We describe the design, development, analytical performance, and a limited clinical evaluation of the 10-color Xpert MTB/XDR assay (CE-IVD only, not for sale in the United States). This assay is intended as a reflex test to detect resistance to isoniazid (INH), fluoroquinolones (FLQ), ethionamide (ETH), and second-line injectable drugs (SLIDs) in unprocessed sputum samples and concentrated sputum sediments which are positive for Mycobacterium tuberculosis.
KEYWORDS: 10-color, GeneXpert, Mycobacterium tuberculosis, TB, XDR, drug resistance, rapid diagnostics
ABSTRACT
We describe the design, development, analytical performance, and a limited clinical evaluation of the 10-color Xpert MTB/XDR assay (CE-IVD only, not for sale in the United States). This assay is intended as a reflex test to detect resistance to isoniazid (INH), fluoroquinolones (FLQ), ethionamide (ETH), and second-line injectable drugs (SLIDs) in unprocessed sputum samples and concentrated sputum sediments which are positive for Mycobacterium tuberculosis. The Xpert MTB/XDR assay simultaneously amplifies eight genes and promoter regions in M. tuberculosis and analyzes melting temperatures (Tms) using sloppy molecular beacon (SMB) probes to identify mutations associated with INH, FLQ, ETH, and SLID resistance. Results can be obtained in under 90 min using 10-color GeneXpert modules. The assay can differentiate low- versus high-level resistance to INH and FLQ as well as cross-resistance versus individual resistance to SLIDs by identifying mutation-specific Tms or Tm patterns generated by the SMB probes. The assay has a limit of detection comparable to that of the Xpert MTB/RIF assay and successfully detected 16 clinically significant mutations in a challenge set of clinical isolate DNA. In a clinical study performed at two sites with 100 sputum and 214 clinical isolates, the assay showed a sensitivity of 94% to 100% and a specificity of 100% for all drugs except for ETH compared to that of sequencing. The sensitivity and specificity were in the same ranges as those of phenotypic drug-susceptibility testing. Used in combination with a primary tuberculosis diagnostic test, this assay should expand the capacity for detection of drug-resistant tuberculosis near the point of care.
INTRODUCTION
Drug-resistant Mycobacterium tuberculosis remains a significant threat to global tuberculosis (TB) care and public health. The World Health Organization (WHO) has estimated that in 2018, 4.1% of new cases and 19% of previously treated cases had rifampicin-resistant TB (RR-TB) or multidrug-resistant TB (MDR-TB), and as many as 6.2% of MDR cases had extensively drug-resistant TB (XDR-TB) (1). MDR-TB is caused by M. tuberculosis that is resistant to isoniazid (INH) and rifampicin (RIF), and XDR-TB is additionally resistant to at least one of the fluoroquinolones (FLQs) and second-line injectable drugs (SLIDs), including amikacin (AMK), capreomycin (CAP), and kanamycin (KAN). Phenotypic drug susceptibility testing (pDST), the current gold standard for identifying drug resistance in M. tuberculosis, takes 6 to 8 weeks to provide definitive results and poses a biohazard risk for laboratory personnel, especially when working with XDR strains. Thus, treatment is often empirically based on other factors such as past medical history or local prevalence of resistance (2, 3). Delays in appropriate treatment can increase both mortality and transmission of drug-resistant strains.
The Xpert MTB/RIF (in vitro diagnostic use only) assay and its more advanced version, the Xpert MTB/RIF Ultra (CE-IVD only; not for sale in the United States) assay (Cepheid, CA, USA), were designed to simultaneously detect the presence of M. tuberculosis and RIF resistance in an integrated and fully automated system directly from sputum. These assays can be used with only minimal training at point-of-care settings (4, 5), where widespread implementation has led to an overall decrease in TB incidence in some studies (6) and increased notifications of RIF resistance in others (7). However, these assays only detect RIF resistance, and it has been shown that selection of TB treatment regimens based only on detection of RIF resistance can result in suboptimal therapy for 49% of patients with MDR- or XDR-TB compared to that with pDST (8). Thus, additional tests that identify resistance to INH, FLQs, and SLIDs equally rapidly in similar point-of-care settings are also necessary. The MTBDRplus and MTBDRsl version 2.0 (HAIN Life Sciences, Germany) assays are currently recommended by the WHO as molecular tests to detect INH and RIF resistance and FLQ and SLID resistance, respectively. However, these assays are not suitable for near-patient or point-of-care testing, as they require sophisticated laboratory settings (9).
We previously developed and validated a prototype cartridge-based assay that detected M. tuberculosis resistant to INH, the FLQs, and SLIDs directly from sputum using a multiplexed 10-color molecular test. This cartridge was a proof-of-principle prototype, which underscored our technical capability to develop a functional 10-color reflex test on sputum samples positive for M. tuberculosis by primary diagnostic tests, including the Xpert MTB/RIF and Xpert MTB/RIF Ultra assays. The prototype cartridge detected resistance with high sensitivity and specificity directly from the sputum of TB patients in less than 2 h (10, 11). However, since the development of this cartridge, mutations in the M. tuberculosis fabG1 gene have been shown to account for a significant portion of INH resistance not detected by mutations at other gene loci (12). Mutations at the −14 position in the eis promoter region have been shown to confer resistance to both KAN and AMK (other eis promoter mutations only confer resistance to KAN) (13, 14), while the mutation “A1401G” in the rrs gene confers cross-resistance to AMK, KAN, and CAP (15, 16). Furthermore, mutations in the quinolone resistance-determining region (QRDR) of gyrA have been subdivided into mutations that cause low-level resistance (GyrA A90V, S91P, and D94A) and other mutations that elevate MICs to the commonly used FLQ drugs to treat TB more substantially (17–19). The MIC differences caused by these two different categories of mutations have also been shown to have different clinical outcomes when an FLQ is used as part of MDR/XDR therapy (20, 21). Similar MIC differences are also observed with INH. Mutations in regions upstream of the inhA promoter, such as in the fabG1 gene or oxyR-ahpC intergenic regions, or codon 315 of the katG gene have been clinically associated with high-level resistance to INH (22–26). Interestingly, specific mutations in the inhA promoter region shown low-level resistance to INH, but these same inhA mutations in combination with mutations in codon 315 of katG or those upstream of the inhA promoter region confer high-level resistance to INH (22–24). In the present study, we describe a new more advanced test, the Xpert MTB/XDR assay (CE-IVD only, not for sale in the United States), which was redesigned to improve mutation coverage for INH, differentiate low-level INH resistance from higher-level resistance, identify ethionamide (ETH) resistance, distinguish between low and high levels of resistance to FLQs, and identify cross-resistance versus individual resistance to the SLIDs. We have also improved the overall sensitivity of the assay and reduced the time to result to less than 90 min after the run is started. Here, we describe the development and initial clinical evaluation of this assay along with the additional attributes to improve assay performance.
MATERIALS AND METHODS
Description of the assay.
The Xpert MTB/XDR assay is a 9-plex assay consisting of 10 sloppy molecular beacon (SMB) probes (27) that target 8 different M. tuberculosis genes detecting resistance to INH, ETH, FLQ, and SLID. To detect INH resistance, four probes target the inhA promoter (nucleotides −1 to −32), the katG (codons 311 to 319) and fabG1 (codons 199 to 210) genes, and the oxyR-ahpC (ahpC) intergenic region (nucleotides −5 to −50). Identification of inhA promoter mutations in a specific optical channel additionally enables detection of ETH resistance and allows differentiation of low-level INH resistance, as both resistance characteristics are encoded by mutations in the inhA promoter (22, 28, 29). To detect FLQ resistance, three overlapping probes target the gyrA QRDR (codons 87 to 95) and one probe targets the gyrB QRDR (codons 531 to 544). The three gyrA probes used in the assay have 8 defined mutant windows, which enables specific identification of the QRDR mutations A90V, S91P, and D94A associated with low-level FLQ resistance and differentiates them from other QRDR mutations associated with higher-level FLQ resistance, as described in detail in Results. To detect resistance to SLIDs, namely, AMK, KAN, and CAP, one probe targets the rrs gene (nucleotides 1396 to 1417) and a second probe targets the eis promoter region (nucleotides −6 to −42). Cross-resistance between AMK, KAN, and CAP is well documented (14–16, 30) and is captured by the rrs probe. The eis probe can differentiate between C(−14)T which confers cross-resistance to KAN and AMK and other mutations in the eis promoter region which only confer resistance to KAN, since the probe has been specifically designed to generate a higher melting temperature (Tm) shift for the C(−14)T mutation and a lower Tm shift for all the other mutations from the wild-type (WT) Tm. To serve as an internal sample processing and PCR control, an additional TaqMan probe (SPC probe) targets a Bacillus atrophaeus subsp. globigii gene using the same fluorophore as the ahpC probe. This enabled us to accommodate the 11 probes in the assay within the 10 optical channels. Dehydrated B. globigii spore beads are included in the assay cartridge. The SPC probe is a TaqMan probe, which is only detectable during PCR amplification, does not generate a melting curve, and thus will not interfere with the Tm generated by the ahpC SMB probe. Each of the 10 probes in the assay has one defined wild-type (WT) Tm range (defined as a WT Tm window) and one or several Tm ranges that define the presence of mutants (mutant Tm windows). WT or mutant sequences are identified by the WT or mutant Tm values, respectively, for each target, which results in the “resistance not detected” or “resistance detected” calls, respectively, for the related drugs. The PCR assay consists of two phases. The first phase is a conventional symmetric PCR, followed by a second nested asymmetric PCR phase, except for the SPC assay, which is symmetric in both phases. The nested asymmetric assay enables preferential amplification of the target strands to which the SMB probes bind with high efficiency even in this 9-plex assay system. Specific in-cartridge microfluidics allow the products of the first PCR to be added to the second PCR after 31 cycles are complete. The second PCR consists of 40 cycles followed by the melt curve stage. No third stage of linear PCR is used in this assay unlike the earlier prototype XDR cartridge, which reduces the time to result from 120 to <90 min. Using included software, the assay result output shows cycle threshold (CT) values in the “analyte result” tab, Tm values with the specific Tm windows in the “melt” tab, and the resistance “detected/not detected” to the individual drugs in the “test result” tab. A pan-susceptible sample is expected to generate the following “test result” outputs: MTB detected, INH resistance not detected, FLQ resistance not detected, AMK resistance not detected, KAN resistance not detected, CAP resistance not detected, ETH resistance not detected. A pan-resistant sample is expected to generate the following “test result” outputs: MTB detected, INH resistance detected, FLQ resistance detected, AMK resistance detected, KAN resistance detected, CAP resistance detected, ETH resistance detected. Presence or absence of resistance to each target drug will be indicated in the result output depending on the mutation detected. Low resistance to INH and FLQ is also specifically indicated in the “test result” output.
Cartridge configuration, assay composition, and testing procedure.
The Xpert MTB/XDR cartridge is a modified version of the prototype assay cartridge described previously (31). It consists of a multiposition fluidic valve, a bacterial capture chamber, and 11 chambers containing buffers and reagents for sample processing and PCR plus an integrated 50-μl PCR tube. Two sets of two lyophilized beads each are used to amplify resistance-conferring regions of the inhA promoter, katG, fabG1, ahpC, gyrA, gyrB, rrs, and eis promoter as well as an internal control sequence of B. globigii. The first bead set is used to perform 9-plex PCR, followed by a full nested or hemi-nested PCR of the first set of amplicons using the second bead set. The second bead set contains SMBs for 10 gene targets and a TaqMan probe for the internal control. The SMBs for the ahpC target and internal control share the same channel and are designed not to interfere with the other’s detection. The assay performs a post-PCR melt analysis after the second PCR to generate first-derivative Tm curves. The Tm values are identified by the automated GeneXpert Tm calling software (Cepheid, Sunnyvale, CA) and classified as Tm values that identify the WT or mutant amplicon sequence based on predefined Tm parameters (Tm windows). These Tm values are then used to determine the presence or absence of resistance to the target drugs.
To perform a test, each sample (spiked sputum, clinical sputum samples, cultured M. tuberculosis, or Mycobacterium bovis BCG bacterial suspension) was first mixed at a 2:1 ratio with an NaOH- and isopropanol-containing sample reagent (SR) as described previously (32); the sample was then added to the sample loading chamber of the cartridge (for CE-IVD use, sputum is the only recommended sample type currently for diagnostic purposes). The loaded cartridge was placed into a GeneXpert instrument running software developed for the Xpert MTB/XDR assay (Cepheid, Sunnyvale, CA). The assay was then started, and automated processing of the sample for DNA isolation followed by the two-phase PCR assay and melt analysis was performed. The microfluidics were similar to that previously described (31).
Preparation of M. tuberculosis and M. bovis BCG culture stocks and determination of CFU.
M. tuberculosis culture stock preparation and CFU counts were determined as described previously (31). An attenuated strain of M. tuberculosis H37Rv (mc26030) or M. bovis BCG was cultured by inoculating 1:100 in 20 ml of Difco Middlebrook 7H9 medium (BD Biosciences, San Jose, CA, USA) supplemented with 10% BBL Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment (BD Biosciences), 0.05% Tween 80 (Sigma-Aldrich, St. Louis, MO), and 24 μg/ml calcium pantothenate (Sigma-Aldrich) for M. tuberculosis H37Rv (mc26030). Tween 80 is used to disaggregate the M. tuberculosis bacterial suspension by reducing surface tension (33). The strains were grown to an optical density at 600 nm of 0.6 to 0.8 and subcultured twice before performing dilutions for CFU determinations and storage. The cultures were quantified by plating 10−5, 10−6, and 10−7 dilutions in triplicates on 7H10 plates supplemented with 10% OADC and 24 μg/ml calcium pantothenate for M. tuberculosis H37Rv (mc26030). The cultures were divided into 500-μl aliquots and stored at −80°C until use. Colony counts was performed 3 weeks after plating, once the colonies became visible.
Dilutions and spiking in sputum for determining the limit of detection.
To dilute and spike M. tuberculosis or M. bovis BCG in sputum for analytical studies, a frozen aliquot was processed as previously described (31). At the end of the sonication steps, bacterial suspension was serially diluted to 1,000 CFU/ml. The 1,000 CFU/ml dilution was spiked in sputum to obtain the final test concentration. SR was added at a 2:1 ratio to allow sufficient volume to distribute in 2 ml in each cartridge. To determine the limit of detection (LoD), we tested 10, 20, 40, 60, 80, 100, and 200 CFU/ml, 20 replicates each. For the LoD in concentrated sputum, unprocessed sputum samples were first spiked with the target level of M. bovis BCG CFU, and each spiked sputum specimen was processed to obtain concentrated sediment using the BD BBL MycoPrep mycobacterial system digestion/decontamination kits (Becton, Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions.
Preparation of gyrA QRDR mutant library and test protocol.
We have created a repository of plasmids by cloning approximately 100-bp fragment of the gyrA gene in vector pMV306H with the help of gene synthesis and mutagenesis services of GenScript Biotech Inc. (Piscataway, NJ, USA). The cloned fragments contained the individual gyrA QRDR single point mutations that are frequently associated with FLQ resistance (17–19) in each plasmid. Lyophilized recombinant plasmids were resuspended in water and quantified by a NanoDrop 1000 device. To determine the Tm of gyrA probes, each plasmid was tested multiple times at 100 pg/reaction.
Preparation of mixed cells to test for detection of heteroresistance.
Quantified preparations of hardened Escherichia coli cells (Maine Molecular Quality Controls Inc., Saco, ME, USA) that had been transfected with plasmids containing WT or mutant target sequences, including C(−15)T in the inhA promoter, S513T in the katG gene product, L203L in the fabG1 gene product, C(−39)T in oxyR-ahpC region, D94G in the gyrA gene product, E540D in the gyrB product, A1401G in rrs, and C(−14)T in the eis promoter, were used. A series of cell mixtures containing 0%, 10%, 15%, 20%, 25%, 50%, 60%, 75%, 90%, and 100% of mutant was tested against a background of cells with WT sequences. The total amount of cells tested in each mixture was 10,000 cells/ml, containing enough volume to test three replicates. Similarly, mixtures of low-level-FLQ-resistance-conferring mutations A90V, D94A, and S91P in the gyrA gene product were prepared by mixing a series of cell mixtures containing 0%, 10%, 20%, 30%, 40%, 50%, and 100% of mutant cells with cells with the WT gyrA gene sequence at final cell counts of 5,000 cells/ml, in replicates of three, and tested on a GeneXpert instrument as described previously (31).
Mutant panel challenge.
DNA was extracted from a panel of 14 clinical isolates with canonical mutations in the target genes and promoter regions known to be associated with clinical INH, FLQ, ETH, and SLID resistance by a boiling preparation using InstaGene Matrix (Bio-Rad, Hercules, CA USA) or using a phenol-chloroform method, each described previously (27, 34). All the mutations were confirmed by Sanger sequencing of each of the target genes and promoter regions. All isolates were quantified using the Qubit double-stranded DNA (dsDNA) HS assay kit (Thermo Fisher Scientific, Waltham, MA, USA). All DNA extracts were tested at a concentration that was approximately 3× a predetermined BCG DNA LoD (in terms of genome equivalents) or higher in four instances when we observed indeterminate resistance calls resulting from absence of Tm values due to a poor quality of isolated DNA. The diluent solution (Tris-EDTA-Tween buffer) was used as a negative control, and BCG DNA at 3× the LoD was used as a positive control. Each isolate was tested in replicates of three and run on a GeneXpert instrument as described previously (31).
Analytical specificity and cross-reactivity.
Thirty species of non-tuberculous Mycobacterium (NTM) (see Table S1 in the supplemental material), either purchased from the American Type Culture Collection (ATCC) or kindly provided by National Jewish Health (Denver, CO, USA), were cultured and quantified similar to that as described for M. tuberculosis in 7H9 medium supplemented with 10% Middlebrook OADC growth supplement and 0.05% Tween 80. We were able to cultivate and quantify all 30 NTM species except for Mycobacterium genavense, for which we could only record the optical density. Gram-negative and Gram-positive bacteria obtained from the University Hospital Microbiology Lab (University Hospital, Newark, NJ, USA) were cultivated on blood agar plates. DNA was isolated by a boiling preparation using an InstaGene matrix (27). Both NTM and Gram-negative and Gram-positive bacteria were tested at final concentrations equivalent to 106 to 107 CFU/ml. Dilution buffer was used as the negative control, and BCG (cells and DNA) at 3× LoD was used as the positive control.
Twelve clinically relevant NTM species were used in a mixture with M. bovis BCG to test for possible interference with M. tuberculosis detection by high concentrations of NTM. The NTMs were diluted to a final test concentration 106 CFU/ml and mixed with a low concentration of M. bovis BCG (at 408 CFU/ml, i.e., approximately 3× LoD of the stock) (see Table S3) with enough volume for four replicates. Dilution buffer was used as the negative control and M. bovis BCG, at 3× the LoD concentration, was used as the positive control. To all test samples, SR reagent was added and incubated for 15 min. Two milliliters of sample was aliquoted to each cartridge and loaded into the GeneXpert instrument.
Clinical study protocol.
A small clinical study was performed with well-characterized and archived frozen sputum and culture isolates from deidentified TB patients with a variety of drug-resistant phenotypes provided by the Foundation of Innovative New Diagnostics (FIND) (35). The samples were obtained from Georgia, Moldova, Peru, and Vietnam, representing three different continents, and were chosen to represent all the common clinically relevant mutations present in the target genes considering the global estimate of prevalence. The study was performed at two different sites at New Jersey Medical School, Rutgers University, Newark, NJ, USA, and San Raffaele Hospital, Milan, Italy. The study consisted of 214 clinical isolates and 100 sputum samples from TB patients, which were equally distributed between the sites (107 clinical isolates and 50 sputum samples at each site). pDST results were available for INH, ETH, at least one or more of the FLQs (ofloxacin, levofloxacin and moxifloxacin), and the SLIDs (AMK, CAP, and KAN). Sanger sequencing results were available for katG, inhA promoter, fabG1, oxyR-ahpC intergenic region, gyrA, gyrB, rrs, and eis promoter, i.e., all the target genes in the assay along with several other nontarget genes (including pncA, ethA, ethA upstream, rpsl, tlyA, ndh, etc.) associated with resistance to other first-line drugs. Each sputum sample consisted of 0.5-ml duplicate aliquots, which were thawed at room temperature, pooled, and vortexed thoroughly to ensure homogenization at each site before processing. The pooled 1-ml sputum sample was transferred to a new tube, and 2 volumes of sample reagent (SR) was added to it, mixed, and incubated for 15 min before performing the test. The frozen clinical isolates contained approximately 400 to 500 µl of cell suspension at a concentration of approximately 106 CFU/ml. The isolates were thawed, and sterile Tris (pH 8.0) or phosphate-buffered saline (PBS) was added until the total volume of cell suspension was equivalent to 1 ml; the samples were vortexed well for 30 s followed by standing for 5 min. Two volumes of sample reagent (SR) was added to the 1.0-ml suspension, mixed, and incubated for 15 min before performing the test. All the operators at each site as well as personnel performing the result interpretation and data analysis were completely blinded to the sequencing and the pDST results. The aim of this study was to determine the diagnostic performance (sensitivity and specificity) of the Xpert MTB/XDR assay for INH, ETH, FLQ, and SLID resistance detection compared to those of the individual reference standards pDST (phenotypic reference standard) and sequencing (molecular reference standard). We could not perform analysis for sputum and isolates separately, since the sputum samples in our study had a very low representation of drug-susceptible strains, which would fail to generate statistically significant performance values for the assay. The study was thus supplemented with isolates which had a larger representation of drug-susceptible strains, and a composite analysis was performed combining both sample types. Additionally, “indeterminate” rates for each drug type and rates of “nondeterminate” runs (run aborts due to errors) of the assay were also calculated for each sample type at each site. The nondeterminate samples were repeated only for the isolates, since a second aliquot was available. No repeat runs were performed for nondeterminate sputum samples, since the entire sample was used for the first run.
Statistical analysis.
LoD was calculated using the percentages of the replicates resulting in successful TB detection and drug-susceptibility calls at each input CFU concentration in sputum for both Xpert MTB/XDR and Xpert MTB/RIF assays using probit analysis on R studio version 1.2.5019, “Elderflower” (R Studio, PBC, Boston, MA). Binary probit regression results were fitted through the tested concentrations, and lower and upper 95% confidence intervals (95% CIs) were generated for the curve. The 95% CI for the minimum input concentration was determined by where the 95% probability level crossed the upper and lower 95% CIs, which indicated the LoD. Mean Tm values and standard deviations were calculated in Microsoft Excel.
RESULTS
Detection and differentiation of gyrA QRDR mutations.
We redesigned our previous assay in order to be able to specifically identify the gyrA QRDR mutations A90V, S91P, and D94A that are associated with low-level FLQ resistance (18, 36) and to distinguish them from the other QRDR mutations that are associated with higher-level resistance. We designed three overlapping sloppy molecular beacon (SMB) probes with slightly varied sequences against the gyrA QRDR. These three gyrA probes were designed to generate specific “three-Tm window” patterns, which identify and distinguish each of the above-mentioned QRDR mutations when they occur in the absence of other gyrA QRDR mutations. We designated one wild-type (WT) window and multiple mutant windows for each of the gyrA probes. The gyrA1 and gyrA3 probes were assigned three mutant windows (MutA, MutB, and MutC), and the gyrA2 probe was assigned two mutant windows (MutA and MutB). The multiple-mutant and the single WT Tm for each probe can theoretically generate nearly 48 different three-Tm window combinations specific to the QRDR mutations and the WT sequence. The WT QRDR sequence generates a “WT-WT-WT” Tm window pattern for the three probes, and the mutant sequences have one or more of the WT Tm values replaced by a mutant Tm (Table 1). Thus, the three overlapping probes with different binding affinities to the gyrA QRDR enabled us to generate tri-window Tm patterns specific to each A90V, S91P, or D94A mutation, namely, “MutB-MutA-MutB,” “MutB-MutA-MutC,” or “MutA-MutA-WT”, respectively, for the “gyrA1-gyrA2-gyrA3” probes. Each pattern is specific for one of these three mutations, enabling the identification of low FLQ resistance. Additionally, we designed the probes to be agnostic to the Ser/Thr polymorphism in codon 95 so that identical Tm patterns for the WT and all the mutant sequences were generated for both these polymorphisms. We tested gyrA QRDR-containing plasmids bearing 11 different gyrA mutation types for both the 95S and 95T polymorphisms as a challenge set. The probes successfully identified and differentiated the individual low-resistance-conferring mutations from other mutations by their different Tm signatures (falling in different Tm windows).
TABLE 1.
Tm values for gyrA probes and resistance detection
| Genotypea | Tm category | Probe Tm (°C) (±SD), Δ, or window type for associated phenotypeb |
FLQ resistance output | ||
|---|---|---|---|---|---|
| Probe 1 | Probe 2 | Probe 3 | |||
| WT | Mean | 76.2 (±0.2) | 70 (±0.2) | 70.8 (±0.2) | Not detected |
| Window | WT | WT | WT | ||
| G88C | Mean | 72.7 (±0.3) | 65.2 (±0.4) | 66.4 (±0.3) | Detected |
| Δ | −3.4 | −4.8 | −4.4 | ||
| Window | Mut B | Mut B | Mut C | ||
| G88A | Mean | 71.7 (±0.5) | 63.9 (±0.3) | 65.4 (±0.3) | Detected |
| Δ | −4.4 | −6.1 | −5.4 | ||
| Window | Mut B | Mut B | Mut C | ||
| A90V | Mean | 72.2 (±0.2) | 75.6 (±0.3) | 76.2 (±0.2) | Low resistance detected |
| Δ | −4.1 | 5.6 | 5.4 | ||
| Window | Mut B | Mut A | Mut B | ||
| S91P | Mean | 72.2 (±0.1) | 74.8 (±0.1) | 66.1 (±0.4) | Low resistance detected |
| Δ | −3.9 | 4.8 | −4.7 | ||
| Window | Mut B | Mut A | Mut C | ||
| D94A | Mean | 78.9 (±0.2) | 73.4 (±0.2) | 71.4 (±0.1) | Low resistance detected |
| Δ | 2.7 | 3.4 | 0.6 | ||
| Window | Mut A | Mut A | WT | ||
| D94G | Mean | 76 (±0.2) | 69.5 (±0.3) | 75.8 (±0.2) | Detected |
| Δ | −0.1 | −0.5 | 5 | ||
| Window | WT | WT | Mut B | ||
| D94N | Mean | 72.9 (±0.3) | 66.1 (±0.4) | 68.9 (±0.3) | Detected |
| Δ | −3.2 | −3.9 | −1.9 | ||
| Window | Mut B | Mut B | WT | ||
| D94Y | Mean | 72.5 (±0.3) | 65.1 (±0.4) | 68.6 (±0.3) | Detected |
| Δ | −3.6 | −4.9 | −2.2 | ||
| Window | Mut B | Mut B | Mut C | ||
| D94H | Mean | 73.2 (±0.3) | 65.6 (±0.3) | 68.9 (±0.3) | Detected |
| Δ | −2.9 | −4.4 | −1.9 | ||
| Window | WT | Mut B | WT | ||
| A90V+S91P | Mean | 67.5 (±0.3) | 79.3 (±0.2) | 71.7 (±0.1) | Detected |
| Δ | −8.6 | 9.3 | 0.9 | ||
| Window | Mut C | Mut A | WT | ||
| A90V+G88C | Mean | 67.8 (±0.5) | 71.3 (±0.2) | 72.2 (±0.2) | Detected |
| Δ | −8.3 | 1.3 | 1.4 | ||
| Window | Mut C | WT | WT | ||
All include 95S or T.
Tm values are means and standard deviations (SDs) from 3 to 163 replicates from multiple experiments depending on genotype for the three gyrA probes with representative gyrA mutant plasmids and their corresponding windows. WT windows are indicated, and the three mutant windows, MutA, MutB, and MutC, are light gray, dark gray, and dark gray with boldface, respectively.
Mutant panel challenge.
We assessed the ability of the Xpert MTB/XDR assay to detect drug-resistance-associated mutations in clinical M. tuberculosis isolates. A panel of 14 clinical isolates with canonical mutations in the target genes and promoter regions known to be associated with clinical INH, ETH, FLQ, and SLID resistance were tested (Table 2). All mutations were confirmed by pDST and Sanger sequencing of each of the target genes and promoter regions. The assay was able to detect all of the mutations targeted by the assay (except a single gyrB mutation), and the assay correctly determined the specific drug resistance profile for all the isolates tested. The assay correctly detected low INH and ETH resistance-conferring mutation [inhA C(−15)T] and all the other INH resistance-conferring mutations in the katG gene product (S315T), fabG1 (G603A), and ahpC [G(−48)A, G(−6)A]. The assay also detected all the gyrA QRDR mutations, including a triple and a double mutant. The assay resulted in correct “low FLQ resistance detected” calls for A90V, S91P, and D94A mutations that are associated with low-level FLQ resistance and resulted in the correct “FLQ resistance detected” call for D94G and D94Y mutations. Isolates with mutations associated with SLID resistance in eis [G(−10)A, C(−12)T] and rrs (a1401g) genes were also identified correctly. Although the assay was able to correctly determine the resistance profile of all 14 isolates, it was not able to identify the gyrB product mutation T539N because the Tm difference (dTm) between the WT Tm and the Tm for this mutation was 1.1°C, which did not result in the Tm falling into the mutant window for the gyrB probe. However, as the isolate also had an A90V mutation, it was identified as a low-FLQ-resistance sample. The gyrB T539N mutation has been reported to be present at a very low frequency in FLQ-resistant isolates (37), and functional genetic studies have demonstrated that introduction of this mutation into the wild-type M. tuberculosis genome does not result in any appreciable increase in MIC to FLQ (38). Thus, the assay’s failure to detect gyrB T539N would not be expected to affect the sensitivity for detecting FLQ resistance.
TABLE 2.
Xpert MTB/XDR mutant DNA panel challenge
| Strain type | pDSTa | Gene conferring resistance | Resistance-conferring variantb |
Tm (°C) (SDc) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| inhA | katG | fabG | ahpC | gyrA1 | gyrA2 | gyrA3 | gyrB2 | rrs | eis | ||||
| H37Rv | NAd | NA | None | 76.3 (±0.0) | 73.7 (±0.0) | 71.4 (±0.1) | 69.3 (±0.0) | 76.1 (±0.1) | 70.3 (±0.1) | 71.1 (±0.1) | 69.6 (±0.0) | 75 (±0.0) | 68.5 (±0.1) |
| Clinical isolate | INHr | katG | S315T | 76.5 (±0.0) | 68.4 (± 0.0) | 71.6 (±0.0) | 0 (±0.0) | 76.5 (±0.0) | 70.4 (±0.0) | 71.3 (±0.0) | 69.7 (±0.1) | 75.2 (±0.0) | 68.6 (±0.0) |
| Clinical isolate | OFXr, MOXr | gyrA | D94G | 76.5 (±0.1) | 68.4 (±0.1) | 71.5 (±0.0) | 0.0 | 76.2 (±0.1) | 69.7 (±0.0) | 75.7 (±0.0) | 69.7 (±0.1) | 71.0 (±0.0) | 68.6 (±0.1) |
| INHr | katG | S315T | |||||||||||
| KANr, AMKr | rrs | a1401g | |||||||||||
| Clinical isolate | OFLr, MOXr | gyrB | C539A | 71.1 (±0.0) | 73.8 (±0.0) | 71.6 (±0.0) | 67.2 (±0.0) | 72.3 (±0.0) | 75.9 (±0.1) | 76.5 (±0.0) | 68.6 (±0.0) | 71.1 (±0.0) | 68.6 (±0.0) |
| gyrA | A90V | ||||||||||||
| KANr, AMKr | rrs | a1401g | |||||||||||
| INHr | OxyR-ahpC | g(−6)a | |||||||||||
| inhA promoter | c(−15)t | ||||||||||||
| Clinical isolate | OFXr, MOXr | gyrA | D94Y | 76.3 (±0.0) | 68.2 (±0.0) | 75.6 (±0.0) | 0.0 | 72.7 (±0.0) | 0.0 | 69 (±0.0) | 69.6 (±0.0) | 75 (±0.0) | 68.5 (±0.0) |
| INHr | katG | S315T | |||||||||||
| fabG1 | g609a | ||||||||||||
| Clinical isolate | OFXr, MOXr | gyrA | S91P | 71.1 (±0.0) | 73.9 (±0.1) | 71.7 (±0.1) | 0.0 | 72.2 (±0.0) | 75.1 (±0.0) | 66.6 (±0.0) | 69.7 (±0.0) | 75.2 (±0.0) | 68.6 (±0.0) |
| INHr | inhA promoter | c(−15)t | |||||||||||
| Clinical isolate | OFXr, MOXr | gyrA | A90A/V, D94D/G | 71.03 (±0.1) | 73.7 (±0.1) | 71.6 (±0.0) | 0.0 | 76.13 (±0.1) | 69.4 (±0.2) | 76 (±0.10) | 69.7 (±0.0) | 75.1 (±0.0) | 68.5 (±0.0) |
| INHr | inhA promoter | c(−15)t | |||||||||||
| OxyR-ahpC | g(−6)a | ||||||||||||
| Clinical isolate | OFXr, MOXr | gyrA | G88G/A, A90V, S91S/P | 76.43 (±0.1) | 68.3 (±0.1) | 71.5 (±0.1) | 69.3 (±0.0) | 72.2 (±0.1)/67.6 (±0.1) | 70.5 (±0.0)/79.4 (±0.0) | 71.6 (±0.0)/76.7 (±0.0) | 69.7 (±0.1) | 71 (±0.1) | 68.6 (±0.1) |
| INHr | katG | S315T | |||||||||||
| KANr, AMKr | rrs | a1401g | |||||||||||
| Clinical isolate | INHr | katG | S315T | 76.4 (±0.1) | 68.3 (±0.0) | 71.5 (±0.0) | 67.1 (±0.0) | 76.4 (±0.0) | 70.3 (±0.0) | 71.3 (±0.0) | 69.6 (±0.0) | 75.1 (±0.0) | 68.6 (±0.0) |
| OxyR-ahpC | a(−48)g | ||||||||||||
| Clinical isolate | INHr | katG | S315T | 76.4 (±0.1) | 68.3 (±0.1) | 71.5 (±0.1) | 66.03 (±0.1) | 76.3 (±0.0) | 70.3 (±0.0) | 71.2 (±0.0) | 69.6 (±0.1) | 75.03 (±0.1) | 68.6 (±0.1) |
| OxyR-ahpC | a(−48)g | ||||||||||||
| Clinical isolate | OFXr, MOXr | gyrA | D94A | 70.9 (±0.0) | 68.2 (±0.0) | 71.5 (±0.0) | 69.1 (±0.0) | 78.9 (±0.0) | 73.5 (±0.0) | 72.1 (±0.0) | 69.6 (±0.0) | 75 (±0.0) | 62.6 (±0.0) |
| INHr, ETHr | katG | S315T | |||||||||||
| inhA promoter | c(−15)t | ||||||||||||
| Clinical isolate | INHr | katG | S315T | 76.2 (±0.0) | 68.2 (±0.0) | 71.5 (±0.0) | 68.9 (±0.1) | 76.2 (±0.0) | 70.3 (±0.1) | 71.2 (±0.0) | 69.5 (±0.1) | 74.9 (±0.0) | 64.1 (±0.0) |
| KANr | eis promoter | g(−10)a | |||||||||||
| Clinical isolate | INHr | katG | S315T | 76.4 (±0.1) | 68.3 (±0.1) | 71.6 (±0.0) | 69.2 (±0.1) | 76.3 (±0.1) | 70.4 (±0.0) | 71.3 (±0.0) | 69.7 (±0.0) | 75.1 (±0.1) | 64.2 (±0.0) |
| KANr | eis promoter | g(−10)a | |||||||||||
| Clinical isolate | INHr, ETHr | katG | S315T | 71.1 (±0.1) | 68.4 (±0.1) | 71.7 (±0.1) | 69.1 (±0.1) | 76.5 (±0.1) | 70.3 (±0.1) | 71.4 (±0.0) | 69.7 (±0.1) | 75.2 (±0.1) | 62.7 (±0.0) |
| inhA promoter | c(−15)t | ||||||||||||
| KANr | eis promoter | c(−12)t | |||||||||||
| Clinical isolate | INHr, ETHr | katG | S315T | 70.9 (±0.0) | 68.2 (±0.0) | 71.5 (±0.0) | 69 (±0.0) | 76.3 (±0.0) | 70.2 (±0.2) | 71.4 (±0.0) | 69.6 (±0.1) | 75 (±0.1) | 62.6 (±0.0) |
| inhA promoter | c(−15)t | ||||||||||||
| KANr | eis promoter | c(−12)t | |||||||||||
INH, isoniazid; OFX, ofloxacin; MOX, moxifloxacin; KAN, kanamycin; AMK, amikacin; ETH, ethionamide.
Capital letters indicate amino acid change, and lowercase letters indicate nucleotide change.
SD, standard deviation calculated from replicates of three. All mutant Tm values are in boldface font.
NA, not applicable.
The Xpert MTB/XDR assay has a LoD that is comparable to that of Xpert MTB/RIF for M. tuberculosis detection.
The Xpert MTB/XDR assay includes a separate call-out for M. tuberculosis detection, which occurs when the assay’s inhA probe produces an identifiable Tm in either WT or mutant windows. Positive detection of M. tuberculosis is required for the assay software to generate a resistance “detected” or “not detected” call. Thus, if the inhA probe does not result in a detectable Tm (WT or mutant), the result will be “MTB not detected” and no resistance result output will be available irrespective of whether Tm values are generated by the other targets in the assay. The Xpert MTB/XDR assay is designed to be run as a reflex test after initial testing has identified the presence of M. tuberculosis in the sample. Thus, our preference was to ensure that the Xpert MTB/XDR assay was at least as sensitive as the Xpert MTB/RIF assay in its M. tuberculosis detection function. The LoD of M. tuberculosis for the Xpert MTB/XDR assay was determined by performing a head-to-head comparison with the Xpert MTB/RIF assay, using the same M. tuberculosis H37Rv stock cultures. Six concentrations (200, 100, 80, 60, 20, and 10 CFU/ml) of M. tuberculosis strain H37Rv mc26030 were spiked into pooled sputum samples collected from leftover clinical samples obtained at Rutgers University Hospital, Newark, NJ, that were confirmed to be M. tuberculosis negative by the Xpert MTB/RIF Ultra assay and tested in parallel by the Xpert MTB/RIF and the Xpert MTB/XDR assays in 20 replicates per concentration. The LoD calculated by Probit analysis was 71.9 CFU/ml (95% CI. 58 to 100 CFU/ml) for the Xpert MTB/XDR assay and 86.9 CFU/ml (95% CI, 72 to 110 CFU/ml) for the Xpert MTB/RIF assay (Fig. 1). The LoD analyzed for each drug susceptibility call separately was 79.8 CFU/ml for INH, 95.5 CFU/ml for FLQ, 92.2 CFU/ml for AMK, 74.5 CFU/ml for KAN, 74.8 CFU/ml for CAP, and 71.9 CFU/ml for ETH. Separate LoD studies were also performed at a different laboratory setting with two different lots of Xpert MTB/XDR cartridges and M. bovis BCG stock instead of H37Rv to address the reproducibility of the initial LoD estimate. This study resulted in higher LoD estimates (126 to 136 CFU/ml) than the initial study using H37Rv stock, but they were still comparable to the initially published Xpert MTB/RIF LoD (31). These minor differences in LoD estimates may be attributed to differences in cartridge lots and our use of two different CFU stocks to generate contrived samples. When we tested the LoD on N-acetyl-l-cysteine (NALC)-NaOH concentrated sputum samples spiked with our M. bovis BCG stock, the LoD was observed to be 86 CFU/ml, which is similar to the LoD obtained with direct sputum using H37Rv mc26030 stock. Both studies with the M. bovis BCG stock are described in detail in the “Supplementary Results” section of the supplemental material.
FIG 1.
Limits of detection of the Xpert MTB/XDR and the Xpert MTB/RIF assays. The two assays were performed side by side, with a minimum of 20 replicates for each cell concentration. Both assays were tested at 10, 20, 40, 60, 80, 100, and 200 CFU/ml, and probit analysis was performed to calculate the LoD using R studio.
Ability to detect a genetically diverse set of M. tuberculosis complex strains.
To assess the capacity of the Xpert MTB/XDR assay to detect different M. tuberculosis lineages and different species in the M. tuberculosis complex (MTBC), we tested M. tuberculosis H37Rv, M. bovis, M. africanum, M. canettii, and M. microti as well as a phylogenetically diverse set of M. tuberculosis strains representing all major M. tuberculosis lineages (39) using the Xpert MTB/XDR assay. All samples were detected as M. tuberculosis positive (Table 3). M. canettii caused the assay’s gyrB probe to shift −1.8°C to a Tm of 67.8°C from the mean WT Tm of 69.6°C due to a C/T polymorphism in gyrB codon 533. This mutation was previously reported for M. canettii but is not associated with drug resistance (40). However, the gyrB probe Tm shift did not cause any false FLQ resistance calls, since the gyrB Tm remained within the defined WT Tm window for gyrB probes. All of the other M. tuberculosis complex species tested generated WT Tm values identical to those of H37Rv and a “resistance not detected” result output for all the drugs.
TABLE 3.
Melting temperature values generated by the Xpert MTB/XDR assay tested for major M. tuberculosis complex lineages and species
| RFLPa | Lineage (39) |
Tm (°C) (mean value [SDb]) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| inhA | katG | fabG | ahpC | gyrA1 | gyrA2 | gyrA3 | gyrB2 | rrs | eis | ||
| AH1 | 4 | 76.3 (±0.2) | 73.7 (±0.1) | 71.5 (±0.1) | 69.1 (±0.2) | 76.3 (±0.1) | 70.3 (±0.1) | 71.2 (±0.1) | 69.6 (±0.1) | 75 (±0.1) | 68.5 (±0.1) |
| HR36 (M. africanum) | 5 | 76.4 (±0.2) | 73.7 (±0.1) | 71.5 (±0.1) | 69 (±0.1) | 76.3 (±0.1) | 70.3 (±0.1) | 71.2 (±0.1) | 69.6 (±0.2) | 75.0 (±0.1) | 68.6 (±0.1) |
| AR2 | 2 | 76.2 (±0.1) | 73.6 (±0.1) | 71.4 (±0.1) | 68.8 (±0.1) | 76.3 (±0.0) | 70.3 (±0.1) | 71.2 (±0.0) | 69.5 (±0.1) | 74.9 (±0.1) | 68.5 (±0.0) |
| GD139 | 3 | 76.3 (±0.2) | 73.7 (±0.1) | 71.5 (±0.1) | 70.3 (±0.2) | 76.4 (±0.1) | 70.3 (±0.1) | 71.2 (±0.1) | 69.6 (±0.2) | 75.0 (±0.2) | 68.6 (±0.1) |
| BCG | 1 | 76.2 (±0.0) | 73.6 (±0.1) | 71.5 (±0.0) | 68.6 (±0.0) | 76.3 (±0.0) | 70.3 (±0.1) | 71.15 (±0.1) | 69.5 (±0.0) | 74.9 (±0.0) | 68.5 (±0.0) |
| H37Rv | 4 | 76.3 (±0.0) | 73.7 (±0.0) | 71.4 (±0.1) | 69.3 (±0.0) | 76.1 (±0.1) | 70.3 (±0.1) | 71.1 (±0.1) | 69.6 (±0.0) | 75 (±0.0) | 68.5 (±0.1) |
| M. bovis | Animal strain | 76.4 (±0.0) | 73.8 (±0.0) | 71.6 (±0.1) | 69.1 (±0.0) | 76.4 (±0.1) | 70.3 (±0.1) | 71.2 (±0.0) | 69.6 (±0.1) | 75.0 (±0.1) | 68.6 (±0.1) |
| M. canettii | NAc | 76.4 (±0.1) | 73.8 (±0.0) | 71.6 (±0.0) | 69.3 (±0.1) | 76.4 (±0.0) | 70.3 (±0.1) | 71.3 (±0.1) | 67.8 (±0.0) | 75.1 (±0.1) | 68.6 (±0.0) |
| M. microti | Animal strain | 76.4 (±0.1) | 73.8 (±0.0) | 71.6 (±0.1) | 69.1 (±0.2) | 76.4 (±0.1) | 70.3 (±0.0) | 71.2 (±0.1) | 69.6 (±0.1) | 75.1 (±0.1) | 68.6 (±0.0) |
RFLP, restriction fragment length polymorphism.
SD, standard deviation calculated from replicates of four.
NA, not available.
Analytical specificity and cross-reactivity.
The specificity of the assay was assessed by testing 30 non-tuberculous Mycobacterium (NTM) species, 19 Gram-positive and Gram-negative bacteria, along with Candida albicans at 106 to 107 CFU/ml (see Tables S1 and S2 in the supplemental material). All of the samples generated “MTB not detected” results by the Xpert MTB/XDR assay, which specifically requires that the inhA probe generate a Tm in either the WT or MUT window to be called M. tuberculosis positive. All of the NTM species tested, except M. triviale, generated rrs WT Tm values, which was expected because the target region of the rrs gene is conserved among most Mycobacterium species (Table S1). No NTM was misidentified as M. tuberculosis, since the inhA promoter SMB probe did not produce a Tm for any of the NTM samples tested. M. gastri, M. gordonae, and M. xenopi showed weak Tm peaks in the gyrA1 MutB Tm window and M. interjectum generated a gyrB2 WT Tm. WT rrs Tm values were obtained in tests of Citrobacter freundii, Corynebacterium xerosis, Enterobacter cloacae, Nocardia asteroides, Staphylococcus epidermidis, Streptococcus pyogenes, and Candida albicans, indicating the rrs primer/probe sequence overlaps the 16S ribosomal gene in these strains (Table S2). Very weak gyrA probe cross-reactivity was observed with a few additional bacterial species. The rest of the assay probes did not cross-react with any of the samples in the specificity panel.
Since weak gyrA mutant Tm peaks were observed for some of the NTM species, we performed spiking experiments with BCG and NTM mixtures to simulate the clinical scenario of a tuberculosis patient who is also infected with an NTM. Studies were undertaken to test whether this type of dual infection could generate a false-positive FLQ resistance call. None of the NTMs tested in these mixtures generated a false FLQ resistance calls. However, we did observe that one strain of M. marinum (ATCC 0927) interfered with the gyrA signal produced by the M. tuberculosis target, resulting in the suppression of the Tm generated by at least one of the gyrA probes and an “FLQ resistance indeterminate” call. At 106 CFU/ml, all 4 replicates tested generated indeterminate calls for FLQ, and at 105 CFU/ml, 2 of 4 replicates resulted in indeterminate calls. With M. marinum ATCC 0927 spiked at 104 CFU/ml, no interference was observed, and the correct “FLQ resistance not detected” call was observed. Therefore, the M. marinum-induced suppression of FLQ resistance identification only occurred when samples were spiked with at least 105 M. marinum CFU/ml. To the best of our knowledge, there have been no reports of pulmonary infections caused by coinfection with M. tuberculosis and M. marinum; thus, this interference may not be clinically relevant, at least for pulmonary TB cases.
Detection of heteroresistance.
It is estimated that 20% to 45% of XDR-TB cases contain a mixed population of susceptible and resistant strains, i.e., are heteroresistant (41–44). We have shown previously that SMB assays can efficiently detect WT and mutant DNA mixtures by generating double Tm peaks corresponding to WT and mutant DNA sequences (10). To assess the performance of the Xpert MTB/XDR assay for detecting mutations in heteroresistant samples, a series of cell mixtures containing 0%, 10%, 15%, 20%, 25%, 50%, 60%, 75%, 90%, and 100% of mutant cells were tested against a background of cells with WT sequences. We used this approach to test mixtures of WT cells and cells containing the mutations C(−15)T in the inhA promoter, S513T in the katG gene product, G609A in the fabG1 product, C(−39)T in the oxyR-ahpC region, D94G in the gyrA product, E540D in the gyrB product, A1401G in rrs, and C(−14)T in the eis promoter. Resistance was detected when the mutant Tm was detected in the presence of the WT background, and “resistance not detected” calls were made when the mutant Tm was undetectable and only WT Tm was detected (Fig. 2). For detection of fabG1, katG, or inhA promoter mutations, our results showed that INH resistance (INRr) was detected in mixtures containing as little as 20% mutant cells with 80% WT cells. However, cells containing an ahpC mutant could not be detected unless they were present in at least 75% of the mixture. FLQr was detected in mixtures that contained as little as 25% of the D94G mutation; however, the gyrB mutation was only detected in mixtures that contained 60% or more of the mutant sequence. SLID resistance was detected in mixtures that contained as little as 60% of the rrs or the eis promoter mutations. Below these levels, resistance was not detected, since the mutant Tms could not be identified against the WT Tm background, as shown in Fig. 2.
FIG 2.
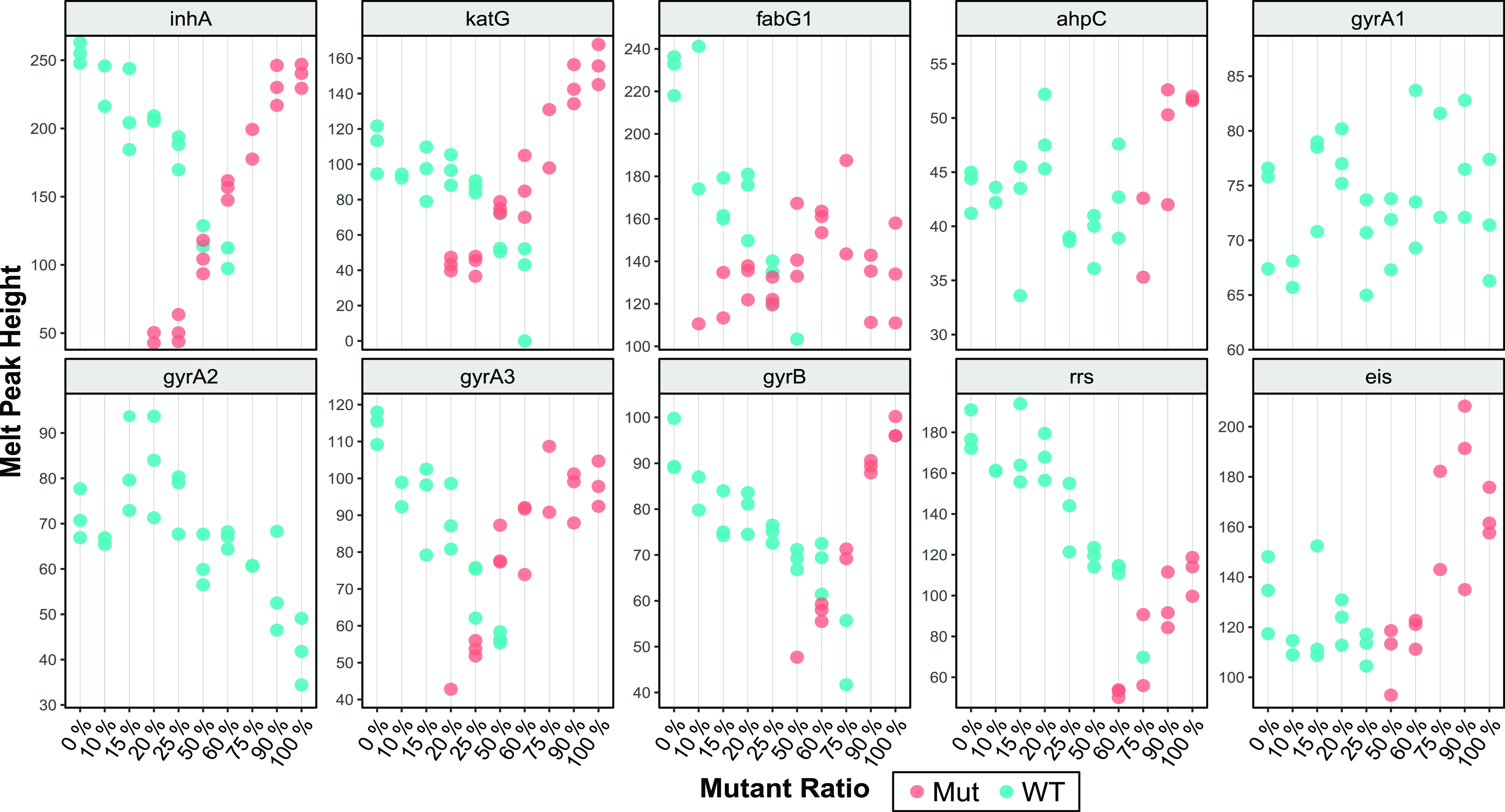
Mutation detection in mixtures of WT and mutant DNA. Melt peak heights of each target in cell mixtures containing different ratios of WT and mutant plasmids, where blue dots indicate a susceptible call and red dots indicate mutant calls based on their Tm and melt peak height. The melt peak height is determined by highest distance between the peak of the first-derivative melt curve and baseline. The presence of blue and red dots for any concentration designates detection of both a WT and a mutant Tm. In such cases, the result obtained was “resistance detected” for the corresponding drug. The QRDR mutation D94G generates a mutant Tm only with the gyrA3 probe. Each mixture was tested in triplicates, except the 10% and 75% mixtures, which had two valid replicates.
We also evaluated the ability of the assay to accurately detect the three low-FLQ-resistance-associated mutations, A90V, S91P, and D94A, when present as a mixture with the WT sequence. We found that the assay was not able to detect low FLQ resistance for all the A90V and S91P mixtures we tested. Instead, these mixtures caused the assay to generate either a “FLQ resistance detected” call or “FLQ resistance not detected” call. In the former case, the presence of Tm values in both WT and MUT windows produced a Tm pattern that was consistent with a “FLQ resistance detected” call, and in the latter case, there was only WT Tm present. A “FLQ resistance detected” call was made for S91P-WT mixtures down to as little as 20% S91P. Below that concentration, S91P-WT mixtures produced a “FLQ resistance not detected” call, since no mutant Tm was detected. A “FLQ resistance detected” call was made for A90V-WT mixtures down to as little as 20% to 50% A90V, and mixtures with less than 20% A90V produced a “FLQ resistance not detected” call. In contrast, with the D94A mutation, the assay was able to correctly detect low-level FLQ resistance in heteroresistant samples containing at least 50% D94A mutant cells mixed with 50% WT cells in all three replicates tested, due to the correct D94A specific Tm signature (MutA-MutA-WT) being present. Two of three replicates containing 40% D94A produced a “low FLQ resistance detected” call, and one replicate produced a “FLQ resistance detected” call. At D94A/WT mixtures below 40% D94A, the assay was unable to detect the presence of FLQ-specific mutations (data not shown). Thus, the assay demonstrates a substantial loss in the ability to distinguish low FLQ resistance conferring A90V and S91P mutations from other QRDR mutations when these two mutations are present along with the WT sequence, but its overall ability to identify FLQ resistance is not affected.
Performance on sputum samples and clinical isolates.
A limited clinical study was performed at two different testing sites with a total of 100 M. tuberculosis-positive frozen sputum samples and 214 clinical isolates from deidentified patients with various types of drug resistance. The sensitivity and the specificity of the assay for detecting resistance to INH, ETH, FLQ, and SLIDs on this sample set were estimated by individually comparing assay results to those from the two different reference standards: pDST and DNA sequencing. The capacity of the assay to accurately detect the mutations in the target genes was also estimated. The results from 105 of 107 clinical isolates and all 50 sputum samples were available from site 1, and the results from 106 of 107 isolates and 49 of the 50 sputum samples were available from site 2, which allowed us to include 310 of the 314 samples for the final analysis. Any “indeterminate” result for any drug targets and samples with missing or uninterpretable pDST and/or sequencing results, due to culture contamination or confounding MIC results and/or absence of a clean sequence chromatogram, respectively, were excluded from the analysis. Excluding such samples, pDST results were available for 309 samples for INH, 306 samples for KAN, 305 samples each for FLQ and CAP, 303 samples for AMK, and 265 samples for ETH. Similarly, sequencing results were available for all 310 samples for INH- and ETH-specific targets, 309 samples for FLQ-specific targets, and 306, 307, and 308 samples for AMK-, CAP-, and KAN-specific targets, respectively. Compared to pDST, the assay showed a sensitivity and specificity of 98.3% and 95%, respectively, for INH resistance, 91.4% and 98.5% for FLQ resistance, 91% and 99% for AMK resistance, 98.1% and 97% for KAN resistance, 70% and 99.7% for CAP resistance, and 65.4% and 97.3% for ETH resistance (Table 4). Compared to sequencing, the assay showed sensitivity of 99.7%, 97.5%, 100%, 96.5%, 94.1%, and 88.5% for detection of INH, FLQ, AMK, KAN, CAP, and ETH resistance, respectively, with a specificity of 100% for all the drugs except for ETH, where the specificity was 97.3%. In this sample set, the mutations present in the key target genes were as follows: G(−17)T, C(−15)T, T(−8)A, and T(−8)C in inhA promoter region, S315T, S315N, and S315G in the katG gene product, L203L in the fabG1 gene product, G88C, D89N, A90V, D94A, D94G, D94Y in the gyrA gene product, A1401G in the rrs gene, and G(−37)T, C(−14)T, C(−12)T, G(−10)A, and C(−8)A in the eis promoter region. All the gyrB or ahpC mutations present in this sample set were in combination with mutations in the gyrA gene and inhA promoter or katG genes, respectively. The assay detected all the mutations present in the inhA promoter region and the katG gene as well as all the low-FLQ-resistance A90V and D94A mutations and differentiated them from high FLQ resistance caused by other gyrA QRDR mutations. The assay also correctly detected SLID cross-resistance and individual resistance to KAN by correctly identifying the mutations in the rrs and eis promoter genes, respectively. As shown in Fig. 3, the assay was able to clearly and independently cluster the WT and mutant Tm values for all the targets, resulting in unequivocal identification of these mutations with a high degree of accuracy. A very few “indeterminate” results were obtained for FLQ (0.3%), AMK (1.3%), KAN (0.6%), and CAP (0.9%) due to missing Tm values from one or more of the SMB probes used for detection of resistance to these drugs. We observed that in all these “indeterminate” calls, the respective Tm peaks were present, but they were not high enough to cross their predefined Tm peak height threshold; thus, the Tm values were not calculated by the assay algorithm, which indicates possible sub-LoD concentrations of the targets. In at least one case of CAP indeterminate results, the missing Tm from the rrs probe could be attributed to unexpected signal distortions, very likely due to a bubble in the reaction tube, which prevented determination of the melt peak. We did not perform separate analysis for sputum and culture isolate sample types due to a lower number of susceptible samples represented in the sputum group, which failed to generate statistically significant values. However, we did not observe any difference in performance between the two sample types with respect to assay inhibition, indeterminate rates, pressure aborts, or general assay performance. Both the sample types resulted in overall identical assay performance.
TABLE 4.
Xpert MTB/XDR assay’s concordance with pDST and sequencing on drug resistance detection on clinical isolates and sputum samples
| Drug | No. of:a |
Sensitivity |
Specificity |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | TP | FN | TN | FP | % | 95% CI | % | 95% CI | |
| pDST | |||||||||
| INH | 309 | 284 | 5 | 19 | 1 | 98.3 | 95.8–99.3 | 95.0 | 73.1–99.7 |
| FLQ | 305 | 32 | 3 | 266 | 4 | 91.4 | 78.9–98.9 | 98.5 | 95.9–99.5 |
| AMK | 303 | 20 | 2 | 278 | 3 | 91.0 | 69.4–98.4 | 98.9 | 96.6–99.7 |
| KAN | 306 | 101 | 2 | 197 | 6 | 98.1 | 92.5–99.7 | 97.4 | 93.4–98.8 |
| CAP | 305 | 14 | 6 | 284 | 1 | 70.0 | 45.6–87.2 | 99.7 | 97.7–99.9 |
| ETH | 265 | 102 | 54 | 106 | 3 | 65.4 | 57.3–72.7 | 97.3 | 91.6–99.3 |
| Sequencing | |||||||||
| INH | 310 | 286 | 1 | 23 | 0 | 99.6 | 97.8–99.9 | 100 | 82.2–100 |
| FLQ | 309 | 39 | 1 | 269 | 0 | 97.5 | 85.3–99.8 | 100 | 98.2–100 |
| AMK | 306 | 24 | 0 | 282 | 0 | 100 | 82.8–100 | 100 | 98.3–100 |
| KAN | 308 | 109 | 4 | 195 | 0 | 96.4 | 90.6–98.9 | 100 | 97.6–100 |
| CAP | 307 | 16 | 1 | 290 | 0 | 94.1 | 69.2–99.6 | 100 | 98.4–100 |
| ETH | 310 | 108 | 14 | 183 | 5 | 88.5 | 81.2–93.4 | 97.3 | 93.6–99.0 |
TP, true-positive results; FN, false-negative results; TN, true-negative results; FP, false-positive results.
FIG 3.

Scatterplot showing clustering of WT and mutant Tm values from samples tested in the clinical study. One hundred M. tuberculosis-positive frozen sputum samples and 214 clinical isolates for all Xpert MTB/XDR targets were tested and plotted using ggplot in R studio. To prevent overplotting, a degree of jitter was introduced. All green dots are WT Tm, while brown, purple, blue, and pink are mutant Tm values.
DISCUSSION
We describe here the development of an in vitro diagnostic cartridge for INH, FLQ, and SLID resistance detection (10). Compared to an earlier prototype assay, the new assay has better coverage for INH resistance, the capacity to detect low- versus high-level resistance for INH and FLQ and to identify individual versus cross-resistance to SLID, has better analytical sensitivity, and has a reduced time to result. The SMB probe design and chemistry of the new Xpert MTB/XDR assay are similar to those for the prototype cartridge, with additional probes added to detect new targets indicative of INH and ETH resistance, an additional probe and modified probe designs for the gyrA gene to differentiate and identify low- versus high-level FLQ resistance, and a modified eis probe design to identify KAN resistance only, versus KAN-AMK cross-resistance. An additional gyrB probe in the prototype version, which targets codon 500, was removed from this assay to accommodate the new probes on account of the very low frequency of mutations present in codon 500 of gyrB that have a high-confidence association with FLQ resistance (24). This modified assay version eliminates the three-stage PCR amplification used in the prototype cartridge and closely approximates the PCR cycling strategy used in the Xpert MTB/RIF Ultra assay, where the first stage of symmetric PCR is followed by a second stage of asymmetric PCR preferentially amplifying the target strands, followed by a melt stage. We have also utilized the strategy of including a TaqMan probe (SPC) and an SMB probe (ahpC) with the same fluorophore to emit signal in a single channel, which allowed us to develop a 10-color 11-probe assay. The ahpC probe was designed to have a probe-target hybrid Tm close to the annealing temperature of the assay, while the TaqMan probe was designed to have a probe-target hybrid Tm at least 5°C above the annealing temperature. This allowed us to generate good real-time signals preferentially from the SPC TaqMan probe during the amplification stage and obtain clear melt curves from the ahpC SMB probe during the melt stage, without any interference of the melt signal from the TaqMan probe.
Designing probes to distinguish high- and low-level FLQ resistance was especially challenging, since we had to ensure that the Ser/Thr polymorphism at codon 95 was not recognized as a mutation, while all of the three low-level-FLQ-resistance-inducing mutations were individually identified and differentiated from all of the other QRDR mutations. Several iterative probe designs were tested based on the probes present in our prototype cartridge, and a combination of three overlapping probes were chosen to generate a series of Tm signatures, which not only individually identified the three different mutations and differentiated them from other QRDR mutations but also generated the same WT Tm values for the codon 95 polymorphism. We successfully used this Tm signature principle to identify these mutations by placing the mutant Tm values in carefully chosen specific WT and mutant Tm windows for each probe, which underscores the previously described capacity of SMB probe tiling to accurately identify DNA sequences (45). The Xpert MTB/XDR assay targeted two regions in the M. tuberculosis genome that contain mutations spread over relatively long stretches, which would normally be very difficult for a single probe to query. Resistance-associated mutations in the oxyR-ahpC intergenic region were spread over 46 bp, and resistance-associated mutations in the eis promoter region were spread over 37 bp. We used poly(dT) and poly(dA) to link two different probes for the ahpC target region to generate a 49-bp-long probe, and we used special proprietary Cepheid linkers to combine two of the eis probes from the prototype cartridge assay to create a 50-bp-long probe (including the linker sequence). We introduced mismatches in the probes to enhance the delta Tm between the WT and mutant sequences to ensure that clearly separated Tm values were generated between mutant and WT sequences. These and other probe design principles were used to ensure that there was a ≥2°C separation between WT and mutant Tm values for most clinically relevant mutations.
As an INH and second-line resistance detection assay designed to be used as a reflex test in Xpert MTB/RIF or Xpert MTB/RIF Ultra positive assays, our preference was to design Xpert MTB/XDR so that it had an analytical sensitivity at least matching that of the Xpert MTB/RIF assay, keeping in mind that the analytical sensitivity of the Xpert MTB/RIF assay is roughly equivalent to the analytic sensitivity of the rpoB component of the Xpert MTB/RIF Ultra assay (31). The prototype cartridge LoD was 300 CFU/ml, which was in the range of, but not as good as, that of the Xpert MTB/RIF assay (130 CFU/ml). Our new Xpert MTB/XDR assay showed a comparable LoD to that of the Xpert MTB/RIF for M. tuberculosis detection. We confirmed the reliability of our LoD estimation with multiple cartridge lots and analytical studies performed at two laboratories. We did not perform any head-to-head comparisons between Xpert MTB/XDR and Xpert MTB/RIF Ultra, since we expect that Xpert MTB/XDR will perform well with samples that test positive by Xpert MTB/RIF Ultra as long as the Xpert MTB/RIF Ultra does not produce a “trace” call with “indeterminate” rifampin resistance results due to low bacillary load.
We performed a limited clinical study on a panel of frozen sputum samples and clinical isolates from three different continents, representing a wide range of clinically relevant mutations. The clinical isolates and sputum samples represent considerable geographical variation and thus enabled us to assess the performance of the assay as a reflex test on both TB-positive sputum samples as well as M. tuberculosis clinical isolates. The performance of the assay compared to that of pDST generated very high sensitivity and specificity values except for ETH, which showed a sensitivity of 65.4%. The assay showed 100% specificity and 94% to 100% sensitivity in detecting WT and mutant sequence types for all other drug targets. The low sensitivity for ETH compared to that of either reference standards can be explained by the fact that this assay only targets mutations in the inhA promoter among the several other possible gene mutations which may be associated with ETH resistance (15). Our study sample contained several ETH resistance-associated mutations in ethA and the ethA upstream region, which are not targeted by our assay, explaining the low sensitivity of the assay for detecting ETH resistance. Detection of ETH resistance was not an original aim of the assay and was included later, since ETH resistance has been reported to show significant association with mutations in inhA promoter that are also associated with low-level INH resistance, as tested by our assay (28, 46). We have designed the assay to detect all the clinically important and commonly prevalent mutations strongly associated with phenotypic drug resistance for all target drugs. However, rare uncommon mutations, which may be present outside the regions targeted by our assay, can also account for the small number of false-negative results that we noted in our studies of clinical samples and isolates. We can expect that the assay will show similar performance when tested in a larger multicentric clinical study. Given the limited scope of the study and uneven distribution of resistant and susceptible strains in sputum samples and culture isolates, we were unable to evaluate the performance of the assay separately on these two different sample types. Larger clinical studies are currently being undertaken which will address if there are any differences in performance between these two class of samples. We did not observe any difference with respect to assay inhibition or indeterminate rates between these two sample types in our limited study. Currently, the assay is recommended to be utilized only for unprocessed sputum or concentrated sputum sediments.
The Xpert MTB/XDR assay is intended to be used as a reflex test for a specimen that is determined to be M. tuberculosis positive and to serve as an aid in the diagnosis of the main types of resistance that exist in M/XDR-TB when used in conjunction with clinical and other laboratory findings. To address the global MDR-TB crisis and expedite diagnosis, WHO has determined that expanding rapid testing and the detection of drug-resistant TB is a top priority (47) and recently intends to endorse a 4-month treatment regimen, with promising results from study 31/A5349, a clinical trial (48). Access to fast, sensitive, and safer genotypic assays such as Xpert MTB/RIF Ultra and Xpert MTB/XDR, which detect resistance by identifying mutations known to confer resistance to the first- and second-line drugs in a majority of clinical strains, will minimize the biohazard and reduce sample preparation to a few manual steps that are more amenable to use at the point of care. When used as a reflex assay in conjunction with Xpert MTB/RIF or Xpert MTB/RIF Ultra, the Xpert MTB/XDR assay can expand TB and drug resistance detection to medically underserved populations.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI111397 and a grant from the Foundation for Innovative New Diagnostics. Research support was also provided by Cepheid.
Cepheid collaborated in assay design, analytical study design, and performance, while FIND was involved in the clinical study planning, design, and providing samples. The NIH had no role in study design, planning, or manuscript preparation. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
D.A. receives income from license payments from Cepheid to Rutgers University. D.A. also reports receiving research contracts and support from Cepheid. D.A. and S.C. report the filing of patents for primers and probes for detecting drug resistance in M. tuberculosis. R.L.G., D.L., S.R., N.V., R.K., D.P., and S.C. are employed by Cepheid.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2019. Global tuberculosis report 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Dookie N, Rambaran S, Padayatchi N, Mahomed S, Naidoo K. 2018. Evolution of drug resistance in Mycobacterium tuberculosis: a review on the molecular determinants of resistance and implications for personalized care. J Antimicrob Chemother 73:1138–1151. doi: 10.1093/jac/dkx506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georghiou SB, Schumacher SG, Rodwell TC, Colman RE, Miotto P, Gilpin C, Ismail N, Rodrigues C, Warren R, Weyer K, Zignol M, Arafah S, Cirillo DM, Denkinger CM. 2019. Guidance for studies evaluating the accuracy of rapid tuberculosis drug-susceptibility tests. J Infect Dis 220:S126–S135. doi: 10.1093/infdis/jiz106. [DOI] [PubMed] [Google Scholar]
- 4.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, Hall SL, Chakravorty S, Cirillo DM, Tukvadze N, Bablishvili N, Stevens W, Scott L, Rodrigues C, Kazi MI, Joloba M, Nakiyingi L, Nicol MP, Ghebrekristos Y, Anyango I, Murithi W, Dietze R, Lyrio Peres R, Skrahina A, Auchynka V, Chopra KK, Hanif M, Liu X, Yuan X, Boehme CC, Ellner JJ, Denkinger CM. 2018. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks GB, Nguyen NV, Nguyen PTB, Nguyen T-A, Nguyen HB, Tran KH, Nguyen SV, Luu KB, Tran DTT, Vo QTN, Le OTT, Nguyen YH, Do VQ, Mason PH, Nguyen V-AT, Ho J, Sintchenko V, Nguyen LN, Britton WJ, Fox GJ. 2019. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 381:1347–1357. doi: 10.1056/NEJMoa1902129. [DOI] [PubMed] [Google Scholar]
- 7.Sachdeva KS, Raizada N, Sreenivas A, Van't Hoog AH, van den Hof S, Dewan PK, Thakur R, Gupta RS, Kulsange S, Vadera B, Babre A, Gray C, Parmar M, Ghedia M, Ramachandran R, Alavadi U, Arinaminpathy N, Denkinger C, Boehme C, Paramasivan CN. 2015. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One 10:e0126065. doi: 10.1371/journal.pone.0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyckendorf J, Andres S, Koser CU, Olaru ID, Schon T, Sturegard E, Beckert P, Schleusener V, Kohl TA, Hillemann D, Moradigaravand D, Parkhill J, Peacock SJ, Niemann S, Lange C, Merker M. 2017. What Is resistance? Impact of phenotypic versus molecular drug resistance testing on therapy for multi- and extensively drug-resistant tuberculosis. Antimicrob Agents Chemother 62:e01550-17. doi: 10.1128/AAC.01550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddabra R, Ait Benhassou H. 2018. Rapid molecular assays for detection of tuberculosis. Pneumonia (Nathan) 10:4. doi: 10.1186/s41479-018-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravorty S, Roh SS, Glass J, Smith LE, Simmons AM, Lund K, Lokhov S, Liu X, Xu P, Zhang G, Via LE, Shen Q, Ruan X, Yuan X, Zhu HZ, Viazovkina E, Shenai S, Rowneki M, Lee JS, Barry CE, III, Gao Q, Persing D, Kwiatkawoski R, Jones M, Gall A, Alland D. 2017. Detection of isoniazid-, fluoroquinolone-, amikacin-, and kanamycin-resistant tuberculosis in an automated, multiplexed 10-color assay suitable for point-of-care use. J Clin Microbiol 55:183–198. doi: 10.1128/JCM.01771-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie YL, Chakravorty S, Armstrong DT, Hall SL, Via LE, Song T, Yuan X, Mo X, Zhu H, Xu P, Gao Q, Lee M, Lee J, Smith LE, Chen RY, Joh JS, Cho Y, Liu X, Ruan X, Liang L, Dharan N, Cho SN, Barry CE, III, Ellner JJ, Dorman SE, Alland D. 2017. Evaluation of a rapid molecular drug-susceptibility test for tuberculosis. N Engl J Med 377:1043–1054. doi: 10.1056/NEJMoa1614915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ando H, Miyoshi-Akiyama T, Watanabe S, Kirikae T. 2014. A silent mutation in mabA confers isoniazid resistance on Mycobacterium tuberculosis. Mol Microbiol 91:538–547. doi: 10.1111/mmi.12476. [DOI] [PubMed] [Google Scholar]
- 13.Zaunbrecher MA, Sikes RD, Jr, Metchock B, Shinnick TM, Posey JE. 2009. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 106:20004–20009. doi: 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georghiou SB, Magana M, Garfein RS, Catanzaro DG, Catanzaro A, Rodwell TC. 2012. Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to amikacin, kanamycin and capreomycin: a systematic review. PLoS One 7:e33275. doi: 10.1371/journal.pone.0033275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miotto P, Tessema B, Tagliani E, Chindelevitch L, Starks AM, Emerson C, Hanna D, Kim PS, Liwski R, Zignol M, Gilpin C, Niemann S, Denkinger CM, Fleming J, Warren RM, Crook D, Posey J, Gagneux S, Hoffner S, Rodrigues C, Comas I, Engelthaler DM, Murray M, Alland D, Rigouts L, Lange C, Dheda K, Hasan R, Ranganathan UDK, McNerney R, Ezewudo M, Cirillo DM, Schito M, Koser CU, Rodwell TC. 2017. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur Respir J 50:1701354. doi: 10.1183/13993003.01354-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, Posey JE. 2011. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother 55:2032–2041. doi: 10.1128/AAC.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farhat MR, Jacobson KR, Franke MF, Kaur D, Sloutsky A, Mitnick CD, Murray M. 2016. Gyrase mutations are associated with variable levels of fluoroquinolone resistance in Mycobacterium tuberculosis. J Clin Microbiol 54:727–733. doi: 10.1128/JCM.02775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien JY, Chiu WY, Chien ST, Chiang CJ, Yu CJ, Hsueh PR. 2016. Mutations in gyrA and gyrB among fluoroquinolone- and multidrug-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 60:2090–2096. doi: 10.1128/AAC.01049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Groll A, Martin A, Jureen P, Hoffner S, Vandamme P, Portaels F, Palomino JC, da Silva PA. 2009. Fluoroquinolone resistance in Mycobacterium tuberculosis and mutations in gyrA and gyrB. Antimicrob Agents Chemother 53:4498–4500. doi: 10.1128/AAC.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farhat MR, Jacobson KR, Franke MF, Kaur D, Murray M, Mitnick CD. 2017. Fluoroquinolone resistance mutation detection is equivalent to culture-based drug sensitivity testing for predicting multidrug-resistant tuberculosis treatment outcome: a retrospective cohort study. Clin Infect Dis 65:1364–1370. doi: 10.1093/cid/cix556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigouts L, Coeck N, Gumusboga M, de Rijk WB, Aung KJ, Hossain MA, Fissette K, Rieder HL, Meehan CJ, de Jong BC, Van Deun A. 2016. Specific gyrA gene mutations predict poor treatment outcome in MDR-TB. J Antimicrob Chemother 71:314–323. doi: 10.1093/jac/dkv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghodousi A, Tagliani E, Karunaratne E, Niemann S, Perera J, Koser CU, Cirillo DM. 2019. Isoniazid resistance in Mycobacterium tuberculosis is a heterogeneous phenotype composed of overlapping mic distributions with different underlying resistance mechanisms. Antimicrob Agents Chemother 63:e00092-19. doi: 10.1128/AAC.00092-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Jiang F, Chen L, Zhao B, Dong J, Sun L, Zhu Y, Liu B, Zhou Y, Yang J, Zhao Y, Jin Q, Zhang X. 2018. The impact of combined gene mutations in inhA and ahpC genes on high levels of isoniazid resistance amongst katG non-315 in multidrug-resistant tuberculosis isolates from China. Emerg Microbes Infect 7:183. doi: 10.1038/s41426-018-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Park YJ, Kim WI, Lee SH, Ludgerus Chang C, Kang SJ, Kang CS. 2003. Molecular analysis of isoniazid resistance in Mycobacterium tuberculosis isolates recovered from South Korea. Diagn Microbiol Infect Dis 47:497–502. doi: 10.1016/s0732-8893(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 25.Nagel S, Streicher EM, Klopper M, Warren RM, Van Helden PD. 2017. Isoniazid resistance and dosage as treatment for patients with tuberculosis. Curr Drug Metab 18:1030–1039. doi: 10.2174/1389200218666171031121905. [DOI] [PubMed] [Google Scholar]
- 26.Riviere E, Whitfield MG, Nelen J, Heupink TH, Van Rie A. 2020. Identifying isoniazid resistance markers to guide inclusion of high-dose isoniazid in tuberculosis treatment regimens. Clin Microbiol Infect 26:1332–1337. doi: 10.1016/j.cmi.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Chakravorty S, Aladegbami B, Thoms K, Lee JS, Lee EG, Rajan V, Cho EJ, Kim H, Kwak H, Kurepina N, Cho SN, Kreiswirth B, Via LE, Barry CE, III, Alland D. 2011. Rapid detection of fluoroquinolone-resistant and heteroresistant Mycobacterium tuberculosis by use of sloppy molecular beacons and dual melting-temperature codes in a real-time PCR assay. J Clin Microbiol 49:932–940. doi: 10.1128/JCM.02271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. 2015. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One 10:e0119628. doi: 10.1371/journal.pone.0119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC. 2003. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 47:3799–3805. doi: 10.1128/aac.47.12.3799-3805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. 2018. Technical report on critical concentrations for TB drug susceptibility testing of medicines used in the treatment of drug-resistant TB. WHO/CDS/TB/20185. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 31.Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, Banada PP, Deshpande S, Shenai S, Gall A, Glass J, Krieswirth B, Schumacher SG, Nabeta P, Tukvadze N, Rodrigues C, Skrahina A, Tagliani E, Cirillo DM, Davidow A, Denkinger CM, Persing D, Kwiatkowski R, Jones M, Alland D. 2017. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 8:e00812-17. doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-Lopez EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. 2010. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubos RJ, Davis BD. 1946. Factors affecting the growth of tubercle bacilli in liquid media. J Exp Med 83:409–423. doi: 10.1084/jem.83.5.409. [DOI] [PubMed] [Google Scholar]
- 34.van Soolingen D, Hermans PW, de Haas PE, Soll DR, van Embden JD. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol 29:2578–2586. doi: 10.1128/JCM.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tessema B, Nabeta P, Valli E, Albertini A, Collantes J, Lan NH, Romancenco E, Tukavdze N, Denkinger CM, Dolinger DL. 2017. FIND tuberculosis strain bank: a resource for researchers and developers working on tests to detect Mycobacterium tuberculosis and related drug resistance. J Clin Microbiol 55:1066–1073. doi: 10.1128/JCM.01662-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Gao X, Luo T, Wu J, Sun G, Liu Q, Jiang Y, Zhang Y, Mei J, Gao Q. 2014. Association of gyrA/B mutations and resistance levels to fluoroquinolones in clinical isolates of Mycobacterium tuberculosis. Emerg Microbes Infect 3:e19. doi: 10.1038/emi.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avalos E, Catanzaro D, Catanzaro A, Ganiats T, Brodine S, Alcaraz J, Rodwell T. 2015. Frequency and geographic distribution of gyrA and gyrB mutations associated with fluoroquinolone resistance in clinical Mycobacterium tuberculosis isolates: a systematic review. PLoS One 10:e0120470. doi: 10.1371/journal.pone.0120470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik S, Willby M, Sikes D, Tsodikov OV, Posey JE. 2012. New insights into fluoroquinolone resistance in Mycobacterium tuberculosis: functional genetic analysis of gyrA and gyrB mutations. PLoS One 7:e39754. doi: 10.1371/journal.pone.0039754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gagneux S. 2018. Ecology and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol 16:202–213. doi: 10.1038/nrmicro.2018.8. [DOI] [PubMed] [Google Scholar]
- 40.Supply P, Marceau M, Mangenot S, Roche D, Rouanet C, Khanna V, Majlessi L, Criscuolo A, Tap J, Pawlik A, Fiette L, Orgeur M, Fabre M, Parmentier C, Frigui W, Simeone R, Boritsch EC, Debrie AS, Willery E, Walker D, Quail MA, Ma L, Bouchier C, Salvignol G, Sayes F, Cascioferro A, Seemann T, Barbe V, Locht C, Gutierrez MC, Leclerc C, Bentley SD, Stinear TP, Brisse S, Medigue C, Parkhill J, Cruveiller S, Brosch R. 2013. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat Genet 45:172–179. doi: 10.1038/ng.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Zhao B, Liu L, Zhu Y, Zhao Y, Jin Q. 2012. Subpopulation analysis of heteroresistance to fluoroquinolone in Mycobacterium tuberculosis isolates from Beijing, China. J Clin Microbiol 50:1471–1474. doi: 10.1128/JCM.05793-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eilertson B, Maruri F, Blackman A, Herrera M, Samuels DC, Sterling TR. 2014. High proportion of heteroresistance in gyrA and gyrB in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother 58:3270–3275. doi: 10.1128/AAC.02066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Operario DJ, Koeppel AF, Turner SD, Bao Y, Pholwat S, Banu S, Foongladda S, Mpagama S, Gratz J, Ogarkov O, Zhadova S, Heysell SK, Houpt ER. 2017. Prevalence and extent of heteroresistance by next generation sequencing of multidrug-resistant tuberculosis. PLoS One 12:e0176522. doi: 10.1371/journal.pone.0176522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kargarpour Kamakoli M, Sadegh HR, Farmanfarmaei G, Masoumi M, Fateh A, Javadi G, Rahimi Jamnani F, Vaziri F, Siadat SD. 2017. Evaluation of the impact of polyclonal infection and heteroresistance on treatment of tuberculosis patients. Sci Rep 7:41410. doi: 10.1038/srep41410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao Y, Parmar H, Simmons AM, Kale D, Tong K, Lieu D, Persing D, Kwiatkowski R, Alland D, Chakravorty S. 2019. Automatic identification of individual rpoB gene mutations responsible for rifampin resistance in Mycobacterium tuberculosis using melting temperature signatures generated by the Xpert(R) MTB/RIF Ultra assay. J Clin Microbiol 58:e00907-19. doi: 10.1128/JCM.00907-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazbon MH, Brimacombe M, Bobadilla del Valle M, Cavatore M, Guerrero MI, Varma-Basil M, Billman-Jacobe H, Lavender C, Fyfe J, Garcia-Garcia L, Leon CI, Bose M, Chaves F, Murray M, Eisenach KD, Sifuentes-Osornio J, Cave MD, Ponce de Leon A, Alland D. 2006. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 50:2640–2649. doi: 10.1128/AAC.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 48.World Health Organization. 2020. New study 31/A5349 on the treatment of drug-susceptible TB. World Health Organization, Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



