Abstract
Sleep and fatigue-associated symptoms are prominent during chemotherapy. The purpose of this pilot study was to examine bright light effects on sleep disruption, fatigue, daytime sleepiness, depression, and quality of life (QOL) in women with stage I-III breast cancer undergoing chemotherapy. In this 2-group randomized controlled trial (NCT02658708), participants were randomized to receive either blue-green light of 12,000 lux (experimental) or dim red light of 5 lux (control). Light therapy was self-administered using a light visor cap at home. Both groups received 30-minute daily light therapy for 21 consecutive days following the 2nd cycle of chemotherapy. Sleep quality, fatigue, daytime sleepiness, depression, and QOL were self-reported, and nocturnal sleep was monitored by ambulatory polysomnography before the initiation of chemotherapy (baseline) and following the light intervention (post-test). Relative change was assessed at post-test controlling for pretest scores. At post-test, the experimental group self-reported significantly shorter sleep latency than controls (10 vs. 20 min, p=.045) consistent with polysomnography findings (14 vs. 63 min). Polysomnography also revealed longer total sleep time (467 vs. 315 min) and higher sleep efficiency (74% vs. 58%) in experimental vs. controls. Participants receiving bright light experienced a 30% relative decrease in depression, while there was a 24% increase in the controls. The experimental group reported substantially fewer increases in symptom intensity than controls (33% vs. 166%). These findings suggest that bright light likely improved sleep quality and depression and mitigated worsening intensity of symptoms during the first three cycles of chemotherapy. However, features of bright light, e.g., treatment duration, frequency, and timing in relation to chemotherapy treatment require further investigation.
Keywords: bright light, sleep quality, cancer, quality of life, depression, Fatigue
Introduction
Fatigue is prevalent among individuals with breast cancer. As many as 91% of breast cancer patients undergoing chemotherapy report cancer-related fatigue (Manir et al. 2012). Fatigue negatively affects cancer patients’ daily functions and quality of life (QOL) (Alexander et al. 2009; Byar et al. 2006; Davidson et al. 2002). Disrupted sleep/wake patterns often co-occur with fatigue (Alexander et al. 2009; Berger and Higginbotham 2000; Cheng and Lee 2011; Davidson et al. 2002) and heighten symptom distress (Sarna 1993). Although a wide range of interventions have been employed to manage cancer-associated fatigue and disrupted sleep, these two symptoms, alone or in combination, remain devastating and unmitigated in the oncology population, especially during aggressive cancer treatment.
Therapeutic bright light has shown effectiveness in treating circadian rhythm sleep disorders (Dijk et al. 1995; Gooley 2008; Kuller 2002; Terman et al. 1995) and has alleviated fatigue and insomnia in conditions such as seasonal affective disorder, shift work, and jet-lag (Eastman and Martin 1999; Meesters and Lambers 1990; Petrie et al. 1989; Rastad et al. 2011; Tanaka et al. 2011). The underlying rationale for therapeutic bright light is that optimal sleep/wakefulness patterns require proper alignment of endogenous circadian rhythms and sleep/wake cycles (Kanathur et al. 2010). A misalignment of the cycles results in various sleep/wake disorders, which can be treated by appropriately timed exposure to bright light (Dijk et al. 1995; Gooley 2008; Terman et al. 1995).
The efficacy of light therapy depends on the time of day the light is administered (Dijk et al. 1995; Gooley 2008). According to circadian physiology, light exposure in the morning induces sleep onset at an earlier time, eliciting phase advance of the circadian system. Conversely, light exposure in the evening suppresses sleep onset until a later time, delaying the phases. Existing studies have shown that morning bright light prevents fatigue and QOL from worsening during chemotherapy (Ancoli-Israel et al. 2012; Jeste et al. 2013). However, these studies did not consider the differences in the individuals’ circadian chronotype (morningness-eveningness). Without considering differences in individuals’ circadian chronotypes, light can induce changes in an unwanted direction, further disrupting or worsening their circadian aberrations. The purpose of this pilot study was to estimate the effects of a home-based bright light intervention on sleep disruption, fatigue, daytime sleepiness, depression, and QOL in women with breast cancer undergoing chemotherapy. In this study, the timing of the bright light intervention was tailored to each individual’s circadian chronotype.
Methods
In this 2-group randomized controlled trial (NCT02658708), participants were randomized to receive either blue-green light of 12,000 lux (intervention group) or dim red light of 5 lux (control group). The 21d light intervention was implemented across the second cycle of chemotherapy. Baseline data were collected before the first cycle; the post-test data were collected following the light intervention, which was on the day the individual received the third chemotherapy treatment.
Sample and Settings
Data collection took place at the Siteman Cancer Center in St. Louis, Missouri. Eligible participants were female, age 21 y of age or older, newly diagnosed with stage I-III breast cancer, and scheduled to receive 21 d cycles of intravenous chemotherapy; sighted, mentally competent to consent, and able to understand English as subjectively evaluated by an on-site research staff. Individuals were excluded if they had a concurrent malignancy; were undergoing other cancer treatments; engaged in shift work or traveled across more than three time zones within two weeks prior to the study; had a known history of seasonal affective disorder or substance abuse; had a current diagnosis of major Axis I psychiatric disorders, neurological impairments, or muscular dystrophies; regularly used steroid or other immunosuppressive medications; took prescribed sedative hypnotics or sleep medications; had eye conditions (glaucoma or retinal disease) or problems triggered by bright light (e.g., migraine); or took photosensitizing medications (e.g., some porphyrin drugs, antipsychotics, antiarrhythmic agents). The study was approved by the Human Investigation Committee at Washington University in St. Louis, Missouri and the study protocol conformed to international ethical standards for human chronobiology research (Portaluppi et al. 2010).
Light Treatment
Participants were randomized using a computer-generated list to receive either bright blue-green light (~515nm; 12,000 lux) (experimental group) or dim red light (5 lux) (control group) for 30 min once a day during the second cycle of chemotherapy. Both experimental and control groups followed the same treatment protocol consisting of a 21d light intervention at the individual’s home. Light therapy was self-administered using a light visor cap (Physician Engineered Products, Fryeburg, ME). The cap visor-mounted device controls the distance of the light exposure and positions the light source above eye level to target the lower retina, allowing participants to continue daily activities at home. Side effects (e.g., eyestrain, headache) were assessed via three telephone call follow-ups and a daily log across the entire treatment phase.
The timing of light administration was tailored to the individual’s circadian chronotype. Participants who were identified as evening types (Horne-Ostberg Morningness-Eveningness Questionnaire [MEQ] score ≤ 41) (Horne and Ostberg 1976; Smith et al. 1989) were instructed to apply the light within 30 min of waking in the morning. Participants who were morning types (MEQ score ≥ 59) were instructed to apply the light between 19:00 – 20:00h in the evening. For those with a MEQ score of 42 to 58, the timing of light administration was based on the individual’s response to question 19 on the MEQ asking which chronotype, morningness vs. eveningness, the person considers herself to be.
Variables and Measures
Fatigue.
Fatigue was measured by Patient-Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 Fatigue 8a (Garcia et al. 2007). The instrument consists of eight items with a five-point rating scale (1 = not at all or never, 5 = very much or always) measuring fatigue experience (frequency, duration, and intensity) and fatigue impact (physical, mental, and social activities). Higher scores indicate worse fatigue. The PROMIS-Fatigue 8a was developed based on rigorous methodologies. Psychometric properties have been established across chronic illnesses including cancer (Garcia et al. 2007).
Disrupted sleep patterns.
Sleep patterns were measured subjectively and objectively.
Subjective sleep measure.
Sleep quality was measured by the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al. 1989). The PSQI contains 19 self-report items measuring seven sleep characteristics, including sleep quality, latency, duration, efficiency, disturbance, medication use, and daytime dysfunction. The total global PSQI score ranges from 0–21; a higher score suggests worse sleep quality. The cut-off score of five was found to have a sensitivity of 89.6% and a specificity of 86.5% in differentiating good and poor sleepers (Buysse et al. 1989). In a sample of cancer patients, internal consistency reliability was α=0.81 for the global sleep quality score (Berger et al. 2005).
Objective sleep measure.
Nocturnal sleep patterns were measured by ambulatory polysomnography (PSG) using a four-channel electroencephalograph (EEG) for brain waves, a two-channel electrooculograph (EOG) for extraocular eye movements, and a one-channel electromyograph (EMG) for chin muscle movements. The electrodes were placed in accordance with the International 10–20 System of Electrode Placement (Jasper 1958) and connected to an Easy Ambulatory 2 System (Cadwell, Kennewick, WA). Data were recorded using Easy 2 System software and visually scored by a registered polysomnographer blind to the group assignment following the American Academy of Sleep Medicine Manual (Iber et al. 2007).
Daytime sleepiness.
Global daytime sleepiness was measured using the Epworth Sleepiness Scale (ESS) (Johns 1991). The ESS consists of eight common daily activities (e.g., reading, watching TV, and sitting) and asks the respondent to rate each activity on a scale of 0 (would never doze) to 3 (high chance of dozing). Studies demonstrated that ESS scores significantly distinguished groups of patients with known differences in their levels of sleepiness as measured by the multiple sleep-latency test (Richardson et al. 1978; Roehrs et al. 1989).
Depression.
Severity of depression was measured by the Patient Health Questionnaire (PHQ-9) (Kroenke et al. 2001). The PHQ-9 consists of 9 items scoring the nine DSM-IV criteria of depression with a four-point rating scale (0 = not at all, 3 = nearly every day). A higher score suggests worse depression. The reliability and validity of PHQ-9 were established in studies involving 6000 patients in eight primary care and seven obstetrics-gynecology clinics (Kroenke et al. 2001).
Quality of life.
QOL was measured by the European Organization for Research and Treatment of Cancer–Quality of Life Questionnaire (EORTC QLQ-C30) (Aaronson et al. 1993). The EORTC QLQ-C30 consists of 30 items with a four-point rating scale (1 = not at all, 4 = very much) measuring functioning, symptom intensity, and global health status. Internal consistency α of the functioning and symptoms subscales ranged from 0.54 to 0.86 in lung cancer patients before and during cancer treatments (Aaronson et al. 1993). Known-group comparisons showed differences between patients differing in clinical status.
Procedure
Potential subjects were referred to the principal investigator by clinic nurses or oncologists before starting their chemotherapy treatment. Once the referral was made, the principal investigator or a research assistant contacted the study candidate by telephone and/or in-person meeting at a location convenient to the candidate. The study was explained to the candidate in detail, and all questions were answered. After giving informed consent, candidates were screened for suicidal ideation and depressive disorders using the PHQ-9 (Kroenke et al. 2001). Next, candidates completed the PSQI (Buysse et al. 1989), and MEQ (Horne and Ostberg 1976; Smith et al. 1989) to determine chronotype. Each candidate was then individually interviewed using a list of criteria to further determine eligibility.
Participants who met the study criteria were scheduled for the subsequent study activities. During the baseline data collection, participants completed a battery of self-reported instruments, including the demographic information sheet, the PROMIS-Fatigue 8a, ESS, and EORTC QLQ-C30 scales. Polysomnographic (PSG) data were also collected from a subset of participants over a 24h period.
Each participant who agreed to PSG checked into a hospital-based sleep center for electrode placement between 1400–17:00h on a weekday. The BraiNet ® (Jordan NeuroScience, Inc.) was placed on the individual’s head to determine EEG electrode placement. The electrodes were then applied by a registered sleep technician following a standard sleep montage, which included central and occipital EEG electrodes right and left referenced to a mastoid, right and left eye EOG electrodes referenced to the opposite mastoid, and chin EMG electrodes referenced to a mentum electrode. All electrodes were connected to the Easy Ambulatory 2 System. After activating the recording, participants returned home with the ambulatory devices. Once the individual completed 24h of continuous recording, the investigator visited the participant at home to remove the electrodes and collect the device.
The light treatment was initiated following the second cycle of chemotherapy treatment to prevent fatigue from worsening during the third cycle of chemotherapy when a majority of patients typically experience marked fatigue and sleep/wake cycle disruption (Berger & Farr 2019; Piper 1992). Based on one’s chronotype, participants were instructed to wear the light visor cap with the light on for 30 min, either between 19:00h – 20:00h or within 30 min of waking in the morning, for 21 consecutive days. Daily telephone calls were made to all participants on the first three days after they started the light treatment, and then weekly telephone calls were made to remind them to follow the treatment protocol.
Baseline data collection was repeated for the post-test data collection that started on the day the individual received the third cycle of chemotherapy treatment. Each participant completed the battery of self-reporting instruments while receiving chemotherapy infusion. Right after the completion of chemotherapy, each participant, accompanied by the investigator, travelled between 15:00 – 17:00h to the sleep center where they prepared for ambulatory PSG monitoring commencing that day. The participant was then sent home with the ambulatory PSG devices for 24h recording. The investigator visited each participant at home to collect the devices the following afternoon. Throughout the study, the participants were instructed to record sleep and wake-up times in a daily log.
Data Analysis
All data were de-identified using three-digit codes and double entered into SPSS version 25.0 (IBM Corp., Armonk, N.Y., USA). Descriptive statistics were computed to describe the sample. Two-sample t-tests and chi-squared tests were used to examine differences in demographic characteristics between experimental and control groups. We compared the relative change of outcomes from baseline (prior to chemotherapy) to post-test (the third chemotherapy cycle) between groups using two-sample t-tests. We also performed Wilcoxon rank-sum tests to examine differences between participants who received the intervention (n=9) or controls (n=9) on the day the individual received the third cycle of chemotherapy for post-test comparisons. All statistical tests were two-sided at a significance level 0.05. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
A convenience sample of 22 women newly-diagnosed with breast cancer consented to participate in this pilot study. Among those, four were excluded because they did not meet eligibility criteria, and two withdrew. A total of 18 women completed the baseline assessments; of those, 16 completed the study. The study analyses were based on the data from 18 women, ages 29 to 68 (mean=51.1 ± 11.7) y of age. The majority were White/Caucasian (66.7%), college-educated (77.8%), treated for stage II breast cancer (77.8%), and morning type (61.1%; n=11). Table 1 summarizes the demographics of the study participants. There were no significant differences in the demographic characteristics between the experimental and control groups. Among those who completed the study (n=16), 79% of the intervention arm (132 intervention sessions) and 65% of the control arm (82 intervention sessions) adhered to the three-week daily light protocol. No adverse events from the bright light intervention were reported, except one participant reported that the light visor cap irritated her skin.
Table 1.
Characteristics of Samples (N = 18)
| Treatment (n = 9) | Control (n = 9) | ||||
|---|---|---|---|---|---|
| Number (valid %) | Number (valid %) | p | |||
| Age (years) | .57 | ||||
| Race | |||||
| White | 7 (78) | 5 (56) | .34 | ||
| Black | 1 (11) | 3 (33) | |||
| Others | 1 (11) | 1 (11) | |||
| Education (years) | .74 | ||||
| Marital Status | .38 | ||||
| Single | 1 (11) | ||||
| Married/Partnered | 6 (67) | 7 (78) | |||
| Divorced | 2 (22) | 0 | |||
| Widowed | 1 (11) | 1 (11) | |||
| Employment | .42 | ||||
| Full-time | 2 (22) | 2 (22) | |||
| Part-time | 1 (11) | ||||
| Self-employed | 2 (22) | ||||
| Unemployed | 4 (44) | 2 (22) | |||
| Retired | 3 (33) | 2 (22) | |||
| Living Arrangement | .38 | ||||
| Live alone | 1 (11) | 0 | |||
| Live with others | 8 (89) | 9 (100) | |||
| Tumor Stage | |||||
| Stage I | 1 (11) | 0 | .45 | ||
| Stage II | 6 (67) | 8 (89) | |||
| Stage III | 2 (22) | 1 (11) | |||
Fatigue and Daytime Sleepiness
The PROMIS-Fatigue 8a was scored using item-level calibrations by the HealthMeasures Scoring Service (HealthMeasures 2020). The PROMIS-Fatigue measure was scored on a T score metric with mean = 50 and standard deviation (SD) = 10 (HealthMeasures 2019). A higher PROMIS T-score represented more fatigue. The median post-treatment fatigue level for the experimental group was 53.6 (SD=10.3), and the median comparison fatigue was 49.2 (SD=10.0), similar to the general population mean of 50.0. Although the relative change from baseline (prior to chemotherapy) to the post-test (the third cycle of chemotherapy) was not significantly different between groups (p=.77), the fatigue level increased by 7% in controls compared to a lessor 4% increase in the experimental group after the light treatment.
At the post-test, both groups reported median total daytime sleepiness scores of 8.0, which were considered normal (≤9). Even though differences in the relative change were not statistically significant (p=.64), the relative change from baseline to post-test showed that daytime sleepiness decreased by 4% in the experimental group, while it increased by 7% in the control group. Further analysis showed that on the day participants received the third chemotherapy treatment, 57% of the control group compared with 33% in the experimental group reported moderate to high chance of dozing during sitting and reading. Almost twice as many controls as those who received bright light (86% vs. 44%) reported they would doze when sitting quietly after lunch.
Depression
At the post-test, women who received bright light had slightly lower depression scores than controls (median=4.0 vs. 5.5). Although the relative changes were not significantly different between groups (p=.42), Figure 1 illustrates a trend indicating depression changed over time in opposite directions for the two study conditions. The median severity of depression relatively increased by 24% in the controls compared to a 30% relative decrease in the experimental group.
Figure 1.
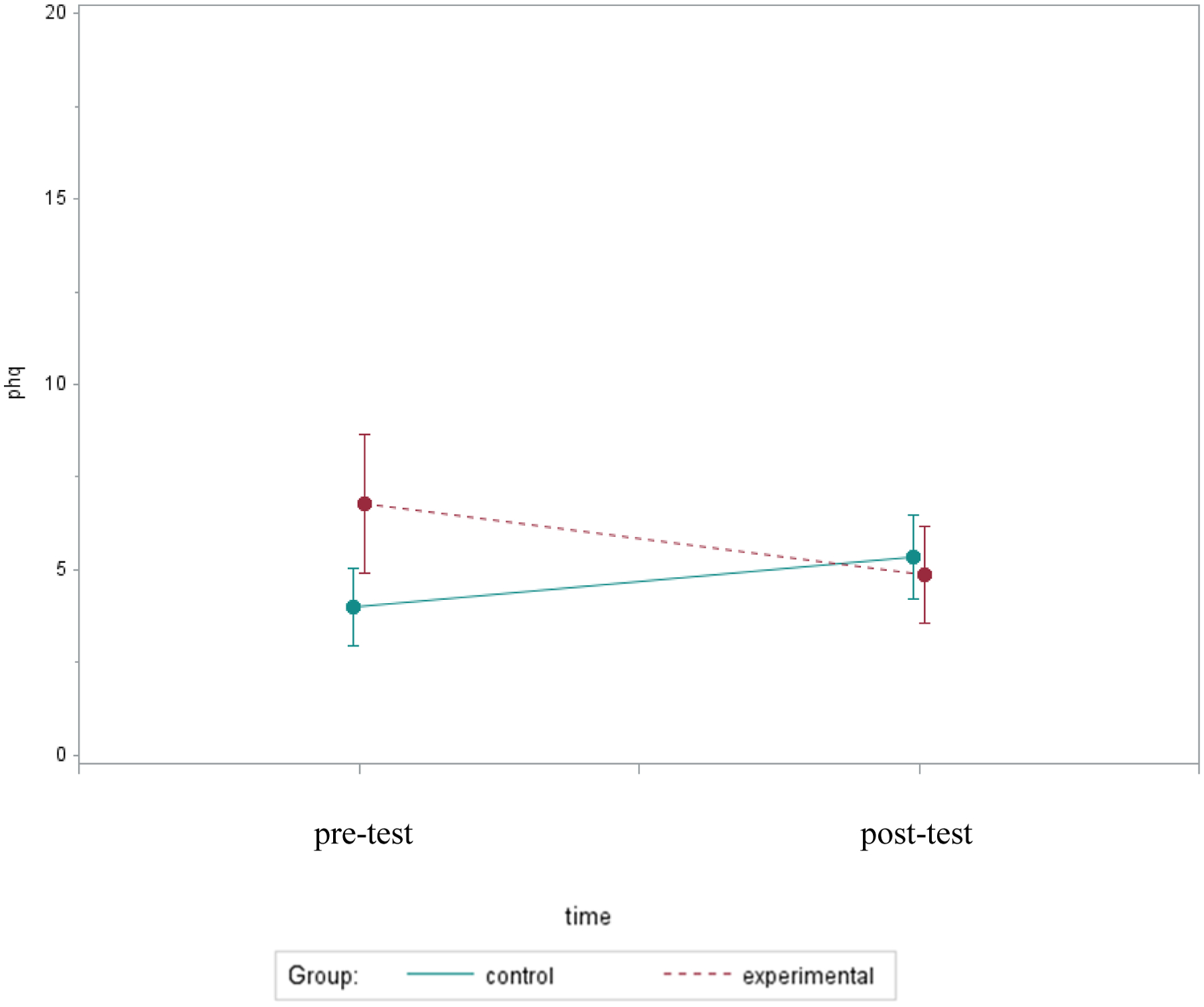
Mean PHQ Depression Scores Over Two Assessments
Quality of Life
The raw scores on the three subscales of the EORTC QLQ-C30 representing functioning, symptom intensity, and global health status were linearly transformed to the standardized scores ranging from 0 to 100. A higher score indicates a better level of functioning, lower level of symptomology, and a better level of health status. At the post-test, although the participants who received bright light reported slightly better functioning and less symptomology (median=87 vs. 80 for functioning; median= 24 vs. 28 for symptom intensity), their global health status was similar to the controls (median = 75 vs. 75).
Even though the relative change in symptomology did not differ significantly by group (p=.17), Figure 2 illustrates a pattern showing possible group differences in symptoms worsening over time. The post-test symptomology was 116% worse in controls compared to 33% worse in those who received bright light, suggesting that bright light may preclude overall symptomology from worsening during chemotherapy. A similar pattern was observed in functioning; functional status decreased by 8% in the control group compared to only a 1% decrease in the experimental group (p=.50). Figure 3 illustrates a pattern showing that functional status remained at the same level in the experimental group, while it declined over time in the control group.
Figure 2.
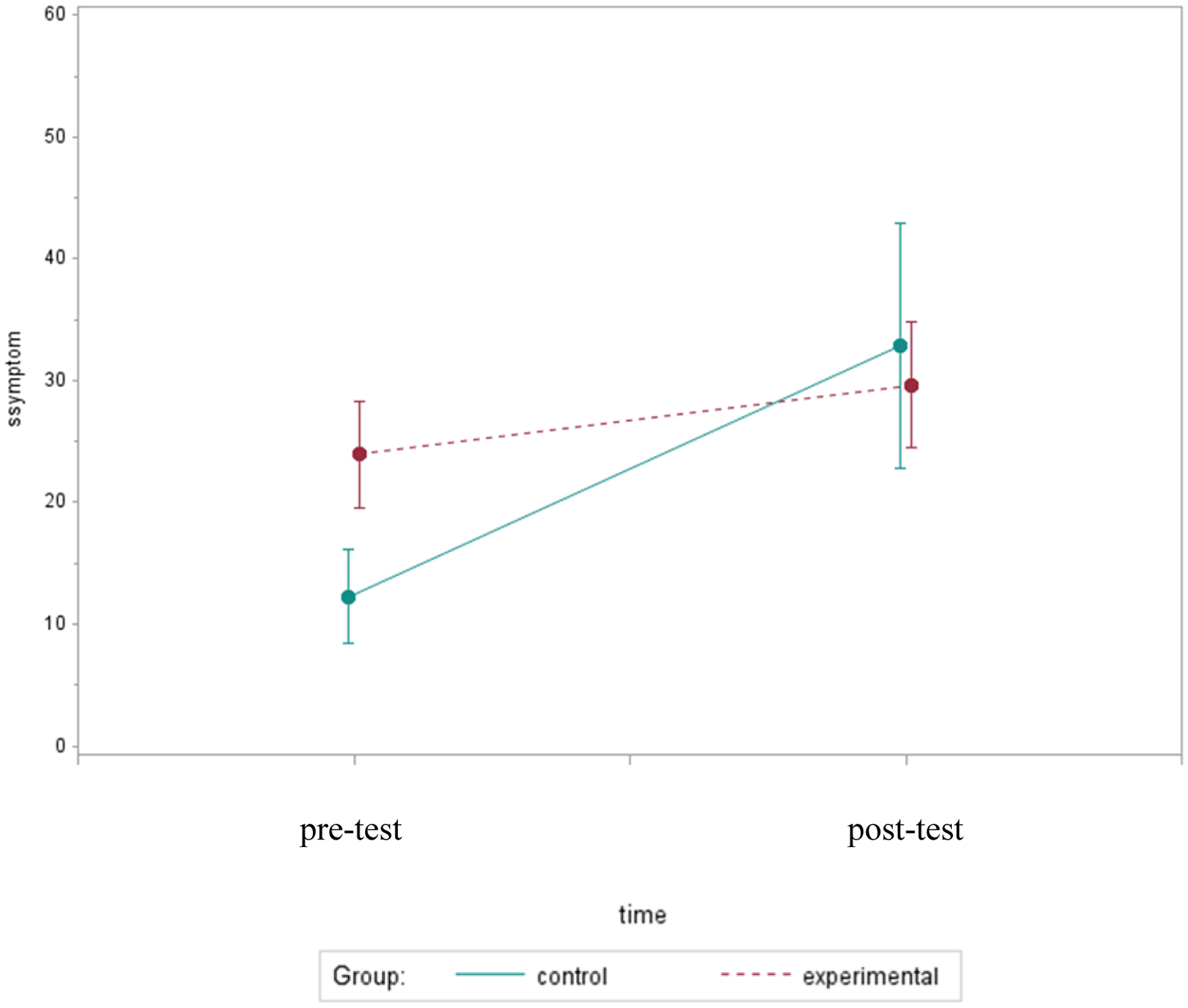
Mean Symptom Scores Over Two Assessments
Figure 3.
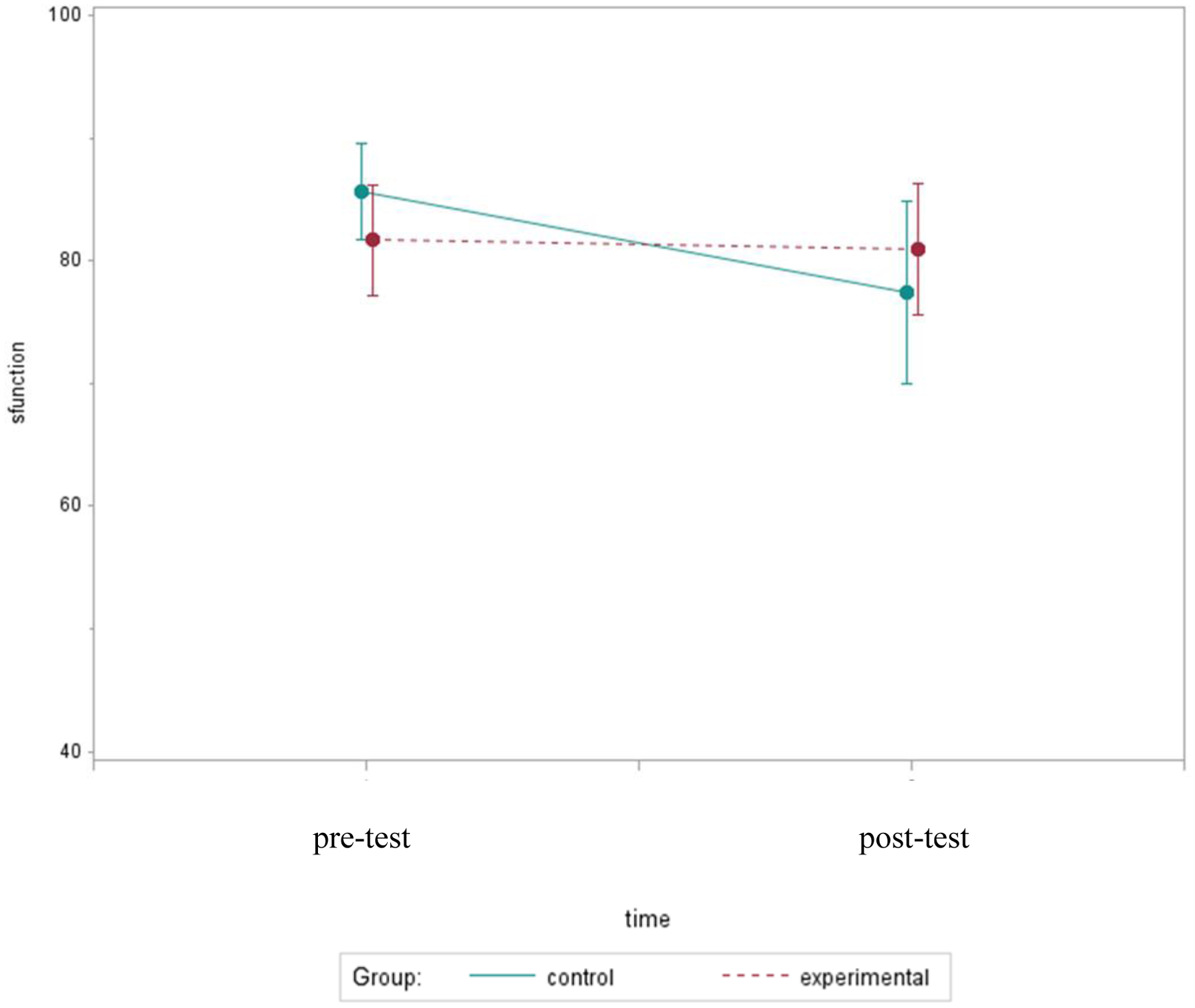
Mean Functional Scores Over Two Assessments
Subjective Sleep Quality
At the post-test, both groups reported sleeping for a median of 7.0h each night; however, total sleep time increased by 4% in the experimental group, while there was no relative change (0%) in the controls (p=.75). The post-test comparison showed that participants who received bright light self-reported higher sleep efficiency (the percentage of time spent asleep while in bed) than controls (median 86% vs. 74%, p=.34). Although group differences in sleep efficiency were not statistically significant (p=.50), those who received the bright light intervention reported a 4% improvement in sleep efficiency, while it became worse by 4% among the controls. Using the Wilcoxon rank-sum tests, participants who received bright light gave subjective reports of significantly shorter sleep onset latency (length of time required to fall asleep) than the controls (median 10 vs. 20 min, p=.045). Figure 4 illustrates a trend indicating sleep onset latency changed over time in opposite directions for the two study conditions. Sleep onset latency was shortened by 7% in the experimental group compared to a 21% increase in the controls (p=.31).
Figure 4.
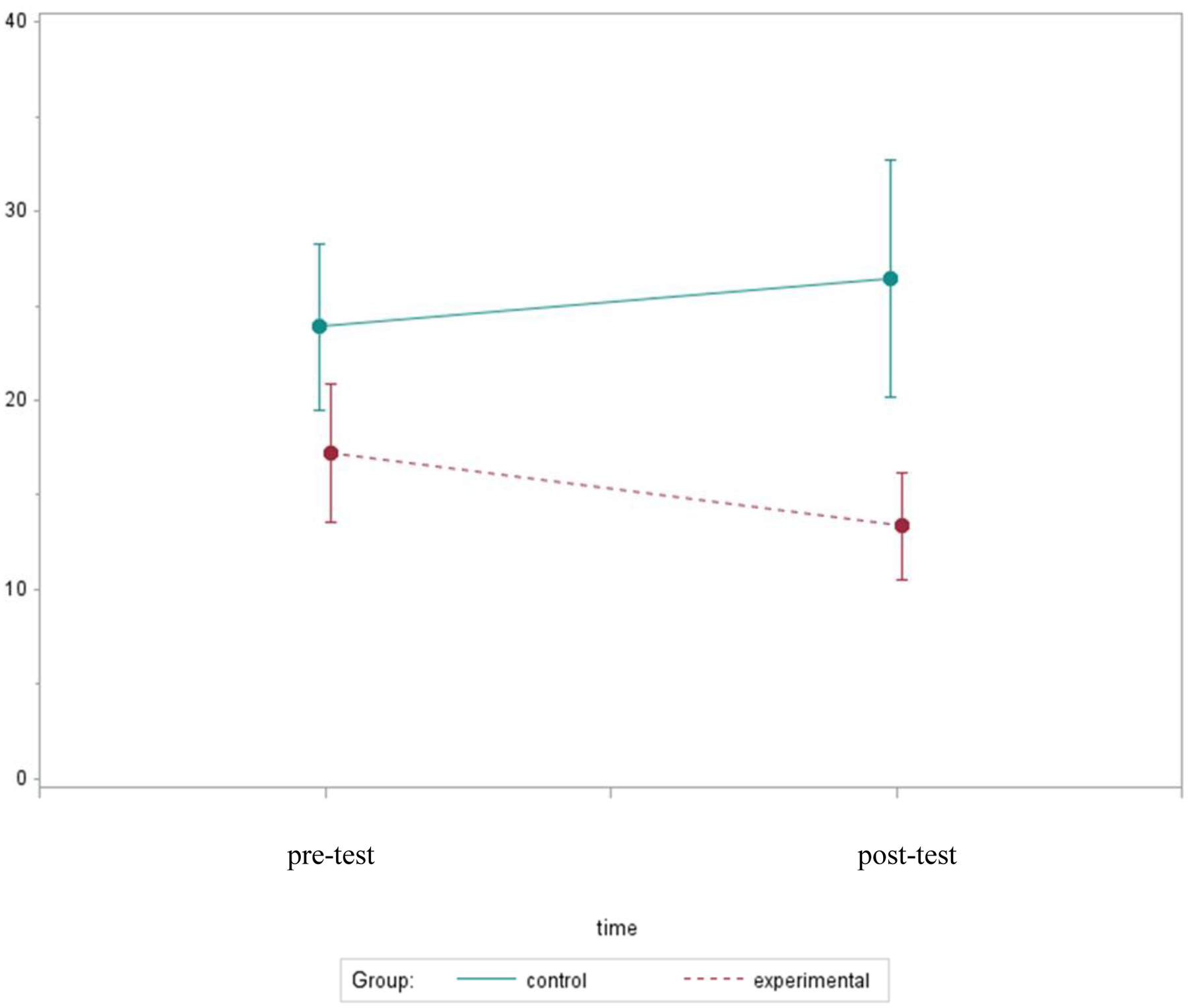
Mean Sleep Onset Latency Over Two Assessments
Objective Sleep Characteristics: Polysomnographic Findings
A subset of the participants (n=7) participated in the 24h at-home polysomnographic (PSG) recordings. Only nocturnal sleep data are reported in this paper. In this study, lights out and final wake times for the PSG sleep-scoring were based on the self-reported sleep and final wake times in the daily log. Table 2 provides sleep parameter definitions and the corresponding descriptive findings. The PSG findings are considered as preliminary because of the small sample size. Wilcoxon rank-sum tests examined the differences in overnight sleep between participants who received the intervention (n=5) and controls (n=2). Although the differences were not statistically significant, the objective PSG data showed that those who received bright light had much shorter sleep latency (median=14 vs. 63 min), longer total sleep time (median=467 vs. 315 min), and higher sleep efficiency (74% vs. 58%) than the controls.
Table 2.
Nocturnal Polysomnography Sleep Parameters at Post-Test (n = 7)
| Treatment (n = 5) | Control (n = 2) | ||
|---|---|---|---|
| Description | Median (SD) | Median (SD) | |
|
Total Time in Bed (TIB; minutes) |
From lights out to final wake time by self-report in diary | 632.5 (158.2) | 528.5 (57.3) |
|
Total Sleep Time (TST; minutes) |
The total time spent asleep during the sleep period time | 467.0 (106.3) | 314.5 (213.5) |
|
Sleep Onset Latency
(minutes) |
From light out to first 30-second epoch scored as sleep from lights out | 14 (27.9) | 63 (64.3) |
|
Sleep Efficiency
(%) |
TST/TIB × 100 | 74 (1.9) | 58 (33.9) |
| Stage 1 Sleep | |||
| Stage 2 Sleep | |||
| Stage 3 Sleep | |||
| REM Sleep | 6.4 (3.2) | 2.7 (3.9) | |
| Wake after Sleep Onset (minutes) | Total number of minutes spent awake after sleep onset | 155.0 (334.7) | 150.5 (91.2) |
|
Arousals
(counts) |
Total number of abrupt interruptions in sleep lasting 3 to15 seconds ending in lighter sleep stage | 56.0 (15.9) | 55.5 (54.4) |
| Awakenings (counts) | Total number of interruptions in sleep lasting longer than 15 seconds scored as wake | 41.0 (23.8) | 15.5 (16.3) |
Bright light did not significantly affect sleep architecture in the subset of women in the study. The women who served as controls spent 3% of their total sleep time in REM sleep compared with 6% among those who received bright light. Time spent in Stage 1 sleep (median = 10% vs. 11%) and Stage 2 sleep (median = 78% vs. 78%) were equivalent between the two groups. The controls experienced more Stage 3 sleep than those who received bright light (median 9% vs. 0%, respectively). Although the duration of wake after sleep onset was similar between the experimental and control groups (median = 155 vs. 151 min, respectively), those who received bright light experienced more frequent nighttime awakenings than controls (41 vs. 16 wakings).
Discussion
Although the findings from this study are mostly non-significant statistically, several findings are encouraging and may be of clinical significance. The descriptive findings showed an improvement in daytime sleepiness, depression, self-reported and objectively measured sleep onset latency, and sleep efficiency among those who received bright light, while these aspects became worse in those who received dim light. The findings suggest that the therapeutic bright light likely reversed worsening course of the sleep-related variables during the first three cycles of chemotherapy. The therapeutic bright light also mitigated worsening of QOL-related symptomology, as those who received bright light experienced substantially fewer relative increases in symptomology than controls (33% vs. 166%). Similar effects were also observed for fatigue, QOL-related functioning, and self-reported total sleep time, although the group differences were less noticeable for these variables.
Despite the small sample size, the women who received bright light took significantly less time to fall asleep, which was well supported by both subjective (10 vs. 20 min) and objective (14 vs. 63 min) sleep data. The effect sizes of subjective and objective sleep onset latency were considered as large with d=1.04 and d=0.86, respectively (Cohen 1988; Sawilowsky 2009). These findings are especially encouraging as women with breast cancer are known to suffer difficulty of falling asleep. Therapeutic bright light may provide a non-pharmacological option to help cancer patients who have trouble falling asleep. On the other hand, compared to sleep of healthy women (Sahlin et al. 2009), our participants showed more Stage 2 sleep but substantially less REM and Stage 3 sleep at post-test. Their total duration of wake time after sleep onset was three times longer than experienced by women without cancer (Sahlin et al. 2009). These findings showing those who received bright light experienced more nighttime awakenings and less Stage 3 sleep were unexpected.
In this study, the beneficial effects of bright light on sleep onset latency, total sleep time, and sleep efficiency are supported by both objective and subjective findings. While the subjective and objective sleep findings were overall consistent, the participants tended to self-report better sleep quality than indicated by the objective PSG measures. Regardless of group assignment, participants self-reported longer total sleep time, better sleep efficiency, and shorter sleep onset latency compared to the objective PSG sleep data except, for sleep onset latency at baseline. All participants reported approximately two times longer, or worse, sleep onset latency, than their PSG sleep findings at the baseline.
This study is unique in that the timing of light exposure was tailored to the individual’s chronotype. Different from previous studies in which bright light was uniformly delivered in the morning, the beneficial effects of bright light on sleep were pertinent in this study. It is known that appropriately timed exposure to bright light synchronizes internal biological rhythms to the external 24h light/dark cycle and corrects misalignments of the sleep/wake cycle (Kanathur et al. 2010). Personalized timings for light exposure induce circadian phase shift in the direction desirable for the individual and avoids inducing unwanted changes that may worsen the existing circadian aberrations. A study further examining the effect of bright light according to the individual’s chronotype is already underway (NCT03304587).
The small sample size (no power analysis) is a major limitation. In addition, although previous studies found that morning bright light prevented fatigue from worsening during chemotherapy (Ancoli-Israel et al. 2012; Jeste et al. 2013), the beneficial effect of bright light on fatigue was not supported in this study. The PROMIS fatigue measure asked participants on the first day of chemotherapy to rate their fatigue levels during the past7 d. With 21 d cycles of intravenous chemotherapy, most patients start recouping and experience less symptom intensity toward the end of the cycle. Retrospectively, assessing fatigue for the past 7 d on the day of chemotherapy may not reflect the severity of fatigue, which typically peaks 24–48h after chemotherapy administration (Schwartz 2000; Wu et al. 2008). This timing offset may also explain why fatigue did not differ between the groups after receiving bright light. A daily fatigue measure can better capture the fluctuation and, therefore, is recommended for future studies. Furthermore, the study assessments were limited to the first three cycles of chemotherapy. The long-term effect of bright light is unknown.
Our descriptive analyses illustrate favorable trends that show the potential benefits of using the tailored bright light intervention to abate symptoms (i.e., daytime sleepiness, depression, sleep disturbance, and QOL decline) that often deteriorate during chemotherapy. Even though the findings of this study are preliminary, they provide a starting point to further examine the efficacy of tailored bright light on symptoms. Future studies with larger sample sizes are needed to validate the findings. Further research using established circadian measures is needed to investigate the hypothesized biological mechanisms underlying therapeutic bright light. To maximize the effectiveness of therapeutic bright light, features, such as treatment duration, frequency, and timing in relation to chemotherapy treatment trajectory require further investigation. With further testing, bright light may provide cancer patients with a nonpharmacological, inexpensive, and simple to use treatment option with minimal to no side effects for alleviating disrupted sleep and associated symptoms during cancer treatment.
Funding details:
This study was supported by NIH CTSA under Grant #UL1 TR000448
Footnotes
Disclosure statement.
The authors report no conflict of interest.
References
- 1.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. 1993. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 2.Alexander S, Minton O, Andrews P, Stone P. 2009. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 45(3):384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, Lawton S, Desan P, Liangi L. 2012. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer. 20(6):1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AM & Farr L. 1999. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 26(10):1663–1671. [PubMed] [Google Scholar]
- 5.Berger AM, Higginbotham P. 2000. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: A pilot study. Oncol Nurs Forum. 27(9):1443–1448. [PubMed] [Google Scholar]
- 6.Berger AM, Parker KP, Young-McCaughan S, Mallory GA, Barsevick AM, Beck SL, Carpenter JS, Carter PA, Hinds PS, Lee KA, et al. 2005. Sleep wake disturbances in people with cancer and their caregivers: State of the science. Oncol Nurs Forum. 32(6):E98–126. [DOI] [PubMed] [Google Scholar]
- 7.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. 1989. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 8.Byar KL, Berger AM, Bakken SL, Cetak MA. 2006. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 33(1):E18–26. [DOI] [PubMed] [Google Scholar]
- 9.Cheng KK, Lee DT. 2011. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. 78(2):127–137. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J 1998. Statistical power analysis for the behavioral sciences. Hillsdale (NJ): Lawrence Erlbaum Associates. [Google Scholar]
- 11.Davidson JR, MacLean AW, Brundage MD, Schulze K. 2002. Sleep disturbance in cancer patients. Soc Sci Med. 54(9):1309–1321. [DOI] [PubMed] [Google Scholar]
- 12.Dijk DJ, Boulos Z, Eastman CI, Lewy AJ, Campbell SS, Terman M. 1995. Light treatment for sleep disorders: Consensus report. II. Basic properties of circadian physiology and sleep regulation. J Biol Rhythms. 10(2):113–125. [DOI] [PubMed] [Google Scholar]
- 13.Eastman CI, Martin SK. 1999. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 31(2):87–98. [DOI] [PubMed] [Google Scholar]
- 14.Garcia SF, Cella D, Clauser SB, Flynn KE, Lad T, Lai J-S, Reeve BB, Smith AW, Stone AA, Weinfurt K. 2007. Standardizing patient-reported outcomes assessment in cancer clinical trials: A patient-reported outcomes measurement information system initiative. J Clin Oncol. 25(32):5106–5112. [DOI] [PubMed] [Google Scholar]
- 15.Gooley JJ. 2008. Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singapore. 37(8):669–676. [PubMed] [Google Scholar]
- 16.HealthMeasures. 2020. HealthMeasures Scoring Service powered by Assessment Center. [accessed 2020 Dec 20]; https://www.assessmentcenter.net/ac_scoringservice
- 17.HealthMeasures. 2019. PROMIS fatigue scoring manual. [accessed 2020 Dec 20]; http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Fatigue_Scoring_Manual.pdf
- 18.Horne JA, Ostberg O. 1976. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 4(2):97–110. [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A. 2007. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester (NY): American Academy of Sleep Medicine. [Google Scholar]
- 20.Jasper HH. 1958. The ten twenty system of the International Federation. Electroencephalogr Clin Neurophysiol. 10:371–375. [PubMed] [Google Scholar]
- 21.Jeste N, Liu L, Rissling M, Trofimenko V, Natarajan L, Parker BA, Ancoli-Israel S. 2013. Prevention of quality-of-life deterioration with light therapy is associated with changes in fatigue in women with breast cancer undergoing chemotherapy. Qual Life Res. 22(6):1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns MW. 1991. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 23.Kanathur N, Harrington J, Lee-Chiong T Jr. 2010. Circadian rhythm sleep disorders. Clin Chest Med. 31(2):319–325. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. 2001. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuller R 2002. The influence of light on circarhythms in humans. J Physiol Anthropol Appl Human Sci. 21(2):87–91. [DOI] [PubMed] [Google Scholar]
- 26.Manir KS, Bhadra K, Kumar G, Manna A, Patra NB, Sarkar SK. 2012. Fatigue in breast cancer patients on adjuvant treatment: Course and prevalence. Indian J Palliat Care. 18(2):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meesters Y, Lambers PA. 1990. Light therapy in patient with seasonal fatigue. Lancet. 336(8717):745. [DOI] [PubMed] [Google Scholar]
- 28.Petrie K, Conaglen JV, Thompson L, Chamberlain K. 1989. Effect of melatonin on jet lag after long haul flights. BMJ. 298(6675):705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piper B 1993. Subjective fatigue in women receiving six cycles of adjuvant chemotherapy for breast care. (Doctoral dissertation, University of California, San Francisco). Dissertation Abstracts International. 9303553. [Google Scholar]
- 30.Rastad C, Ulfberg J, Lindberg P. 2011. Improvement in fatigue, sleepiness, and health-related quality of life with bright light treatment in persons with seasonal affective disorder and subsyndromal SAD. Depress Res Treat. 2011:543906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson GS, Carskadon MA, Flagg W, Van den Hoed J, Dement WC, Mitler MM. 1978. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 45(5):621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roehrs T, Zorick F, Wittig R, Conway W, Roth T. 1989. Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders. Chest. 95(6):1202–1206. [DOI] [PubMed] [Google Scholar]
- 33.Sahlin C, Franklin KA, Stenlund H, Lindberg E. 2009. Sleep in women: Normal values for sleep stages and position and the effect of age, obesity, sleep apnea, smoking, alcohol and hypertension. Sleep Med. 10(9):1025–1030. [DOI] [PubMed] [Google Scholar]
- 34.Sarna L 1993. Correlates of symptom distress in women with lung cancer. Cancer Pract 1(1):21–28. [PubMed] [Google Scholar]
- 35.Sawilowsky S 2009. New effect size rules of thumb. J Mod Appl Stat Meth. 8:597–599. [Google Scholar]
- 36.Schwartz AL. 2000. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract. 8(1):16–24. [DOI] [PubMed] [Google Scholar]
- 37.Smith CS, Reilly C, Midkiff K. 1989. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Takahashi M, Tanaka M, Takanao T, Nishinoue N, Kaku A, Kato N, Tagaya H, Miyaoka H. 2011. Brief morning exposure to bright light improves subjective symptoms and performance in nurses with rapidly rotating shifts. J Occup Health. 53(4):258–266. [DOI] [PubMed] [Google Scholar]
- 39.Terman M, Lewy AJ, Dijk DJ, Boulos Z, Eastman CI, Campbell SS. 1995. Light treatment for sleep disorders: consensus report. IV. Sleep phase and duration disturbances. J Biol Rhythms. 10(2):135–147. [DOI] [PubMed] [Google Scholar]
- 40.Wu HS, Dodd M, Cho MH. 2008. Patterns of fatigue and effect of exercise in breast cancer patients receiving chemotherapy. Oncol Nurs Forum. 35(5):E90–E99. [Google Scholar]


