Tuberculous meningitis (TBM) is the most devastating form of tuberculosis (TB), causing high mortality or disability. Clinical management of the disease is challenging due to limitations of the existing diagnostic approaches. Our knowledge on the immunology and pathogenesis of the disease is currently limited. More research is urgently needed to enhance our understanding of the immunopathogenesis of the disease and guide us toward the identification of targets that may be useful for vaccines or host-directed therapeutics.
KEYWORDS: biomarker, central nervous system infections, diagnosis, immune response, meningitis, pathogenesis, tuberculosis, tuberculous meningitis
ABSTRACT
Tuberculous meningitis (TBM) is the most devastating form of tuberculosis (TB), causing high mortality or disability. Clinical management of the disease is challenging due to limitations of the existing diagnostic approaches. Our knowledge on the immunology and pathogenesis of the disease is currently limited. More research is urgently needed to enhance our understanding of the immunopathogenesis of the disease and guide us toward the identification of targets that may be useful for vaccines or host-directed therapeutics. In this review, we summarize the current knowledge about the immunology and pathogenesis of TBM and summarize the literature on existing and new, especially biomarker-based, approaches that may be useful in the management of TBM. We identify research gaps and provide directions for research which may lead to the development of new tools for the control of the disease in the near future.
INTRODUCTION
Tuberculosis (TB) is the leading cause of death from a single infectious agent (Mycobacterium tuberculosis) and killed nearly 1.5 million people in 2018 (1). TB mostly manifests as a pulmonary disease but also affects other body sites, causing extrapulmonary TB (EPTB). About 5% of all EPTB cases are tuberculous meningitis (TBM), which results from the spread of M. tuberculosis into the meninges and cerebrospinal fluid (CSF) (2). It is unclear what proportion of all TB cases are TBM, as it varies across studies by local TB prevalence, with high proportions (about 10%) suggested in high TB burden settings compared to low TB prevalence settings (around 1%) (3). It is estimated that at least 100,000 individuals develop TBM annually (3). TBM is the most devastating form of TB and continues to cause high morbidity and mortality (4), with an estimated 50% of patients dying or suffering neurological sequelae and complications (5, 6). TBM is mostly common in young children (2 to 4 years old) and individuals infected with HIV (4, 7). Besides TBM, infectious meningitis is also commonly caused by viruses, bacteria, and fungi, which are often challenging to differentiate from meningitis caused by TB (8). In both children and adults, viral meningitis is more common, followed by bacterial and fungal meningitis (9–11). Streptococcus pneumoniae is the most common cause of bacterial meningitis worldwide in both adults and children, followed by Neisseria meningitidis (8, 12). TB (22%) was reported as the most common form of bacterial meningitis in children from a high TB burden setting, followed by Streptococcus pneumoniae (4%) and Klebsiella pneumoniae (3%) (11). The diagnosis of TBM is challenging and often delayed, with deleterious outcomes for patients. These challenges are even more serious in very young infants (13).
The methods currently used for diagnosing TBM in children are unreliable. Symptoms and signs of the disease are not specific, and the tests used for diagnosis of the disease are highly invasive and time-consuming. General diagnostic tests, including CSF white cell count (WBC) with differential, total protein, and glucose level measurements, are performed for the diagnosis of meningitis (14). Typical CSF findings in TBM include increased total protein, decreased CSF-to-serum glucose ratio, and increased total WBC with lymphocytic pleocytosis (14, 15). Bacterial meningitis is characterized by mild-to-marked elevated total protein, mild-to-marked decreased CSF-to-serum glucose ratio, and increased total WBC with neutrophil predominance (16). In viral meningitis, there are normal-to-elevated levels of total protein, usually normal CSF-to-serum glucose ratio, and minimal total WBC, with lymphocyte predominance; while fungal meningitis is characterized by elevated total protein, low CSF-to-serum glucose ratio, and minimal total WBC with lymphocyte predominance (14). Various diagnostic algorithms that take into account the symptoms and signs, in conjunction with results from laboratory and imaging tests, have been suggested for use in classifying individuals suspected of having TBM, at least for research purposes (17, 18). The clinical management of TBM is challenging due to an incomplete understanding of the immunopathogenesis underlying the disease. Further investigations are required to update and refresh the body of knowledge for management of the disease, including the development of effective TB drugs, host-directed therapies, vaccines, and diagnostics. In the current review, we summarize evidence published in the literature on different diagnostic approaches for TBM in children, the pathogenesis and immunology of TBM, and the recent advances in the search for novel approaches, mainly biomarkers, in the diagnosis of the disease.
IMMUNOPATHOGENESIS OF TBM
Pathogenesis.
The development of TBM begins with respiratory infection, followed by hematogenous spread to the central nervous system (CNS). Within the lungs, a localized infection is initiated following inhalation of aerosol droplets containing M. tuberculosis bacilli, and the alveolar macrophages, neutrophils, and dendritic cells (DCs) are activated and release numerous cytokines, chemokines, and antimicrobial peptides (19). Infected DCs migrate to the local draining lymph node under the influence of cytokines and chemokines to stimulate the differentiation of T helper 1 cells. The T helper 1 cells release cytokines (interferon gamma [IFN-γ] and tumor necrosis factor alpha [TNF-α]) at the site of infection and activate macrophages and DCs to produce cytokines and antimicrobial peptides for containment of the infection (20). Ultimately, a granuloma is formed, containing the bacilli in a latent state.
Hematogenous spread to other organ systems, including the CNS, may occur after one of two processes, as follows: (i), a short bacteremia may occur when M. tuberculosis is filtered into the local draining lymph nodes during primary TB infection, before granuloma formation; or (ii) the latent infection stage may progress to active TB disease due to a lapse or decrease in the immune response, especially in the elderly, immunocompromised, or very young individuals, thus leading to lung tissue destruction (19). M. tuberculosis bacilli bypass the alveolar epithelium through infected phagocytes or as free bacteria, and the latter has been linked to two bacterial proteins, namely, early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 kDA (CFP-10), together with heparin-binding hemagglutinin adhesin (HBHA). TB bacilli may migrate across the blood-brain barrier (BBB) and blood-CSF barrier (BCSFB) through the following suggested mechanisms: (i) “Trojan horse,” in which M. tuberculosis bypasses the barriers via infected macrophages and neutrophils (19); or (ii) bacillary invasion of brain endothelium, mediated by M. tuberculosis pknD (Rv0931c) (21).
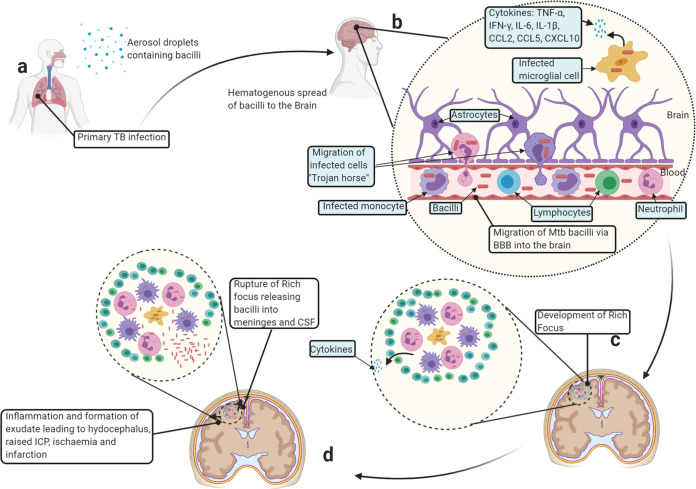
In the brain, the TB bacilli initiate the development of tuberculous lesions (known as Rich foci) in the meninges or the subpial or subependymal surface (22). Rich and McCordock demonstrated through postmortem experiments that the rupture of these lesions releases M. tuberculosis into the subarachnoid space or ventricular system, causing granulomatous infection and subsequent inflammation of meninges (23). Recently, the role of miliary spread in addition to the generally accepted pathogenetic mechanism of the Rich focus has been revised, based on more recent clinical, postmortem, and epidemiological data (24). The onset of TBM takes less than 12 months from the time of primary infection in 75% of children (25). Poor outcomes in TBM are due to host inflammatory responses, which result in the formation of a thick exudate at the base of the brain. The dense basal exudate blocks the basal subarachnoid cisterns by the formation of adhesions, obstructing CSF flow and resulting in hydrocephalus and raised intracranial pressure. Further extension of the exudate results in (i) obliterative vasculitis of small proliferating blood vessels, leading to the development of focal and diffuse ischemic brain changes, whereas blockage of larger arteries results in infarction; and (ii) perineuritis, resulting in cranial nerve palsies; and in severe cases, (iii) direct parenchymal involvement (25, 26). A schematic representation of the route from inhalation of M. tuberculosis to the development of TBM is shown in Fig. 1.
FIG 1.
The generalized pathogenesis of tuberculous meningitis. (a) The host inhales aerosol droplets containing M. tuberculosis (Mtb) bacilli. Within the lungs, the bacilli may infect the alveolar macrophages, resulting in the formation of granuloma. The bacilli may then escape from a damaged granuloma or from the lungs during primary TB causing bacteremia, resulting in hematogenous spread of the bacteria into the brain. (b) Extracellular bacteria and infected cells may migrate through the blood-brain barrier (BBB) into the brain. Once in the brain, the bacilli infect microglial cells, which then together with infiltrating cells release cytokines and chemokines, leading to disruption of the BBB and influx of other uninfected immune cells into the brain. (c) This results in the formation of the granuloma “Rich focus.” (d) When the Rich focus ruptures, the bacteria are released into the subarachnoid space, leading to dissemination of the infection to the CSF and meninges. The release of bacteria into the meninges and CSF leads to meningeal inflammation and the formation of thick exudate. The thick exudate precipitates TBM signs.
Clinical manifestation.
TBM typically presents as a subacute disease with many days or weeks (an average of 5 to 30 days) of nonspecific symptoms, including low-grade fever, malaise, headache, dizziness, vomiting, personality changes, and symptoms related to pulmonary TB (such as cough) (27, 28). Patients with advanced disease may present with more severe headache, altered mental status, stroke, hydrocephalus, and cranial neuropathies (27).
In children, clinical signs may include initial apathy or irritability that progresses to meningism, signs of raised intracranial pressure (such as abducens nerve palsy), and focal neurological signs (29). In adults, clinical signs may include neck stiffness, cranial nerve palsies (cranial nerve III, IV, VI, and VIII), confusion, and coma (29). Clinical motor deficits (monoplegia, hemiplegia, or paraplegia) occur in about 10% to 20% of cases (29, 30). Death is invariably inevitable if TBM is not treated.
Immune response.
The host inflammatory response plays an important role in TBM pathology (31). Within the CNS, microglia are resident macrophages and are arguably the most prominent immune effector cells responsible for recognition and internalization of M. tuberculosis (32). Microglial cells and migrated infected neutrophils and macrophages become rapidly activated and can proliferate and increase the expression of different molecules and secrete cytokines and chemokines, which in turn modulate immune responses within the CNS (33). Such cytokines and chemokines released by infected microglial cells include TNF-α, IFN-γ, interleukin-6 (IL-6), IL-1β, CCL2, CCL5, and CXCL10 (34). The cytokines and chemokines may disrupt the BBB, allowing the influx of other uninfected cells (monocytes, neutrophils, and lymphocytes) (30). Infected cells (microglial cells and migrated cells) together with migrating uninfected cells lead to the formation of Rich focus.
Following the rupture of the Rich focus, release of M. tuberculosis into the subarachnoid space elicits a local T cell-mediated response characterized by caseating granulomatous inflammation (35). The role of T cells in TBM has been supported by several studies. The predominance of αβT cells and NK cells in the CSF of TBM patients was associated with better survival (36). Whole blood transcriptome analysis of children with TBM at different time points demonstrated that reduced T cell proliferation and immune responses is associated with disease progression (37). A recent study reported a similar reduction pattern; however, this study did not assess the changes over time (31).
Inflammatory mediators (cytokines and chemokines) including TNF-α, IFN-γ, IL-1β, IL-6, IL-8, and IL-10 are increased in the CSF of patients with TBM (17, 38–40). TNF-α has been linked to a protective role against M. tuberculosis, through the formation of granulomata. Studies on rabbit models of TBM have demonstrated that high levels of TNF-α in CSF were associated with worse outcomes (41). The use of TNF-α antagonists in combination with antibiotics improved survival and outcomes in rabbits (42). In contrast, CSF TNF-α levels of children treated for TBM did not show a significant decline over a 4-week period (43).
Other host mediators implicated in the pathology of TBM include matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF). Elevated levels of MMP-9, MMP-2, tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-2 were reported in CSF samples of pediatric patients with TBM (44). MMP-9 levels decreased significantly early in treatment; however, there was an increase during hospital stay, which was associated with better outcomes (44). MMP-2 and MMP-9 may be involved in the pathology of TBM due to their key role in the disruption of BBB and the BCSF barrier by breaking down the extracellular matrix that constitutes the barriers, which may in turn cause brain edema, tissue damage, and migration of blood-derived inflammatory cells (45). Therefore, the inhibition of MMPs, as proposed in one study, may be a potential strategy for management of complications seen in TBM (45). VEGF is a potent factor of vascular permeability and angiogenesis (46), is vasculotoxic, is prothrombotic, reduces cerebral blood flow, and produces nitric oxide as well as oxygen free radicals (47). In TBM, VEGF has been associated with brain edema and the disruption of BBB (48), with its induction being mediated by TNF-α (49).
In a recent study, transcriptional responses of pediatric TBM at a systemic level revealed upregulated innate cell populations, such as neutrophils, macrophages, resting dendritic cells, and plasma B cells, in the blood (31). Similarly, van Laarhoven et al. (36) observed increased numbers of mature neutrophils and classical monocytes in the blood of adult TBM patients versus healthy controls. Gene expression associated with CD4 and CD8 T cells was more predominant in healthy controls, while genes associated with T cell activation and their signaling were significantly downregulated in the TBM cases (31). Furthermore, TBM was also associated with proinflammatory inflammasome signaling pathways (31). This could suggest that innate and inflammasome responses play an important role in TBM and that reduced T cell response is associated with the disease. Although the current findings suggest a role for neutrophils and other innate cells in TBM immunopathogenesis, the roles of different immune cell subpopulations, including the mucosa-associated invariant T (MAIT) cells, a T cell subset that displays innate-like characteristics and which have only recently been described in the CSF of TBM patients (36), remain unclear. Further investigations are needed to assess the frequencies, characteristics, and responsiveness of immune cells in patients with TBM in order to understand the host responses underlying the disease pathology.
CURRENT DIAGNOSTIC APPROACHES FOR TBM
Clinical diagnostic criteria.
Owing to the inadequate performance of microbiological tests, there have been attempts to establish clinical diagnostic criteria for the diagnosis of TBM based on a combination of all the evidence from medical history, clinical assessment, and other relevant investigations (including CSF investigations and neuroimaging) (13). Despite numerous efforts to create clinical prediction rules to differentiate TBM from other meningitis based on these tests, standardized diagnostic criteria are still lacking. Thwaites et al. proposed a scoring system for the diagnosis of TBM in adults on the basis of clinical and basic laboratory findings (18). In 2010, Marais et al. developed TBM diagnostic criteria for clinical case definition in research, incorporating the findings of Thwaites et al., among others (17). This uniform research case definition classifies patients as “definite,” “probable,” “possible,” and “not TBM” and is based on a composite score of clinical findings, CSF findings, neuroimaging, evidence of TB elsewhere, and exclusion of alternative diagnosis (17). It is important to note that the uniform research case definition criteria were not designed for use in clinical practice. Hence, caution is needed when applying the research case definitions for TBM patient care (50).
Microbiological diagnosis.
Smear microscopy is the most widely used rapid and inexpensive diagnostic test for TB; however, staining of CSF smears for acid-fast bacilli has poor sensitivity (about 10% to 15%) (51). Smear microscopy is therefore not reliable for the diagnosis of TBM. Mycobacterial culture, the gold standard for the diagnosis of TB, is recommended by the World Health Organization (WHO) for use in both adults and children, including for TBM. Although culture has a higher sensitivity (about 50% to 60%) for the diagnosis of TBM than that of other TB tests, its turnaround time (up to 8 weeks with solid media such as Lowenstein-Jensen [25, 51]) is a limitation. Although automated systems such as Bactec MGIT 960 have shown reduced average time to yield results (18 days versus 38 days), clinicians cannot afford to wait for culture results before treating patients, as death is a distinct possibility if empirical therapy is not initiated (30, 52). That notwithstanding, M. tuberculosis culture is still important for recovery of the bacilli needed for downstream phenotypic drug susceptibility testing (DST), as well as epidemiologic and sequencing-based studies.
Molecular tests.
To overcome the limitations of the conventional laboratory diagnostic approaches, commercial nucleic acid amplification tests (NAATs) have emerged. These tests have the advantage of rapidity while simultaneously detecting drug resistance. In a recent systemic review and meta-analysis of 18 studies, NAATs were shown to provide better performance with pooled sensitivity and specificity of 96% and 92%, respectively (53). However, the diagnostic accuracy of NAATs is different depending on the specimen type, with respiratory specimens associated with better accuracy (53). These tests are therefore not reliable for ruling out TB from nonrespiratory specimens due to the lower sensitivity. For the diagnosis of TBM specifically, a systematic review and meta-analysis on NAATs reported a pooled sensitivity of 82% and specificity of 99% against culture (54) and sensitivity and specificity of 68% and 98%, respectively, against a composite reference standard (54). In line with these results, another meta-analysis reported a lower sensitivity of 64% but high specificity of 98% against CSF M. tuberculosis culture for commercial NAATs (55).
The Xpert MTB/RIF test (Cepheid, Sunnyvale, CA, USA), arguably the game changer, was developed for the rapid diagnosis of TB. It is an automated closed-cartridge system that allows the rapid (within 2 h) detection of both M. tuberculosis and rifampin (RIF) resistance simultaneously. The WHO recommends the GeneXpert test for the diagnosis of EPTB, including TBM with CSF specimen in both adults and children. The sensitivity of the GeneXpert test for TBM ranges from approximately 50% to 60% (56), with various performances reported. A study from Uganda reported that sensitivity improved from 28% to 72% when larger volumes (6 ml) of concentrated CSF were used, compared with 2 ml of uncentrifuged CSF (56). In another study, the GeneXpert test showed an overall sensitivity of 59.3% compared with clinical diagnosis (based on uniform case definition [17]) in adult TBM suspects (57), with another meta-analysis (1 retrospective study and 4 prospective studies) reporting pooled sensitivity and specificity values of 70% and 97%, respectively (55).
Xpert MTB/RIF Ultra (Xpert Ultra) was developed to overcome some of the shortcomings of the initial Xpert test, including the inadequate sensitivity. In a recent prospective cohort study, Xpert Ultra demonstrated a sensitivity of 70% for probable or definite TBM (diagnosed based on uniform case definition [17]) compared with 43% obtained by either Xpert or culture (58). Compared with either Xpert or culture, Xpert Ultra diagnosed TBM in HIV-infected adults with a sensitivity of 95% (21/22 cases) (58). Although Ultra demonstrated improved performance, it does not appear to be adequate to rule out TBM due to concerns over low negative predictive value (NPV).
Another promising NAAT which will potentially be suitable for use in resource-limited settings, as it does not require expensive instruments or expertise and yields results in 60 minutes, is the loop-mediated isothermal amplification (LAMP) test (59). When investigated as a diagnostic test for TBM, LAMP showed potential with sensitivity between 88% and 96% and specificity of 80% to 100% (59, 60). Another NAAT that is commercially available (the Amplicor TB PCR test) has a sensitivity of ∼40% and specificity of ∼90% to 100% in the diagnosis of TBM, as reported in a recent review (14). The requirements of trained laboratory staff and high costs limit the wide use of the test (14). The Gen-probe amplified M. tuberculosis direct test (MTD), another NAAT, was initially intended for the detection of M. tuberculosis in respiratory specimens. When CSF samples were used in the test, it showed potential in the diagnosis of TBM, with pooled sensitivity of 86% and specificity of 99% (54). Another commercial NAAT, the Genotype MTBDRplus is a molecular line probe assay that targets specific genes for M. tuberculosis complex detection, as well as rifampin (RIF) and isoniazid (INH) susceptibility (61). When evaluated in a few TBM cases, the sensitivity of the test was 33%, with specificity of 98% against a clinical reference standard (61). While most NAATs have shown potential as diagnostic tests for TBM, more data on the performance of the tests are still required. Overall, commercial NAATs have demonstrated high specificity but, generally, suboptimal sensitivity for TBM, whereas Xpert Ultra, although promising, can still miss up to 30% of TBM patients. Despite improved diagnostic performance, both the GeneXpert and Xpert Ultra tests cannot rule out TBM due to their low negative predictive value (51, 62). Although NAATs are a major diagnostic advance, they are still inadequate to replace culture methods. The CRISPR-M. tuberculosis and metagenomic next-generation sequencing (mNGS) technologies (reviewed in reference 63), may improve the detection of M. tuberculosis in CSF samples with low bacillary load. However, further investigations are required to ascertain the performance of these methods.
Brain imaging.
Brain imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), are part of the clinical diagnostic assessment of TBM. Contrast-enhanced CT imaging reveals basal meningeal exudates specific for TBM and predicts poor outcomes (30). However, neurological signs or features (such as infarcts and hydrocephalus) revealed by CT imaging lack diagnostic specificity for TBM, mainly because similar features are seen in other infectious and noninfectious diseases (30). MRI has been found to have superior diagnostic abilities compared with CT (64). This includes better detection of basal meningeal enhancement and infarcts (especially in the brain stem) and early infection (64). The main common limitation to these brain imaging techniques is that CT scans are normal in about 30% of individuals at an early stage of TBM, while MRI scans are normal in about 15% (30). Furthermore, both CT and MRI evaluations are usually carried by medical experts in a tertiary care setting and are mostly not available in primary care settings or resource-limited settings (MRI more so than CT) (65).
Immune response-based diagnosis.
Immunodiagnostic tests, such as interferon gamma release assays (IGRAs), are primarily used for the diagnosis of M. tuberculosis infection. IGRAs measure the IFN-γ produced by lymphocytes when stimulated with M. tuberculosis-specific antigens. As these tests cannot differentiate latent TB infection (LTBI) from active TB disease, their use in the diagnosis of active TB is discouraged (66). The WHO recommends the use of IGRAs for LTBI testing in individuals who are at risk, including people living with HIV and infants and children aged 5 years and younger who are household contacts of pulmonary TB patients in both low- and high-TB burden settings (66). In TBM, a moderate diagnostic accuracy was reported for CSF IGRA, with sensitivity of 77% and specificity of 88% (67). The most commonly used immunodiagnostic modality in TBM management is the measurement of adenosine deaminase (ADA). ADA is an enzyme that is released by lymphocytes and plays an important role in the proliferation and differentiation of T cells. As the release of ADA from T cells has been associated with cell-mediated immune responses to tubercle bacilli, the measurement of levels of the enzyme in CSF is being done as an approach for the diagnosis of TBM (68–70). A meta-analysis of 20 studies on the accuracy of ADA reported a pooled sensitivity of 89% and specificity of 91% in the diagnosis of TBM (71). However, evidence about the clinical usefulness of CSF ADA is contradictory. Ekermans and colleagues reported that at an optimal cutoff of 2.0 U/liter, the sensitivity and specificity of CSF ADA was 85.9% and 77.7%, respectively (72). The study further showed that an optimal cutoff value for the routine diagnosis of TBM could not be established, as many cases were missed (72). Furthermore, high numbers of false positives and limited utility were reported for CSF ADA in another study on HIV-infected individuals (73). The main concern with the use of ADA in practice is the fact that similar levels of the protein have been documented in CSF from patients with other infective pathologies, including bacterial meningitis and ventriculitis, thereby making interpretation of the results difficult. ADA is therefore not useful in settings where the differential diagnosis is broad (72). As CSF ADA results may be misleading, clinicians should be aware of its limitations when making TBM diagnostic decisions. The poor standardization of ADA assays and the fact that ADA results are dependent on the integrity of the specimen (72) are further concerns. In summary, none of the currently available methods is adequate for use as a stand-alone test for the diagnosis of TBM. New and improved diagnostic methods are therefore urgently needed.
POTENTIAL OF NOVEL BIOMARKER-BASED APPROACHES
In the search of better TB diagnostic tools, recent studies have investigated several alternative approaches, including the measurement of protein concentrations in biological fluids, transcriptional molecules, and metabolites as biomarkers for TB. Several attempts are being made at detecting such biomarkers in easily obtainable specimens, such as blood, urine, and saliva, among others, with the need for such nonsputum-based approaches being deemed a high priority by the WHO (74).
Host protein biomarkers.
Several studies have proven that the measurement of inflammatory proteins, such as cytokines, chemokines, acute phase proteins, and growth factors, can differentiate TB from other infections (reviewed in reference 75). Earlier studies evaluated the value of alternative proteins other than IFN-γ that were detected in supernatants following the stimulation of blood cells with M. tuberculosis-specific antigens using multiplex immunoassays, mainly the Luminex platform (76–78). As such studies were based on overnight stimulation assays, most of the recent studies have focused on the evaluation of host markers in unstimulated specimens, including serum (79), plasma (80), urine (81), and saliva (82), given that such biomarkers may be more easily translated into point-of-care tests.
In a study conducted in China on adult patients with CNS infection, including TBM (n = 17), purulent meningitis (n = 13), and cryptococcal meningitis (n = 13), CSF levels of IL-1β, TNF-α, IFN-γ, IL-6, IL-4, IL-10, IL-17A, IL-17F, and CD40L were ≥2-fold higher in the TBM group than in the control group (83), with IL-6 reported as the most important cytokine for differentiating CNS infection from controls (83). CSF glucose and the CSF/blood glucose ratio were negatively correlated with CSF IL-6 levels in patients with CNS infection, thus revealing the potential of combining CSF IL-6 and CSF glucose as a biomarker for CNS infection (83). In another Chinese study that included patients with viral meningitis, encephalitis, and bacterial meningitis and patients with intracranial metastatic tumor as controls, CSF Delta-like 1 ligand (DLL) levels showed promise in diagnosing TBM with a sensitivity of 87.1%, specificity of 99.1%, negative predictive value (NPV) of 92.2%, and positive predictive value (PPV) of 98.2%, at a cutoff value of >1.0 ng/ml (84). Similarly, serum DLL levels were also higher in the TBM group and diagnosed TBM (cutoff value of >6.0 ng/ml) with sensitivity of 82.3%, specificity of 91.0%, PPV of 83.6%, and NPV of 90.2% (84). In contrast, a Ugandan study conducted in HIV-infected patients reported poor sensitivity (32%) but high specificity (98%) (cutoff value of 1,150 pg/ml) for DLL1 in the diagnosis of TBM (85). Another protein (high mobility box-1; HMGB1), a damage-associated molecular pattern (DAMP) protein that plays a role in inflammation, was also shown to have potential in the diagnosis of TBM (sensitivity and specificity of 61.02% and 89.94%), respectively, at a (cutoff value of 3.4 ng/ml) in another study (86). Other studies that evaluated the value of various protein biomarkers as TBM diagnostic candidates include a South African study by Visser et al. (87), which identified a three-marker CSF biosignature of IL-13, VEGF, and cathelicidin LL-37, that showed potential (sensitivity of 52.0%), specificity of 95.0%, PPV of 91.0%, and NPV of 66.0% in the diagnosis of TBM in young children (87). When assessed in a more recent study, this three-marker biosignature diagnosed TBM with improved sensitivity of 95.7% at the cost of specificity (37.5%), with better results obtained (sensitivity of 91.3% and specificity of 100%) when IL-13 and LL-37 were replaced by IFN-γ and myeloperoxidase (MPO), which was also for the diagnosis of TBM in children (88). Multiple new biomarkers, including a new four-marker biosignature (soluble intracellular adhesion molecule 1 [sICAM-1], MPO, IL-8, and IFN-γ) and various individual biomarkers, including IFN-γ, MIP-4, CXCL9, CCL1, RANTES, IL-6, TNF-α, MPO, MMP-9, MMP-8, complement component 2 (CC2), IL-10, PAI-1, CXCL8, IL-1b, A1AT, CXCL10, granulocyte colony-stimulating factor (G-CSF), CC4, CC4b, granulocyte-macrophage-CSF (GM-CSF), platelet-derived growth factor (PDGF) AB/BB, apolipoprotein (Apo)-AI, metallo-β-lactamase (MBL), ferritin, CC5a, SAP, and CC5, were shown to have potential for childhood TBM diagnosis in the same study (88).
Despite the promise shown by the CSF host inflammatory biomarker-based studies described above, the procedure for the collection of CSF (lumbar puncture) may be a limitation in the implementation of CSF-based tests in resource-limited settings. Blood-based biomarkers, which have shown potential and are being developed into point-of-care tests for the diagnosis of pulmonary TB (79, 89), may be alternatives in TBM diagnosis. When evaluated as a tool for the diagnosis of TBM in children, a modified version of an adult serum seven-marker signature (C-reactive protein [CRP], IFN-γ, IP-10, CFH, Apo-A1, SAA, and NCAM) diagnosed TBM with moderate accuracy (sensitivity of 73.9% and specificity of 66.7%) (90). In the same South African study, a new three-marker serum biosignature (adipsin, Aβ42, and IL-10), which diagnosed childhood TBM with sensitivity of 82.6% and specificity of 75.0%, alongside several individual candidate biomarkers were identified (90). Given the potential shown in these studies, there is a need for further discovery and validation of similar biomarkers, followed by incorporation of the most promising candidates into point-of-care tests. The added benefit of blood-based biomarkers is the possibility of detecting them in fingerprick blood, as is currently being done for new pulmonary TB-based prototype tests (https://www.triagetb.com/).
Host transcriptional biomarker-based signatures.
Transcriptomics has become a popular approach for biomarker discovery, with several infectious diseases, including TB biosignatures, being discovered using techniques such as RNA sequencing, quantitative real-time PCR, and microarrays. It is suggested that quantifying the shifts in RNA abundances triggered by diseases could help in identifying diagnostic, disease-associated, and treatment response biomarkers. Recent studies have identified gene signatures that predict the onset of active TB several months before the onset of symptoms (91), signatures for the prediction of progression from latent TB infection to active TB in household contacts (92), diagnosis of TB (93, 94), and monitoring of TB treatment response (95). Most of these investigations have been adult, pulmonary TB-based studies, with one study reporting on the up-, or downregulation of 796 genes (398 and 398, respectively) in brain tissues of TBM patients who were coinfected with HIV (96). Of importance, four gene products, namely, glial fibrillary acidic protein (GFAP), serpin peptidase inhibitor clade A member 3 (SERPINA3), thymidine phosphorylase (TYMP/ECGF1), and heat shock 70 kDA protein 8 (HSPA8), were confirmed to be abundant in TBM patients with HIV coinfection (96). As this study compared TBM patients with individuals who succumbed to road traffic accidents, the utility of these genes as candidate TBM diagnostic biomarkers is unknown. In addition to evaluating the usefulness of these genes as TBM diagnostic candidates, further work is required for the identification and validation of TBM-specific biosignatures in well-designed TBM diagnostic studies. Given that prototype fingerprick blood-based mRNA signature tests currently exist (97), validated TBM transcriptomic biosignatures could be further incorporated into such platforms, followed by large-scale field trials against acceptable reference standards.
Host miRNA biosignatures.
MicroRNAs (miRNAs) are a class of conserved noncoding small RNAs (21 to 25 nucleotides long), which play an important role in the regulation of gene expression and other biological processes, including cell proliferation, cell differentiation, organ development, apoptosis, immune response, angiogenesis, and onset of disease (98, 99). Altered expression of miRNAs has been associated with TB (100). In a study including 112 children with TBM and 130 healthy controls, miR-29a expression in peripheral blood mononuclear cells (PBMCs) showed potential in the diagnosis of TBM, with a sensitivity of 67.2% and specificity of 88.5 and a sensitivity of 81.1% and specificity of 90% when evaluated in CSF (101). When used in combination, CSF plus PBMC miR-29a expression diagnosed pediatric TBM with sensitivity of 84.4% and specificity of 95.4% (101).
In a recent genome-wide miRNA analysis study performed on adult PBMCs and CSF samples (99), a combination of four miRNAs (miR-126-3p, miR-130a-3p, miR-151a-3p, and miR-199a-5p) discriminated TBM from viral meningitis (VM) in PBMCs with sensitivity of 90.6% and specificity of 86.7% and discriminated TBM from healthy controls with sensitivity of 93.5% and specificity of 70.6% (99). Three CSF-based miRNAs (miR-126-3p, miR-130a-3p, and miR-151a-3p) also showed potential in discriminating between TBM and VM (99), with miR-199a-5p levels undetectable in CSF (99). The four-marker PBMC miRNA signature (miR-126-3p, miR-130a-3p, miR-151a-3p, and miR-199a-5p) was validated in an independent sample set in the same study with sensitivity of 81.8% (9/11) and specificity of 90.0% (9/10) in distinguishing TB and VM and sensitivity of 81.8% (9/11) and a specificity of 84.6% (11/13) in discriminating TBM from other non-TBM patients (99). Three exosomal miRNAs (miR-20b, miR-191, and miR486) also showed potential as biomarkers for discriminating TBM from non-TBM disease when used in combination with electronic health records (EHRs) in another study, diagnosing TBM with sensitivity of 94% and specificity of 95% (102). Taken together, these studies show that miRNA-based biosignatures have potential as candidate TBM diagnostic biomarkers. However, more studies on their potential value are required, including studies done at multiple field sites in both adults and children.
Metabolic biosignatures.
Metabolomics is an emerging powerful and advanced omics platform, which may be useful in the identification of novel diagnostic biomarkers. The technique is used to identify metabolites that are associated with certain physiological or pathological conditions. Several studies have demonstrated significant differences in amino acids and energy metabolism in CSF samples of TBM patients compared with other groups, including patients with viral, bacterial, and cryptococcal meningitis (103–106). However, the diagnostic accuracy of the metabolites identified was not reported. In a study that investigated urine metabolic biomarkers in 12 children with TBM and 29 controls, a host biosignature (SUM-4) generated from the sum of urinary concentrations of methylcitric acid, 2-ketoglutaric acid, quinolinic acid, and 4-hydroxyhippuric acid, separated TBM from other groups with an area under the receiver operator characteristic (ROC) curve (AUC) of 96.6% (107). These largely small, proof-of-concept studies provide evidence that host metabolomic biomarkers may be useful in the diagnosis of TBM. However, further work is required in this field, coupled with work focusing on the development of end user-friendly detection devices for the measurement of any candidate metabolites, preferably at the point of care.
CONCLUSIONS
The diagnosis of TBM remains challenging, mainly due to difficulties in the direct detection of M. tuberculosis bacilli in CSF and other specimens from patients who are suspected of having the disease. The recently introduced Xpert MTB/RIF Ultra has shown promise in detecting paucibacillary TB, including diagnosing more TBM cases than the previous version. However, it may still fail to rule out TBM due to inadequate negative predictive value. Furthermore, its implementation in resource-limited settings will be hampered by the same issues that hindered the successful roll out of the GeneXpert MTB/RIF test in such settings (108, 109). None of the currently available diagnostic tools is adequate as a stand-alone method for the definite diagnosis of TBM. Therefore, CSF microscopy, mycobacterial culture, and molecular tests, such as GeneXpert, Xpert Ultra, and other NAATs, should all be performed for the diagnosis of TBM, in settings where this is possible. The TB field has recently seen much activity in the discovery, validation, and development of novel biomarker-based tests, but most of this activity is for the management of pulmonary TB, especially in adults. The few host biomarker-based projects that have focused on TBM have shown that the targets described in the WHO target-product profiles (TPPs) for a nonsputum biomarker test for the diagnosis of extrapulmonary TB (sensitivity of at least 80% in CSF samples for microbiologically confirmed TB and specificity of 98%, or as specific as the Xpert MTB/RIF) (74) may be achievable or approachable given the recent promising findings (88, 90, 99, 101, 102). However, much work is still required in the refinement and validation of the proposed biomarkers. Furthermore, such biomarker-based approaches will make the most impact only if further developed into easy-to-use diagnostic tests, especially tools that are implementable at the point of care, in resource-limited settings.
DIRECTIONS FOR FUTURE RESEARCH
Traditionally, TB diagnostic tests have been developed and largely validated in adults with pulmonary TB. Research focusing on the development and validation of tools in children and especially tests that may be useful in the diagnosis of EPTB, including TBM, should be encouraged.
Assessment of biomarkers produced after stimulation of blood cells with M. tuberculosis antigens (110, 111) has been shown to possess diagnostic potential in other extrapulmonary forms of TB (112–114). Such approaches should be explored in other EPTB types, including TBM.
Approaches that have resulted in potentially useful pulmonary TB signatures in noninvasive and easily obtainable specimens, including saliva (82, 115, 116) and urine (81, 117), require evaluation in TBM and should be encouraged.
- Further work needs to be done to validate the different inflammatory host biomarker signatures reviewed in the current study (Table 1). Such future work should focus on the following:
- Evaluation of the signatures in large independent cohorts of both adults and children that are recruited after clinical suspicion of having meningitis, prior to the confirmation of TBM or no TBM.
- Evaluation of the influence of HIV infection and other comorbidities on biomarker accuracy.
- Inclusion of participants from different geographical areas in the evaluation and validation of biomarkers, such as to assess global applicability.
- Incorporation of the most promising globally relevant biosignatures into point-of-care tests, followed by field trials of the tests in multiple settings.
- While validation of the few transcriptomic, metabolomic, and miRNA candidate biomarkers that have so far been identified (Table 1) is ongoing, further work, including new biomarker discovery in new, well-designed TBM studies in which controls are individuals suspected of having TBM as would be obtained in routine clinical practice, is encouraged.
- If the goal of developing a useful TBM biomarker-based point-of-care test remains elusive, the inclusion of different biomarker-based tests in a uniform research omics-based case definition may be beneficial. A uniform research omics-based case definition could be based on different blood and CSF validated biomarker signatures/omics-based tests, which may diagnose TBM with optimal accuracy when combined.
- To further enhance our knowledge of the immunology and pathogenesis of TBM (Fig. 1), further investigations of the immune cell populations, characteristics, and responses at both the site of disease (CSF) and peripheral (blood) in TBM patients and appropriate controls should be encouraged. Such knowledge may shed light on new potential vaccine and host-directed therapeutic targets.
- Following the development of new tools for TBM, evaluation of the accuracy of the tools against appropriate benchmarks, e.g., the TPPs proposed by the WHO, is encouraged (74).
TABLE 1.
Host biomarkers with potential for use in diagnosis of tuberculous meningitis
| Category | Biomarker | Sample | Sample size of: |
Location | Approach | Sensitivity (%) | Specificity (%) | Intended applicationa | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TBM cases | Controls | Total | |||||||||
| Host protein markers | Delta-like 1 ligand | CSFb | 62 | 111 | 173 | China | ELISAc | 87.1 | 99.1 | TBM vs VM, BM, nondiagnosed group | Peng et al. (84) |
| Delta-like 1 ligandd | CSF | 62 | 111 | 173 | China | ELISA | 82.3 | 91.0 | TBM vs VM, BM, nondiagnosed group | Peng et al. (84) | |
| HMGB1 | CSF | 59 | 169 | 228 | China | ELISA | 61.02 | 89.94 | TBM vs control patients | Chen et al. (86) | |
| 3-host marker signature (VEGF + IL-13 + cathelicidin LL-37) | CSF | 56 | 55 | 111 | South Africa | Multiplex cytokine assay, ELISA (LL-37) | 52.0 | 95.0 | TBM vs Non-TBM | Visser et al. (87) | |
| 3-host marker signature (VEGF + IL-13 + cathelicidin LL-37) | CSF | 23 | 24 | 47 | South Africa | Multiplex cytokine assay, ELISA (LL-37) | 95.7 | 37.5 | TBM vs no TBM | Manyelo et al. (88) | |
| 3-host marker signature (VEGF + IFN-γ + MPO) | CSF | 23 | 24 | 47 | South Africa | Multiplex cytokine assay | 91.3 | 100.0 | TBM vs no TBM | Manyelo et al. (88) | |
| 4-host marker signature (sICAM + MPO + IL-8 + IFN-γ) | CSF | 23 | 24 | 47 | South Africa | Multiplex cytokine assay | 96.0 | 96.0 | TBM vs no TBM | Manyelo et al. (88) | |
| 7-host marker signature (CRP + IFN-γ + IP-10 + CFH + Apo-A1 + SAA + NCAM1) | Blood | 23 | 24 | 47 | South Africa | Multiplex cytokine assay | 73.9 | 66.7 | TBM vs no TBM | Manyelo et al. (90) | |
| 3-host marker signature (adipsin + Aβ42 + IL-10) | Blood | 23 | 24 | 47 | South Africa | Multiplex cytokine assay | 82.6 | 75.0 | TBM vs no TBM | Manyelo et al. (90) | |
| Host RNA | 792 up- or downregulated genes (of importance: GFAP, SERPINA3, TYMP/ECGF1, and HSPA8) | Brain tissues | 5 | 4 | 9 | India | Microarray and immunohistochemistry validation | NRe | NR | TBM vs individuals who succumbed to road traffic accidents | Kumar et al. (96) |
| Host microRNA | Mir-29a | PBMCs | 122 | 130 | 252 | China | qRT-PCRf | 67.2 | 88.5 | TBM vs HC | Pan et al. (101) |
| CSF | 122 | 130 | 252 | China | qRT-PCR | 81.1 | 90.0 | TBM vs HC | Pan et al. (101) | ||
| 4-host miRNA marker signature (miR-126-3p + miR-130a-3p + miR-151a-3p + miR-199a-5p) | PBMCs | 32 | 64 (30 VM, 34 HC) | 96 | China | Genome-wide microarray, qPCR independent validation | 90.6 | 86.7 | TBM vs VM | Pan et al. (99) | |
| 93.5 | 70.6 | TBM vs HC | |||||||||
| Metabolic markers | 16 NMRg metabolites | CSF | 33 | 73 (30 nonmeningitis controls from South Africa and 43 neurological controls from the Netherlands) | 106 | South Africa | Untargeted magnetic resonance (1H NMR)-based metabolomics analysis | NR | NR | TBM vs controls | Mason et al. (103) |
| Alanine, glycine, lysine, proline, and asparagine | CSF | 33 | 34 | 67 | South Africa | GC-MSh | NR | NR | TBM vs controls (suspected meningitis) | Mason et al. (104) | |
| 25 key metabolites | CSF | 18 | 20 | 38 | China | 1H NMR-based metabolomics | NR | NR | TBM vs VM | Li et al. (105) | |
TBM, tuberculous meningitis; VM, viral meningitis; BM, bacterial meningitis; HC, healthy controls.
CSF, cerebrospinal fluid.
ELISA, enzyme-linked immunosorbent assay.
At a different cutoff value.
NR, not reported.
qRT-PCR, quantitative real-time PCR.
NMR, nuclear magnetic resonance.
GC-MS, gas chromatography-mass spectrometry.
ACKNOWLEDGMENTS
This review was written as part of an EDCTP2 project, supported by the European Union (grant number TMA2018SF-2470-TBMBIOMARKERS). The project was also made possible through funding by the South African Medical Research Council through its Division of Research Capacity Development under the Internship Scholarship Program, from funding received from the South African National Treasury.
The views and opinions of authors expressed herein do not necessarily state or reflect those of the EDCTP and the SAMRC.
REFERENCES
- 1.World Health Organization. 2019. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Thakur K, Das M, Dooley K, Gupta A. 2018. The global neurological burden of tuberculosis. Semin Neurol 38:226–237. doi: 10.1055/s-0038-1651500. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JA, Tugume L, Solomons R, Prasad K, Bahr NC, Tuberculous Meningitis International Research Consortium. 2019. The current global situation for tuberculous meningitis: epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res 4:167. doi: 10.12688/wellcomeopenres.15535.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thwaites G. 2017. Tuberculous meningitis. Medicine (Baltimore) 45:670–673. doi: 10.1016/j.mpmed.2017.08.010. [DOI] [Google Scholar]
- 5.Chiang SS, Khan FA, Milstein MB, Tolman AW, Benedetti A, Starke JR, Becerra MC. 2014. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 14:947–957. doi: 10.1016/S1473-3099(14)70852-7. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DT, Agarwal S, Graviss EA. 2019. Trends of tuberculosis meningitis and associated mortality in Texas, 2010–2017, a large population-based analysis. PLoS One 14:e0212729. doi: 10.1371/journal.pone.0212729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thwaites GE, van Toorn R, Schoeman J. 2013. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 12:999–1010. doi: 10.1016/S1474-4422(13)70168-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang AY, Machicado JD, Khoury NT, Wootton SH, Salazar L, Hasbun R. 2014. Community-acquired meningitis in older adults: clinical features, etiology, and prognostic factors. J Am Geriatr Soc 62:2064–2070. doi: 10.1111/jgs.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulaiman T, Salazar L, Hasbun R. 2017. Acute versus subacute community-acquired meningitis: analysis of 611 patients. Medicine (Baltimore) 96:e7984. doi: 10.1097/MD.0000000000007984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasbun R, Rosenthal N, Balada-Llasat JM, Chung J, Duff S, Bozzette S, Zimmer L, Ginocchio CC. 2017. Epidemiology of meningitis and encephalitis in the United States, 2011–2014. Clin Infect Dis 65:359–363. doi: 10.1093/cid/cix319. [DOI] [PubMed] [Google Scholar]
- 11.Wolzak NK, Cooke ML, Orth H, van Toorn R. 2012. The changing profile of pediatric meningitis at a referral centre in Cape Town, South Africa. J Trop Pediatr 58:491–495. doi: 10.1093/tropej/fms031. [DOI] [PubMed] [Google Scholar]
- 12.Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A. 2011. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 364:2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 13.van Toorn R, Solomons R. 2014. Update on the diagnosis and management of tuberculous meningitis in children. Semin Pediatr Neurol 21:12–18. doi: 10.1016/j.spen.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Poplin V, Boulware DR, Bahr NC. 2020. Methods for rapid diagnosis of meningitis etiology in adults. Biomark Med 14:459–479. doi: 10.2217/bmm-2019-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahr NC, Boulware DR. 2014. Methods of rapid diagnosis for the etiology of meningitis in adults. Biomark Med 8:1085–1103. doi: 10.2217/bmm.14.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seehusen DA, Reeves M, Fomin D. 2003. Cerebrospinal fluid analysis. Am Fam Physician 68:1103–1108. [PubMed] [Google Scholar]
- 17.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ, Marais BJ. 2010. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 10:803–812. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 18.Thwaites G, Chau T, Stepniewska K, Phu N, Chuong L, Sinh D, White N, Parry C, Farrar J. 2002. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 360:1287–1292. doi: 10.1016/S0140-6736(02)11318-3. [DOI] [PubMed] [Google Scholar]
- 19.Davis AG, Rohlwink UK, Proust A, Figaji AA, Wilkinson RJ. 2019. The pathogenesis of tuberculous meningitis. J Leukoc Biol 105:267–280. doi: 10.1002/JLB.MR0318-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. 2013. The immune response in tuberculosis. Annu Rev Immunol 31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 21.Be NA, Bishai WR, Jain SK. 2012. Role of Mycobacterium tuberculosis pknD in the Pathogenesis of central nervous system tuberculosis. BMC Microbiol 12:7. doi: 10.1186/1471-2180-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isabel BE, Rogelio HP. 2014. Pathogenesis and immune response in tuberculous meningitis. Malays J Med Sci 21:4–10. [PMC free article] [PubMed] [Google Scholar]
- 23.Rich AR, McCordock HA. 1933. The pathogenesis of tuberculous meningitis. Bull Johns Hopkins Hosp 52:2–37. [Google Scholar]
- 24.Donald PR, Schaaf HS, Schoeman JF. 2005. Tuberculous meningitis and miliary tuberculosis: the Rich focus revisited. J Infect 50:193–195. doi: 10.1016/j.jinf.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Thwaites G, Chau TTH, Mai NTH, Drobniewski F, McAdam K, Farrar J. 2000. Tuberculous meningitis. J Neurol Neurosurg Psychiatry 68:289–299. doi: 10.1136/jnnp.68.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dastur DK, Manghani DK, Udani PM. 1995. Pathology and pathogenetic mechanisms in neurotuberculosis. Radiol Clin North Am 33:733–752. [PubMed] [Google Scholar]
- 27.Marx GE, Chan ED. 2011. Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat 2011:798764–798769. doi: 10.1155/2011/798764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniel BD, Grace GA, Natrajan M. 2019. Tuberculous meningitis in children: clinical management & outcome. Indian J Med Res 150:117–130. doi: 10.4103/ijmr.IJMR_786_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Méchaï F, Bouchaud O. 2019. Tuberculous meningitis: challenges in diagnosis and management. Rev Neurol (Paris) 175:451–457. doi: 10.1016/j.neurol.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson RJ, Rohlwink U, Misra UK, van Crevel R, Mai NTH, Dooley KE, Caws M, Figaji A, Savic R, Solomons R, Thwaites GE, on behalf of the Tuberculous Meningitis International Research Consortium. 2017. Tuberculous meningitis. Nat Rev Neurol 13:581–598. doi: 10.1038/nrneurol.2017.120. [DOI] [PubMed] [Google Scholar]
- 31.Rohlwink UK, Figaji A, Wilkinson KA, Horswell S, Sesay AK, Deffur A, Enslin N, Solomons R, Toorn RV, Eley B, Levin M, Wilkinson RJ, Lai RPJ. 2019. Tuberculous meningitis in children is characterized by compartmentalized immune responses and neural excitotoxicity. Nat Commun 10:3767. doi: 10.1038/s41467-019-11783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spanos JP, Hsu N-J, Jacobs M. 2015. Microglia are crucial regulators of neuro-immunity during central nervous system tuberculosis. Front Cell Neurosci 9:182. doi: 10.3389/fncel.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almolda B, González B, Castellano B. 2015. Are microglial cells the regulators of lymphocyte responses in the CNS? Front Cell Neurosci 9:440–440. doi: 10.3389/fncel.2015.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rock RB, Hu S, Gekker G, Sheng WS, May B, Kapur V, Peterson PK. 2005. Mycobacterium tuberculosis–induced cytokine and chemokine expression by human microglia and astrocytes: effects of dexamethasone. J Infect Dis 192:2054–2058. doi: 10.1086/498165. [DOI] [PubMed] [Google Scholar]
- 35.Török ME. 2015. Tuberculous meningitis: advances in diagnosis and treatment. Br Med Bull 113:117–131. doi: 10.1093/bmb/ldv003. [DOI] [PubMed] [Google Scholar]
- 36.van Laarhoven A, Dian S, van Dorp S, Purnama F, Koeken VACM, Diandini E, Utami F, Livia R, Apriani L, Ardiansyah E, ter Horst R, Netea MG, Achmad TH, Hill PC, Ruslami R, Alisjahbana B, Ussher JE, Indrati A, Verrall A, Ganiem AR, van Crevel R. 2019. Immune cell characteristics and cytokine responses in adult HIV-negative tuberculous meningitis: an observational cohort study. Sci Rep 9:884. doi: 10.1038/s41598-018-36696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemingway C, Berk M, Anderson ST, Wright VJ, Hamilton S, Eleftherohorinou H, Kaforou M, Goldgof GM, Hickman K, Kampmann B, Schoeman J, Eley B, Beatty D, Pienaar S, Nicol MP, Griffiths MJ, Waddell SJ, Newton SM, Coin LJ, Relman DA, Montana G, Levin M. 2017. Childhood tuberculosis is associated with decreased abundance of T cell gene transcripts and impaired T cell function. PLoS One 12:e0185973. doi: 10.1371/journal.pone.0185973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagesh Babu G, Kumar A, Kalita J, Misra UK. 2008. Proinflammatory cytokine levels in the serum and cerebrospinal fluid of tuberculous meningitis patients. Neurosci Lett 436:48–51. doi: 10.1016/j.neulet.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 39.Misra UK, Kalita J, Srivastava R, Nair PP, Mishra MK, Basu A. 2010. A study of cytokines in tuberculous meningitis: clinical and MRI correlation. Neurosci Lett 483:6–10. doi: 10.1016/j.neulet.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz E, Gürgöze MK, Ilhan N, Doğan Y, Aydinoğlu H. 2002. Interleukin-8 levels in children with bacterial, tuberculous and aseptic meningitis. Indian J Pediatr 69:219–221. doi: 10.1007/BF02734223. [DOI] [PubMed] [Google Scholar]
- 41.Tsenova L, Bergtold A, Freedman VH, Young RA, Kaplan G. 1999. Tumor necrosis factor is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc Natl Acad Sci U S A 96:5657–5662. doi: 10.1073/pnas.96.10.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsenova L, Sokol K, Freedman VH, Kaplan G. 1998. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis 177:1563–1572. doi: 10.1086/515327. [DOI] [PubMed] [Google Scholar]
- 43.Donald PR, Schoeman JF, Beyers N, Nel ED, Carlini SM, Olsen KD, McCracken GH. 1995. Concentrations of interferon γ, tumor necrosis factor α, and interleukin-1β in the cerebrospinal fluid of children treated for tuberculous meningitis. Clin Infect Dis 21:924–929. doi: 10.1093/clinids/21.4.924. [DOI] [PubMed] [Google Scholar]
- 44.Li YJ, Wilkinson KA, Wilkinson RJ, Figaji AA, Rohlwink UK. 2020. Elevated matrix metalloproteinase concentrations offer novel insight into their role in pediatric tuberculous meningitis. J Pediatr Infect Dis Soc 9:82–86. doi: 10.1093/jpids/piy141. [DOI] [PubMed] [Google Scholar]
- 45.Lee KY, Kim EH, Yang WS, Ryu H, Cho S-N, Lee BI, Heo JH. 2004. Persistent increase of matrix metalloproteinases in cerebrospinal fluid of tuberculous meningitis. J Neurol Sci 220:73–78. doi: 10.1016/j.jns.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Misra UK, Kalita J, Singh AP, Prasad S. 2013. Vascular endothelial growth factor in tuberculous meningitis. Int J Neurosci 123:128–132. doi: 10.3109/00207454.2012.743127. [DOI] [PubMed] [Google Scholar]
- 47.Misra UK, Kalita J, Maurya PK. 2011. Stroke in tuberculous meningitis. J Neurol Sci 303:22–30. doi: 10.1016/j.jns.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 48.M van der F, Hoppenreijs S, Rensburg A van, Ruyken M, Kolk A, Springer P, Hoepelman A, Geelen S, Kimpen J, Schoeman J. 2004. Vascular endothelial growth factor and blood-brain barrier disruption in tuberculous meningitis. Pediatr Infect Dis J 23:608–613. doi: 10.1097/01.inf.0000131634.57368.45. [DOI] [PubMed] [Google Scholar]
- 49.Ryuto M, Ono M, Izumi H, Yoshida S, Weich HA, Kohno K, Kuwano M. 1996. Induction of vascular endothelial growth factor by tumor necrosis factor α in human glioma cells possible roles of SP-1. J Biol Chem 271:28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 50.Solomons RS, Wessels M, Visser DH, Donald PR, Marais BJ, Schoeman JF, van Furth AM. 2014. Uniform research case definition criteria differentiate tuberculous and bacterial meningitis in children. Clin Infect Dis 59:1574–1578. doi: 10.1093/cid/ciu665. [DOI] [PubMed] [Google Scholar]
- 51.Bahr NC, Meintjes G, Boulware DR. 2019. Inadequate diagnostics: the case to move beyond the bacilli for detection of meningitis due to Mycobacterium tuberculosis. J Med Microbiol 68:755–760. doi: 10.1099/jmm.0.000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thakur R, Goyal R, Sarma S. 2010. Laboratory diagnosis of tuberculous meningitis—is there a scope for further improvement? J Lab Physicians 2:21–24. doi: 10.4103/0974-2727.66705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei Z, Zhang X, Wei C, Yao L, Li Y, Zhang X, Xu H, Jia Y, Guo R, Wu Y, Yang K, Gao X. 2019. Diagnostic accuracy of in-house real-time PCR assay for Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis 19:701. doi: 10.1186/s12879-019-4273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pormohammad A, Nasiri MJ, McHugh TD, Riahi SM, Bahr NC. 2019. A systematic review and meta-analysis of the diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis. J Clin Microbiol 57:e01113-18. doi: 10.1128/JCM.01113-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solomons RS, van Elsland SL, Visser DH, Hoek KGP, Marais BJ, Schoeman JF, van Furth AM. 2014. Commercial nucleic acid amplification tests in tuberculous meningitis—a meta-analysis. Diagn Microbiol Infect Dis 78:398–403. doi: 10.1016/j.diagmicrobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Bahr NC, Tugume L, Rajasingham R, Kiggundu R, Williams DA, Morawski B, Alland D, Meya DB, Rhein J, Boulware DR. 2015. Improved diagnostic sensitivity for TB meningitis with Xpert MTB/Rif of centrifuged CSF: a prospective study. Int J Tuber Lung Dis 19:1209–1215. doi: 10.5588/ijtld.15.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nhu NTQ, Heemskerk D, Thu DDA, Chau TTH, Mai NTH, Nghia HDT, Loc PP, Ha DTM, Merson L, Thinh TTV, Day J, Chau NVV, Wolbers M, Farrar J, Caws M. 2014. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol 52:226–233. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahr NC, Nuwagira E, Evans EE, Cresswell FV, Bystrom PV, Byamukama A, Bridge SC, Bangdiwala AS, Meya DB, Denkinger CM, Muzoora C, Boulware DR, ASTRO-CM Trial Team. 2018. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect Dis 18:68–75. doi: 10.1016/S1473-3099(17)30474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagdev KJ, Kashyap RS, Parida MM, Kapgate RC, Purohit HJ, Taori GM, Daginawala HF. 2011. Loop-mediated isothermal amplification for rapid and reliable diagnosis of tuberculous meningitis. J Clin Microbiol 49:1861–1865. doi: 10.1128/JCM.00824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Modi M, Sharma K, Sharma M, Sharma A, Sharma N, Sharma S, Ray P, Varma S. 2016. Multitargeted loop-mediated isothermal amplification for rapid diagnosis of tuberculous meningitis. Int J Tuber Lung Dis 20:625–630. doi: 10.5588/ijtld.15.0741. [DOI] [PubMed] [Google Scholar]
- 61.Solomons RS, Visser DH, Friedrich SO, Diacon AH, Hoek KGP, Marais BJ, Schoeman JF, van Furth AM. 2015. Improved diagnosis of childhood tuberculous meningitis using more than one nucleic acid amplification test. Int J Tuber Lung Dis 19:74–80. doi: 10.5588/ijtld.14.0394. [DOI] [PubMed] [Google Scholar]
- 62.Bahr NC, Marais S, Caws M, van Crevel R, Wilkinson RJ, Tyagi JS, Thwaites GE, Boulware DR, Tuberculous Meningitis International Research Consortium. 2016. GeneXpert MTB/Rif to diagnose tuberculous meningitis: perhaps the first test but not the last. Clin Infect Dis 62:1133–1135. doi: 10.1093/cid/ciw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cresswell FV, Davis AG, Sharma K, Basu Roy R, Ganiem AR, Kagimu E, Solomons R, Wilkinson RJ, Bahr NC, Thuong NTT, Tuberculous Meningitis International Research Consortium. 2020. Recent developments in tuberculous meningitis pathogenesis and diagnostics. Wellcome Open Res 4:164. doi: 10.12688/wellcomeopenres.15506.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pienaar M, Andronikou S, van Toorn R. 2009. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Childs Nerv Syst 25:941–947. doi: 10.1007/s00381-008-0785-3. [DOI] [PubMed] [Google Scholar]
- 65.Kurien R, Sudarsanam TD, Samantha S, Thomas K. 2013. Tuberculous meningitis: a comparison of scoring systems for diagnosis. Oman Med J 28:163–166. doi: 10.5001/omj.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Global Tuberculosis Programme. 2018. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 67.Yu J, Wang Z-J, Chen L-H, Li H-H. 2016. Diagnostic accuracy of interferon-gamma release assays for tuberculous meningitis: a meta-analysis. Int J Tuber Lung Dis 20:494–499. doi: 10.5588/ijtld.15.0600. [DOI] [PubMed] [Google Scholar]
- 68.Moghtaderi A, Niazi A, Alavi-Naini R, Yaghoobi S, Narouie B. 2010. Comparative analysis of cerebrospinal fluid adenosine deaminase in tuberculous and non-tuberculous meningitis. Clin Neurol Neurosurg 112:459–462. doi: 10.1016/j.clineuro.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Ghosh GC, Sharma B, Gupta BB. 2016. CSF ADA determination in early diagnosis of tuberculous meningitis in HIV-infected patients. Scientifica (Cairo) 2016:5820823–5820824. doi: 10.1155/2016/5820823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta BK, Bharat A, Debapriya B, Baruah H. 2010. Adenosine deaminase levels in CSF of tuberculous meningitis patients. J Clin Med Res 2:220–224. doi: 10.4021/jocmr429w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pormohammad A, Riahi S-M, Nasiri MJ, Fallah F, Aghazadeh M, Doustdar F, Pouriran R. 2017. Diagnostic test accuracy of adenosine deaminase for tuberculous meningitis: a systematic review and meta-analysis. J Infect 74:545–554. doi: 10.1016/j.jinf.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Ekermans P, Dusé A, George J. 2017. The dubious value of cerebrospinal fluid adenosine deaminase measurement for the diagnosis of tuberculous meningitis. BMC Infect Dis 17:104. doi: 10.1186/s12879-017-2221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corral I, Quereda C, Navas E, Martín-Dávila P, Pérez-Elías M-J, Casado J-L, Pintado V, Cobo J, Pallarés E, Rubí J, Moreno S. 2004. Adenosine deaminase activity in cerebrospinal fluid of HIV-infected patients: limited value for diagnosis of tuberculous meningitis. Eur J Clin Microbiol Infect Dis 23:471–476. doi: 10.1007/s10096-004-1110-z. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization. 2014. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 75.Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. 2014. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J 43:1472–1486. doi: 10.1183/09031936.00151413. [DOI] [PubMed] [Google Scholar]
- 76.Awoniyi DO, Teuchert A, Sutherland JS, Mayanja-Kizza H, Howe R, Mihret A, Loxton AG, Sheehama J, Kassa D, Crampin AC, Dockrell HM, Kidd M, Rosenkrands I, Geluk A, Ottenhoff THM, Corstjens PLAM, Chegou NN, Walzl G, Walzl G, Chegou NN, Kriel M, van der Spuy G, Loxton AG, Stanley K, Malherbe S, Mcanda S, Kriel B, Phalane KG, Essone P, Sutherland JS, Owolabi O, Sillah A, Mendy J, Gindeh A, Donkor S, Togun T, Ota M, Crampin AC, Simukonda F, Amberbir A, Chilongo F, Houben R, Kassa D, Gebrezgeabher A, Mesfin G, Belay Y, Gebremichael G, Alemayehu Y, van der Vyver M, Amutenya FN, Nelongo JN, Monye L, AE-TBC Consortium. 2016. Evaluation of cytokine responses against novel Mtb antigens as diagnostic markers for TB disease. J Infect 73:219–230. doi: 10.1016/j.jinf.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 77.Essone PN, Chegou NN, Loxton AG, Stanley K, Kriel M, van der Spuy G, Franken KL, Ottenhoff TH, Walzl G. 2014. Host cytokine responses induced after overnight stimulation with novel M. tuberculosis infection phase-dependent antigens show promise as diagnostic candidates for TB disease. PLoS One 9:e102584. doi: 10.1371/journal.pone.0102584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chegou NN, Essone PN, Loxton AG, Stanley K, Black GF, van der Spuy GD, van Helden PD, Franken KL, Parida SK, Klein MR, Kaufmann SHE, Ottenhoff THM, Walzl G. 2012. Potential of host markers produced by infection phase-dependent antigen-stimulated cells for the diagnosis of tuberculosis in a highly endemic area. PLoS One 7:e38501. doi: 10.1371/journal.pone.0038501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chegou NN, Sutherland JS, Malherbe S, Crampin AC, Corstjens PLAM, Geluk A, Mayanja-Kizza H, Loxton AG, van der Spuy G, Stanley K, Kotzé LA, van der Vyver M, Rosenkrands I, Kidd M, van Helden PD, Dockrell HM, Ottenhoff THM, Kaufmann SHE, Walzl G, AE-TBC consortium. 2016. Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax 71:785–794. doi: 10.1136/thoraxjnl-2015-207999. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs R, Malherbe S, Loxton AG, Stanley K, van der Spuy G, Walzl G, Chegou NN. 2016. Identification of novel host biomarkers in plasma as candidates for the immunodiagnosis of tuberculosis disease and monitoring of tuberculosis treatment response. Oncotarget 7:57581–57592. doi: 10.18632/oncotarget.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrone L, Cannas A, Vanini V, Cuzzi G, Aloi F, Nsubuga M, Sserunkuma J, Nazziwa RA, Jugheli L, Lukindo T, Girardi E, Antinori A, Pucci L, Reither K, Goletti D. 2016. Blood and urine inducible protein 10 as potential markers of disease activity. Int J Tuber Lung Dis 20:1554–1561. doi: 10.5588/ijtld.16.0342. [DOI] [PubMed] [Google Scholar]
- 82.Jacobs R, Tshehla E, Malherbe S, Kriel M, Loxton AG, Stanley K, van der Spuy G, Walzl G, Chegou NN. 2016. Host biomarkers detected in saliva show promise as markers for the diagnosis of pulmonary tuberculosis disease and monitoring of the response to tuberculosis treatment. Cytokine 81:50–56. doi: 10.1016/j.cyto.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Liu Q, Gao Y, Zhang B, Sun F, Yang Q, Liu Y, Wu J, Chen K, Weng X, Zhang W, Huang R, Shao L. 2020. Cytokine profiles in cerebrospinal fluid of patients with meningitis at a tertiary general hospital in China. J Microbiol Immunol Infect 53:216–224. doi: 10.1016/j.jmii.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Peng T, Zhou Y, Li J, Li J, Wan W, Jia Y. 2014. Detection of Delta-like 1 ligand for the diagnosis of tuberculous meningitis: an effective and rapid diagnostic method. J Int Med Res 42:728–736. doi: 10.1177/0300060513498669. [DOI] [PubMed] [Google Scholar]
- 85.Bahr NC, Halupnick R, Linder G, Kiggundu R, Nabeta HW, Williams DA, Musubire AK, Morawski BM, Sreevatsan S, Meya DB, Rhein J, Boulware DR. 2018. Delta-like 1 protein, vitamin D binding protein and fetuin for detection of Mycobacterium tuberculosis meningitis. Biomark Med 12:707–716. doi: 10.2217/bmm-2017-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Zhang J, Wang X, Wu Y, Zhu L, Lu L, Shen Q, Qin Y. 2016. HMGB1 level in cerebrospinal fluid as a complimentary biomarker for the diagnosis of tuberculous meningitis. SpringerPlus 5:1775. doi: 10.1186/s40064-016-3478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Visser DH, Solomons RS, Ronacher K, van Well GT, Heymans MW, Walzl G, Chegou NN, Schoeman JF, van Furth AM. 2015. Host immune response to tuberculous meningitis. Clin Infect Dis 60:177–187. doi: 10.1093/cid/ciu781. [DOI] [PubMed] [Google Scholar]
- 88.Manyelo CM, Solomons RS, Snyders CI, Manngo PM, Mutavhatsindi H, Kriel B, Stanley K, Walzl G, Chegou NN. 2019. Application of cerebrospinal fluid host protein biosignatures in the diagnosis of tuberculous meningitis in children from a high burden setting. Mediators Inflamm 2019:7582948. doi: 10.1155/2019/7582948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chegou NN, Sutherland JS, Namuganga A-R, Corstjens PL, Geluk A, Gebremichael G, Mendy J, Malherbe S, Stanley K, van der Spuy GD, Kriel M, Loxton AG, Kriel B, Simukonda F, Bekele Y, Sheehama JA, Nelongo J, van der Vyver M, Gebrexabher A, Hailu H, Esterhuyse MM, Rosenkrands I, Aagard C, Kidd M, Kassa D, Mihret A, Howe R, Cliff JM, Crampin AC, Mayanja-Kizza H, Kaufmann SHE, Dockrell HM, Ottenhoff THM, Walzl G, AE-TBC consortium. 2018. Africa-wide evaluation of host biomarkers in QuantiFERON supernatants for the diagnosis of pulmonary tuberculosis. Sci Rep 8:2675. doi: 10.1038/s41598-018-20855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manyelo CM, Solomons RS, Snyders CI, Mutavhatsindi H, Manngo PM, Stanley K, Walzl G, Chegou NN. 2019. Potential of host serum protein biomarkers in the diagnosis of tuberculous meningitis in children. Front Pediatr 7:376. doi: 10.3389/fped.2019.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zak DE, Penn-Nicholson A, Scriba TJ, Thompson E, Suliman S, Amon LM, Mahomed H, Erasmus M, Whatney W, Hussey GD, Abrahams D, Kafaar F, Hawkridge T, Verver S, Hughes EJ, Ota M, Sutherland J, Howe R, Dockrell HM, Boom WH, Thiel B, Ottenhoff THM, Mayanja-Kizza H, Crampin AC, Downing K, Hatherill M, Valvo J, Shankar S, Parida SK, Kaufmann SHE, Walzl G, Aderem A, Hanekom WA. 2016. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 387:2312–2322. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suliman S, Thompson EG, Sutherland J, Weiner J, Ota MOC, Shankar S, Penn-Nicholson A, Thiel B, Erasmus M, Maertzdorf J, Duffy FJ, Hill PC, Hughes EJ, Stanley K, Downing K, Fisher ML, Valvo J, Parida SK, van der Spuy G, Tromp G, Adetifa IMO, Donkor S, Howe R, Mayanja-Kizza H, Boom WH, Dockrell HM, Ottenhoff THM, Hatherill M, Aderem A, Hanekom WA, Scriba TJ, Kaufmann SHE, Zak DE, Walzl G, Walzl G, Black GF, van der Spuy G, Stanley K, Kriel M, Du Plessis N, Nene N, Roberts T, Kleynhans L, Gutschmidt A, Smith B, Nene N, Loxton AG, Chegou NN, Tromp G, Tabb D, GC6-74 cohort study team, the ACS cohort study team. 2018. Four-gene pan-African blood signature predicts progression to tuberculosis. Am J Respir Crit Care Med 197:1198–1208. doi: 10.1164/rccm.201711-2340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sweeney TE, Braviak L, Tato CM, Khatri P. 2016. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. Lancet Respir Med 4:213–224. doi: 10.1016/S2213-2600(16)00048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Darboe F, Mbandi SK, Thompson EG, Fisher M, Rodo M, van Rooyen M, Filander E, Bilek N, Mabwe S, Hatherill M, Zak DE, Penn-Nicholson A, Scriba TJ, Matiwane S, Jaxa L, Xoyana N, Schreuder C, Botes J, Africa H, Makhethe L, Steyn M, SATVI Clinical Immunology Team. 2018. Diagnostic performance of an optimized transcriptomic signature of risk of tuberculosis in cryopreserved peripheral blood mononuclear cells. Tuberculosis (Edinb) 108:124–126. doi: 10.1016/j.tube.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Thompson EG, Du Y, Malherbe ST, Shankar S, Braun J, Valvo J, Ronacher K, Tromp G, Tabb DL, Alland D, Shenai S, Via LE, Warwick J, Aderem A, Scriba TJ, Winter J, Walzl G, Zak DE, Du Plessis N, Loxton AG, Chegou NN, Lee M, Catalysis TB–Biomarker Consortium. 2017. Host blood RNA signatures predict the outcome of tuberculosis treatment. Tuberculosis (Edinb) 107:48–58. doi: 10.1016/j.tube.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar GSS, Venugopal AK, Kashyap MK, Raju R, Marimuthu A, Palapetta SM, Subbanayya Y, Goel R, Chawla A, Dikshit JB, Tata P, Harsha HC, Maharudraiah J, Ramachandra YL, Satishchandra P, Prasad TSK, Pandey A, Mahadevan A, Shankar SK. 2012. Gene expression profiling of tuberculous meningitis co-infected with HIV. J Proteomics Bioinform 5:235–244. doi: 10.4172/jpb.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sodersten E, Ongarello S, Mantsoki A, Wyss R, Persing DH, Banderby S, Meuzelaar LS, Prieto J, Gnanashanmugam D, Khatri P, Schumacher S, Denkinger C. 2020. Diagnostic accuracy study of a novel blood-based assay for identification of TB in people living with HIV. medRxiv 10.1101/2020.06.10.20127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wahid F, Shehzad A, Khan T, Kim YY. 2010. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta 1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Pan L, Liu F, Zhang J, Li J, Jia H, Huang M, Liu X, Chen W, Ding Z, Wang Y, Du B, Wei R, Sun Q, Xing A, Zhang Z. 2019. Genome-wide miRNA analysis identifies potential biomarkers in distinguishing tuberculous and viral meningitis. Front Cell Infect Microbiol 9:323. doi: 10.3389/fcimb.2019.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sabir N, Hussain T, Shah SZA, Peramo A, Zhao D, Zhou X. 2018. miRNAs in tuberculosis: new avenues for diagnosis and host-directed therapy. Front Microbiol 9:602–602. doi: 10.3389/fmicb.2018.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan D, Pan M, Xu Y-M. 2017. Mir-29a expressions in peripheral blood mononuclear cell and cerebrospinal fluid: diagnostic value in patients with pediatric tuberculous meningitis. Brain Res Bull 130:231–235. doi: 10.1016/j.brainresbull.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 102.Hu X, Liao S, Bai H, Wu L, Wang M, Wu Q, Zhou J, Jiao L, Chen X, Zhou Y, Lu X, Ying B, Zhang Z, Li W. 2019. Integrating exosomal microRNAs and electronic health data improved tuberculosis diagnosis. EBioMedicine 40:564–573. doi: 10.1016/j.ebiom.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mason S, van Furth AM, Mienie LJ, Engelke UFH, Wevers RA, Solomons R, Reinecke CJ. 2015. A hypothetical astrocyte–microglia lactate shuttle derived from a 1H NMR metabolomics analysis of cerebrospinal fluid from a cohort of South African children with tuberculous meningitis. Metabolomics 11:822–837. doi: 10.1007/s11306-014-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mason S, Reinecke CJ, Solomons R. 2017. Cerebrospinal fluid amino acid profiling of pediatric cases with tuberculous meningitis. Front Neurosci 11:534–534. doi: 10.3389/fnins.2017.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z, Du B, Li J, Zhang J, Zheng X, Jia H, Xing A, Sun Q, Liu F, Zhang Z. 2017. Cerebrospinal fluid metabolomic profiling in tuberculous and viral meningitis: screening potential markers for differential diagnosis. Clin Chim Acta 466:38–45. doi: 10.1016/j.cca.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Dai Y-N, Huang H-J, Song W-Y, Tong Y-X, Yang D-H, Wang M-S, Huang Y-C, Chen M-J, Zhang J-J, Ren Z-Z, Zheng W, Pan H-Y. 2017. Identification of potential metabolic biomarkers of cerebrospinal fluids that differentiate tuberculous meningitis from other types of meningitis by a metabolomics study. Oncotarget 8:100095–100112. doi: 10.18632/oncotarget.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mason S, van Furth AMT, Solomons R, Wevers RA, van Reenen M, Reinecke CJ. 2016. A putative urinary biosignature for diagnosis and follow-up of tuberculous meningitis in children: outcome of a metabolomics study disclosing host–pathogen responses. Metabolomics 12:110. doi: 10.1007/s11306-016-1053-2. [DOI] [Google Scholar]
- 108.Trébucq A, Enarson DA, Chiang CY, Van Deun A, Harries AD, Boillot F, Detjen A, Fujiwara PI, Graham SM, Monedero I, Rusen ID, Rieder HL. 2011. Xpert MTB/RIF for national tuberculosis programmes in low-income countries: when, where and how? Int J Tuber Lung Dis 15:1567–1572. doi: 10.5588/ijtld.11.0392. [DOI] [PubMed] [Google Scholar]
- 109.Amicosante M, D'Ambrosio L, Munoz M, Mello F. C d Q, Tebruegge M, Chegou NN, Seghrouchni F, Centis R, Goletti D, Bothamley G, Migliori GB, TB Diagnostic Survey Working Group. 2017. Current use and acceptability of novel diagnostic tests for active tuberculosis: a worldwide survey. J Bras Pneumol 43:380–392. doi: 10.1590/S1806-37562017000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manngo PM, Gutschmidt A, Snyders CI, Mutavhatsindi H, Manyelo CM, Makhoba NS, Ahlers P, Hiemstra A, Stanley K, McAnda S, Kidd M, Malherbe ST, Walzl G, Chegou NN. 2019. Prospective evaluation of host biomarkers other than interferon gamma in QuantiFERON Plus supernatants as candidates for the diagnosis of tuberculosis in symptomatic individuals. J Infect 79:228–235. doi: 10.1016/j.jinf.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chegou NN, Black GF, Kidd M, van Helden PD, Walzl G. 2009. Host markers in Quantiferon supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm Med 9:21. doi: 10.1186/1471-2466-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andreae CD, Smit DP, Makhoba NS, Kidd M, Walzl G, Chegou NN. 2019. Identification of potential biomarkers in peripheral blood supernatants of South African patients with syphilitic and herpetic uveitis. Ocul Immunol Inflamm 7:1–9. doi: 10.1080/09273948.2019.1674341. [DOI] [PubMed] [Google Scholar]
- 113.Makhoba NS, Smit DP, Walzl G, Chegou NN. 2020. Evaluation of potential antigen-specific host biomarkers in Quantiferon supernatants as candidates for the diagnosis of ocular tuberculosis. Ocul Immunol Inflamm 7:1–9. doi: 10.1080/09273948.2020.1763404. [DOI] [PubMed] [Google Scholar]
- 114.Chegou NN, Walzl G, Bolliger CT, Diacon AH, van den Heuvel MM. 2008. Evaluation of adapted whole-blood interferon-gamma; release assays for the diagnosis of pleural tuberculosis. Respiration 76:131–138. doi: 10.1159/000128575. [DOI] [PubMed] [Google Scholar]
- 115.Jacobs R, Maasdorp E, Malherbe S, Loxton AG, Stanley K, van der Spuy G, Walzl G, Chegou NN. 2016. Diagnostic potential of novel salivary host biomarkers as candidates for the immunological diagnosis of tuberculosis disease and monitoring of tuberculosis treatment response. PLoS One 11:e0160546. doi: 10.1371/journal.pone.0160546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Namuganga AR, Chegou NN, Mubiri P, Walzl G, Mayanja-Kizza H. 2017. Suitability of saliva for tuberculosis diagnosis: comparing with serum. BMC Infect Dis 17:600. doi: 10.1186/s12879-017-2687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J, Zhu X, Xiong X, Ge P, Liu H, Ren N, Khan FA, Zhou X, Zhang L, Yuan X, Chen X, Chen Y, Hu C, Robertson ID, Chen H, Guo A. 2018. Identification of potential urine proteins and microRNA biomarkers for the diagnosis of pulmonary tuberculosis patients. Emerg Microbes Infect 7:1–13. doi: 10.1038/s41426-018-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]