Abstract
Background
In the Eurotransplant, 12.6% of kidney transplantations are a repeat procedure. Third transplants are significantly more complex than first and second ones. We compared the results of first (PRT) versus third (TRT) transplantations.
Methods
Between 2011 and 2016, we performed 779 deceased donor adult kidney transplantations, 14.2% out of them were second, 2.6% (20) third, and 0.3% fourth. We compared the pre-, intra-, and postoperative data, kidney function, and survival rate.
Results
Recipients of TRT were younger (53.4 vs. 47.3 p = 0.02). HCV infection rate (20%, p = 0.00) is ten times higher. The operation time is longer (132 vs. 152 min, p = 0.02), and delayed graft function is much more frequent (22.4% vs. 60%, p = 0.00). Induction therapy was given to every TRT (7.9% vs.100%), but as a result, the rejection rate was the same (~ 15%). Hospital stay is a week longer. Patient’s survival at 1, 3, and 5 years for PRT is 96.4%, 93.9%, and 91.2% and for TRT is 90%, 85%, and 78.4%, respectively (p = 0.023). TRT’s odds ratio of fatal outcome is 4.35 (1.5–12.5). Graft survival at 1, 3, and 5 years for PRT is 93.1%, 91.4%, and 90.3% and for TRT is 75%, 75%, and 75%, respectively (p = 0.020). TRT’s odds ratio of graft loss is 3.14 (1.1–8.9). Of PRT 85.76%, out of PRT 85.76%, while out of TRT 60% live with a functioning graft, p=0.00149.
Conclusion
In a third transplant, both graft and patient survival are significantly inferior to primer ones. Careful selection is required to minimize the patient risk and graft loss.
Keywords: Kidney transplantation; Patient survival; Graft survival; Graft loss; Retransplantation; Survival; Mortality, propensity score matching analysis
Introduction
Despite significant improvements in the last five decades, the real half-life of kidney grafts is still around 8 years, substantially shorter than projected half-lives [1]. Chronic graft failure is still a major problem, and especially younger recipients of the primer graft might need a second or third transplantation. The rate of repeat transplantation was increasing in the USA from 1996 to 2005, reaching 12.4%, while potential retransplant recipients represented 16.1% of all kidney candidates [2, 3].
In the Eurotransplant, 17.9% of those on the waiting list are listed for repeat kidney transplantation, and 13.7% of the procedures performed was a repeat one in 2019 (http://statistics.eurotransplant.org).
While mortality after a failing graft is considered to be high, the first retransplantation is associated with significantly reduced mortality rates [4–6].
Moreover, in several papers, the outcome of a second graft has been reported to be similar to the first one [7–9]. Others observed that the graft survivals for repeat deceased donor transplants were all significantly lower; the relative risk of graft loss was 1.18–1.24 [2].
Recipients of a third graft constitute a unique population among kidney patients. Patients are often highly sensitized, have limited surgical options, suffer from comorbidities e.g. atherosclerosis, virologic infections, and all other consequences of previous operations, immunosuppression, and long-lasting dialysis. These patients accumulate several risk factors associated with poor patient and graft outcome [10].
The surgery of these patients is always challenging. Several surgical approaches exist but there is no standard technique [11].
Reported results of third transplantations are a bit inconsistent. Some studies demonstrated a similar survival rate to that of primary transplants, at least for patient survival [10, 12, 13]. However, the majority of the literature agrees on inferior graft survival with a higher complication rate [11, 14–16].
The kidney transplantation program started in 1973 in Hungary, and today there is a constant need for repeat transplantations [17]. Accurate knowledge of prognosis may help in the judicious and responsible use of deceased donor kidneys.
Aims
The aim of our study was to analyse our results of third transplantations and compare them with primary ones.
Materials and methods
This is a single-centre, retrospective, observational study from the largest Hungarian kidney transplant centre within the Eurotransplant community, with an institutional experience of more than 5000 kidney transplants since 1973.
Patients
Between 2011 and 2016, 779 adult, kidney alone, brain-dead deceased donor transplantations were performed and included. Out of them, 82.9% (646) were first, 14.2% (111) second, 2.6% (20) third, and 0.3% (2) fourth transplantations.
Nine TRT recipients shared the donor with a first or second recipient. We compared the outcomes of these nine pairs as “selected third” (sTRT) vs. “non-third” (nTRT), as a mixed group of 7 first and 2 second recipients. We have no data about the kidney pair of the other eleven third recipients.
We prospectively registered the pre-, intra-, and postoperative data:
Sex, age, BMI, ECD, virology
PRA, HLA mismatch, immunosuppressive therapy, rejection
Surgical details and complications, hospital stay
DGF, kidney function, graft, and patient survival. Graft survival was recorded from transplantation to graft failure: graftectomy or return to dialysis. Death censored graft survival was counted.
The end of the observation period is March 2019.
Immunosuppression
Immunosuppression was given as per protocol. Maintenance therapy generally consists of tacrolimus, mycophenolic acid, and prednisolone triple therapy.
Induction therapy was given to all third and 7.9% of primer recipients, considering previous immunization, p = 0.000.
Surgical considerations
According to our centre policy, we require preoperative pelvic angio-CT to visualize the vascular anatomy of the potential TRT recipient. At least one of the previous grafts has to be removed prior to waitlisting. Removal of the specific kidney graft depends on clinical circumstances. Our centre’s preferred site is the right iliac fossa for primer, and if possible, for third transplant as well. Third transplantations were performed by experienced senior transplant surgeons.
Statistics
For descriptive statistical analysis, mean and median values, standard deviations, and absolute and relative frequencies were calculated. Qualitative data were compared by the Pearson Chi-square test. Quantitative variables were compared using Mann-Whitney U or Kruskal-Wallis test. Survival was analysed by the Kaplan-Meier method and compared with log-rank test. A p value of less than 0.05 was considered to be significant. Propensity score matching analysis was performed using logistic regression analysis, and then thirds were matched to primers with a 1:1 matching in propensity scores without replacement. The match tolerance was set to 0.1. Statistics were calculated by TIBCO Software Inc. (2018), Statistica (data analysis software system), version 13, and by IBM SPSS version 25.
Results
Donors
The demographic data of donors are presented in Table 1. TRT donors were younger (47.4 vs.52); there was no difference in BMI, CMV infection rate, or sex. The CMV infection rate represents Hungarian population data, amounting to 86% [18].
Table 1.
Demographics of donors
| Donors | PRT (646) | TRT (20) | p |
|---|---|---|---|
| Age, y (SD) | 52.0 (11.7) | 47.4 (9.8) | n.s. 0.073 |
| female, % | 44.7 | 40.0 | n.s. |
| BMI | 26.2 | 25.7 | n.s. |
| CMV IgG pos % | 82.8 | 80.0 | n.s. |
| ECD, % | 34 | 0 | 0.01 |
None of our third recipients received an ECD graft, while this rate is about 34% for primer grafts.
Recipients
Recipients’ data are presented in Table 2. TRT recipients are significantly younger than PRT recipients, (47.3 years vs. 53.4 years). Third recipients got significantly more HCV (20% vs. 2.1%), and slightly more CMV infection. The PRA level was much higher (34.4% vs. 2.5%) in the TRT group.
Table 2.
Demographics, virology, and immunology of the recipients
| All recipients | Paired recipients | |||||
|---|---|---|---|---|---|---|
| PRT (646) | TRT (20) | p | nTRT (9) | sTRT (9) | p | |
| Age, y (SD) | 53.4 (12.6) | 47.3 (9.8) | 0.016 | 53.7 | 48.9 | n.s. (0.27) |
| Female, % | 40.1 | 30,0 | n.s. | 66.7 | 22.2 | n.s. |
| BMI | 26.4 | 25.0 | 0.045 | 27.8 | 26.0 | n.s. |
| HCV pos, % | 2.1 | 20.0 | 0.000 | 0 | 11.1 | n.s. |
| CMV IgG pos, % | 81.5 | 95.0 | n.s. | 62.5 | 100 | n.s. |
|
CMV mismatch donor pos. Recipient neg. |
14.2% (92) | 5% (1) | n.s. | 22.2 (2) | 0 | 0.023 |
| Antigen mismatch | 3.0 | 3.1 | n.s. | 2.4 | 2.8 | n.s. |
| PRA level, % mean/median | 2.5/0.0 | 34.4/44.0 | 0.0000 | 4.7/0 | 30.0/7.0 | 0.0042 |
Considered significant if p < 0.05
Surgery, postop course, rejection
Details and exact numbers are presented in Table 3. The cold ischaemic time is the same (~ 14–15 h), or even shorter in the TRT group. The whole operative time is significantly longer in TRT (132 vs. 152 min) (verified by propensity matching analysis as well), with a significantly shorter handling time (42 vs. 35 min).
Table 3.
Surgical details and postop. course
| All recipients | Paired recipients | |||||
|---|---|---|---|---|---|---|
| PRT | TRT | p | nTRT | sTRT | p | |
| CIT (h) | 14.7 (SD: 4.3) | 14.4 (SD: 4.8) | n.s. | 14.9 | 12.8 | n.s. |
| HT (min.) | 42 (SD: 17) | 35 (SD: 13) | 0.02 | 38 | 36 | n.s. |
| OT (min.) | 132 (SD: 40) | 152 (SD: 37) | 0.02 | 118 | 142 | n.s. |
| Stent use % | 13.3 | 25.0 | n.s. | 11.1 | 11.1 | n.s. |
| DGF % | 22.4 | 60.0 | 0.00009 | 22.2 | 44.4 | n.s. |
| Haematoma % | 16.6 | 35.0 | 0.031 | 22.2 | 55.6 | n.s. |
| Lymphocele % | 4.0 | 0 | n.s. | 11.1 | 0 | n.s. |
| Vascular complications % | 2.94 | 5.0 | n.s. | 0 | 11.1 | n.s. |
| Ureter complications % | 8.7 | 20.0 | n.s (0.08) | 11.1 | 33.3 | n.s. |
| Reoperations within 30 days, % | 8.5 | 10.0 | n.s. | 11.1 | 22.2 | n.s. |
| Biopsy % | 22.4 | 40.0 | n.s. (0.06) | 33.3 | 66.7 | n.s. |
| Acute rejection % | 15.9 | 15.0 | n.s. | 22.2 | 22.2 | n.s. |
| Hospital stay (days) | 14.8 | 21.4 | 0.003 | 11.7 | 28.8 | 0.0213 |
Considered signficant if p < 0.05
We did not observe differences in vascular or ureter-related complications, and there were no more lymphoceles. In the TRT group, one arterial thrombosis caused graft loss; in the PRT group, we could save three kidneys out of 19 having vascular complications. The follow-up US revealed several perirenal hematomas, but they did not require more reoperation.
There were no differences in acute rejection rate (PRT: 15.9% vs. TRT: 15%).
The DGF rate proved to be much more frequent: 22.4% in the PRT and 60% in the TRT group, which is highly significant. In case of TRT, the OR of DGF is 5.2 (2.1–12.9).
Hospital stay proved to be roughly a week longer (PRT 14.8 vs. TRT 21.4 days); propensity analysis verified the significance.
Out of PRT, 78.2% got end-to-side uretero-ureteral anastomosis, while in the TRT group, 70% of recipients received neocystostomy; this difference is highly significant (see Table 4).
Table 4.
Types of ureter anastomosis
| ureteric | Neocystostomy | p | |
|---|---|---|---|
| PRT | 78.2% (504) | 21.7% (140) | 0.00 |
| TRT | 30% (6) | 70% (14) |
Considered significant if p < 0.05
Patient survival
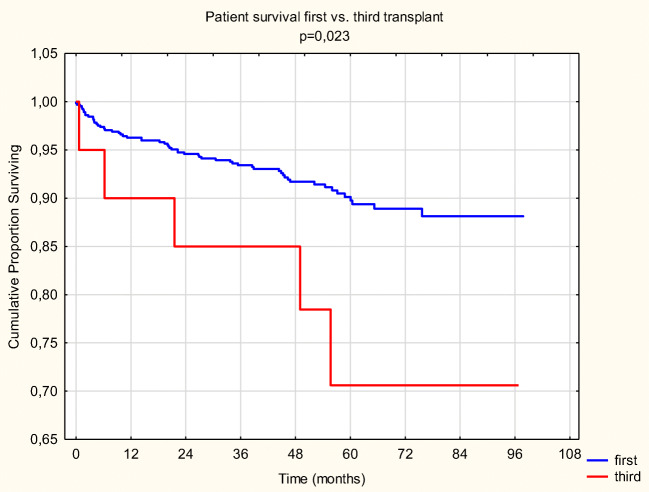
Patient survival at 1, 3, and 5 years for PRT is 96.4%, 93.9%, and 91.2%, and for TRT it is 90%, 85%, and 78.4%, respectively (p = 0.023) (Fig. 1). Propensity score matching analysis reconfirmed the significance of the difference.
Fig. 1.
Patient survival PRT vs. TRT
In the first 30 days, there were 3 (0.46%) deaths in the PRT and 1 (5%) in the TRT group (p = 0.0014). Until March 2019, we lost 56 more patients in the PRT group (∑: 59, which is 9.13%) and 4 more in the TRT group (∑: 5, which is 25%), resulting in a significant difference (p = 0.01).
The OR of a fatal outcome for TRT patient is 3.3 (1.16–9.4) compared to PRT.
Patient survival at 1, 3, and 5 years for nTRT is 100% and for sTRT is 77.7%, 66.7%, and 66.7% (p = 0.065).
Graft survival
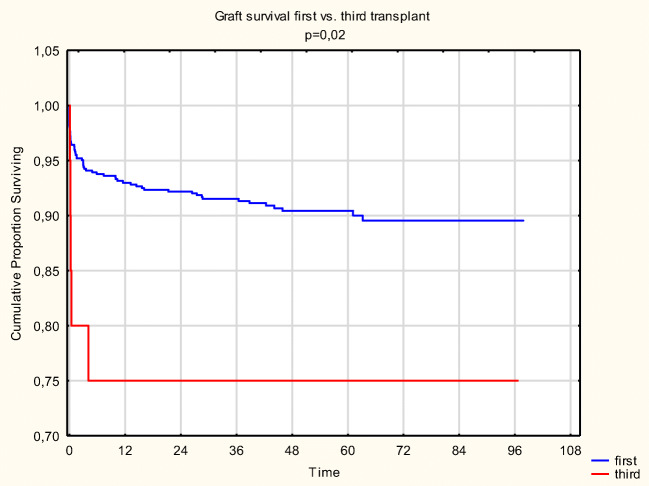
Death censored graft survival at 1, 3, and 5 years for PRT is 93.1%, 91.4%, and 90.3%, and for TRT, it is 75%, 75%, and 75%, respectively (p = 0.020) (Fig. 2). Propensity analysis revealed better graft survival for PRT; however, this was not significant.
Fig. 2.
Graft survival PRT vs. TRT
In the first 30 days, there were 26 (4.02%) graft losses in the PRT and 4 (20%) in the TRT group (p = 0.00069). One graft had arterial thrombosis caused by acute accelerated rejection; one had acute irreversible rejection; in the others, ischaemic lesions were observed even though ultrasound showed proper circulation.
We lost one more graft about 4 months later due to acute bacterial nephritis following several reoperations. In the long run, there were no more TRT graft losses.
From 2011 to March 2019, graft loss in surviving patients was 9.6% in the PRT and 25% in the TRT group (p = 0.024).
The odds ratio of graft loss for TRT recipients is 3.14 (1.1–8.9) compared to PRT.
Graft survival at 1, 3, and 5 years for nTRT is 100% and for sTRT is 55.6% (p = 0.028).
Patient and graft losses are summarized in Table 5.
Table 5.
Graft and patient loss
| All recipients | Paired recipients | |||||
|---|---|---|---|---|---|---|
| PRT | TRT | p | nTRT | sTRT | p | |
| Graft loss within 30 days % | 4.02 | 20 | 0.00069 | 0 | 33.3 | n.s. (0.058) |
| Graftectomy within 30 days, % | 3.25 | 5.0 | n.s. | 0 | 11.1 | n.s. |
| Graft loss total, % | 9.6 | 25 | 0.02411 | 0 | 44.4 | 0.02334 |
| Death within 30 days, % | 0.46 | 5.0 | 0.0014 | 0 | 11.1 | n.s. |
| Death total, % | 9.13 | 25 | 0.01 | 0 | 33.3 | n.s. (0.058) |
| Efficiency (working grafts) % | 85.76 | 60.0 | 0.00149 | 100 | 44.4 | 0.00851 |
Considered significant if p < 0.05
Kidney function
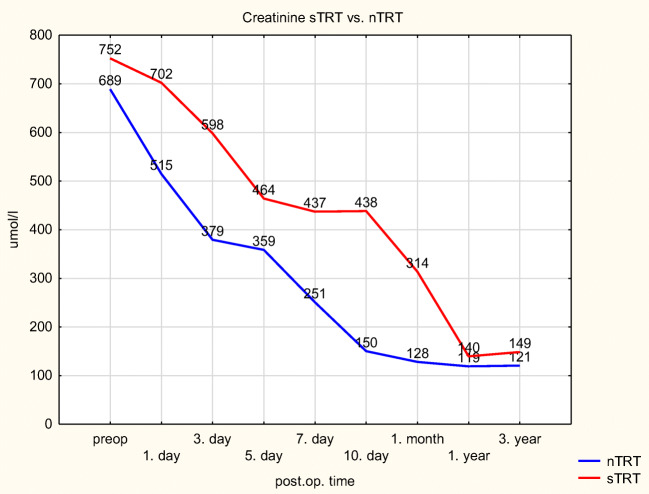
In the TRT group, the function of surviving kidneys is moderately and continuously inferior (see Table 6).
Table 6.
Postoperative serum creatinine (μmol/l)
| PRT | TRT | p | |
|---|---|---|---|
| Preop | 659 | 738 | 0,096 |
| Day 1 | 544 | 665 | 0,002 |
| Day 3 | 397 | 597 | 0,001 |
| Day 5 | 330 | 453 | 0,016 |
| Day 7 | 280 | 380 | 0,065 |
| Day 10 | 223 | 316 | 0,076 |
| Month 1 | 154 | 219 | 0,107 |
| Year 1 | 140 | 138 | 0,631 |
Comparing the sTRT and nTRT groups, who share the same donor, this difference is present, but it does not reach statistical significance either (see Fig. 3).
Fig. 3.
Kidney function nTRT vs. sTRT
Discussion
Donors
We did not perform any TRT from an ECD. We acknowledge that a selection bias is likely to exist at that point, favouring the TRT group. This practice is justified by the observation of Miles et al.: The survival of ECD retransplant recipients was not different from those remaining on dialysis [19]. The quality of kidneys used for TRT is the same or even better than the quality of those used for PRT [3, 16].
Recipients
Our rate of TRT, 2.6%, belongs into the upper range according to published data, varying from 1–1.4% [16, 20] to 2.4–2.9% [10, 14].
There were no really obese patients in our TRT group. A selection bias might exist at that point, too; a real obese candidate (BMI > 35) would be refused, as obesity poses a significant risk of surgical complications [21–23].
Many centres have observed that TRT patients are younger [2, 3, 14, 16, 20]. Third recipients at a younger age already have a long, chronic medical history. These repeated Uremia-dialysis-immunosuppression-surgery sequences obviously alter the patient’s body structure, cardiac and vascular status, etc. [11, 16, 24, 25]. This factor is probably highly underestimated as it is almost impossible to objectify. Kousoulas et al. presented a cut value, 43 years, as an independent risk factor for mortality [26]. Further efforts are to be taken to prevent early graft loss in these young patients with long predicted lifespan.
The rate of HCV positivity in the PRT group is slightly higher than the population-based frequency in Hungary (0.5–0.7%) [27, 28]. The infection rate reaches a tenfold higher rate in the TRT group, resulting in a highly significant difference.
The PRA level, a marker of prior sensitization, proved to be much higher in the TRT population. This is expected and consistent with all the known literature. Several papers report a trend that a PRA of more than 80% is associated with poor long-term graft function [15, 20, 29]. In our cohort, we could not demonstrate a significant relationship between the PRA level and the outcome. Out of twenty, only two of our TRT recipients had PRA > 80%, and both are doing well. This remains a controversial area of transplantation.
Surgery, postop course, rejection
The mean cold ischaemic time is the same for PRT and TRT. Comparing the nTRT and sTRT groups, there is an almost 2-h difference in favour of third transplantations.
TRT needs significantly longer operative time [3, 26, 30].
Surgical opinions vary in the question of previous transplant nephrectomy. Kienzl-Wagner et al. claim it is not necessary at all [25]. Another opinion is that it can be performed in the same setting prior to retransplantation [15, 24, 31].
Our policy is that at least one of the previous grafts has to be removed prior to waitlisting. We strongly believe that this is rational as both presence and removal might cause unexpected complications. TRT itself is a demanding long operation performed on a comorbid patient, and there is no need for any extension of the procedure.
Another potential surgical challenge is creating ureter anastomosis. The majority of centres perform neocystostomy for TRT patients [20, 25, 32], and uretero-ureteric anastomosis is reserved for technical difficulties [33]. Historically, a specialty of our centre is the creation of end-to-side uretero-ureteral anastomosis [34, 35]. But in the third transplant procedures, the native ureter of the recipient is more likely to be scaring or tight, and because of that, in most of the cases, neocystostomy has been performed. Yet, more ureter complications occurred in TRT than in PRT, not being significant, but seeming to be remarkable clinically.
Induction therapy was given after immunologic consideration in a few cases in PRT and to everyone in the TRT group with the likely result that there was no difference in acute rejection rates. This is important as the negative impact of even a single episode of acute rejection is well documented [20, 36–39]. However, this finding is in contrast with others [3, 12, 20].
The DGF rate proved to be significantly higher in TRT. Most authors assume that the increased rate of DGF is driven by recipient factors, namely, sensitization, rather than by donor factors [3, 16, 20, 25, 30, 40, 41].
Remarkably more indicative biopsies were obtained in TRT. We do not perform protocol biopsies.
Induction therapy, prolonged DGF, more frequent biopsy, and other factors resulted to a 1-week longer hospital stay, reaching a high significance.
All these factors together count for much higher expenses in case of a third transplantation [42, 43].
Outcome
Patient survival
Death in the first 30 days occurred in very few cases, due to cardiac failure or pneumonia with sepsis and ARDS. On the long run, cerebro- and cardiovascular events caused the death of our TRT patients, who are at a higher risk of fatal outcome. Our finding corresponds to the multicentre ET study [14]. Many other groups reported similar patient survival as compared to first and second transplants, but the leading causes of death, cardiovascular events and sepsis, correlate with our findings [3, 10, 12, 13, 24, 25, 44].
On the other hand, even third transplantation is associated with a significant survival advantage relative to remaining on dialysis, provided that an SCD donor organ was used [4, 6, 11, 16, 19].
Graft survival
Graft losses occurred in the first 6 months, and in our paired subpopulation (sTRT vs. nTRT), the same was observed. This early graft loss is likely the cause of inferior graft survival [2, 16, 25, 30, 31]. Assfalg et al. in the ET study observed significantly worse patient and graft survival, and the authors found this issue so pronounced that they question the current policy of repeated retransplantations, especially the forth ones [14]. However, Horovitz et al., who compared the kidneys from paired donors, demonstrated only insignificant differences [3].
We had no TRT graft loss due to surgical reasons. Graft loss occurred as the result of either early rejection or chronic allograft dysfunction, which is in agreement with others [3, 10, 20, 25, 30].
We introduced a not too scientific, but practical parameter to assess the efficiency of our labour investment into third transplantations. The rate of working kidneys in living recipients per performed transplantations is much higher following PRT.
TRT and sTRT recipients show a lower GFR until the end of the first post-transplant year. This might have a clinical importance, although mathematically it is not significant.
Summary
In a third transplant, younger recipients receive a younger, good quality kidney, and still both graft and patient survival are significantly inferior to primer ones. Third kidney transplantations may be performed safely by experienced, senior surgeons, but they represent an intense surgical challenge. The main cause of graft loss is rather immune mediated than surgical. This sensitized patient population requires profound immunosuppression with all its risks and consequences.
Patients are at a high mortality risk receiving a third transplant, but this is probably less than remaining on dialysis. A meticulous patient selection is mandatory with a view to reducing post-transplant mortality. Immunotherapy, postoperative dialysis, and prolonged hospital stay cause remarkable expenses. Further prospective studies should be performed to compare the third transplantation with the continuation of dialysis.
Acknowledgement
Open Access funding provided by Semmelweis University. We say special thanks to János Fekete from the Department of Bioinformatics, Semmelweis University, for the statistical help and calculations.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ATG
Anti-thymocyte globulin
- BMI
Body mass index
- CIT
Cold ischaemic time
- CMV
Cytomegalovirus
- CT
Computed tomography
- DBD
Donation after brain death
- DCD
Donation after cardiac death
- DGF
Delayed graft function
- ECD
Expanded criteria donor
- ET
Eurotransplant
- HCV
Hepatitis C
- HLA
Human leukocyte antigen
- HT
Handling, or “anastomosis” time
- KDPI
Kidney donor profile index
- KDRI
Kidney donor risk index
- MA
Mycophenolic acid
- nTRT
Non-third renal transplant
- OR
Odds ratio
- OT
Operation time
- PRA
Panel-reactive antibodies
- PRT
Primer renal transplant
- SCD
Standard criteria donor
- SD
Standard deviation
- sTRT
Selected third renal transplant
- TAC
Tacrolimus
- TRT
Third renal transplant
- US
Ultrasound
Authors’ contributions
Each author participated in the transplantations, collection and analysis of data, writing parts of the paper, and approving the final version.
Data availability
Data are available at the corresponding author.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meier-Kriesche HU, Schold JD. Kaplan B long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4(8):1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 2.Magee JC, Barr ML, Basadonna GP, Johnson MR, Mahadevan S, McBride MA, Schaubel DE, Leichtman AB. Repeat organ transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1424–1433. doi: 10.1111/j.1600-6143.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 3.Horovitz D, Caumartin Y, Warren J, Sheikh AA, Bloch M, Kapoor A, Jevnikar AM, Luke PPW (2009) Outcome of third renal allograft retransplants versus primary transplants from paired donors. Transplantation. 87(8):1214–1220. 10.1097/TP.0b013e31819f0f5c [DOI] [PubMed]
- 4.Ojo A, Wolfe RA, Agodoa LY, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States renal data system. Transplantation. 1998;66(12):1651–1659. doi: 10.1097/00007890-199812270-00014. [DOI] [PubMed] [Google Scholar]
- 5.McCaughan JA, Patterson CC, Maxwell AP, Courtney AE. Factors influencing survival after kidney transplant failure. Transplant Res. 2014;3:18. doi: 10.1186/2047-1440-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao PS, Schaubel DE, Wei G, Fenton SS. Evaluating the survival benefit of kidney retransplantation. Transplantation. 2006;82(5):669–674. doi: 10.1097/01.tp.0000235434.13327.11. [DOI] [PubMed] [Google Scholar]
- 7.Coupel S, Giral-Classe M, Karam G, Morcet JF, Dantal J, Cantarovich D, Blancho G, Bignon JD, Daguin P, Soulillou JP, Hourmant M. Ten-year survival of second kidney transplants: impact of immunologic factors and renal function at 12 months. Kidney Int. 2003;64(2):674–680. doi: 10.1046/j.1523-1755.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 8.Barocci S, Valente U, Fontana I, Tagliamacco A, Santori G, Mossa M, Ferrari E, Trovatello G, Centore C, Lorenzi S, Rolla D, Nocera A. Long-term outcome on kidney retransplantation: a review of 100 cases from a single center. Transplant Proc. 2009;41(4):1156–1158. doi: 10.1016/j.transproceed.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 9.Yeo SM, Kim Y, Kang SS, Park WY, Jin K, Park SB, Park UJ, Kim HT, Cho WH, Han S. Long-term clinical outcomes of kidney re-transplantation. Transplant Proc. 2017;49(5):997–1000. doi: 10.1016/j.transproceed.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Loupy A, Anglicheau D, Suberbielle C, Mejean A, Martinez F, Zuber J, Mamzer-Bruneel MF, Kreis H, Thervet E, Thiounn N, Legendre C. Long-term outcome of third kidney transplants. Nephrol Dial Transplant. 2007;22(9):2693–2700. doi: 10.1093/ndt/gfm226. [DOI] [PubMed] [Google Scholar]
- 11.Halawa A. The third and fourth renal transplant, technically challenging, but still a valid option. Ann Transplant. 2012;17(4):125–132. doi: 10.12659/aot.883703. [DOI] [PubMed] [Google Scholar]
- 12.Matas AJ, Gillingham KJ, Payne WD, et al. A third kidney transplant: cost-effective treatment for end-stage renal disease? Clin Transpl. 1996;10(6 Pt 1):516–520. [PubMed] [Google Scholar]
- 13.Friedersdorff F, Patabendhi S. Busch J et al Outcome of patients after third and fourth kidney transplantation. Urol Int. 2016;97(4):445–449. doi: 10.1159/000445216. [DOI] [PubMed] [Google Scholar]
- 14.Assfalg V, Selig K, Tolksdorf J, Meel M, Vries E, Ramsoebhag AM, Rahmel A, Renders L, Novotny A, Matevossian E, Schneeberger S, Rosenkranz AR, Berlakovich G, Ysebaert D, Knops N, Kuypers D, Weekers L, Muehlfeld A, Rump LC, Hauser I, Pisarski P, Weimer R, Fornara P, Fischer L, Kliem V, Sester U, Stippel D, Arns W, Hau HM, Nitschke M, Hoyer J, Thorban S, Weinmann-Menke J, Heller K, Banas B, Schwenger V, Nadalin S, Lopau K, Hüser N, Heemann U. Repeated kidney re-transplantation - the Eurotransplant experience: a retrospective multicenter outcome analysis. Transpl Int. 2020;33(6):617–631. doi: 10.1111/tri.13569. [DOI] [PubMed] [Google Scholar]
- 15.Blanco M, Medina J, Gonzalez E, Dominguez M, Rodriguez A, Pamplona M, Andres A, Leiva O, Morales JM. Third kidney transplantation: a permanent medical-surgical challenge. Transplant Proc. 2009;41(6):2366–2369. doi: 10.1016/j.transproceed.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 16.Redfield RR, Gupta M, Rodriguez E, Wood A, Abt PL, Levine MH. Graft and patient survival outcomes of a third kidney transplant. Transplantation. 2015;99(2):416–423. doi: 10.1097/TP.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perner F, Megyaszai S, Járay J, Faller J, Szécsény A. Successful kidney transplantation in Hungary. Orv Hetil. 1974;115(35):2067–2068. [PubMed] [Google Scholar]
- 18.Varga M, Gorog D, Kari D, et al. Cytomegalovirus seroprevalence among solid organ donors in Hungary: correlations with age, gender, and blood group. Transplant Proc. 2011;43(4):1233–1235. doi: 10.1016/j.transproceed.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 19.Miles CD, Schaubel DE, Jia X, Ojo AO, Port FK, Rao PS. Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys. Am J Transplant. 2007;7(5):1140–1147. doi: 10.1111/j.1600-6143.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- 20.Hagan C, Hickey DP. Little DM a single-center study of the technical aspects and outcome of third and subsequent renal transplants. Transplantation. 2003;75(10):1687–1691. doi: 10.1097/01.TP.0000062536.34333.BB. [DOI] [PubMed] [Google Scholar]
- 21.Kamdoum Nanfack ML, Bayoud Y, Marchand C, Cholley I, Leon P, Fournier R, Lassere T, Larre S. Is obesity a barrier to kidney transplantation? Prog Urol. 2015;25(1):40–46. doi: 10.1016/j.purol.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Veasey TM, Fleming JN, Strout SE, Miller R, Pilch NA, Meadows HB, Mardis CR, Mardis BA, Shenvi S, McGillicuddy J, Chavin KD, Baliga P, Taber DJ. Morbid obesity and functional status as predictors of surgical complication after renal transplantation. Am J Surg. 2018;215(4):663–668. doi: 10.1016/j.amjsurg.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Dabare D, Kassimatis T, Hodson J, Khurram MA, Papadakis G, Rompianesi G, Shaw O, Karydis N, Callaghan C, Olsburgh J, Mamode N, Kessaris N, Loukopoulos I. Outcomes in third and fourth kidney transplants based on the type of donor. Transplantation. 2019;103(7):1494–1503. doi: 10.1097/TP.0000000000002428. [DOI] [PubMed] [Google Scholar]
- 24.Izquierdo L, Peri L, Piqueras M, Revuelta I, Alvarez-Vijande R, Musquera M, Oppenheimer F, Alcaraz A. Third and fourth kidney transplant: still a reasonable option. Transplant Proc. 2010;42(7):2498–2502. doi: 10.1016/j.transproceed.2010.04.064. [DOI] [PubMed] [Google Scholar]
- 25.Kienzl-Wagner K, Mark W, Maglione M, Brandacher G, Öllinger R, Margreiter R, Pratschke J, Bösmüller C. Single-center experience with third and fourth kidney transplants. Transpl Int. 2011;24(8):780–786. doi: 10.1111/j.1432-2277.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- 26.Kousoulas L, Vondran FW, Syryca P, Klempnauer J, Schrem H, Lehner F. Risk-adjusted analysis of relevant outcome drivers for patients after more than two kidney transplants. J Transp Secur. 2015;2015:712049. doi: 10.1155/2015/712049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barna TK, Ozsvár Z, Szendrényi V, Gál G. Hepatitis C virus antibody in the serum of blood donors. Orv Hetil. 1996;137(10):507–511. [PubMed] [Google Scholar]
- 28.Pár A, Pár G. Three decades of the hepatitis C virus from the discovery to the potential global elimination: the success of translational researches. Orv Hetil. 2018;159(12):455–465. doi: 10.1556/650.2018.30997. [DOI] [PubMed] [Google Scholar]
- 29.Hirata M, Terasaki PI (1994) Renal retransplantation. Clin Transpl 419–433 [PubMed]
- 30.Mazzucchi E, Danilovic A, Antonopoulos IM, Piovesan AC, Nahas WC, Lucon AM, Srougi M. Surgical aspects of third and subsequent renal transplants performed by the extraperitoneal access. Transplantation. 2006;81(6):840–844. doi: 10.1097/01.tp.0000203559.57088.f6. [DOI] [PubMed] [Google Scholar]
- 31.Gutiérrez Baños JL, Rodrigo Calabia E, Rebollo Rodrigo M d H, et al. Surgical details and complications from retransplantation into the iliac fossa for third and fourth kidney transplants. Arch Esp Urol. 2005;58(2):121–129. doi: 10.4321/s0004-06142005000200005. [DOI] [PubMed] [Google Scholar]
- 32.Nourbala MH, Ghaheri H, Kardavani B. Our experience with third renal transplantation: results, surgical techniques and complications. Int J Urol. 2007;14(12):1057–1059. doi: 10.1111/j.1442-2042.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 33.Musquera M, Peri LL, Alvarez-Vijande R, Oppenheimer F, Gil-Vernet JM. Alcaraz a Orthotopic kidney transplantation: an alternative surgical technique in selected patients. Eur Urol. 2010;58(6):927–933. doi: 10.1016/j.eururo.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Perner F, Járay J, Alföldy F, Makó E, Hernády T, Szécsény A. Experiences with ureteroureteral anastomosis in renal transplant: early complications and late results. Transplant Proc. 1984;16(5):1335–1336. [PubMed] [Google Scholar]
- 35.Perner F, Járay J, Alföldy F, et al. The results of 1009 kidney transplantations performed in Hungary. Surg Today. 1996;26:561. doi: 10.1007/BF00311568. [DOI] [PubMed] [Google Scholar]
- 36.Meier-Kriesche HU, Ojo AO, Hanson JA, Cibrik DM, Punch JD, Leichtman AB, Kaplan B. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation. 2000;70(7):1098–1100. doi: 10.1097/00007890-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 37.Joseph JT, Kingsmore DB, Junor BJ, Briggs JD, Mun Woo Y, Jaques BC, Hamilton DN, Jardine AG, Jindal RM. The impact of late acute rejection after cadaveric kidney transplantation. Clin Transpl. 2001;15(4):221–227. doi: 10.1034/j.1399-0012.2001.150401.x. [DOI] [PubMed] [Google Scholar]
- 38.Koo EH, Jang HR, Lee JE, Park JB, Kim SJ, Kim DJ, Kim YG, Oh HY, Huh W. The impact of early and late acute rejection on graft survival in renal transplantation. Kidney Res Clin Pract. 2015;34(3):160–164. doi: 10.1016/j.krcp.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benkö T, Halfmann P, Gäckler A, Radünz S, Treckmann JW, Kaiser GM, Hoyer DP. Long-term outcome of third, fourth and fifth kidney transplantation: technical aspects and immunological challenges. Clin Kidney J. 2019;12(6):895–900. doi: 10.1093/ckj/sfz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghods AJ, Savaj S, Abbasi M, Heidari H. Rokhsatyazdi H The incidence and risk factors of delayed graft function in 689 consecutive living unrelated donor renal transplantation. Transplant Proc. 2007;39(4):846–847. doi: 10.1016/j.transproceed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 41.López-Hoyos M, Fernández-Fresnedo G, Rodrigo E, Ruiz JC, Arias M. Effect of delayed graft function in hypersensitized kidney transplant recipients. Hum Immunol. 2005;66(4):371–377. doi: 10.1016/j.humimm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 42.Barreto MFC, Dellaroza MSG, Fernandes KBP, Pissinati PSC, Galdino MJQ, Haddad MDCFL. Hospitalization costs and their determining factors among patients undergoing kidney transplantation: a cross-sectional descriptive study. Sao Paulo Med J. 2019;137(6):498–504. doi: 10.1590/1516-3180.2018.055117092019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaib-Eddour D, Chaib-Eddour H, Malaise J, Mourad M, Squifflet JP. Cost of renal transplant in Belgium. Transplant Proc. 2005;37(6):2819–2820. doi: 10.1016/j.transproceed.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 44.Reboux AH, Kamar N, Fort M, Rischmann P, Malavaud B, Cointault O, Abbal M, Durand D, Rostaing L. A third renal transplantation: is it relevant and is it worth it? Transplant Proc. 2005;37(10):4199–4202. doi: 10.1016/j.transproceed.2005.11.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at the corresponding author.