ABSTRACT
Background
Trimethylamine N-oxide (TMAO), a diet-derived, gut microbial-host cometabolite, has been linked to cardiometabolic diseases. However, the relations remain unclear between diet, TMAO, and cardiometabolic health in general populations from different regions and ethnicities.
Objectives
To examine associations of circulating TMAO with dietary and cardiometabolic factors in a pooled analysis of 16 population-based studies from the United States, Europe, and Asia.
Methods
Included were 32,166 adults (16,269 white, 13,293 Asian, 1247 Hispanic/Latino, 1236 black, and 121 others) without cardiovascular disease, cancer, chronic kidney disease, or inflammatory bowel disease. Linear regression coefficients (β) were computed for standardized TMAO with harmonized variables. Study-specific results were combined by random-effects meta-analysis. A false discovery rate <0.10 was considered significant.
Results
After adjustment for potential confounders, circulating TMAO was associated with intakes of animal protein and saturated fat (β = 0.124 and 0.058, respectively, for a 5% energy increase) and with shellfish, total fish, eggs, and red meat (β = 0.370, 0.151, 0.081, and 0.056, respectively, for a 1 serving/d increase). Plant protein and nuts showed inverse associations (β = −0.126 for a 5% energy increase from plant protein and −0.123 for a 1 serving/d increase of nuts). Although the animal protein–TMAO association was consistent across populations, fish and shellfish associations were stronger in Asians (β = 0.285 and 0.578), and egg and red meat associations were more prominent in Americans (β = 0.153 and 0.093). Besides, circulating TMAO was positively associated with creatinine (β = 0.131 SD increase in log-TMAO), homocysteine (β = 0.065), insulin (β = 0.048), glycated hemoglobin (β = 0.048), and glucose (β = 0.023), whereas it was inversely associated with HDL cholesterol (β = −0.047) and blood pressure (β = −0.030). Each TMAO-biomarker association remained significant after further adjusting for creatinine and was robust in subgroup/sensitivity analyses.
Conclusions
In an international, consortium-based study, animal protein was consistently associated with increased circulating TMAO, whereas TMAO associations with fish, shellfish, eggs, and red meat varied among populations. The adverse associations of TMAO with certain cardiometabolic biomarkers, independent of renal function, warrant further investigation.
Keywords: trimethylamine N-oxide, diet, biomarker, cardiovascular disease, Consortium of Metabolomics Studies
Introduction
The gut microbiota and its metabolites are increasingly recognized as playing important roles in human nutrition and cardiometabolic health (1, 2). Particularly, trimethylamine N-oxide (TMAO), a gut microbial-host cometabolite of dietary choline and carnitine, has received much attention (3, 4). Epidemiological studies have linked high circulating TMAO concentrations to multiple cardiometabolic diseases, including diabetes, cardiovascular disease (CVD), and chronic kidney disease (CKD) (4–7). Animal studies have shown that increasing TMAO promotes thrombosis, atherosclerosis, renal damage, and metabolic dysfunction (3–5, 8–10), whereas inhibiting TMAO reduces the formation of atherosclerotic lesions and improves glucose tolerance (8, 10, 11). TMAO has thus been proposed as a possible link between high intakes of dietary choline/carnitine or foods high in those nutrients (e.g., red meat and eggs) and poor cardiometabolic health (2, 3, 5, 12).
However, despite the existing evidence from animal models and epidemiological studies (mostly in patients or populations at high risk of CVD), the relations between diet, TMAO, and cardiometabolic health in general populations remain unclear (6, 13). The uncertainty could be due to variations in habitual diets that determine the TMAO precursors and gut microbial profiles, and also nondietary factors that might modulate TMAO concentrations and its cardiometabolic effects. Dietary sources of TMAO vary across populations. In typical Western diets, choline and carnitine mostly come from red meat, poultry, eggs, and dairy (14–16). Yet, choline is also abundant in fish, shellfish, legumes, and nuts (17), and TMAO is naturally abundant in fish and shellfish (no microbial metabolism required) (13). Studies have suggested that TMAO concentrations are associated with dairy and/or fish intakes in some European and Asian populations, rather than red meat in US populations (18–22). Studies have also reported associations of TMAO with choline from animal foods but not choline from plant foods, and with low adherence to the plant-based Mediterranean diet (23, 24), indicating that differences in habitual diets can result in variations in the food-TMAO associations. Besides dietary factors, increased TMAO has been linked to older age, male sex, obesity, dyslipidemia, inflammation, renal impairment, and history of diabetes, CVD, and CKD (18, 25–33), although those associations remain inconsistent and mostly unexplored in general populations. Collaborative epidemiological studies involving demographically diverse populations with different habitual diets and characteristics can further clarify the associations between diet and TMAO and cardiometabolic diseases.
To this end, we conducted an international pooled analysis to examine associations of circulating TMAO with demographics, diet, lifestyle, and cardiometabolic diseases and biomarkers in >32,000 participants from 16 studies conducted in the United States, Europe, and Asia.
Methods
Study populations and variables
This project is based on the COnsortium of METabolomics Studies (COMETS). Details of the design and studies within the COMETS have been described (34). Additionally, we reached out to studies not part of COMETS but with available blood TMAO data. A total of 16 studies joined the “TMAO Pooling Project,” by alphabetical order, including 9 in the United States: the Coronary Artery Risk Development in Young Adults Study (CARDIA) (35), Framingham Heart Study (FHS) (36), Health Professionals Follow-Up Study (37), Insulin Resistance Atherosclerosis Family Study (38), Multi-Ethnic Study of Atherosclerosis (MESA) (39), Nurses' Health Study (NHS) (40), Nurses' Health Study II (NHS2) (40), Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (41), and Women's Health Initiative; 3 in Europe: the Airwave Health Monitoring Study (Airwave) (42), Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (43), and TwinsUK Registry (44); and 4 in Asia: the Guangzhou Nutrition and Health Study (45), Shanghai Men's Health Study (SMHS) (46), Shanghai Women's Health Study (SWHS) (47), and Tsuruoka Metabolomics Cohort Study (TMCS) (48). The TMAO Pooling Project was approved by the COMETS steering committee, committees of the participating studies, and the Institutional Review Board of Vanderbilt University Medical Center.
Participants with available data on blood TMAO, age (>18 y), and sex were included. To minimize the influence of certain diseases and their treatments on TMAO concentration, we excluded participants if they had a self-reported history of cancer (except nonmelanoma skin cancer), coronary artery disease, stroke, heart failure, inflammatory bowel disease, or CKD [self-reported diagnosis or if without the information, a 1-time estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m²]. Moreover, participants above the study- and sex-specific 99th percentile of the TMAO concentration were excluded to minimize the influence of extreme values on linear regression results. Our final dataset included 32,166 adults, comprised of 16,269 white, 13,293 Asian, 1247 Hispanic/Latino, 1236 black, and 121 others (Supplemental Figure 1).
Survey-based study variables
A broad range of data, including demographics, lifestyle, dietary habits, anthropometrics, and disease history, were collected in each study at blood sample collection. A “data dictionary” was developed to harmonize variables across studies, including age at blood draw, sex, race and ethnicity (self-reported), BMI, waist-to-hip ratio (WHR), smoking status, alcohol consumption, physical activity, menopausal status and hormone therapy in women only, and history of diabetes, hypertension, dyslipidemia, and nonalcoholic fatty liver disease (NAFLD), which was defined by self-reported physician diagnosis or current use of medications to treat those conditions.
Habitual food intakes were assessed in 14 studies using FFQs that captured commonly consumed foods in the study population (Airwave and TMCS did not provide dietary information and were therefore excluded from all diet-related analyses). Intakes of total energy and nutrients were calculated using the country/region-specific food composition tables. Intakes of foods and nutrients were all standardized to intakes per 2000 kcal/d (49). Macronutrients, for example, carbohydrate, protein, and fat, were modeled as per 5% energy increment. Dietary fiber was modeled as 5 g/d increase. Food intakes were harmonized based on portion sizes (i.e., per 1 serving/d increase); included were: red meat, processed meat, poultry, total fish, and shellfish [1 serving = 4 oz (113.4 g)]; eggs (50 g); dairy foods—milk/cottage cheese [8 fluid oz (240 g)], firm cheese (50 g), and ice cream (100 g); soy products—soy milk [8 fluid oz (240 g)] and tofu/soybeans/soy meats [4 oz (113.4 g)]; legumes (50 g in dry weight); nuts (30 g in dry weight); vegetables (80 g); fruits (80 g); and whole grains (50 g in dry weight).
Metabolites and cardiometabolic biomarkers
Blood concentrations of TMAO were measured in each study using targeted or untargeted assays. Targeted assays were performed by the Broad Institute (50), Keio University (51), University of North Carolina Nutrition Obesity Research Center (52), and Sun Yat-sen University (45); untargeted assays were performed by Imperial College London's National Phenome Centre (ICL-NPC) (53) and Metabolon Inc. (54). The correlation of TMAO concentrations between different platforms was high (Spearman correlation r = 0.93 for the Broad Institute and Metabolon Inc. measures, and r = 0.96 for Metabolon Inc. and ICL-NPC measures) (34, 53). TMAO concentrations were log-transformed and normalized by study-specific mean and SD.
Cardiometabolic biomarkers were assessed per each study protocol. We harmonized data on glucose (milligrams per deciliter), insulin (microunits per milliliter), glycated hemoglobin (HbA1c; percentage of total hemoglobin), systolic blood pressure (millimeters of mercury), diastolic blood pressure (millimeters of mercury), total cholesterol (milligrams per deciliter), HDL cholesterol (milligrams per deciliter), LDL cholesterol (milligrams per deciliter), triglycerides (milligrams per deciliter), C-reactive protein (milligrams per liter), IL-6 (picograms per milliliter), creatinine (milligrams per deciliter), and homocysteine (micromoles per liter). Given usually skewed distributions of biomarkers, they were log-transformed and standardized by the mean and SD of each study. Additionally, we collected data on fasting status in all participating studies and recent use of antibiotics in 5 studies (i.e., FHS, MESA, SMHS, SWHS, and TMCS).
Statistical analysis
The analytic protocol was developed with inputs from all studies. A “standard statistical program” was developed based on harmonized variables and sent to each study for data analysis. The final numbers of harmonized variables varied due to the difference in data availability across studies. Study-specific results were combined using random-effects meta-analyses, considering differences in the study time period, metabolomics platform, and participant characteristics. The degree of heterogeneity across studies was assessed by the Q and I2 statistics; P for heterogeneity <0.10 was considered significant (55, 56). Linear regression was used to estimate β coefficients and 95% CIs for the associations of standardized log-TMAO with demographics (age, sex, and race and ethnicity), lifestyles (smoking, alcohol drinking, and physical activity), obesity and central obesity, metabolic conditions (diabetes, hypertension, dyslipidemia, and NAFLD), dietary intakes (per 5% energy increment for macronutrients and per 1 serving/d increment for foods), and cardiometabolic biomarkers (per 1 SD change in log-transformed value). A separate linear regression model was built for each variable of interest. Covariates were selected a priori and added to the model sequentially. The basic model included age (years), sex (men and women), race and ethnicity (white, black, Hispanic, Asian, and others), and fasting status (<6 h and ≥6 h). A second model further adjusted for education (less than high school, high-school graduation, post–high-school training or some college, and college graduation or higher), BMI (<18.5, 18.5–24.9, 25.0–29.9, and ≥30.0 kg/m2), central obesity (normal, moderate, high, and very high defined per WHO criteria; WHR: <0.90, 0.90–0.94, 0.95–0.99, and ≥1.00 for non-Asian men; <0.85, 0.85–0.89, 0.90–0.94, and ≥0.95 for Asian men; and <0.75, 0.75–0.79, 0.80–0.84, and ≥0.85 for all women), tobacco smoking status (never, former, and current), total physical activity (study- and sex-specific tertiles of total physical activity level), alcohol consumption (none, >0 to ≤1, >1 to ≤2, and >2 drinks/d; 1 drink = 14 g ethanol), use of multivitamins (yes and no), menopausal status and hormone therapy in women (yes and no), and intakes of major TMAO-contributing foods: red meat, eggs, and total fish (study- and sex-specific quintiles). In the final model, we further adjusted for metabolic disease status, including diabetes, hypertension, dyslipidemia, and NAFLD (yes and no). Missing covariates were coded as an unknown category and included in the models. For diet-related analyses, we further excluded participants with extreme energy intake (beyond ±5 SDs of study- and sex-specific mean) and adjusted for total energy intake. To evaluate the possible variations in TMAO associations due to differences in habitual diet, we conducted stratified analyses by region (United States, Europe, and Asia). Sensitivity analyses were conducted by excluding participants who reported recent antibiotic use (data were available in 5 studies, mostly defined as antibiotic use in the last 7 d) and then comparing results with those using data from all participants of these 5 studies. For the biomarker-TMAO associations, further stratified analyses were conducted by age, sex, race and ethnicity, fasting status, obesity status, metabolic disease status, and intakes of red meat, fish, fiber, and vegetable/fruits. P values were corrected for multiple comparisons using the Benjamini–Hochberg false discovery rate (FDR); FDR-q <0.10 was considered significant to embrace all potentially meaningful results, because FDR-q <0.05 has been suggested as being too low for many clinical settings (57). All results described in the following section had FDR-q <0.10, unless specified. Analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute Inc.) and Stata version 12 (StataCorp).
Results
Table 1 presents the characteristics of the participating studies. Among a total of 32,166 adults aged 19–84 y, 19,632 (61.0%) were women, 16,269 (50.6%) were white, 13,293 (41.3%) were Asian, 1247 (3.9%) were Hispanic/Latino, and 1236 (3.8%) were black. More details on baseline characteristics across studies are given in Supplemental Tables 1 and 2.
TABLE 1.
Characteristics of the participating studies: the TMAO Pooling Project1
| No. of participants by race and ethnicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline2 | No. of participants3 | Age4 | No. of women | White | Black | Hispanic | Asian | Metabolomics platform5 | |
| United States | |||||||||
| Coronary Artery Risk Development in Young Adults Study | 2000–2001 | 784 | 33–55 | 373 | 400 | 384 | 0 | 0 | UNC NORC |
| Framingham Heart Study | 1971 | 2255 | 26–84 | 1200 | 2255 | 0 | 0 | 0 | Broad Institute |
| Health Professionals Follow-Up Study | 1993–1995 | 1179 | 40–75 | 0 | 1109 | 2 | 0 | 2 | Broad Institute |
| Insulin Resistance Atherosclerosis Family Study | 1999–2002 | 1630 | 28–70 | 961 | 0 | 548 | 1082 | 0 | Metabolon Inc. |
| Multi-Ethnic Study of Atherosclerosis | 2000–2002 | 734 | 44–84 | 360 | 255 | 187 | 165 | 127 | ICL-NPC |
| Nurses' Health Study | 1989–1990 | 2993 | 43–69 | 2993 | 2953 | 27 | 0 | 7 | Broad Institute |
| Nurses' Health Study II | 1996–1999 | 2990 | 32–54 | 2990 | 2910 | 29 | 0 | 38 | Broad Institute |
| Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial | 1993–2001 | 1123 | 55–74 | 1123 | 1018 | 59 | 0 | 10 | Metabolon Inc. |
| Women's Health Initiative | 1993–1998 | 320 | 62–72 | 320 | 320 | 0 | 0 | 0 | Metabolon Inc. |
| Europe | |||||||||
| Airwave Health Monitoring Study | 2004 | 1938 | 19–65 | 666 | 1938 | 0 | 0 | 0 | Metabolon Inc. |
| Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study | 1985–1988 | 2072 | 50–69 | 0 | 2072 | 0 | 0 | 0 | Metabolon Inc. |
| UK Adult Twin Registry | 1992 | 1039 | 32–73 | 1039 | 1039 | 0 | 0 | 0 | Metabolon Inc. |
| Asia | |||||||||
| Guangzhou Nutrition and Health Study | 2008–2010 | 1732 | 40–80 | 1186 | 0 | 0 | 0 | 1732 | Sun Yat-sen University |
| Shanghai Men's Health Study | 2002–2006 | 699 | 40–75 | 0 | 0 | 0 | 0 | 699 | Metabolon Inc. |
| Shanghai Women's Health Study | 1996–2000 | 1423 | 40–71 | 1423 | 0 | 0 | 0 | 1423 | Metabolon Inc. |
| Tsuruoka Metabolomics Cohort Study | 2012–2015 | 9255 | 34–75 | 4996 | 0 | 0 | 0 | 9255 | Keio University |
| Total | 32,166 | 19–84 | 19,632 | 16,269 | 1236 | 1247 | 13,293 | ||
1ICL-NPC, Imperial College London's National Phenome Centre; TMAO, trimethylamine N-oxide; UNC NORC, University of North Carolina Nutrition Obesity Research Center.
The time period of blood collection used for metabolomics study.
Included were only participants who were eligible for the TMAO Pooling Project.
Age range at blood collection.
Targeted assays included the Broad Institute (United States), Keio University (Japan), University of North Carolina Nutrition Obesity Research Center (United States), and Sun Yat-sen University (China); untargeted assays included the ICL-NPC (United Kingdom) and Metabolon Inc. (Unites States).
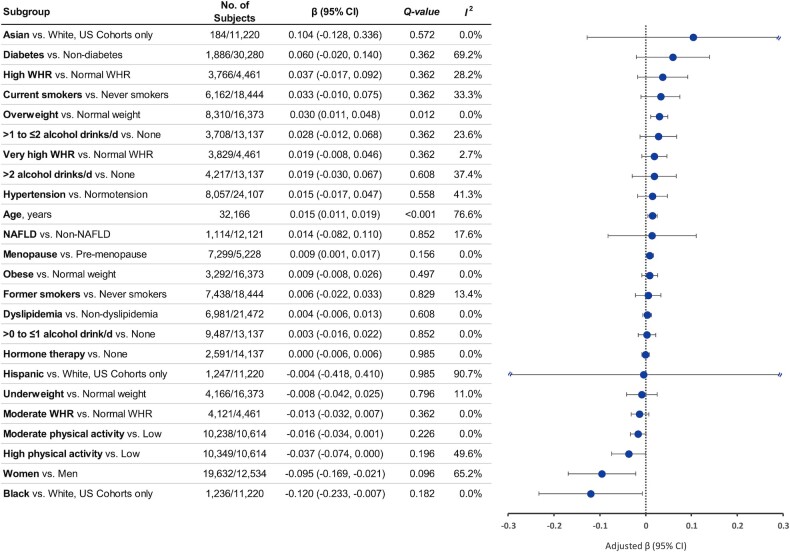
Circulating TMAO was associated with older age, male sex, and overweight (Figure 1). In our final model, each year of age was associated with a 0.015 SD increase in log-TMAO (95% CI: 0.011, 0.019). Women had lower TMAO concentrations than men (β: −0.095; 95% CI: −0.169, −0.021). We found no significant associations of TMAO with race or ethnicity, lifestyles, or metabolic disease history. Results of all 3 models can be found in Supplemental Table 3.
FIGURE 1.
Circulating trimethylamine N-oxide (TMAO) in relation to demographics, lifestyle, and medical history. Linear regression coefficients (β) and 95% CIs were adjusted for age, sex, race and ethnicity, education, fasting time, obesity, central obesity, smoking status, alcohol consumption, physical activity, use of multivitamins, menopausal status and hormone therapy in women, intakes of red meat, egg, and fish, and history of diabetes, hypertension, dyslipidemia, and nonalcoholic fatty liver disease. β indicates the increase or decrease in SD units of TMAO on the log-scale. Q-values represent corrected P values for multiple comparisons by controlling the false discovery rate. I2 represents the degree of heterogeneity. NAFLD, nonalcoholic fatty liver disease; WHR, waist-to-hip ratio.
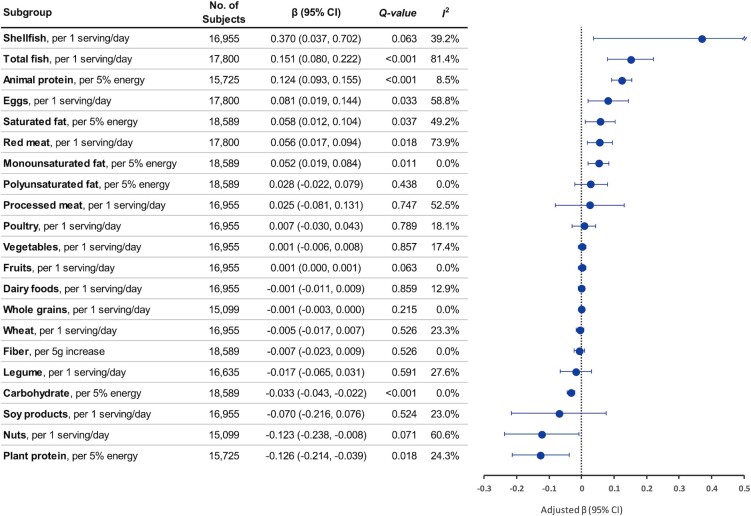
Circulating TMAO was positively associated with intakes of animal protein, saturated fat, monounsaturated fat, fish, shellfish, eggs, and red meat, whereas it was inversely associated with intakes of plant protein, carbohydrate, and nuts (Figure 2, Supplemental Table 4). In our final model, a 5% energy increase in animal protein or plant protein was associated with a 0.124 (95% CI: 0.093, 0.155) and a −0.126 (95% CI: −0.214, −0.039) SD increase in log-TMAO, respectively. With mutual adjustment for major TMAO-contributing foods (red meat, eggs, and fish), a 1 serving/d increase in total fish and shellfish was associated with a 0.151 (95% CI: 0.080, 0.222) and a 0.370 (95% CI: 0.037, 0.702) SD increase in log-TMAO, respectively, whereas a 1 serving/d increase in nuts was associated with a −0.123 (95% CI: −0.238, −0.008) SD increase in log-TMAO. The associations of TMAO with saturated fat, monounsaturated fat, eggs, and red meat were modest: β ranged from 0.05 to 0.08 for each 5% energy or 1 serving/d increment.
FIGURE 2.
Circulating trimethylamine N-oxide (TMAO) in relation to dietary factors. Linear regression coefficients (β) and 95% CIs were adjusted for age, sex, race and ethnicity, education, fasting time, obesity, central obesity, smoking status, alcohol consumption, physical activity, use of multivitamins, menopausal status and hormone therapy in women, intakes of red meat, egg, and fish, and history of diabetes, hypertension, dyslipidemia, and nonalcoholic fatty liver disease. β indicates the increase or decrease in SD units of log-TMAO by dietary changes. Q-values represent corrected P values for multiple comparisons by controlling the false discovery rate. I2 represents the degree of heterogeneity.
The positive association between animal protein and circulating TMAO was found in all regions (Table 2, Supplemental Table 5). However, associations of eggs, red meat, saturated fat, and plant protein with TMAO were only significant in US studies: β = 0.153 (95% CI: 0.068, 0.238) and 0.093 (95% CI: 0.021, 0.165) for 1 serving/d of eggs and red meat; 0.070 (95% CI: 0.028, 0.113) and −0.161 (95% CI: −0.248, −0.075) for a 5% energy increment from saturated fat and plant protein, respectively; all P-heterogeneity values by region <0.10. However, stronger associations of fish and shellfish with TMAO were found in Asian studies than in US or European studies (both P-heterogeneity by region <0.10). In Asian studies, a 1 serving/d of total fish and shellfish was associated with β = 0.285 (95% CI: 0.163, 0.407) and 0.578 (95% CI: 0.166, 0.990), respectively; whereas, no significant associations were found in US and European studies, except for total fish in US studies (β: 0.161; 95% CI: 0.081, 0.241).
TABLE 2.
Circulating TMAO in relation to dietary factors and cardiometabolic biomarkers: stratified analysis by region1
| United States | Europe2 | Asia2 | ||||
|---|---|---|---|---|---|---|
| β (95% CI)3 | Q-value4 | β (95% CI)3 | Q-value4 | β (95% CI)3 | Q-value4 | |
| Macronutrients, per 5% energy increase | ||||||
| Animal protein | 0.110 (0.079, 0.141) | <0.001 | 0.208 (0.053, 0.362) | 0.051 | 0.194 (0.102, 0.286) | <0.001 |
| Plant protein5 | −0.161 (−0.248, −0.075) | <0.001 | 0.190 (−0.086, 0.467) | 0.378 | −0.107 (−0.295, 0.081) | 0.559 |
| Saturated fat5 | 0.070 (0.028, 0.113) | 0.004 | −0.049 (−0.107, 0.009) | 0.240 | 0.112 (0.007, 0.217) | 0.117 |
| Monounsaturated fat | 0.057 (0.020, 0.094) | 0.006 | 0.034 (−0.126, 0.194) | 0.778 | 0.033 (−0.041, 0.107) | 0.655 |
| Carbohydrate | −0.035 (−0.047, −0.023) | <0.001 | −0.002 (−0.050, 0.046) | 0.933 | −0.031 (−0.055, −0.008) | 0.048 |
| Food items, per 1 serving/d increase | ||||||
| Red meat5 | 0.093 (0.021, 0.165) | 0.029 | 0.011 (−0.014, 0.036) | 0.677 | −0.013 (−0.166, 0.141) | 0.884 |
| Eggs5 | 0.153 (0.068, 0.238) | <0.001 | −0.008 (−0.045, 0.030) | 0.778 | 0.120 (0.010, 0.231) | 0.117 |
| Total fish5 | 0.161 (0.081, 0.241) | <0.001 | 0.040 (−0.038, 0.117) | 0.595 | 0.285 (0.163, 0,407) | <0.001 |
| Shellfish | 0.290 (−0.122, 0.703) | 0.294 | NA | 0.578 (0.166, 0.990) | 0.038 | |
| Nuts | −0.123 (−0.238, −0.008) | 0.086 | NA | NA | ||
| Biomarkers, per 1 SD log-biomarker increase | ||||||
| Creatinine, mg/dL | 0.129 (0.068, 0.189) | <0.001 | 0.216 (0.058, 0.374) | 0.063 | 0.123 (0.066, 0.181) | <0.001 |
| Homocysteine, µmol/L | 0.065 (0.019, 0.112) | 0.020 | NA | NA | ||
| Insulin, uU/mL | 0.036 (0.009, 0.063) | 0.023 | NA | 0.094 (0.042, 0.146) | <0.001 | |
| HbA1c, % of total hemoglobin | 0.049 (0.014, 0.084) | 0.020 | 0.007 (−0.037, 0.052) | 0.847 | 0.076 (0.051, 0.101) | <0.001 |
| Glucose, mg/dL | 0.040 (0.006, 0.075) | 0.050 | 0.032 (0.000, 0.063) | 0.117 | 0.012 (−0.010, 0.034) | 0.346 |
| Systolic blood pressure, mmHg | −0.015 (−0.047, 0.017) | 0.459 | −0.058 (−0.117, 0.000) | 0.117 | −0.024 (−0.045, −0.004) | 0.038 |
| Diastolic blood pressure, mmHg | −0.028 (−0.057, 0.002) | 0.117 | −0.037 (−0.101, 0.026) | 0.445 | −0.027 (−0.047, −0.007) | 0.024 |
| HDL cholesterol, mg/dL | −0.056 (−0.083, −0.030) | <0.001 | −0.042 (−0.124, 0.039) | 0.462 | −0.031 (−0.052, −0.010) | 0.009 |
1HbA1c, glycated hemoglobin; NA, not available; TMAO, trimethylamine N-oxide.
Dietary data were not available in the Airwave Health Monitoring Study (Europe) and the Tsuruoka Metabolomics Cohort Study (Asia).
Linear regression coefficients (β) and 95% CIs were adjusted for total energy, age, sex, race and ethnicity, education, fasting time, obesity, central obesity, smoking status, alcohol consumption, physical activity level, use of multivitamins, menopausal status and hormone therapy in women, intakes of red meat, egg, and fish, and history of diabetes, hypertension, dyslipidemia, and nonalcoholic fatty liver disease. β indicates the increase or decrease in SD units of log-TMAO.
Q-value represents corrected P value for multiple comparisons by controlling the false discovery rate.
P values for heterogeneity across the United States, Europe, and Asia: 0.058 for plant protein, 0.002 for saturated fat, 0.099 for red meat, 0.001 for eggs, and 0.003 for total fish.
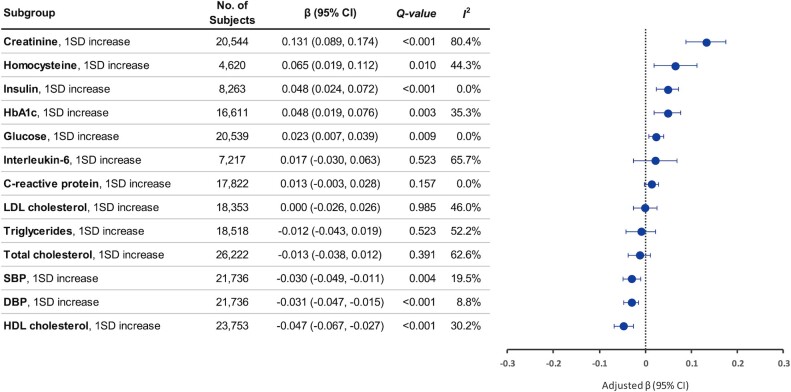
Circulating TMAO was positively associated with creatinine, homocysteine, and glycemic control biomarkers, whereas it was inversely associated with HDL and blood pressure (Figure 3, Supplemental Table 6). After adjustment for dietary intakes and metabolic disease status, a 1 SD increase in log-creatinine was related to a 0.131 (95% CI: 0.089, 0.174) SD increase in log-TMAO. Increasing TMAO concentrations were also related to homocysteine (0.065; 95% CI: 0.019, 0.112), insulin (0.048; 95% CI: 0.024, 0.072), HbA1c (0.048; 95% CI: 0.019, 0.076), and glucose (0.023; 95% CI: 0.007, 0.039). Meanwhile, TMAO was associated with lower HDL (−0.047; 95% CI: −0.067, −0.027) and blood pressure (−0.030; 95% CI: −0.049, −0.011, for systolic blood pressure). The biomarker-TMAO associations seemed similar across the US, European, and Asian studies (Table 2, Supplemental Table 7).
FIGURE 3.
Circulating trimethylamine N-oxide (TMAO) in relation to cardiometabolic biomarkers. Linear regression coefficients (β) and 95% CIs were adjusted for age, sex, race and ethnicity, education, fasting time, obesity, central obesity, smoking status, alcohol consumption, physical activity, use of multivitamins, menopausal status and hormone therapy in women, intakes of red meat, egg, and fish, and history of diabetes, hypertension, dyslipidemia, and nonalcoholic fatty liver disease. β indicates the increase or decrease in SD units of log-biomarkers by per SD change in log-TMAO. Q-values represent corrected P values for multiple comparisons by controlling the false discovery rate. I2 represents the degree of heterogeneity. DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; SBP, systolic blood pressure.
To evaluate whether the biomarker-TMAO associations are independent of, or confounded by, renal function (given a strong TMAO-creatinine association), in 5 studies (2 US and 3 Asian studies), we further adjusted for creatinine and found no appreciable changes in results, whereas associations for insulin/HbA1c/glucose became even stronger (Supplemental Table 8). In addition, we conducted stratified analyses by renal function—reduced (eGFR <90) compared with normal (eGFR ≥90)—and found no significant interactions (Supplemental Table 9; all P-interaction >0.15). The TMAO-HbA1c association was significant in individuals with normal or reduced renal function: β = 0.069 (95% CI: 0.040, 0.098) and 0.085 (95% CI: 0.045, 0.125), respectively. All biomarker-TMAO associations remained robust in stratified analyses by other baseline characteristics, including metabolic disease history and dietary intakes of meat, fish, fiber, and fruits/vegetables (Supplemental Tables 10 and 11).
Results did not change after excluding recent antibiotic users (Supplemental Tables 12, 13, and 14). Study-specific results are shown in forest plots (Supplemental Figures 2 and 3). Results remained robust if any study was excluded from the meta-analysis one at a time (data not shown).
Discussion
In this international collaborative project including 16 population-based studies from the United States, Europe, and Asia, circulating TMAO was positively associated with intakes of animal protein, saturated fat, monounsaturated fat, fish, shellfish, eggs, and red meat, and inversely associated with intakes of plant protein, carbohydrate, and nuts. Whereas the animal protein–TMAO association was consistent, TMAO associations with eggs, red meat, saturated fat, and plant protein were mainly observed in US studies, and associations with fish and shellfish were more evident in Asian studies. Furthermore, circulating TMAO was associated with multiple cardiometabolic risk factors after comprehensive adjustments, including biomarkers of renal function (creatinine), glycemic control (insulin/HbA1c/glucose), methylation (homocysteine), and dyslipidemia (HDL), although the effect sizes were generally small. Meanwhile, TMAO was not associated with inflammatory markers and other blood lipids.
TMAO is a gut microbial-host cometabolite derived from dietary choline and carnitine, abundant in red meat, eggs, fish, dairy, and some nuts and legumes. Habitual diets determine not only the amount of TMAO precursors from different foods but also gut microbial composition and possibly the ability of the gut microbiome to generate TMAO (5, 11, 22, 58), which in turn could affect host cardiometabolic health. In the current study, we found a positive association between animal protein intake and TMAO concentrations, consistent across ethnic groups and geographic regions. This finding agrees with results from previous studies that high intakes of animal foods (i.e., red meat, eggs, dairy, or fish) and animal-sourced choline were associated with increased TMAO concentrations (18–22, 24). Although TMAO was related to overall animal food intakes, we found varied associations between specific animal foods and TMAO concentrations by population/region (e.g., US compared with Asian studies). Variations in the consumption of TMAO-contributing foods have been reported across populations. For most Americans, the main sources of choline/carnitine were red meat, poultry, eggs, and dairy (14–16), although Hispanic/Latino Americans, on average, consumed more choline from legumes than non-Hispanic white Americans (59). For many Asians, most dietary choline was from eggs and soy foods (60). TMAO concentrations have been associated with intakes of dairy and/or fish in some European and Asian populations (18–21), rather than meat in US populations (19, 22). A recent international study—the INTERMAP study, which measured urinary TMAO concentrations in 4680 adults from Japan, China, the United States, and the United Kingdom—also reported population-specific associations between foods and TMAO: Japanese showed stronger associations of TMAO with fish and shellfish than Westerners (19). Overall, findings from our and other population-based studies have supported that high animal food intakes increased TMAO concentrations, but the impacts of different animal foods on TMAO concentrations vary among populations. The TMAO pathway could be one of the mechanisms underlying the associations of red meat/eggs with CVD risk in US populations (3, 5, 12, 22), but this is less likely in European or Asian populations.
We also observed significant inverse associations of plant protein, carbohydrate, and nuts with TMAO concentrations, even though nuts and legumes (a major source of plant protein) contain considerable amounts of choline. These findings were in line with previous reports that TMAO concentrations were lower in individuals with a habitual plant-based Mediterranean diet (23) and were decreased after a nut-supplemented diet (61). The seemingly incongruous association of nuts with reduced TMAO concentrations implies that the gut microbiota can convert choline to trimethylamine at different rates under different habitual diets (e.g., animal- compared with plant-based diets). So far, a few microbial genes have been identified for choline-trimethylamine conversion, for example, choline utilization C/D (CutC/D) (62, 63), and some taxa have been associated with TMAO concentration, for example, Clostridium and Fusobacterium (58, 64), although their contributions to TMAO production in humans remain unclear. Meanwhile, accumulating evidence has shown distinct gut microbial profiles in individuals with animal- compared with plant-based diets, including taxa that could be related to TMAO production (23, 65). For the current analysis, microbiome data were not available. Nevertheless, a few of our participating cohorts have recently collected stool samples from participants. Future studies will enable further investigations of the relations between diet, gut microbiota, microbial metabolite TMAO, and cardiometabolic health and potential diet–microbiota interactions that might affect TMAO concentrations and risk of cardiometabolic diseases.
Among biomarkers, we found significant associations of TMAO with creatinine, homocysteine, insulin, HbA1c, glucose, HDL, and blood pressure, but null associations for inflammatory markers and other blood lipids. Many studies have reported elevated TMAO concentrations in patients with diabetes, CKD, or CVD (4–7, 26–29, 66, 67) and associations of baseline TMAO or changes in TMAO concentrations with the development of those diseases (24, 25, 68–70). Evidence from animal models has indicated a causal role for TMAO in the development and/or progression of diabetes and CKD (9), in addition to CVD. For example, knockdown of Flavin Containing Dimethylaniline Monoxygenase 3 (FMO3) in mice, the host gene for generating TMAO, led to improved insulin sensitivity and glycemic control (10, 71, 72); conversely, supplementation with TMAO or choline promoted renal fibrosis and functional impairment (9). Yet, some studies suggested that elevated TMAO is an indication of renal impairment and/or underlying diabetes, which could confound the associations of TMAO with other diseases (28, 73, 74). In the current study, we found a strong association between TMAO and creatinine. However, the associations between TMAO and other cardiometabolic biomarkers remained similar after further controlling for creatinine or in stratified analyses by renal function. Notably, the TMAO associations with insulin/HbA1c/glucose became even stronger after the creatinine adjustment, and the TMAO-HbA1c association was significant in individuals with normal renal function. Consistent with our findings, the Multiethnic Cohort Study recently reported a significant association of circulating TMAO with HOMA-IR in 1653 US adults without severe diseases (58). The overall evidence so far seems to support bidirectional associations between elevated TMAO concentrations and cardiometabolic disorders. Given our cross-sectional design, prospective studies and intervention studies are needed to investigate the potential role of reducing TMAO [e.g., via plant-based or Mediterranean-style diets or supplementation with nuts or other bioactive compounds (64)] in the prevention or treatment of diabetes, CKD, or CVD. Finally, the inverse association of TMAO with blood pressure seems contradictory with the adverse cardiometabolic effect of TMAO and with previous findings in US adults (19); however, this association was weak and only significant in Asian studies (β = −0.03 SD), and thus would have minimal clinical significance.
Our current study has several limitations besides a cross-sectional design. First, this is a secondary analysis of existing data collected in studies using different instruments. To address this issue, we developed a data dictionary for variable harmonization and a standard analytic program for statistical analyses. We applied random-effects meta-analysis to combine study-specific results and found no substantial changes in overall results if any study was excluded from the meta-analysis. Second, a one-time measurement of TMAO might not reflect its usual concentration, and other measurement errors could affect our findings, in spite of all participating studies using validated questionnaires and metabolite/biomarker assays. Generally, measurement errors are likely to attenuate the exposure-outcome association. Third, we cannot rule out residual confounding due to uncontrolled or imperfectly controlled variables. For example, due to a lack of data, we could not control the potential effect of refined grains, which are often consumed with TMAO-contributing foods and have adverse cardiovascular effects; although in an exploratory analysis with additional adjustments for total carbohydrates or wheat products (mostly refined), the overall results remained virtually the same (data not shown). Finally, our large sample size resulted in high precision for detecting small effects, which could be statistically significant but with unclear clinical or public health interpretation. Future intervention studies are needed to determine to what extent TMAO concentrations can be reduced by certain dietary or microbial changes and whether a reduction in TMAO concentrations confers clinically meaningful results.
Nevertheless, to our knowledge, this is the first large-scale, epidemiological study evaluating associations between diet, TMAO, and cardiometabolic biomarkers in ethnically and geographically diverse populations. Comprehensive data on demographics, diet, lifestyle, and blood concentrations of TMAO and biomarkers were harmonized among >32,000 participants from 16 studies in 3 continents. A series of adjustments for potential confounders and stratified analyses by individual characteristics allowed for in-depth analyses to provide insights into relations between diet, TMAO, and cardiometabolic health.
In summary, in a large-scale, international pooled analysis, we observed associations of circulating TMAO with high intakes of animal protein, saturated fat, monounsaturated fat, fish, shellfish, eggs, and red meat, and with low intakes of plant protein, carbohydrate, and nuts. The association of TMAO with animal protein was consistent across populations, but its associations with fish, shellfish, eggs, and red meat varied among the US, European, and Asian populations. Circulating TMAO was associated with multiple cardiometabolic risk factors, including impaired renal function and poor glycemic control (independent of renal function), but not with inflammation and blood lipids except HDL. Whereas prospective studies and mechanistic investigations are needed to elucidate potential causality and underlying mechanisms further, our study shows that certain dietary and cardiometabolic factors are linked to TMAO metabolism, supporting the role of diet–microbiota–host interactions in human cardiometabolic health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Krista A Zanetti (Epidemiology and Genomics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA), Jessica Lasky-Su (Chair of the COMETS Steering Committee; Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA), and Marinella Temprosa (Vice-Chair of the COMETS Steering Committee; Department of Epidemiology and Biostatistics, Milken Institute School of Public Health, George Washington University, Washington, DC, USA) for their support.
The authors’ responsibilities were as follows—DY, X-OS: designed the study; DMH, SCM, KAM, JO, CM, NDP, HE, SH, IT, HZ, DA, TJW, WZ, HC, CMU, MG-F, IK, MF, QC, CEM, LEW, PE, REG, X-OS: provided essential reagents or essential materials; DY, JJY: performed statistical analysis and drafted the manuscript; DY: had primary responsibility for final content; and all authors: reviewed and approved the final manuscript.
The authors report no conflicts of interest.
Notes
This study was supported by R21 HL140375 to DY from the National Heart, Lung, and Blood Institute (NHLBI) of the NIH. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). The Framingham Heart Study (FHS) is supported by contract number HHSN268201500001I from the NHLBI with additional support from other sources. The Multi-Ethnic Study of Atherosclerosis is supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. The Insulin Resistance Atherosclerosis Family Study was funded by R01 HL060944 and R01 DK118062 from the NIH. The Health Professionals Follow-up Study is funded by grants U01 CA167552 and P50 090381 from the National Cancer Institute (NCI). The Nurses’ Health Study is funded by grants CA186107 and CA49449, and the Nurses’ Health Study II is funded by grants CA176726 and CA067262 from the NCI. MG-F is supported by American Diabetes Association grant #1-18-PMF-029. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial is funded by the NCI. This research also was supported by contracts from the Division of Cancer Prevention of the NCI and by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the NCI, National Institutes of Health (NIH). The Women's Health Initiative is funded by the NHLBI (contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and HHSN268201300008C). The Airwave Health Monitoring Study is funded by the Home Office (grant no. 780-TETRA) with additional support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre. PE is Director of the Medical Research Council (MRC)-Public Health England (PHE) Centre for Environment and Health and acknowledges support from the MRC and PHE (MR/L01341X/1). PE acknowledges support from the NIHR Imperial Biomedical Research Centre and the Health Protection Research Unit in Health Impact of Environmental Hazards (HPRU-2012-10141). The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study is supported by the Intramural Research Program of the National Cancer Institute, NIH. The Department of Twin Research receives support from grants from the Wellcome Trust (212904/Z/18/Z) and the Medical Research Council (MRC)/British Heart Foundation Ancestry and Biological Informative Markers for Stratification of Hypertension (AIMHY; MR/M016560/1), European Union, Chronic Disease Research Foundation (CDRF), Zoe Global Ltd, NIH and the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust in partnership with King's College London. CM is funded by the CDRF and by the MRC Aim-Hy project grant. The Shanghai Women's Health Study and Shanghai Men's Health Study are funded by grants UM1 CA182910 and UM1 CA173640 from the NCI of the NIH. The Guangzhou Nutrition and Health Study was supported by the National Natural Science Foundation of China (nos. 81472966, 81273050, and 81472965) and the Sun Yat-sen University, Guangzhou, China (no. 2007032). The Tsuruoka Metabolomics Cohort study was supported in part by research funds from the Yamagata Prefectural Government and the city of Tsuruoka and by the Grant-in-Aid for Scientific Research (B) (grants JP24390168 and JP15H04778), Grant-in-Aid for Challenging Exploratory Research (grant 25670303), and Grant-in-Aid for Young Scientists (B) (grant JP15K19231) from the Japan Society for the Promotion of Science.
This manuscript has been reviewed and approved by CARDIA for the scientific content. The opinions and conclusions in this publication are solely those of the authors and are not endorsed by the FHS or NHLBI and should not be assumed to reflect the opinions or conclusions of either. The funders had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
The data and materials that support the findings of this study are available upon request and committee approval.
Supplemental Tables 1–14 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: Airwave, Airwave Health Monitoring Study; CKD, chronic kidney disease; COMETS, COnsortium of METabolomics Studies; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FDR, false discovery rate; FHS, Framingham Heart Study; HbA1c, glycated hemoglobin; ICL-NPC, Imperial College London's National Phenome Centre; MESA, Multi-Ethnic Study of Atherosclerosis; NAFLD, nonalcoholic fatty liver disease; NHS, Nurses' Health Study; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study; TMAO, trimethylamine N-oxide; TMCS, Tsuruoka Metabolomics Cohort Study; WHR, waist-to-hip ratio.
Contributor Information
Jae Jeong Yang, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Xiao-Ou Shu, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
David M Herrington, Section on Cardiology, Department of Internal Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Steven C Moore, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Katie A Meyer, Department of Nutrition and Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA.
Jennifer Ose, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA; Huntsman Cancer Institute, Salt Lake City, UT, USA.
Cristina Menni, Department of Twin Research and Genetic Epidemiology, King's College London, London, United Kingdom.
Nicholette D Palmer, Department of Biochemistry, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Heather Eliassen, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Sei Harada, Department of Preventive Medicine and Public Health, Keio University School of Medicine, Tokyo, Japan.
Ioanna Tzoulaki, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, United Kingdom; MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, London, United Kingdom; Dementia Research Institute, Imperial College London, London, United Kingdom; Department of Hygiene and Epidemiology, University of Ioannina Medical School, Ioannina, Greece.
Huilian Zhu, Department of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou, China.
Demetrius Albanes, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Thomas J Wang, Division of Cardiovascular Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA; Department of Internal Medicine, UT Southwestern Medical Center, Dallas, TX, USA.
Wei Zheng, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Hui Cai, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Cornelia M Ulrich, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA; Huntsman Cancer Institute, Salt Lake City, UT, USA.
Marta Guasch-Ferré, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Ibrahim Karaman, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, United Kingdom; MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, London, United Kingdom; Dementia Research Institute, Imperial College London, London, United Kingdom.
Myriam Fornage, Brown Foundation Institute of Molecular Medicine, McGovern Medical School, University of Texas Health Science Center, Houston, TX, USA.
Qiuyin Cai, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Charles E Matthews, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Lynne E Wagenknecht, Public Health Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Paul Elliott, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, United Kingdom; MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, London, United Kingdom; Dementia Research Institute, Imperial College London, London, United Kingdom.
Robert E Gerszten, Cardiovascular Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA; Broad Institute of MIT and Harvard, Cambridge, MA, USA.
Danxia Yu, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
References
- 1. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. [DOI] [PubMed] [Google Scholar]
- 2. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–89. [DOI] [PubMed] [Google Scholar]
- 3. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-Met al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li Let al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao M-E, Liao P-D, Zhao X-J, Wang L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: a meta-analysis and dose-response analysis. BMC Cardiovasc Disord. 2020;20:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MKet al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, Schell M, Sandoval-Espinola WJ, Tao J, Sha Bet al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30:1141. [DOI] [PubMed] [Google Scholar]
- 11. Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BSet al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao Let al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321:1081–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landfald B, Valeur J, Berstad A, Raa J. Microbial trimethylamine-N-oxide as a disease marker: something fishy? Microb Ecol Health Dis. 2017;28:1327309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GA, Willett WC. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang JJ, Lipworth LP, Shu X-O, Blot WJ, Xiang Y-B, Steinwandel MD, Li H, Gao Y-T, Zheng W, Yu D. Associations of choline-related nutrients with cardiometabolic and all-cause mortality: results from 3 prospective cohort studies of blacks, whites, and Chinese. Am J Clin Nutr. 2020;111:644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM.. USDA database for the choline content of common foods. Release two. [Internet]. USDA; 2008; [cited July 12, 2018]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Choline/Choln02.pdf. [Google Scholar]
- 18. Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Müller D. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr. 2016;146:283–9. [DOI] [PubMed] [Google Scholar]
- 19. Gibson R, Lau C-HE, Loo RL, Ebbels TMD, Chekmeneva E, Dyer AR, Miura K, Ueshima H, Zhao L, Daviglus MLet al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am J Clin Nutr. 2020;111:280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost Get al. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017;105:600–8. [DOI] [PubMed] [Google Scholar]
- 21. Gessner A, di Giuseppe R, Koch M, Fromm MF, Lieb W, Maas R. Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: distribution and correlates in the population-based PopGen cohort. Clin Chem Lab Med. 2020;58:733–40. [DOI] [PubMed] [Google Scholar]
- 22. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHWet al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi Cet al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–21. [DOI] [PubMed] [Google Scholar]
- 24. Yu D, Shu X-O, Rivera ES, Zhang X, Cai Q, Calcutt MW, Xiang Y-B, Li H, Gao Y-T, Wang TJet al. Urinary levels of trimethylamine-N-oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc. 2019;8:e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, Yang W, Yang X, Yao P, Cheng Jet al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106:888–94. [DOI] [PubMed] [Google Scholar]
- 26. Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, Hartmane D, Pugovics O, Erglis A, Liepinsh E. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes. 2016;124:251–6. [DOI] [PubMed] [Google Scholar]
- 27. Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015;31:1189–94. [DOI] [PubMed] [Google Scholar]
- 28. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–44. [DOI] [PubMed] [Google Scholar]
- 29. Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, Stenvinkel P, Bergman P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11:e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Svingen GFT, Schartum-Hansen H, Pedersen ER, Ueland PM, Tell GS, Mellgren G, Njølstad PR, Seifert R, Strand E, Karlsson Tet al. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem. 2016;62:755–65. [DOI] [PubMed] [Google Scholar]
- 31. Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, de Alteriis G, Tenore GC, Colao A, Savastano S. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. 2018;10:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, Lovejoy JC, Magis AT. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24:935–46. [DOI] [PubMed] [Google Scholar]
- 33. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TDet al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu B, Zanetti KA, Temprosa M, Albanes D, Appel N, Barrera CB, Ben-Shlomo Y, Boerwinkle E, Casas JP, Clish Cet al. The consortium of metabolomics studies (COMETS): metabolomics in 47 prospective cohort studies. Am J Epidemiol. 2019;188:991–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer KA, Benton TZ, Bennett BJ, Jacobs DR, Lloyd-Jones DM, Gross MD, Carr JJ, Gordon-Larsen P, Zeisel SH. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA). J Am Heart Assoc. 2016;5:e003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, Stampfer MJ, Giovannucci E, Mucci LA. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. J Natl Cancer Inst. 2011;103:876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF. The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol. 1995;5:464–72. [DOI] [PubMed] [Google Scholar]
- 39. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu Ket al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 40. Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu CS, Pinsky PF, Kramer BS, Prorok PC, Purdue MP, Berg CD, Gohagan JK. The prostate, lung, colorectal, and ovarian cancer screening trial and its associated research resource. J Natl Cancer Inst. 2013;105:1684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elliott P, Vergnaud A-C, Singh D, Neasham D, Spear J, Heard A. The Airwave Health Monitoring Study of police officers and staff in Great Britain: rationale, design and methods. Environ Res. 2014;134:280–5. [DOI] [PubMed] [Google Scholar]
- 43. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 44. Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort profile: TwinsUK and healthy ageing twin study. Int J Epidemiol. 2013;42:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen Y, Liu Y, Zhou R, Chen X, Wang C, Tan X, Wang L, Zheng R, Zhang H, Ling Wet al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shu X-O, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang Y-B. Cohort profile: the Shanghai Men's Health Study. Int J Epidemiol. 2015;44:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng W, Chow W-H, Yang G, Jin F, Rothman N, Blair A, Li H-L, Wen W, Ji B-T, Li Qet al. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–31. [DOI] [PubMed] [Google Scholar]
- 48. Harada S, Takebayashi T, Kurihara A, Akiyama M, Suzuki A, Hatakeyama Y, Sugiyama D, Kuwabara K, Takeuchi A, Okamura Tet al. Metabolomic profiling reveals novel biomarkers of alcohol intake and alcohol-induced liver injury in community-dwelling men. Environ Health Prev Med. 2016;21:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S.; discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 50. Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SSet al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Soga T, Igarashi K, Ito C, Mizobuchi K, Zimmermann H-P, Tomita M. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal Chem. 2009;81:6165–74. [DOI] [PubMed] [Google Scholar]
- 52. daCosta KA, Vrbanac JJ, Zeisel SH. The measurement of dimethylamine, trimethylamine, and trimethylamine N-oxide using capillary gas chromatography-mass spectrometry. Anal Biochem. 1990;187:234–9. [DOI] [PubMed] [Google Scholar]
- 53. Tzoulaki I, Castagné R, Boulangé CL, Karaman I, Chekmeneva E, Evangelou E, Ebbels TMD, Kaluarachchi MR, Chadeau-Hyam M, Mosen Det al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur Heart J. 2019;40:2883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bridgewater BR, Evans A. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. [Internet]2014;4(132). doi:10.4172/2153-0769.1000132. [Google Scholar]
- 55. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 56. Higgins JPT, Green S . Cochrane handbook for systematic reviews of interventions version 5.1.0. [Internet]. The Cochrane Collaboration; 2011, [cited October 3, 2019]. Available from: www.handbook.cochrane.org. [Google Scholar]
- 57. McDonald JH. Multiple comparisons. In: Handbook of biological statistics. 3rd ed. Baltimore (MD):Sparky House Publishing; 2014. p. 254–60. [Google Scholar]
- 58. Fu BC, Hullar MAJ, Randolph TW, Franke AA, Monroe KR, Cheng I, Wilkens LR, Shepherd JA, Madeleine MM, Le Marchand Let al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am J Clin Nutr. 2020;111:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yonemori KM, Lim U, Koga KR, Wilkens LR, Au D, Boushey CJ, Le Marchand L, Kolonel LN, Murphy SP. Dietary choline and betaine intakes vary in an adult multiethnic population. J Nutr. 2013;143:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yu D, Shu X-O, Xiang Y-B, Li H, Yang G, Gao Y-T, Zheng W, Zhang X. Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J Nutr. 2014;144:2034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hernández-Alonso P, Cañueto D, Giardina S, Salas-Salvadó J, Cañellas N, Correig X, Bulló M. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in prediabetic subjects. J Nutr Biochem. 2017;45:48–53. [DOI] [PubMed] [Google Scholar]
- 62. Skye SM, Zhu W, Romano KA, Guo C-J, Wang Z, Jia X, Kirsop J, Haag B, Lang JM, DiDonato JAet al. Microbial transplantation with human gut commensals containing CutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circ Res. 2018;123:1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simó C, García-Cañas V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020;11:6745–76. [DOI] [PubMed] [Google Scholar]
- 65. Aron-Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12:169–81. [DOI] [PubMed] [Google Scholar]
- 66. Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto Cet al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89:1144–52. [DOI] [PubMed] [Google Scholar]
- 67. Tang WHW, Wang Z, Li XS, Fan Y, Li DS, Wu Y, Hazen SL. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik Aet al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FB, Rexrode KM, Manson JE, Qi L. Long-term changes in gut microbial metabolite trimethylamine N-oxide and coronary heart disease risk. J Am Coll Cardiol. 2020;75:763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Croyal M, Saulnier P-J, Aguesse A, Gand E, Ragot S, Roussel R, Halimi J-M, Ducrocq G, Cariou B, Montaigne Det al. Plasma trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J Clin Endocrinol Metab. 2020;105(7):2371. [DOI] [PubMed] [Google Scholar]
- 71. Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB, Morbid Obesity Study Group et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schugar RC, Shih DM, Warrier M, Helsley RN, Burrows A, Ferguson D, Brown AL, Gromovsky AD, Heine M, Chatterjee Aet al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017;19:2451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, Jorsal A, Theilade S, Parving H-H, Hansen TWet al. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care. 2019;42:1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jia J, Dou P, Gao M, Kong X, Li C, Liu Z, Huang T.. Assessment of causal direction between gut microbiota-dependent metabolites and cardiometabolic health: a bidirectional mendelian randomization analysis. Diabetes. 2019;68:1747–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.