Child Sleep Wake Scale by parent: 3–6 Child Sleep Wake Scale: 7–12 Adolescent Sleep Wake Scale: 13–18 |
Cronbach’s alpha = 0.81–0.91 (Storfer-Isser et al., 2013; LeBourgeois & Harsh, 2016) | 6-point Likert scale Total score: Average reversed score ranging from 25–168 |
| Pain | Wong-Baker Faces Scale by parent: 3–6 Wong-Baker Faces Scale: 7–18 |
Pearson coefficient = 0.62–0.96 (Tsze et al., 2017) Kappa coefficient = 0.846 (Wood et al, 2011) |
6-point visual analog scale Total score: Raw score ranging from 0–10 |
| Nausea | Visual Analog Scale by parent: 3–6 Visual Analog Scale: 7–18 |
Spearman =0.90 (Meek, Kelly, & Hu, 2009) | 100-point visual analog scale Total score: Raw score ranging from 0–100 |
| Depression | Child Depression Inventory (CDI-2) Parent Report: 3–6 CDI-2: 7–18 |
Cronbach alpha = 0.91 Test-retest reliability = 0.89 (Kovacs, 2011) | 3 choice response (absence, mild, or definite symptom) Total score: T-score ranging from 20–80 |
| Health-related quality of life | Peds QL Cancer Module Parent Report: 3–6 Peds QL Cancer Module Child Report: 7–12 PedsQL Cancer Module Teen Report: 13–18 |
Cronbach’s alpha = 0.87 for parent report and 0.72 for child/teen (Varni et al., 2002) | 5-point Likert scale Total score: Mean score ranging from 0–100 |
In the current authors’ previous work, exploratory factor analysis demonstrated significant relationships among all the symptoms in the cluster (factor loadings from .37 to .91) throughout post-induction and the start of maintenance therapy establishing the Childhood Cancer Symptom Cluster - Leukemia (CCSC-L) (Hockenberry et al., 2017). The factor score of the CCSC-L was a composite score (or weighted average) of the values in the five symptom measures (e.g., fatigue, sleep disturbance, pain, nausea, and depression). The weights were determined by the one-factor solution in the exploratory factor analysis of the five symptom measures (Hockenberry et al., 2017). The factor score of the CCSC-L is a continuous variable; and the higher factor score is, the more severe the CCSC-L.
HRQOL was measured with a self-reported questionnaire or a parent-proxy questionnaire (Table 1). Higher scores on the HRQOL questionnaire signified better health-related quality of life.
Procedure
After obtaining approval by the Institutional Review Board at each site, eligible patients and their parents were introduced to the study, initially by a provider known to them then by a member of the study team. If the patient and parent agreed to participate, parents of patients less than 18 years of age provided written consent while patients 7–11 years provided verbal assent and patients 12–17 years provided written assent. Patients 18 years of age were consented.
All four time points for data collection occurred during a routine cancer center clinic visit or while hospitalized. At each time point, parents and patients were asked if they were willing to continue with the study and completed the questionnaires if agreed. Parent or patients who were unwilling to complete the questionnaires were asked if data could be collected at another time and a future date was determined. All questionnaires were administered electronically either in English or Spanish on a tablet computer (i.e., iPad). Demographic and treatment information was obtained from the medical record.
Data Analysis
Descriptive statistics were used to summarize the sample characteristics, symptom scores, and HRQOL scores over time. The initial sample consisted of 329 participants; however, two participants had missing data on all symptom measures and were excluded from the analysis. The remaining 327 participants had no missing data on demographic variables, but some intermittent missing data across the four time points on CCSC-L and HRQOL scores. The missing pattern was verified by the Little’s (1988) test as missing completely at random and no further missing data treatment was necessary. Thus, the intermittent missing data were automatically dealt with by the multilevel modeling technique in the two-step LPP described in detail below.
Relationships between the CCSC-L and HRQOL scores were examined using longitudinal parallel-process (LPP) (Cheong et al., 2003). LPP is a two-step modeling technique on longitudinal data. In the first step, the growth parameters of each longitudinal variable, e.g., the intercept (or initial status) and the slope (or rate of change), are estimated from multilevel modeling. In this step, intermittent missing data across time can be easily handled by the expectation-maximization algorithm in the multilevel modeling technique, and if necessary, covariates can be controlled in the multilevel modeling. In this study, sociodemographic variables (e.g., age, sex, and race/ethnicity) and leukemia risk levels were controlled when estimating the growth parameters. In the second step of LPP, structural equation modeling (SEM) (Kline, 2010) was used for examining the longitudinal relationships among the variables. The longitudinal relationships are captured by the causal paths among the growth parameters of each longitudinal variable (e.g., the intercept and the slope).
Guided by the literature on SEM (Hu & Bentler, 1999; Kline, 2010), the model-fit indices used for testing the model fit for the SEM were: chi-square of the estimated model (χ2), goodness of fit index (GFI), normed fit index (NFI), incremental fit index (IFI), relative fit index (RFI), comparative fit index (CFI), and root mean square error of approximation (RMSEA). A nonsignificant chi-square value (e.g., p > .05) suggests a good overall model fit to the data, whereas RMSEA should be below .06. For GFI, NFI, IFI, RFI, and CFI, values higher than .90 indicate a good fit to the data.
Results
Characteristics of the 327 participants have been previously described (Hooke et al., 2018), but primarily consisted of non-Hispanic ethnicity (53%) and males (52%). Approximately 45% (n=148) of participants were 3 to 6 years old, 34% (n=110) of participants were 7 to 12 years old, and 21% (n=69) of participants were 13 to 18 years old. These sample characteristics are similar to children diagnosed with ALL throughout the United States; ALL is more common in those with Hispanic ethnicity, has a slight male predominance, and is less common among adolescents (Rabin, Gramatges, Margolin, & Poplack, 2016).
Health-Related Quality of Life
Overall, the mean HRQOL significantly increased from the start of post-induction therapy (T1) to the start of maintenance therapy (T4). The mean HRQOL at T1 was 70.08 (SD =15.66) and the mean HRQOL at T4 was 75.77 (SD = 14.36) (p < .001, Table 2) with a close to medium effect (Cohen’s d = 0.4). This relationship is also demonstrated by the positive, significant path coefficient of HRQOL from T1 to T4 noted in Figure 2 (β = 0.22, p < .008).
Table 2.
Mean (M) and Standard Deviation (SD) of Individual Symptoms and Health-Related Quality of Life by Time (N = 327)
| Variable | Start of post-induction therapy (T1) |
4 months post- induction therapy (T2) |
8 months post- induction therapy (T3) |
Start of maintenance therapy (T4) |
||||
|---|---|---|---|---|---|---|---|---|
| n | M (SD) | n | M (SD) | n | M (SD) | n | M (SD) | |
| Fatigue | 300 | 53.87 (10.01) |
292 | 50.03 (10.56) |
241 | 48.22 (9.23) |
222 | 46.60 (8.11) |
| Sleep Disturbance | 296 | 2.66 (0.75) |
286 | 2.75 (0.79) |
240 | 2.75 (0.78) |
219 | 2.69 (0.81) |
| Pain | 297 | 2.67 (2.33) |
287 | 1.67 (2.22) |
239 | 1.37 (1.81) |
220 | 1.34 (1.85) |
| Nausea | 295 | 15.12 (21.67) |
286 | 14.83 (21.84) |
237 | 15.33 (21.35) |
221 | 11.24 (18.33) |
| Depression | 297 | 51.46 (9.88) |
287 | 50.66 (10.52) |
234 | 49.33 (10.23) |
217 | 47.92 (8.75) |
| Health-Related Quality of Life | 292 | 70.08 (15.66) |
n/a | n/a | n/a | n/a | 219 | 75.77 (14.36) |
Figure 2.
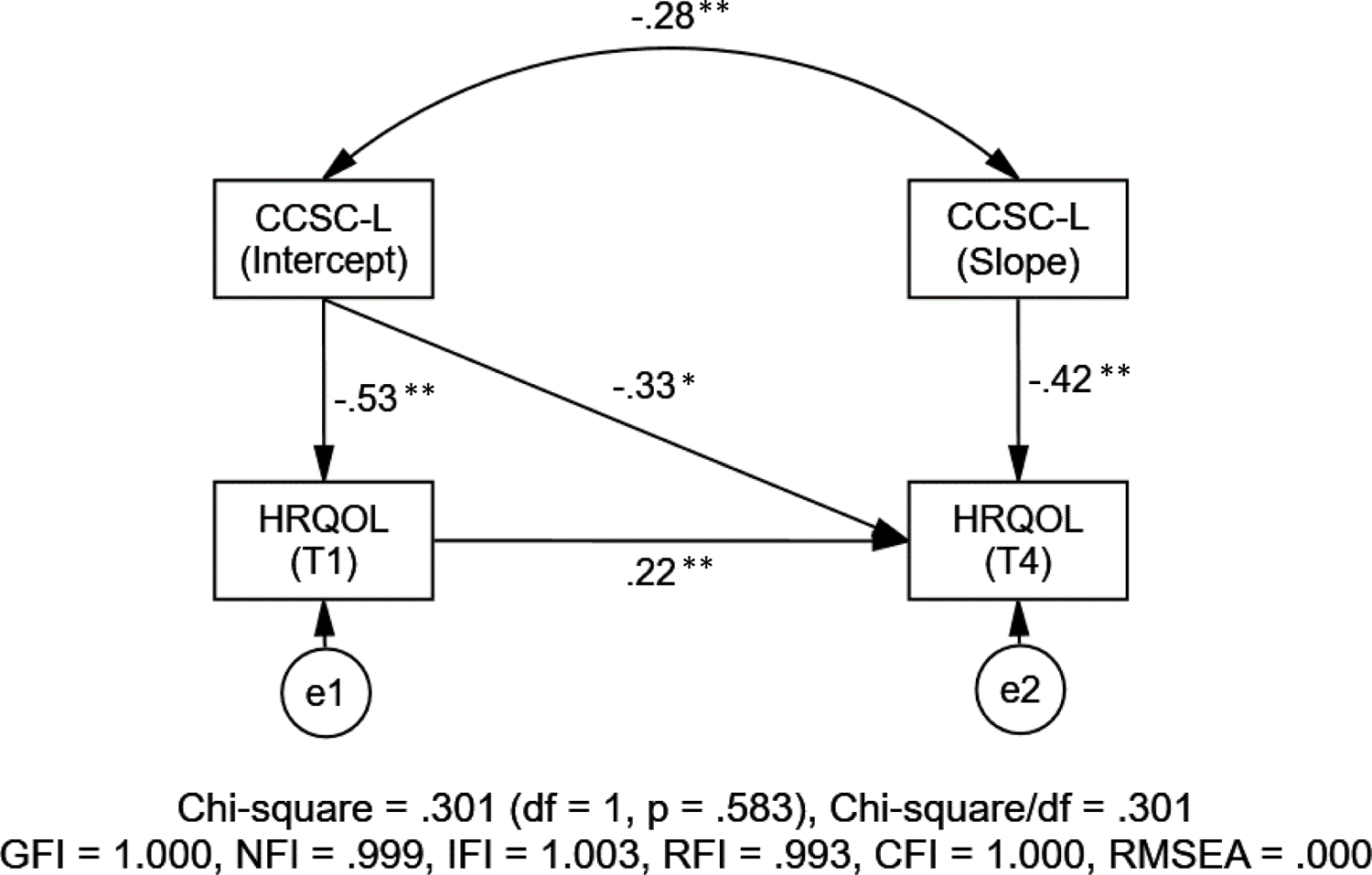
Health-Related Quality of Life and the Childhood Cancer Symptom Cluster – Leukemia (N = 327; all the estimated path coefficients are standardized; *p < .05, **p < .01)
Relationships between CCSC-L and Health-Related Quality of Life
As illustrated in Figure 2, the results from the structural equation modeling demonstrated a good model fit to the data with satisfactory model-fit indices (χ2(1) = 0.30, p = .583; GFI = 1.00, NFI = 1.00, IFI = .99, RFI = .96, CFI = 1.00; and RMSEA < .001). First of all, for participants who had more severe symptoms in the CCSC-L at the start of post-induction therapy (intercept), the severity of their symptoms within the CCSC-L reduced faster (slope) than those who had less severe symptoms in the CCSC-L at the start of post-induction therapy (r = −0.28, p < .007). As for the relationships between the symptom cluster and HRQOL, a significant negative association was noted for HRQOL scores with the intercept of the CCSC-L at both the start of post-induction therapy (T1) (β = −0.53, p < .005) and the start of maintenance therapy (T4) (β = −0.33, p < .015). Participants with more severe symptoms in the CCSC-L at the start of post-induction therapy experienced lower HRQOL at the start of post-induction therapy and the start of maintenance therapy. Similarly, the slope of the CCSC-L was negatively associated HRQOL scores at the start of maintenance therapy (T4) (β = −0.42, p < .005). Participants with more severe symptoms in the CCSC-L over time had significantly lower HRQOL at the start of post-induction therapy; this rate of change over time in the symptom cluster is represented in the slope of the CCSC-L.
Discussion
This study demonstrated a statistically significant improvement in HRQOL among children with ALL during 12 months of treatment. Improvement in HRQOL is similar to other studies that report a low HRQOL among children with ALL during induction therapy that slowly improves during subsequent phases of therapy (Eiser et al., 2017; Furlong et al., 2012; Mitchell et al., 2016). This improvement would be expected as leukemia chemotherapy treatment becomes less intensive over the first 12 to 14 months of treatment after the more intensive early phases of induction and consolidation. In the current study, the 5.69 change in the PedsQL HRQOL score was clinically significant as it was greater than the published change threshold of 4.5 needed for a minimal clinically meaningful difference (Varni, Limbers, Burwinkle, 2007). Despite the improvement, the improved mean score of 75.77 was still lower than a healthy sample of children who report HRQOL with a mean score of 83.00 on a generic scale (Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002). Overall, children receiving treatment for ALL have a poor HRQOL during post-induction therapy through the start of maintenance therapy. These children require an ongoing assessment to identify factors negatively influencing their quality of life.
This study identified a significant longitudinal relationship between the CCSC-L and HRQOL. The LPP model was instrumental in evaluating the longitudinal relationships between two processes over time (i.e., CCSC-L and HRQOL) as it allowed an evaluation of the relationship between rates of change in the two variables which can be measured at different time points (Cheong, MacKinnon, & Khoo, 2003; Sousa, Kwok, Schmiege, & West, 2014). This analysis revealed that children who experienced more severe symptoms within the CCSC-L at baseline had lower HRQOL scores at the start of post-induction therapy and the start of maintenance therapy. Likewise, children with more severe symptoms within the CCSC-L over time had lower HRQOL at the start of maintenance therapy. Sleep disturbances over one month have been associated with worse HRQOL in children receiving treatment for ALL (Daniel et al., 2016) but symptoms rarely occur in isolation (Aktas, 2013). Of importance from these findings is the fact that a symptom cluster, not just a single symptom, was negatively associated with HRQOL. Awareness of and response to symptom clusters allows the healthcare provider to provide comprehensive interventions to minimize or alleviate the factors negatively affecting the patient.
Findings from this study are significant because the variable influencing HRQOL in this study can be managed with appropriate intervention strategies. Symptom severity within the CCSC-L can be minimized through symptom management interventions, such as medication and/or psychoeducation (Nunes et al., 2018; Lopes-Junior et al., 2016). Other factors known to negatively influence HRQOL among children with ALL include older age (Eiser et al., 2017; van Litsenburg et al., 2014) and corticosteroid therapy (Daniel et al., 2016; Fardell et al., 2017) that cannot be altered. Therefore, clinical and research efforts should focus on relieving the modifiable variable (i.e., the symptom cluster) by implementing strategies early in treatment that will minimize or ameliorate the symptom cluster thus improving the HRQOL.
Limitations
A limitation of this study includes a sample consisting of a wide range of ages. Recent studies have found negative associations with older age and HRQOL (Eiser et al., 2017; van Litsenburg et al., 2014); however, additional studies are needed to identify symptom clusters among specific age groups (Rodgers et al., 2016). The researchers also recognize that other variables may influence HRQOL and symptom severity as children begin the maintenance phase of leukemia treatment and these merit investigation in the future. Potential variables include but are not limited to adaptation to the diagnosis of cancer, improved ability and confidence in managing symptoms, and relief at maintaining remission and entering less demanding cycles of treatment. In addition, the sample in this study excluded children with cognitive disabilities. While this study was designed to obtain self-reported symptom data among the participants, future studies could obtain objective and proxy symptom data among children with cognitive disabilities to evaluate symptom clusters and HRQOL. Ultimately, future studies should evaluate the relationship of symptom clusters and HRQOL during cancer therapy among specific groups of children with defined characteristics (i.e. age, pre-existing conditions).
A strength of this study is the evaluation of symptom clusters and HRQOL during a specific phase of cancer therapy; however, future studies should be conducted in children with ALL beyond the start of maintenance therapy to evaluate the duration of the relationship of the CCSC-L and HRQOL. Evaluation of symptom clusters and HRQOL in children during all phases of treatment can identify if changes occur over time, which can assist healthcare providers who are providing symptom management.
The differences in adherence of symptom management strategies may have affected the symptom cluster and HRQOL in this study. While supportive care for symptoms is similar for patients during ALL therapy, the uptake of a symptom management plan is highly individualized among patients. For example, the same symptom treatment may have been provided to two patients in this study with nausea but one patient may have been adherent to the regimen while the patient could have ignored the plan. The potential influence of symptom management strategies should be considered in future studies.
Implications for Nursing Practice
Symptom assessment and management is integral to nursing care. Nurses are often familiar with the incidence of individual symptoms but may not be aware of the interaction of multiple symptoms or how symptom cluster is affecting the child’s life. Questions during the assessment about the interaction of multiple symptoms can provide a more comprehensive understanding of the symptom experience. For example, if a child with ALL is reporting nausea and pain during post-induction therapy, the nurse should ask about fatigue, sleep disturbances, and depression because these symptoms commonly cluster together. Furthermore, an understanding of how the symptom cluster is affecting the child’s lifestyle will identify important areas for symptom management. For example, rather than asking the child to rate the severity of a specific symptom, questions such as “what is bothering you most during the day (or night)” or “what is keeping you from doing what you want” can provide an opportunity for the child to discuss multiple symptoms and start a dialogue to help distinguish the most distressing symptoms. With this information, nurses can advocate for symptom management strategies focused on the most bothersome symptoms for the child. Customizing symptom management strategies may increase the child’s HRQOL more than the typical improvement.
Conclusion
Children receiving treatment for ALL have a low HRQOL, but healthcare providers can focus efforts on management strategies that can improve HRQOL. The CCSC-L was negatively associated with HRQOL during the post-induction treatment. In order to improve HRQOL, symptom management strategies should focus on reducing or alleviating fatigue, sleep disturbances, pain, nausea, and depression. Future research should focus on the effectiveness of the symptom management strategies and also identify other modifiable factors that significantly affect HRQOL.
Knowledge Translation.
Mean health-related quality of life (HRQOL) for children with acute lymphoblastic leukemia (ALL) is low at the start of post induction therapy but improves over time.
Children who have severe symptoms at the start of post-induction therapy experience lower HRQOL at that time and at the start of maintenance therapy.
Children receiving ALL treatment who have more severe symptoms throughout post-induction therapy have lower HRQOL at the start of maintenance therapy.
Acknowledgments
Disclosures: This was supported by a National Institutes of Health RO1CA1693398 and the Alex’s Lemonade Stand Foundation.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Cheryl Rodgers, Associate Professor, Duke University School of Nursing.
Mary C. Hooke, Associate Professor, University of Minnesota School of Nursing, Clinical Nurse Specialist, Children’s Minnesota Cancer and Blood Disorders Program.
Olga Taylor, Clinical Research Manager, Texas Children’s Cancer and Hematology Centers/Baylor College of Medicine.
Kari M. Koerner, Senior Research Specialist, College of Nursing, University of Arizona.
Pauline Mitby, Clinical Research Manager, Children’s Minnesota Cancer and Blood Disorders Program.
Ida Moore, Ann Furrow Professor of Nursing and Director of Biobehavioral Health Science Division, College of Nursing, University of Arizona.
Michael E. Scheurer, Associate Professor and Director of Population Sciences Biorepository Shared Resources, Texas Children’s Cancer and Hematology Centers/Baylor College of Medicine.
Marilyn J. Hockenberry, Bessie Baker Professor of Nursing, Professor of Pediatrics and Associate Dean for Research Affairs, Duke University School of Nursing.
Wei Pan, Associate Professor, Duke University School of Nursing.
References
- Aktas A (2013). Cancer symptom clusters: Current concepts and controversies. Current Opinion in Supportive and Palliative Care, 7(1), 38–44. doi: 10.1097/SPC.0b013e32835def5b [DOI] [PubMed] [Google Scholar]
- Al-Gamal E & Long T (2016). Health-related quality of life and its association with self-esteem and fatigue among children diagnosed with cancer. Journal of Clinical Nursing, 25(21–22), 3391–3399. doi: 10.1111/jocn.13467 [DOI] [PubMed] [Google Scholar]
- Ameringer S, Elswick RK, Shockey DP, & Dillon R (2013). A pilot exploration of symptom trajectories in adolescents with cancer during chemotherapy. Cancer Nursing, 36(1), 60–71. doi: 10.1097/NCC.0b013e318250da1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atay S, Conk Z, & Bahar Z (2012). Identifying symptom clusters in paediatric cancer patients using the Memorial Symptom Assessment Scale. European Journal of Cancer Care, 21(4), 460–468. doi: 10.1111/j.1365-2354.2012.01324.x [DOI] [PubMed] [Google Scholar]
- Baggott C, Cooper BA, Marina N, Matthay KK, & Miaskowski C (2012). Symptom cluster analyses based on symptom occurrence and severity ratings among pediatric oncology patients during myelosuppressive chemotherapy. Cancer Nursing, 35(1), 19–28. doi: 10.1097/NCC.0b013e31822909fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott CR, Dodd M, Kennedy C, Marina N, Matthay KK, Cooper B, & Miaskowski C (2011). An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy. Supportive Care in Cancer, 19(3), 353–361. doi: 10.1007/s00520-010-0824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner TW, Wang J, DeWalt DA, Jacobs S Reeve BB & Hinds PS (2014). Patterns of symptoms and functional impairments in children with cancer. Pediatric Blood & Cancer, 61(7), 1282–1288. doi: 10.1002/pbc.25029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KK, Lee V, Li CH, Yuen HL & Epstein JB (2012). Oral mucositis in pediatric and adolescent patients undergoing chemotherapy: The impact of symptoms on quality of life. Supportive Care in Cancer, 20(10), 2335–2342. doi: 10.1007/s00520-011-1343-1 [DOI] [PubMed] [Google Scholar]
- Cheong J, MacKinnon DP, & Khoo ST (2003). Investigation of mediational processes using parallel process latent growth curve modeling. Structural Equation Modeling: A Multidisciplinary Journal, 10(2), 238–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel LC, Li Y, Kloss JD, Reilly AF, & Barakat LP (2016). The impact of dexamethasone and prednisone on sleep in children with acute lymphoblastic leukemia. Supportive Care in Cancer, 24,1–10. doi: 10.1007/s00520-016-3234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiser C, Stride CB, Vora A, Goulden N, Mitchell C, Buck G, … UK Childhood Leukaemia Clinicians Network. (2017). Prospective evaluation of quality of life in children treated in UK ALL 2003 for acute lymphoblastic leukaemia: A cohort study. Pediatric Blood & Cancer, 64(11). doi: 10.1002/pbc.26615 [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vetsch J, Trahair T, Mateos MK, Grootenhuis MA, Touyz LM, … Wakefield CE (2017). Health-related quality of life of children on treatment for acute lymphoblastic leukemia: A systematic review. Pediatric Blood & Cancer, 64(9), 1–13. doi: 10.1002/pbc.26489 [DOI] [PubMed] [Google Scholar]
- Furlong W, Rae C, Feeny D, Gelber RD, Laverdiere C, Michon B, … Barr R (2012). Health-related quality of life among children with acute lymphoblastic leukemia. Pediatric Blood & Cancer, 59(4), 717–724. doi: 10.1002/pbc.24096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P, Yan J, Gattuso J, Hockenberry M, Jones H, Zupanec S, … Srivastava DK (2010). Psychometric and clinical assessment of the 10-item reduced version of the fatigue scale – child instrument. Journal of Pain and Symptom Management, 39(3), 572–578. doi: 10.1016/j.jpainsymman [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenberry M, Hinds PS, Barrera P, Bryant R, Adams-McNeill J, Hooke C, … Manteuffel B (2003). Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. Journal of Pain and Symptom Management, 25(4), 319–328. [DOI] [PubMed] [Google Scholar]
- Hockenberry MJ, Hooke MC, McCarthy K, & Gregurich MA (2011). Sickness behavior clustering in children with cancer. Journal of Pediatric Oncology Nursing, 28(5),263–272. doi: 10.1177/1043454211409586 [DOI] [PubMed] [Google Scholar]
- Hockenberry MJ, Hooke MC, Rodgers C, Taylor O, Koerner KM, Mitby P, … Pan W (2017). Symptom trajectories in children receiving treatment for leukemia: A latent class growth analysis with multitrajectory modeling. Journal of Pain and Symptom Management, 54(1), 1–8. doi: 10.1016/j.jpainsymman.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenberry MJ, Taylor OA, Pasvogel A, Rodgers C, McCarthy K, Gundy P, … Moore IM (2014). The influence of oxidative stress on symptom occurrence, severity, and distress during childhood leukemia treatment. Oncology Nursing Forum, 41(4), E238–47. doi: 10.1188/14.ONF.E238-E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooke MC, Rodgers C, Taylor O, Koerner K, Mitby P, Moore I, … Pan W (2018). Physical activity, the Childhood Cancer Symptom Cluster–Leukemia, and cognitive function: A longitudinal mediation analysis. Cancer Nursing, 41, 434–440. 10.1097/NCC.0000000000000634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. [Google Scholar]
- Kestler SA & LoBiondo-Wood G (2012). Review of symptom experiences in children and adolescents with cancer. Cancer Nursing, 35(2), E31–49. doi: 10.1097/NCC.0b013e3182207a2a [DOI] [PubMed] [Google Scholar]
- Kline RB (2010). Principles and Practice of Structural Equation Modeling, 3rd ed. New York, NY: The Guilford Press. [Google Scholar]
- Kovacs M (2011). Children’s Depression Inventory, 2nd ed. Toronto, ON, Canada: Multi-Health Systems Inc. [Google Scholar]
- LeBourgeois MK, & Harsh JR (2016). Development and psychometric evaluation of the children’s sleep-wake scale. Sleep Health, 2(3), 198–204. doi: 10.1016/j.sleh.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83(404), 198–1202. doi: 10.2307/2290157 [DOI] [Google Scholar]
- Lopes-Junior LC, Bomfim EO, Nascimento LC, Nunes MD, Pereira-da-Silva B, & Lima RA (2016). Non-pharmacological interventions to manage fatigue and psychological stress in children and adolescents with cancer: An integrative review. European Journal of Cancer Care, 25(6), 921–935. doi: 10.1111/ecc.12381 [DOI] [PubMed] [Google Scholar]
- Mandrell B, Yang J, Hooke MC, Wang C, Gattuso JS, Hockenberry M, … Hinds PS (2011). Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. Journal of Pediatric Oncology Nursing, 28(5), 287–294. doi: 10.1177/1043454211418667 [DOI] [PubMed] [Google Scholar]
- Margolin JF, Rabin KR, Steuber CP, & Poplack DG (2016). Acute lymphoblastic leukemia. In Pizzo PA, Poplack DG, editors: Principles and practices of pediatric oncology, ed 7, Philadelphia, Lippincott. [Google Scholar]
- Meek R, Kelly AM, & Hu XF (2009). Use of the visual analog scale to rate and monitor severity of nausea in the emergency department. Academic Emergency Medicine, 16(12), 1304–1310. doi: 10.1111/j.1553-2712.2009.00581.x [DOI] [PubMed] [Google Scholar]
- Mitchell HR, Lu X, Myers RM, Sung L, Balsamo LM, Carroll WL, … Kadan-Lottick N (2016). Prospective, longitudinal assessment of quality of life in children from diagnosis to 3 months off treatment for standard risk acute lymphoblastic leukemia: Results of Children’s Oncology Group study AALL0331. International Journal of Cancer, 138, 332–339. doi: 10.1002/ijc.29708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MDR, Bomfim EO, Olson K, Lopes-Junior LC, Silva-Rodriques FM, Garcia de Lima RA, & Nascimento LC (2018). Interventions minimizing fatigue in children/adolescents with cancer: An integrative review. Journal of Child Health Care, 22(2), 186–204. doi: 10.1177/1367493517752498 [DOI] [PubMed] [Google Scholar]
- Nunes MDR, Jacob E, Bomfim EO, Lopes-Junior LC, de Lima RAG, Floria-Santos M, & Nascimento LC (2017). Fatigue and health related quality of life in children and adolescents with cancer. European Journal of Oncology Nursing, 29, 39–46. doi: 10.1016/j.ejon.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HT, Wu LM, & Wen SH (2017). Quality of life and its predictors among children and adolescents with cancer. Cancer Nursing, 40(5), 343–351. doi: 10.1097/NCC.0000000000000433 [DOI] [PubMed] [Google Scholar]
- Rabin KR, Gramatges MM, Margolin JF, & Poplack DG (2016). Acute lymphoblastic leukemia. In Pizzo PA, Poplack DG, editors: Principles and practices of pediatric oncology, ed 7, Philadelphia, Lippincott. [Google Scholar]
- Rodgers C, Hooke MC, Ward J, & Linder L (2016). Symptom clusters in children and adolescents with cancer. Seminars in Oncology Nursing, 32(4), 394–404. doi: 10.1016/j.soncn.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Scheurer ME, Lupo PJ, & Bondy ML (2016). Epidemiology of childhood cancer. In Pizzo PA, Poplack DG, editors: Principles and practices of pediatric oncology, ed 7, Philadelphia, Lippincott. [Google Scholar]
- Sousa KH, Kwok OM, Schmiege SJ, & West SG (2014). A longitudinal approach to understanding the relationship between symptom status and QOL. Western Journal of Nursing Research, 36(6), 732–747. doi: 10.1177/0193945913510980 [DOI] [PubMed] [Google Scholar]
- Storfer-Isser A, Lebourgeois MK, Harsh J, Tompsett CJ, & Redline S (2013). Psychometric properties of the adolescent sleep hygiene scale. Journal of Sleep Research, 22(6), 707–716. doi: 10.1111/jsr.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L, Yanofsky R, Klaassen RJ, Dix D, Pritchard S, Winick N, … Klassen A (2011). Quality of life during active treatment for pediatric acute lymphoblastic leukemia. International Journal of Cancer, 128(5), 1213–1120. doi: 10.1002/ijc.25433 [DOI] [PubMed] [Google Scholar]
- Tsze DS, von Baeyer CL, Pahalyants V, & Dayan PS (2017). Validity and reliability of the verbal numerical rating scale for children aged 4 to 17 years with acute pain. Annals of Emergency Medicine, 71(6), 691–702. doi: 10.1016/j.annemergmed.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Litsenburg RR, Huisman J, Pieters R, Verhaak C, Kaspers GJ, & Gemke RJ (2014). Determinants of quality of life during induction therapy in pediatric acute lymphoblastic leukemia. Supportive Care in Cancer, 22(12), 3235–3242. doi: 10.1007/s00520-014-2349-2 [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Katz ER, Meeske K, & Dickinson P (2002). The PedsQL in pediatric cancer: reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer, 94(7), 2090–2106. [DOI] [PubMed] [Google Scholar]
- Varni JW, Limbers C, Burwinkle TM (2007). Literature review: Health-related quality of life measurement in pediatric oncology: Hearing the voices of the children. Journal of Pediatric Psychology 32(9), 1151–1163. doi: 10.1093/jpepsy/jsm008 [DOI] [PubMed] [Google Scholar]
- Wood C, von Baeyer CL, Falinower S, Moyse D, Annequin D, & Legout V (2011). Electronic and paper versions of a faces pain intensity scale: concordance and preference in hospitalized children. BMC Pediatrics, 11, 87. doi: 10.1186/1471-2431-11-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgate R (2008). Feeling states: A new approach to understanding how children and adolescents with cancer experience symptoms. Cancer Nursing, 31(3), 229–238. doi: 10.1097/01.NCC.0000305731.95839.ca [DOI] [PubMed] [Google Scholar]


