Abstract
Background.
Impairments in inhibitory control and its underlying brain networks (control/salience areas) are associated with substance misuse. Research often assumes a causal substance exposure effect on brain structure. This assumption remains largely untested and other factors (e.g., familial risk) may confound exposure effects. We leveraged a genetically-informative sample of 24-year-old twins and a quasi-experimental cotwin control design to separate alcohol or cannabis exposure effects during emerging adulthood from familial risk on control/salience network cortical thickness.
Methods.
In a population-based sample of 436 24-year-old twins, dimensional measures of alcohol and cannabis use (e.g., frequency, density, quantity, intoxications) across emerging adulthood were assessed. Cortical thickness of control/salience network areas were assessed using MRI and defined by a fine-grained cortical atlas.
Results.
Greater alcohol, but not cannabis, misuse was associated with reduced thickness of prefrontal (e.g., dorso/ventrolateral, right frontal operculum) and frontal medial cortices, as well as temporal lobe, intraparietal sulcus, insula, parietal operculum, precuneus, and parietal medial areas. Effects were predominately (pre)frontal and right lateralized. Cotwin control analyses suggested the effects likely reflect both the familial predisposition to misuse alcohol, and specifically for lateral prefrontal, frontal/parietal medial, and right frontal operculum, an alcohol exposure effect.
Conclusions.
This study provides novel evidence that alcohol-related reductions in cortical thickness of control/salience brain networks likely represent the effects of alcohol exposure and premorbid characteristics of the genetic predisposition to misuse alcohol. The dual effects of these two alcohol-related causal influences have important and complementary implications regarding public health and prevention efforts to curb youth drinking.
Keywords: Alcohol, cannabis, control network, cortical thickness, cotwin control, salience network
Introduction
Substance misuse is a leading concern for young adults. Alcohol and cannabis are the two most commonly used recreational substances among emerging adults aged 18 to 25; according to a 2018 United States national survey (1), approximately 55% were regular drinkers, 17% of those were heavy drinkers, and 35% were cannabis users. Emerging adulthood is an important developmental period with continued changes in structural brain development (2,3) (particularly in various prefrontal cortex [PFC] areas) occurring alongside peak lifetime levels of alcohol and cannabis (mis)use (4,5). This may create a vulnerable period where the structure (e.g., cortical thickness) of the still-developing young adult brain is particularly sensitive to deleterious effects of alcohol or cannabis exposure.
Impairments in inhibitory/cognitive control, salience/ventral attention, and their proposed brain-based substrates reflect core attributes of substance misuse (6–9). Cortical grey matter reductions are observed across several forms of substance use in regions such as the dorsolateral PFC, anterior cingulate/frontal medial cortex, insula, and temporal cortex (7,10–12). All of these areas have been implicated in specific large-scale cortical networks (13) thought to support cognitive control (e.g., behavioral adaptation) and salience (e.g., ventral attentional orienting toward salient stimuli) processes (14–17), which are ‘two sides of the same (inhibitory control) coin’ (18,19). Individual differences in the structure of control/salience areas may reflect neural substrates of substance-related deficits in inhibitory control (20). The greatest effects on cortical grey matter structure are typically observed for alcohol, with less consistent findings for cannabis (21,22). Recent studies report alcohol-specific cortical grey matter reductions but no evidence for cannabis-specific effects (11,23), though others report decreases in certain prefrontal areas (e.g., anterior cingulate; (24)). Contributing to the lack of cannabis findings in prior work may be the use of limited phenotypes (e.g., use vs. no use) or small samples (typically N < 100).
Research has often assumed a causal deleterious exposure effect of alcohol, cannabis, or other substance use on control/salience brain networks (25,26). Experimental non-human primate models suggest that alcohol exposure during the rhesus macaque equivalent of young adulthood causes grey matter shrinkage (27,28). Prospective studies of genetically-unrelated adolescents have reported accelerated decline in frontal grey matter after drinking initiation (29–31). This is often explicitly or implicitly interpreted as reflective of an exposure effect, however, those who misuse substances typically differ from those who do not on important characteristics that may influence both substance use and brain outcomes (32,33). Rather than reflecting exposure, substance-related brain anomalies may instead be due to familial risk (e.g., genetic liability, rearing environment) influences. For example, high-risk adolescents with a family history of alcohol use disorder exhibit reduced thickness in right frontal regions (34), suggesting a premorbid risk effect. Despite significant public health implications (e.g., policy decisions, targeted prevention efforts), there remains a lack of evidence separating these sources of causality related to alcohol and cannabis use on the emerging adult brain. This is largely because the overwhelming majority of prior research is correlational in nature and has been unable to disentangle familial risk from exposure. Novel research designs approximating true experiments (35,36) are needed in human research to make stronger causal inferences regarding the effects of alcohol and cannabis on the structure of control/salience brain networks.
One such research design is the cotwin control (CTC) analysis (37,38), a “natural” quasi-experiment that uses twins as ideal genetic and shared environmental controls, to more appropriately and stringently evaluate for causal alcohol/cannabis exposure effects (unconfounded by familial influences) than is possible with cross-sectional or longitudinal studies of genetically-unrelated individuals. In this design, outcomes of the lesser-using twin provide a close approximation of the expected outcomes for the heavier-using twin had they had less exposure (e.g., used less alcohol or cannabis). For example, if reduced thickness of control/salience network areas reflects a substance-related exposure effect, the heavier-using twin would be expected to have decreased thickness relative to their lesser-using cotwin. In contrast, if familial risk accounts for the observed association, thickness should be comparable between the cotwins.
The current study was designed to address this important gap in the literature by testing the causal relationship between alcohol and cannabis (mis)use in emerging adulthood and thickness of control and salience networks in a large population-based, etiologically-informative sample of 436 24-year-old twins. Of primary interest was the use of the CTC to separate familial risk influence from exposure effects on cortical thickness.
A secondary interest of this study was to provide greater localization of alcohol/cannabis effects relative to most prior work in this area that used brain atlases to segment the cortex into a small number of spatially large/coarse areas based solely on structural boundaries (e.g., Desikan-Killiany atlas (39)). To make more nuanced inferences regarding the spatial localization and potential functional significance of any observed alcohol/cannabis effects, we used a recent empirically derived atlas (40) to define fine-grained cortical areal boundaries within specific spatial networks clustered within well-established functional systems (13). This approach is well suited to this study because it allowed us to select a priori cortical areas of interest within predefined control/salience networks related to response inhibition.
Given prior work, we hypothesized that substance misuse across emerging adulthood would be associated with reduced thickness in control/salience network areas including the dorsolateral/ventrolateral prefrontal cortex, anterior cingulate/medial frontal cortex, and insula, all of which are central to neurocognitive models of inhibitory/cognitive control, salience detection (18,41,42), and substance misuse (8,20). Greater individual-level phenotypic associations were expected for alcohol than cannabis based on previous work suggesting a strong link between alcohol and grey matter reductions and less consistent findings for cannabis-specific effects (11,22,23). For all significant associations, we tested whether associations differed between sexes given suggestive evidence of an increased liability for substance-related effects in women (43–46) and the need for more well-powered neuroimaging studies evaluating substance-related sex differences (8). Follow-up CTC analysis models were conducted for significant alcohol or cannabis phenotypic associations to assess the relative contribution of familial risk (e.g., genetic propensity) and potential exposure-related effects on cortical thickness.
Methods and Materials
Sample
Participants were same-sex twins assessed at the target age of 24 from the population-based Minnesota Twin Family Study Enrichment Sample (47). By design (participants were able to complete in-person MRI assessments; met standard MRI safety exclusions), 441 individuals underwent structural MRI scans. Four individuals with clinically significant brain anomalies (determined by a clinical radiologist) and one with coil failure during scanning were excluded. The final sample contained 436 individuals (age: mean [SD] = 24.3 [0.8] years; 254 women) from 120 complete MZ pairs (i.e., 240 MZ twins), 30 unpaired MZ twins, 66 complete DZ pairs (i.e., 132 DZ twins), and 34 unpaired DZ twins. See Supplemental Section S1 for racial composition. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Substance use assessment
Substance use history was assessed by trained interviewers using an expanded version of the Substance Abuse Module of the Composite International Diagnostic Interview (48). Measures used in this report were dimensional index scores of alcohol and cannabis use (e.g., frequency; quantity; intoxications) designed to capture variations in normative patterns of alcohol/cannabis exposure (i.e., spanning no use to heavy misuse) across emerging adulthood in this population-based sample. This approach has been used in several other studies from our group (49–54).
A drink index (55) (possible range: 0.00–5.75) was derived by taking the average of four dimensional alcohol use items: frequency of drinking (last seven years); typical number of drinks per occasion (quantity; last seven years); maximum number of drinks consumed in 24 hours (last seven years); and number of intoxications (lifetime) (Cronbach’s α = 0.78; average pairwise r = 0.47, range = 0.22–0.61). A cannabis index (possible range: 0.00–5.00) was derived by taking the average of two dimensional cannabis use items (both last seven years): frequency of use; number of uses (pairwise r = 0.94). See Table S1 for details.
MRI acquisition and processing
Acquisition parameters are detailed in Supplemental Section S2.
All images were normalized and manually reviewed for artifacts/structural anomalies prior to processing in Freesurfer (version 5.3.0) (56,57) and segmentation according to the 400-area cortical parcellation atlas from Schaefer et al. (40) mapped to the 17-networks from Yeo et al. (13).
For our study, analyses were restricted to cortical thickness of areas with functional/anatomical relevance to prominent models of inhibitory/cognitive control and salience detection (15,17,42,58) and neurobiological models of substance use/addiction (8). As shown in Figure 1, cortical thickness from 112 individual areas of the control and salience/ventral attention networks were calculated for analysis.
Figure 1.
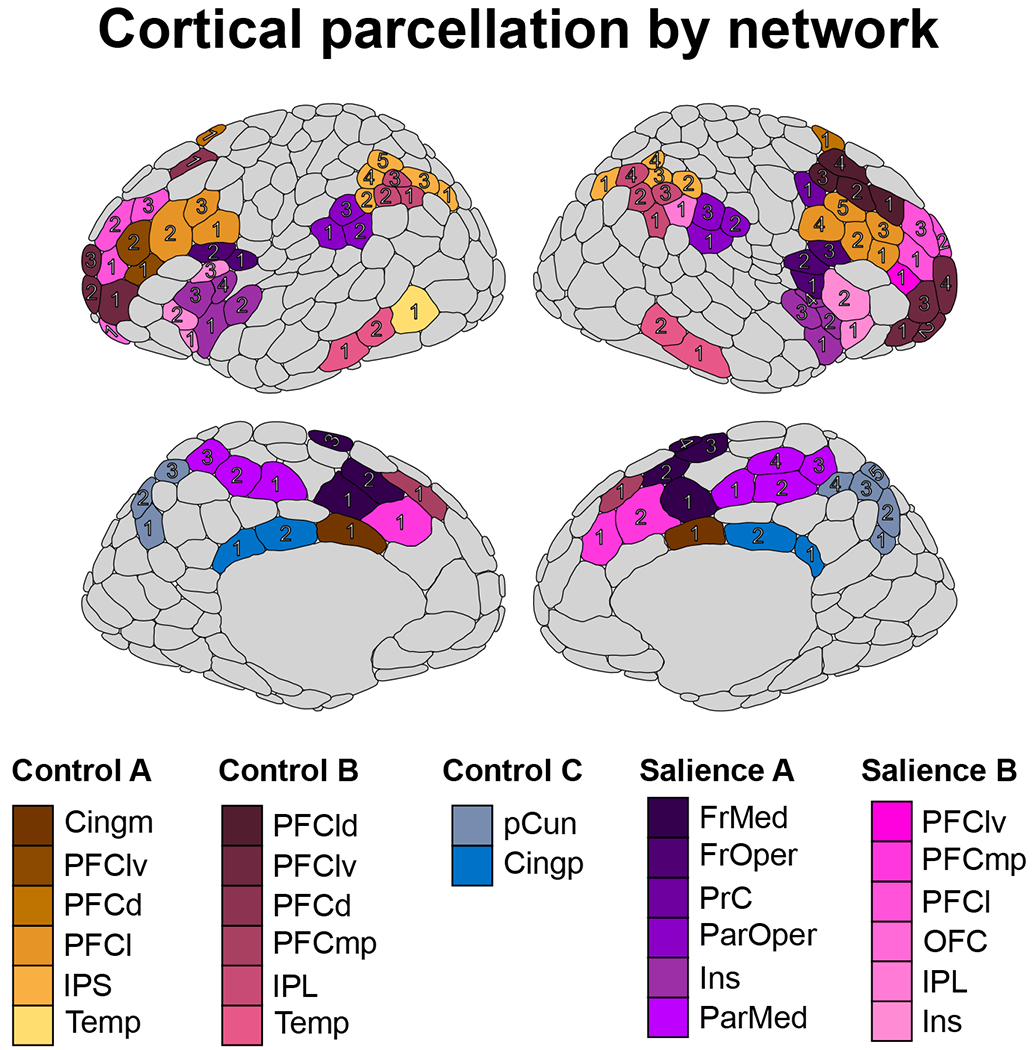
The 400-area cortical parcellation from Schaefer et al. (2018) displaying the 112 areas within the three control networks (A, B, and C) and two salience networks (A and B) chosen for analysis in the current report. Each area is identified by a specific shade of its network color (assigned based on the networks from Yeo et al. (2011)), and the numbers for each area correspond to the assignment from the Schaefer et al. (2018) atlas. For example, the four right hemisphere frontal medial areas within the Salience A network are colored in dark purple. Areas in grey are not members of the control/salience networks and were not analyzed in this report. Figure was created using the ggseg (82) R package. Abbreviations: Cing, cingulate; m, medial; IPS, intraparietal sulcus; PFC, prefrontal cortex; d, dorsal; l, lateral; v, ventral; Temp, temporal; IPL, inferior parietal lobule; p, posterior; pCun, precuneus; FrMed, frontal medial; FrOper, frontal operculum; Ins, insula; ParMed, parietal medial; ParOper, parietal operculum; PrC, precentral gyrus; OFC, orbitofrontal cortex.
Statistical analysis
Linear mixed models (LMMs; (59)) were fit in R (60) with Kenward-Roger approximated denominator degrees of freedom (61) and random intercepts at the family level to adjust for within-twin-pair correlations in dependent measures. All models included sex, age, zygosity, scanner, and acquisition software as covariates.
First, we tested the individual-level phenotypic associations between drink or cannabis index scores (independent variable) and thickness of each area (dependent measure). Comparisons across the 112 areas were adjusted using a false discovery rate (FDR) (62,63) at q-value < 0.05 (64). For significant associations, sex-related differences were tested by adding a sex (coded for women) by drink/cannabis index interaction term.
For each significant effect, follow-up CTC analyses (37) evaluated causal exposure and familial risk effects. Outcomes (e.g., thickness) were compared between members of a twin pair; if a twin used alcohol (or cannabis) more than their cotwin, the outcome of the lesser-using twin provides a close approximation of the expected outcome (unobserved counterfactual (65)) for the heavier-using twin had she/he used less. The index score was decomposed into two orthogonal components: (1) the twin-pair mean score (between-pair effect), indexing all familial risk influences (genetic; shared environmental), whether measured or unmeasured, holding constant twin differences in drinking; and (2) an individual’s deviation from their respective twin-pair mean (within-pair effect), reflecting the nonshared environmental substance exposure effect (66). A significant between-pair effect would be consistent with familial risk influencing both use and thickness. A significant within-pair effect would be consistent with the potential effect of alcohol (or cannabis) exposure (unconfounded by all measured and unmeasured familial factors influencing use (66)) on thickness. A zygosity by within-pair interaction compared the magnitude of within-pair effects between MZ (100% genetic control) and DZ (50% genetic control) twins; statistically comparable MZ/DZ effects would be strongly consistent with an exposure effect (37).
Results
Alcohol and cannabis use scores
Descriptive statistics for the drink and cannabis index scores are presented in Table 1. There were moderate familial influences and substantial within-pair differences in alcohol/cannabis exposure, which supported the use of the CTC analysis in this sample. See Supplemental Section S3 for further details.
Table 1.
Descriptive statistics for the drink and cannabis index measures.
| Measure | Total Sample | Men | Women | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Drink Index | 2.79 (0.97) | 0–4.75 | 3.17 (0.91) | 0–4.75 | 2.52 (0.92) | 0–4.50 |
| Frequency | 2.82 (0.83) | 0–5.00 | 3.03 (0.85) | 0–5.00 | 2.67 (0.78) | 0–4.00 |
| Amount | 1.59 (0.81) | 0–5.00 | 1.84 (0.86) | 0–5.00 | 1.42 (0.72) | 0–5.00 |
| Max drinks | 3.39 (1.22) | 0–6.00 | 3.91 (1.13) | 0–6.00 | 3.01 (1.15) | 0–6.00 |
| Intoxications | 3.32 (1.93) | 0–6.00 | 3.86 (1.83) | 0–6.00 | 2.93 (1.91) | 0–6.00 |
| Cannabis Index | 1.72 (1.76) | 0–5.00 | 2.31 (1.85) | 0–5.00 | 1.30 (1.57) | 0–5.00 |
| Frequency | 1.69 (1.78) | 0–5.00 | 2.28 (1.87) | 0–5.00 | 1.28 (1.59) | 0–5.00 |
| Amount | 1.75 (1.79) | 0–5.00 | 2.33 (1.87) | 0–5.00 | 1.33 (1.60) | 0–5.00 |
Note: All alcohol and cannabis use scores were greater for men compared to women (p-values < 0.0001).
Individual-level analyses
As reported in Table 2 and shown in Figure 2, greater drinking index scores were significantly associated with reduced thickness in 31 cortical areas after FDR adjustment (see Table S2 for all results). Significant associations were found for the control network right dorsolateral PFC, precuneus, and temporal lobe; left medial posterior PFC; and bilateral lateral PFC, ventrolateral PFC, and intraparietal sulcus. Significant salience network areas were the right frontal operculum, insula, ventrolateral and medial posterior PFC, and parietal medial cortex; left lateral PFC and parietal operculum; and bilateral frontal medial. The majority of effects were right lateralized (65%) and located in (pre)frontal areas (68%). For all 31 areas, the alcohol effects did not statistically differ as a function of sex (interaction p-values range: 0.0806–0.9956).
Table 2.
Individual-level associations between alcohol use and cortical thickness.
| Area | Beta (SE) | t (df) | p-value | q-value |
|---|---|---|---|---|
| Control A | ||||
| PFCl_1 (r) | −0.021 (0.009) | −2.321 (403) | 0.0208 | 0.0439 |
| PFCl_4 (r) | −0.018 (0.008) | −2.388 (404) | 0.0174 | 0.0412 |
| PFCl_2 (l) | −0.020 (0.007) | −3.063 (421) | 0.0023 | 0.0140 |
| IPS_1 (r) | −0.023 (0.010) | −2.370 (392) | 0.0182 | 0.0412 |
| IPS_3 (l) | −0.022 (0.008) | −2.748 (417) | 0.0063 | 0.0260 |
| Control B | ||||
| PFCld_2 (r) | −0.026 (0.010) | −2.636 (426) | 0.0087 | 0.0301 |
| PFCld_3 (r) | −0.035 (0.012) | −3.026 (403) | 0.0026 | 0.0140 |
| PFCld_4 (r) | −0.025 (0.010) | −2.507 (416) | 0.0126 | 0.0327 |
| PFClv_4 (r) | −0.016 (0.007) | −2.298 (425) | 0.0221 | 0.0439 |
| PFClv_1 (l) | −0.022 (0.009) | −2.510 (423) | 0.0124 | 0.0327 |
| PFClv_2 (l) | −0.019 (0.008) | −2.238 (423) | 0.0258 | 0.0475 |
| PFClv_3 (l) | −0.021 (0.008) | −2.580 (418) | 0.0102 | 0.0301 |
| PFCmp_1 (l) | −0.032 (0.012) | −2.686 (428) | 0.0075 | 0.0287 |
| Temp_1 (r) | −0.028 (0.012) | −2.302 (391) | 0.0219 | 0.0439 |
| Temp_2 (r) | −0.048 (0.014) | −3.496 (390) | 0.0005 | 0.0080 |
| Control C | ||||
| pCun_1 (r) | −0.035 (0.010) | −3.353 (404) | 0.0009 | 0.0087 |
| pCun_4 (r) | −0.028 (0.011) | −2.578 (414) | 0.0103 | 0.0301 |
| Salience A | ||||
| FrMed_3 (r) | −0.028 (0.013) | −2.171 (422) | 0.0305 | 0.0489 |
| FrMed_4 (r) | −0.076 (0.015) | −4.975 (404) | <0.0001 | <0.0001 |
| FrMed_2 (l) | −0.038 (0.011) | −3.285 (421) | 0.0011 | 0.0092 |
| FrMed_3 (l) | −0.047 (0.015) | −3.135 (413) | 0.0018 | 0.0131 |
| FrOper_2 (r) | −0.022 (0.008) | −2.596 (428) | 0.0097 | 0.0301 |
| Ins_4 (r) | −0.025 (0.011) | −2.237 (421) | 0.0258 | 0.0475 |
| ParMed_1 (r) | −0.027 (0.009) | −3.006 (411) | 0.0028 | 0.0140 |
| ParMed_3 (r) | −0.017 (0.008) | −2.194 (402) | 0.0288 | 0.0483 |
| ParMed_4 (r) | −0.026 (0.007) | −3.437 (422) | 0.0006 | 0.0080 |
| ParOper_2 (l) | −0.023 (0.010) | −2.189 (407) | 0.0291 | 0.0483 |
| Salience B | ||||
| PFCl_1 (l) | −0.025 (0.010) | −2.491 (411) | 0.0131 | 0.0327 |
| PFCl_2 (l) | −0.018 (0.008) | −2.219 (413) | 0.0270 | 0.0480 |
| PFClv_1 (r) | −0.026 (0.009) | −2.759 (408) | 0.0061 | 0.0260 |
| PFCmp_1 (r) | −0.033 (0.009) | −3.776 (426) | 0.0002 | 0.0045 |
Notes: All tests survived false discovery rate adjustment (q < 0.05). The numbers in each parcel name denote the parcel number from the Schaefer et al. (2018) atlas (see Figure 2 for a visual depiction), and parentheses denote hemisphere (l, left; r, right).
Abbreviations: PFC, prefrontal cortex; IPS, intraparietal sulcus; Temp, temporal; pCun, precuneus; FrMed, frontal medial; FrOper, frontal operculum; Ins, insula; ParMed, parietal medial; ParOper, parietal operculum; l, lateral; d, dorsal; v, ventral; mp, medial posterior.
Figure 2.
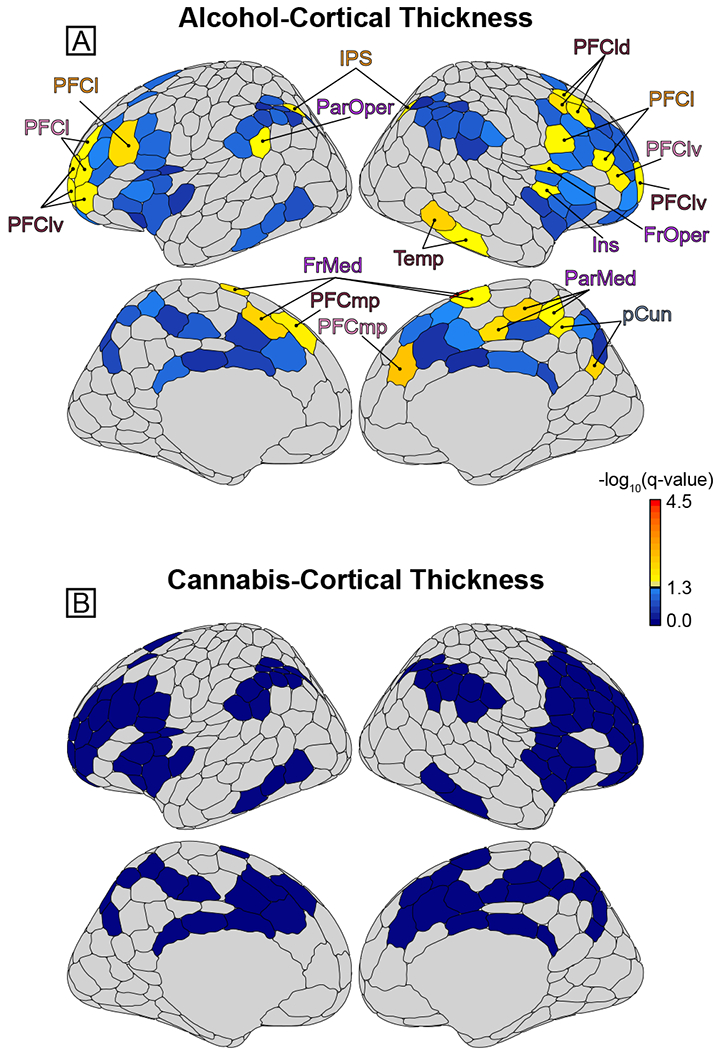
Association between cortical thickness and alcohol or cannabis use. [A] Brain maps plotting the significance (false discovery rate [FDR]-adjusted −log10(q) values; larger value = greater significance/smaller q-value) of the drink index effect for each area. Greater alcohol use was associated with decreased thickness in a collection of prefrontal cortex (lateral [PFCl], dorsolateral [PFCld]; ventrolateral [PFClv]; medial posterior [PFCmp]), frontal operculum (FrOper), and frontal medial cortex (FrMed) areas. Significant alcohol effects were also found in the parietal medial cortex (ParMed), precuneus (pCun), temporal lobe (Temp), insula (Ins), parietal operculum (ParOper), and intraparietal sulcus (IPS). The color scaling is such that yellow-red signifies those areas showing a significant negative association with drink index scores that survived FDR multiple comparison adjustment, whereas blue denotes areas that were not significant/did not survive FDR adjustment. [B] The same as A, but for the cannabis index. No effects survived FDR adjustment for cannabis use. Note that the coloring of the area labels (e.g., PFCl) corresponds to the network color assignments in Figure 1 (i.e., orange = Control A network). Figure was created using the ggseg (82) R package.
For cannabis, only three areas were nominally significant at p < 0.05 and none survived FDR adjustment (see Figure 2, Table S3).
Alcohol-thickness associations were by and large robust to the inclusion of various potential confounders (e.g., psychopathology, trauma; Supplemental Section S4, Table S4–5).
Post-hoc analyses tested whether cortical thickness deviations in areas showing significant alcohol use relate to behavioral disinhibition, where positive findings would suggest a link between cortical thickness, disinhibitory traits, and drinking. Behavioral disinhibition was nominally associated (p < 0.05) with reduced thickness in four salience network areas (frontal medial, parietal medial, insula, frontal operculum cortex) (Supplement Section S5, Table S6); however, while all tests were in the expected direction (greater externalizing – decreased thickness), small effect sizes precluded significance after FDR adjustment.
Cotwin control analysis of alcohol use
As reported in Table 3, the between-pair effect (reflecting familial influences shared by cotwins) had a significant negative association with thickness in nearly all areas. The within-pair effect (reflecting nonshared environmental exposure unconfounded by familial influences) had a significant negative association with the control network lateral PFC, and the frontal medial, frontal operculum, and parietal medial areas of the salience network. That is, after adjusting for all measured and unmeasured sources of potential familial risk affecting these brain outcomes, heavier-drinking twins showed decreased thickness of the lateral PFC, frontal/parietal medial cortex, and frontal operculum relative to their lesser-drinking cotwins. Although in the expected negative direction, neither the salience network insula nor the ventrolateral PFC produced significant within- or between-pair effects. Scatterplots for three illustrative sets of CTC effects are depicted in Figure 3. Consistent with expectations for a within-pair effect (37), the MZ and DZ within-pair effects were statistically equivalent (in all cases, the zygosity by within-effect interaction terms were non-significant [p-values ≥ 0.1379]).
Table 3.
Cotwin control analysis between alcohol use (drink index) and cortical thickness.
| Cotwin control | ||||||
|---|---|---|---|---|---|---|
| Within-pair | Between-pair | |||||
| Area | Beta (SE) | t (df) | p-value | Beta (SE) | t (df) | p-value |
| Control A | ||||||
| PFCl | −0.081 (0.026) | −3.096 (185) | 0.0023 | −0.051 (0.025) | −2.059 (179) | 0.0409 |
| IPS | −0.040 (0.024) | −1.629 (185) | 0.1050 | −0.047 (0.020) | −2.348 (179) | 0.0200 |
| Control B | ||||||
| PFCld | −0.052 (0.032) | −1.608 (185) | 0.1096 | −0.111 (0.031) | −3.616 (179) | 0.0004 |
| PFClv | −0.033 (0.033) | −0.964 (185) | 0.3363 | −0.125 (0.037) | −3.390 (179) | 0.0009 |
| PFCmp | −0.017 (0.019) | −0.935 (185) | 0.3509 | −0.044 (0.018) | −2.462 (179) | 0.0148 |
| Temp | −0.058 (0.039) | −1.509 (185) | 0.1331 | −0.092 (0.028) | −3.330 (179) | 0.0011 |
| Control C | ||||||
| pCun | −0.035 (0.027) | −1.319 (185) | 0.1888 | −0.080 (0.023) | −3.430 (179) | 0.0007 |
| Salience A | ||||||
| FrMed | −0.113 (0.049) | −2.285 (185) | 0.0234 | −0.205 (0.054) | −3.825 (179) | 0.0002 |
| FrOper | −0.031 (0.013) | −2.431 (185) | 0.0160 | −0.012 (0.012) | −0.945 (179) | 0.3458 |
| Ins | −0.021 (0.018) | −1.151 (185) | 0.2514 | −0.018 (0.015) | −1.176 (179) | 0.2412 |
| ParMed | −0.068 (0.027) | −2.547 (185) | 0.0117 | −0.063 (0.026) | −2.443 (179) | 0.0156 |
| ParOper | 0.006 (0.018) | 0.349 (185) | 0.7278 | −0.040 (0.014) | −2.845 (179) | 0.0050 |
| Salience B | ||||||
| PFCl | −0.032 (0.023) | −1.411 (185) | 0.1599 | −0.052 (0.020) | −2.560 (179) | 0.0113 |
| PFClv | −0.027 (0.016) | −1.627 (185) | 0.1054 | −0.025 (0.013) | −1.954 (179) | 0.0523 |
| PFCmp | −0.015 (0.014) | −1.065 (185) | 0.2881 | −0.036 (0.013) | −2.806 (179) | 0.0056 |
Notes: Significant effects (p < 0.05) are in bold. Cortical thickness was summed across parcels within the same network and cortical regions that showed significant individual-level associations with alcohol (e.g., the three Control A PFCl parcels in Table 2) prior to the cotwin control analysis to reduce multiple comparisons.
Abbreviations: PFC, prefrontal cortex; IPS, intraparietal sulcus; Temp, temporal; pCun; precuneus; FrMed, frontal medial; FrOper, frontal operculum; Ins, insula; ParMed, parietal medial; ParOper, parietal operculum; l, lateral; d, dorsal; v, ventral; mp, medial posterior.
Figure 3.
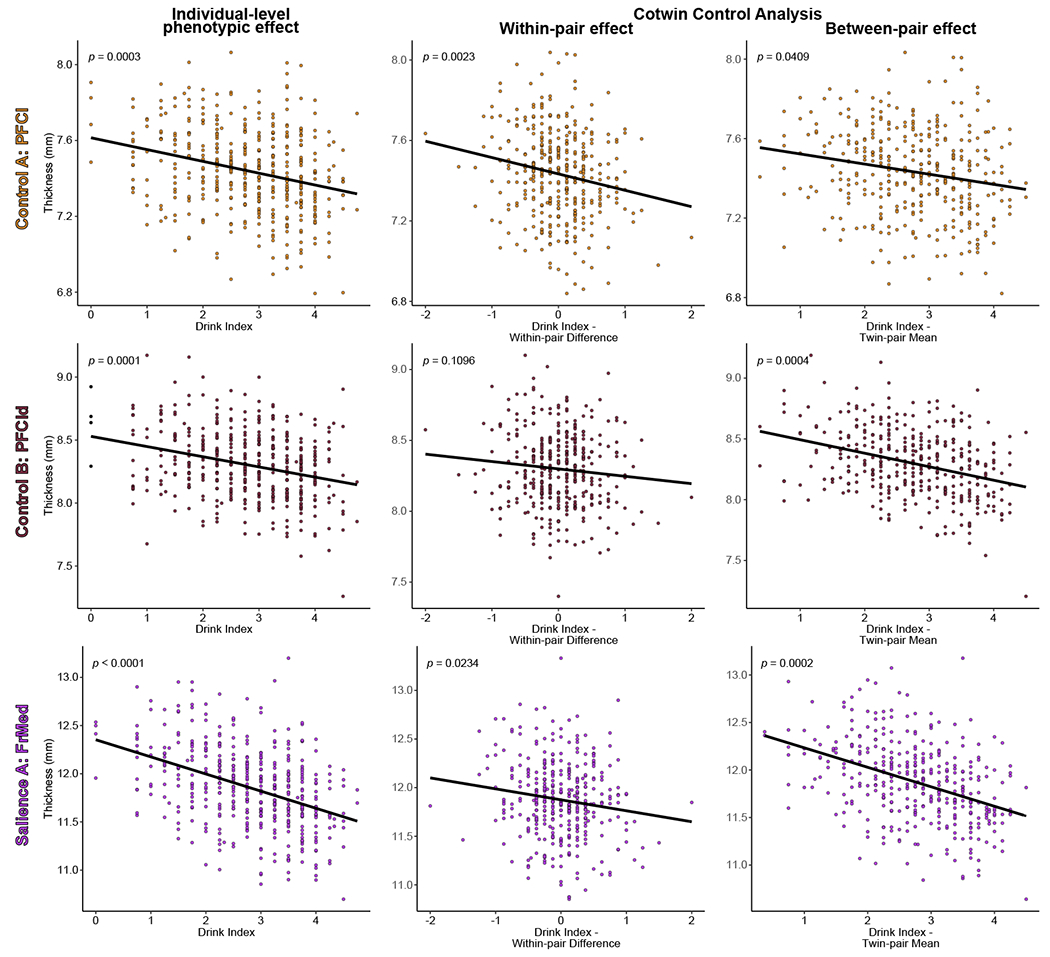
Partial residual plots of alcohol use and cortical thickness for three illustrative cortical areas. The individual-level phenotypic effects (i.e., without regard to twin-pair membership, analogous to a standard regression of outcome on exposure) and cotwin control analysis within-pair and between-pair effects are depicted along with model fit lines and p-values from the linear mixed models of drink index scores on cortical thickness (adjusting for all covariates). For the within-pair effects, the model fit lines illustrate that heavier-drinking twins (positive within-pair difference scores) exhibited significantly reduced thickness of the lateral prefrontal and frontal medial, but not dorsolateral prefrontal, cortices relative to their lesser-drinking cotwins (negative within-pair difference scores), consistent with an exposure effect (after accounting for all familial confounding; see Methods). For the between-pair scores, model fit lines illustrate the significant relationship between cortical thickness of all three areas and the mean level of alcohol use within a twin pair (twin-pair mean score), consistent with a premorbid familial risk effect. The visreg R package (83) was used to create the figure. Abbreviations: PFCl, lateral prefrontal cortex; PFCld; dorsolateral prefrontal cortex; FrMed, frontal medial cortex.
The CTC design controls all shared confounders but cannot control for individual-specific unshared factors that may confound within-pair effects. Supplemental analyses indicated that the observed within-pair effects are robust and cannot be attributed to the heavier-drinking twins differing from the lesser-drinking twin on potentially confounding unshared factors (e.g., internalizing, externalizing, trauma history) (Supplemental Section S6).
To complement the CTC, supplemental etiologically-informative bivariate biometric models were fit to test whether significant familial (between-pair) effects are better explained by genetic vs. shared environmental influence and to calculate the relative genetic/environmental contributions to alcohol-thickness phenotypic correlations (see Supplemental Section S7 for details). Results suggest that additive genetic influences, not shared environment, underlie the familial associations observed between drinking and thickness (Table S7). As shown in Figure 4, genetic influence was the largest contributor to phenotypic correlations across a majority of areas, consistent with the ubiquity of significant between-pair effects. Turning to areas with significant between-pair and within-pair effects, the control network lateral PFC phenotypic correlation was largely explained by nonshared environmental influence, whereas salience network frontal/parietal medial effects were primarily driven by genetic contribution in the context of significant nonshared environmental influence.
Figure 4.
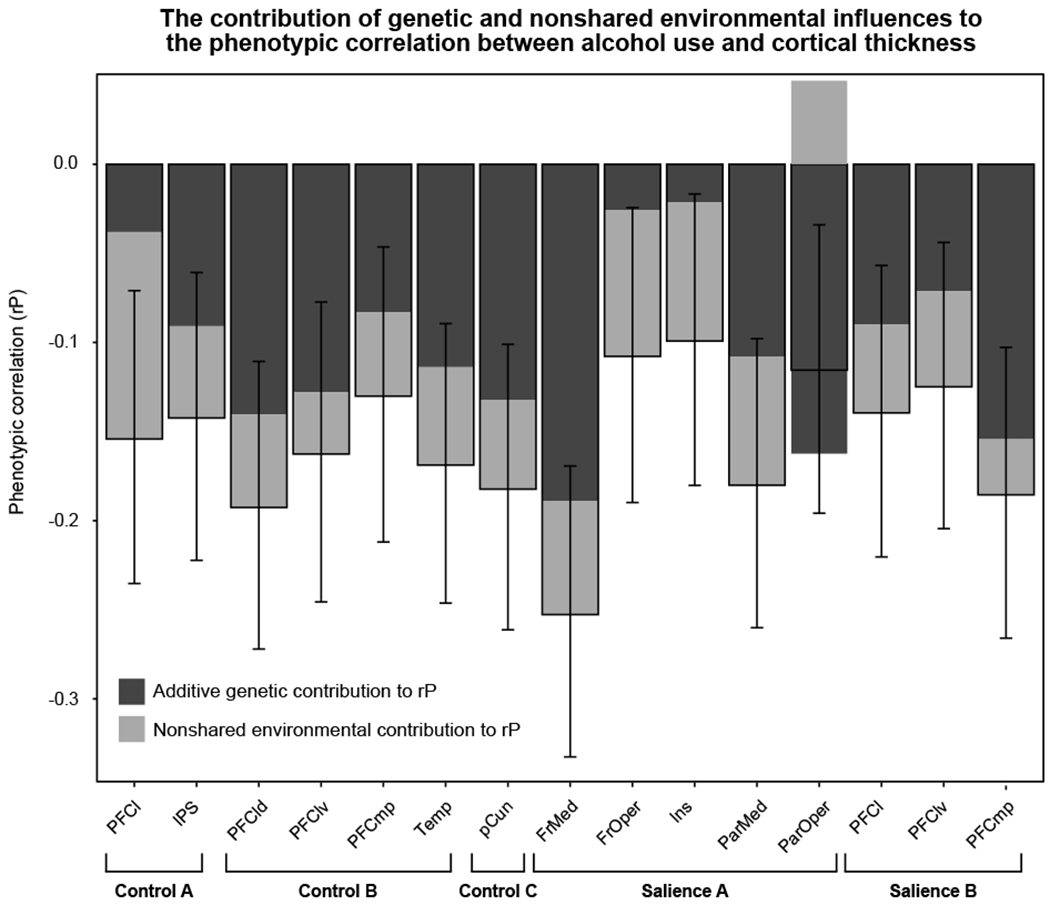
Bar graph of the biometric model-implied phenotypic correlations between drink index scores and cortical thickness. Bars are filled according to the relative contribution of genetic (dark grey) and nonshared environmental influences (light grey) to the phenotypic correlation. See Supplemental Section S7 and Table S7 for details. Whiskers depict likelihood-based 90% confidence intervals corresponding to one-tailed, α = 0.05 hypothesis tests given expectations set by the individual-level phenotypic linear mixed model results of negative phenotypic correlations between alcohol use and thickness. Note that for the parietal operculum, because the genetic and nonshared environmental influences were in opposing directions (as were the between-pair and within-pair cotwin control effects), their relative contributions to the phenotypic correlation are also in opposing directions but still sum to the phenotypic correlation. Abbreviations: PFC, prefrontal cortex; IPS, intraparietal sulcus; Temp, temporal; pCun; precuneus; FrMed, frontal medial; FrOper, frontal operculum; Ins, insula; ParMed, parietal medial; ParOper, parietal operculum; l, lateral; d, dorsal; v, ventral; mp, medial posterior.
Overall, the pattern of etiologically-informative results suggest that the observed alcohol effects likely reflect both the brain manifestation of the genetic predisposition to misuse alcohol, and specifically for control network lateral PFC, and salience network frontal medial cortex, parietal medial cortex, and frontal operculum areas, an alcohol exposure effect.
Discussion
The current report investigated the causal association between alcohol and cannabis use during emerging adulthood and cortical thickness of control and salience brain network areas related to inhibitory control in a large population-based sample of 24-year-old twins. While associations between grey matter reductions in various cortical regions implicated in cognitive control and salience processes (e.g., PFC, insula, cingulate cortex) and alcohol use (to a lesser extent, cannabis use) have been documented, the causal nature of these associations has remained largely unknown. Using a quasi-experimental CTC analysis that builds on other work using the CTC (67), the present study provides important novel evidence regarding the potential exposure-related consequences of alcohol use on the emerging adult brain in a field where true experimentation is often unfeasible.
Using dimensional measures to capture several complementary aspects of alcohol exposure across the emerging adulthood period, the current study supports previous findings of alcohol-related reductions in a collection of areas implicated in cognitive control and salience processing (11,23,26). We observed that greater scores on the drink index (indicative of increased alcohol misuse) were associated with decreased thickness in a collection of subregions spanning the frontal and association cortices, including lateral, dorso/ventrolateral, and medial posterior aspects of the bilateral PFC, right frontal operculum and insula, frontal medial cortex, and the intraparietal sulcus (among others). Collectively, these regions are thought to contribute to flexible behavioral adaptation and salience detection processes to facilitate successful inhibitory/cognitive control (16,17,68). Effects were predominantly right-lateralized, which is interesting given evidence that inhibitory control is strongly right-lateralized (18). Evidence suggests that specific areas (i.e., frontal medial; precuneus; frontal operculum) related to alcohol use in this report reflect structural and functional hubs that play a central role in information transfer and higher-order cognitive functioning (69), while others, particularly the right frontal operculum, have preeminent roles in successful inhibitory control (17). Individual differences in these areas may result in a decreased ability to detect the need for control (e.g., frontal medial cortex), which as a result, could lead to a decreased likelihood that prefrontal areas (e.g., frontal operculum, insula, lateral PFC) will be recruited to initiate the ‘braking’ system to pause or outright suppress an inappropriate response (18,68), which may contribute to the impulse control problems often observed in alcohol/substance misuse (7,20,32,33,70). Interestingly, frontal medial, parietal medial, insula, and frontal operculum thickness were nominally associated with disinhibitory externalizing traits, offering some preliminary support for this interpretation. However, small effect sizes precluded significance robust to FDR adjustment and further work in larger samples is needed to more strongly link alcohol-related cortical thickness variations and problematic behaviors. Contrary to some prior work suggesting more pronounced alcohol-related grey matter reductions in women (10,44,46), alcohol-thickness effects were statistically comparable between sexes in this relatively large sample.
No significant associations between cannabis use and thickness were observed. The lack of cannabis-specific effects is consistent with literature reviews (21,22), large sample studies (11,23,71), and evidence that observed cannabis effects may be accounted for by comorbid alcohol (72,73). As noted elsewhere (23,74), previous null/inconclusive findings may be partially due to limitations/variations in cannabis use assessments across studies (e.g., users vs. non-users; past 30-day use). However, even with a measure capturing both frequency and amount of cannabis use across emerging adulthood, we did not find any associations with control or salience network cortical thickness. We noted that there is evidence that cannabis use is associated with decreased volume of the hippocampus, ventral striatum, nucleus accumbens, and amygdala (71,74); perhaps cannabis is most strongly associated with structures containing high densities of endocannabinoid receptors, such as the aforementioned subcortical areas (22).
Evidence from the CTC design suggested a causal effect of alcohol exposure (within-pair effect) on the thickness of the lateral PFC, frontal medial, parietal medial, and frontal operculum areas. After controlling for all sources of potential familial confounding shared by members of a twin pair, thickness reductions in these areas were observed in twins who drank more relative to their lesser-drinking cotwin. This is consistent with the interpretation that alcohol exposure in emerging adulthood confers a detrimental effect to the structure of inhibitory/cognitive control and salience/ventral attention brain networks. Exposure effects appear to develop as early as age 24; emerging adulthood may represent a ‘window of vulnerability’ for the deleterious consequences of alcohol exposure on the still-developing young adult brain. If confirmed through further research, the finding that even normative levels of alcohol use during emerging adulthood produce exposure effects on key brain networks of response inhibition, cognitive control, and salience processing has potentially significant public health implications given the ubiquity and high rates of alcohol use during this developmental period (1). Given evidence that the frontal medial cortex plays a central role in inhibitory/cognitive control by detecting the need for behavioral adaptation (salience/ventral attention processes) then broadcasting that signal to recruit lateral prefrontal/frontal operculum regions to execute control/inhibition processes (16,17,75), the observed exposure-related changes in these three particular areas may reflect brain-based correlates/mediators of alcohol-related disinhibition (see Supplemental Section S5).
In addition, we also observed alcohol effects in the CTC analysis that are likely not due to drinking, but instead reflect the brain-based expression of the genetic vulnerability to misuse alcohol (between-pair effect). The same genetic risk factors that influence drinking also appear to influence the thickness of nearly all control/salience brain areas associated with drinking in this study, and individual differences in these areas likely reflect premorbid characteristics that would be observed prior to, and confer risk for, drinking escalation. As no evidence was found for a cannabis-related risk signal, effects likely reflect genetic risk for alcohol use specifically. Because alcohol misuse is comorbid with the externalizing spectrum (32,33), individuals with these brain-based predispositions may also at heightened risk for other negative outcomes including addiction, illicit drug use, and antisocial behavior. Recent evidence suggests only partial genetic overlap between alcohol use disorder (AUD) and alcohol use (76,77); it is unclear whether these familial effects would extend to AUD.
Similar findings have been reported in a high-risk offspring study (34) and an independent sample of adolescent twins (52), where the strongest evidence was for alcohol-related familial risk causing reduced grey matter volume/thickness. A recent study (67) reported evidence for a genetic relationship between drinking and dorsolateral PFC volume in a sample spanning adolescence to middle age, agreeing with our findings. However, the two aforementioned studies using CTC and discordant twin/sibling designs, respectively, did not find strong evidence for an exposure effect, whereas the current report did. This may be due to several factors, including our hypothesis-driven focus on the narrow developmental period of emerging adulthood and rich phenotyping of alcohol use during that timeframe that made this study well suited to identifying exposure effects. Seeing as the current report observed both familial and exposure effects on the brain, more research should focus on emerging adulthood as a vulnerable period for insults to the still-developing young adult brain.
A major strength of this study is its potential generalizability to the community at large through use of a population-based representative sample of 24-year-old emerging adults whose level and range of drinking (and cannabis use) is comparable to that observed in the general United States population (1). The twin sample strengthened our ability to draw stronger inferences regarding the causal nature of alcohol-related structural brain deviations than the typical cross-sectional or prospective study of unrelated individuals. However, there are limitations to the current work. Measurement error in the exposure can attenuate within-pair effects more strongly than individual-level effects (37), meaning the observed within-pair effects may be underestimated. While potentially relevant unshared confounders had minimal impact on the observed within-pair effects, the full extent of other unshared confounders is unknown. Results offer evidence consistent with an alcohol exposure effect but do not necessarily imply direct neurotoxicity. The role of additional substances cannot be excluded; however, there is a lack of evidence for non-alcohol-related substance-specific effects (e.g., unique to stimulants) on thickness (11). Future work will benefit from exploring other complementary measures (e.g., surface area, density) and using prospective genetically-informative samples to understand how alcohol may impact normative cortical developmental trajectories. This sample was designed to reflect the demographics of Minnesota in the target birth years and is predominantly White/Caucasian; replication is needed in diverse samples. Potential causal effects of prolonged heavy alcohol or cannabis exposure on other cortical areas cannot be ruled out.
Results suggest that premorbid cortical thickness reductions in control and salience brain regions related to inhibitory control may predispose an individual toward alcohol misuse during emerging adulthood, which then confers an additional alcohol exposure effect on certain cortical areas. Both the exposure and genetic risk effects reported in this report have important implications in terms of etiological/developmental models of alcohol misuse, public health policy, and targeted preventions. A potential consequence of these dual alcohol-related causal effects is that efforts should be focused on both a) reducing emerging adult drinking to prevent the development of exposure effects on the brain (e.g., public health messaging), and b) targeting individuals with the brain-based predisposition (endophenotypes; (78,79)) toward alcohol misuse to help identify and intervene with high-risk individuals before they begin drinking. Results may be used to inform neuromodulation/cognitive-behavioral training interventions to target these particular alcohol risk/consequence areas (80) and future work on the premorbid brain-based predictors of substance use development using longitudinal twin designs (e.g., Adolescent Brain Cognitive Development study; (81)).
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health grants R01 DA036216 (W.G.I.), R21 AA026919 (S.M.M.), K01 DA037280 (S.W.), and R21 AA026632 (S.W.). J.H. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. 00039202, University of Minnesota Eva O. Miller Fellowship, and the National Institute on Drug Abuse of the National Institutes of Health under Award Number T32DA037183. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This report is based on work completed by the first author (J.H.) in partial fulfillment of the requirements for the degree of Doctor of Philosophy, under the supervision of the senior author (W.G.I.).
The authors acknowledge the Minnesota Supercomputing Institute (MSI; http://www.msi.umn.edu) and the Center for Magnetic Resonance Research (supported by grants NIBIB P41 EB027061 and 1S10OD017974-01) at the University of Minnesota for providing resources that contributed to the research results reported within this paper. We thank the four anonymous reviewers for their helpful comments on an earlier version of this article.
Finally, we extend our gratitude to the twins for their participation in our studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Substance Abuse and Mental Health Services Administration (2019): Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- 2.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. (2008): Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28: 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci 6: 309–315. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Patricia Chou S, Saha TD, Pickering RP, Kerridge BT, June Ruan W, et al. (2017): Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013. JAMA Psychiatry, vol. 74. p 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrieze SI, Hicks BM, Iacono WG, McGue M (2012): Decline in Genetic Influence on the Co-Occurrence of Alcohol, Marijuana, and Nicotine Dependence Symptoms From Age 14 to 29. Am J Psychiatry 169: 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everitt BJ, Robbins TW (2016): Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol 67: 23–50. [DOI] [PubMed] [Google Scholar]
- 7.Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL (2010): Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev 35: 248–275. [DOI] [PubMed] [Google Scholar]
- 8.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ (2018): Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron 98: 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koob GF, Volkow ND (2010): Neurocircuitry of Addiction. Neuropsychopharmacology 35: 1051–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch KA, Carson A, Lawrie SM (2013): Brain Structure in Adolescents and Young Adults with Alcohol Problems: Systematic Review of Imaging Studies. Alcohol Alcohol 48: 433–444. [DOI] [PubMed] [Google Scholar]
- 11.Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, et al. (2018): Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry 176: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bühler M, Mann K (2011): Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res 35: 1771–1793. [DOI] [PubMed] [Google Scholar]
- 13.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS (2004): Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 56: 129–140. [DOI] [PubMed] [Google Scholar]
- 15.Botvinick M, Braver TS, Barch DM, Carter CS, Cohen JD (2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- 16.Botvinick M, Braver T (2015): Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol 66: 83–113. [DOI] [PubMed] [Google Scholar]
- 17.Ullsperger M, Danielmeier C, Jocham G (2014): Neurophysiology of Performance Monitoring and Adaptive Behavior. Physiol Rev 94: 35–79. [DOI] [PubMed] [Google Scholar]
- 18.Aron AR, Robbins TW, Poldrack RA (2014): Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18: 177–185. [DOI] [PubMed] [Google Scholar]
- 19.Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS (2013): Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int J Psychophysiol 87: 217–233. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein RZ, Volkow ND (2011): Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12: 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, et al. (2010): Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med 40: 383–398. [DOI] [PubMed] [Google Scholar]
- 22.Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, et al. (2019): The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol Ther 195: 132–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thayer RE, YorkWilliams S, Karoly HC, Sabbineni A, Ewing SF, Bryan AD, Hutchison KE (2017): Structural neuroimaging correlates of alcohol and cannabis use in adolescents and adults. Addiction 112: 2144–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill SY, Sharma V, Jones BL (2016): Lifetime use of cannabis from longitudinal assessments, cannabinoid receptor (CNR1) variation, and reduced volume of the right anterior cingulate. Psychiatry Res Neuroimaging 255: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobus J, Tapert SF (2013): Neurotoxic Effects of Alcohol in Adolescence. Annual Review of Clinical Psychology 9: 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thayer RE, Hagerty SL, Sabbineni A, Claus ED, Hutchison KE, Weiland BJ (2016): Negative and interactive effects of sex, aging, and alcohol abuse on gray matter morphometry. Hum Brain Mapp 37: 2276–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA (2014): Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology 39: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shnitko TA, Liu Z, Wang X, Grant KA, Kroenke CD (2019): Chronic alcohol drinking slows brain development in adolescent and young adult nonhuman primates. eNeuro 6: ENEURO.0044-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luciana M, Collins PF, Muetzel RL, Lim KO (2013): Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse 39: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, et al. (2018): Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am J Psychiatry 175: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A (2015): Brain development in heavy-drinking adolescents. Am J Psychiatry 172: 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacono WG, Malone SM, McGue M (2008): Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol 4: 325–348. [DOI] [PubMed] [Google Scholar]
- 33.Zucker RA, Heitzeg MM, Nigg JT (2011): Parsing the Undercontrol-Disinhibition Pathway to Substance Use Disorders: A Multilevel Developmental Problem. Child Dev Perspect 5: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson KE, Vaidya JG, Kramer JR, Kuperman S, Langbehn DR, O’Leary DS (2018): Cortical thickness in adolescents with a family history of alcohol use disorder. Alcohol Clin Exp Res 42: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thapar A, Rutter M (2019): Do natural experiments have an important future in the study of mental disorders? Psychol Med 49: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin DB (2007): The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med 26: 20–36. [DOI] [PubMed] [Google Scholar]
- 37.McGue M, Osler M, Christensen K (2010): Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci 5: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T (2005): Regression models for twin studies: a critical review. Int J Epidemiol 34: 1089–1099. [DOI] [PubMed] [Google Scholar]
- 39.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 40.Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. (2018): Local Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex 28: 3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy BJ, Wagner AD (2011): Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 1224: 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gratton G, Cooper P, Fabiani M, Carter CS, Karayanidis F (2017): Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology. 10.1111/psyp.13016 [DOI] [PubMed] [Google Scholar]
- 43.Becker JB, Koob GF (2016): Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68: 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erol A, Karpyak VM (2015): Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend 156: 1–13. [DOI] [PubMed] [Google Scholar]
- 45.Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR, Wiren KM (2016): Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathol 26: 433–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo S, Beck A, Matthis C, Genauck A, Banaschewski T, Bokde ALW, et al. (2019): Risk profiles for heavy drinking in adolescence: differential effects of gender. Addict Biol 24: 787–801. [DOI] [PubMed] [Google Scholar]
- 47.Keyes MA, Malone SM, Elkins IJ, Legrand LN, McGue M, Iacono WG (2009): The Enrichment Study of the Minnesota Twin Family Study: Increasing the Yield of Twin Families at High Risk for Externalizing Psychopathology. Twin Res Hum Genet 12: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robins LN, Babor TF, Cottler LB (1987): Composite international diagnostic interview: expanded substance abuse module. St Louis: Authors. [Google Scholar]
- 49.Harper J, Malone SM, Iacono WG (2018): Impact of alcohol use on EEG dynamics of response inhibition: a cotwin control analysis. Addict Biol 23: 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harper J, Malone SM, Iacono WG (2017): Testing the effects of adolescent alcohol use on adult conflict-related theta dynamics. Clin Neurophysiol 128: 2358–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harper J, Malone SM, Iacono WG (2019): Parietal P3 and midfrontal theta prospectively predict the development of adolescent alcohol use. Psychol Med 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson S, Malone SM, Thomas KM, Iacono WG (2015): Adolescent drinking and brain morphometry: A co-twin control analysis. Dev Cogn Neurosci 16: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson S, Malone SM, Hunt RH, Thomas KM, Iacono WG (2017): Problematic alcohol use and hippocampal volume in a female sample: disentangling cause from consequence using a co-twin control study design. Psychol Med 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malone SM, Luciana M, Wilson S, Sparks JC, Hunt RH, Thomas KM, Iacono WG (2014): Adolescent Drinking and Motivated Decision-Making: A Cotwin-Control Investigation with Monozygotic Twins. Behav Genet 44: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGue M, Malone S, Keyes M, Iacono WG (2014): Parent-Offspring Similarity for Drinking: A Longitudinal Adoption Study. Behav Genet 44: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 57.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14: 11–22. [DOI] [PubMed] [Google Scholar]
- 58.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004): The role of the medial frontal cortex in cognitive control. Science 306: 443–447. [DOI] [PubMed] [Google Scholar]
- 59.Bates D, Machler M, Bolker BM, Walker SC (2015): Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67: 1–48. [Google Scholar]
- 60.R Core Team (2019): R: A Language and Environment for Statistical Computing, version 3.6.1. Vienna, Austria: R Foundation for Statistical Computing. Retrieved July 5, 2019, from https://www.R-project.org/ [Google Scholar]
- 61.Kuznetsova A, Brockhoff PB, Christensen RHB (2017): lmerTest package: Tests in linear mixed effects models. J Stat Softw 82: 1–26. [Google Scholar]
- 62.Storey JD, Tibshirani R (2003): Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100: 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storey JD (2003): The positive false discovery rate: a Bayesian interpretation and the q-value. Ann Stat. Retrieved from https://projecteuclid.org/euclid.aos/1074290335 [Google Scholar]
- 64.Storey JD, Bass AJ, Dabney A, Robinson D (2019): qvalue: Q-value estimation for false discovery rate control. R package version 2.18.0. Retrieved from http://github.com/jdstorey/qvalue [Google Scholar]
- 65.Rutter M (2007): Proceeding From Observed Correlation to Causal Inference The Use of Natural Experiments. Perspect Psychol Sci 2: 377–395. [DOI] [PubMed] [Google Scholar]
- 66.Begg MD, Parides MK (2003): Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med 22: 2591–2602. [DOI] [PubMed] [Google Scholar]
- 67.Baranger DAA, Demers CH, Elsayed NM, Knodt AR, Radtke SR, Desmarais A, et al. (2020): Convergent evidence for predispositional effects of brain gray matter volume on alcohol consumption. Biol Psychiatry 87: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavanagh JF, Frank MJ (2014): Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 18: 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Heuvel MP, Sporns O (2013): Network hubs in the human brain. Trends in Cognitive Sciences, vol. 17. pp 683–696. [DOI] [PubMed] [Google Scholar]
- 70.Volkow ND, Wang G-J, Tomasi D, Baler RD (2013): Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol 23: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orr JM, Paschall CJ, Banich MT (2016): Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin 12: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE (2015): Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci 35: 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malone SM, Wilson S, Bair JL, McGue M, Iacono WG (2020): A Cotwin-Control Analysis of Adolescent and Young Adult Drinking Effects on Learning and Memory. Addiction. Retrieved from https://onlinelibrary.wiley.com/doi/abs/10.1111/add.15334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pagliaccio D, Barch DM, Bogdan R, Wood PK, Lynskey MT, Heath AC, Agrawal A (2015): Shared Predisposition in the Association Between Cannabis Use and Subcortical Brain Structure. JAMA Psychiatry 72: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen MX (2014): A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci 37: 480–490. [DOI] [PubMed] [Google Scholar]
- 76.Kranzler HR, Zhou H, Kember RL, Vickers Smith R, Justice AC, Damrauer S, et al. (2019): Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun 10: 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez-Roige S, Palmer AA, Clarke T-K (2020): Recent efforts to dissect the genetic basis of alcohol use and abuse. Biol Psychiatry 87: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iacono WG, Malone SM, Vrieze SI (2017): Endophenotype best practices. Int J Psychophysiol 111: 115–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gottesman II, Gould TD (2003): The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry 160: 636–645. [DOI] [PubMed] [Google Scholar]
- 80.Naish KR, Vedelago L, MacKillop J, Amlung M (2018): Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: A systematic review. Drug Alcohol Depend 192: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iacono WG, Heath AC, Hewitt J, Neale M, Banich MT, Luciana M, et al. (2017): The Utility of Twins in Developmental Clinical Neuroscience Research: How Twins Strengthen the ABCD Research Design. Dev Cogn Neurosci. 10.1016/j.dcn.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mowinckel AM, Vidal-Piñeiro D (2019): Visualisation of Brain Statistics with R-packages ggseg and ggseg3d. ArXiv. Retrieved from http://arxiv.org/abs/1912.08200 [Google Scholar]
- 83.Breheny P, Burchett W (2017): Visualization of Regression Models Using visreg. The R Journal 9: 56–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


