See corresponding article on page 1332.
Intermittent fasting, a dietary approach featuring alternating periods of fasting and eating of differing durations, has recently emerged as a popular means of dieting aimed at tackling obesity and its complications, but has yielded conflicting clinical results. On the one hand, 10 h of time-restricted eating (TRE) for 12 wk was recently found to promote weight loss, reduce percentage body fat and visceral fat, and lower blood pressure, atherogenic lipids, and glycated hemoglobin (HbA1c) in participants that were previously diagnosed with the metabolic syndrome (1). A shorter TRE daily interval followed for 2 mo was likewise associated with similarly beneficial effects, including weight loss, improved insulin resistance, and reduced oxidative stress (2). Similar intermittent fasting–associated health benefits were observed in healthy nonobese humans (3). Conversely, a recent clinical trial noted a significant weight decrease (postintervention compared with preintervention) in the TRE group, but featured no excess weight reduction in comparison with an age- and BMI-matched control group consuming 3 structured daily meals (4). In addition, in this trial, no significant within-group or intergroup differences were noted with respect to intermittent fasting effects on fasting glucose, fasting insulin, HbA1c, triglyceride, total cholesterol, LDL cholesterol, or HDL cholesterol concentrations, or HOMA-IR (4). These seemingly conflicting results may point to, among other things, interindividual differences in TRE-induced metabolic effects in different individuals and populations. Such individually varying TRE-mediated mechanisms may include altered induction of an elevated concentration of ketone bodies during the fasting state (5), reduction in oxidative and metabolic stress, potentially through enhanced expression of antioxidant defenses and DNA repair proteins (5, 6), and differential and potentially personalized induction of alterations in gut microbiome composition (7).
In this issue of The American Journal of Clinical Nutrition, Su et al. (8) took advantage of a variant of intermittent fasting practiced by Muslims worldwide during the holy month of Ramadan, in which fasting starts before sunrise and extends to sunset (∼16 h of daily fasting in this cohort from China). Ramadan fasting resulted in reduced body weight and an increased liver aminotransferase activity ratio (aspartate aminotransferase:alanine aminotransferase), potentially reflecting improved nonalcoholic fatty liver disease in the TRE group. The authors further explored whether Ramadan fasting-associated beneficial impacts on clinical features were associated with modulation of gut microbiome composition. 16S ribosomal DNA analysis of fecal samples obtained from 2 cohorts of healthy nonobese Ramadan followers revealed taxonomic changes in the microbiome to be associated with fasting, while being independent of living area and dietary composition. Among the changes noted with fasting were increased microbiome diversity, and upregulation of the relative abundance of the butyric acid-producing Lachnospiraceae. Interestingly, both Lachnospiraceae and butyric acid have been previously suggested to be linked to human health benefits, including reduced incidence of cancer, improvement in inflammatory bowel disease, and even improved mental health (9–13). The authors therefore propose that increased TRE-associated abundance of Lachnospiraceae species may participate in the mediation of the beneficial metabolic effects noted during the Ramadan intermittent fasting, potentially through production of butyric acid. Of note, the observed taxonomic changes returned to baseline upon cessation of intermittent feeding, thereby supporting such a diet–microbiome association.
These results are consistent with other studies showing that the interplay between diet and microbiome may affect outcomes to host metabolic health (14). For example, the gut microbiome may modulate the postprandial glycemic response (15). Moreover, a microbiome signature in mice was associated with faster weight regain and metabolic aberrations upon re-exposure to obesity-promoting conditions (the yo-yo effect) (16). The interesting observations reported by Su et al. may pave the way to future studies further exploring the links between intermittent fasting, microbiome alterations, and metabolic consequences, while focusing on unravelling causal links potentially driving these effects. Such mechanistic explorations, in animals and humans, should take into consideration ethnic, dietary, and other lifestyle variables (Figure 1), and should include shotgun metagenomic and untargeted metabolomics analysis (including direct measurements of SCFAs such as butyrate), which may collectively help to identify molecular drivers of these effects. Causality could be further established in such future studies, by using fecal microbiota transfers of whole microbiome configurations, distinct signatures, or single commensals from individuals after TRE into germ-free mice. Altogether, the study by Su et al. utilizes a widespread human cultural practice to highlight an exciting new potential link between dietary habits, microbiome changes, and host metabolic outcomes. Understanding the molecular details of such interactions may shed light on microbiome regulation of host health, while potentially identifying microbiome-associated therapeutic targets able to be exploited in the prevention of obesity and its common complications.
FIGURE 1.
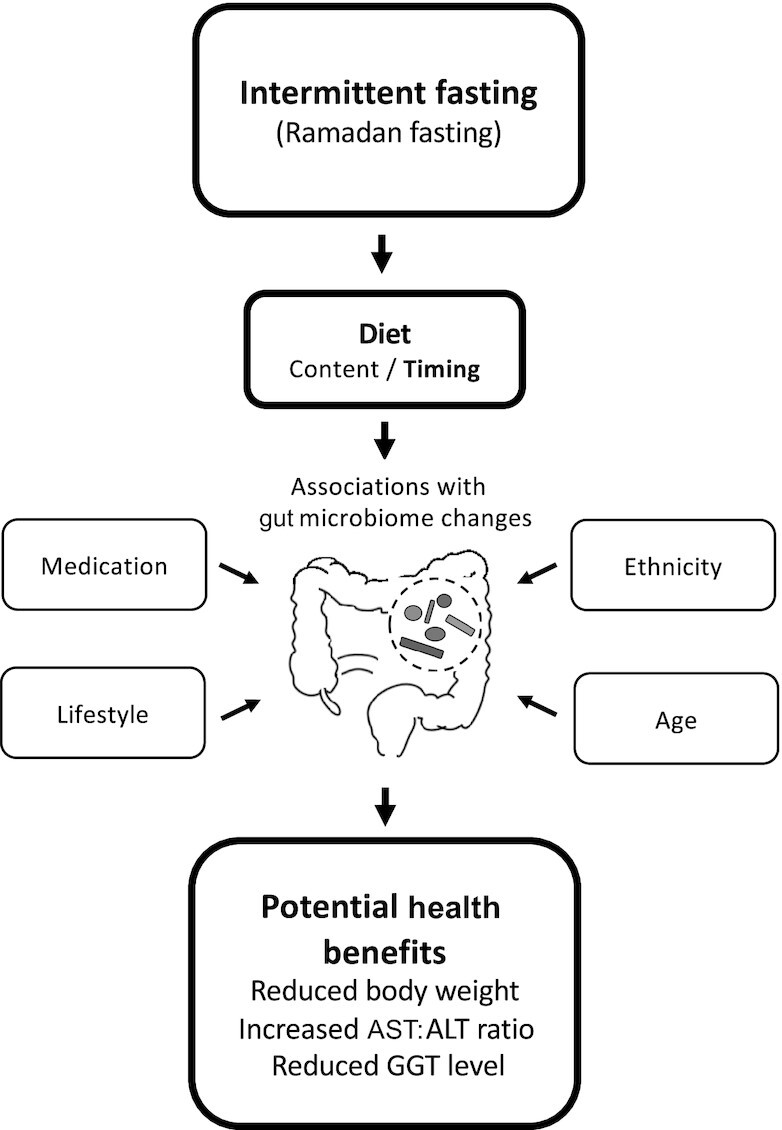
Several factors contribute to potential gut microbiome modulation by intermittent dieting, including diet, ethnicity, age, medication, and lifestyle, affecting human health. Ramadan fasting, a human cultural model of intermittent dietary intervention, is associated with metabolic health benefits and associated microbiome alterations. ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—both authors: drafted the paper and read and approved the final manuscript. EE is a scientific cofounder and paid consultant at DayTwo, a company focused on development of personalized nutritional approaches, and BiomX, a company focused on development of phage cocktails for manipulation of the microbiome. The other author reports no conflicts of interest.
Notes
SKA is supported by the Israeli Ministry of Science and Technology Zvi Yanai Fellowship. EE is the incumbent of the Sir Marc and Lady Tania Feldmann Professorial Chair; a senior fellow, Canadian Institute for Advanced Research; and an international scholar, The Bill & Melinda Gates Foundation and Howard Hughes Medical Institute.
EE is a member of the Editorial Board of The American Journal of Clinical Nutrition.
Abbreviations used: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase; HbA1c, glycated hemoglobin; TRE, time-restricted eating.
Contributor Information
Suhaib K Abdeen, Department of Immunology, Weizmann Institute of Science, Rehovot, Israel.
Eran Elinav, Department of Immunology, Weizmann Institute of Science, Rehovot, Israel; Division of Cancer-Microbiome Research, German Cancer Research Center (DKFZ), Heidelberg, Germany.
References
- 1. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda Set al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31:92–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32:366–78.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url Jet al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019;30:462–76.e5. [DOI] [PubMed] [Google Scholar]
- 4. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JEet al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180:1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541–51. [DOI] [PubMed] [Google Scholar]
- 6. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan Met al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–94. [DOI] [PubMed] [Google Scholar]
- 8. Su J, Wang Y, Zhang X, Ma M, Xie Z, Ma Z, Peppelenbosch MP. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting, Am J Clin Nutr. 2021;(): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. [DOI] [PubMed] [Google Scholar]
- 10. Flemer B, Warren RD, Barrett MP, Cisek K, Das A, Jeffery IB, Hurley E, O'Riordain M, Shanahan F, O'Toole PW. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67:1454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Y, Fournier N, Michetti P, Mueller C, Geuking Met al. Microbial network disturbances in relapsing refractory Crohn's disease. Nat Med. 2019;25:323–36. [DOI] [PubMed] [Google Scholar]
- 12. Humbel F, Rieder JH, Franc Y, Juillerat P, Scharl M, Misselwitz B, Schreiner P, Begré S, Rogler G, von Känel Ret al. Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in remission. Clin Gastroenterol Hepatol. 2020;18:2019–29.e11. [DOI] [PubMed] [Google Scholar]
- 13. Simpson CA, Mu A, Haslam N, Schwartz OS, Simmons JG. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome. J Affect Disord. 2020;266:429–46. [DOI] [PubMed] [Google Scholar]
- 14. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. [DOI] [PubMed] [Google Scholar]
- 15. Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, Capdevila J, Hadjigeorgiou G, Davies R, Al Khatib Het al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, Dohnalová L, Braverman S, Rozin S, Malitsky Set al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–51. [DOI] [PubMed] [Google Scholar]


