Abstract
Background
Influenza and pneumococcal pneumonia are serious health problems among elderly people and a major cause of death in long-term care facilities. We describe the results of serial surveys of vaccination coverage and influenza outbreak management in Canadian long-term care facilities over the last decade.
Methods
Cross-sectional surveys consisting of questionnaires mailed to all Canadian residential long-term care facilities for elderly people in 1991 and to a random sample of respondents in 1995 and 1999.
Results
The response rates were 83% (430/515) in 1995 and 75% (380/506) in 1999. In 1999 the mean reported rates of influenza vaccination were 83% among residents and 35% among staff, and the mean rate of pneumococcal vaccination among residents was 71%; all 3 rates were significantly higher than those in 1991. The rates were also higher in facilities with an infection control practitioner than in those without such an individual (88% v. 82% for influenza vaccination among residents [p < 0.001], 42% v. 35% for influenza vaccination among staff [p = 0.008] and 75% v. 63% for pneumococcal vaccination among residents [p < 0.001]). Obtaining consent for vaccination on admission to the facility was associated with higher influenza and pneumococcal vaccination rates among residents (p = 0.04 and p < 0.001 respectively). Facilities with higher influenza vaccination rates among residents and staff reported lower rates of influenza outbreaks (p = 0.08 and 0.03 respectively). Despite recommendations from the National Advisory Committee on Immunization, only 50% of the facilities had policies for amantadine prophylaxis during influenza A outbreaks. Amantadine was judged effective in controlling 76% of the influenza A outbreaks and was discontinued because of side effects in 3% of the residents.
Interpretation
Influenza and pneumococcal vaccination rates among residents and staff in Canadian long-term care facilities have increased over the last decade but remain suboptimal. Vaccination of residents and staff against influenza is associated with a reduced risk of influenza outbreaks. Amantadine is effective in controlling influenza outbreaks in long-term care facilities.
Influenza and pneumococcal pneumonia are serious health problems among elderly people. Each year in Canada up to 75 000 people are admitted to hospital with influenza and 6700 die.1,2 Ninety percent of deaths involve people 65 years of age or older,3 and about half of these occur in long-term care facilities.4 Residents in such facilities are especially vulnerable, not only because of their advanced age and underlying illness, but also because they live in close mutual proximity and have extensive contact with a range of caregivers. In outbreaks in nursing homes, attack rates of 25%–60% and case-fatality rates of 10%–20% have been measured.5 Pneumococcal pneumonia is the most common serious infectious complication of influenza and a significant independent source of illness and death.6 The annual incidence of bacteremic pneumococcal disease in people over the age of 75 years is 1 per 1000, and the case-fatality rate in this age group is 39%.7
In Canada the National Advisory Committee on Immunization recommends annual influenza vaccination and one-time pneumococcal vaccination for all people over the age of 65 years.8 The influenza vaccine has variable efficacy, depending on the host's immune response and how closely the vaccine matches the virus strain.3,9,10 Among elderly residents in long-term care facilities, the efficacy of the vaccine in preventing any respiratory illness due to influenza is only 30%–40%; however, the vaccine is 50%–60% effective in preventing pneumonia and hospital admission, and up to 80% effective in preventing death from influenza-related complications.3 The pneumococcal vaccine is 60%–80% effective in preventing pneumococcal bacteremia in middle-aged and elderly people.9,10,11 In 1999 amantadine was the only antiviral agent licensed in Canada for prophylaxis against influenza A, and the National Advisory Committee on Immunization recommends its routine use for this purpose in outbreaks in long-term care facilities.8
To assess progress in vaccination coverage and influenza outbreak management in Canadian long-term care facilities for elderly people, we conducted surveys in 1991, 1995 and 1999.
Methods
A list of Canadian residential long-term care facilities serving a primarily elderly population was compiled in 1991 for the initial survey in this series. The methodology and results of that survey have been previously published.12,13 Using a modification of Dillman's survey design method,14 we repeated the survey in 1995 and 1999: questionnaires were sent to all long-term care facilities in Prince Edward Island and Newfoundland and to 40% of randomly selected facilities in the other provinces that responded to the 1991 survey. French questionnaires were sent to Quebec facilities, French and English questionnaires were sent to New Brunswick facilities, and English questionnaires were sent to the remaining institutions.
The questionnaire asked for basic demographic information, influenza vaccination rates among staff and residents for the influenza season preceding the survey, pneumococcal vaccination rates among residents, institutional policies regarding influenza and pneumococcal vaccination, and the number of outbreaks and the frequency of amantadine use during the 2 previous influenza seasons. Data were entered in duplicate.
Univariate analysis of factors associated with vaccination rates was performed using the Wilcoxon rank-sum test, Fisher's exact test and the Mantel–Haenszel χ2 test. Multivariate analysis of the impact of facility size and influenza vaccination rates was performed by stratifying according to facility size and by logistic regression analysis, with facility size and vaccination rates as continuous variables. Because some facilities did not complete all of the questions on the survey, the totals for certain analyses are not equal to the total number of surveys. Analyses were conducted on an annual basis (using all facilities responding in a given year) and overall (using only facilities responding in all 3 years [n = 354]); because there was no significant difference between the annual and overall results, we are presenting data from the annual analysis.
Results
The response rates were 84% (1270/1520) in 1991, 83% (430/515) in 1995 and 75% (380/506) in 1999. Nine facilities closed between 1995 and 1999. In 1999 the mean facility size was 108 beds (range 16–1004), with 9 physicians on average (range 1–70) providing medical care to residents in each facility. The median overall staff:resident ratio was 1.1 (5%–95% range 0.6–2.5) in 1999 (no difference in 1995). In 1999, 64% of the facilities reported having an infection control practitioner, as compared with 54% in 1995 (p < 0.001); these practitioners spent 4.4 hours per week on average (range 0–38) on infection control activities (no difference in 1995). In 1999 data were provided by 361 facilities (95%) on influenza vaccination rates among residents, by 322 (85%) on influenza vaccination rates among staff and by 330 (87%) on pneumococcal vaccination rates among residents; the corresponding rates in 1995 were 93%, 56% and 82% respectively.
Influenza vaccination rates
The overall vaccination rate among residents was 83% for the 1998/99 influenza season (median rate by facility 90%, range 10%–100%), representing a significant increase over the vaccination rate of 79% for the 1990/91 season (p < 0.001) (Fig. 1). Rates increased in all provinces from 1990/91 to 1998/99; however, differences among provinces persisted, with rates being significantly lower than the national average in Quebec and Newfoundland (p < 0.001, Fig. 2).
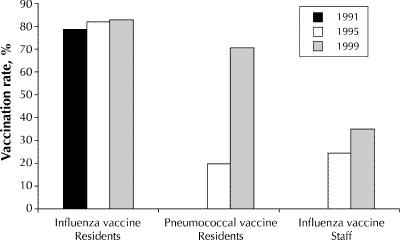
Fig. 1: Influenza and pneumococcal vaccination rates among residents and staff in Canadian long-term care facilities reported in 1991, 1995 and 1999. Quantitative data on pneumococcal vaccination rates and staff influenza vaccination rates were not collected in 1991.
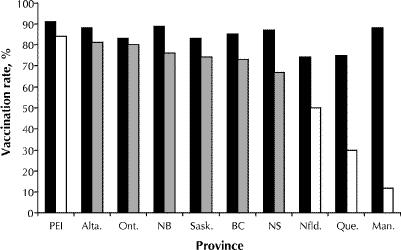
Fig. 2: Influenza (black bars) and pneumococcal (dark and light grey bars) vaccination rates among residents reported in 1999, by province. Dark grey bars = provinces with publicly funded pneumococcal vaccination programs for residents of long-term care facilities introduced before 1998; light grey bars = provinces with programs introduced in 1998 or later.
The overall staff vaccination rate for the 1998/99 season was 35% (median rate by facility 40%, range 0%–100%). Most (71%) of the facilities reported staff vaccination rates of 25% or greater, but only 9% reported rates greater than 75%. Differences among provinces were significant (Fig. 3). Overall, 46% (170/369) of the facilities reported that they offer or recommend vaccination to casual or agency staff, and 28% (96/342) reported that they offer vaccine or require proof of vaccination of new staff hired during the influenza season. Of the 186 facilities that provided information on their most recent influenza A outbreak, only 47% (88/186) indicated that they re-offered vaccine to staff during the outbreak; however, 36% (22/61) of these reported that no staff agreed to vaccination at that time.
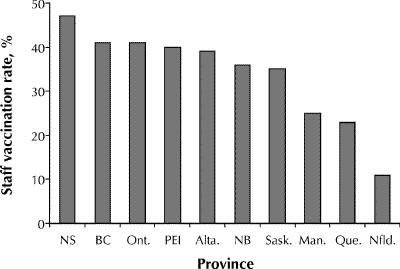
Fig. 3: Influenza vaccination rates among staff in long-term care facilities for the 1998/99 influenza season, by province.
Pneumococcal vaccination rates
Between 1991 and 1999 the proportion of facilities able to provide data on pneumococcal vaccination rates among residents increased, from 58% to 87% (p < 0.001). In 1999 the overall vaccination rate was 71% (median rate per facility 82%, range 0%–100%). There was substantial interprovincial variation, from 12% in Manitoba and 31% in Quebec to 81% in Alberta and 84% in PEI (Fig. 2). The overall rate in 1999 was substantially higher than the rate of 20% reported in 1995. In 1999, only 17% (61/366) of the facilities reported that the pneumococcal vaccine is not routinely offered to residents.
Factors associated with vaccination
Higher influenza vaccination rates among residents were reported in facilities with an infection control practitioner (88% v. 82% in those without such a practitioner, p < 0.001), in facilities obtaining consent for vaccination on admission for current and future years (88% v. 85% in those obtaining consent annually, p = 0.04) and in facilities offering the vaccine to residents admitted during the winter (88% v. 82% in those without such a policy, p < 0.001). Offering of the vaccine to residents admitted during the winter was carried out in 70% (254/363) of the facilities.
Higher staff influenza vaccination rates were reported in facilities with an infection control practitioner (42% v. 35% in those without such a practitioner, p = 0.008) and in facilities with a smaller than average number of beds (42% v. 34% in those with more beds, p = 0.003). Rates were also higher in facilities that offered the vaccine to staff hired during the winter (48% v. 36% in those without such a policy, p < 0.001) and in facilities that offered vaccine to nonemployees active in the facility (43% v. 31% in those without such a policy, p < 0.001) and to agency or casual staff (49% v. 31% in those without such a policy, p < 0.001).
Pneumococcal vaccination rates among residents were higher in facilities with an infection control practitioner (75% v. 63% in those without such a practitioner, p < 0.001), in those with a higher than average number of physicians (78% v. 68% in those with fewer physicians, p = 0.03) and in those that obtained consent for vaccination from the residents on admission (83% v. 56% in those without such a policy, p < 0.001).
Influenza outbreaks
Overall, 69% (264/380) of the facilities that responded to the 1999 questionnaire provided data on the number and types of outbreaks in the 1997/98 and 1998/99 influenza seasons. The response rates varied by province, with 93% of the facilities in Ontario and 81% in Nova Scotia providing data, as compared with 26% in Quebec and 38% in PEI. The 264 facilities reported 349 outbreaks of respiratory illness over the 2 seasons (0.66 outbreaks per year); 247 (0.47 outbreaks per year) were due to influenza A or B. The number of reported outbreaks was similar in the 2 seasons, and the annual reported rate of outbreaks did not differ among the provinces (data not shown). Larger facilities were more likely than smaller facilities to report influenza outbreaks: during the 1998/99 season, 31% of the facilities with fewer than 50 beds reported at least 1 outbreak, as compared with 38% of the facilities with 50–199 beds and 64% of those with 200 or more beds (p = 0.002). The risk of an outbreak was inversely related to vaccination rates among the residents (Fig. 4 [p = 0.08, univariate χ2 for trend by categories; p = 0.01, logistic regression model]) and to vaccination rates among the staff (Fig. 5 [p = 0.03, univariate χ2 for trend by category; p = 0.08, logistic regression model]). In an analysis stratified by facility size, the association between risk of an outbreak and staff vaccination rates remained statistically significant in facilities with fewer than 100 beds (p = 0.02), but not in larger facilities.
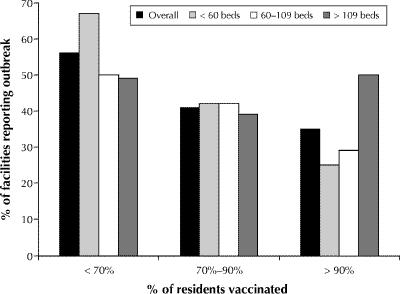
Fig. 4: Proportion of long-term care facilities reporting an influenza outbreak in the 1998/99 influenza season, by vaccination rates among residents and by size of facility.
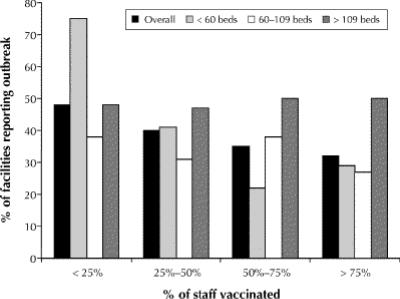
Fig. 5: Proportion of long-term care facilities reporting an influenza outbreak in the 1998/99 influenza season, by staff vaccination rates and by size of facility.
Amantadine use
In 1999, 50% (185/370) of the facilities reported having a policy recommending amantadine prophylaxis to residents during influenza A outbreaks. There was substantial interprovincial variation in the proportion of facilities with such a policy: 85% in Ontario, 56% in Nova Scotia, 49% in British Columbia, 48% in Alberta and less than 20% in the remaining 6 provinces. Of the facilities with a policy for amantadine prophylaxis, 77% (142/185) reported that they routinely assess renal function and calculate individualized amantadine doses before each influenza season, and most (76%) stated that they test creatinine clearance annually for this purpose. The presence of an infection control practitioner in the facility was associated with a higher likelihood of having a policy recommending amantadine for residents (60% v. 32% of facilities without such a practitioner, p < 0.001).
Of the facilities whose most recent influenza A outbreak occurred in the 1997/98 season, 84% (46/55) reported offering amantadine prophylaxis to the residents, and 65% (33/51) reported recommending amantadine prophylaxis for staff. Among the facilities with an influenza A outbreak in the 1998/99 season, the rates were similar: 88% (84/95) and 66% (57/86) respectively. Of the facilities with an outbreak in the 1998/99 season that reported recommending amantadine prophylaxis to staff, 13% (11/82) indicated that they required staff to take amantadine before they were allowed to work.
Most (76% [99/131]) of the facilities that reported using mass amantadine prophylaxis among the residents during their most recent influenza A outbreak indicated that the prophylaxis was effective in controlling the outbreak, in that new cases stopped occurring within 2 days after the start of the amantadine prophylaxis. Outbreaks reported to be controlled with amantadine involved significantly fewer residents (median 20, range 2–96) than did those in which amantadine failed (median 36, range 5–96, p = 0.008). Only 3% of the residents were reported to have experienced side effects that required discontinuation of the amantadine.
Interpretation
In Canada the National Advisory Committee on Immunization has set a 90% target vaccination rate for people aged 65 years or older or at high risk of influenza-related complications.8 Furthermore, in a closed setting such as a long-term care facility, it is recommended that at least 80% of the residents and staff be vaccinated to achieve herd immunity.5 Our data suggest that, although influenza vaccination rates among residents and staff of Canadian long-term care facilities have increased over the last decade, they remain suboptimal. In the facilities responding to our survey, 83% of the residents were vaccinated for the 1998/99 influenza season. Several pieces of evidence suggest that this is an overestimate of the true rate. Validation of the 1991 survey data by chart review found lower than reported rates (by 7% and 30%) in 2 of 12 facilities.13 A recent audit of 7 facilities in Ontario with influenza outbreaks in 1999/2000 revealed that vaccination rates among residents measured by chart review were a median of 2% (range 0%–7%) lower than the rates reported to the provincial ministry of health (unpublished data). In addition, survey response rates were higher in provinces with higher overall vaccination rates, which suggests that the vaccination rates in the nonresponding facilities may have been lower than those in the facilities that responded to the survey. In summary, these data suggest that the overall influenza vaccination rate among residents in Canadian long-term care facilities remains below 80%. This, combined with the fact that there was a mean staff:resident ratio of 1.1 and that only 40% of staff were vaccinated for the 1998/99 season, substantially explains why there is inadequate herd immunity against influenza in our long-term care facilities.
Staff vaccination against influenza is important because staff can transmit influenza to residents. The estimated efficacy of the vaccine in preventing illness in staff is as high as 88%,4,15,16,17 with benefits including lower rates of influenza-like illness and complications, fewer physician visits and lost work days, and decreased antibiotic use.15,16,17,18,19 Two randomized controlled trials have shown that staff vaccination reduces influenza-related morbidity and death among facility residents.20,21 Our findings suggest that the risk of influenza outbreaks is substantially reduced in facilities with higher staff vaccination rates. The apparently greater effect of vaccination among staff and residents in smaller facilities than in larger ones may be because the absolute number of susceptible people is important, or it may be due to lack of power to detect a difference within subgroups (e.g., only 5 of 101 facilities with more than 100 beds reported staff vaccination rates greater than 75%).
The variation in staff vaccination rates among provinces suggests that provincial policies have a substantial impact on vaccine use,12 although the small numbers of facilities in some provinces suggests that these results be interpreted with caution. The impact of provincial public health policies was clearly demonstrated in 1999 in Ontario. The Ontario Ministry of Health and Long-Term Care began paying for influenza vaccine for staff in long-term care facilities in 1993. In the fall of 1999, the ministry issued an influenza prevention and surveillance protocol that recommended policies restricting unvaccinated staff from work during outbreaks unless they were taking antiviral prophylaxis. The policy recommendation, and its enforcement by some local public health units, resulted in an increase in median staff vaccination rates, from 45% in 1998/99 to 86% in 1999/2000 (Ontario Ministry of Health and Long-Term Care: unpublished data).
Pneumococcal vaccination increased substantially over the last decade, but this vaccine remains underused compared to influenza vaccine, and pneumococcal vaccination rates among elderly people in Canada are significantly lower than those in the United States.22,23 The low rates are associated with physician doubts about the vaccine's effectiveness.24,25,26,27,28,29 However, there is good evidence that vaccination is associated with significant protection against bacteremic pneumococcal disease and that its use reduces costs to the health care system.30,31,32 Currently, the National Advisory Committee on Immunization recommends that the pneumococcal vaccine be given to all residents of long-term care facilities who have not been previously vaccinated.8 Our findings showed that vaccination rates among residents were higher in facilities with an infection control practitioner, those with a higher than average number of physicians physicians and those in provinces with established, publicly funded pneumococcal vaccination programs. Infection control practitioners likely influence rates through their promotion of preventive practices. Facilities with such individuals also reported higher resident and staff influenza vaccination rates and increased use of amantadine. An increased number of physicians might increase the probability that at least one will promote vaccination. The effect of publicly funded programs re-emphasizes the importance of public health programs in promoting vaccination.
The National Advisory Committee on Immunization recommends that amantadine prophylaxis be offered to all asymptomatic residents for the duration of an influenza A outbreak.8 Amantadine is 60%–90% effective in preventing influenza in exposed individuals,8,33 and although there are no randomized controlled trials of outbreak control, experience suggests that mass amantadine prophylaxis is usually very effective in controlling outbreaks.34,35,36,37 The reported failure rate of amantadine identified in our survey is similar to that reported by Tamblyn.38 Reasons for failure could include suboptimal dosing regimens, mixed outbreaks involving influenza and noninfluenza viruses or the emergence of amantadine-resistant strains of influenza virus, a phenomenon estimated to occur in up to 30% of amantadine-treated patients.39 A better understanding of the reasons for failure is important in determining how outbreak control can be improved. The fact that a median of 22 residents were involved in outbreaks controlled with amantadine highlights another problem in influenza management: early detection of outbreaks. Gomolin and associates40 have suggested that a cluster of 3 ill residents on a unit within 72 hours deserves investigation. If outbreaks were consistently detected at this point, and amantadine prophylaxis started promptly, the median number of involved residents should be no more than 10.
Prior studies have identified rates of adverse events associated with amantadine use as high as 47%,34 and the proportion of residents who stopped taking amantadine because of side effects has ranged from 7%–19%.34,41,42,43 Most of these studies used amantadine doses of 100 mg/d. In our study, a majority of the facilities measured creatinine clearance to determine individual doses, a practice recommended by the National Advisory Committee on Immunization and one that has been shown to reduce the incidence of side effects.44 Although we did not collect data on overall rates of side effects, only 3% of the residents were reported to have stopped taking amantadine because of adverse effects, which emphasizes that amantadine prophylaxis in this setting poses a lesser risk than exposure to influenza. It is unclear how usage patterns will change with the advent of neuraminidase inhibitors, which are active against both influenza A and B, have a lower incidence of side effects and have less potential for the emergence of resistance.45
In summary, our study shows that influenza and pneumococcal vaccination rates in Canadian long-term care facilities have risen over the last decade but remain suboptimal. Vaccination of both staff and residents appears to be important in preventing influenza outbreaks, and the use of mass antiviral prophylaxis is effective in controlling outbreaks when they do occur. Our data explain why the National Advisory Committee on Immunization now states that “healthcare workers and their employers have a duty to actively promote, implement and comply with influenza immunization.”12 These data should also encourage all those responsible for the care of residents in long-term care facilities, and for the accreditation and regulation of such facilties, to establish, support and expand vaccination programs and antiviral prophylaxis policies in order to reduce the impact of influenza and pneumococcal pneumonia in this fragile population.
Footnotes
This article has been peer reviewed.
Acknowledgements: We are grateful to the administrators, directors of care and infection control practitioners in facilities across Canada who so willingly collaborated with us on our survey, and to Jeannette Huberty-Edwards and Hana Zivnickova for their assistance with form design, mailings and data entry.
Competing interests: None declared.
Reprint requests to: Dr. Allison J. McGeer, Rm. 1460, Department of Microbiology, Mount Sinai Hospital, 600 University Ave., Toronto ON M5G 1X5; fax 416 586-3140; amcgeer@mtsinai.on.ca
References
- 1.Fedson DS. Influenza and pneumococcal vaccination in Canada and the United States, 1980–1993: What can the two countries learn from each other? Clin Infect Dis 1995;20:1371-6. [DOI] [PubMed]
- 2.Canadian Consensus Conference on Influenza. Can Commun Dis Rep 1993;19: 136-47. [PubMed]
- 3.Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999;48(RR-04):1-28. [PubMed]
- 4.Nicholson KG. Should staff in long-stay hospitals for elderly patients be vaccinated against influenza? Lancet 2000;355:83-4. [DOI] [PubMed]
- 5.Nichol KL, Grimm MB, Peterson DC. Immunizations in long-term care facilities: policies and practice. J Am Geriatr Soc 1996;44(4):349-55. [DOI] [PubMed]
- 6.Pneumococcal vaccines: World Health Organization position paper. Can Commun Dis Rep 1999;25(17):150-1. [PubMed]
- 7.McGeer A, Green K, Landry L, Talbot J, Goldenberg E, TIBDN. Assessing the potential impact of vaccination programs on invasive pneumococcal disease: data from population-based surveillance. Can J Infect Dis 1999;10(Suppl):24A-6A.
- 8.National Advisory Committee on Immunization. Statement on influenza vaccination for the 2000–2001 season. Can Commun Dis Rep 2000;26(ACS-2):1-16. [PubMed]
- 9.Influenza vaccine, 1999–2000. Med Lett Drugs Ther 1999;41:82-3. [PubMed]
- 10.Pneumococcal vaccine. Med Lett Drugs Ther 1999;41:84. [PubMed]
- 11.Squires SG, Spika JS. Protecting against invasive pneumococcal disease: Be wise — immunize! CMAJ 1998;159(7):826-7. Available: www.cma.ca/cmaj/vol-159/issue-7/0826e.htm [PMC free article] [PubMed]
- 12.McArthur MA, Simor AE, Campbell B, McGeer A. Influenza vaccination in long-term care facilities: structuring programs for success. Infect Control Hosp Epidemiol 1999;20:499-503. [DOI] [PubMed]
- 13.McArthur MA, Simor AE, Campbell B, McGeer A. Influenza and pneumococcal vaccination and tuberculin skin testing programs in long-term care facilities: Where do we stand? Infect Control Hosp Epidemiol 1995;16:18-24. [DOI] [PubMed]
- 14.Dillman D. Mail and telephone surveys: the total design method. New York: Wiley Interscience; 1978.
- 15.Wilde JA, McMillan JA, Serwint J, Butta J, O'Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999;281:908-13. [DOI] [PubMed]
- 16.Nichol KL, Lind A, Margolis KL, Murdoch M, McFadden R, Hauge M, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med 1995;333:889-93. [DOI] [PubMed]
- 17.Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB. Effectiveness of live, attenuated intranasal influenza vaccine in healthy, working adults: a randomized controlled trial. JAMA 1999;282:137-44. [DOI] [PubMed]
- 18.Dille JH. A worksite influenza immunization program: impact on lost work days, health care utilization, and health care spending. AAOHN J 1999;47:301-9. [PubMed]
- 19.Yassi A, Kettner J, Hammond G, Cheang M, McGill M. Effectiveness and cost-benefit of an influenza vaccination program for health care workers. Can J Infect Dis 1991;2:101-8. [DOI] [PMC free article] [PubMed]
- 20.Carman WF, Elder AG, Wallace LA, McAulay K, Walker A, Murray GD, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000;355:93-7. [DOI] [PubMed]
- 21.Potter J, Stott DJ, Roberts MA, Elder AG, O'Donnell B, Knight PV, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 1997;175:1-6. [DOI] [PMC free article] [PubMed]
- 22.Fedson DS. Pneumococcal vaccination in the United States and 20 other developed countries, 1981–1996. Clin Infect Dis 1998;26:1117-23. [DOI] [PubMed]
- 23.Influenza and pneumococcal vaccination levels among adults aged greater than or equal to 65 years — United States. MMWR 1998;47:797-802. [PubMed]
- 24.Gaillat J, Zmirou D, Mallaret MR, Rouhan D, Bru JP, Stahl JP, et al. Essai clinique du vaccin antipneumococcique chez personnes âgées vivant en institution. Rev Epidemiol Sante Publique 1985;33:437-44. [PubMed]
- 25.Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med 1997;103:281-90. [DOI] [PubMed]
- 26.Ortqvist A, Hedlund J, Burman LA, Elbel E, Hofer M, Leinonen M, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet 1998;351: 399-403. [DOI] [PubMed]
- 27.Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, et al. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med 1986,315:1318-27. [DOI] [PubMed]
- 28.Honkanen PO, Keistinen T, Miettinen L, Herva E, Sankilampi U, Laara E, et al. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia among persons aged 65 years or older. Vaccine 1999;17:2493-500. [DOI] [PubMed]
- 29.Farr BM, Johnston BL, Cobb DK, Fisch MJ, Germanson TP, Adal KA, et al. Preventing pneumococcal bacteremia in patients at risk. Arch Intern Med 1995; 155;2336-40. [PubMed]
- 30.Sisk JE, Moskowitz AJ, Whang W, Lin JD, Fedson DS, McBean AM et al. Cost effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA 1997;278:1333-9. [PubMed]
- 31.Fine MJ, Smith MA, Carson CA, Meffe F, Sankey SS, Weissfeld LA, et al. Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomized controlled trials. Arch Intern Med 1994;154:2666-77. [DOI] [PubMed]
- 32.Green K, Landry L, Goldenberg E. Effectiveness of a pneumococcal vaccination program in preventing invasive pneumococcal disease [abstract 633]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sept 26–29; San Francisco.
- 33.Jefferson TO, Demicheli V, Deeks JJ, Rivetti D. Amantadine and rimantadine for preventing and treating influenza A in adults [Cochrane review]. In: The Cochrane Library; Issue 1, 2000. Oxford: Update Software. [DOI] [PubMed]
- 34.Arden NH, Patriarca PA, Fasano MB, Lui KJ, Harmon MW, Kendal AP, et al. The roles of vaccination and amantadine prophylaxis in controlling an outbreak of influenza A (H3N2) in a nursing home. Arch Intern Med 1988;148:865-8. [PubMed]
- 35.Control of influenza A outbreaks in nursing homes: amantadine as an adjunct to vaccine — Washington, 1989–90. MMWR 1991;40:841-4. [PubMed]
- 36.Staynor K, Foster G, McArthur M, McGeer A, Petric M, Simor AE. Influenza A outbreak in a nursing home: the value of early diagnosis and the use of amantadine hydrochloride. Can J Infect Control 1994;9:109-11. [PubMed]
- 37.Libow LS, Neufeld RR, Olson E, Breuer B, Starer P. Sequential outbreak of influenza A and B in a nursing home: efficacy of vaccine and amantadine. J Am Geriatr Soc 1996;44:1153-7. [DOI] [PubMed]
- 38.Tamblyn SE. Influenza control in long-term care facilities: the Perth County experience [abstract R4-7]. Options for the Control of Influenza III; 1996 May 4–9; Cairns, Australia.
- 39.Hayden FG. Antivirals for pandemic influenza. J Infect Dis 1997;176(Suppl 1):S56-61. [DOI] [PubMed]
- 40.Gomolin IH, Leib HB, Arden NH, Sherman FT. Control of influenza outbreaks in the nursing home: guidelines for diagnosis and management. J Am Geriatr Soc 1995;43:71-4. [DOI] [PubMed]
- 41.Degelau J, Somani S, Cooper SL, Irvine PW. Occurrence of adverse effects and high amantadine concentrations with influenza prophylaxis in the nursing home. J Am Geriatr Soc 1990;38:428-32. [DOI] [PubMed]
- 42.Peters NL, Oboler S, Hair C, Laxson L, Kost J, Meiklejohn G. Treatment of an influenza A outbreak in a teaching nursing home. Effectiveness of a protocol for prevention and control. J Am Geriatr Soc 1989;37:210-8. [DOI] [PubMed]
- 43.Stange KC, Little DW, Blatnik B. Adverse reactions to amantadine prophylaxis of influenza in a retirement home. J Am GeriatrSoc 1991;39(7):700-5. [DOI] [PubMed]
- 44.Tamblyn SE. Amantadine use in influenza outbreaks in long-term care facilities. CMAJ 1997;157(11):1573-4. Available: www.cma.ca/cmaj/vol-157/issue-11/1573.htm [PMC free article] [PubMed]
- 45.Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet 2000;355:827-35. [DOI] [PubMed]


