Abstract
Objective:
To determine drivers of the racial disparity in stage at diagnosis and overall survival (OS) between black and white patients with HPV-negative head and neck squamous cell carcinoma (HNSCC).
Methods:
Data were examined from of a population based HNSCC study in North Carolina. Multivariable logistic regression and Cox proportional hazards models were used to assess racial disparities in stage at diagnosis and OS with sequential adjustment sets.
Results:
A total of 340 black patients and 864 white patients diagnosed with HPV-negative HNSCC were included. In the unadjusted model, black patients had increased odds of advanced T stage at diagnosis (OR 2.0; 95% CI [1.5–2.5]) and worse OS (HR 1.3, 95% CI 1.1 to 1.6) compared to white patients. After adjusting for age, sex, tumor site, tobacco use, and alcohol use, the racial disparity persisted for advanced T-stage at diagnosis (OR 1.7; 95% CI [1.3–2.3]) and showed a non-significant trend for worse OS (HR 1.1, 95% CI 0.9 to 1.3). After adding SES to the adjustment set, the association between race and stage at diagnosis was lost (OR: 1.0; 95% CI [0.8–1.5]). Further, black patients had slightly favorable OS compared to white patients (HR 0.8, 95% CI [0.6 to 1.0]; p=0.024).
Conclusions:
SES has an important contribution to the racial disparity in stage at diagnosis and OS for HPV-negative HNSCC. Low SES can serve as a target for interventions aimed at mitigating the racial disparities in head and neck cancer.
Keywords: Head and neck neoplasms, race, disparities, access to care, socioeconomic status, survival
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) accounted for approximately 65,410 new cases and 14,620 deaths in 2019 in the United States.1,2 HNSCC predominantly involves cancers of the oral cavity, oropharynx, and larynx. Prognosis for head and neck cancer is relatively poor with 5-year overall survival estimates ranging from 50% to 66% based on large population studies.3,4 Stage at diagnosis is one of the strongest predictors of overall survival, with greater than three times the 5-year risk of mortality in patients diagnosed with advanced stage HNSCC.3,5 Identifying disparities in stage at diagnosis and overall survival (OS) will help guide interventions to reduce the burden of disease from HNSCC in the United States.
Over the past two decades, it has become well established that black race is a predictor for advanced stage at diagnosis and poor OS in patients with HNSCC in the United States.6–10 Low socioeconomic status (SES) has also been shown to predict more advanced stage at diagnosis and worse OS in HNSCC.11,12,13,14,15,16 Large population studies have demonstrated that black patients with HNSCC in the United States are more likely to be uninsured, have lower median household income, and receive less education than white patients.6,7,17,18 Based on these findings, SES is a potential confounder or mediator for the association between race and poor outcomes in HNSCC.
Human papillomavirus (HPV) is a validated risk factor for HNSCC and leads to tumors in the oropharyngeal subsite.19 HPV tumor status is also an established confounder of the association between race and HNSCC outcomes.20–25 Racial disparities in HNSCC are largely driven by the oropharyngeal subsite.26–28 In the United States, white patients with oropharyngeal cancer are more likely than black patients to have HPV-positive tumors (67.6% vs. 42.3%, respectively; p<0.001).29 HPV-positivity is a favorable prognostic factor for patients with HNSCC,30,31 which may partially explain the racial differences in overall survival. Given the extensive differences in tumor biology, natural history, and treatment response between HPV-positive and HPV-negative tumors, they should be considered distinct clinicopathological entities.19 For these reasons, studies assessing racial disparities in HNSCC should either stratify by HPV-status or adjust for it in the analysis.
Despite evidence in current literature to suggest that SES and HPV tumor status may influence the relationship between race and HNSCC outcomes, no studies have examined racial disparities while accounting for both of these variables in a large HNSCC population-based study.
Our objective was to evaluate racial disparities in stage at diagnosis and OS in a large HPV-negative HNSCC cohort using data from the Carolina Head and Neck Cancer Epidemiology Study (CHANCE). We sought to determine the relative contributions of demographic, clinical, behavioral, and SES variables to racial disparities between black patients and white patients. Patients with HPV-positive oropharyngeal tumors were excluded given evidence in literature that HPV-positive oropharyngeal cancer confounds the association under investigation and acts as a distinct clinicopathologic entity.
Materials and Methods
Study population
The study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Data for this analysis were obtained from the Carolina Head and Neck Cancer Epidemiology Study (CHANCE); a population based study in North Carolina.32,33 Cases were eligible to participate in CHANCE if diagnosed with a first primary squamous cell carcinoma of the oral cavity, pharynx, or larynx between January 1, 2002, and February 28, 2006; were ages 20 to 80 years at diagnosis; and resided in a 46-county region in central North Carolina. Case ascertainment relied on rapid identification of newly diagnosed cancer cases through the North Carolina Central Cancer Registry (NCCCR). The cancer registrars of 54 hospitals in the study area were contacted monthly to identify potentially eligible cases. Potentially eligible study subjects were mailed a brochure describing the purpose of the study, and upon consent, a study nurse conducted an at-home in-person interview. There were 1,381 cases in CHANCE. Patients were excluded from this study if they had p16+ oropharyngeal cancer (n=157) and if race was classified as other or unknown (n=28).
Exposure Assessment
Trained nurse-interviewers used a structured questionnaire during an in-home visit to obtain information about the demographics, health behaviors, and socioeconomic status from the cases. Cases were interviewed soon after cancer diagnosis (the average time between diagnosis and interview was 5.3 months).33 Demographic and socioeconomic data obtained during the interview was verified by hospital records, which were sent to the coordinating center at the University of North Carolina at Chapel Hill within 4–8 weeks of diagnosis.
SES variables were defined as individual-level income, education, and insurance status. Income was ascertained via the following question: “What was your gross household income category in the calendar year before [reference date of diagnosis], including salaries, wages, social security, welfare, and other income?” Options were categorized in $10,000 increments from zero to ≥$80,000 (i.e. $10,001 to $20,000, $20,001 to $30,000, etc.) and included don’t know or refuse to answer. Level of educational attainment was ascertained via the following question: “How many years of education have you completed? Please include any education you received at a vocational or technical college.” Options included <8 years, 8–11 years, 12 years or completed high school, vocational or technical training, some college, graduated college, post-graduate, don’t know, and refuse to answer. Health insurance status was ascertained via the following question: “At the time of diagnosis of your recent head and neck problem, which of the following types of health insurance did you have?” Options included Medicare, TRICARE/military health, VA insurance, Medicaid, other public assistance/welfare-type program, Indian Health Service, private health insurance (purchased on your own or through your employer), insurance that pays only for certain illnesses such as stroke or cancer, “extra cash” policies that pay cash only if you go into the hospital, any other insurance that covered a portion of medical bills not specified in this survey (e.g. Medi-Gap or other supplemental insurance programs), none, don’t know, and refuse to answer. Patients with health insurance classified as “federal” consisted of patients with TRICARE/military health or VA insurance. Patients with health insurance classified as “other” consisted of patients that selected one of the following options: other public assistance/welfare-type program, insurance that pays only for certain illnesses such as stroke or cancer, “extra cash” policies that pay cash only if you go into the hospital, any other insurance that covered a portion of medical bills not specified in this survey, and refuse to answer.
Smoking was dichotomized at 10 pack-years and alcohol use as any versus none based on the survey design. Race was self-identified from an interview question that gave the following options: white, black, American Indian, Alaskan Native, Asian/Pacific Islander, other, and don’t know. Given the low number of other and unknown race (n=28), we limited our analysis to patients who self-identified as white or black. Clinical information such as tumor site was abstracted from participants’ medical records and reviewed independently by a pathologist and a head neck cancer surgeon. Tumors were classified by site according to International Classification of Diseases for Oncology, third edition (ICD-O-3).34 In order to assess for HPV-status, p16 immunohistochemistry was retrospectively performed using a previously described protocol.35,36
Outcomes Assessment
The primary outcomes were early (T1-T2) and advanced (T3-T4) T stage at diagnosis. Stage at diagnosis was abstracted from medical records specifying the initial treatment plan. All staging used 7th edition AJCC guidelines as these were in use at the time of data collection. A secondary outcome was overall survival (OS). CHANCE data were linked to the National Death Index (NDI) based on name, social security number, date of birth, sex, race, and state of residence to identify deaths through December 31, 2013. More than 75% of the CHANCE cases were perfect or near-perfect NDI matches on social security number, date of birth, and sex. The remaining near matches were confirmed by examining the United States Social Security Death Index and obituaries on newspaper websites. A 5-year survival endpoint was chosen because it is thought that after this timeframe the initial tumor likely plays a diminished role in OS.33
Statistical Analysis
Descriptive statistics were calculated for cases and stratified by race; bivariate testing methods included two-sided t and chi-squared tests. All variables were examined for missing data and excluded from the analysis if there were >10% missing observations. Of note, rural/urban household location was only available for a subset of the sample (n=826) but significance of the association between race and T stage at diagnosis or overall survival was unchanged when excluding this variable in a sensitivity analysis. A conventional P<0.05 statistical significance criterion was used for all testing. Stata 16.0 was used for all statistical analyses (StataCorp LP, College Station, TX).
Univariate and multivariable logistic regression models were used to estimate ORs and 95% CIs for advanced T stage at diagnosis (the primary outcome) in relation to demographic, clinical, behavioral, and SES variables. Cox proportional hazards models were used to estimate the HRs and 95% CIs for OS in relation to demographic, clinical, behavioral, and SES variables. We developed an a priori set of models to isolate the relative effects of demographic, clinical, behavioral, and SES variables on the observed racial disparity. Model 1 adjusted for demographic (age and sex) and clinical (tumor site, stage) variables. Model 2 added health-related behaviors (alcohol and tobacco use) to the previous adjustment set. Model 3 added individual-level SES variables (education, insurance status, household income level) to the previous adjustment set. Model 4 added rural/urban household location and treatment to the previous adjustment set. Treatment was omitted from the analysis for T stage at diagnosis given that conceptually we were most interested in predictors of advanced T stage at diagnosis, and treatment decisions were made after the initial diagnosis and workup. Of note, inclusion of treatment in Model 4 for T stage at diagnosis did not change the significance of the racial disparity in a sensitivity analysis. We found no evidence of multicollinearity on variance inflation factor testing. The proportional hazards assumption was met for all variables.
Results
Baseline Characteristics
A total of 340 black patients and 864 white patients diagnosed with HPV-negative HNSCC were included (n=1204). Compared to white patients, black patients were more likely to be younger, have equal to or less than a high school education, lack private insurance, have an income < $20,000, not drink alcohol, have a history of tobacco use, be diagnosed at a more advanced T stage, and have nodal disease or metastases at diagnosis (Table 1).
Table 1:
Baseline characteristics of HPV-negative HNSCC patients by race
| White patients (n=864) | Black patients (n=340) | Total (n=1,204) | P-Value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age category | |||||||
| < 50 (n=237) | 146 | 17% | 91 | 27% | 237 | 20% | < 0.001* |
| 50–65 (n=558) | 382 | 44% | 176 | 52% | 558 | 46% | |
| 65+ (n=409) | 336 | 39% | 73 | 21% | 409 | 34% | |
| Sex | |||||||
| Male (n=909) | 639 | 74% | 270 | 79% | 909 | 75% | 0.048 |
| Female (n=295) | 225 | 26% | 70 | 21% | 295 | 25% | |
| Education | |||||||
| Less Than High School (n=442) | 253 | 29% | 189 | 56% | 442 | 37% | < 0.001** |
| High School Grad (n=345) | 232 | 27% | 113 | 33% | 345 | 29% | |
| Greater than High School (n=417) | 379 | 44% | 38 | 11% | 417 | 35% | |
| Insurance Type | |||||||
| Private (n=383) | 317 | 38% | 66 | 20% | 383 | 33% | < 0.001*** |
| Medicaid/Medicare (n=442) | 284 | 34% | 158 | 47% | 442 | 38% | |
| None (n=149) | 74 | 9% | 75 | 22% | 149 | 13% | |
| Federal (n=74) | 51 | 6% | 23 | 7% | 74 | 6% | |
| Other (n=130) | 116 | 14% | 14 | 4% | 130 | 11% | |
| Income | |||||||
| Income > $50,000 (n=287) | 267 | 32% | 20 | 6% | 287 | 25% | < 0.001**** |
| Income $20,000 – $50,000 (n=412) | 324 | 39% | 88 | 28% | 412 | 36% | |
| Income < $20,000 (n=448) | 237 | 29% | 211 | 66% | 448 | 39% | |
| Area of Residence | |||||||
| Metropolitan Area (> 50,000) (n=642) | 456 | 78% | 186 | 77% | 642 | 78% | 0.578 |
| Micropolitan Area (10,000 – 49,999) (n=130) | 94 | 16% | 36 | 15% | 130 | 16% | |
| Rural or Small Town (<10,000) (n=54) | 35 | 6% | 19 | 8% | 54 | 7% | |
| Alcohol Use | |||||||
| Any (n=162) | 148 | 18% | 14 | 4% | 162 | 14% | < 0.001 |
| None (n=990) | 683 | 82% | 307 | 96% | 990 | 86% | |
| Smoking History | |||||||
| < 10 Years (n=219) | 170 | 20% | 49 | 15% | 219 | 18% | 0.044 |
| > 10 Years (n=972) | 689 | 80% | 283 | 85% | 972 | 82% | |
| Tumor Site | |||||||
| Larynx/Hypopharynx (n=540) | 384 | 45% | 156 | 46% | 540 | 45% | 0.011 |
| Oral cavity (n=405) | 309 | 36% | 96 | 28% | 405 | 34% | |
| Oropharynx (n=256) | 168 | 20% | 88 | 26% | 256 | 21% | |
| T Stage | |||||||
| T1 (n=387) | 311 | 36% | 76 | 22% | 387 | 32% | 0.001***** |
| T2 (n=374) | 274 | 32% | 100 | 29% | 374 | 31% | |
| T3 (n=223) | 145 | 17% | 78 | 23% | 223 | 19% | |
| T4 (n=220) | 134 | 16% | 86 | 25% | 220 | 18% | |
| N Stage | |||||||
| N0 (n=702) | 524 | 61% | 178 | 52% | 702 | 58% | 0.009****** |
| N1 (n=140) | 101 | 12% | 39 | 11% | 140 | 12% | |
| N2 (n=317) | 211 | 24% | 106 | 31% | 317 | 26% | |
| N3 (n=45) | 28 | 3% | 17 | 5% | 45 | 4% | |
| M Stage | |||||||
| M0 (n=1,194) | 860 | 100% | 334 | 98% | 1194 | 99% | 0.025 |
| M1 (n=10) | 4 | 0% | 6 | 2% | 10 | 1% | |
| Treatment Category | |||||||
| Radiation Only (n=243) | 180 | 21% | 63 | 19% | 243 | 21% | NA |
| Surgery Only (n=301) | 241 | 28% | 60 | 18% | 301 | 26% | |
| Radiation + Chemotherapy (n=283) | 187 | 22% | 96 | 29% | 283 | 24% | |
| Surgery + Chemotherapy (n=2) | 2 | <1% | 0 | 0% | 2 | 0% | |
| Surgery + Radiation (n=228) | 149 | 18% | 79 | 24% | 228 | 19% | |
| Surgery + Chemoradiation (n=120) | 90 | 11% | 30 | 9% | 120 | 10% | |
P-value for 65+ vs. <65
P-value for >high school education vs. high school or less
P-value for private insurance vs. any other type
P-value for income <20,000 vs. >20,0000
P-value for early stage (T1,T2) vs. advanced stage (T3,T4)
P-value for presence of nodal metastases vs. none
Advanced T Stage at Diagnosis
In the unadjusted model for T stage at diagnosis, black patients had a two-fold increased odds of advanced T stage at diagnosis compared to white patients (OR 2.0; 95% CI [1.5–2.5]) (Table 2). The racial disparity in T stage at diagnosis persisted after adjustment for age, sex, and tumor site (Model 1; OR 1.8; 95% CI [1.4–2.3]) (Table 2). Addition of alcohol and tobacco use to the previous adjustment set did not significantly change the racial disparity (Model 2; OR 1.7; 95% CI [1.3–2.3]) (Table 2). After adding education, insurance status, and household income to the adjustment set, the association between race and T stage at diagnosis was lost (Model 3; OR 1.0; 95% CI [0.8–1.5]) (Table 2). After addition of rural/urban household location to the previous adjustment set, the relationship remained not statistically significant (Model 4; OR 1.0; 95% CI [0.7–1.4]) (Table 2).
Table 2:
Race and T-Stage at Diagnosis: Results for sequential adjustment sets
| Unadjusted Model | Model 1 (Demographics and Tumor Site) | Model 2 (Addition of Alcohol and Tobacco Use) | Model 3 (Addition of SES Variables) | Model 4 (Addition of Rural/Urban Household Location) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | OR | 95% CI | P-Value | OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| Black race (vs. white race) | 2.0 | 1.5 – 2.5 | < 0.001 | 1.8 | 1.4 – 2.3 | < 0.001 | 1.7 | 1.3 – 2.3 | < 0.001 | 1.0 | 0.8 – 1.5 | 0.790 | 1.0 | 0.7 – 1.4 | 0.864 |
| Age Category (Relative to < 50) | |||||||||||||||
| 50–65 | 0.8 | 0.6 – 1.1 | 0.138 | 0.7 | 0.5 – 1.0 | 0.071 | 0.8 | 0.6 – 1.1 | 0.181 | 0.8 | 0.5 – 1.2 | 0.189 | |||
| 65+ | 0.6 | 0.4 – 0.8 | 0.002 | 0.6 | 0.4 – 0.8 | 0.001 | 0.5 | 0.3 – 0.9 | 0.010 | 0.4 | 0.2 – 0.8 | 0.005 | |||
|
Female sex (relative to male) |
0.8 | 0.6 – 1.1 | 0.105 | 0.8 | 0.6 – 1.1 | 0.236 | 0.8 | 0.6 – 1.2 | 0.326 | 0.8 | 0.5 – 1.2 | 0.358 | |||
| Site (Relative to larynx/hypopharynx) | |||||||||||||||
| Oral cavity | 0.8 | 0.6 – 1.0 | 0.062 | 0.8 | 0.6 – 1.1 | 0.162 | 0.8 | 0.6 – 1.1 | 0.270 | 1.1 | 0.8 – 1.7 | 0.488 | |||
| Oropharynx | 0.9 | 0.7 – 1.2 | 0.510 | 0.9 | 0.7 – 1.3 | 0.613 | 1.0 | 0.7 – 1.4 | 0.942 | 1.1 | 0.7 – 1.6 | 0.765 | |||
| Smoking (> 10 pack-years) | 1.6 | 1.1 – 2.3 | 0.008 | 1.4 | 1.0 – 2.1 | 0.070 | 1.3 | 0.8 – 2.0 | 0.336 | ||||||
| Alcohol use (any vs. none) | 1.0 | 0.7 – 1.6 | 0.820 | 1.2 | 0.7 – 1.8 | 0.516 | 1.3 | 0.8 – 2.2 | 0.339 | ||||||
| Education (relative to less than high school) | |||||||||||||||
| High school graduate | 1.1 | 0.8 – 1.6 | 0.431 | 1.1 | 0.7– 1.6 | 0.689 | |||||||||
| Additional education past high school | 0.8 | 0.5 – 1.1 | 0.133 | 0.6 | 0.4 – 0.9 | 0.012 | |||||||||
| Insurance (relative to private insurance) | |||||||||||||||
| Medicaid/Medicare | 1.3 | 0.8 – 2.0 | 0.328 | 1.4 | 0.8 – 2.4 | 0209 | |||||||||
| None | 2.5 | 1.6 – 4.0 | <0.001 | 3.1 | 1.8 – 5.4 | <0.001 | |||||||||
| Federal | 1.8 | 1.0–3.1 | 0.047 | 2.3 | 1.2–4.4 | 0.011 | |||||||||
| Other | 1.6 | 0.9 – 2.8 | 0.108 | 1.2 | 0.6– 2.4 | 0.612 | |||||||||
| Income (relative to > 50 K) | |||||||||||||||
| Income $20,000 – $50,000 | 1.1 | 0.7 – 1.5 | 0.799 | 1.0 | 0.6 – 1.5 | 0.859 | |||||||||
| Income < $20,000 | 1.7 | 1.1 – 2.7 | 0.013 | 1.3 | 0.8 – 2.1 | 0.366 | |||||||||
| Rural Location (relative to metropolitan area) | |||||||||||||||
| Micropolitan Area | 1.6 | 1.0 – 2.4 | 0.046 | ||||||||||||
| Rural Area | 1.1 | 0.6 – 2.2 | 0.688 | ||||||||||||
Treatment Strategy
Given that black patients were more likely to present with advanced T stage at diagnosis, differences in treatment strategy by race were examined before and after adjusting for tumor stage. In the unadjusted analysis, black race associated with significantly increased odds of receiving multimodal therapy compared to single-modality treatment (OR 1.64, 95% CI 1.26–2.13; p<0.001). Specifically, 205 (62.5%) of black patients received multimodal therapy compared to 428 (50.4%) of white patients. The distribution for exact type of treatment received by race is detailed in Table 1. When adjusting for T stage, nodal disease, and distant metastases at diagnosis, the association between race and receipt of multimodal therapy was lost (OR 1.26, 95% CI 0.91–1.74; p=0.171). This non-significant effect persisted when adding tumor site to the adjustment set (OR 1.19, 95% CI 0.85–1.65; p=0.304).
In a subset analysis among patients receiving surgery (n=651 total, 169 black patients and 482 white patients), black race was associated with significantly increased odds of receiving adjuvant chemotherapy or radiation (OR 1.82, 95% CI 1.26–2.61; p=0.001) in the unadjusted analysis. When adjusting for T stage, nodal disease, and distant metastases at diagnosis, the association between race and receipt of adjuvant therapy in patients receiving surgery was lost (OR 1.51, 95% CI 0.98–2.32; p=0.060). This non-significant effect persisted when adding tumor site to the adjustment set (OR 1.22, 95% CI 0.77–1.92; p=0.391).
Overall Survival
In the unadjusted model for OS, black patients had significantly worse OS compared to white patients (HR 1.3; 95% CI [1.1–1.6]) (Table 3). The racial disparity for OS showed a non-significant trend after adjustment for age, sex, tumor site, and stage (Model 1; HR 1.2; 95% CI [1.0–1.4]; p=0.103) (Table 3). After adding tobacco use and alcohol use to the adjustment set, there was no statistically significant difference between white and black patients (Model 2; HR 1.1; 95% CI [0.9–1.3]) (Table 3). After adding education, insurance status, and household income to the adjustment set, the racial disparity OS was modified, with black patients trending towards superior OS compared to white patients (Model 3; HR 0.8; 95% CI [0.6–1.0], p=0.020) (Table 3). The association between black race and favorable OS persisted after adding rural/urban household location and treatment to the adjustment set (Model 4; HR 0.7; 95% CI [0.5–0.9], p=0.015) (Table 3 and Figure 1).
Table 3:
Race and Overall Survival: Results for sequential adjustment sets
| Unadjusted Model | Model 1 (Demographics, Tumor Site, and Stage) | Model 2 (Addition of Alcohol and Tobacco Use) | Model 3 (Addition of SES Variables) | Model 4 (Addition of Rural/Urban Household Location and Treatment) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-Value | HR | 95% CI | P-Value | HR | 95% CI | P-Value | HR | 95% CI | P-Value | HR | 95% CI | P-Value | |
| Black race (vs. white race) | 1.3 | 1.1 – 1.6 | 0.005 | 1.2 | 1.0 – 1.4 | 0.103 | 1.1 | 0.9 – 1.3 | 0.422 | 0.8 | 0.6 – 1.0 | 0.020 | 0.7 | 0.5 – 0.9 | 0.015 |
| Age Category (Relative to < 50) | |||||||||||||||
| 50–65 | 1.3 | 1.0 – 1.7 | 0.031 | 1.2 | 0.9 – 1.6 | 0.165 | 1.2 | 0.9 – 1.5 | 0.229 | 1.2 | 0.9 – 1.7 | 0.300 | |||
| 65+ | 2.0 | 1.5 – 2.5 | <0.001 | 1.9 | 1.5 – 2.5 | <0.001 | 1.3 | 0.9 – 1.8 | 0.130 | 1.1 | 0.7 – 1.6 | 0.797 | |||
| Female sex (relative to male) | 1.0 | 0.9 – 1.3 | 0.679 | 1.1 | 0.9 – 1.4 | 0.281 | 1.0 | 0.8 – 1.3 | 0.779 | 1.1 | 0.8 – 1.4 | 0.599 | |||
| T Stage (relative to T1) | |||||||||||||||
| T2 | 1.6 | 1.2 – 2.0 | <0.001 | 1.5 | 1.2 – 1.9 | 0.001 | 1.4 | 1.1 – 1.8 | 0.014 | 1.3 | 0.9 – 1.8 | 0.118 | |||
| T3 | 2.0 | 1.5 – 2.6 | <0.001 | 1.9 | 1.4 – 2.5 | <0.001 | 1.6 | 1.2 – 2.2 | 0.001 | 1.7 | 1.2 – 2.5 | 0.007 | |||
| T4 | 2.6 | 2.0 – 3.4 | <0.001 | 2.5 | 1.9 – 3.3 | <0.001 | 2.1 | 1.6 – 2.8 | <0.001 | 1.9 | 1.3 – 2.8 | 0.002 | |||
| Nodal disease (relative to N0) | 1.5 | 1.3 – 1.8 | <0.001 | 1.6 | 1.3 – 1.9 | <0.001 | 1.6 | 1.3 – 1.9 | <0.001 | 1.8 | 1.3 – 2.3 | <0.001 | |||
| Distant Metastases (relative to M0) | 6.4 | 3.4 – 12.4 | <0.001 | 5.6 | 2.6 – 12.0 | <0.001 | 6.3 | 2.9 – 14.0 | <0.001 | 8.1 | 3.4 – 19.5 | <0.001 | |||
| Site (Relative to larynx/hypopharynx) | |||||||||||||||
| Oral cavity | 1.0 | 0.8 – 1.3 | 0.776 | 1.1 | 0.9 – 1.3 | 0.429 | 1.1 | 0.9 – 1.4 | 0.423 | 1.0 | 0.7 – 1.4 | 0.934 | |||
| Oropharynx | 1.1 | 0.8 – 1.3 | 0.637 | 1.1 | 0.8 – 1.4 | 0.613 | 1.2 | 0.9 – 1.5 | 0.244 | 1.3 | 1.0 – 1.8 | 0.061 | |||
| Smoking (> 10 pack-years) | 1.4 | 1.1 – 1.9 | 0.009 | 1.3 | 1.0 – 1.7 | 0.107 | 1.2 | 0.9 – 1.7 | 0.276 | ||||||
| Alcohol use (any vs. none) | 1.3 | 0.9 – 1.7 | 0.137 | 1.2 | 0.9 – 1.6 | 0.341 | 1.2 | 0.8 – 1.8 | 0.352 | ||||||
| Education (relative to less than high school) | |||||||||||||||
| High school graduate | 0.9 | 0.7 – 1.2 | 0.495 | 0.9 | 0.7 – 1.2 | 0.347 | |||||||||
| Additional education past high school | 0.7 | 0.5 – 0.9 | 0.002 | 0.7 | 0.5 – 0.9 | 0.022 | |||||||||
| Insurance (relative to private insurance) | |||||||||||||||
| Medicaid/Medicare | 1.9 | 1.4 – 2.6 | <0.001 | 2.3 | 1.6 – 3.6 | <0.001 | |||||||||
| None | 1.7 | 1.2 – 2.4 | 0.003 | 2.0 | 1.3 – 3.0 | 0.001 | |||||||||
| Federal | 1.5 | 1.0 – 2.3 | 0.039 | 1.3 | 0.7 – 2.1 | 0.398 | |||||||||
| Other | 1.8 | 1.2 – 2.7 | 0.004 | 1.8 | 1.1 – 2.9 | 0.025 | |||||||||
| Income (relative to > 50 K) | |||||||||||||||
| Income $20,000 – $50,000 | 1.4 | 1.0 – 1.8 | 0.032 | 1.4 | 0.9 – 1.9 | 0.094 | |||||||||
| Income < $20,000 | 1.5 | 1.1 – 2.0 | 0.021 | 1.3 | 0.9 – 2.0 | 0.136 | |||||||||
| Rural Location (relative to metropolitan area) | |||||||||||||||
| Micropolitan Area | 0.9 | 0.7 – 1.3 | 0.576 | ||||||||||||
| Rural Area | 1.4 | 0.9 – 2.3 | 0.105 | ||||||||||||
| Treatment (relative to surgery alone)* | |||||||||||||||
| Radiation only | 1.7 | 1.1 – 2.6 | 0.014 | ||||||||||||
| Radiation and Chemotherapy | 1.1 | 0.7 – 1.8 | 0.550 | ||||||||||||
| Surgery and adjuvant chemoradiation | 0.7 | 0.4 – 1.2 | 0.225 | ||||||||||||
| Surgery and adjuvant radiation | 1.6 | 1.0 – 2.5 | 0.041 | ||||||||||||
Surgery with adjuvant chemotherapy was excluded due to an insufficient number of observations (n=2)
Figure 1:
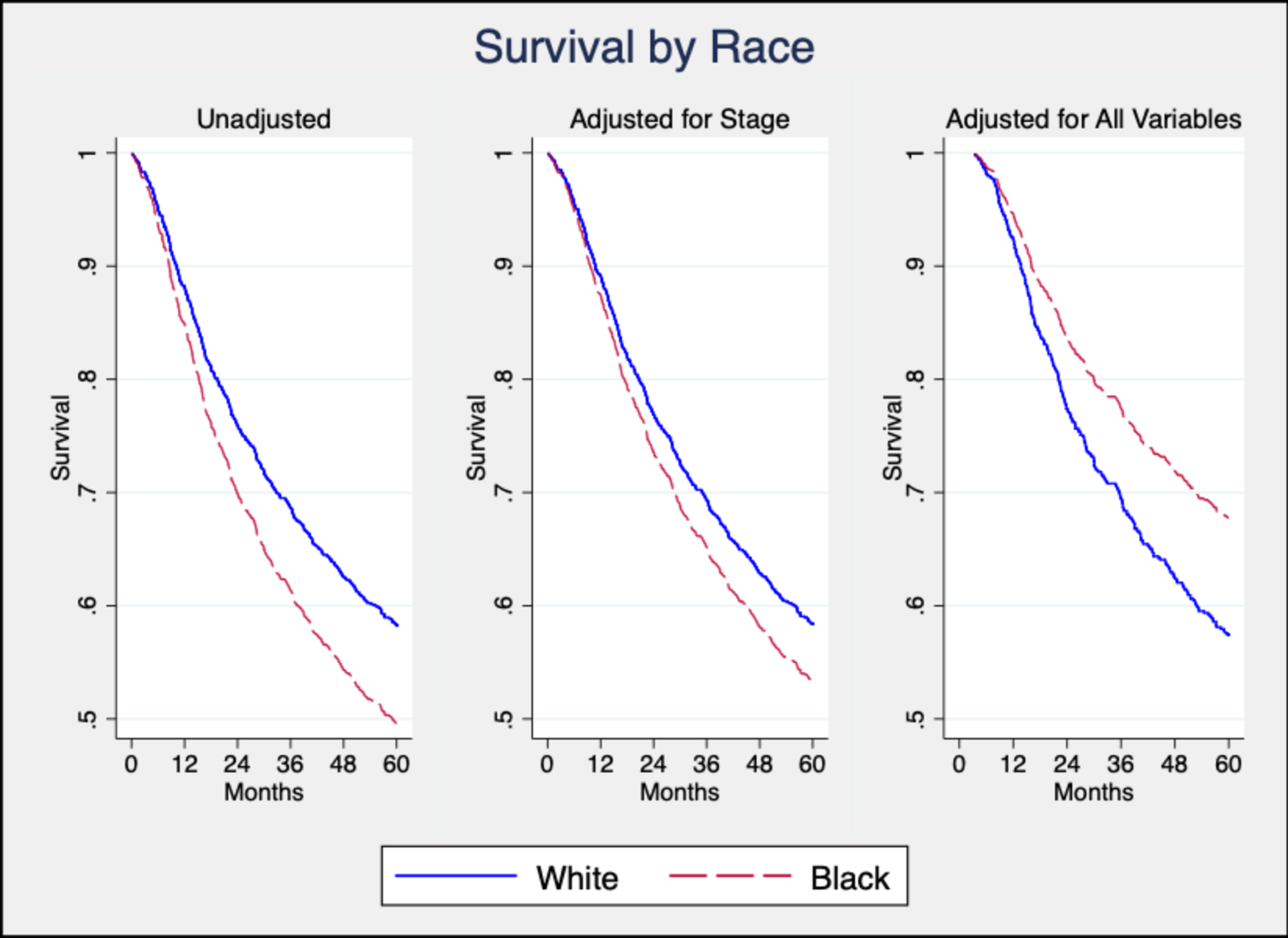
Overall Survival by Race in Limited and Full Adjustment Sets
Discussion
Our results suggest that racial disparities in stage at diagnosis and OS for HPV-negative HNSCC may be driven by differences in SES. Our findings are supported by several studies reporting that black patients with HNSCC in the United States are more likely to live in economically-deprived neighborhoods, have lower median household income, and lack medical insurance.7,11,12 Our study builds on prior studies by isolating the effects of SES using sequential adjustment sets accounting for different types of demographic, behavioral, clinical, and socioeconomic factors in a population-based cohort.
Our findings may have several explanations. It is plausible that low income patients who lack medical insurance face economic barriers to the detection and treatment of HNSCC. This could result in more advanced stage at diagnosis and worse survival outcomes. This is supported by a recent study which found that low-income patients and patients lacking private insurance were less likely to receive HPV testing for oropharyngeal cancer.37 Additionally, low education levels are known to correlate with poor health literacy,38,39 which could plausibly contribute to delays in seeking care or adhering to treatment recommendations. A recent meta-analysis found that patients with low health literacy were less likely to adhere to treatment recommendations across a range of illnesses, and health literacy interventions showed the greatest improvement on treatment adherence among ethnic minorities and low-income individuals.40
Our finding that SES is a key driver of racial disparities in HNSCC outcomes can help inform targeted interventions. Interventions that expand insurance coverage have the potential to reduce the burden of HNSCC in the United States and address this well-documented racial disparity. One potential policy solution is Medicaid expansion. North Carolina is one of 14 states that has not adopted Medicaid expansion through the Affordable Care Act (ACA), which would expand Medicaid eligibility to all individuals below 138% of the federal poverty line ($17,240 for a family of two in 2020).41 It is estimated that an additional 4.4 million individuals in the U.S. and 357,000 in North Carolina would gain health insurance coverage if the remaining states expanded Medicaid eligibility under the ACA.42
Other interventions may include policies that improve income and education levels. The minimum wage in North Carolina in 2019 was $7.25 per hour, which falls well below the calculated living wage even for a single adult with no children.43 Raising the minimum wage threshold is one potential solution. Additionally, a large percentage of our sample had less than a high school education (37%), which may contribute to poor health literacy as previously discussed. Policies that expand public school resources, health literacy curriculums, and student career counseling services may help address these gaps in education. Overall, we suspect that addressing the insurance, income, and educational shortcomings in our state would greatly reduce the observed racial disparity based on our findings.
Our findings are consistent with the growing understanding of race in the United States as a socially-defined construct rather than a biological entity.44 According to a 2019 executive summary on race by the American Association of Physical Anthropologists (AAPA), biological and genetic clusters do not map clearly onto socially-recognized racial groups as once thought.45 Rather, race must be examined in the context of United States history.46 For example, slavery in the United States created structural barriers with lasting ramifications on access to housing, education, employment, and health care among black patients, as evidenced by racial disparities in each of these domains.46
Importantly, our findings support the concept that race is a proxy for underlying differences in SES. Lack of adjustment for SES is thought to be one of the most important sources of potential bias in studies on racial differences in health outcomes.44 Our findings emphasize the importance of including SES in the adjustment set when examining racial disparities. Racial disparities found in prior HNSCC studies can be misleading, as the differences may be attributable to unmeasured confounders related to differences in SES that correlate with race.26,47
It is important to note that OS was actually favorable for black patients compared to white patients in the fully adjusted model. To our knowledge, this has never before been reported by a study in the United States. It suggests that there may be unmeasured racial differences in treatment or tumor characteristics. It is possible that this effect has been masked in previous studies by lack of adjustment for SES or access to care. More research is warranted to confirm and investigate potential drivers of this survival difference.
Strengths of our study include its large sample size and the collection of data through in-home interviews. This allowed us to obtain accurate information on self-identified race and individual-level indicators of SES. Furthermore, our sample used a population-based rather than an institutional based study design, so it provides a more representative sample of patients in North Carolina with HNSCC. Most importantly, it is the first study to sequentially control for indicators of SES while assessing racial disparities in HPV-negative HNSCC.
Our study as several limitations. The geographic area was restricted to a single state and may not be representative of the United States as a whole. It is also limited by its use of the 7th edition AJCC staging guidelines. Variables included in the 8th edition update, such as extranodal extension and depth-of-invasion, were not routinely included in pathology reports during the time period of this study. Another major limitation of this study was that it lacked enough data to include other traditional race categories such as Hispanic/Latino and Native American/Alaskan Indian. Only 28 (2.0%) of the 1,381 cases in the CHANCE database self-identified as “other” or “unknown” race based on the questionnaire administered by trained nurse-interviewers. It is possible that many of these 28 patients would identify as Hispanic/Latino, but a major limitation of our retrospective analysis is that the CHANCE questionnaire did not include Hispanic/Latino as a selection option under race category. Based on data from the U.S. Census Bureau, Hispanic and Latino individuals make up 9.6% of the total North Carolina population.48 We would expect more Hispanic/Latino cases in CHANCE based on this data. There was likely selection bias arising from both survey design (lack of inclusion of a traditional race category for Hispanic/Latino) as well as case recruitment (brochure mailed to home address), which may have disproportionately excluded patients with poor English-literacy or those without a home address. This greatly limits the generalizability of our findings. It is known that other racial and ethnic groups may also experience structural bias, and more research is needed to characterize potential health disparities.46
Finally, it is important to recognize that using race as an exposure variable has limited value in medical literature given the diversity of individuals within each socially-defined racial category.49 Race is a proxy for complex factors that interact and can be hard to define. We hope that by illustrating racial disparities in HNSCC, we can help inform future public health interventions aimed at improving outcomes in the affected populations while recognizing that race does not define any one individual.
Policies that expand health insurance coverage and improve income and education levels may begin to address this racial disparity observed in HNSCC. Policy development at the national, state and local level should include input from the community members and people affected by this disparity. Additional studies are needed to further uncover the mechanisms underlying this racial disparity, including using other measures of SES and racial factors such as discrimination which may provide more targets for intervention. Future studies should ideally report how information on race was obtained and adjust for indicators of SES.44
Conclusions:
Our findings indicate that racial disparities in stage at diagnosis and OS for HPV-negative head and neck cancer may be attributable to lower SES among black patients compared to white patients. Strategies focused on improving insurance coverage, income, and education have the potential to address this racial disparity while reducing the overall burden of HNSCC in the United States.
Acknowledgements:
This study was made possible by the help of Paul Brennan, Devasena Anantharaman, and Behnoush Abedi-Ardekani from the International Agency for Research on Cancer (IARC), who helped with the creation of the CHANCE database. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Funding:
This study was supported in part by grants R01- CA90731 from the National Cancer Institute and T32 – DC005360–12 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
Conflicts of Interest: None to disclose
Meeting Information: This abstract was presented as a poster for the Triological Society at the Combined Otolaryngology Spring Meetings (COSM) Virtual Poster Session from May 15th to June 15th, 2020.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. 4th edition. International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2019. ACS; 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf [Google Scholar]
- 3.Giraldi L, Leoncini E, Pastorino R, et al. Alcohol and cigarette consumption predict mortality in patients with head and neck cancer: a pooled analysis within the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Ann Oncol. 2017;28(11):2843–2851. doi: 10.1093/annonc/mdx486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 5.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–816. doi: 10.1002/ijc.20740 [DOI] [PubMed] [Google Scholar]
- 6.Peterson CE, Khosla S, Chen LF, et al. Racial differences in head and neck squamous cell carcinomas among non-Hispanic black and white males identified through the National Cancer Database (1998–2012). J Cancer Res Clin Oncol. 2016;142(8):1715–1726. doi: 10.1007/s00432-016-2182-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116(7):1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83 [DOI] [PubMed] [Google Scholar]
- 8.Subramanian S, Chen A. Treatment patterns and survival among low-income medicaid patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139(5):489–495. doi: 10.1001/jamaoto.2013.2549 [DOI] [PubMed] [Google Scholar]
- 9.Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head Neck. 2011;33(8):1092–1098. doi: 10.1002/hed.21584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahal BA, Inverso G, Aizer AA, Bruce Donoff R, Chuang S-K. Impact of African–American race on presentation, treatment, and survival of head and neck cancer. Oral Oncology. 2014;50(12):1177–1181. doi: 10.1016/j.oraloncology.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Reitzel LR, Nguyen N, Zafereo ME, Li G, Wei Q, Sturgis EM. Neighborhood deprivation and clinical outcomes among head and neck cancer patients. Health Place. 2012;18(4):861–868. doi: 10.1016/j.healthplace.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: An examination of 20,915 patients. Cancer. 2008;113(10):2797–2806. doi: 10.1002/cncr.23889 [DOI] [PubMed] [Google Scholar]
- 13.Shin JY, Yoon JK, Shin AK, Diaz AZ. The influence of insurance status on treatment and outcomes in oral cavity cancer: an analysis on 46,373 patients. International Journal of Oral and Maxillofacial Surgery. 2018;47(10):1250–1257. doi: 10.1016/j.ijom.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 14.Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, Taioli E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116(2):476–485. doi: 10.1002/cncr.24774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta V, Shi Z, Mills GM, Nathan C-AO, Shi R. Effect of Payer Status on Relative Survival of Patients with Laryngeal Cancer. Anticancer Res. 2016;36(1):327–333. [PubMed] [Google Scholar]
- 16.Chu KP, Habbous S, Kuang Q, et al. Socioeconomic status, human papillomavirus, and overall survival in head and neck squamous cell carcinomas in Toronto, Canada. Cancer Epidemiology. 2016;40:102–112. doi: 10.1016/j.canep.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Arbes SJ, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States). Cancer Causes Control. 1999;10(6):513–523. [DOI] [PubMed] [Google Scholar]
- 18.Shavers VL, Harlan LC, Winn D, Davis WW. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx, sinuses, and salivary glands. Cancer Metastasis Rev. 2003;22(1):25–38. [DOI] [PubMed] [Google Scholar]
- 19.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillison ML, Restighini C. Anticipation of the Impact of Human Papillomavirus on Clinical Decision Making for the Head and Neck Cancer Patient. Hematology/Oncology Clinics of North America. 2015;29(6):1045–1060. doi: 10.1016/j.hoc.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS. Human papillomavirus-related squamous cell carcinoma of the oropharynx: A comparative study in whites and African Americans. Archives of Otolaryngology - Head and Neck Surgery. 2011;137(2):163–169. doi: 10.1001/archoto.2010.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiron J, Sethi S, Ali-Fehmi R, et al. Racial disparities in Human Papillomavirus (HPV) associated head and neck cancer. American journal of otolaryngology. 2014;35(2):147–153. doi: 10.1016/j.amjoto.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragin C, Liu JC, Jones G, et al. Prevalence of HPV infection in racial-ethnic subgroups of head and neck cancer patients. Carcinogenesis. 2017;38(2):218–229. doi: 10.1093/carcin/bgw203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrank TP, Han Y, Weiss H, Resto VA. Case-matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States--possible role for human papillomavirus in survival disparities. Head Neck. 2011;33(1):45–53. doi: 10.1002/hed.21398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenze NR, Farquhar DR, Mazul AL, Masood MM, Zevallos JP. Racial disparities and human papillomavirus status in oropharyngeal cancer: A systematic review and meta-analysis. Head Neck. Published online December 18, 2018. doi: 10.1002/hed.25414 [DOI] [PubMed] [Google Scholar]
- 26.Zandberg DP, Liu S, Goloubeva O, et al. Oropharyngeal cancer as a driver of racial outcome disparities in squamous cell carcinoma of the head and neck: 10-year experience at the University of Maryland Greenebaum Cancer Center. Head Neck. 2016;38(4):564–572. doi: 10.1002/hed.23933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrank TP, Han Y, Weiss H, Resto VA. Case-matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States--possible role for human papillomavirus in survival disparities. Head Neck. 2011;33(1):45–53. doi: 10.1002/hed.21398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila). 2009;2(9):776–781. doi: 10.1158/1940-6207.CAPR-09-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liederbach E, Kyrillos A, Wang C-H, Liu JC, Sturgis EM, Bhayani MK. The national landscape of human papillomavirus-associated oropharynx squamous cell carcinoma. International Journal of Cancer. 2017;140(3). doi: 10.1002/ijc.30442 [DOI] [PubMed] [Google Scholar]
- 30.Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566–1575. doi: 10.1002/cncr.30353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 32.Divaris K, Olshan AF, Smith J, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010;21(4):567–575. doi: 10.1007/s10552-009-9486-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farquhar DR, Divaris K, Mazul AL, Weissler MC, Zevallos JP, Olshan AF. Poor oral health affects survival in head and neck cancer. Oral Oncol. 2017;73:111–117. doi: 10.1016/j.oraloncology.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritz AG, Percy, Constance, Jack, Andrew, et al. , eds. International Classification of Diseases for Oncology: ICD-O. Third edition, First revision. World Health Organization; 2013. [Google Scholar]
- 35.Mazul AL, Taylor JM, Divaris K, et al. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2017;123(1):71–80. doi: 10.1002/cncr.30312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer. 2017;140(9):1968–1975. doi: 10.1002/ijc.30608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazul AL, Colditz GA, Zevallos JP. Factors associated with HPV testing in oropharyngeal cancer in the National Cancer Data Base from 2013 to 2015. Oral Oncol. 2020;104:104609. doi: 10.1016/j.oraloncology.2020.104609 [DOI] [PubMed] [Google Scholar]
- 38.Mantwill S, Monestel-Umaña S, Schulz PJ. The Relationship between Health Literacy and Health Disparities: A Systematic Review. PLoS ONE. 2015;10(12):e0145455. doi: 10.1371/journal.pone.0145455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker DW, Gazmararian JA, Williams MV, et al. Functional health literacy and the risk of hospital admission among Medicare managed care enrollees. Am J Public Health. 2002;92(8):1278–1283. doi: 10.2105/ajph.92.8.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: A meta-analysis. Patient Educ Couns. 2016;99(7):1079–1086. doi: 10.1016/j.pec.2016.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. 2020 Poverty Guidelines.; 2020. https://aspe.hhs.gov/poverty-guidelines [Google Scholar]
- 42.Garfield Rachel, Orgera Kendal, Damico Anthony. The Coverage Gap: Uninsured Poor Adults in States That Do Not Expand Medicaid. Kaiser Family Foundation; 2020:10. http://files.kff.org/attachment/Issue-Brief-The-Coverage-Gap-Uninsured-Poor-Adults-in-States-that-Do-Not-Expand-Medicaid [Google Scholar]
- 43.Amy Glasmeier. Living Wage Calculation for North Carolina. Massachusetts Institute of Technology; Accessed June 14, 2020. https://livingwage.mit.edu/states/37 [Google Scholar]
- 44.Kaplan JB. Use of Race and Ethnicity in Biomedical Publication. JAMA. 2003;289(20):2709. doi: 10.1001/jama.289.20.2709 [DOI] [PubMed] [Google Scholar]
- 45.American Association of Physical Anthropologists. AAPA Statement on Race & Racism.; 2019. Accessed October 25, 2019. https://physanth.org/about/position-statements/aapa-statement-race-and-racism-2019/
- 46.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet. 2017;389(10077):1453–1463. doi: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 47.Megwalu UC, Ma Y. Racial Disparities in Oropharyngeal Cancer Stage at Diagnosis. Anticancer Res. 2017;37(2):835–839. doi: 10.21873/anticanres.11386 [DOI] [PubMed] [Google Scholar]
- 48.United States Census Bureau. QuickFacts North Carolina. Accessed June 14, 2020. https://www.census.gov/quickfacts/NC
- 49.Hammonds EM, Herzig RM. Nature of Difference Sciences of Race in the United States from Jefferson to Genomics. MIT Press; 2009. [Google Scholar]


