Abstract
With tyrosine kinase inhibitor (TKI) therapy, chronic myelogenous leukemia (CML) is now a chronic disease. CML patients treated with TKIs (n=1,200) were identified from the OptumLabs® Data Warehouse (de-identified claims and electronic health records) between 2000-2016 and compared with a non-cancer cohort (n=7,635). The 5-year cumulative incidence of all organ system outcomes was significantly greater for the TKI versus non-cancer group. In the first year, compared with imatinib, later generation TKIs were associated with primary infections (hazard ratios [HR] 1.43, 95% CI 1.02-2.00), circulatory events (HR 1.15, 95% CI 1.01-1.31), and skin issues (HR 1.43, 95% CI 1.13-1.80); musculoskeletal and nervous system/sensory issues were less common (HRs 0.83-0.84, p<0.05). Increased risk of infections, cardiopulmonary and skin issues associated with later generation TKIs persisted in subsequent years. In this real-world population, TKI therapy was associated with a high burden of adverse events. Later generation TKIs may have greater toxicity than imatinib.
Keywords: chronic myelogenous leukemia, tyrosine kinase inhibitor, complications, epidemiology, insurance
INTRODUCTION
Chronic myelogenous leukemia (CML) comprises approximately 15% of leukemia diagnoses in the United States and nearly 10,000 new patients are diagnosed with CML annually [1]. Since imatinib was approved as first-line therapy for CML in 2001, annual CML mortality has decreased significantly, with 10-year overall survival from the initial IRIS study of imatinib exceeding 80% [2]. Presently, in addition to imatinib, several newer tyrosine kinase inhibitors (TKIs; dasatinib, nilotinib, bosutinib, ponatinib) have been widely approved for CML treatment. While allogeneic hematopoietic cell transplantation (HCT) remains the only definitively curative treatment for CML, its treatment-related toxicity limits its application, particularly among older patients and those with more pre-existing comorbidities. Even among younger, relatively healthy patients, including children, TKIs have supplanted HCT as the initial treatment of choice.
While most imatinib adverse events tend to be mild and often resolve spontaneously or can be reduced by brief drug holidays, rare, but serious side effects have been reported more commonly with later generation TKIs [3]. These include: an increased risk of bleeding independent of platelet count, and pleural effusions (especially dasatinib); arrhythmia (especially nilotinib); edema and possibly heart failure, vascular disease, thrombosis, and hepatic toxicity (especially ponatinib) [3]. Patients on long-term TKI treatment, particularly those who are younger, also have reported significantly lower health-related quality of life versus age-matched population controls [4].
Therefore, due to the current need for long-term, and for many, indefinite TKI therapy, significant questions remain concerning its impact on long-term health outcomes in CML patients beyond mortality. Even though TKI discontinuation recommendations are beginning to be issued, most patients would be expected to remain on therapy for at least four to five years, and many who stop TKIs need to resume at a later date [5]. Thus, to address this gap in knowledge, we used de-identified administrative claims data from a longitudinal, real-world data asset to examine adverse events in CML patients treated with TKI and without HCT, compared with the general population without cancer.
METHODS
Study Population
This retrospective study used de-identified data (not classified as human subjects research) from the OptumLabs® Data Warehouse (OLDW), which includes claims for commercially insured and Medicare Advantage enrollees, representing a diverse mixture of ages, ethnicities and geographical regions across the US [6-8]. Eligible patients were those who had a non-diagnostic medical claim for CML (International Classification of Diseases, 9th revision [ICD-9], code 205.1, or ICD-10 code C92.1) beginning January 1, 2000 through October 31, 2016. These codes do not provide information on disease phase. We defined the initial CML claim date as our “index” date. To reduce the possibility of misclassification, eligible CML patients were required to have at least one additional CML code 30 to 365 days after the initial claim. Although the OLDW cannot provide a definitive cancer diagnosis date, we required eligible patients to be continuously enrolled for at least 180 days prior to the index date, increasing the likelihood that the index date represents the initial CML diagnosis. We also excluded any individual with a solid tumor diagnosis code during the 180 days pre-index and 5-years post-index time periods. Within the CML population we focused on those with any TKI prescriptions (CML+TKI). TKIs of interest included first generation (imatinib) and later generation (dasatinib, nilotinib, bosutinib, ponatinib) agents. CML patients who received HCT regardless of TKI exposure (defined using ICD-9 codes 279.5, 996.85, V42.81, V42.82, and ICD-10 codes T86.0, Z94.8; n=159) were excluded from this analysis. CML patients who did not have either TKI prescriptions or HCT codes (n=473) were also excluded from further analysis.
We identified enrollees without any cancer diagnoses over their enrollment period to serve as a general population (GEN) comparison group. GEN was frequency-matched on an approximately 4:1 ratio based on age (10-year increments), sex, index year (3-year increments), geographic region (10 US census divisions), and insurance (commercial versus Medicare) to the entire CML group. Because GEN patients do not have a natural diagnosis date, a proxy index date was chosen for these individuals defined as their health plan enrollment date plus a randomly generated number of days, based upon a gamma distribution. A gamma distribution was chosen because it best represented the distribution of days between enrollment date and diagnosis date in the CML group. All GEN enrollees (similar to the CML group) were required to be continuously enrolled in the OLDW for at least 180 days prior to their proxy index date and at least 365 days post-index.
Adverse Events and Explanatory Variables
The OLDW contains claims information for all prescription medications and medical services submitted to a health plan for payment. For this analysis, similar to our prior work [9], we assigned ICD-9 and equivalent ICD-10 codes to major organ system-based categories, as well as a potential TKI side effect category focused on several of the more unique TKI-associated adverse effects (Supplemental Table 1). In addition to the demographic characteristics mentioned above, the OLDW also provided information on each individual’s race/ethnicity, household income, and baseline Charlson Comorbidity Index (CCI). Race/ethnicity, household income, and educational level are sourced from a national supplier of consumer marketing data. Household income was imputed based on a model using both public and private consumer data. The CCI was defined using claims data from the initial 120 days pre-index (i.e., we excluded the two-month period immediately prior to the index date given concern that the presentation and diagnosis of cancer may inflate one’s baseline’s CCI) and excluded cancer-related items. From the OLDW, we also determined annualized variables for healthcare utilization (number of ambulatory and emergency room visits, and inpatient days) and percentage of days with a TKI prescription in the 6 months preceding the adverse event of interest (excluding the month immediately prior to the event).
Statistical Analyses
We examined the distribution of baseline demographic and healthcare utilization characteristics. For continuous variables, differences were assessed using ANOVA and pairwise T-test if normally distributed, or Wilcoxon rank sum or Kruskal-Wallis tests if not normally distributed. For categorical variables, Chi-square tests were used. For each adverse events category, we then estimated the 5-year cumulative incidence and associated differences with 95% CIs among the CML+TKI and GEN cohorts starting from the index time point. Follow-up of individuals without events was censored on October 31, 2017 or health plan disenrollment date if earlier.
For analysis of risk factors within the CML-TKI group, we used Cox proportional hazards models with time since index as the time scale to estimate the hazard ratios (HRs) and 95% CIs for each event category through five years adjusted for sex, age at index date, index year, race/ethnicity, and CCI. Because these are multiple-failure data, we used the Prentice-Williams-Peterson regression method, which stratifies the baseline hazard by event number without assuming that events are independent, and uses the total time since index as the time scale [10]. We also explored the potential influence of different TKI agents (imatinib versus later generation agents), the percentage of preceding days with TKI prescription coverage (approximate tertiles: <65%, 65-90%, >90%), and the impact of prior healthcare utilization. For all Cox models, hazard assumptions were assessed visually by log-log plots and no prominent departures from proportionality were noted [11]. Analyses were completed using Stata/SE 15 (College Station, Texas).
RESULTS
As key demographic characteristics were frequency-matched between GEN and the CML group, the median diagnosis age of the CML+TKI (55 years [IQR 44-66]) and GEN (56 years [IQR 44-69]) groups were similar (Table 1). Both groups had similar median follow-up of approximately three years, with 24.3% of the CML+TKI group and 21.9% of the GEN group having continuous follow-up exceeding five years. Within the CML+TKI group, 73.8% received imatinib (51.7% did not receive any other TKI), 31.0% received dasatinib (16.0% as the initial agent), and 26.3% received nilotinib (13.6% as the initial agent). Bosutinib and ponatinib usage was rare (2.5% and 1.8% respectively; n<11 receiving these agents as the initial drug).
TABLE 1.
Baseline demographic characteristics of chronic myelogenous leukemia (CML) patients treated with tyrosine kinase inhibitors (TKI) only and those who received hematopoietic cell transplantation (HCT) and the general (GEN) population
| Characteristics (%) | GEN N=7,635 |
CML+TKI N=1,200 |
||
|---|---|---|---|---|
| Female | 3459 | (45.3) | 524 | (43.7) |
| Age at diagnosis, y | ||||
| <20 | 175 | (2.3) | 16 | (1.3) |
| 20-39 | 1164 | (15.2) | 206 | (17.2) |
| 40-59 | 2924 | (38.3) | 537 | (44.8) |
| 60-69 | 1512 | (19.8) | 238 | (19.8) |
| ≥70 | 1860 | (24.4) | 203 | (16.9) |
| Race | ||||
| White | 5502 | (72.1) | 878 | (73.2) |
| Black | 794 | (10.4) | 125 | (10.4) |
| Asian | 290 | (3.8) | 43 | (3.6) |
| Hispanic | 636 | (8.3) | 102 | (8.5) |
| Unknown | 413 | (5.4) | 52 | (4.3) |
| Household income | ||||
| <$50,000 | 1950 | (25.5) | 280 | (23.3) |
| $50,000-$74,999 | 1185 | (15.5) | 177 | (14.8) |
| $75,000-$99,999 | 938 | (12.3) | 158 | (13.2) |
| ≥$100,000 | 2194 | (28.7) | 390 | (32.5) |
| Unknown | 1368 | (17.9) | 195 | (16.3) |
| Index year | ||||
| 2000-2004 | 1246 | (16.3) | 151 | (12.6) |
| 2005-2009 | 2213 | (29.0) | 357 | (29.8) |
| 2010-2014 | 3088 | (40.4) | 496 | (41.3) |
| 2015-2016 | 1088 | (14.3) | 196 | (16.3) |
| Health plan type | ||||
| Commercial | 5543 | (72.6) | 951 | (79.3) |
| Medicare Advantage | 2092 | (27.4) | 249 | (20.8) |
| Charlson comorbidity index | ||||
| 0 | 6709 | (87.9) | 1023 | (85.3) |
| 1 | 582 | (7.6) | 96 | (8.0) |
| ≥2 | 344 | (4.5) | 81 | (6.8) |
| Median enrollment, y (interquartile range) | 2.8 | (1.7-4.6) | 2.9 | (1.7-4.9) |
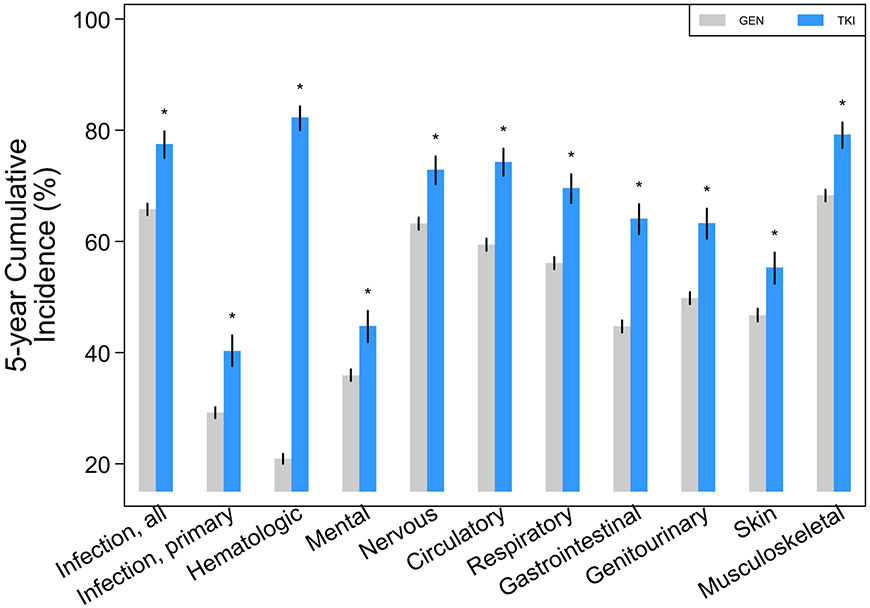
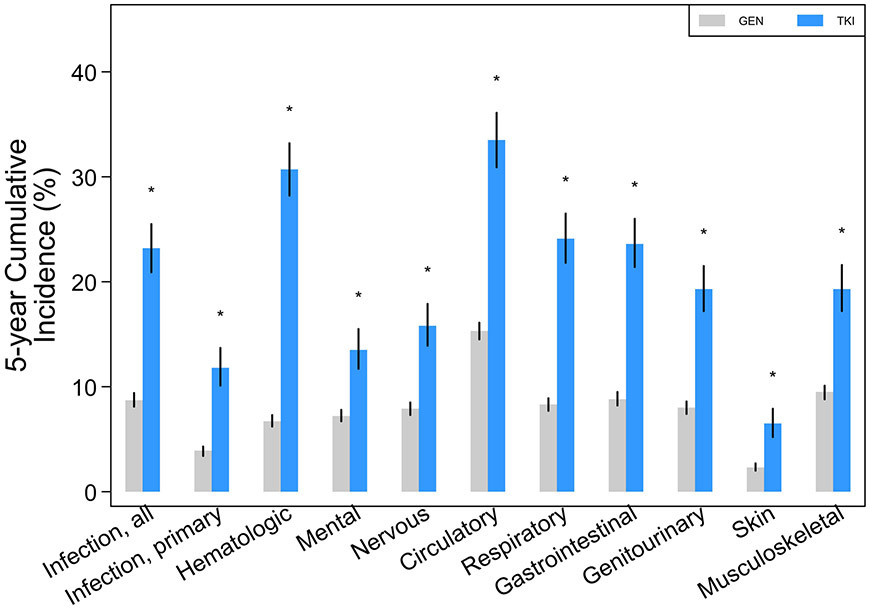
The 5-year cumulative incidence of almost all major organ system outcomes was significantly greater for the CML+TKI group compared with GEN (Figure 1A; Table 2). The differences remained significant even when cumulative incidences were limited to events occurring only in the first-year post-index (Supplemental Table 2), if the first year was excluded and only events occurring in years 2 to 5 were analyzed (Supplemental Table 3), and if claims were limited to only those occurring as an inpatient (Figure 1B; Supplemental Table 4).
FIGURE 1.
Five-year cumulative incidence with 95% CIs (spikes) of adverse events among chronic myelogenous leukemia patients treated with tyrosine kinase inhibitors (TKI) compared with the general population (GEN): (a) all events, inclusive of ambulatory, emergency room, and inpatient claims; (b) events limited to inpatient claims alone. “*” denotes that rates were significantly different (p<0.05) versus GEN.
TABLE 2.
Five-year cumulative incidence with 95% CIs of all adverse events (ambulatory, emergency room, inpatient) among CML patients treated with tyrosine kinase inhibitors (TKI) compared with the general population (GEN)
| Events category | GEN |
CML+TKI |
||
|---|---|---|---|---|
| Cum. Incid. | (95% CI) | Cum. Incid. | (95% CI) | |
| Infection, all | 65.8 | (64.7-66.8) | 77.5 | (75.0-79.8)* |
| Infection, primary | 29.2 | (28.2-30.2) | 40.3 | (37.6-43.1)* |
| Hematologic | 20.9 | (20.0-21.8) | 82.3 | (80.0-84.3)* |
| Mental | 35.9 | (34.9-37.0) | 44.8 | (41.9-47.5)* |
| Nervous/sensory | 63.2 | (62.1-64.3) | 72.9 | (70.3-75.3)* |
| Circulatory | 59.4 | (58.3-60.5) | 74.3 | (71.8-76.7)* |
| Respiratory | 56.1 | (55.0-57.2) | 69.6 | (66.9-72.1)* |
| Gastrointestinal | 44.7 | (43.6-45.8) | 64.1 | (61.3-66.7)* |
| Genitourinary | 49.8 | (48.7-50.9) | 63.3 | (60.5-65.9)* |
| Skin | 46.7 | (45.6-47.9) | 55.3 | (52.4-58.0)* |
| Musculoskeletal | 68.3 | (67.2-69.3) | 79.2 | (76.8-81.4)* |
Significantly (p<0.05) different from GEN rates
Among CML+TKI patients in the first year of treatment, compared with imatinib, later generation TKIs were more likely to be associated with primary infections (HR 1.43, 95% CI 1.02-2.00), circulatory events (HR 1.15, 95% CI 1.01-1.31), skin issues (HR 1.43, 95% CI 1.13-1.80), and potential TKI-specific side effects in general (HR 1.37, 95% CI 1.06-1.77; Table 3). Among infection subtypes, later generation TKIs were associated with an approximate two-fold or greater increased risk of fungal and viral infections compared with imatinib. Among specific circulatory and respiratory side effects, later generation TKIs were associated with a significantly increased risk of pleural effusions and a borderline risk of hypertension. However, nervous system/sensory and musculoskeletal issues were less common with later generation TKIs versus imatinib (HR 0.83-0.84, p<0.05).
TABLE 3.
Relative hazards (HR) of adverse events in relation to TKI type among CML patients treated with tyrosine kinase inhibitors (TKI)*
| 1st year of therapy† |
Years 2-5 of therapy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events category | Imatinib | Later generation TKI‡ | Imatinib | Later generation TKI‡ | ||||||||
| Claims | Rate per person-y |
Claims | Rate per person-y |
HR | (95% CI) | Claims | Rate per person-y |
Claims | Rate per person-y |
HR | (95% CI) | |
| Infection, all | 519 | 1.45 | 285 | 1.68 | 1.11 | (0.92-1.33) | 2174 | 1.53 | 1806 | 2.28 | 1.17 | (1.06-1.29) |
| Infection, primary | 145 | 0.41 | 90 | 0.53 | 1.43 | (1.02-2.00) | 609 | 0.43 | 520 | 0.66 | 1.16 | (0.96-1.40) |
| Bacterial | 59 | 0.17 | 22 | 0.13 | 1.02 | (0.58-1.79) | 248 | 0.18 | 189 | 0.24 | 0.96 | (0.68-1.36) |
| Fungal | 30 | 0.08 | 26 | 0.15 | 2.76 | (1.47-5.17) | 202 | 0.14 | 207 | 0.26 | 1.38 | (1.02-1.87) |
| Viral | 57 | 0.16 | 43 | 0.25 | 1.81 | (1.20-2.74) | 177 | 0.13 | 168 | 0.21 | 1.34 | (1.02-1.76) |
| Hematologic | 1266 | 3.55 | 611 | 3.60 | 1.00 | (0.88-1.13) | 3542 | 2.50 | 2122 | 2.68 | 0.95 | (0.86-1.04) |
| Mental | 502 | 1.41 | 225 | 1.32 | 1.04 | (0.86-1.26) | 1880 | 1.33 | 1308 | 1.65 | 1.11 | (0.95-1.30) |
| Nervous/sensory | 845 | 2.37 | 360 | 2.12 | 0.83 | (0.71-0.98) | 3289 | 2.32 | 1970 | 2.49 | 1.09 | (0.99-1.20) |
| Circulatory§ | 1191 | 3.34 | 751 | 4.42 | 1.15 | (1.01-1.31) | 5104 | 3.60 | 3550 | 4.48 | 1.10 | (1.02-1.19) |
| Myocardial ischemia | 220 | 0.62 | 132 | 0.78 | 1.18 | (0.85-1.65) | 1103 | 0.78 | 764 | 0.96 | 1.11 | (0.92-1.33) |
| Stroke | 22 | 0.06 | 68 | 0.40 | 1.65 | (0.86-3.18) | 129 | 0.09 | 251 | 0.32 | 1.95 | (1.39-2.73) |
| Venous thrombosis | 41 | 0.11 | <11 | -- | 0.60 | (0.27-1.33) | 156 | 0.11 | 30 | 0.04 | 0.45 | (0.23-0.88) |
| Hypertension | 690 | 1.93 | 455 | 2.68 | 1.17 | (0.99-1.37) | 2788 | 1.97 | 2013 | 2.54 | 1.09 | (1.00-1.20) |
| Respiratory | 511 | 1.43 | 420 | 2.47 | 1.14 | (0.94-1.37) | 2035 | 1.44 | 2205 | 2.78 | 1.19 | (1.06-1.34) |
| Pleural effusion | 34 | 0.10 | 136 | 0.80 | 3.59 | (2.04-6.32) | 136 | 0.10 | 508 | 0.64 | 2.92 | (2.14-3.97) |
| Gastrointestinal | 400 | 1.12 | 212 | 1.25 | 0.98 | (0.80-1.20) | 1654 | 1.17 | 980 | 1.24 | 0.94 | (0.83-1.06) |
| Genitourinary | 601 | 1.68 | 296 | 1.74 | 0.91 | (0.75-1.11) | 2116 | 1.49 | 1545 | 1.95 | 1.05 | (0.93-1.18) |
| Skin | 275 | 0.77 | 262 | 1.54 | 1.43 | (1.13-1.80) | 1151 | 0.81 | 1064 | 1.34 | 1.42 | (1.23-1.65) |
| Musculoskeletal | 1444 | 4.05 | 580 | 3.41 | 0.84 | (0.71-0.98) | 5750 | 4.06 | 3493 | 4.41 | 1.04 | (0.94-1.14) |
| Potential TKI side effect** | 252 | 0.71 | 271 | 1.60 | 1.37 | (1.06-1.77) | 963 | 0.68 | 1120 | 1.41 | 1.38 | (1.19-1.60) |
Adjusted for age at index, sex, race/ethnicity, index year, and baseline Charlson Comorbidity Index; imatinib group as referent
Beginning 6 months after index date, through 12 months post-index
Dasatinib, nilotinib, bosutinib, and ponatinib
The numbers of claims for arterial disease and long QT interval were <11 and HRs were not estimable
Erythema multiforme, peripheral edema, pleural effusion, pulmonary hypertension, and thyroid disease
During years 2 to 5 of treatment, later generation agents were still associated with an increased risk of infections overall, although primary infection risk, including fungal and viral causes, appeared attenuated. Circulatory, skin, and potential TKI-specific side effects remained more common among patients treated with later generation agents compared with those on imatinib during years 2 to 5. Among specific circulatory side effects, stroke was significantly more common (HR 1.95, 95% CI 1.39-2.73) along with hypertension (HR 1.09, 95% CI 1.00-1.20). However, later generation TKIs were associated with a lower risk of venous thrombosis versus imatinib (HR 0.45, 95% CI 0.23-0.88). During this time, there was no longer a difference in nervous system/sensory and musculoskeletal issues, but respiratory issues were more common with later generation TKIs versus imatinib, including a continued increased risk of pleural effusions. Overall, these results were similar if only second generation TKIs were compared with imatinib, excluding ponatinib (data not shown).
When those on nilotinib were compared with dasatinib, dasatinib was associated with a 40-50% greater risk of respiratory issues across all time periods (Table 4). This includes a four-fold increased risk of pleural effusions during years 2-5 of treatment. However, we did not observe any other significant differences in adverse events between these two later generation TKIs. Other patient characteristics associated with an increased risk of multiple organ system adverse events included female sex, older age (≥55 versus <55 years), and baseline comorbidity (CCI>0 versus CCI=0; Table 5). Notably, proportion of time on TKIs was not associated with a differential risk of subsequent events (Supplemental Table 5). Overall, results were similar if models were also adjusted for prior healthcare utilization. Utilization by the CML+TKI group was significantly greater compared with GEN for the number of annualized ambulatory claims, inpatient days, and emergency room visits, with the greatest difference being annualized ambulatory claims (median 19.3 visits [IQR 12.7-28.7] versus 6.4 visits [IQR 2.8-12.4]; p<0.001; Supplemental Table 6).
TABLE 4.
Relative hazards (HR) of adverse events among CML patients treated with nilotinib (referent) versus dasatinib*
| Events category | 1st year of therapy† |
Years 2-5 of therapy |
||||||
|---|---|---|---|---|---|---|---|---|
| Nilotinib rate per person-y |
Dasatinib rate per person-y |
HR | (95% CI) | Nilotinib rate per person-y |
Dasatinib rate per person-y |
HR | (95% CI) | |
| Infection, all | 1.69 | 1.73 | 1.10 | (0.85-1.43) | 2.42 | 1.98 | 0.96 | (0.82-1.11) |
| Infection, primary | 0.61 | 0.48 | 0.89 | (0.57-1.38) | 0.55 | 0.55 | 0.86 | (0.66-1.11) |
| Hematologic | 2.92 | 3.97 | 1.03 | (0.87-1.21) | 2.35 | 2.81 | 1.08 | (0.94-1.25) |
| Mental | 1.41 | 1.17 | 1.00 | (0.75-1.33) | 2.35 | 1.04 | 0.83 | (0.68-1.02) |
| Nervous/sensory | 2.08 | 2.14 | 0.93 | (0.73-1.18) | 2.43 | 2.46 | 1.04 | (0.90-1.21) |
| Circulatory | 4.82 | 4.09 | 0.95 | (0.79-1.14) | 5.02 | 3.77 | 1.05 | (0.94-1.19) |
| Myocardial ischemia | 0.49 | 1.04 | 1.36 | (0.87-2.13) | 1.02 | 0.89 | 1.13 | (0.89-1.42) |
| Stroke | 0.72 | 0.15 | 0.79 | (0.40-1.59) | 0.41 | 0.23 | 0.77 | (0.48-1.24) |
| Venous thrombosis‡ | -- | -- | -- | -- | 0.05 | -- | 1.16 | (0.48-2.82) |
| Hypertension | 3.09 | 2.29 | 0.89 | (0.72-1.09) | 2.82 | 2.13 | 0.95 | (0.84-1.08) |
| Respiratory | 1.86 | 3.03 | 1.46 | (1.14-1.87) | 2.46 | 3.07 | 1.39 | (1.18-1.64) |
| Pleural effusion | 0.45 | 1.16 | 2.27 | (0.72-7.16) | 0.42 | 0.81 | 4.16 | (2.62-6.60) |
| Gastrointestinal | 1.02 | 1.24 | 1.21 | (0.91-1.60) | 1.28 | 1.19 | 1.18 | (0.96-1.44) |
| Genitourinary | 1.27 | 2.20 | 0.95 | (0.69-1.31) | 1.77 | 2.06 | 1.05 | (0.88-1.25) |
| Skin | 2.12 | 1.02 | 0.87 | (0.62-1.22) | 1.63 | 1.09 | 1.01 | (0.83-1.24) |
| Musculoskeletal | 3.88 | 2.93 | 0.78 | (0.60-1.00) | 4.01 | 4.48 | 1.13 | (0.98-1.31) |
Adjusted for age at index, sex, race/ethnicity, index year, and baseline Charlson Comorbidity Index
Beginning 6 months after index date, through 12 months post-index
The number of claims for venous thrombosis during the 1st year of therapy was too rare to allow estimation of a HR
TABLE 5.
Relative hazards (HR) of adverse events associated with baseline characteristics among CML patients treated with tyrosine kinase inhibitors (TKI)*
| 1st year of therapy† |
Years 2-5 of therapy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events category | Female vs. Male | Age ≥55 vs. <55 y | CCI >0 vs. 0 | Female vs. Male | Age ≥55 vs. <55 y | CCI >0 vs. 0 | ||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Infection, all | 1.07 | (0.92-1.24) | 1.11 | (0.94-1.30) | 1.49 | (1.26-1.76) | 1.10 | (1.01-1.20) | 1.16 | (1.07-1.26) | 1.32 | (1.19-1.47) |
| Infection, primary | 0.83 | (0.64-1.07) | 1.18 | (0.88-1.58) | 2.09 | (1.51-2.89) | 1.09 | (0.93-1.27) | 1.26 | (1.07-1.47) | 1.52 | (1.25-1.84) |
| Hematologic | 1.12 | (1.01-1.24) | 1.13 | (1.02-1.26) | 1.20 | (1.07-1.35) | 1.13 | (1.04-1.22) | 1.28 | (1.19-1.38) | 1.17 | (1.07-1.29) |
| Mental | 1.07 | (0.91-1.25) | 0.92 | (0.78-1.08) | 1.42 | (1.19-1.71) | 1.13 | (0.99-1.28) | 1.01 | (0.90-1.14) | 1.18 | (1.00-1.38) |
| Nervous/sensory | 1.09 | (0.96-1.24) | 1.36 | (1.17-1.58) | 1.31 | (1.14-1.51) | 1.08 | (1.00-1.16) | 1.32 | (1.21-1.44) | 1.14 | (1.04-1.25) |
| Circulatory | 0.90 | (0.81-1.00) | 1.77 | (1.53-2.04) | 1.33 | (1.18-1.51) | 1.01 | (0.95-1.08) | 1.36 | (1.26-1.47) | 1.25 | (1.16-1.35) |
| Respiratory | 0.97 | (0.84-1.13) | 1.29 | (1.09-1.52) | 1.56 | (1.32-1.84) | 0.98 | (0.90-1.07) | 1.30 | (1.19-1.42) | 1.24 | (1.11-1.39) |
| Gastrointestinal | 1.07 | (0.90-1.27) | 1.25 | (1.04-1.50) | 1.28 | (1.03-1.59) | 1.13 | (1.03-1.25) | 1.30 | (1.17-1.45) | 1.17 | (1.03-1.33) |
| Genitourinary | 1.23 | (1.05-1.45) | 1.41 | (1.17-1.69) | 1.44 | (1.21-1.73) | 1.17 | (1.05-1.30) | 1.44 | (1.29-1.60) | 1.15 | (1.02-1.29) |
| Skin | 1.05 | (0.87-1.26) | 1.46 | (1.18-1.81) | 1.27 | (1.01-1.61) | 1.04 | (0.92-1.16) | 1.32 | (1.18-1.49) | 1.14 | (0.97-1.33) |
| Musculoskeletal | 1.10 | (0.97-1.25) | 1.21 | (1.06-1.39) | 1.10 | (0.97-1.26) | 1.04 | (0.96-1.13) | 1.14 | (1.04-1.25) | 1.07 | (0.97-1.18) |
| Potential TKI side effect‡ | 1.40 | (1.13-1.73) | 1.81 | (1.45-2.28) | 1.33 | (1.07-1.66) | 1.14 | (1.00-1.31) | 1.49 | (1.27-1.74) | 1.25 | (1.08-1.46) |
CCI, Charlson Comorbidity Index
Adjusted for variables shown (male, <55 y, and CCI=0 as referent groups), plus race/ethnicity, index year, and TKI type (imatinib vs. later generation agent)
Beginning 6 months after index date, through 12 months post-index
Erythema multiforme, peripheral edema, pleural effusion, pulmonary hypertension, and thyroid disease
DISCUSSION
Based on a longitudinal, real-world data asset of commercial and Medicare enrollees, we reported that CML patients treated on TKIs had a significantly greater burden of adverse events across most organ system categories compared with the general non-cancer population. These differences were apparent within the first year of treatment and persisted through five years. We also were able to characterize the burden of these adverse events by TKI type, and found that compared with imatinib, later generation TKIs were associated with an increased risk of infections, cardiopulmonary and skin issues, with dasatinib associated with more respiratory events compared with nilotinib.
Most published studies to date have focused on CML survival, recurrence, and selected adverse events captured in the context of randomized clinical trials. For example, the landmark IRIS study of imatinib showed that while the efficacy benefits favoring imatinib over conventional therapy persisted after >10 years of median follow-up, serious adverse events related to imatinib were relatively uncommon and most frequently occurred in the first year of therapy [2]. Pooled data from multiple frontline TKI-based trials at MD Anderson have reported excellent overall and conditional survival [12]. Outside of clinical trials, most observational cohorts have also focused on conventional oncology metrics like overall survival or recurrence-free survival, but generally have limited insights into other outcomes [13-16].
A large multi-institutional registry-based study followed over 800 long-term CML survivors treated with imatinib for nearly 4 years and reported that only 2% of patients discontinued drug because of side effects (overall, 9% discontinued imatinib for any reason) [17]. In this study of mainly Italian patients, only 3% of patients had severe adverse events thought to be linked imatinib, although over 50% of patients had some non-serious event recorded, mostly attributed to imatinib. However, similar to the clinical trial data, interpretation of adverse burden and attribution can be challenging in the absence of comparative information to a non-cancer or non-TKI group [5].
Outcomes based on clinical trials or pooled from larger academic centers may also differ from community-based settings. For example, a recent US-based SEER analysis found that CML patients had a greater than two-fold increased risk of death compared with the general population, in contrast to data from clinical trials and large academic centers reporting similar, or near-similar survival to the general population [18]. The authors suggested that the mortality difference may be due to less optimal compliance with and monitoring of TKI-therapy in the general population [18].
There is also a growing literature that shows that CML patients treated with TKIs have poorer health-related quality of life compared with non-cancer peers [4]. In particular, female and younger patients (<60 years) have reported greater impairment compared with same-sex and same-age non-cancer peers, respectively. Among imatinib-treated patients, fatigue, edema, and musculoskeletal pain were common complaints [19]. However, these symptoms were also present and prominent in cohorts inclusive of later generation TKIs [20]. Overall, while rates varied, in some larger studies up to one-third of patients had persistent moderate-severe symptoms [21] and up to 90% had mild persistent symptoms [22].
Our results are consistent with these findings, with musculoskeletal events being more common among imatinib-exposed patients versus later generation agents in the first year of treatment but not subsequently [3]. Neurologic symptoms such as headache are commonly reported with TKIs, but more serious neurologic toxicities are uncommon, and a differential risk between TKIs has not been clearly established [3]. In contrast, circulatory/respiratory complications overall were more common among later generation agents compared with imatinib in our study throughout the observation period, even after excluding ponatinib [23]. This included significantly increased risks of stroke and pleural effusions, but not a significant difference in myocardial ischemia. Ponatinib has been clearly associated with an increased risk of vascular events, but its use was extremely limited in our study cohort [23]. However, we observed that imatinib was associated with a greater likelihood of venous thrombosis compared with later generation agents, which appears to differ from clinical trial results [24].
We also found an increased risk of infections with later generation TKIs versus imatinib. While our analysis excluded patients treated with HCT, we did not have information about the disease phase, and it is possible that later generation TKIs could have been preferentially used among patients with more advanced disease, or given to patients who did not initially respond to imatinib. Notably, the relative infection risk appeared attenuated in years 2-5 vs. the initial year of therapy. Skin issues are common with all TKIs, although some studies have reported nilotinib to be associated with rashes more frequently than imatinib and dasatinib [3]. In contrast, pleural effusions (and less often, pulmonary hypertension) are reportedly much more common with dasatinib compared with other TKIs used for CML, consistent with our results [3,5]. However, studies directly comparing outcomes between later generation TKIs have been rare, particularly studies not sponsored by the pharmaceutical industry [3,5].
A strength of this analysis is that it represents a real-world sample, providing a different perspective from carefully selected clinical trial populations and not limited to those treated at academic centers. We also examined a broad range of outcomes, beyond what more limited case report forms collect in a clinical trial or a registry-based setting. Our findings for the TKI group were also presented in relation with that of the general population. However, our study has several limitations. With all administrative datasets, there is potential misclassification of codes and outcomes. However, any misclassification should apply similarly across groups, and if non-differential, biases results towards the null. Any adverse event could also be more likely to be recorded among those with greater healthcare encounters, but adjustment for utilization did not materially impact our results. We also lacked information on clinical characteristics such as initial disease phase and molecular response to therapy, which could have provided more context to our findings if available. Finally, our sample was limited those in the OLDW, an insured US population. Those who are less well-insured may experience worse outcomes [25].
Nevertheless, this study, even with its limitations, provides useful information in evaluating the full spectrum of CML outcomes, in a setting where chronic therapy is now the norm and where traditional adverse events reporting is often limited [26]. Given increasing cancer-related costs, a better understanding of the side effect profiles of CML TKI therapy can inform policy makers, particularly the differential profiles of imatinib versus later generation agents [27,28]. While later generation TKIs are more potent and achieve faster, deeper molecular responses compared with imatinib, these advantages have not translated to differences in progression-free or overall survival [5]. A better appreciation of these adverse events is also important should discontinuation strategies become more widely adopted, by determining if TKI-related side effects resolve completely and health outcomes, including patient-reported quality of life, normalize [5,29].
Supplementary Material
ACKNOWLEDGEMENTS
Funding provided by Stand Up To Cancer, the American Association for Cancer Research, and the US National Institutes of Health (P30 CA015704). The authors report no potential disclosures related to this research.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, Larson RA, Guilhot F, et al. . Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med 2017;376:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016;30:1648–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efficace F, Cardoni A, Cottone F, Vignetti M, Mandelli F. Tyrosine-kinase inhibitors and patient-reported outcomes in chronic myeloid leukemia: a systematic review. Leuk Res 2013;37:206–213. [DOI] [PubMed] [Google Scholar]
- 5.Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020;34:966–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics 2007;25:481–496. [DOI] [PubMed] [Google Scholar]
- 7.Henk HJ, Woloj M, Shapiro M, Whiteley J. Real-world analysis of tyrosine kinase inhibitor treatment patterns among patients with chronic myeloid leukemia in the United States. Clin Ther 2015;37:124–133. [DOI] [PubMed] [Google Scholar]
- 8.Wilkes JJ, Lyman GH, Doody DR, et al. Health care cost associated with contemporary chronic myelogenous leukemia therapy compared with that of other hematologic malignancies. JCO Oncol Pract 2020:OP2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow EJ, Cushing-Haugen KL, Cheng GS, et al. Morbidity and mortality differences between hematopoietic cell transplantation survivors and other cancer survivors. J Clin Oncol 2017;35:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prentice RL, Williams BJ, Peterson AV. On the Regression-Analysis of Multivariate Failure Time Data. Biometrika 1981;68:373–379. [Google Scholar]
- 11.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995;14:1707–1723. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, Kantarjian HM, Jain P, et al. Conditional survival in patients with chronic myeloid leukemia in chronic phase in the era of tyrosine kinase inhibitors. Cancer 2016;122:238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzin J, Lang K, Earle CC, Glendenning A. Treatment patterns, outcomes and costs among elderly patients with chronic myeloid leukaemia: a population-based analysis. Drugs Aging 2004;21:737–746. [DOI] [PubMed] [Google Scholar]
- 14.Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol 2016;34:2851–2857. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann VS, Baccarani M, Hasford J, et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia 2017;31:593–601. [DOI] [PubMed] [Google Scholar]
- 16.Kizaki M, Takahashi N, Iriyama N, et al. . Efficacy and safety of tyrosine kinase inhibitors for newly diagnosed chronic-phase chronic myeloid leukemia over a 5-year period: results from the Japanese registry obtained by the New TARGET system. Int J Hematol 2019;109:426–439. [DOI] [PubMed] [Google Scholar]
- 17.Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 2011;103:553–561. [DOI] [PubMed] [Google Scholar]
- 18.Radivoyevitch T, Weaver D, Hobbs B, et al. Do persons with chronic myeloid leukaemia have normal or near normal survival? Leukemia 2020;34:333–335. [DOI] [PubMed] [Google Scholar]
- 19.Efficace F, Baccarani M, Breccia M, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 2011;118:4554–4560. [DOI] [PubMed] [Google Scholar]
- 20.Phillips KM, Pinilla-Ibarz J, Sotomayor E, et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer 2013;21:1097–1103. [DOI] [PubMed] [Google Scholar]
- 21.Williams LA, Garcia Gonzalez AG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 2013;122:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zulbaran-Rojas A, Lin HK, Shi Q, et al. A prospective analysis of symptom burden for patients with chronic myeloid leukemia in chronic phase treated with frontline second- and third-generation tyrosine kinase inhibitors. Cancer Med 2018;7:5457–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 24.Haguet H, Douxfils J, Mullier F, Chatelain C, Graux C, Dogne JM. Risk of arterial and venous occlusive events in chronic myeloid leukemia patients treated with new generation BCR-ABL tyrosine kinase inhibitors: a systematic review and meta-analysis. Expert Opin Drug Saf 2017;16:5–12. [DOI] [PubMed] [Google Scholar]
- 25.Perry AM, Brunner AM, Zou T, et al. Association between insurance status at diagnosis and overall survival in chronic myeloid leukemia: A population-based study. Cancer 2017;123:2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thanarajasingam G, Minasian LM, Baron F, et al. Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies. Lancet Haematol 2018;5:e563–e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laviana AA, Luckenbaugh AN, Resnick MJ. Trends in the cost of cancer care: beyond drugs. J Clin Oncol 2020;38:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huntington SF, Davidoff AJ, Gross CP. Precision medicine in oncology II: economics of targeted agents and immuno-oncology drugs. J Clin Oncol 2020;38:351–358. [DOI] [PubMed] [Google Scholar]
- 29.Dulucq S, Astrugue C, Etienne G, Mahon FX, Benard A. Risk of molecular recurrence after tyrosine kinase inhibitor discontinuation in chronic myeloid leukaemia patients: a systematic review of literature with a meta-analysis of studies over the last ten years. Br J Haematol 2020;189:452–468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.