Abstract
Obsessive-compulsive disorder (OCD) is disabling and often treatment-refractory. Host immunity and gut microbiota have bidirectional communication with each other and with the brain. Perturbations to this axis have been implicated in neuropsychiatric disorders, but immune-microbiome signaling in OCD is relatively underexplored. We review support for further pursuing such investigations in OCD, including: 1) gut microbiota has been associated with OCD, but causal pathogenic mechanisms remain unclear; 2) early environmental risk factors for OCD overlap with critical periods of immune-microbiome development; 3) OCD is associated with increased risk of immune-mediated disorders and changes in immune parameters, which are separately associated with the microbiome; and 4) gut microbiome manipulations in animal models are associated with changes in immunity and some obsessive-compulsive symptoms. Theoretical pathogenic mechanisms could include microbiota programming of cytokine production, promotion of expansion and trafficking of peripheral immune cells to the CNS, and regulation of microglial function. Immune-microbiome signaling in OCD requires further exploration, and may offer novel insights into pathogenic mechanisms and potential treatment targets for this disabling disorder.
Keywords: Obsessive-compulsive disorder, obsessive-compulsive behavior, gut microbiome, microbiota, immune, inflammation, neuroinflammation, gut-brain-immune axis
1. Introduction
Obsessive-compulsive disorder (OCD) is a disabling and chronic neuropsychiatric disorder, characterized by obsessions, compulsions, or both, which significantly interfere with an individual’s functioning (APA, 2013). Several lines of inquiry have offered insights into possible pathogenic mechanisms in OCD, and serotonergic, dopaminergic, and glutamatergic dysfunction in brain cortical-striatal-thalamic-cortical (CSTC) circuitry is consistently implicated (Pauls et al., 2014; Stein et al., 2019). However, the exact causes of OCD remain unclear, and with current first-line treatments, a majority of individuals will have at least some symptoms persist throughout life, and one-third or more of all patients with OCD are treatment-resistant (Pittenger et al., 2005; Skoog and Skoog, 1999; Stewart et al., 2004). Therefore, reliable biomarkers of disease, predictors of treatment response, and individualized treatments are critically needed for OCD.
Immune-mediated mechanisms may be involved in at least some cases of OCD (Gerentes et al., 2019; Lamothe et al., 2018; Marazziti et al., 2018; Pérez-Vigil et al., 2016). Epidemiologic studies suggest that OCD is associated with immunologic comorbidities (Isung et al., 2020; Mataix-Cols et al., 2018; Orlovska et al., 2017; Wang et al., 2019), and genetic studies have implicated immune-related genes in OCD (Cappi et al., 2016, 2012; Den Braber et al., 2016; Hounie et al., 2008; Rodriguez et al., 2017). The immune system has become a treatment target in several psychiatric disorders including OCD, but identifying whom to treat, appropriate immune-modifying agents that will be safe and effective for individual patients, and at what timepoints in disease, remain major challenges (Gerentes et al., 2019; Köhler et al., 2014; Roman and Irwin, 2020; Zheng et al., 2017). Notably, fast growing evidence has implicated immune regulation, the microbiome, and host immune-microbiome interactions in normal neurodevelopment and in a number of neuropsychiatric and neurodegenerative disorders (Dinan and Cryan, 2017; Fung et al., 2017; Petra et al., 2015; Pronovost and Hsiao, 2019), which present novel areas of inquiry for OCD and may offer mechanistic and therapeutic clues.
The human microbiome refers to the collection of trillions of microorganisms including bacteria, viruses, and fungi, which together with their genetic material inhabit human hosts (Turnbaugh et al., 2007). Bidirectional communication occurs between host and microbiome via multiple mechanisms, and microbes have the potential to influence host physiology, as they participate in the production and degradation of various short chain fatty acids (SCFAs), vitamins, hormones, and neurotransmitters (Cho and Blaser, 2012; Cryan and Dinan, 2012; Rogers et al., 2016). Changes in microbial diversity or composition, sometimes termed “dysbiosis,” have been implicated in a number of human disease states, including obesity, autoimmune disorders, inflammatory bowel disease (IBD), cancer, and some neuropsychiatric disorders (Bastiaanssen et al., 2018; Gilbert et al., 2018; O’ Mahony et al., 2015; Wu and Wu, 2012). Studies of microbiome-targeted therapies such as prebiotics, probiotics, antibiotics, and fecal microbiota transplant (FMT) in the context of neuropsychiatric disorders are in their infancy, with mixed results and ambiguous mechanisms of action (Kang et al., 2019, 2017; Van Ameringen et al., 2019). The microbiome has a bidirectional relationship with immune development and homeostasis (Belkaid and Hand, 2014; Belkaid and Harrison, 2017; Chen and Stappenbeck, 2019; Robertson et al., 2018). While the gut microbiota participates in bidirectional communication with the nervous system by various mechanisms, immune-microbiome signaling is of particular interest to OCD, where immunologic etiologies have been suspected but exact mechanisms remain unclear.
1.1. Approaches and Inclusion of Literature:
In this comprehensive narrative review, we aim to provide a critical appraisal of the extant literature in immune and microbiome investigations in OCD to integrate and scrutinize the evidence of their associations. We conducted an inclusive compilation of the literature through PubMed and EMBASE of articles available in English, through January 31, 2021, without limitation to publication date. Key search terms included obsessive, compulsive, immune, immunologic, inflammation, microbiome, microbiota, and germ-free. Reference lists of relevant publications were also manually searched for additional references that did not result from those search engines, and both human and pre-clinical studies were included. The inclusive nature of this review was necessary given the infancy of this literature in order to decipher the complex nature of immune-microbiome interactions in the context of OCD. Thus, we evaluated evidence of immune dysregulation in OCD, which has been systematically reviewed elsewhere (Gerentes et al., 2019; Lamothe et al., 2018; Marazziti et al., 2018; Pérez-Vigil et al., 2016), in combination with literature related to the gut microbiome and OCD, which remains relatively less explored (Turna et al., 2017, 2016). Given the current paucity of direct evidence implicating gut microbiome changes in the pathophysiology of OCD, the evidence of microbiome associations in immune-mediated processes that had been separately associated with OCD, was also evaluated. The role of the microbiome in immune and inflammatory disorders, outside of OCD, has been extensively reviewed elsewhere (Fitzgibbon and Mills, 2020; Peroni et al., 2020; Trøseid et al., 2020; Xuan Zhang et al., 2020), which offered insight. One study to date has examined immune and gut microbiome biomarkers concurrently in the context of OCD (Turna et al., 2020), and to our knowledge there were no systematic or quantitative (i.e., meta-analysis) reviews published on the topic of host immune and microbiome associations related to OCD symptomatology at the time of this review.
In the following sections, we critically synthesize scientific premise and rationale for examining host immune and microbiome correlates in OCD in future studies, including the following: 1) The gut microbiome has been associated with OCD symptomatology in a small number of pre-clinical and human studies, and causal pathogenic mechanisms remain unclear. 2) Early environmental risk factors for OCD overlap with critical periods of immune-microbiome development. 3) OCD is associated with increased risk of immune-mediated disorders and changes in immune parameters, which have been separately associated with the microbiome. 4) Gut microbiome manipulations in animal models are associated with changes in immunity and some obsessive-compulsive symptom domains. Theoretical mechanisms by which host immune and microbiome signaling could influence OCD symptomatology are also reviewed, including the microbiota potentiating various neuroinflammatory processes. Further studies are needed in this novel area, and finally, considerations for such investigations and potential treatment implications are discussed.
2. Evidence in Support of Microbiome Involvement in OCD Symptomatology
Initial evidence from animal and human studies together suggest OCD symptomatology could be associated with gut microbial composition changes, although direct evidence supporting a causal role for aberrant immune-microbiome signaling in OCD remains lacking (see Table 1). In rodent models of both experimentally induced and naturally occurring obsessive compulsive behavior (OCB), gut microbiota alterations have been described (Jung et al., 2018; Scheepers et al., 2019). For example, in Long-Evans male rats exposed to experimental induction of OCB with quinpirole, onset of compulsive checking and locomotor sensitization was associated with changes in 25 fecal microbiota operational taxonomic units (OTUs), with several belonging to the Lachnospiraceae and Ruminococcaceae families (Jung et al., 2018). Large nest-building (LNB) deer mice that naturally develop obsessive-compulsive behaviors have altered gut microbiome composition compared to normal-nest building (NNB) deer mice, characterized by decreased abundances of Prevotella and Anaeroplasma, which have anti-inflammatory effects (Scheepers et al., 2019). In a human study, OCD was associated with decreased gut microbiome richness, along with lower relative abundance of Oscillospira, Odoribacter, and Anaerostipes (Turna et al., 2020). Changes in microbiome composition have also been described in youth with Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS), a condition which can present with obsessions and compulsions (Quagliariello et al., 2018).
Table 1. Animal and human studies relating gut microbiome to OCD symptomatology.
ng = nanograms; mL = milliliters; 5-HT = serotonin; wks = weeks; D = dopamine; mg = milligrams; kg = kilogram; rRNA = ribosomal ribonucleic acid; OTUs = operational taxonomic units; PANDAS = pediatric autoimmune neuropsychiatric disorders associated with streptococcus; PANS = pediatric acute-onset neuropsychiatric syndrome; yo = years old; LLB = large nest-building; NNB = normal nest-building; IBD = inflammatory bowel disease; CRP = C-reactive protein; IL-6 = interleukin 6; TNF = tumor necrosis factor.
| Authors | Study Population | Study Design | Intervention | Analyses | Results |
|---|---|---|---|---|---|
| (Messaoudi et al., 2011) | Healthy human adult volunteers (n=25), with low baseline urinary free cortisol (10–50 ng/mL) | 30-day controlled clinical trial | Daily probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) (n=10) v. placebo (n=15) | Percentage change from pre- to post-intervention on the “obsessive compulsive” (OC) subscale of the Hopkins Symptoms Checklist (HSCL-90) | Treatment group with significantly greater decrease in HSCL-90 OC subscale scores compared to placebo group |
| (Kantak et al., 2014) | Male Balb/cJ mice at 8 weeks of age, exposed to experimental induction of obsessive compulsive behavior (OCB) using RU 24969 (a 5-HT1A/1B receptor agonist) | Controlled experiments |
Experiment 1: Pre-treatment with daily Lactobacillus
rhamnosus GG gavage (n=12) v. daily saline gavage (n=6), followed by treatment with RU 24969 at 2 weeks and 4 weeks later; Experiment 2: Pre-treatment with daily L. rhamnosus x2wks (n=12) v. pre-treatment with daily fluoxetine x4wks (n=12) v. pre-treatment with daily saline x2wks (n=6) v. pre-treatment with daily saline x4wks (n=6), followed by RU 24969 |
Open field test (OFT), marble burying test (MBT) |
Experiment 1: At both 2 and 4 weeks, L.
rhamnosus GG treated mice with ↓ OCBs (↓ open field perseverative hyperlocomotion, ↓ marbles buried), compared to salinetreated controls; Experiment 2: Salinetreated controls with ↑ OCBs compared to L. rhamnosus GG and fluoxetine-treated mice; no significant differences between L. rhamnosus GG and fluoxetine-treated groups |
| (Jung et al., 2018) | Long-Evans male rats exposed to experimental induction of OCB using chronic administration of quinpirole (a D2/D3 receptor agonist) (n=15) v. saline control (n=16) | Controlled experiment | Twice-weekly subcutaneous injection of quinpirole (0.25 mg/kg) v. saline solution, for a total of 9 injections | OFT after each injection; fecal microbiome by 16s rRNA amplicon sequencing (V3 region) after injections 1, 5, and 9 | Quinpirole-treated rats with ↑ compulsive checking and ↑ locomotor sensitization, accompanied by changes in 25 OTUs, most from Firmicutes phylum, Clostridiales order, specifically Lachnospiraceae and Ruminococcaceae families |
| (Quagliariello et al., 2018) | Human youth, 4–16 years of age, with PANDAS/PANS (total n=30, male n=20, female n=10), v. healthy controls (n=70) | Observational, case-control | Not applicable | 16s rRNA amplicon sequencing (V1–V3 region) | PANDAS/PANS: ↓ α- diversity (Observed and Chao1 indices); young PANDAS/PANS (4–8yo): ↑ Bacteroidetes and ↓ Firmicutes at phylum level; ↑ Bacteroidaceae, Rikencellaceae, Odoribacteriaceae, ↓ Turicibacteraceae, Tissierellaceae, Gemellaceae, Carnobacteriaceae, Corynebacteriaceae, Lachnospiraceae, Erysipelotrichaceae, Actinobacteria at family level; ↑ Odoribacter, Bacteroides, Oscillospira; ↓ Dorea, Roseburia, Coprococcus, Turicibacter at genus level; older PANDAS/PANS (9–16yo): ↑ Peptostreptococcaceae, Erysipelotrichaceae, ↓ Rikencellaceae, Barnesiellaceae at family Level |
| (Scheepers et al., 2019) | Specific-pathogen- free large v. normal nest-building deer mice (LNB and NNB Peromyscus maniculatus bairdii), total n=11, male n=3, female n=8 (per group) | Observational, case-control | Not applicable | Nest-building behavior, fecal microbiota by 16s rRNA amplicon sequencing (V3–V4 region) | No differences in α- diversity; β-diversity (Aitchison distance at genus level) revealed clustering of LLB v. NNB deer mice; LLB with non-significant ↑ Desulfovermiculus, Aestuariispira, Peptococcus, Holdemanella; NNB with non-significant ↑ Prevotella, Anaeroplasma |
| (Zhang et al., 2020) | Male C57BL/6 mice at 7–8 weeks of age with dextran sulfate sodium (DSS)-induced colitis | Controlled experiment | Food ad libitum v. alternate-day fasting (ADF) v. time-restricted feeding (TRF) v. intermittent energy fasting (IEF) (n=12 per group) | Colitis disease activity, MBT, elevated-plus maze (EPM), brain immunohistochemistry, fecal microbiota by 16s rRNA amplicon sequencing (V3–V4 region), fecal metabolomics by gas chromatography | ADF: ↓ survival; TRF & IER: ↓ colitis disease activity, ↑ survival, ↓ marble burying, ↑ time in open arm of EPM, ↓ neuroinflammation, ↓ Gammaproteobacteria, ↑ Christensenellaceae, ↓ Enterobacteriaceae, ↓ Shigella, ↓ Escherichia coli, ↑ Rikenellaceae, ↑ acetate, ↑ butyrate, ↑ isobutyrate |
| (Kilinçarslan and Evrensel, 2020) | Human adults (n=10) receiving fecal microbiota transplant (FMT) for IBD (UC n=6, CD n=4), psychiatric disorders excluded | Observational | FMT for IBD | Symptom Checklist- 90-Revised (SCL-90- R), Beck Depression Inventory (BDI), and Maudsley Obsessive Compulsive Inventory (MOCI) at baseline and 1-month post-FMT | Significant pre- to post- FMT ↓ SCL-90-R scores, ↓ BDI scores, and ↓ MOCI scores (effect sizes 0.81–0.84) |
| (Turna et al., 2020) | Human adults with OCD, who were medication- and depression-free (n=21) v. healthy controls (n=22) | Observational, case-control | Not applicable | Blood inflammatory markers (CRP, IL-6, TNF-α), fecal microbiota by 16s rRNA amplicon sequencing (V3 region) | OCD with ↑ CRP but no difference in IL-6 or TNF- α, ↓ α-diversity (Inverse Simpson) but no significant differences in β-diversity metrics, ↓ relative abundance of Oscillospira, Odoribacter, Anaerostipes; CRP correlated with OCD symptom severity but not microbiome metrics |
Studies of microbiome-based and dietary interventions also provide support for a potential role of gut microbiota in obsessive-compulsive symptomatology (OCS) (see Table 1). Pre-treatment with Lactobacillus rhamnosus GG effectively attenuated experimental induction of OCB in mice, an effect comparable to that of pre-treatment with fluoxetine (Kantak et al., 2014). In an animal model of colitis, exposure to select intermittent fasting schedules has not only more favorable colitis-related outcomes, but also attenuated colitis-related changes in compulsive behavior, neuroinflammation, and fecal microbiome composition (Zhang et al., 2020). Healthy humans treated with a probiotic formulation containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 had decreased sub-clinical OCS (Messaoudi et al., 2011), and in a clinical study of adults with IBD, FMT was associated with significant pre- to post-treatment decrease in obsessive symptoms (Kilinçarslan and Evrensel, 2020). Notably, studies have not evaluated the effect of microbiome-based interventions on psychiatric symptoms in humans with clinically diagnosed OCD.
The mechanisms by which microbiota composition and function could influence OCD symptomatology remain unclear, and only one study to date has examined gut microbiome features concurrently with other biomarkers in OCD (Turna et al., 2020). Compared to healthy controls, OCD was associated with a significantly different gut microbiome composition and greater peripheral C-reactive protein (CRP) levels. Elevated CRP was associated with OCD symptomatology, but not microbiome features, which could have been related to the study’s small sample size (OCD n=21, control n=22) (Turna et al., 2020). While microbiome studies of OCD are in their infancy, further examining host immune-microbiome correlates, and their relationships with genetic, neuroimaging, neuropsychological, and other clinical variables in larger samples of individuals with OCD, across developmental time periods, has the potential to increase our understanding of pathogenic mechanisms in OCD, and to inform novel treatment targets for this disabling disorder.
3. Overlapping Critical Periods of Immune, Microbiome, and Brain Development
Initial microbial colonization occurs during birth and is subsequently shaped during the first three years of life, thus setting the stage for the lifelong, relatively stable adult microbiome (Dominguez-Bello et al., 2019; Yatsunenko et al., 2012). In animal models, this early colonization event regulates the developmental programming of several systems, including the central nervous system (CNS) (Bruce-Keller et al., 2018; Galland, 2014). For example, germ-free (GF) animals show developmental derangements in brain structure and behavior (Desbonnet et al., 2014; Heijtz et al., 2011; Hoban et al., 2016; Luczynski et al., 2016; Ogbonnaya et al., 2015; Stilling et al., 2015). Additionally, microbial colonization of GF animals in early life can restore normal CNS function, while colonization in adulthood cannot, which further supports the crucial role of early gut microbiota for normal CNS function in adulthood (Borre et al., 2014).
In addition to its role in CNS development, microbial colonization of the gut is inextricably linked to the maturation of the immune system, and is also likely to have a critical developmental period (Gensollen et al., 2016; Knoop et al., 2017). For instance, GF mice have evidence of invariant natural killer T (iNKT) cell accumulation, which results in increased morbidity in models of inflammatory bowel disease and allergic asthma, while neonatal colonization of GF mice leads to markedly reduced number of iNKT cells, and to development of immune homeostasis (Olszak et al., 2014). In mice, host immune maturation within the CNS is also guided by the gut microbiome, which in early life is thought to ‘educate’ developing CNS immune cells such as microglia and astrocytes, and in turn shape normal neurodevelopmental processes such as synaptic pruning and myelination (Erny et al., 2015). In animal models of adulthood, the gut microbiome continues to stimulate inflammatory signaling in the host, as intestinal microbial antigens trigger release of cytokines by intestinal macrophages and T cells (Caspani et al., 2019; Fung et al., 2014). Peptidoglycans from bacterial cell walls can also enter the CNS in mice, where they stimulate central pattern-recognition receptors (PRRs) to trigger the innate immune system and modify behavior (Arentsen et al., 2017). Taken together, these observations suggest that both early and sustained immune-microbiome signaling are likely necessary for protection from certain diseases, and that the immune system is a key component of microbiome-to-brain communication throughout the lifespan.
Because the development of host immunity, gut microbiome, and normal CNS function occur in tandem and are inter-dependent, early disruptions to microbial colonization resulting from environmental insults can lead to persistent, and in some cases, irreversible changes in specific immune parameters, behavior, and cognition (Bailey et al., 2011; Crumeyrolle-Arias et al., 2014; Gensollen et al., 2016; Sudo et al., 2004). However, most evidence in support of this is gleaned from pre-clinical models. Associations among early life exposures, host immunity, gut microbial colonization, and neurodevelopment are starting to be identified in human studies, but causative pathogenic mechanisms largely remain to be established (Borre et al., 2014; Pronovost and Hsiao, 2019).
During gestation and early development, exposure to certain environmental factors could influence immune-microbiome parameters in humans (Codagnone et al., 2019), and recent studies have suggested some of the same early environmental exposures may also confer risk for later development of OCD symptoms in humans (see Table 2). For example, a Swedish population-based cohort study demonstrated that perinatal events such as in utero exposure to maternal smoking, pre-term birth, and Cesarean section (C-section) delivery are associated with increased risk of OCD (Brander et al., 2016). In a community-based sample of Brazilian youth, self-reported prenatal maternal stress and lack of early breast feeding were associated with subclinical OCS severity scores (Macul Ferreira de Barros et al., 2020). These environmental exposures are also associated with changes in immune and microbiome parameters; therefore, early immune-microbiome development in OCD warrants further investigation. Future studies could elucidate if other early threats to early immune-microbiome development, such as maternal high-fat diet, early antibiotic exposure, or early immigration to a developed country, among others, are associated with risk for OCD (Rook et al., 2014). Longitudinal studies of immune and microbiome development in offspring of parents with OCD, or youth at high risk for OCD, would shed further light on these potential associations, as would assessment of OCS and other psychiatric symptoms in longitudinal studies of immune-microbiome development in other pediatric populations.
Table 2. Early environmental exposures which confer risk for OCD, and also influence immune-microbiome development.
OCD = obsessive compulsive disorder; HR = hazard ratio; CI = confidence interval; Th2 = T-helper 2 cell; IL = interleukin; OCS = obsessive compulsive symptom; TNFα = tumor necrosis factor alpha; IgG = immunoglobulin G; Th1 = T-helper 1 cell; TLR = toll-like receptor.
| Early Life Events | Association with OCD | Immune Associations | Gut Microbiome Associations |
|---|---|---|---|
| Prenatal maternal smoking | ↑ OCD risk (HR 1.27, 95% CI 1.02–1.58) (Brander et al., 2016) | ↑ Neonatal Th2 responses to allergens (Noakes et al., 2003); ↑ IL-8 in offspring at birth (Chahal et al., 2017); ↑ offspring risk of asthma (Ng & Zelikoff, 2007) and Crohn’s disease (Roberts et al., 2011) | ↑ Enterobacteriaceae in meconium (Gosalbes et al., 2013); ↑ Bacteroides and ↑ Staphylococcus at 6 months of age (Levin et al., 2016) |
| Prenatal maternal stress | ↑ OCS score (β 0.056, 95% CI 0.006–0.106) (Macul Ferreira de Barros et al., 2020) | ↓ CD4+ lymphocytes, ↑ TNFα, IL-1β, IL-6, IL-4, IL-13 (Veru et al., 2015); altered neuroendocrine-immune regulation in females (Riis et al., 2020); ↑ health outcomes requiring antibiotic use (Zijlmans et al., 2017) | ↑ Proteobacteria (e.g., Escherichia, Enterobacter), ↓ Actinobacteria, ↓ lactic acid producers (e.g., Lactobacillus) (Zijlmans et al., 2015) |
| Pre-term birth | ↑ OCD risk (HR 1.24, 95% CI 1.07–1.43) (Brander et al., 2016) | ↓ Complement activity, ↓ neutrophil count/function, ↓ monocyte count/function, ↓ IgG, ↓ cytokine production (Amélie Collins et al., 2018; Melville & Moss, 2013) | Delayed colonization, ↓ diversity (Rougé et al., 2010); ↑ Enterobacteriaceae, Enterococcus, Staphylococcus, Escherichia, Klebsiella, ↓ Bifidobacterium species (Arboleya et al., 2012; Schwiertz et al., 2003; Wandro et al., 2018) |
| Cesarean section delivery | ↑ OCD risk (HR 1.17, 95% CI 1.01–1.34) (Brander et al., 2016) | ↓ Th1 responses (Jakobsson et al., 2014); ↓ perinatal cytokine production (Malamitsi-Puchner et al., 2005); ↓ TNFα and IL-6 in response to TLR stimulation (Liao et al., 2017); ↑ risk of immune-mediated disorders (Bager et al., 2008; Cardwell et al., 2008; Kristensen & Henriksen, 2016; Słabuszewska-Jóźwiak et al., 2020; Thavagnanam et al., 2008) | ↓ Lactobacillus, Bifidobacterium, Bacteroides, Prevotella, ↑ Staphylococcus, Streptococcus, Klebsiella, Enterococcus, Clostridium (Akagawa et al., 2019; Biasucci et al., 2010; Dominguez-Bello et al., 2010; Shao et al., 2019) |
| Formula feeding | ↑ OCS score (β 0.209, 95% CI 0.037–0.380) (Macul Ferreira de Barros et al., 2020) | ↑ Early respiratory symptoms, mixed evidence for asthma risk (Oddy, 2017); ↑ early antibiotic use (Di Mario et al., 2019) | ↑ Escherichia coli, Clostridium difficile, Bacteroides, lactobacilli (Penders et al., 2006); ↓ Bacteroides, Bifidobacterium (Hesla et al., 2014) |
(Akagawa et al., 2019; Amélie Collins et al., 2018; Arboleya et al., 2012; Bager et al., 2008; Biasucci et al., 2010; Brander et al., 2016; Cardwell et al., 2008; Chahal et al., 2017; Di Mario et al., 2019; Dominguez-Bello et al., 2010; Gosalbes et al., 2013; Hesla et al., 2014; Jakobsson et al., 2014; Kristensen and Henriksen, 2016; Levin et al., 2016; Liao et al., 2017; Macul Ferreira de Barros et al., 2020; Malamitsi-Puchner et al., 2005; Melville and Moss, 2013; Ng and Zelikoff, 2007; Noakes et al., 2003; Oddy, 2017; Penders et al., 2006; Riis et al., 2020; Roberts et al., 2011; Rougé et al., 2010; Schwiertz et al., 2003; Shao et al., 2019; Słabuszewska-Jóźwiak et al., 2020; Thavagnanam et al., 2008; Veru et al., 2015; Wandro et al., 2018; Zijlmans et al., 2017, 2015)
4. Microbiome Implicated in Immune-Mediated Processes Associated with OCD
4.1. Pathogenic Infections:
A healthy microbiome can provide protection against pathogenic infection, as commensal microorganisms compete with potential pathogens for resources, produce metabolic byproducts such as SCFAs which help maintain the integrity of epithelial barriers, and promote differentiation of immune cells and maturation of secondary lymphoid tissues (Belkaid and Hand, 2014; Belkaid and Harrison, 2017; Wu and Wu, 2012). Notably, disrupted immune-microbiome development is associated with increased susceptibility to infection in animal models (Deshmukh et al., 2014; Khosravi et al., 2014). In humans, OCS can spontaneously emerge following pathogen exposure, such as is the case in Sydenham’s chorea (SC), PANDAS, or Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) (Freeman et al., 1965; Swedo et al., 2012, 1998, 1989). However, controversy has long surrounded the issue of whether or not the PANDAS/PANS phenotypes represent distinct clinical entities. More recent studies have suggested the broader OCD phenotype may also be associated with increased susceptibility to infection, and to increased risk of symptom onset following pathogenic infection (Isung et al., 2020; Mell et al., 2005; Orlovska et al., 2017; Wang et al., 2016). Thus far, gut microbial composition in youth with PANDAS has been explored in one study (Quagliariello et al., 2018), and future longitudinal investigations of immune-microbiome correlates in pathogen-associated OCD symptomatology could shed light on this complex and poorly understood phenomenon.
It remains unclear if individuals with OCD have increased susceptibility to infection itself, to the deleterious neuroimmune consequences of pathogenic infection, or both. In support of the former, a Swedish population- and sibling-based cohort study showed that history of any primary humoral immunodeficiency is associated with increased risk of any psychiatric disorder, including OCD (population-based adjusted odds ratio [aOR] 2.19, 95% confidence interval [CI] 1.68–2.86; sibling-based adjusted aOR 1.55, 95% CI 0.95–2.56) (Isung et al., 2020). The most common humoral immunodeficiency, selective immunoglobulin A (IgA) deficiency was also associated with risk for OCD (population-based aOR 2.27, 95% CI 1.53–3.37, sibling-based aOR 1.50, 95% CI 0.74–3.04) (Isung et al., 2020). Clinically, selective IgA deficiency can be asymptomatic, or associated with increased infections and with autoinflammatory and autoimmune processes. Secretory IgA is important for maintaining host-microbiome homeostasis at gut epithelium (Yang and Palm, 2020). Changes in gut microbial composition have been demonstrated in individuals with selective IgA deficiency, including increased relative abundance of Eubacterium dolichum and Ruminococcus bromii, and decreased relative abundance of unclassified Paraprevotellaceae (Catanzaro et al., 2019) The clinical relevance of primary humoral immunodeficiencies to OCD symptomatology remains to be established. Further studies are warranted, to determine if these are causally related, and if treatments targeting immunodeficiency syndromes and/or their associated microbial dysbiosis could offer preventive or therapeutic targets for OCD.
Evidence supporting a temporal relationship between pathogenic infections and onset of OCD symptomatology has been mixed, particularly in prospective clinical studies (Leckman et al., 2011; Luo et al., 2004; Murphy and Pichichero, 2002). Larger population studies suggest that Streptococcus infection may be associated not only with PANDAS/PANS, but also with the broader OCD and other psychiatric phenotypes, including obsessive-compulsive personality disorder (OCPD), tic disorders, and/or attention-deficit hyperactivity disorder (ADHD), with streptococcal infection history having the strongest association with incidence of tic disorders (Mell et al., 2005; Wang et al., 2016). In addition, history of non-streptococcal throat infections is associated with increased risk of OCD, tic disorder, or any psychiatric disorder (Orlovska et al., 2017), and in a nationwide pediatric study, any treated infection since birth was associated with increased risk of subsequent treatment for any psychiatric disorder in adolescence, including OCD (Köhler-Forsberg et al., 2019). Associations between multiple non-streptococcal pathogens and onset of OCD symptoms have been suggested, including Borna disease viruses, Borrelia burgdorferi, Herpes simplex virus 1, Mumps orthorubulavirus, Mycoplasma pneumoniae, Toxoplasma gondii, and varicella-zoster virus, with no association with human immunodeficiency viruses (Akaltun et al., 2018; Dietrich et al., 2005; Flegr and Horáček, 2017; Johnco et al., 2018; Khanna et al., 1997b, 1997a; Miman et al., 2010, 2018; Murphy et al., 2015; Sutterland et al., 2015; Ursoiu et al., 2018; Yaramiş et al., 2009). To date, only one study of gut microbial ecology in youth with a history of PANDAS/PANS has been completed (see Table 1). When compared with healthy controls, children aged 4 to 8 years with history of PANDAS/PANS had decreased microbial diversity, along with increased relative abundance of members of the Bacteroidetes phylum (Quagliariello et al., 2018). Youth 9 to 12 years of age did not have evidence of specific microbiome changes when compared with healthy controls, possibly related to age or maturational effects. While children were not exposed to antibiotics in the 2 to 4 months immediately prior to enrollment, history of repeated antibiotic exposure is not uncommon in youth with PANDAS/PANS, and this could also confound microbiome studies in this group (Quagliariello et al., 2018).
Collectively, studies suggest that susceptibility to and/or infection by a diverse set of bacterial and viral pathogens could be associated with at least some cases of OCD, but immunodeficiency syndromes and pathogen exposures might also increase risk for psychiatric disorders more non-specifically. The mechanisms by which immune responses could lead to gradual versus acute onset of OCD symptoms also remain largely unclear, and the role of the gut microbiome in this context remains unknown. If immune-microbiome processes are involved in some cases of pathogen-associated OCD, directions of causation cannot be inferred based on existing knowledge. It might be the case that infection and immune responses shape microbial composition, microbiome dysbiosis may predispose to pathogenic infection and immune dysregulation, or other genetic and environmental factors could influence both host immunity and microbial ecology. Further studies are needed to elucidate potential mechanisms, particularly in the case of acute-onset OCD following pathogenic infection, where immune and microbiome biomarkers could facilitate development of more precise guidelines for use of immunomodulatory and antimicrobial treatment strategies.
4.2. Atopy, Autoimmunity, and Inflammation:
In clinical and epidemiologic studies, OCD has been associated with a diverse set of other immune-mediated processes including autoimmune, inflammatory, and atopic disorders; separately the microbiome has been associated with or causally implicated in several immune-mediated disorders. In a clinical study, significantly higher frequency of asthma, recurrent bronchitis, recurrent sinusitis, arthritis, recurrent infections, and/or recurrent diarrheal illness was seen in adults with OCD compared to controls with other psychiatric diagnoses (Dinn et al., 2005). Children and adolescents with OCD with and without Tourette’s syndrome (TS) also had significantly higher rates of atopic disorders such as asthma, allergic rhinitis, and eczema compared to controls (Yuce et al., 2014). Among individuals with childhood-onset OCD, frequency of ear and throat infections has been positively correlated with severity of contamination-related OCD symptoms (Westwell-Roper et al., 2019).
Population-based studies have also addressed the relationship between OCD and immune dysregulation. In a Swedish birth cohort study, OCD and tic disorders were associated with significantly increased risk of any immune-mediated disorder in probands and their relatives, when compared to population controls (Mataix-Cols et al., 2018). In the OCD group, the strongest associations were found (in order of strength of association) with Sjogren’s syndrome, celiac disease, Guillain-Barre syndrome, Crohn’s disease, Hashimoto’s thyroiditis, diabetes mellitus type I (DM I), scarlet fever, idiopathic thrombocytopenic purpura (ITP), ulcerative colitis (UC), multiple sclerosis (MS), and psoriasis vulgaris (Mataix-Cols et al., 2018). In a population-based cohort study in Taiwan, any immune-mediated disorder was associated with higher incidence of subsequent OCD diagnosis, and risk of developing OCD was greatest for individuals with dermatomyositis, Sjogren’s syndrome, and systemic lupus erythematosus (SLE) (Wang et al., 2019). Notably, history of immunosuppressive steroid treatment for the immune-mediated disorder was protective against OCD development, further indicating the role of significant immune activation as a pathogenic mechanism in OCD (Wang et al., 2019). Associations have also been identified with OCD and increased metabolic and cardiovascular comorbidity, thus expanding the range of inflammatory processes in OCD (Isomura et al., 2018).
OCD is not only associated with individual risk of immune-mediated disease, but first-degree relatives of probands with OCD also have increased odds of any immune or inflammatory disorder (Mataix-Cols et al., 2018). Among mothers, fathers, and full siblings of individuals with OCD, odds of immune-mediated disease were similar. However, in first-degree relatives of probands with tic disorders, which are frequently comorbid with OCD, risk for immune-mediated disorder was greatest for mothers (Mataix-Cols et al., 2018), suggesting maternal immune factors could be particularly relevant to offspring with OCD and comorbid tics. Indeed, in a clinical study of pediatric OCD and tic disorders, prevalence of both confirmed maternal autoimmune disease and maternal pro-inflammatory state (e.g., asthma, smoking) were significantly greater in the OCD/tic group compared to both neurological autoimmune and healthy control groups (Jones et al., 2021). Further, mothers with immune-mediated disorders and children with OCD/tics had evidence of peripheral cytokine network dysregulation and enriched transcription of genes related to innate immune function (e.g., neutrophil degranulation, toll-like receptor signaling), when compared to control mothers (Jones et al., 2021).
While immune-microbiome studies in OCD are in their infancy, microbiome changes have been implicated in several immune-mediated disorders associated with OCD risk in humans (see Table 3). Microbiome correlates of various immune-mediated disorders have been extensively reviewed elsewhere (Fitzgibbon and Mills, 2020; Peroni et al., 2020; Trøseid et al., 2020; Xuan Zhang et al., 2020), and Table 3 is therefore not exhaustive. Taken together, clinical and epidemiologic associations between OCD and several immune-mediated processes, in which the microbiome has been separately implicated, provide indirect support for further characterizing host immune and microbiome interactions in OCD. However, as illustrated in Table 3, OCD has been associated with a heterogeneous set of immunologic disorders, which have partially overlapping but distinct pathogenic mechanisms and microbiome correlates. It may also be the case that not only personal but also family history of immune-mediated disorders, of maternal versus paternal origin, could influence microbiome composition and neuropsychiatric symptomatology differently, but this remains unclear. It will therefore be important for future investigations to disentangle immune-microbiome relationships in homogeneous subgroups of OCD with and without personal and family history of autoimmune, inflammatory, and atopic comorbidities.
Table 3. Immune and microbiome features of select immune-mediated disorders associated with OCD risk.
IBD = inflammatory bowel disease; UC = ulcerative colitis; CD = Crohn’s disease; OR = odds ratio; CI = confidence interval; Th1 = T-helper 1 cell; Th17 = T-helper 17 cell; Treg = T-regulatory cell; IFN = interferon; IL = interleukin; NK = natural killer; TNF = tumor necrosis factor; BAFF = B-cell activating factor; SSA = Sjogren’s syndrome-associated antigen-A; SSB = Sjogren’s syndrome-associated antigen-B; RF = rheumatoid factor; Th2 = T-helper 2 cell; Ig = immunoglobulin; HR = hazard ratio; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate.
| Immune-Mediated Disorder | Associations with OCD | Host Immune Features | Gut Microbiome Features |
|---|---|---|---|
| Autoinflammatory disorders (e.g. IBD, UC, CD) |
OCD: ↑ CD risk (OR 1.66, 95% CI 1.44–1.91) and ↑ UC risk (OR 1.41, 95% CI 1.24–1.59) (Mataix-Cols et al., 2018) | IBD: ↑ Th1, ↑ Th17, ↓ Treg, ↑ IFNγ, ↑ IL-17, ↑ IL-22 (Friedrich et al., 2019) | IBD: ↓ Roseburia hominis, ↓ Escherichia coli (Lloyd-Price et al., 2019); ↓ Ruminococcus torques, ↓ Ruminococcus gnavus, ↓ Prevotella copri, ↓ Faecalibacterium prauznnitzii (Halfvarson et al., 2017; Rajca et al., 2014; Sokol et al., 2008) |
| Autoimmune disorders (e.g. Sjogren’s syndrome) |
OCD: ↑ Sjogren’s syndrome risk (OR 1.94, 95% CI 1.41–2.66) (Mataix-Cols et al., 2018); Sjogren’s syndrome also associated with ↑ OCD risk (adjusted HR 2.38, 95% CI 1.53–3.72) (Wang et al., 2019) | Sjogren’s: ↑ Th1, ↑ Th17, ↑ NK cells, ↑ IL-1, ↑ IL-2, ↑ IL-6, ↑ IL-10, ↑ IL-17, ↑ IFNγ, ↑ TNFα (García-Carrasco et al., 2006); ↑ BAFF, +anti-SSA/Ro, +SSB/La antibodies, +RF (Nocturne & Mariette, 2018) | Sjogren’s: ↓ Romboutsia, ↓ Turicibacter, ↓ Actinomyces, ↓ Slackia, ↓ Enterorhabdus, ↓ Senegalimassalia, ↓ Parasutterella, ↑ Alistipes, ↑ Lachnoclostridium, ↑ Barnesiella, ↑ Lachnospira, ↑ Bacteroides vulgatus, uniformis, ovatus (van der Meulen et al., 2019); ↑ Veillonella, ↓ Blautia, ↓ Dorea, ↓ Bifidobacterium, ↓ Agathobacter (Moon et al., 2020) |
| Atopic disorders (e.g. Eczema) |
OCD: ↑ Frequency of atopic disorders (p=0.018) and eczema (p=0.03) compared to controls (Yuce et al., 2014) | Eczema: ↑ Th2, ↑ IgE, ↑ IL- 4, ↑ IL-5, ↑ IL-13, ↑ IL-17, ↑ IL-22, ↑ IL-31 (Boguniewicz & Leung, 2011; Kuo et al., 2013; Leung, 2013) | Eczema: ↑ Clostridium difficile (Penders et al., 2007); ↑ Faecalibacterium, ↑ Oscillospira, ↑ Bacteroides, ↑ Parabacteroides, ↑ Sutterella, ↓ Bifidobacterium, ↓ Blautia, ↓ Coprococcus, ↓ Eubacterium, ↓ Propionibacterium (Reddel et al., 2019); ↑ Escherichia coli, ↑ Klebsiella pneumoniae, ↓ Bacteroides fragilis (Ta et al., 2020); ↓ Lachnobacterium, ↓ Faecalibacterium (Galazzo et al., 2020) |
| Chronic inflammatory states (e.g. Obesity) |
OCD: ↑ Cardiometabolic disease risk, including obesity (adjusted HR 1.45, 95% CI 1.42–1.49) (Isomura et al., 2018) | Obesity: ↑ CRP (Choi et al., 2013); ↑ ESR (De Rooij et al., 2009); ↑ TNFα, ↑ IL-6 (Bahceci et al., 2007; Marques-Vidal et al., 2012); ↓ NK cell activity (Michelet et al., 2018) | Obesity: ↑ Lactobacillus reuteri, ↓ Bifidobacterium animalis, ↓ Methanobrevibacter smithii (Million et al., 2012); ↓ Bacteroides, ↑ Prevotella (Hu et al., 2015); ↑ Blautia hydrogenotorophica, ↑ Coprococcus catus, ↑ Eubacterium ventriosum, ↑ Ruminococcus bromii, ↑ Ruminococcus obeum (Kasai et al., 2015); ↓ Akkermansia muciniphila (Dao et al., 2019) |
(Bahceci et al., 2007; Boguniewicz and Leung, 2011; Choi et al., 2013; Dao et al., 2019; De Rooij et al., 2009; Friedrich et al., 2019; Galazzo et al., 2020; García-Carrasco et al., 2006; Halfvarson et al., 2017; Hu et al., 2015; Isomura et al., 2018; Kasai et al., 2015; Kuo et al., 2013; Leung, 2013; Lloyd-Price et al., 2019; Marques-Vidal et al., 2012; Mataix-Cols et al., 2018; Michelet et al., 2018; Million et al., 2012; Moon et al., 2020; Nocturne and Mariette, 2018; Penders et al., 2007; Rajca et al., 2014; Reddel et al., 2019; Sokol et al., 2008; Ta et al., 2020; van der Meulen et al., 2019; Wang et al., 2019; Yuce et al., 2014)
5. Immune Parameters, the Microbiome, and OCD
The gut microbiome has been shown to participate in immune development and homeostasis, largely in animal models, (Belkaid and Hand, 2014; Belkaid and Harrison, 2017; Chen and Stappenbeck, 2019; Robertson et al., 2018), and relationships between gut microbial composition and peripheral immune parameters are starting to be elucidated in humans (Schirmer et al., 2016). In individuals with OCD, peripheral immune biomarkers have been an area of ongoing investigation, but findings are not widely replicated, and at times contradictory (Cosco et al., 2019; Lamothe et al., 2018), likely due to significant heterogeneity in patient samples and methodologies. Despite this, existing findings suggest there might be overlap in some of the immune parameters separately associated with microbiome changes, and with OCD symptomatology (see Table 4).
Table 4. Select immune parameters which are separately associated with OCD, and with gut microbial composition changes in animals or humans.
OCD = obsessive-compulsive disorder; CSTC = cortical-striatal-thalamic-cortical; PET = positron emission tomography; HoxB8 = homeobox B8; GF = germ-free; MDD = major depressive disorder; NK = natural killer; iNKT = invariant natural killer T-lymphocyte; Th = T-helper lymphocyte; SFB = segmented filamentous bacteria; Treg = regulatory T-lymphocyte; IgA = immunoglobulin A; IL = interleukin; IFN = interferon; TNF = tumor necrosis factor; CNS = central nervous system; SC = Sydenham chorea; PANDAS = pediatric autoimmune neuropsychiatric disorders associated with streptococcus; PANS = pediatric acute-onset neuropsychiatric syndrome; MS = multiple sclerosis; EAE = experimental autoimmune encephalomyelitis.
| Microglia | - Adults (human) with OCD evidence microglial activation in brain CSTC circuitry structures on PET imaging (Attwells et al., 2017) - Loss of HoxB8-lineage microglia function is associated with obsessive-compulsive behaviors in mice (Chen et al., 2010; Tränkner et al., 2019) |
- GF and antibiotic-treated mice have abnormal microglial phenotype (Erny et al., 2015) - Gut microbiome influences on microglia are sex-specific in mice (Thion et al., 2018) |
| Neutrophils | - Adults (human) with OCD have decreased neutrophil count compared to healthy controls (Atmaca et al., 2011) | - Maternal antibiotic exposure associated with less abundant gut microbiota, decreased neutrophil count, and increased susceptibility to infection in mice offspring (Deshmukh et al., 2014) |
| Natural killer cells | - Adult (human) males with MDD and OCD demonstrate increased circulating NK cells, which normalizes with treatment in MDD but not in OCD (Ravindran et al., 1999) - Adults (human) with OCD have decreased ex vivo NK cell activity (Denys et al., 2004) |
- GF animals have increased accumulation of iNKT cells, and increased susceptibility to inflammatory bowel and respiratory pathologies (Olszak et al., 2014) |
| Th17 cells | - Pediatric (human) OCD is associated with increased frequency of Th17 cells, which is correlated with symptom severity and disease duration (Rodríguez et al., 2019) | - Certain microbes, such as SFB, promote Th17 differentiation in mice (Lécuyer et al., 2014) |
| Treg cells | - Pediatric (human) OCD is associated with decreased frequency of Treg cells, and Treg frequency is negatively correlated with disease duration (Rodríguez et al., 2019) | - Certain microbes, such as Bacteroides species, promote Treg differentiation in mice (Faith et al., 2014) |
| IgA | - Children/adolescents (human) with OCD have decreased IgA concentration compared to adults with OCD, and compared to pediatric psychiatric controls (Williams et al., 2019) - Selective IgA deficiency associated with increased odds of OCD in human population- based study (Isung et al., 2020) |
- Microbiota promote expansion of IgA- producing plasma cells in mice (Lécuyer et al., 2014) - Secretory IgA participates in host- microbiome homeostasis (Pabst et al., 2016; Yang and Palm, 2020) |
| Cytokines and chemokines | - Meta-analysis of cytokine studies found no difference in IL-1β, IL-4, IL-6, IL-10, IFNγ, or TNFα in OCD (human) compared to controls (Cosco et al., 2019) - Smaller studies in homogeneous subgroups suggest possible differences (Karagüzel et al., 2019; Şimşek et al., 2016) |
- Microbially-derived metabolites are associated with host cytokine production capacity in humans (Schirmer et al., 2016) |
| CNS-reactive autoantibodies | - OCD (human) associated with CNS-reactive autoantibody positivity (Bhattacharyya et al., 2009; Cox et al., 2015; Dale et al., 2005; Pearlman et al., 2014) | - Microbiota are necessary for autoantibody production in animal models of MS (EAE) (Berer et al., 2011; Petta et al., 2018) |
(Atmaca et al., 2011; Attwells et al., 2017; Berer et al., 2011; Bhattacharyya et al., 2009; Chen et al., 2010; Cosco et al., 2019; Cox et al., 2015; Dale et al., 2005; Denys et al., 2004; Deshmukh et al., 2014; Erny et al., 2015; Faith et al., 2014; Isung et al., 2020; Karagüzel et al., 2019; Lécuyer et al., 2014; Olszak et al., 2014; Pabst et al., 2016; Pearlman et al., 2014; Petta et al., 2018; Ravindran et al., 1999; Rodríguez et al., 2019; Schirmer et al., 2016; Şimşek et al., 2016; Thion et al., 2018; Tränkner et al., 2019; Williams et al., 2019; Yang and Palm, 2020)
5.1. Innate Immune Parameters:
The innate arm of the immune system represents the first line of defense against pathogens, and is in constant bidirectional communication with microbiota at epithelial barriers (Thaiss et al., 2016). A diverse microbiome is required for normal innate immune development, as evidenced by GF status and antibiotic exposure leading to impaired myelopoiesis and granulocyte homeostasis in pre-clinical models (De Agüero et al., 2016; Deshmukh et al., 2014; Khosravi et al., 2014). The function of tissue-resident macrophages including microglia is also altered in GF mice (Erny et al., 2015). Genetic changes in components of the innate immune system, such as PRR polymorphisms, can also predispose mice to both microbial dysbiosis and increased risk of inflammatory sequelae (Elinav et al., 2011; Vijay-Kumar et al., 2010).
Microglia are the resident immune cells of the brain, and a diverse microbiome is essential for normal microglia development and function in animal models (Erny et al., 2017; Lebovitz et al., 2018). In human OCD, microglial activation is a potential CNS biomarker (e.g., neuroinflammation) (Attwells et al., 2017). Changes in peripheral innate immune cell counts and function have also been identified in human OCD, including lower neutrophil counts (Atmaca et al., 2011), and also higher natural killer (NK) cell counts, but only in males (Ravindran et al., 1999). Medication-free adults with OCD had decreased ex vivo NK cell activity when compared to the control group, with no differences in NK cell count (Denys et al., 2004). In the same study, NK cell activity was associated with familial and childhood-onset OCD, with lower NK cell cytotoxic activity compared with adult-onset OCD (Denys et al., 2004). Pediatric OCD has been associated with a significantly higher proportion of total and intermediate monocytes compared to healthy controls (Rodríguez et al., 2017). The OCD group did not show any difference in cultured monocytes’ basal cytokine production or sensitivity to an anti-inflammatory stimulus (e.g., dexamethasone), but ex vivo stimulation of monocyte cultures with lipopolysaccharide (LPS) was associated with significantly increased inflammatory cytokine production in the OCD group. The study further demonstrated that youth taking psychotropic medication for OCD had a lesser degree of ex vivo inflammatory cytokine release, compared with unmedicated OCD youth (Rodríguez et al., 2017).
Taken together, the literature suggests that markers of altered innate immune function may be associated with OCD but could be specific to different OCD subgroups (e.g., male versus female, adult versus pediatric). Gut microbiota have complex relationships with the innate immune system, but these have largely been established in animal studies. The degree to which the microbiome could be a possible source of innate immune dysregulation in human OCD is therefore unknown. Existing evidence suggests that examining microbiome and immune parameters concurrently, in homogeneous human OCD populations, will be necessary to elucidate any potential associations.
5.2. Adaptive Immune Parameters:
The adaptive arm of the immune system serves to provide long-term protection against specific pathogens through development of immune memory. Lymphoid-derived T- and B-lymphocytes comprise the cellular components of the adaptive immune system, and B-cells mediate humoral immunity through antibody production. As with the innate immune system, adaptive immunity is in part shaped by the microbiome, and intact adaptive immune function is also required to control microbiota (Belkaid and Hand, 2014; Belkaid and Harrison, 2017). Relationships have been identified between specific microbes and adaptive immune processes (Geva-Zatorsky et al., 2017). For example, in animal studies, segmented filamentous bacteria (SFB) promote differentiation of T-helper 17 (Th17) cells and immunoglobulin A (IgA) production (Lécuyer et al., 2014), while Bacteroides and other species support differentiation of regulatory T-cells (Tregs) (Faith et al., 2014). However, understanding of microbiome relationships with adaptive immunity are largely gleaned from pre-clinical models, and caution is therefore required in translating this to humans, where such associations remain less well elucidated.
In human OCD, cellular components of the adaptive immune system have been examined, and adults with OCD had significantly increased CD8+ T-cells and decreased CD4+ T-cells compared to healthy controls, while no other cell counts differed between groups (Marazziti et al., 1999). Other studies have failed to demonstrate differences in T- or B-cell subsets between OCD and control groups (Barber et al., 1996; Denys et al., 2004). Most recently, children and adolescents with OCD were shown to have normal distribution of Th1 and Th2 lymphocyte subsets compared to healthy controls, but greater frequency of pro-inflammatory Th17 lymphocytes compared to healthy controls (Rodríguez et al., 2019). Youth with OCD also had lower frequency of Tregs, which play an anti-inflammatory function. Th17 level positively correlated with both symptom severity and disease duration, while Treg level was negatively correlated with disease duration (Rodríguez et al., 2019).
Investigations of humoral adaptive immunity in OCD have primarily centered on the search for autoantibodies, as discussed in a following section ‘Brain Autoantibodies.’ More recently however, immunoglobulin profiles have been investigated, and groups have reported selective IgA deficiency in pediatric OCD, tic disorders, and PANDAS/PANS (Bos-Veneman et al., 2011; Frankovich et al., 2015; Kawikova et al., 2010). In a medical record review study of youth and adults with OCD compared to medical and psychiatric controls, youth with OCD were significantly more likely to demonstrate decreased IgA concentration compared to adults with OCD, and compared to youth with ASD and anxiety disorders (Williams et al., 2019). Pediatric OCD was associated with a comparable rate of decreased IgA concentration when compared to the celiac disease group, with celiac disease being a disorder in which IgA deficiency is a recognized feature (Williams et al., 2019).
Available but limited evidence therefore suggests changes in adaptive immunity could be of relevance to OCD, with some parameters possibly having associations with specific subgroups of OCD. For example, adaptive immune parameter changes in OCD, such as increased Th17 frequency and decreased Treg frequency have been demonstrated in pediatric OCD, but not explored in adult OCD (Rodríguez et al., 2019), while decreased IgA concentration was present in youth but not adults with OCD, and was most pronounced in male youth (Williams et al., 2019). The microbiota has been shown to communicate with adaptive immune parameters in animal models; however, adaptive immune-microbiome relationships in human OCD remain to be investigated.
5.3. Cytokines and Inflammation:
Cytokines and chemokines are the primary proteins involved in immune signaling and can be pro-inflammatory or anti-inflammatory depending on the context. Cytokines are also involved in neuroimmune signaling, and abnormal cytokine profiles have been associated with a number of clinical neuropsychiatric disorders (Dantzer, 2018; Khandaker et al., 2017). Cytokine production is influenced by a number of factors including age, sex, and genetics, and microbiome function also accounts for some inter-individual differences in human cytokine profiles (Schirmer et al., 2016). Specific microbially-derived metabolites, such as tryptophol and palmitoleic acid, for example, are associated with decreased interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) production, respectively (Schirmer et al., 2016).
Several groups have sought to characterize cytokine and chemokine profiles in clinical OCD samples, though results have been inconclusive. In a meta-analysis of 12 studies of interleukin 1 beta (IL-1β), IL-6, and TNFα in OCD, IL-1β levels were significantly decreased in OCD compared to controls with no differences in IL-6 or TNFα concentrations (Gray and Bloch, 2012). A more recent meta-analysis of 14 studies examining IL-1β, IL-4, IL-6, IL-10, IFNγ, and TNFα in OCD found no differences in any of the parameters in the OCD group compared to controls (Cosco et al., 2019). Similarly, in a meta-analysis of immune biomarkers in pediatric anxiety disorders, including OCD, no significant differences were demonstrated (Parsons et al., 2020). Significant heterogeneity likely limits such meta-analyses, as individual studies vary in terms of the OCD phenotype studied (pediatric versus adult), participants’ psychotropic medication status, inclusion and exclusion of psychiatric comorbidities, reporting of factors known to affect inflammatory markers such as body mass index (BMI), time of sample collection, and immunoassay methods. OCD subtype may also be related to variability in cytokine profiles. To this end, higher TNFα, IL-1β, and IL-6 concentrations in medication-exposed adult outpatients with OCD were higher than healthy controls, and further, IL-1β concentration was significantly higher in the “reactive” OCD subtype group compared to the “autogenous” OCD subtype group (Karagüzel et al., 2019), supporting the notion that phenotypic heterogeneity in OCD likely contributes to discrepant findings in cytokine and chemokine studies.
Taken together, changes in immune biomarkers might be associated with at least some cases of OCD, but most results have not been replicated and the implications of peripheral immune abnormalities for psychopathology are not fully understood. The cause of these immune abnormalities is also unknown, and microbiome studies are needed to determine if microbial composition or function contributes to immune dysregulation in OCD. If replicated, findings in pediatric OCD, such as increased peripheral IL-17a concentration (Şimşek et al., 2016), increased Th17 cell frequency, and decreased Tregs (Rodríguez et al., 2019), suggest specific types of microbes such as SFB might be relatively more abundant in OCD, while Prevotella species might be relatively less abundant (Mangalam et al., 2017). The age- and sex-specific nature of some immune abnormalities in OCD, such as increased frequency of selective IgA deficiency in pediatric but not adult OCD (Williams et al., 2019), and increased NK cell frequency in adult males but not females (Ravindran et al., 1999), also suggest microbiome correlates in OCD could vary depending on age and biologic sex. In neuroimaging studies of OCD, developmentally- and sex-specific biomarkers have been identified, such as increased thalamic volume in pediatric but not adult OCD (Boedhoe et al., 2017), and decreased pituitary volume which is most pronounced in males with pediatric OCD (MacMaster et al., 2006). It is therefore likely that, looking forward, large studies with homogeneous samples will be needed to identify subtypes of OCD for which specific immune-microbiome correlates are potentially relevant.
6. Toward Immune-Microbiome-Brain Mechanisms in OCD
6.1. Brain Autoantibodies:
Autoantibodies which are cross-reactive with streptococcal antigens and basal ganglia underlie the onset of neuropsychiatric symptoms in the context of SC (Ben-Pazi et al., 2013; Dale et al., 2012, 2006; Doyle et al., 2012; Kirvan et al., 2007, 2003). Similar processes of molecular mimicry have been proposed to underlie PANDAS/PANS, but findings of autoantibodies in PANDAS/PANS are less consistent (Brilot et al., 2011; Dale et al., 2012; Frick et al., 2018; Kirvan et al., 2006; Loiselle et al., 2004; Morer et al., 2008; Pavone et al., 2004; Singer et al., 2005; Xu et al., 2020). Idiopathic OCD is also associated with anti-neuronal antibody positivity (Bhattacharyya et al., 2009; Cox et al., 2015; Dale et al., 2005; Pearlman et al., 2014), thus calling into question the specificity of the proposed association between PANDAS/PANS and brain autoantibodies.
Gut microbial composition may be of relevance to cases of OCD in which autoantibody production is suspected, as the microbiome has been shown to be necessary for autoantibody production in animal models of autoimmunity, such as experimental autoimmune encephalitis (EAE) (Berer et al., 2011; Petta et al., 2018). Commensal microbial peptides may also share sequence homology with autoantigens, and therefore participate in autoimmune processes via molecular mimicry (Xuan Zhang et al., 2020). However, a potential role for the microbiome in OCD symptoms associated with autoimmunity remains speculative.
6.2. Neuroinflammation:
Aside from autoimmunity, neuroinflammation is increasingly recognized as pathogenic in psychiatric disorders (Dantzer, 2018; Khandaker et al., 2017). Inflammatory cytokines, for example, from the periphery can influence brain and behavior, and are also produced by glial cells in the CNS (Dantzer et al., 2008; Miller and Raison, 2016). While findings in regard to circulating cytokine concentrations in individuals with OCD have been mixed (Cosco et al., 2019; Gray and Bloch, 2012; Parsons et al., 2020), genetic studies have suggested TNFα polymorphisms (Cappi et al., 2012; Hounie et al., 2008; Jiang et al., 2018) and single nucleotide variants (SNVs) in genes related to transforming growth factor beta (TGFβ) signaling are associated with risk for OCD (Cappi et al., 2016). Gut microbiome-derived metabolic pathways have also been shown to Influence cytokine production capacity in humans (Schirmer et al., 2016). Complex host immunogenetic-microbiome relationships may therefore mediate the pathogenesis of OCD, but this requires further investigation.
Trafficking of immune cells into the CNS is another means by which peripheral immunity can influence behavior. Monocytes, for example, can cross the BBB under circumstances of stress or inflammation and propagate neuroinflammation (Wohleb et al., 2015). Auto-reactive Th17 lymphocytes can also enter the CNS, and have been shown to induce obsessive-compulsive behaviors in a mouse model of MS, namely EAE (Kant et al., 2018). Female mice affected with chronic EAE following myelin oligodendrocyte glycoprotein (MOG) immunization exhibited increased grooming behavior, marble burying, and nestlet shredding early in their disease course. While adoptive transfer of both Th1 and Th17 cells could induce demyelination, only Th17 cell transfer was associated with onset of obsessive-compulsive behaviors, which were successfully treated with either Th17 depletion or fluoxetine (Kant et al., 2018). Separately, GF rearing and antibiotic treatment have been shown to be protective against development of EAE in mice by attenuating microbially-driven Th17 responses (Lee et al., 2011; Ochoa-Repáraz et al., 2009). Taken together, immune-microbiome studies in the context of EAE suggest that microbiome-dependent expansion and infiltration of auto-reactive Th17 cells, among other activated immune cells, into the CNS may be a novel neuroimmune mechanism of relevance to OCD symptomatology.
6.3. Microglial Dysregulation:
Recently, attention has also been given to microglial dysregulation for its role in the pathogenesis of OCD (Frick and Pittenger, 2016). Microglia, the resident macrophages of the brain, arise from yolk sac-derived progenitors during embryonic development and may have more heterogeneous developmental trajectories and functions than previously recognized (De et al., 2018). Microglia are required for multiple aspects of normal neurodevelopment and synaptic plasticity, and the microbiome is associated with microglial development and functioning throughout the lifespan in pre-clinical models (Lebovitz et al., 2018). In humans with OCD, a positron emission tomography (PET) neuroimaging study demonstrated increased microglial activation in CSTC structures in adults with OCD compared to healthy controls (Attwells et al., 2017), and in mice, loss of homeobox B8 (Hoxb8)-lineage microglia function causes obsessive-compulsive symptoms (Nagarajan et al., 2018). Interestingly, the Hoxb8-null behavioral phenotype is marked by excessive self-grooming in both males and females, but increased anxiety only in female mice starting at sexual maturity (Chen et al., 2010; Tränkner et al., 2019). Abnormalities in CSTC have been identified in Hoxb8-null mice, which are consistent with those found in OCD, and treatment with fluoxetine has also demonstrated efficacy in mitigating the Hoxb8-null phenotype (Nagarajan et al., 2018). Taken together, these studies suggest that microglial dysregulation in OCD warrants further study. Microglial loss of function, as is the case in Hoxb8-null mice, and activation, as demonstrated by PET imaging findings in human adults with OCD, could be of relevance to OCD symptomatology. Further understanding of separate microglial subsets, with differential anatomic locations and specializations, separate but overlapping critical periods of development, and differential responses to the effects of gonadal hormones, could also help account for the sexual dimorphism and developmentally specific features of OCD in humans.
In pre-clinical models, it is increasingly recognized that the microbiome is essential for normal microglia development and function (Lebovitz et al., 2018); changes in microbiome composition may therefore represent a means by which microglial dysregulation can develop. In adulthood, GF mice have an increase in microglial density and rate of proliferation, but an immature phenotype characterized by impaired responses to viral and bacterial challenges (Erny et al., 2015). A similar immature microglial phenotype could also be induced with antibiotic treatment of adult mice (Erny et al., 2015). Microbiome-associated microglial dysregulation may also manifest differently depending on developmental stage and biologic sex. For example, both male and female GF mice exhibit increased microglial density, but this is more pronounced in utero in males, and more so in adulthood in females (Thion et al., 2018). Further, while both male and female GF mice had altered microglial transcriptomic signatures, this manifested earlier in development for male compared to female GF mice, and differentially expressed genes were also sexually dimorphic (Thion et al., 2018). Treatment of non-GF mice with antibiotics in adulthood was not associated with altered microglial colonization, but with changes in microglia transcriptomic signatures, most markedly in adult male mice (Thion et al., 2018). Taken together, studies in mice demonstrate that both genetic and environmental insults can alter microglial function, resulting in different developmental trajectories depending on timing of the insult, developmental stage, and host sex.
In humans, early-onset OCD is more frequently associated with male sex and tic-related comorbidity, while later-onset OCD has a more equal sex distribution (Taylor, 2011); the developmentally-specific sexual dimorphism of microglial dysregulation seen in pre-clinical studies therefore offers potential for compelling translational models. However, while the Hoxb8-null mouse is a valid genetic model of microglial dysfunction leading to OCD-like behavior, analogous genetic associations have not been identified in human OCD, and Hoxb8-null mice have physical deformities not observed in humans with OCD (Chen et al., 2010). Microbial dysbiosis may lead to microglial dysregulation in animal models, but whether or not this could also lead to an OCD-related phenotype remains less clear. To this end, studies have demonstrated that animals exposed to early microbiome manipulations, such as GF rearing or maternal high-fat diet, can subsequently develop compulsive behaviors, including increased self-grooming and marble burying (Bruce-Keller et al., 2017; Desbonnet et al., 2014). Impaired fear extinction learning is also a feature of human OCD (Milad et al., 2013), and both GF and antibiotic-treated adult mice have normal fear acquisition, but impaired fear extinction learning (Chu et al., 2019). This impaired fear extinction was associated with immature microglial morphology, defective dendritic spine remodeling, and decreased expression of the post-synaptic density protein-95 (PSD-95) in medial prefrontal cortex (mPFC) (Chu et al., 2019). GF mice have increased PSD-95 expression also in striatum (Heijtz et al., 2011). SAP90/PSD95-associated protein 3 (SAPAP3) is a post-synaptic scaffolding protein at glutamatergic synapses previously implicated in human genetic studies of OCD (Bienvenu et al., 2009; Boardman et al., 2011), and SAPAP3-null mice are an existing animal model of OCD (Welch et al., 2007). Finally, normal fear extinction learning could be restored in ex-GF mice by recolonization with a diverse microbiota only if recolonization occurred immediately after birth, but not at weaning or in adulthood (Chu et al., 2019). In summary, in pre-clinical models, early microbiome composition and function are relevant not only for long-term microglial function, but also for normal excitatory signaling and synaptic pruning in the mPFC and striatum, and for normal fear extinction learning, all of which have been implicated in OCD. Microbiome programming of microglia therefore represents a novel area of research with promise for elucidating the mechanisms underlying immune-mediated and environmental influences in the pathophysiology of OCD symptomatology.
7. Future Directions
Further studies of host immunity and microbiota in OCD are warranted, and a translational systems biology approach will be necessary for realizing the clinical impact of such investigations. Animal models of microbial manipulation at multiple developmental timepoints (e.g., GF rearing, antibiotic treated, maternal high-fat diet) are likely relevant to certain symptom domains in OCD, and microbiome analyses in existing genetic and behavioral animal models of OCD are also thus far under-explored (see Table 5). In humans, gut-brain-immune axis dysregulation is a plausible etiopathogenic mechanism in neuropsychiatric disorders, but thus far this is only indirectly supported. Therefore, more patient-oriented studies are critically needed, in which immune and microbiome parameters are systematically investigated together, while also connecting the dots with and among existing genetic, neuroimaging, neuropsychological, and other clinical findings, in order to fully understand complex gut-brain-immune relationships in OCD.
Table 5. Animal models with translational potential for immune-microbiome investigations in OCD symptomatology.
GF = germ-free; PSD-95 = post-synaptic density protein 95; mPFC = medial prefrontal cortex; iNKT = invariant natural killer T-lymphocytes; SRI = serotonin reuptake inhibitor; MS = multiple sclerosis; EAE = experimental autoimmune encephalomyelitis; MOG = myelin oligodendrocyte glycoprotein; Th17 = T-helper 17 cells; HoxB8 = homeobox B8; CSTC = cortical-striatal-thalamic-cortical.
| Animal model | Obsessive-compulsive features | Immune parameters | Microbiome correlates |
|---|---|---|---|
| Microbial manipulations | GF and maternal high-fat diet-exposed mice have increased self-grooming and marble burying (Bruce-Keller et al., 2017; Desbonnet et al., 2014); GF mice have impaired fear-extinction learning, decreased expression of PSD-95 in mPFC (Chu et al., 2019), and increased PSD-95 expression in striatum (Heijtz et al., 2011) |
GF animals have increased accumulation of iNKT cells, increased susceptibility to inflammatory bowel and respiratory pathologies (Olszak et al., 2014), and impaired myelopoiesis and granulocyte homeostasis (De Agüero et al., 2016; Deshmukh et al., 2014; Khosravi et al., 2014); GF and antibiotic-treated mice have sex-specific microglial changes (Erny et al., 2015; Thion et al., 2018) |
GF animals are raised in a sterile environment without microbial exposures and therefore lack a microbiome, while antibiotic-treated mice demonstrate gut microbiome composition changes which are dependent on the type of antibiotic (Luczynski et al., 2016); in utero exposure to maternal high-fat diet is associated with decreased relative abundances of members of the Firmicutes phylum (Bruce-Keller et al., 2017) |
| Large nest- building (LNB) mice | LNB mice naturally develop obsessive-compulsive behaviors including stereotypy and large nest-building, which are ameliorated by SRI treatment (Wolmarans et al., 2016) | Unknown | LNB and normal nest-building mice have significantly different gut microbial composition, and decreased relative abundances of Prevotella and Anaeroplasma (Scheepers et al., 2019) |
| Animal models of MS (EAE) | Following MOG immunization, and early in demyelinating disease course, female mice demonstrate increased grooming behavior, marble burying, and nestlet shredding (Kant et al., 2018) |
Auto-reactive Th17 cells (to MOG) cause obsessive-compulsive behaviors in female mice, and effects are attenuated with SRI treatment or Th17 depletion (Kant et al., 2018) |
GF-rearing and antibiotic treatment are associated with attenuated Th17 responses in animal models of EAE (Lee et al., 2011; Ochoa-Repáraz et al., 2009) |
| HoxB8-null mice | Male and female HoxB8-null mice have excessive selfgrooming, females have increased anxiety at sexual maturity, both sexes have CSTC circuit abnormalities, and the phenotype is ameliorated with SRI treatment (Chen et al., 2010; Nagarajan et al., 2018; Tränkner et al., 2019) |
Mice have loss of HoxB8-lineage microglial function (Chen et al., 2010; Nagarajan et al., 2018; Tränkner et al., 2019) |
Unknown |
(Bruce-Keller et al., 2017; Chen et al., 2010; Chu et al., 2019; De Agüero et al., 2016; Desbonnet et al., 2014; Deshmukh et al., 2014; Erny et al., 2015; Heijtz et al., 2011; Kant et al., 2018; Khosravi et al., 2014; Lee et al., 2011; Luczynski et al., 2016; Nagarajan et al., 2018; Ochoa-Repáraz et al., 2009; Olszak et al., 2014; Scheepers et al., 2019; Thion et al., 2018; Tränkner et al., 2019; Wolmarans et al., 2016)
Several potential confounders will need to be taken into account in such investigations. In epidemiologic studies, OCD has been associated with diverse immune-mediated processes, which have distinct but partially overlapping influences on immune and microbiome parameters. OCD is also frequently comorbid with other neurodevelopmental and psychiatric disorders, including tic disorders, attention-deficit hyperactivity disorder, anxiety, and depressive disorders (Janowitz et al., 2009; Politis et al., 2017; Ruscio et al., 2010), many of which have separately been associated with diverse immune abnormalities and/or microbiome changes (Halverson and Alagiakrishnan, 2020; Leckman and Vaccarino, 2015; Martino et al., 2020; Yuan et al., 2019). Several types of comorbidity will therefore need to be taken into account in studies of human OCD of a large sample. Within the OCD phenotype, there is also significant heterogeneity, which has contributed to mixed and at times contradictory findings in immunologic studies, and the extent to which this will influence microbiome studies remains unknown but could be significant. In both animal models and human studies, careful attention to biologic sex and age will be necessary, as sexual dimorphism and developmental effects are observed in features of immunity, microbial colonization, and in pre-clinical models of OCD and human OCD. Further, in human studies, clinical features such as predominant OCD symptom domain, acuity of symptom onset, duration of illness, and others have been shown to be associated with diverse biomarkers and may therefore also be relevant to microbiome investigations. Antibiotic and other medications, including psychotropic medications, can be both metabolized by the microbiome, and may also influence microbial communities; the extent to which this is the case in humans is only starting to be appreciated (Cussotto et al., 2019). Finally, factors such as diet, weight, exercise, ethnicity, and psychosocial stress can influence immune parameters and the microbiota, and must also be taken into account (Van Ameringen et al., 2019).
Looking forward, understanding host immune-microbiome signaling in OCD could help guide novel, targeted treatment approaches. It remains to be established whether microbiome modulation with prebiotics, probiotics, antibiotics, or FMT are viable treatments for psychiatric disorders such as OCD. In mice, pre-treatment with Lactobacillus rhamnosus GG effectively attenuated experimental induction of compulsive behavior (Kantak et al., 2014), and in a study of healthy human volunteers, treatment with a probiotic formulation containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 was associated with a decrease in sub-clinical obsessive-compulsive symptoms (Messaoudi et al., 2011). However, the mechanisms by which such therapies exert their effects remain largely unclear, and data regarding the safety, efficacy, and dosing of different probiotic strains for specific conditions or individuals are lacking.
Microbiome data may also help guide more precise use of existing immune-modulating or psychotropic medications, or to monitor their effects. In the context of IBD, for example, specific microbially-derived metabolic pathways have been associated with the clinical efficacy of TNFα antagonists (Aden et al., 2019). In individuals treated with risperidone for first-episode schizophrenia, change in copy number of Bifidobacterium species was associated with weight gain over the course of the study (Yuan et al., 2018). In the context of OCD, in addition to various classes of psychotropic medications, several antibiotic or immune-modulating treatments have been studied, including penicillin, cephalosporins, minocycline, amantadine, celecoxib, corticosteroids, and IVIG, to name a few (Esalatmanesh et al., 2016; Gerentes et al., 2019; Murphy et al., 2014; Rodriguez et al., 2010; Shalbafan et al., 2015). The positive or deleterious effects of such treatments on immune and microbiome markers in individuals with OCD are unknown, and reliable predictors or biomarkers of treatment response for specific agents remain elusive for OCD in general. Better understanding of gut-brain-immune axis function in OCD could therefore reveal immune-microbiome markers with potential to help guide more precise use of treatments for this condition.
8. Conclusion
OCD is a disabling and often treatment-refractory disorder. Affected individuals are in critical need of reliable biomarkers of disease, predictors of treatment response, and novel treatment discovery. Aberrant immune-microbiome signaling has now been implicated in several human neuropsychiatric disorders, which represents a novel line of inquiry through which to understand and develop treatments for OCD with attention to demographic and clinical parameters.
Support for further pursuing such investigations in OCD comes from observations that 1) the gut microbiome has been associated with OCD symptomatology in a small number of pre-clinical and human studies, but causal pathogenic mechanisms have not been established; 2) early environmental risk factors for OCD overlap with critical periods of immune-microbiome development; 3) OCD is associated with increased risk of immune-mediated disorders and changes in immune parameters, which have been separately associated with the microbiome; and 4) gut microbiome manipulations in animal models are associated with changes in immunity and some obsessive-compulsive symptom domains. As shown in Figure 1, existing pre-clinical studies suggest possible mechanisms by which host immune-microbiome signaling could elicit obsessive-compulsive symptoms, including microbiota programming of cytokine production capacity, expansion and trafficking of peripheral immune cell populations to the CNS, and microglial dysregulation; however, these remain to be established in humans.
Figure 1. Select theoretical mechanisms by which host immune-microbiome signaling could influence OCD symptomatology.
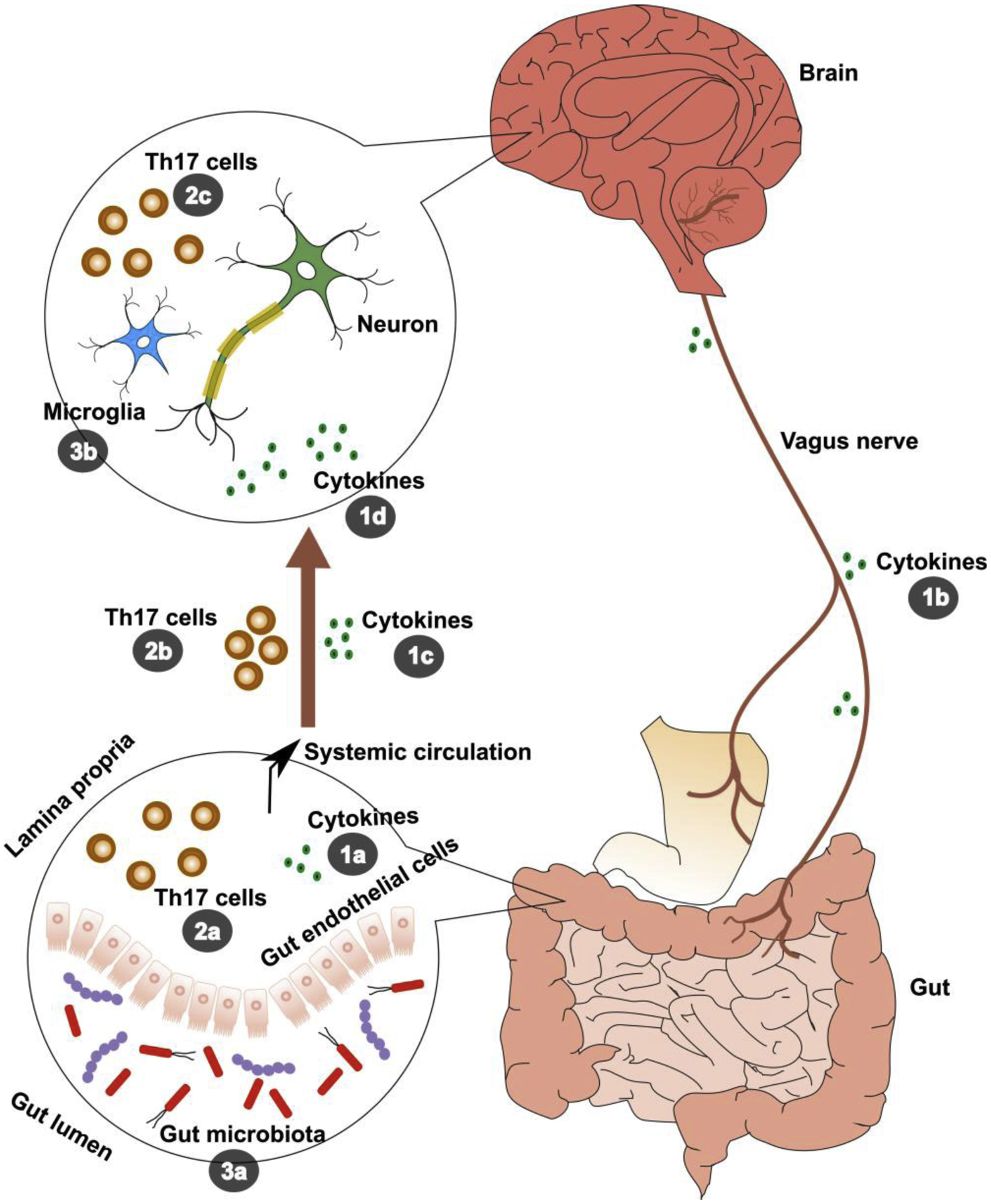
1. Gut microbiome can influence cytokine production capacity (1a) (Schirmer et al., 2016). Peripheral cytokines can influence afferent signaling to the central nervous system (CNS) via the vagus nerve (1b), or cross the blood brain barrier directly (1c), and cytokines are also produced by glial cells within the CNS. Cytokines of either peripheral or central origin can influence brain and behavior (1d) (Dantzer et al., 2008; Miller and Raison, 2016). Based on genetic studies, TNFα might be of particular relevance to OCD, but the relationship between TNFα and OCD remains to be fully elucidated (Cappi et al., 2012; Hounie et al., 2008; Jiang et al., 2018). 2. Gut microbes, specifically segmented filamentous bacteria (SFB), can participate in inflammatory and autoimmune processes by promoting expansion of T-helper 17 (Th17) cells (2a) (Lécuyer et al., 2014). In mice, auto-reactive Th17 cells can enter the CNS (2b) and have been shown to induce obsessive-compulsive behaviors (2c) (Kant et al., 2018). Other peripheral immune cell types, such as monocytes, may also exhibit dysregulated inflammatory responses in humans with OCD (Rodríguez et al., 2017), and can traffic to the CNS under conditions of increased blood brain barrier permeability (Wohleb et al., 2015), but are not included here, as their relationships with microbiome composition are less clear in the current literature. 3. Gut microbiota are needed for development and homeostasis of mature microglia (3a) (Erny et al., 2017). Microbiome manipulations, such as germ-free (GF) rearing and antibiotic treatment, are associated with microglial dysregulation and cognitive and behavioral changes in animal models, in an age- and sex-dependent manner (3b) (Thion et al., 2018).
With a thoughtful, translational, systems-biology approach, future host immune-microbiome investigations in OCD offer not only to further our understanding of gut-brain-immune axis function in OCD, but also to help guide more precise treatment for individuals with OCD.
Highlights.
Current literature suggests immune-microbiome signaling could be relevant to OCD.
We discuss mechanisms by which gut-brain-immune axis dysfunction could underlie OCD.
OCD is heterogeneous, and future microbiome investigations should take this into account.
This offers to be a novel area of inquiry to further our understanding and treatment of OCD.
Acknowledgements
Dr. Troyer is supported by the National Institute of Mental Health (NIMH, T32MH018399) and the American Academy of Child and Adolescent Psychiatry (AACAP) Pilot Research Award for Junior Faculty and Child and Adolescent Psychiatry Fellows, Dr. Kohn by the National Center for Advancing Translational Studies (TL1TR001443) and NIMH (T32MH019934), Dr. Aleti by the Kavli Institute for Brain and Mind (KIBM) Innovative Research Grant, Dr. Rosenberg by NIMH (R01MH059299), and Dr. Hong by the National Heart, Lung, and Blood Institute (NHLBI, R01HL126056) and the National Institute on Aging (NIA, R03AG063328).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Drs. Troyer, Kohn, Ecklu-Mensah, Aleti, Rosenberg and Hong have no commercial financial conflicts of interest to disclose.
References
- Aden K, Rehman A, Waschina S, Pan WH, Walker A, Lucio M, Nunez AM, Bharti R, Zimmerman J, Bethge J, Schulte B, Schulte D, Franke A, Nikolaus S, Schroeder JO, Vandeputte D, Raes J, Szymczak S, Waetzig GH, Zeuner R, Schmitt-Kopplin P, Kaleta C, Schreiber S, Rosenstiel P, 2019. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology 157, 1279–1292.e11. 10.1053/j.gastro.2019.07.025 [DOI] [PubMed] [Google Scholar]
- Akagawa S, Tsuji S, Onuma C, Akagawa Y, Yamaguchi T, Yamagishi M, Yamanouchi S, Kimata T, Sekiya SI, Ohashi A, Hashiyada M, Akane A, Kaneko K, 2019. Effect of delivery mode and nutrition on gut microbiota in Neonates. Ann. Nutr. Metab 74, 132–139. 10.1159/000496427 [DOI] [PubMed] [Google Scholar]
- Akaltun İ, Kara T, Sertan Kara S, Ayaydın H, 2018. Seroprevalance Anti-Toxoplasma gondii antibodies in children and adolescents with tourette syndrome/chronic motor or vocal tic disorder: A case-control study. Psychiatry Res 263, 154–157. 10.1016/j.psychres.2018.03.020 [DOI] [PubMed] [Google Scholar]
- Collins Amélie, Weitkampb Jörn-Hendrik, Wynnc James L., D., 2018. Why are preterm newborns at increased risk of infection? Arch. Dis. Child. - Fetal Neonatal Ed 103, F391–F394. 10.1136/archdischild-2017-313595.Why [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA, 2013. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth. ed. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Arboleya S, Ang L, Margolles A, Yiyuan L, Dongya Z, Liang X, Solís G, Fernández N, de los Reyes-Gavilán CG, Gueimonde M, 2012. Deep 16S rRNA metagenomics and quantitative PCR analyses of the premature infant fecal microbiota. Anaerobe 18, 378–380. 10.1016/j.anaerobe.2012.04.013 [DOI] [PubMed] [Google Scholar]
- Arentsen T, Qian Y, Gkotzis S, Femenia T, Wang T, Udekwu K, Forssberg H, Diaz Heijtz R, 2017. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 22, 257–266. 10.1038/mp.2016.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M, Kilic F, Koseoglu F, Ustundag B, 2011. Neutrophils are decreased in obsessive-compulsive disorder: Preliminary investigation. Psychiatry Investig 8, 362–365. 10.4306/pi.2011.8.4.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwells S, Setiawan E, Wilson AA, Rusjan PM, Mizrahi R, Miler L, Xu C, Richter MA, Kahn A, Kish SJ, Houle S, Ravindran L, Meyer JH, 2017. Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry 74, 833–840. 10.1001/jamapsychiatry.2017.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bager P, Wohlfahrt J, Westergaard T, 2008. Caesarean delivery and risk of atopy and allergic disesase: Meta-analyses. Clin. Exp. Allergy 38, 634–642. 10.1111/j.1365-2222.2008.02939.x [DOI] [PubMed] [Google Scholar]
- Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S, 2007. The correlation between adiposity and adiponectin, tumor necrosis factor α, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J. Endocrinol. Invest 30, 210–214. 10.1007/BF03347427 [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M, 2011. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain. Behav. Immun 25, 397–407. 10.1016/j.bbi.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber Y, Toren P, Achiron A, Noy S, Wolmer L, Weizman R, Laor N, 1996. T cell subsets in obsessive-compulsive disorder. Neuropsychobiology 34, 63–66. 10.1159/000119293 [DOI] [PubMed] [Google Scholar]
- Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF, 2018. Making sense of ⋯ the microbiome in psychiatry. Int. J. Neuropsychopharmacol 22, 37–52. 10.1093/ijnp/pyy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW, 2014. Role of the microbiota in immunity and inflammation. Cell 157, 121–141. 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Harrison OJ, 2017. Homeostatic immunity and the microbiota. Immunity 46, 562–576. 10.1016/j.immuni.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Pazi H, Stoner JA, Cunningham MW, 2013. Dopamine receptor autoantibodies correlate with symptoms in Sydenham’s chorea. PLoS One 8. 10.1371/journal.pone.0073516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, AlRasbi Z, Boziki M, Johner C, Wekerle H, Krishnamoorthy G, 2011. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK, 2009. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology 34, 2489–2496. 10.1038/npp.2009.77 [DOI] [PubMed] [Google Scholar]
- Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C, 2010. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev 86, 13–15. 10.1016/j.earlhumdev.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, Rauch SL, McCracken JT, Rasmussen SA, Murphy DL, Cullen B, Valle D, Hoehn-Saric R, Greenberg BD, Pinto A, Knowles JA, Piacentini J, Pauls DL, Liang KY, Willour VL, Riddle M, Samuels JF, Feng G, Nestadt G, 2009. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet 150, 710–720. 10.1002/ajmg.b.30897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L, Van Der Merwe L, Lochner C, Kinnear CJ, Seedat S, Stein DJ, Moolman-Smook JC, Hemmings SMJ, 2011. Investigating SAPAP3 variants in the etiology of obsessive-compulsive disorder and trichotillomania in the South African white population. Compr. Psychiatry 52, 181–187. 10.1016/j.comppsych.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Boedhoe PSW, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, Benedetti F, Beucke JC, Bollettini I, Bose A, Brem S, Calvo A, Cheng Y, Cho KIK, Dallaspezia S, Denys D, Fitzgerald KD, Fouche J-P, Giménez M, Gruner P, Hanna GL, Hibar DP, Hoexter MQ, Hu H, Huyser C, Ikari K, Jahanshad N, Kathmann N, Kaufmann C, Koch K, Kwon JS, Lazaro L, Liu Y, Lochner C, Marsh R, Martínez-Zalacaín I, Mataix-Cols D, Menchón JM, Minuzzi L, Nakamae T, Nakao T, Narayanaswamy JC, Fabrizio Piras, Federica Piras, Pittenger C, Reddy YCJ, Sato JR, Simpson HB, Soreni N, Soriano-Mas C, Spalletta G, Stevens MC, Szeszko PR, Tolin DF, Venkatasubramanian G, Walitza S, Wang Z, van Wingen GA, Xu J, Xu X, Yun J-Y, Zhao Q, Thompson PM, Stein DJ, van den Heuvel OA, Abe Y, Alonso P, Ameis SH, Arnold PD, Bargalló N, Batistuzzo MC, Benedetti F, Beucke JC, Boedhoe PSW, Bollettini I, Bose A, Brem S, Busatto GF, Calvo A, Calvo R, Cath DC, Cheng Y, Cho KIK, Dallaspezia S, de Vries FE, de Wit SJ, Denys D, Fang Y, Fitzgerald KD, Fontaine M, Fouche J-P, Giménez M, Gruner P, Hanna GL, Hibar DP, Hoexter MQ, Hu H, Huyser C, Ikari K, Jahanshad N, Kathmann N, Kaufmann C, Khadka S, Koch K, Kwon JS, Lazaro L, Liu Y, Lochner C, Marsh R, Martínez-Zalacaín I, Mataix-Cols D, Menchón JM, Miguel EC, Minuzzii L, Morer A, Nakamae T, Nakao T, Narayanaswamy JC, Piras Fabrizio, Piras Federica, Pittenger C, Reddy YCJ, Sato JR, Simpson HB, Schmaal L, Soreni N, Soriano-Mas C, Spalletta G, Stein DJ, Stevens MC, Szeszko PR, Thompson PM, Tolin DF, Veltman DJ, Venkatasubramanian G, van den Heuvel OA, van der Werf YD, van Wingen GA, Walitza S, Wang Z, Xu J, Xu X, Yun J-Y, Zhao Q, 2017. Distinct subcortical volume alterations in pediatric and adult OCD: A worldwide meta- and mega-analysis. Am. J. Psychiatry 174, 60–69. 10.1176/appi.ajp.2016.16020201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DYM, 2011. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev 242, 233–246. 10.1111/j.1600-065X.2011.01027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF, 2014. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med 20, 509–518. 10.1016/j.molmed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Bos-Veneman NGP, Olieman R, Tobiasova Z, Hoekstra PJ, Katsovich L, Bothwell ALM, Leckman JF, Kawikova I, 2011. Altered immunoglobulin profiles in children with Tourette syndrome. Brain. Behav. Immun 25, 532–538. 10.1016/j.bbi.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander G, Rydell M, Kuja-Halkola R, Fernández de la Cruz LF, Lichtenstein P, Serlachius E, Rk C, Almqvist C, D’Onofrio BM, Larsson H, Mataix-Cols D, 2016. Association of perinatal risk factors with obsessive-compulsive disorder a population-based birth cohort, sibling control study. JAMA Psychiatry 73, 1135–1144. 10.1001/jamapsychiatry.2016.2095 [DOI] [PubMed] [Google Scholar]
- Brilot F, Merheb V, Ding A, Murphy T, Dale RC, 2011. Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology 76, 1508–1513. 10.1212/WNL.0b013e3182181090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, Kruger C, Carmouche R, Newman S, Salbaum JM, Berthoud HR, 2017. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One 12, 1–20. 10.1371/journal.pone.0175577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Salbaum JM, Berthoud HR, 2018. Harnessing gut microbes for mental health: Getting from here to there. Biol. Psychiatry 83, 214–223. 10.1016/j.biopsych.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappi C, Brentani H, Lima L, Sanders SJ, Zai G, Diniz BJ, Reis VNS, Hounie AG, Conceição Do Rosário M, Mariani D, Requena GL, Puga R, Souza-Duran FL, Shavitt RG, Pauls DL, Miguel EC, Fernandez TV, 2016. Whole-exome sequencing in obsessive-compulsive disorder identifies rare mutations in immunological and neurodevelopmental pathways. Transl. Psychiatry 6. 10.1038/tp.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappi C, Muniz RK, Sampaio AS, Cordeiro Q, Brentani H, Palácios SA, Marques AH, Vallada H, Miguel EC, Guilherme L, Hounie AG, 2012. Association study between functional polymorphisms in the TNF-alpha gene and obsessive-compulsive disorder. Arq. Neuropsiquiatr 70, 87–90. 10.1590/S0004-282X2012000200003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell CR, Stene LC, Joner G, Cinek O, Svensson J, Goldacre MJ, Parslow RC, Pozzilli P, Brigis G, Stoyanov D, Urbonaitė B, Šipetić S, Schober E, Ionescu-Tirgoviste C, Devoti G, De Beaufort CE, Buschard K, Patterson CC, 2008. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: A meta-analysis of observational studies. Diabetologia 51, 726–735. 10.1007/s00125-008-0941-z [DOI] [PubMed] [Google Scholar]
- Caspani G, Kennedy S, Foster JA, Swann J, 2019. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 6, 454–481. 10.15698/mic2019.10.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro JR, Strauss JD, Bielecka A, Porto AF, Lobo FM, Urban A, Schofield WB, Palm NW, 2019. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci. Rep 9, 1–10. 10.1038/s41598-019-49923-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal N, McLain AC, Ghassabian A, Michels KA, Bell EM, Lawrence DA, Yeung EH, 2017. Maternal smoking and newborn cytokine and immunoglobulin levels. Nicotine Tob. Res 19, 789–796. 10.1093/ntr/ntw324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Stappenbeck TS, 2019. Microbiome control of innate reactivity. Curr. Opin. Immunol 56, 107–113. 10.1016/j.coi.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR, 2010. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785. 10.1016/j.cell.2010.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Blaser MJ, 2012. The human microbiome: At the interface of health and disease. Nat. Rev. Genet 13, 260–270. 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Joseph L, Pilote L, 2013. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev 14, 232–244. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, Tsaava T, Addorisio ME, Putzel GG, Zhou L, Bessman NJ, Yang R, Moriyama S, Parkhurst CN, Li A, Meyer HC, Teng F, Chavan SS, Tracey KJ, Regev A, Schroeder FC, Lee FS, Liston C, Artis D, 2019. The microbiota regulate neuronal function and fear extinction learning. Nature 574, 543–548. 10.1038/s41586-019-1644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codagnone MG, Stanton C, O’Mahony SM, Dinan TG, Cryan JF, 2019. Microbiota and Neurodevelopmental Trajectories: Role of Maternal and Early-Life Nutrition. Ann. Nutr. Metab 74, 16–27. 10.1159/000499144 [DOI] [PubMed] [Google Scholar]
- Cosco TD, Pillinger T, Emam H, Solmi M, Budhdeo S, Prina AM, Maes M, Stein DJ, Stubbs B, Carvalho AF, 2019. Immune aberrations in obsessive-compulsive disorder: A systematic review and meta-analysis. Mol. Neurobiol 56, 4751–4759. 10.1007/s12035-018-1409-x [DOI] [PubMed] [Google Scholar]
- Cox CJ, Zuccolo AJ, Edwards EV, Mascaro-Blanco A, Alvarez K, Stoner J, Chang K, Cunningham MW, 2015. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol 25, 76–85. 10.1089/cap.2014.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, Rabot S, 2014. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42, 207–217. 10.1016/j.psyneuen.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG, 2012. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci 13, 701–712. 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- Cussotto S, Clarke G, Dinan TG, Cryan JF, 2019. Psychotropics and the Microbiome: a Chamber of Secrets…. Psychopharmacology (Berl) 236, 1411–1432. 10.1007/s00213-019-5185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC, Candler PM, Church AJ, Wait R, Pocock JM, Giovannoni G, 2006. Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J. Neuroimmunol 172, 187–197. 10.1016/j.jneuroim.2005.10.014 [DOI] [PubMed] [Google Scholar]
- Dale RC, Heyman I, Giovannoni G, Church AWJ, 2005. Incidence of anti-brain antibodies in children with obsessive − compulsive disorder. Br. J. Psychiatry 187, 314–319. 10.1192/bjp.187.4.314 [DOI] [PubMed] [Google Scholar]
- Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, Ben-Pazi H, Varadkar S, Aumann TD, Horne MK, Church AJ, Fath T, Brilot F, 2012. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain 135, 3453–3468. 10.1093/brain/aws256 [DOI] [PubMed] [Google Scholar]
- Dantzer R, 2018. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol. Rev 98, 477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MC, Belda E, Prifti E, Everard A, Kayser BD, Bouillot JL, Chevallier JM, Pons N, Le Chatelier E, Ehrlich SD, Doré J, Aron-Wisnewsky J, Zucker JD, Cani PD, Clément K, 2019. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am. J. Physiol. Endocrinol. Metab 317, E446–E459. 10.1152/ajpendo.00140.2019 [DOI] [PubMed] [Google Scholar]
- De Agüero MG, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ, 2016. The maternal microbiota drives early postnatal innate immune development. Science (80-. ) 351, 1296–1302. 10.1126/science.aad2571 [DOI] [PubMed] [Google Scholar]
- De Rooij SR, Nijpels G, Nilsson PM, Nolan JJ, Gabriel R, Bobbioni-Harsch E, Mingrone G, Dekker JM, 2009. Low-grade chronic inflammation in the relationship between insulin sensitivity and cardiovascular disease (RISC) population: Associations with insulin resistance and cardiometabolic risk profile. Diabetes Care 32, 1295–1301. 10.2337/dc08-1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Van Deren D, Peden E, Hockin M, Boulet A, Titen S, Capecchi MR, 2018. Two distinct ontogenies confer heterogeneity to mouse brain microglia. Development 145, dev152306. 10.1242/dev.152306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Braber A, Zilhão NR, Fedko IO, Hottenga JJ, Pool R, Smit DJA, Cath DC, Boomsma DI, 2016. Obsessive–compulsive symptoms in a large population-based twin-family sample are predicted by clinically based polygenic scores and by genome-wide SNPs. Transl. Psychiatry 6, 1–7. 10.1038/tp.2015.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D, Fluitman S, Kavelaars A, Heijnen C, Westenberg H, 2004. Decreased TNF-α and NK activity in obsessive-compulsive disorder. Psychoneuroendocrinology 29, 945–952. 10.1016/j.psyneuen.2003.08.008 [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF, 2014. Microbiota is essential for social development in the mouse. Mol. Psychiatry 19, 146–148. 10.1038/mp.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O’Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS, 2014. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med 20, 524–530. 10.1038/nm.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mario S, Gagliotti C, Donatini A, Battaglia S, Buttazzi R, Balduzzi S, Borsari S, Basevi V, Barbieri L, Barella C, Castellana A, Ghigini C, Maffi R, Maffini I, Milani AM, Pellizzari R, Prazzoli M, Bussolati G, Dodi I, Ferraroni E, Piazza N, Sparano M, Moscara L, Volta A, Sighinolfi G, Vezzani M, Lenzi P, Ricci R, Baldisserri B, Caroli P, Suzzi L, Valenti E, Cornale M, Cuoghi C, Pascoletti F, Baldi L, Cappelli G, Gasperoni O, Loreti A, Monti G, Muratori C, Pelliconi N, Zoffoli I, Baldoni AM, Brunelli A, Farneti M, Lombardi M, Marrone F, Bigi M, Gabellini R, Mazzocchi A, Vanni M, 2019. Formula feeding increases the risk of antibiotic prescriptions in children up to 2 years: results from a cohort study. Eur. J. Pediatr 178, 1867–1874. 10.1007/s00431-019-03462-0 [DOI] [PubMed] [Google Scholar]
- Dietrich DE, Zhang Y, Bode L, Münte TF, Hauser U, Schmorl P, Richter-Witte C, Gödecke-Koch T, Feutl S, Schramm J, Ludwig H, Johannes S, Emrich HM, 2005. Brain potential amplitude varies as a function of Borna disease virus-specific immune complexes in obsessive-compulsive disorder [1]. Mol. Psychiatry 10, 519–520. 10.1038/sj.mp.4001645 [DOI] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF, 2017. Microbes immunity and behavior: Psychoneuroimmunology meets the microbiome. Neuropsychopharmacology 42, 178–192. 10.1038/npp.2016.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinn WM, Harris CL, McGonigal KM, Raynard RC, 2005. Obsessive-compulsive disorder and immunocompetence. Int. J. Psychiatry Med 31, 311–320. 10.2190/f0ba-bn4f-61ka-ud99 [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R, 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A 107, 11971–11975. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ, 2019. Role of the microbiome in human development. Gut 68, 1108–1114. 10.1136/gutjnl-2018-317503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle F, Cardoso F, Lopes L, Mendes M, Dias F, Cruz L, Tavares R, Camargos A, Carneiro M, Dias-Lopes C, Chávez-Olórtegui C, 2012. Infusion of Sydenham’s chorea antibodies in striatum with up-regulated dopaminergic receptors: A pilot study to investigate the potential of SC antibodies to increase dopaminergic activity. Neurosci. Lett 523, 186–189. 10.1016/j.neulet.2012.06.073 [DOI] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA, 2011. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757. 10.1016/j.cell.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M, 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci 18, 965–977. 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Prinz M, 2017. Communicating systems in the body: how microbiota and microglia cooperate. Immunology 150, 7–15. 10.1111/imm.12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esalatmanesh S, Abrishami Z, Zeinoddini A, Rahiminejad F, Sadeghi M, Najarzadegan MR, Shalbafan MR, Akhondzadeh S, 2016. Minocycline combination therapy with fluvoxamine in moderate-to-severe obsessive–compulsive disorder: A placebo-controlled, double-blind, randomized trial. Psychiatry Clin. Neurosci 70, 517–526. 10.1111/pcn.12430 [DOI] [PubMed] [Google Scholar]
- Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI, 2014. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci. Transl. Med 6. 10.1126/scitranslmed.3008051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon G, Mills KHG, 2020. The microbiota and immune-mediated diseases: Opportunities for therapeutic intervention. Eur. J. Immunol 326–337. 10.1002/eji.201948322 [DOI] [PubMed] [Google Scholar]
- Flegr J, Horáček J, 2017. Toxoplasma-infected subjects report an Obsessive-Compulsive Disorder diagnosis more often and score higher in Obsessive-Compulsive Inventory. Eur. Psychiatry 40, 82–87. 10.1016/j.eurpsy.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K, 2015. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: Presenting characteristics of the first 47 consecutive patients. J. Child Adolesc. Psychopharmacol 25, 38–47. 10.1089/cap.2014.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JM, Aron AM, Collard JE, Mackay MC, 1965. The emotional correlates of Sydenham’s chorea. Pediatrics 35, 42–49. [PubMed] [Google Scholar]
- Frick L, Pittenger C, 2016. Microglial dysregulation in OCD, Tourette syndrome, and PANDAS. J. Immunol. Res 2016, 1–8. 10.1155/2016/8606057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Rapanelli M, Jindachomthong K, Grant P, Leckman JF, Swedo S, Williams K, Pittenger C, 2018. Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain. Behav. Immun 69, 304–311. 10.1016/j.bbi.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M, Pohin M, Powrie F, 2019. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50, 992–1006. 10.1016/j.immuni.2019.03.017 [DOI] [PubMed] [Google Scholar]
- Fung TC, Artis D, Sonnenberg GF, 2014. Anatomical localization of commensal bacteria in immune cell homeostasis and disease. Immunol. Rev 260, 35–49. 10.1111/imr.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY, 2017. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci 20, 145–155. 10.1038/nn.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazzo G, van Best N, Bervoets L, Dapaah IO, Savelkoul PH, Hornef MW, Hutton EK, Morrison K, Holloway AC, McDonald H, Ratcliffe EM, Stearns JC, Schertzer JD, Surette MG, Thabane L, Mommers M, Lau S, Hamelmann E, Penders J, 2020. Development of the Microbiota and Associations With Birth Mode, Diet, and Atopic Disorders in a Longitudinal Analysis of Stool Samples, Collected From Infancy Through Early Childhood. Gastroenterology 158, 1584–1596. 10.1053/j.gastro.2020.01.024 [DOI] [PubMed] [Google Scholar]
- Galland L, 2014. The gut microbiome and the brain. J. Med. Food 17, 1261–1272. 10.1089/jmf.2014.7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carrasco M, Fuentes-Alexandro S, Escárcega RO, Salgado G, Riebeling C, Cervera R, 2006. Pathophysiology of Sjögren’s Syndrome. Arch. Med. Res 37, 921–932. 10.1016/j.arcmed.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Gensollen T, Iyer SS, Kasper DL, Blumberg RS, 2016. How colonization by microbiota in early life shapes the immune system. Science (80-. ) 352, 539–544. 10.1126/science.aad9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerentes M, Pelissolo A, Rajagopal K, Tamouza R, Hamdani N, 2019. Obsessive-compulsive disorder: Autoimmunity and neuroinflammation. Curr. Psychiatry Rep 21. 10.1007/s11920-019-1062-8 [DOI] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, Benoist C, Kasper DL, 2017. Mining the human gut microbiota for immunomodulatory organisms. Cell 168, 928–943.e11. 10.1016/j.cell.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R, 2018. Current understanding of the human microbiome. Nat. Med 24, 392–400. 10.1038/nm.4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalbes MJ, Llop S, Vallès Y, Moya A, Ballester F, Francino MP, 2013. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 43, 198–211. 10.1111/cea.12063 [DOI] [PubMed] [Google Scholar]
- Gray SM, Bloch MH, 2012. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr. Psychiatry Rep 14, 220–228. 10.1007/s11920-012-0272-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R, Jansson JK, 2017. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol 2, 1–7. 10.1038/nmicrobiol.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson T, Alagiakrishnan K, 2020. Gut microbes in neurocognitive and mental health disorders. Ann. Med 52, 423–443. 10.1080/07853890.2020.1808239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S, 2011. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci 108, 3047–3052. 10.1073/PNAS.1010529108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesla HM, Stenius F, Jäderlund L, Nelson R, Engstrand L, Alm J, Dicksved J, 2014. Impact of lifestyle on the gut microbiota of healthy infants and their mothers - the ALADDIN birth cohort. FEMS Microbiol. Ecol 90, 791–801. 10.1111/1574-6941.12434 [DOI] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF, 2016. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 6, e774–9. 10.1038/tp.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounie AG, Cappi C, Cordeiro Q, Sampaio AS, Moraes I, do Rosário MC, Palácios SA, Goldberg AC, Vallada HP, Machado-Lima A, Nakano E, Kalil J, Pauls D, Pereira CAB, Guilherme L, Miguel EC, 2008. TNF-alpha polymorphisms are associated with obsessive-compulsive disorder. Neurosci. Lett 10.1016/j.neulet.2008.07.022 [DOI] [PubMed] [Google Scholar]
- Hu HJ, Park SG, Jang HB, Choi MG, Park KH, Kang JH, Park SI, Lee HJ, Cho SH, 2015. Obesity alters the microbial community profile in Korean Adolescents. PLoS One 10, 1–14. 10.1371/journal.pone.0134333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura K, Brander G, Chang Z, Kuja-Halkola R, Rück C, Hellner C, Lichtenstein P, Larsson H, Mataix-Cols D, Fernández de la Cruz L, 2018. Metabolic and cardiovascular complications in obsessive-compulsive disorder: A total population, sibling comparison study with long-term follow-up. Biol. Psychiatry 84, 324–331. 10.1016/j.biopsych.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Isung J, Williams K, Williams K, Isomura K, Gromark C, Hesselmark E, Lichtenstein P, Larsson H, Larsson H, Fernández De La Cruz L, Sidorchuk A, Mataix-Cols D, 2020. Association of Primary Humoral Immunodeficiencies with Psychiatric Disorders and Suicidal Behavior and the Role of Autoimmune Diseases. JAMA Psychiatry 10.1001/jamapsychiatry.2020.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF, 2014. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 63, 559–566. 10.1136/gutjnl-2012-303249 [DOI] [PubMed] [Google Scholar]
- Janowitz D, Grabe HJ, Ruhrmann S, Ettelt S, Buhtz F, Hochrein A, Schulze-Rauschenbach S, Meyer K, Kraft S, Ferber C, Pukrop R, Freyberger HJ, Klosterkötter J, Falkai P, John U, Maier W, Wagner M, 2009. Early onset of obsessive-compulsive disorder and associated comorbidity, in: Depression and Anxiety pp. 1012–1017. 10.1002/da.20597 [DOI] [PubMed] [Google Scholar]
- Jiang C, Ma X, Qi S, Han G, Li Y, Liu Y, Liu L, 2018. Association between TNF -α- 238G/A gene polymorphism and OCD susceptibility. Med. (United States) 97. 10.1097/MD.0000000000009769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnco C, Kugler BB, Murphy TK, Storch EA, 2018. Obsessive-compulsive symptoms in adults with Lyme disease. Gen. Hosp. Psychiatry 51, 85–89. 10.1016/j.genhosppsych.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Jones HF, Han VX, Patel S, Gloss BS, Soler N, Ho A, Sharma S, Kothur K, Nosadini M, Wienholt L, Hardwick C, Barnes EH, Lim JR, Alshammery S, Nielsen TC, Wong M, Hofer MJ, Nassar N, Gold W, Brilot F, Mohammad SS, Dale RC, 2021. Maternal Autoimmunity and Inflammation are Associated with Childhood Tics and Obsessive-Compulsive Disorder: Transcriptomic Data show Common Enriched Innate Immune Pathways. Brain. Behav. Immun 10.1016/j.bbi.2020.12.035 [DOI] [PubMed] [Google Scholar]
- Jung TD, Jung PS, Raveendran L, Farbod Y, Dvorkin-Gheva A, Sakic B, Surette MG, Szechtman H, 2018. Changes in gut microbiota during development of compulsive checking and locomotor sensitization induced by chronic treatment with the dopamine agonist quinpirole. Behav. Pharmacol 29, 211–224. 10.1097/FBP.0000000000000363 [DOI] [PubMed] [Google Scholar]
- Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown R, 2019. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci. Rep 9, 1–9. 10.1038/s41598-019-42183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R, 2017. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 5, 1–16. 10.1186/s40168-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant R, Pasi S, Surolia A, 2018. Auto-reactive Th17-cells trigger obsessive-compulsive-disorder like behavior in mice with experimental autoimmune encephalomyelitis. Front. Immunol 9, 2508. 10.3389/fimmu.2018.02508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak PA, Bobrow DN, Nyby JG, 2014. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol 25, 71–79. 10.1097/FBP.0000000000000013 [DOI] [PubMed] [Google Scholar]
- Karagüzel EÖ, Arslan FC, Uysal EK, Demir S, Aykut DS, Tat M, Karahan SC, 2019. Blood levels of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha and cognitive functions in patients with obsessive compulsive disorder. Compr. Psychiatry 89, 61–66. 10.1016/j.comppsych.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, Takase K, 2015. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol 15, 1–10. 10.1186/s12876-015-0330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawikova I, Grady BPX, Tobiasova Z, Zhang Y, Vojdani A, Katsovich L, Richmand BJ, Park TW, Bothwell ALM, Leckman JF, 2010. Children with Tourette’s syndrome may suffer immunoglobulin A dysgammaglobulinemia: Preliminary report. Biol. Psychiatry 67, 679–683. 10.1016/j.biopsych.2009.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Dantzer R, Jones PB, 2017. Immunopsychiatry: Important facts. Psychol. Med 47, 2229–2237. 10.1017/S0033291717000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Ravi V, Shenoy P, Chandramuki A, Channabasavanna S, 1997a. Cerebrospinal fluid viral antibodies in obsessive—compulsive disorder in an Indian population. Biol. Psychiatry 41, 883–890. 10.1016/S0006-3223(96)00174-6 [DOI] [PubMed] [Google Scholar]
- Khanna S, Ravi V, Shenoy PK, Chandramukhi A, Channabasavanna SM, 1997b. Viral antibodies in blood in obsessive compulsive disorder. Indian J Psychiatry 39, 190–195. [PMC free article] [PubMed] [Google Scholar]
- Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK, 2014. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381. 10.1016/j.chom.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinçarslan S, Evrensel A, 2020. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with inflammatory bowel disease: An experimental study. Actas Esp. Psiquiatr 48, 1–7. [PubMed] [Google Scholar]
- Kirvan CA, Cox CJ, Swedo SE, Cunningham MW, 2007. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J. Immunol 178, 7412–7421. 10.4049/jimmunol.178.11.7412 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW, 2003. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat. Med 9, 914–920. 10.1038/nm892 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW, 2006. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J. Neuroimmunol 179, 173–179. 10.1016/j.jneuroim.2006.06.017 [DOI] [PubMed] [Google Scholar]
- Knoop KA, Gustafsson JK, McDonald KG, Kulkarni DH, Coughlin PE, McCrate S, Kim D, Hsieh C, Hogan SP, Elson CO, Tarr PI, Newberry RD, 2017. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol 2, eaao1314. 10.1126/sciimmunol.aao1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler-Forsberg O, Petersen L, Gasse C, Mortensen PB, Dalsgaard S, Yolken RH, Mors O, Benros ME, 2019. A Nationwide Study in Denmark of the Association between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA Psychiatry 76. 10.1001/jamapsychiatry.2018.3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J, 2014. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71, 1381–1391. 10.1001/jamapsychiatry.2014.1611 [DOI] [PubMed] [Google Scholar]
- Kristensen K, Henriksen L, 2016. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunol 137, 587–590. 10.1016/j.jaci.2015.07.040 [DOI] [PubMed] [Google Scholar]
- Kuo IH, Yoshida T, De Benedetto A, Beck LA, 2013. The cutaneous innate immune response in patients with atopic dermatitis. J. Allergy Clin. Immunol 131, 266–278. 10.1016/j.jaci.2012.12.1563 [DOI] [PubMed] [Google Scholar]
- Lamothe H, Baleyte JM, Smith P, Pelissolo A, Mallet L, 2018. Individualized immunological data for precise classification of OCD patients. Brain Sci 8, 1–30. 10.3390/brainsci8080150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz Y, Ringel-Scaia VM, Allen IC, Theus MH, 2018. Emerging developments in microbiome and microglia research: Implications for neurodevelopmental disorders. Front. Immunol 9, 1–9. 10.3389/fimmu.2018.01993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, King RA, Gilbert DL, Coffey BJ, Singer HS, Dure LS IV, Grantz H, Katsovich L, Lin H, Lombroso PJ, 2011. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: A prospective longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 50, 108–118.e3. 10.1016/j.jaac.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Vaccarino FM, 2015. Editorial commentary: “What does immunology have to do with brain development and neuropsychiatric disorders?” Brain Res 1617, 1–6. 10.1016/j.brainres.2014.09.052 [DOI] [PubMed] [Google Scholar]
- Lécuyer E, Rakotobe S, Lengliné-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, Reynaud CA, Cerf-Bensussan N, Gaboriau-Routhiau V, 2014. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 40, 608–620. 10.1016/j.immuni.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK, 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A 108, 4615–4622. 10.1073/pnas.1000082107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DYM, 2013. New insights into atopic dermatitis: Role of skin barrier and immune dysregulation. Allergol. Int 62, 151–161. 10.2332/allergolint.13-RAI-0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, Kim H, Zoratti EM, Lukacs NW, Boushey HA, Ownby DR, Lynch SV, Johnson CC, 2016. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci. Rep 6, 1–16. 10.1038/srep31775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SL, Tsai MH, Yao TC, Hua MC, Yeh KW, Chiu CY, Su KW, Huang SY, Kao CC, Lai SH, Huang JL, 2017. Caesarean Section is associated with reduced perinatal cytokine response, increased risk of bacterial colonization in the airway, and infantile wheezing. Sci. Rep 7, 1–9. 10.1038/s41598-017-07894-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, Casero D, Courtney H, Gonzalez A, Graeber TG, Hall AB, Lake K, Landers CJ, Mallick H, Plichta DR, Prasad M, Rahnavard G, Sauk J, Shungin D, Vázquez-Baeza Y, White RA, Bishai J, Bullock K, Deik A, Dennis C, Kaplan JL, Khalili H, McIver LJ, Moran CJ, Nguyen L, Pierce KA, Schwager R, Sirota-Madi A, Stevens BW, Tan W, ten Hoeve JJ, Weingart G, Wilson RG, Yajnik V, Braun J, Denson LA, Jansson JK, Knight R, Kugathasan S, McGovern DPB, Petrosino JF, Stappenbeck TS, Winter HS, Clish CB, Franzosa EA, Vlamakis H, Xavier RJ, Huttenhower C, 2019. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662. 10.1038/s41586-019-1237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiselle CR, Lee O, Moran TH, Singer HS, 2004. Striatal microinfusion of Tourette syndrome and PANDAS sera: Failure to induce behavioral changes. Mov. Disord 19, 390–396. 10.1002/mds.10522 [DOI] [PubMed] [Google Scholar]
- Luczynski P, Neufeld KAMV, Oriach CS, Clarke G, Dinan TG, Cryan JF, 2016. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol 19, 1–17. 10.1093/ijnp/pyw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Leckman JF, Katsovich L, Findley D, Grantz H, Tucker DM, Lombroso PJ, King RA, Bessen DE, 2004. Prospective longitudinal study of children with tic disorders and/or obsessive-compulsive disorder: Relationship of symptom exacerbations to newly acquired streptococcal infections. Pediatrics 113, e578–e585. 10.1542/peds.113.6.e578 [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Russell A, Mirza Y, Keshavan MS, Banerjee SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, Moore GJ, Rosenberg DR, 2006. Pituitary volume in pediatric obsessive-compulsive disorder. Biol. Psychiatry 59, 252–257. 10.1016/j.biopsych.2005.06.028 [DOI] [PubMed] [Google Scholar]
- Macul Ferreira de Barros P, do Rosário MC, Szejko N, Polga N, Requena G. de L., Ravagnani B, Fatori D, Batistuzzo MC, Hoexter MQ, Rohde LA, Polanczyk GV, Leckman JF, Miguel EC, de Alvarenga PG, 2020. Risk factors for obsessive–compulsive symptoms. Follow-up of a community-based youth cohort. Eur. Child Adolesc. Psychiatry 10.1007/s00787-020-01495-7 [DOI] [PubMed] [Google Scholar]
- Malamitsi-Puchner A, Protonotariou E, Boutsikou T, Makrakis E, Sarandakou A, Creatsas G, 2005. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum. Dev 81, 387–392. 10.1016/j.earlhumdev.2004.10.017 [DOI] [PubMed] [Google Scholar]
- Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, Choung RS, Ju J, Sompallae R, Gibson-Corley K, Patel R, Rodriguez M, David C, Taneja V, Murray J, 2017. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep 20, 1269–1277. 10.1016/j.celrep.2017.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Mucci F, Fontenelle LF, 2018. Immune system and obsessive-compulsive disorder. Psychoneuroendocrinology 10.1016/j.psyneuen.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Marazziti D, Presta S, Pfanner C, Gemignani A, Rossi A, Sbrana S, Rocchi V, Ambrogi F, Cassano GB, 1999. Immunological alterations in adult obsessive-compulsive disorder. Biol. Psychiatry 46, 810–814. 10.1016/S0006-3223(98)00371-0 [DOI] [PubMed] [Google Scholar]
- Marques-Vidal P, Bochud M, Bastardot F, Lüscher T, Ferrero F, Gaspoz J-M, Paccaud F, Urwyler A, von Känel R, Hock C, Waeber G, Preisig M, Vollenweider P, 2012. Association between inflammatory and obesity markers in a Swiss population-based sample (CoLaus Study). Obes. Facts 5, 734–744. 10.1159/000345045 [DOI] [PubMed] [Google Scholar]
- Martino D, Johnson I, Leckman JF, 2020. What Does Immunology Have to Do With Normal Brain Development and the Pathophysiology Underlying Tourette Syndrome and Related Neuropsychiatric Disorders? Front. Neurol 11. 10.3389/fneur.2020.567407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Frans E, Pérez-Vigil A, Kuja-Halkola R, Gromark C, Isomura K, Fernández de la Cruz L, Serlachius E, Leckman JF, Crowley JJ, Rück C, Almqvist C, Lichtenstein P, Larsson H, 2018. A total-population multigenerational family clustering study of autoimmune diseases in obsessive–compulsive disorder and Tourette’s/chronic tic disorders. Mol. Psychiatry 23, 1652–1658. 10.1038/mp.2017.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell LK, Davis RL, Owens D, 2005. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics 116, 56–60. 10.1542/peds.2004-2058 [DOI] [PubMed] [Google Scholar]
- Melville JM, Moss TJM, 2013. The immune consequences of preterm birth. Front. Neurosci 7, 1–9. 10.3389/fnins.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C, 2011. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2. 10.4161/gmic.2.4.16108 [DOI] [PubMed] [Google Scholar]
- Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, O’Farrelly C, Raverdeau M, Vernon A, Pettee W, O’Shea D, Nikolajczyk BS, Mills KHG, Brenner MB, Finlay D, Lynch L, 2018. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol 19, 1330–1340. 10.1038/s41590-018-0251-7 [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S, 2013. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry 70, 608–618. 10.1001/jamapsychiatry.2013.914 [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D, 2012. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes 36, 817–825. 10.1038/ijo.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miman O, Mutlu EA, Ozcan O, Atambay M, Karlidag R, Unal S, 2010. Is there any role of Toxoplasma gondii in the etiology of obsessive-compulsive disorder? Psychiatry Res 177, 263–265. 10.1016/j.psychres.2009.12.013 [DOI] [PubMed] [Google Scholar]
- Miman Ö, Özcan Ö, Ünal S, Atambay M, 2018. Toxoplasma gondii–obsessive –compulsive disorder relationship: is it different in children? Nord. J. Psychiatry 72, 501–505. 10.1080/08039488.2018.1514421 [DOI] [PubMed] [Google Scholar]
- Moon J, Choi SH, Yoon CH, Kim MK, 2020. Gut dysbiosis is prevailing in Sjögren’s syndrome and is related to dry eye severity. PLoS One 15, 1–14. 10.1371/journal.pone.0229029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morer A, Lázaro L, Sabater L, Massana J, Castro J, Graus F, 2008. Antineuronal antibodies in a group of children with obsessive-compulsive disorder and Tourette syndrome. J. Psychiatr. Res 42, 64–68. 10.1016/j.jpsychires.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Murphy ML, Pichichero ME, 2002. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch. Pediatr. Adolesc. Med 156, 356. 10.1001/archpedi.156.4.356 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Parker-Athill EC, Lewin AB, Storch EA, Mutch PJ, 2014. Cefdinir for Recent-Onset Pediatric Neuropsychiatric Disorders: A Pilot Randomized Trial. J. Child Adolesc. Psychopharmacol 25, 57–64. 10.1089/cap.2014.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Patel PD, McGuire JF, Kennel A, Mutch PJ, Parker-Athill EC, Hanks CE, Lewin AB, Storch EA, Toufexis MD, Dadlani GH, Rodriguez CA, 2015. Characterization of the pediatric acute-onset neuropsychiatric syndrome phenotype. J. Child Adolesc. Psychopharmacol 25, 14–25. 10.1089/cap.2014.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan N, Jones BW, West PJ, Marc RE, Capecchi MR, 2018. Corticostriatal circuit defects in Hoxb8 mutant mice. Mol. Psychiatry 23, 1–10. 10.1038/mp.2017.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SP, Zelikoff JT, 2007. Smoking during pregnancy: Subsequent effects on offspring immune competence and disease vulnerability in later life. Reprod. Toxicol 23, 428–437. 10.1016/j.reprotox.2006.11.008 [DOI] [PubMed] [Google Scholar]
- Noakes PS, Holt PG, Prescott SL, 2003. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy Eur. J. Allergy Clin. Immunol 58, 1053–1058. 10.1034/j.1398-9995.2003.00290.x [DOI] [PubMed] [Google Scholar]
- Nocturne G, Mariette X, 2018. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol 14, 133–145. 10.1038/nrrheum.2018.1 [DOI] [PubMed] [Google Scholar]
- O’ Mahony SM, Stilling RM, Dinan TG, Cryan JF, 2015. The microbiome and childhood diseases: Focus on brain-gut axis. Birth Defects Res. Part C - Embryo Today Rev 105, 296–313. 10.1002/bdrc.21118 [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH, 2009. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol 183, 6041–6050. 10.4049/jimmunol.0900747 [DOI] [PubMed] [Google Scholar]
- Oddy WH, 2017. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab 70, 26–36. 10.1159/000457920 [DOI] [PubMed] [Google Scholar]
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF, 2015. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry 78, e7–e9. 10.1016/j.biopsych.2014.12.023 [DOI] [PubMed] [Google Scholar]
- Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, Lin C-S, Jobin C, Brand S, Sotlar K, Wada K, Katayama K, Nakajima A, Mizuguchi H, Kawasaki K, Nagata K, Müller W, Snapper SB, Schreiber S, Kaser A, Zeissig S, Blumberg RS, 2014. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 509, 497–502. 10.1038/nature13150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovska S, Vestergaard CH, Bech BH, Nordentoft M, Vestergaard M, Benros ME, 2017. Association of streptococcal throat infection with mental disorders: Testing key aspects of the PANDAS hypothesis in a nationwide study. JAMA Psychiatry 74, 740–746. 10.1001/jamapsychiatry.2017.0995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O, Cerovic V, Hornef M, 2016. Secretory IgA in the coordination of establishment and maintenance of the microbiota. Trends Immunol 10.1016/j.it.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Parsons C, Roberts R, Mills NT, 2020. Review: Inflammation and anxiety-based disorders in children and adolescents – a systematic review and meta-analysis. Child Adolesc. Ment. Health 10.1111/camh.12434 [DOI] [PubMed] [Google Scholar]
- Pauls DL, Abramovitch A, Rauch SL, Geller DA, 2014. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci 10.1038/nrn3746 [DOI] [PubMed] [Google Scholar]
- Pavone P, Bianchini R, Parano E, Incorpora G, Rizzo R, Mazzone L, Trifiletti RR, 2004. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr. Neurol 30, 107–110. 10.1016/S0887-8994(03)00413-2 [DOI] [PubMed] [Google Scholar]
- Pearlman DM, Vora HS, Marquis BG, Najjar S, Dudley LA, 2014. Anti-basal ganglia antibodies in primary obsessive-compulsive disorder: Systematic review and meta-analysis. Br. J. Psychiatry 205, 8–16. 10.1192/bjp.bp.113.137018 [DOI] [PubMed] [Google Scholar]
- Penders J, Thijs C, Van Den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, Van Ree R, Stobberingh EE, 2007. Gut microbiota composition and development of atopic manifestations in infancy: The KOALA birth cohort study. Gut 56, 661–667. 10.1136/gut.2006.100164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, Van Den Brandt PA, Stobberingh EE, 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521. 10.1542/peds.2005-2824 [DOI] [PubMed] [Google Scholar]
- Pérez-Vigil A, Fernández de la Cruz L, Brander G, Isomura K, Gromark C, Mataix-Cols D, 2016. The link between autoimmune diseases and obsessive-compulsive and tic disorders: A systematic review. Neurosci. Biobehav. Rev 71, 542–562. 10.1016/j.neubiorev.2016.09.025 [DOI] [PubMed] [Google Scholar]
- Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P, 2020. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol 11, 1–8. 10.3389/fimmu.2020.00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC, 2015. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Ther 37, 984–995. 10.1016/j.clinthera.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petta I, Fraussen J, Somers V, Kleinewietfeld M, 2018. Interrelation of diet, gut microbiome, and autoantibody production. Front. Immunol 9. 10.3389/fimmu.2018.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Kelmendi B, Bloch M, Krystal JH, Coric V, 2005. Clinical treatment of obsessive compulsive disorder. Psychiatry (Edgmont) 2, 34–43. [PMC free article] [PubMed] [Google Scholar]
- Politis S, Magklara K, Petrikis P, Michalis G, Simos G, Skapinakis P, 2017. Epidemiology and comorbidity of obsessive–compulsive disorder in late adolescence: a cross-sectional study in senior high schools in Greece. Int. J. Psychiatry Clin. Pract 21, 188–194. 10.1080/13651501.2017.1324038 [DOI] [PubMed] [Google Scholar]
- Pronovost GN, Hsiao EY, 2019. Perinatal interactions between the microbiome, immunity, and neurodevelopment. Immunity 50, 18–36. 10.1016/j.immuni.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliariello A, Del Chierico F, Russo A, Reddel S, Conte G, Lopetuso LR, Ianiro G, Dallapiccola B, Cardona F, Gasbarrini A, Putignani L, 2018. Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front. Microbiol 9. 10.3389/fmicb.2018.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajca S, Grondin V, Louis E, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M, Flamant M, Savoye G, Jian R, Devos M, Paintaud G, Piver E, Allez M, Mary JY, Sokol H, Colombel JF, Seksik P, 2014. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm. Bowel Dis 20, 978–986. 10.1097/MIB.0000000000000036 [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Griffiths J, Merali Z, Anisman H, 1999. Circulating lymphocyte subsets in obsessive compulsive disorder, major depression and normal controls. J. Affect. Disord 52, 1–10. 10.1016/S0165-0327(98)00072-X [DOI] [PubMed] [Google Scholar]
- Reddel S, Del Chierico F, Quagliariello A, Giancristoforo S, Vernocchi P, Russo A, Fiocchi A, Rossi P, Putignani L, El Hachem M, 2019. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep 9, 1–10. 10.1038/s41598-019-41149-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Granger DA, Woo H, Voegtline K, DiPietro JA, Johnson SB, 2020. Long-Term Associations Between Prenatal Maternal Cortisol and Child Neuroendocrine-Immune Regulation. Int. J. Behav. Med 27, 267–281. 10.1007/s12529-019-09814-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SE, Wotton CJ, Williams JG, Griffith M, Goldacre MJ, 2011. Perinatal and early life risk factors for inflammatory bowel disease. World J. Gastroenterol 17, 743–749. 10.3748/wjg.v17.i6.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Goethel A, Girardin SE, Philpott DJ, 2018. Innate immune influences on the gut microbiome: Lessons from mouse models. Trends Immunol 39, 992–1004. 10.1016/j.it.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Bender J, Marcus SM, Snape M, Rynn M, Simpson HB, 2010. Minocycline Augmentation of Pharmacotherapy in Obsessive-Compulsive Disorder. J. Clin. Psychiatry 71, 1247–1249. 10.4088/JCP.09l05805blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez N, Morer A, González-Navarro EA, Gassó P, Boloc D, Serra-Pagès C, Lafuente A, Lazaro L, Mas S, 2017. Human-leukocyte antigen class II genes in early-onset obsessive-compulsive disorder. World J. Biol. Psychiatry 1–7. 10.1080/15622975.2017.1327669 [DOI] [PubMed] [Google Scholar]
- Rodríguez N, Morer A, González-Navarro EA, Serra-Pages C, Boloc D, Torres T, García-Cerro S, Mas S, Gassó P, Lázaro L, 2017. Inflammatory dysregulation of monocytes in pediatric patients with obsessive-compulsive disorder. J. Neuroinflammation 14, 1–11. 10.1186/s12974-017-1042-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez N, Morer A, González-Navarro EA, Serra-Pages C, Boloc D, Torres T, Martinez-Pinteño A, Mas S, Lafuente A, Gassó P, Lázaro L, 2019. Altered frequencies of Th17 and Treg cells in children and adolescents with obsessive-compulsive disorder. Brain. Behav. Immun 81, 608–616. 10.1016/j.bbi.2019.07.022 [DOI] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S, 2016. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 21, 738–748. 10.1038/mp.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M, Irwin MR, 2020. Novel neuroimmunologic therapeutics in depression: A clinical perspective on what we know so far. Brain. Behav. Immun 83, 7–21. 10.1016/j.bbi.2019.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GAW, Raison CL, Lowry CA, 2014. Microbial “old friends”, immunoregulation and socioeconomic status. Clin. Exp. Immunol 177, 1–12. 10.1111/cei.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougé C, Goldenberg O, Ferraris L, Berger B, Rochat F, Legrand A, Göbel UB, Vodovar M, Voyer M, Rozé JC, Darmaun D, Piloquet H, Butel MJ, de La Cochetière MF, 2010. Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe 16, 362–370. 10.1016/j.anaerobe.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC, 2010. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 15, 53–63. 10.1038/mp.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers IM, Cryan JF, Bastiaanssen TFS, Rea K, Clarke G, Jaspan HB, Harvey BH, Hemmings SMJ, Santana L, van der Sluis R, Malan-Müller S, Wolmarans DW, 2019. Natural compulsive-like behaviour in the deer mouse (Peromyscus maniculatus bairdii) is associated with altered gut microbiota composition. Eur. J. Neurosci 1419–1427. 10.1111/ejn.14610 [DOI] [PubMed] [Google Scholar]
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, ter Horst R, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A, Fu J, Joosten LAB, Zhernakova A, Huttenhower C, Wijmenga C, Netea MG, Xavier RJ, 2016. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167, 1125–1136.e8. 10.1016/j.cell.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiertz A, Gruhl B, Löbnitz M, Michel P, Radke M, Blaut M, 2003. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr. Res 54, 393–399. 10.1203/01.PDR.0000078274.74607.7A [DOI] [PubMed] [Google Scholar]
- Shalbafan M, Mohammadinejad P, Shariat SV, Alavi K, Zeinoddini A, Salehi M, Askari N, Akhondzadeh S, 2015. Celecoxib as an Adjuvant to Fluvoxamine in Moderate to Severe Obsessive-compulsive Disorder: A Double-blind, Placebo-controlled, Randomized Trial. Pharmacopsychiatry 48, 136–140. 10.1055/s-0035-1549929 [DOI] [PubMed] [Google Scholar]
- Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, Kumar N, Stares MD, Rodger A, Brocklehurst P, Field N, Lawley TD, 2019. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121. 10.1038/s41586-019-1560-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şimşek Ş, Yüksel T, Çim A, Kaya S, 2016. Serum cytokine profiles of children with obsessive-compulsive disorder shows the evidence of autoimmunity. Int. J. Neuropsychopharmacol 19, 1–6. 10.1093/ijnp/pyw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Hong JJ, Yoon DY, Williams PN, 2005. Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology 65, 1701 LP–1707. 10.1212/01.wnl.0000183223.69946.f1 [DOI] [PubMed] [Google Scholar]
- Skoog G, Skoog I, 1999. A 40-year follow-up of patients with obsessive-compulsive disorder. Arch. Gen. Psychiatry 56, 121. 10.1001/archpsyc.56.2.121 [DOI] [PubMed] [Google Scholar]
- Słabuszewska-Jóźwiak A, Szymański JK, Ciebiera M, Sarecka-Hujar B, Jakiel G, 2020. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 17, 8031. 10.3390/ijerph17218031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P, 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U. S. A 105, 16731–16736. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, van den Heuvel OA, Simpson HB, 2019. Obsessive–compulsive disorder. Nat. Rev. Dis. Prim 5, 52. 10.1038/s41572-019-0102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SE, Geller DA, Jenike M, Pauls D, Shaw D, Mullin B, Faraone SV, 2004. Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr. Scand 110, 4–13. 10.1111/j.1600-0447.2004.00302.x [DOI] [PubMed] [Google Scholar]
- Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, Dinan TG, Cryan JF, 2015. Microbes & neurodevelopment - Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain. Behav. Immun 50, 209–220. 10.1016/j.bbi.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y, 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol 558, 263–275. 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterland AL, Fond G, Kuin A, Koeter MWJ, Lutter R, van Gool T, Yolken R, Szoke A, Leboyer M, de Haan L, 2015. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: Systematic review and meta-analysis. Acta Psychiatr. Scand 132, 161–179. 10.1111/acps.12423 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leckman JF, Rose NR, 2012. From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatr. Ther 02, 1–8. 10.4172/2161-0665.1000113 [DOI] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK, 1998. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am J Psychiatry 155, 264–271. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Rapoport JL, Cheslow DL, Leonard HL, Ayoub EM, Hosier DM, Wald ER, 1989. High prevalence of obsessive-compulsive symptoms in patients with Sydenham’s chorea. Am. J. Psychiatry 146, 246–249. [DOI] [PubMed] [Google Scholar]
- Ta LDH, Chan JCY, Yap GC, Purbojati RW, Drautz-Moses DI, Koh YM, Tay CJX, Huang CH, Kioh DYQ, Woon JY, Tham EH, Loo EXL, Shek LPC, Karnani N, Goh A, Van Bever HPS, Teoh OH, Chan YH, Lay C, Knol J, Yap F, Tan KH, Chong YS, Godfrey KM, Kjelleberg S, Schuster SC, Chan ECY, Lee BW, 2020. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes 12, 1–21. 10.1080/19490976.2020.1801964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, 2011. Early versus late onset obsessive-compulsive disorder: Evidence for distinct subtypes. Clin. Psychol. Rev 10.1016/j.cpr.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, Elinav E, 2016. The microbiome and innate immunity. Nature 535, 65–74. 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR, 2008. A meta-analysis of the association between Caesarean section and childhood asthma. Clin. Exp. Allergy 38, 629–633. 10.1111/j.1365-2222.2007.02780.x [DOI] [PubMed] [Google Scholar]
- Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo PM, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S, 2018. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16. 10.1016/j.cell.2017.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tränkner D, Boulet A, Peden E, Focht R, Van Deren D, Capecchi M, 2019. A microglia sublineage protects from sex-linked anxiety symptoms and obsessive compulsion. Cell Rep 29, 791–799.e3. 10.1016/j.celrep.2019.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trøseid M, Andersen GØ, Broch K, Hov JR, 2020. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 52, 102649. 10.1016/j.ebiom.2020.102649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turna J, Grosman Kaplan K, Anglin R, Patterson B, Soreni N, Bercik P, Surette MG, Van Ameringen M, 2020. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: a pilot study. Acta Psychiatr. Scand 142, 337–347. 10.1111/acps.13175 [DOI] [PubMed] [Google Scholar]
- Turna J, Grosman Kaplan K, Anglin R, Van Ameringen M, 2016. “What’s bugging the gut in OCD?” A review of the gut microbiome in obsessive-compulsive disorder. Depress. Anxiety 33, 171–178. 10.1002/da.22454 [DOI] [PubMed] [Google Scholar]
- Turna J, Patterson B, Van Ameringen M, 2017. An update on the relationship between the gut microbiome and obsessive-compulsive disorder. Psychiatr. Ann 47, 542–551. 10.3928/00485713-20171013-01 [DOI] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI, 2007. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 449, 804–810. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursoiu F, Moleriu L, Lungeanu D, Puschită M, 2018. The association between HIV clinical disease severity and psychiatric disorders as seen in Western Romania. AIDS Care - Psychol. Socio-Medical Asp. AIDS/HIV 30, 1368–1371. 10.1080/09540121.2018.1455959 [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Turna J, Patterson B, Pipe A, Mao RQ, Anglin R, Surette MG, 2019. The gut microbiome in psychiatry: A primer for clinicians. Depress. Anxiety 36, 1004–1025. 10.1002/da.22936 [DOI] [PubMed] [Google Scholar]
- van der Meulen TA, Harmsen HJM, Vila AV, Kurilshikov A, Liefers SC, Zhernakova A, Fu J, Wijmenga C, Weersma RK, de Leeuw K, Bootsma H, Spijkervet FKL, Vissink A, Kroese FGM, 2019. Shared gut, but distinct oral microbiota composition in primary Sjögren’s syndrome and systemic lupus erythematosus. J. Autoimmun 97, 77–87. 10.1016/j.jaut.2018.10.009 [DOI] [PubMed] [Google Scholar]
- Veru F, Dancause K, Laplante DP, King S, Luheshi G, 2015. Prenatal maternal stress predicts reductions in CD4+ lymphocytes, increases in innate-derived cytokines, and a Th2 shift in adolescents: Project Ice Storm. Physiol. Behav 144, 137–145. 10.1016/j.physbeh.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT, 2010. Metabolic syndrome and altered gut microbiota in mice lacking Toll-Like Receptor 5. Science (80-. ) 328, 228–231. 10.1126/science.1179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandro S, Osborne S, Enriquez C, Bixby C, Arrieta A, Whiteson K, 2018. The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis. mSphere 3. 10.1128/mSphere.00104-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Lau CI, Lin CC, Chang A, Kao CH, 2016. Group a streptococcal infections are associated with increased risk of pediatric neuropsychiatric disorders: A Taiwanese population-based cohort study. J. Clin. Psychiatry 77, e848–e854. 10.4088/JCP.14m09728 [DOI] [PubMed] [Google Scholar]
- Wang LY, Chen SF, Chiang JH, Hsu CY, Shen YC, 2019. Systemic autoimmune diseases are associated with an increased risk of obsessive–compulsive disorder: a nationwide population-based cohort study. Soc. Psychiatry Psychiatr. Epidemiol 54, 507–516. 10.1007/s00127-018-1622-y [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G, 2007. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 448, 894–900. 10.1038/nature06104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwell-Roper C, Williams KA, Samuels J, Bienvenu OJ, Cullen B, Goes FS, Grados MA, Geller D, Greenberg BD, Knowles JA, Krasnow J, McLaughlin NC, Nestadt P, Shugart Y-Y, Nestadt G, Stewart SE, 2019. Immune-related comorbidities in childhood-onset obsessive compulsive disorder: Lifetime prevalence in the Obsessive Compulsive Disorder Collaborative Genetics Association Study. J. Child Adolesc. Psychopharmacol 1–10. 10.1089/cap.2018.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Shorser-Gentile L, Sarvode Mothi S, Berman N, Pasternack M, Geller D, Walter J, 2019. Immunoglobulin A dysgammaglobulinemia is associated with pediatric-onset obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol 29, 268–275. 10.1089/cap.2018.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout JP, 2015. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci 9, 1–17. 10.3389/fnins.2014.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolmarans DW, Stein DJ, Harvey BH, 2016. Excessive nest building is a unique behavioural phenotype in the deer mouse model of obsessive-compulsive disorder. J. Psychopharmacol 30, 867–874. 10.1177/0269881116645554 [DOI] [PubMed] [Google Scholar]
- Wu HJ, Wu E, 2012. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu R-J, Fahey S, Frick L, Leckman J, Vaccarino F, Duman RS, Williams K, Swedo S, Pittenger C, 2020. Antibodies From Children With PANDAS Bind Specifically to Striatal Cholinergic Interneurons and Alter Their Activity. Am. J. Psychiatry appi.ajp.2020.1. 10.1176/appi.ajp.2020.19070698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Palm NW, 2020. Immunoglobulin A and the microbiome. Curr. Opin. Microbiol 56, 89–96. 10.1016/j.mib.2020.08.003 [DOI] [PubMed] [Google Scholar]
- Yaramiş A, Hergüner S, Kara B, Tatli B, Tüzün U, Ozmen M, 2009. Cerebral vasculitis and obsessive-compulsive disorder following varicella infection in childhood. Turk J Pediatr 51, 72–5. [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI, 2012. Human gut microbiome viewed across age and geography. Nature 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan N, Chen Y, Xia Y, Dai J, Liu C, 2019. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 9. 10.1038/s41398-019-0570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Zhang P, Wang Y, Liu Y, Li X, Kumar BU, Hei G, Lv L, Huang XF, Fan X, Song X, 2018. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr. Res 201, 299–306. 10.1016/j.schres.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Yuce M, Guner S, Karabekiroglu K, Baykal S, Kilic M, Sancak R, Karabekiroglu A, 2014. Association of Tourette syndrome and obsessive-compulsive disorder with allergic diseases in children and adolescents: a preliminary study. Eur. Rev. Med. Pharmacol. Sci 18, 303–310. [PubMed] [Google Scholar]
- Zhang Xuan, Chen B. di, Zhao L. dan, Li H, 2020. The Gut Microbiota: Emerging Evidence in Autoimmune Diseases. Trends Mol. Med 26, 862–873. 10.1016/j.molmed.2020.04.001 [DOI] [PubMed] [Google Scholar]
- Zhang Xin, Zou Q, Zhao B, Zhang J, Zhao W, Li Y, Liu R, Liu X, Liu Z, 2020. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol 32. 10.1016/j.redox.2020.101535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Cai D. Bin, Yang XH, Ungvari GS, Ng CH, Müller N, Ning YP, Xiang YT, 2017. Adjunctive celecoxib for schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. J. Psychiatr. Res 92, 139–146. 10.1016/j.jpsychires.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Zijlmans MAC, Beijers R, Riksen-Walraven MJ, de Weerth C, 2017. Maternal late pregnancy anxiety and stress is associated with children’s health: a longitudinal study. Stress 20, 495–504. 10.1080/10253890.2017.1348497 [DOI] [PubMed] [Google Scholar]
- Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C, 2015. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 53, 233–245. 10.1016/j.psyneuen.2015.01.006 [DOI] [PubMed] [Google Scholar]


