ABSTRACT
Background
The carbon isotope ratios (CIRs) of individual amino acids (AAs) may provide more sensitive and specific biomarkers of sugar-sweetened beverages (SSBs) than total tissue CIR. Because CIRs turn over slowly, long-term controlled-feeding studies are needed in their evaluation.
Objective
We assessed the responses of plasma and RBC CIRAA's to SSB and meat intake in a 12-wk inpatient feeding study.
Methods
Thirty-two men (aged 46.2 ± 10.5 y) completed the feeding study at the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix, Arizona. The effects of SSB, meat, and fish intake on plasma and RBC CIRAA's were evaluated in a balanced factorial design with each dietary variable either present or absent in a common weight-maintaining, macronutrient-balanced diet. Fasting blood samples were collected biweekly from baseline. Dietary effects on the postfeeding CIR of 5 nonessential AAs (CIRNEAA's) and 4 essential AAs (CIREAA's) were analyzed using multivariable regression.
Results
In plasma, 4 of 5 CIRNEAA's increased with SSB intake. Of these, the CIRAla was the most sensitive (β = 2.81, SE = 0.38) to SSB intake and was not affected by meat or fish intake. In RBCs, all 5 CIRNEAA's increased with SSBs but had smaller effect sizes than in plasma. All plasma CIREAA's increased with meat intake (but not SSB or fish intake), and the CIRLeu was the most sensitive (β = 1.26, SE = 0.23). CIRs of leucine and valine also increased with meat intake in RBCs. Estimates of turnover suggest that CIRAA's in plasma, but not RBCs, were in equilibrium with the diets by the end of the study.
Conclusions
The results of this study in men support CIRNEAA's as potential biomarkers of SSB intake and suggest CIREAA's as potential biomarkers of meat intake in US diets. This trial was registered at clinicaltrials.gov/ct2/show/NCT01237093 as NCT01237093.
Keywords: amino acid carbon isotope ratios, alanine, controlled-feeding study, NIDDK, sugar-sweetened beverages
Introduction
See corresponding article on page 1073.
Sugar-sweetened beverages (SSBs) have been associated with the risk of multiple chronic diseases (1–4). Most of these associations are based on self-reported intakes, which are prone to error (5, 6), especially for sugar-related intakes (7, 8). Dietary estimates based on objective, unbiased dietary biomarkers may strengthen disease-risk models (9–11). The carbon isotope ratio (CIR) of total tissues has been used as a biomarker of added sugar (AS) and/or SSB intake in the United States (12–16), because the corn and sugarcane constituting the majority of these sweeteners have a naturally occurring CIR that is higher than in most other diet components (17–19). Stable isotope biomarkers, including CIR, have the added benefit of representing usual intake because of their slow turnover rates in blood components (19). However, in several populations, the CIR has been more strongly associated with intakes of meat or animal protein (20–22) due to the high amount of corn in US production (23, 24). Approaches to improve the specificity of the CIR for SSBs are needed.
The CIR of molecules that are metabolically linked to foods of interest may provide biomarkers with improved sensitivity and specificity. For example, the CIR of the nonessential amino acid (NEAA) alanine should proportionally reflect synthesis from sugars, due to its close metabolic link with glucose (25, 26). In an Alaska Native (Yup'ik) population, the CIRAla was associated with intakes of both SSB and AS, but importantly, not meat (27). Other NEAAs may also be synthesized from dietary sugar and so may also have a higher CIR in response to SSB consumption. Essential amino acids (EAAs) cannot derive from dietary sugar; however, their CIRs may be elevated in response to meat intake.
The goal of this study was to identify valid CIRamino acid (CIRAA) biomarkers of SSB intake that are not influenced by meat intake. We measured the responses of CIRAA's in plasma and RBCs to SSBs and meat in participants of the Developing Biomarkers of Diet Study, a highly controlled, 12-wk inpatient feeding study that varied SSBs and meat in combination. We expected that the CIRAla and other CIRNEAA's would be elevated by SSB consumption but not by meat consumption. Conversely, we expected CIREAA's to be unaffected by SSB consumption and instead be elevated with meat intake. We also assessed the change over time in CIRAA's from blood samples collected biweekly. Because of differing rates of protein turnover, we expected plasma CIRAA's to approach equilibrium with the experimental diets more quickly than RBC CIRAA's.
Methods
Subjects and study design
The Developing Biomarkers of Diet Study was designed to evaluate the effects of 3 dietary exposures—SSBs, meat, and fish—on total tissue CIRs and nitrogen isotope ratios (NIRs) in a 12-wk inpatient dietary intervention, as described in detail elsewhere (22). The presence or absence of these 3 foods was varied in all possible combinations, resulting in a full-factorial study with 8 experimental diets that were weight-maintaining and macronutrient balanced as described below. Participants were randomly assigned to diets until n = 4 had been completed for each. This sample size was determined based on a power analysis of the total tissue stable isotope ratio measurements, the primary outcomes of the Developing Biomarkers of Diet Study (22). This power at the level of α = 0.05 was determined using an expected within-group SD of 0.5 and expected effect size of ≥1. All laboratory measurements were analyst-blinded.
Subjects were recruited between 2011 and 2018 at the Obesity and Diabetes Clinical Research Section of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in Phoenix, Arizona (clinicaltrials.gov identifier: NCT01237093). Recruitment was restricted to males, because the fish-containing diets exceeded mercury-exposure recommendations for females of reproductive age. Volunteers were screened for overall health prior to admittance, and participants were excluded during an initial 1-wk period of residence in the facility if they presented with type 2 diabetes, but not impaired glucose tolerance, as described below (Supplemental Figure 1). The study protocol was approved by the NIDDK Institutional Review Board (#11-DK-N018).
Participants were placed on a standard weight-maintaining diet for 1 wk prior to the dietary intervention. On day 4 of the standard diet, we administered a 75-g, 3-h oral-glucose-tolerance test (glucose samples run on an Analox GM9 glucose analyzer; Analox Technologies). Daily fasting weight was measured, and percentage body fat was determined at the start and end of the study using DXA (DPX-L; Lunar Corp and Prodigy, GE) (28, 29). Fasting blood samples were collected biweekly, from the first day of the experimental diet (baseline) to the end of the final week (week 12, postintervention). Blood was centrifuged to isolate plasma and RBC samples, which were frozen at −20°C prior to analysis.
The experimental diets were designed using Food Processor (version 11.0.2; ESHA Research) to maintain body weight with a fixed macronutrient profile of 50% carbohydrate, 30% fat, and 20% protein. Diets were designed to vary as little as possible apart from the presence or absence of SSBs, meat, and fish at 14%, 19%, and 6% of daily energetic requirement (kilocalories), respectively. The SSB treatment comprised cola and lemon lime soda. The meat exposure included servings of hamburgers, hot dogs, chicken, turkey, ham, roast beef, meatloaf, bacon, and sausage. The fish included salmon, tuna, and pollock.
Outcome measures: CIRAA's in plasma and RBCs
CIRAA's are measured and reported as δ13C values with units of per mil (‰), as follows: δ13C = (13C/12Csample/13C/12Creference − 1) × 1000‰, where the reference is Vienna Pee Dee Belemnite (13C/12C = 0.0112372), the established international reference material for δ13C measurements. For continuity, we retained CIRAA's as the variable name when referring to AA δ13C values. We measured CIRAA's in plasma and RBC samples using GC–combustion–isotope ratio MS (GC-C-IRMS). We prepared and analyzed baseline and postintervention samples for all participants (n = 32) in batches of 8 by tissue type (plasma or RBCs). These batches were designed to ensure even representation of experimental diets. To evaluate change in CIRAA's over time, we measured CIRAA's at all time points in a subset of 18 participants whose total plasma CIR changed by ≥0.5‰ over the study duration (hereafter, the “turnover subset”). We used this criterion to exclude participants whose CIR did not change appreciably over the study, presumably due to similarity between the randomly assigned study diet and the participant's usual intake. For analyses of the turnover subset, we batched samples by individual and sample type, and biweekly samples (n = 7) were analyzed in random order.
AAs must be hydrolyzed and derivatized prior to measurement of CIR via GC-C-IRMS. We hydrolyzed aliquots of plasma (12–15 μL) or RBCs (2–5 μL) using 1 mL of HCl (6 mol/L) at 110°C for 20 h. We lipid-extracted the hydrolysates using n-hexane and dichloromethane (6:5 vol:vol) and dried them down under nitrogen. Hydrolyzed AAs were derivatized to N-acetyl methyl esters as follows (30, 31). First, we methylated the AAs with acidified methanol during a 75°C incubation for 1 h. Next, methylated AAs were dried under nitrogen and acetylated by incubation with acetic anhydride, triethylamine, and acetone (1:2:5 vol:vol:vol) at 60°C for 10 min. The derivatized AAs were dried under nitrogen, purified with a phosphate buffer wash (1 mol/L potassium phosphate + 1 mol/L sodium phosphate, pH 7), and extracted using chloroform. After the chloroform was evaporated under nitrogen, derivatized AAs were dissolved in ethyl acetate and were analyzed via GC-C-IRMS within 24 h or stored at −18°C.
The GC-C-IRMS analyses were performed at the Alaska Stable Isotope Facility at the University of Alaska Fairbanks using a GC IsoLink II System (ThermoFisher Scientific). The derivatized AAs were injected onto a VF-35ms column (Agilent) in a TRACE 1310 GC (ThermoFisher Scientific) (32) for peak separation, and the GC effluent was routed through the IsoLink II interface for combustion of each individual peak into carbon dioxide gas and introduction into a Delta V Plus isotope ratio mass spectrometer (ThermoFisher Scientific) for determination of the CIR. CIRAA's are reported in δ notation (δ13C values) with units of per mil (‰), as described above, using calibrated carbon dioxide gas as the proximal reference material for Vienna Pee Dee Belemnite. The 13C:12C ratio is calculated by peak integration in the program Isodat (version 3.0; Thermo Scientific). Correct peak identification and integration (width and background assignment) were visually confirmed, as was adequate separation between peaks.
With each batch we also prepared an external standard, containing a mix of commercial AAs (Supplemental Table 1) for which the nonderivatized CIR has been measured relative to certified reference materials, and a laboratory check sample of the same tissue type as the experimental samples. To all samples and external standards, we added 3 internal standards, which do not co-elute with AA peaks: 1 requiring derivatization (norleucine) and 2 that are volatile (nonadecane and caffeine). Internal standards were used to monitor instrument performance but were not used to adjust measured CIRAA's. In a typical analytical sequence, each sample was analyzed in triplicate injections. An injection of the external standard was made between triplicates (n = 8–9/sequence), and an injection of the check sample was made between every other triplicate (n = 4–5/sequence).
We obtained reliable chromatography for 5 NEAAs: alanine (Ala), serine (Ser), aspartic acid (Asp), proline (Pro), and glutamic acid (Glu). Asparagine and glutamine are deamidated during acid hydrolysis; thus, they are indistinguishable from aspartic acid and glutamic acid, respectively. We also measured the CIR of 5 EAAs: valine (Val), leucine (Leu), isoleucine (Ile), threonine (Thr), and phenylalanine (Phe). The CIRIle was measured only in plasma due to its low concentration in RBCs. The N-acetyl methyl ester derivatization allows the CIR measurement of additional AAs, including glycine, methionine, lysine, tyrosine, and histidine. However, here these AAs were excluded based on unreliable chromatography (insufficient peak height or significant co-elution) in human plasma and RBCs. Supplemental Figure 2 shows typical GC-C-IRMS chromatographs of the external standard and a sample.
Derivatization adds carbon atoms to AAs and causes potential kinetic isotope effects, both of which influence the CIR of derivatized AAs. These influences are accounted for by adjusting the measured CIR of derivatized AAs in samples using the measured CIR of derivatized AAs in the external standard (33). The measured CIRAA of derivatized AAs (termed CIRAA,d) were adjusted using the known CIRAA in the external standard, as follows:
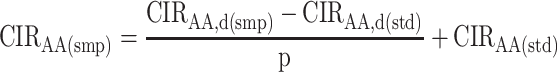 |
(1) |
In this equation, CIRAA,d(smp) and CIRAA,d(std) are the measured CIRs of the derivatized AAs in the sample and external standard, respectively; CIRAA(std) is the known value of the CIRAA in the external standard; p is the proportion of carbon in the derivatized AAs from the un-derivatized AAs; and CIRAA(smp) is the corrected sample CIRAA.
The propagated analytical error (SE) of CIRAA measurements was estimated for replicate injections of the check sample as described elsewhere (34). In plasma, this ranged from 0.08‰ for CIRPhe to 0.34‰ for CIRThr, with an average of 0.16‰. In RBCs, measurement error ranged from 0.09‰ for CIRPhe to 0.29‰ for CIRSer, with an average of 0.12‰. We used measurements of the check sample across batches to evaluate reproducibility. In plasma, this across-batch reproducibility ranged from 0.53‰ for CIRPhe to 1.44‰ for CIRAla, with an average of 1.04‰. In RBCs, the reproducibility across batches ranged from 0.13‰ for CIRAla to 1.21‰ for CIRSer, with an average of 0.76‰. AAs with across-batch reproducibility of SD ≥2.0‰ were considered insufficiently reproducible for inclusion in the paper. In plasma, this included CIRIle (SD = 2.4‰), and in RBCs this included CIRThr (SD = 2.8‰). Thus, for plasma, we present the CIRs of Ala, Asp, Glu, Leu, Pro, Ser, Thr, and Val and, for RBCs, we present the CIRs of Ala, Asp, Glu, Leu, Pro, Ser, and Val. Analytical error and reproducibility of the check sample for all CIRAA's are reported in Supplemental Table 2.
Statistical analyses
We modeled the CIR of each AA in plasma and RBCs separately as a function of 3 dietary factors (SSBs, meat, and fish) with 2 levels each (presence/absence) using multiple linear regressions, with baseline CIRAA as a covariate. Baseline CIRAA values were included to account for potentially incomplete isotopic turnover of CIRAA to equilibrium with the study diets. To account for multiple hypothesis testing across covariates, models, and sample types, we adjusted the false discovery rate of α = 0.05 with a Benjamini-Hochberg procedure to determine the significance of parameter estimates. Data analyses were conducted in R version 4.0.1 (R Core Team, 2020).
We performed both univariable and multivariable logistic regressions to predict the exposures of SSBs and meat separately using CIRAA's as predictors. We assessed the predictive accuracy of the CIRAA biomarkers using the AUC of the receiver operating characteristic curve, as an estimate of the association between the CIRAA and dietary factors, using Harrell's bootstrap estimate of the optimism-corrected AUC (cAUC) to account for potential overfitting by using the same data to fit and test the models, as well as bootstrap 95% CIs for the cAUC using the percentile method (35).
We characterized turnover of CIRAA within individuals by fitting exponential models using nonlinear least squares (36):
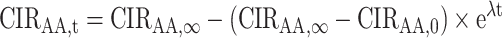 |
(2) |
Here, CIRAA,t is the CIRAA measured at time (wk) t, CIRAA,∞ represents the estimated CIRAA in equilibrium with the diet, CIRAA,0 represents the estimated baseline CIRAA, and λ represents the estimated fractional incorporation rate. The half-life and time to 90% turnover can be calculated as 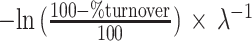 . We used Wilcoxon signed-rank tests to compare estimates of λ between plasma and RBC CIRAA and the number of participant models that converged.
. We used Wilcoxon signed-rank tests to compare estimates of λ between plasma and RBC CIRAA and the number of participant models that converged.
Results
Study participants
We screened 55 male volunteers for the 12-wk controlled-feeding study. Forty-one were admitted to the inpatient study and, of these, 37 were randomly assigned and 32 completed the experimental diet through at least week 8 and provided samples for stable isotope analysis (Supplemental Figure 1), as described elsewhere in detail (22). One participant withdrew after week 8 of the feeding study, another participant withdrew after week 10, and the remaining 30 participants completed the full 12 wk. For the participants who withdrew early, the blood samples collected at the week of withdrawal were used for postintervention CIRAA measurements. The baseline characteristics of the participants are shown in Table 1. The distributions of age, body weight, BMI, percentage body fat, and plasma glucose were similar among participants assigned to each of the 8 diets (22) and between SSB consumers and nonconsumers (Table 1). The largest differences were in age, BMI, and body weight; however, these variables are not expected to affect CIRAA. Participants maintained constant body weights (day of discharge–first full day of study) within 0.5 ± 2.6 kg (0.7% ± 3.2% body weight). Baseline CIRAA measurements were similar across participants when stratified by SSB and meat intake (Supplemental Table 3).
TABLE 1.
Baseline characteristics of study participants, both for study cohort and stratified by SSB intake1
| Characteristic | Total | No SSBs | SSBs |
|---|---|---|---|
| Male sex, n (%) | 32 (100) | 16 (100) | 16 (100) |
| Race/ethnicity, n (%) | |||
| White | 19 (59.4) | 10 (62.5) | 9 (56.2) |
| Native American | 10 (31.3) | 4 (25.0) | 6 (37.5) |
| Hispanic | 2 (6.3) | 1 (6.2) | 1 (6.2) |
| African American | 1 (3.1) | 1 (6.2) | 0 (0.0) |
| Age, y | 46.3 ± 10.5 | 48.8 ± 10.0 | 43.8 ± 10.7 |
| BMI, kg/m2 | 27.2 ± 4.0 | 28.5 ± 4.2 | 25.9 ± 3.5 |
| Weight, kg | 83.9 ± 13.6 | 88.0 ± 14.2 | 79.8 ± 12.1 |
| Body fat, % | 27.9 ± 7.6 | 28.4 ± 8.5 | 27.5 ± 6.9 |
| Glucose, g/dL | 92.7 ± 8.1 | 92.4 ± 7.7 | 92.9 ± 8.8 |
Age and subsequent variables are reported as means ± SDs. SSB, sugar-sweetened beverage.
Effects of SSB and meat intake on postintervention CIRAA
Most CIRNEAA's were elevated in SSB consumers (Table 2, Supplemental Figure 3), including CIRAla, CIRSer, CIRAsp, and CIRGlu in both plasma and RBCs. RBC CIRPro increased with SSB intake, whereas plasma CIRPro did not. CIRAla showed the largest effect of SSB intake in both plasma (βSSB = 2.81, SE = 0.38) and in RBCs (βSSB = 1.66, SE = 0.30). No CIREAA's, in plasma or RBCs, were influenced by SSB consumption.
TABLE 2.
Multivariable linear regression results for postintervention CIRNEAA's and CIREAA's1
| Plasma | RBCs | |||||
|---|---|---|---|---|---|---|
| R 2 | β (SE) | P | R 2 | β (SE) | P | |
| NEAAs | ||||||
| CIRAla | ||||||
| SSBs | 0.65 | 2.81 (0.38) | <0.001 | 0.66 | 1.66 (0.30) | <0.001 |
| Meat | 0.01 (0.37) | 0.980 | 0.20 (0.31) | 0.518 | ||
| Fish | −0.32 (0.37) | 0.390 | −0.25 (0.29) | 0.410 | ||
| Baseline | 0.01 (0.11) | 0.967 | 0.41 (0.10) | <0.001 | ||
| CIRAsp | ||||||
| SSBs | 0.32 | 1.25 (0.36) | 0.002 | 0.59 | 0.73 (0.24) | 0.006 |
| Meat | 0.50 (0.35) | 0.168 | 0.53 (0.25) | 0.042 | ||
| Fish | −0.40 (0.35) | 0.257 | −0.11 (0.23) | 0.650 | ||
| Baseline | 0.14 (0.15) | 0.350 | 0.58 (0.13) | <0.001 | ||
| CIRGlu | ||||||
| SSBs | 0.78 | 1.47 (0.16) | <0.001 | 0.45 | 1.39 (0.33) | <0.001 |
| Meat | 0.37 (0.16) | 0.027 | 0.31 (0.34) | 0.363 | ||
| Fish | −0.43 (0.15) | 0.010 | −0.38 (0.33) | 0.245 | ||
| Baseline | 0.10 (0.09) | 0.249 | 0.30 (0.14) | 0.044 | ||
| CIRPro | ||||||
| SSBs | 0.47 | 0.62 (0.26) | 0.025 | 0.38 | 0.74 (0.22) | 0.003 |
| Meat | 1.23 (0.27) | <0.001 | 0.52 (0.24) | 0.037 | ||
| Fish | 0.06 (0.26) | 0.827 | 0.22 (0.23) | 0.332 | ||
| Baseline | −0.17 (0.15) | 0.268 | 0.30 (0.14) | 0.038 | ||
| CIRSer | ||||||
| SSBs | 0.39 | 1.61 (0.35) | <0.001 | 0.40 | 0.95 (0.26) | 0.001 |
| Meat | 0.40 (0.35) | 0.265 | 0.29 (0.26) | 0.282 | ||
| Fish | −0.30 (0.35) | 0.401 | 0.35 (0.26) | 0.185 | ||
| Baseline | 0.01 (0.12) | 0.938 | 0.24 (0.10) | 0.023 | ||
| EAAs | ||||||
| CIRLeu | ||||||
| SSBs | 0.46 | 0.21 (0.24) | 0.379 | 0.23 | 0.22 (0.20) | 0.262 |
| Meat | 1.26 (0.23) | <0.001 | 0.58 (0.20) | 0.006 | ||
| Fish | 0.05 (0.23) | 0.195 | 0.02 (0.20) | 0.921 | ||
| Baseline | 0.05 (0.16) | 0.777 | 0.27 (0.14) | 0.054 | ||
| CIRPhe | ||||||
| SSBs | 0.43 | 0.08 (0.17) | 0.656 | 0.45 | 0.38 (0.19) | 0.050 |
| Meat | 0.86 (0.17) | <0.001 | 0.29 (0.19) | 0.137 | ||
| Fish | −0.20 (0.17) | 0.244 | 0.05 (0.19) | 0.800 | ||
| Baseline | 0.10 (0.15) | 0.499 | 0.81 (0.17) | <0.001 | ||
| CIRThr2 | ||||||
| SSBs | 0.60 | 0.21 (0.26) | 0.429 | |||
| Meat | 1.23 (0.25) | <0.001 | ||||
| Fish | 0.57 (0.26) | 0.035 | ||||
| Baseline | 0.38 (0.10) | <0.001 | ||||
| CIRVal | ||||||
| SSBs | 0.30 | 0.31 (0.31) | 0.319 | 0.53 | 0.34 (0.24) | 0.163 |
| Meat | 1.08 (0.31) | 0.001 | 0.70 (0.24) | 0.007 | ||
| Fish | 0.40 (0.31) | 0.203 | 0.10 (0.24) | 0.677 | ||
| Baseline | 0.10 (0.15) | 0.480 | 0.58 (0.10) | <0.001 | ||
n = 32. Results are presented as regression coefficients and SEs. Coefficients for dietary intakes refer to presence/absence. R2 presented is the adjusted R2 for the full model. Significant P values (≤0.010) were significant based on the Benjamini-Hochberg correction for the total number of comparisons across both sample types and a false discovery rate of α = 0.05. CIR, carbon isotope ratio; EAA, essential amino acid; NEAA, nonessential amino acid; SSB, sugar-sweetened beverage.
CIRThr reported in plasma only due to high measurement error in RBCs.
All CIREAA's measured in plasma, and 2 out of 3 measured in RBCs, were elevated in meat consumers (Table 2, Supplemental Figure 4): CIRLeu and CIRVal in both plasma and RBCs and CIRThr and CIRPhe in plasma only. Effect sizes were similar across plasma EAAs, with the largest effect in CIRLeu (βmeat = 1.26, SE = 0.23). The CIR of a single NEAA, plasma CIRPro, showed an increase with meat intake, and this increase was similar in magnitude to the increase in CIREAA's.
No CIRAA showed an increase in fish consumers, but there was a small but significant decrease in plasma CIRGlu.
Baseline CIRAA was included as a covariate in these multivariable regression models to account for the potential influence of preintervention diets due to incomplete equilibration of postintervention CIRAA with the study diets. In plasma, only CIRThr was significantly associated with baseline, whereas in RBCs 4 CIRAA's (CIRAla, CIRAsp, CIRVal, CIRPhe) were associated with baseline. Furthermore, the effect sizes measured in RBCs tended to be smaller than in plasma (Table 2).
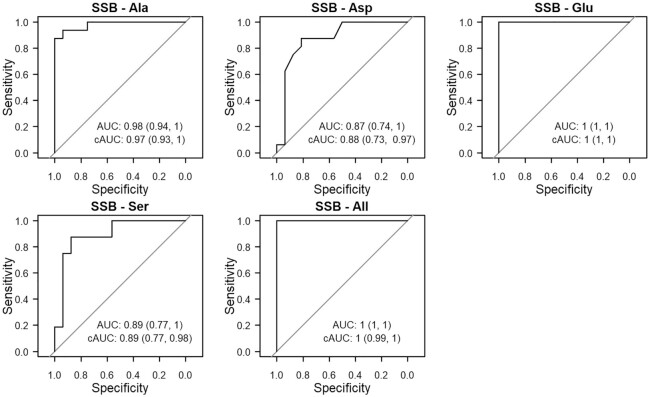
Plasma CIRNEAA's were highly predictive of SSB intake based on their ability to discriminate between consumers and nonconsumers, measured as the AUC of the receiver operating characteristic curves for the logistic regressions (Figure 1). The CIRGlu had the highest predictive accuracy (cAUC = 1; 95% CI: 1, 1), closely followed by the CIRAla (cAUC = 0.97; 95% CI: 0.93, 1). Using all plasma CIRNEAA's as predictors was highly discriminatory for SSB intake (cAUC = 1; 95% CI: 0.99, 1). In RBCs, CIRNEAA's were also highly predictive of SSB intake, achieving, in combination, a cAUC of 0.97 (95% CI: 0.94, 1) (Supplemental Figure 5). Individual CIRNEAA's in RBCs were slightly less discriminatory than in plasma (e.g., RBC CIRAla cAUC = 0.92; 95% CI: 0.81, 0.98). However, even the least discriminatory CIRNEAA's had a moderately high cAUC (RBC CIRPro cAUC = 0.79; 95% CI: 0.57, 0.93).
FIGURE 1.
Receiver operating characteristic curves for logistic regression models (n = 32) predicting SSB consumption using plasma CIRNEAA's as predictors. The cAUC (95% CI) was calculated using Harrell's bootstrap to adjust for optimism. cAUC, corrected AUC; CIR, carbon isotope ratio; NEAA, nonessential amino acid; SSB, sugar-sweetened beverage.
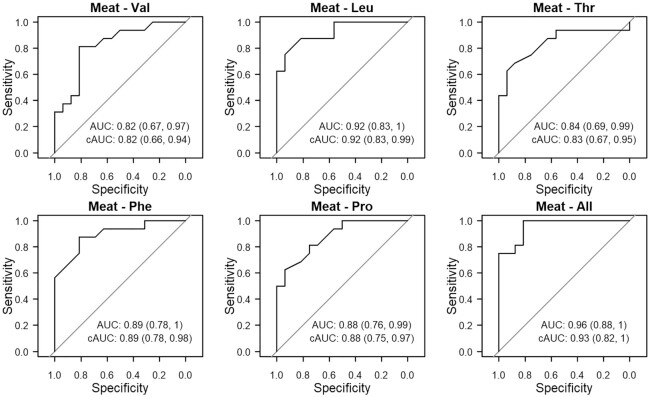
Plasma CIREAA's and CIRPro were highly predictive of meat intake (Figure 2). The CIRLeu achieved the highest predictive accuracy (cAUC = 0.92; 95% CI: 0.83, 0.99) and was as discriminatory as the combination of all plasma predictors of meat intake (cAUC = 0.93; 95% CI: 0.82, 1). All other plasma CIREAA's had cAUC scores >0.8 for meat intake. In contrast, RBC CIRLeu was only moderately predictive of meat intake (cAUC = 0.76; 95% CI: 0.53, 0.93), while RBC CIRVal was less so (cAUC = 0.64; 95% CI: 0.30, 0.85). Using all RBC CIREAA's as predictors did not improve their cAUC (0.73; 95% CI: 0.37, 0.92) (Supplemental Figure 6).
FIGURE 2.
Receiver operating characteristic curves for logistic regression models (n = 32) predicting meat consumption using plasma CIREAA's and 1 CIRNEAA (Pro) as predictors. The cAUC (95% CI) was calculated using Harrell's bootstrap to adjust for optimism. cAUC, corrected AUC; CIR, carbon isotope ratio; EAA, essential amino acid; NEAA, nonessential amino acid.
CIRAA turnover
We characterized the change in CIRAA's over time in individuals by fitting nonlinear models. These models did not converge for all individuals, likely due to the small number of data points and the magnitude of isotopic change, which varied randomly among individuals based on their baseline CIRAA and the treatment assigned. For models that converged, fractional incorporation rates (λ) and weeks to 50% and 90% turnover are presented by AA and specimen type in Table 3. Models of plasma CIRAA tended to converge for a greater number of study participants than did models of RBC CIRAA (a median of 11 compared with 7.5 participants; Wilcoxon signed-rank = 64.5, P = 0.007), and median (25th, 75th percentile) fractional incorporation rates were higher in plasma compared with RBCs [0.38 wk−1 (0.30, 0.39) compared with 0.15 wk−1 (0.12, 0.19); Wilcoxon signed-rank = 69.5, P = 0.0015]. We estimated times to 90% turnover ranging from 5 to 9 wk for plasma AAs and 8–23 wk for RBC AAs. For 75% of RBC CIRAA's the time to 90% turnover exceeded the 12-wk study feeding period.
TABLE 3.
Fractional incorporation rates [median (25th, 75th percentile)] of CIRAA's estimated from biweekly measurements1
| Tissue | CIRAA | Number converged | Median λ2 (25th, 75th) | Median t0.5,3 wk | Median t0.9, wk |
|---|---|---|---|---|---|
| Plasma | Ala | 13 | 0.31 (0.15, 0.53) | 2.2 | 7.4 |
| Plasma | Val | 9 | 0.25 (0.19, 0.37) | 2.8 | 9.2 |
| Plasma | Leu | 13 | 0.38 (0.19, 0.49) | 1.8 | 6.1 |
| Plasma | Thr | 7 | 0.39 (0.23, 0.51) | 1.8 | 5.9 |
| Plasma | Ser | 10 | 0.50 (0.22, 0.87) | 1.4 | 4.6 |
| Plasma | Asp | 11 | 0.42 (0.22, 0.64) | 1.6 | 5.5 |
| Plasma | Pro | 15 | 0.26 (0.13, 0.36) | 2.7 | 8.9 |
| Plasma | Glu | 8 | 0.38 (0.27, 0.59) | 1.8 | 6.1 |
| Plasma | Phe | 14 | 0.30 (0.21, 0.40) | 2.3 | 7.7 |
| RBCs | Ala | 9 | 0.15 (0.11, 0.39) | 4.6 | 15.4 |
| RBCs | Val | 6 | 0.14 (0.05, 0.26) | 5.0 | 16.4 |
| RBCs | Leu | 7 | 0.12 (0.10, 0.52) | 5.8 | 19.2 |
| RBCs | Ser | 5 | 0.30 (0.24, 0.55) | 2.3 | 7.7 |
| RBCs | Asp | 8 | 0.18 (0.15, 0.35) | 3.8 | 12.3 |
| RBCs | Pro | 8 | 0.10 (0.07, 0.16) | 6.9 | 23.0 |
| RBCs | Glu | 8 | 0.20 (0.16, 0.27) | 3.5 | 11.5 |
| RBCs | Phe | 6 | 0.12 (0.06, 0.17) | 5.8 | 19.2 |
Estimated using nonlinear least squares. The number of converged models is out of 18 subjects. CIRAA, carbon isotope ratio of amino acid.
Units of λ, the fractional incorporation rate, are week−1.
Median weeks until 50% turnover (half-life) and 90% turnover calculated as tx = −ln (1 − x)/λ.
Discussion
We measured the responses of plasma and RBC CIRAA's to SSB and meat intakes in a 12-wk fully inpatient controlled-feeding study. Most plasma CIRNEAA's and all RBC CIRNEAA's increased significantly with SSB intake, with the largest effect in plasma CIRAla, and no CIRNEAA's were significantly associated with meat intake. CIRAla and CIRGlu were the most promising individual estimators of SSB intake due to their high predictive accuracy (cAUC ≥0.97). In combination, CIRNEAA's of both plasma and RBCs were highly predictive of SSB intake (cAUC ≥0.98). Meanwhile, most plasma and RBC CIREAA's increased significantly with meat intake. Plasma CIRLeu was the most promising individual predictor of meat intake (cAUC = 0.92) and combining multiple CIREAA's did not improve predictive accuracy. These results further the validation of CIRAA's as biomarkers of SSB and meat intakes.
A key goal of this study was to determine whether CIRAA's were more predictive of SSB intake than total tissue CIR. The effects of SSB intake on CIRNEAA's were 2–5 times larger than on total plasma and RBC CIRs (22), reflecting their greater sensitivity. CIRNEAA's that increased with SSB intake were also more specific, because, unlike total tissue CIR, they were not affected by meat intake. Several prior studies have found that total tissue CIR was more strongly associated with intakes of meat and/or animal protein than AS/SSBs in US populations (13, 20, 21). Although combined total plasma CIR and NIR had moderate success at identifying SSB consumers in the Developing Biomarkers of Diet Study (cAUC = 0.78), the predictive accuracy of CIRNEAA's for SSB intake was higher (22). These findings suggest strong potential for CIRNEAA's as biomarkers of SSB intake.
These findings are broadly consistent with published studies of CIRAA's from a cohort of Alaska Native (Yup'ik) males and females (27) and a cohort of postmenopausal women from the Women's Health Initiative Nutrition and Physical Activity Assessment Study Feeding Study (NPAAS-FS) (37). In the Yup'ik cohort, median intakes of AS and SSBs were high (80 g/d and 1.5 servings/d, respectively) and RBC CIRAla was associated with both AS and SSBs (r = 0.6 and 0.7, respectively) (27). In that study, however, other CIRNEAA's were either not or only weakly associated with SSB intake, including CIRGlu. In the NPAAS-FS, the median intake of AS was low (48 g/d), the median intake of SSBs was very low (<0.5 servings/d), and serum CIRAla was associated with AS only (r = 0.3). Estimation of AS was improved by using multiple CIRAA's: CIRAla; CIRGly, which was inversely associated with AS; and CIRIle, which was associated with animal protein intake (37). Thus, the studies differed in whether single or multiple CIRAA's were required to estimate AS/SSB intake. Comparing all CIRAA responses with diet across these studies is difficult because they used different blood fractions and AA derivatization methods, resulting in different suites of AAs that were reliably measured. Despite these differences, these studies indicate that CIRAla is a robust biomarker of SSB and/or AS intake across diverse US study populations (38) and suggest that using multiple CIRAA's may improve estimation of SSB intake in certain contexts.
We also evaluated whether CIREAA's were more sensitive and specific measures of meat intake in relation to total tissue CIR and NIR. The effects of meat intake on individual CIREAA's were comparable to those on total tissue CIR in both plasma and RBCs (22). Furthermore, the predictive accuracy of CIRLeu, the best CIRAA for detecting meat intake, was identical to that of the total plasma CIR and NIR combined (cAUC = 0.92) (22). Although CIRLeu did not improve upon total tissue CIR and NIR as a measure of meat intake, it would be a useful covariate representing meat intake in models of SSBs using CIRAA's because it is measured in the same analysis. A similar approach was used in the NPAAS-FS, in which a model of AS intake was improved by the inclusion of CIRIle, presumably due to its association with animal protein (37). Similar to the present findings, associations of CIRAA's with animal protein intake in the NPAAS-FS study were not stronger than those with total serum CIR and NIR (21, 37), nor were associations of hair CIRAA's with animal protein stronger than those of total hair CIR and NIR in a German cross-sectional study (39).
A strength of stable isotope biomarkers is that they integrate diet over weeks to months (19, 22, 40), providing estimates of usual intake. This contrasts with other proposed biomarkers of sugar (41) and meat (42) intake, which integrate over the scale of hours or days. The time responses of plasma and RBC CIRAA's have not previously been described. By including baseline CIRAA's as a covariate in models of postintervention CIRAA's, we were able to evaluate whether individual CIRAA's had equilibrated to the study diet within the 12-wk feeding period. With 1 exception, plasma postintervention CIRAA's were not associated with baseline, suggesting that they were at or close to equilibrium with intervention diets. In contrast, all RBC CIRAA's had some association with baseline, suggesting that RBC CIRAA's were not in equilibrium, although several were marginally nonsignificant following correction for multiple testing. These findings are further supported by our estimates of the fractional incorporation rates of CIRAA's, which suggested that plasma CIRAA's were at or near equilibrium with study diets within the 12-wk period, whereas most RBC CIRAA's were not. Thus, dietary effects on RBC CIRAA's are likely underestimated, which may explain the generally higher dietary effect sizes in plasma CIRAA's relative to RBCs. Generally, fractional incorporation rates of CIRAA's in plasma and RBCs were similar to those of total plasma and RBCs (22).
The primary strengths of this study were the high dietary control, factorial design, and 12-wk duration of the fully inpatient feeding study. The factorial design of the experimental diets allowed us to evaluate the responses of CIRAA's to SSB intake in the context of varying meat intake at a similar percentage of energy (and vice versa). The inpatient design gave high confidence in subject compliance, and the 12-wk duration allowed CIRAA's in plasma to achieve equilibrium with experimental diets. This study also had some limitations. One limitation was that we measured responses to dietary variables at only 2 levels (presence or absence). Future work should focus on establishing the dose–response relations between CIRAA's and dietary variables. The 12-wk duration was insufficient for RBC CIRAA's to achieve equilibrium with study diets, potentially attenuating dietary associations. Thus, an explicit comparison of dietary effects in plasma and RBCs would require a longer dietary intervention. There was 15% missingness among randomly assigned participants. While we do not expect the missing data to have a strong effect on our results, we cannot rule out the possibility of some bias in our complete-case analysis. Finally, the lack of an extended run-in period prior to the dietary intervention meant that participants had widely varying baseline CIRAA's, which limited our ability to model the time course of CIRAA turnover to experimental diets. GC-C-IRMS methodology is increasing in availability as it gains popularity in nutrition and other fields (forensics, environmental science) and its costs are intermediate to other biomarker approaches.
In summary, this study in men strongly supports plasma and RBC CIRNEAA's, particularly CIRAla and CIRGlu, as sensitive and specific measures of SSB intake in the US diet. CIRNEAA's will also likely reflect AS intake, based on the high contribution of corn and sugarcane to both AS and SSBs. Furthermore, CIREAA's appear to be discriminatory measures of meat intake. These promising candidate biomarkers warrant further validation, including dose-response assessment (38). Ultimately, the use of long-term intake biomarkers such as CIRAA's may calibrate or replace self-report measures and so reduce error in diet-disease risk models.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Jynene Black, Pat Rivera, and Timothy Howe for their assistance in the laboratory. We also thank the nursing and kitchen staff of the Obesity and Diabetes Clinical Research Section of the NIDDK in Phoenix, Arizona, for helping with the inpatient study and preparing study diets.
The authors’ responsibilities were as follows–––DMO, JK, MJW, and SBV: designed the study; JK and SBV: oversaw the inpatient study; MJW and DMO: oversaw the stable isotope analyses; JJJ and SM: conducted the stable isotope analyses; HYY, SM, and TL: provided essential methods development; DMO, EJO, JJJ, and PAS: conducted data analyses; JJJ and DMO: wrote the manuscript; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH through grant number 1R01DK109946 and intramural NIDDK funding. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIDDK or the NIH.
Data used in this manuscript will be made available upon request at the NIDDK Central Repository (repository.niddk.nih.gov)
Supplemental Tables 1–3 and Supplemental Figures 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Present address for EJO: Social and Decision Analytics Division, Biocomplexity Institute and Initiative, University of Virginia, Arlington, VA 22209.
Present address for SM: Department of Environmental and Radiological Health Sciences, Colorado State University, 350 W. Lake St., Fort Collins, CO 80523, USA.
Present address for HYY: Department of Marine Science and Convergent Technology, Hanyang University, Ansan, Republic of Korea.
Abbreviations used: AA, amino acid; AS, added sugar; cAUC, optimism-corrected AUC; CIR, carbon isotope ratio; EAA, essential amino acid; GC-C-IRMS, GC–combustion–isotope ratio MS; NEAA, nonessential amino acid; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIR, nitrogen isotope ratio; NPAAS-FS, Nutrition and Physical Activity Assessment Study Feeding Study; SSB, sugar-sweetened beverage.
Contributor Information
Jessica J Johnson, Institute of Arctic Biology, Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA.
Pamela A Shaw, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Eric J Oh, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Matthew J Wooller, Alaska Stable Isotope Facility, Water and Environmental Research Center, Institute of Northern Engineering, University of Alaska Fairbanks, Fairbanks, AK, USA; Department of Marine Biology, College of Fisheries and Ocean Sciences, University of Alaska Fairbanks, Fairbanks, AK, USA.
Sean Merriman, Institute of Arctic Biology, Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA.
Hee Young Yun, Institute of Arctic Biology, Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA.
Thomas Larsen, Department of Archaeology, Max Planck Institute for the Science of Human History, Jena, Germany.
Jonathan Krakoff, Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases/NIH, Phoenix, AZ, USA.
Susanne B Votruba, Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases/NIH, Phoenix, AZ, USA.
Diane M O'Brien, Institute of Arctic Biology, Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA.
References
- 1. Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11:1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Connor L, Imamura F, Brage S, Griffin SJ, Wareham NJ, Forouhi NG. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin Nutr. 2018;37:1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma J, Jacques PF, Meigs JB, Fox CS, Rogers GT, Smith CE, Hruby A, Saltzman E, McKeown NM. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J Nutr. 2016;146:2544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braverman-Bronstein A, Camacho-García-Formentí D, Zepeda-Tello R, Cudhea F, Singh GM, Mozaffarian D, Barrientos-Gutierrez T. Mortality attributable to sugar sweetened beverages consumption in Mexico: an update. Int J Obes. 2020;44:1341–9. [DOI] [PubMed] [Google Scholar]
- 5. Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Archer E, Marlow ML, Lavie CJ. Controversy and debate: Memory-Based Methods Paper 1: the fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J Clin Epidemiol. 2018;104:113–24. [DOI] [PubMed] [Google Scholar]
- 7. Tasevska N, Midthune D, Potischman N, Subar AF, Cross AJ, Bingham SA, Schatzkin A, Kipnis V. Use of the predictive sugars biomarker to evaluate self-reported total sugars intake in the Observing Protein and Energy Nutrition (OPEN) study. Cancer Epidemiol Biomarkers Prev. 2011;20:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tasevska N, Midthune D, Tinker LF, Potischman N, Lampe JW, Neuhouser ML, Beasley JM, Van Horn L, Prentice RL, Kipnis V. Use of a urinary sugars biomarker to assess measurement error in self-reported sugars intake in the Nutrition and Physical Activity Assessment Study (NPAAS). Cancer Epidemiol Biomarkers Prev. 2014;23:2874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freedman LS, Kipnis V, Schatzkin A, Tasevska N, Potischman N. Can we use biomarkers in combination with self-reports to strengthen the analysis of nutritional epidemiologic studies?. Epidemiol Perspect Innov. 2010;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedman LS, Tasevska N, Kipnis V, Schatzkin A, Mares J, Tinker L, Potischman N. Gains in statistical power from using a dietary biomarker in combination with self-reported intake to strengthen the analysis of a diet-disease association: an example from CAREDS. Am J Epidemiol. 2010;172:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brouwer-Brolsma EM, Brennan L, Drevon CA, van Kranen H, Manach C, Dragsted LO, Roche HM, Andres-Lacueva C, Bakker SJL, Bouwman Jet al. . Combining traditional dietary assessment methods with novel metabolomics techniques: present efforts by the Food Biomarker Alliance. Proc Nutr Soc. 2017;76:619–27. [DOI] [PubMed] [Google Scholar]
- 12. Fakhouri TH, Jahren AH, Appel LJ, Chen L, Alavi R, Anderson CA. Serum carbon isotope values change in adults in response to changes in sugar-sweetened beverage intake. J Nutr. 2014;144:902–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeung EH, Saudek CD, Jahren AH, Kao WH, Islas M, Kraft R, Coresh J, Anderson CA. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol. 2010;172:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davy BM, Jahren AH, Hedrick VE, Comber DL. Association of delta(1)(3)C in fingerstick blood with added-sugar and sugar-sweetened beverage intake. J Am Diet Assoc. 2011;111:874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hedrick VE, Zoellner JM, Jahren AH, Woodford NA, Bostic JN, Davy BM. A dual-carbon-and-nitrogen stable isotope ratio model is not superior to a single-sarbon stable isotope ratio model for predicting added sugar intake in Southwest Virginian adults. J Nutr. 2015;145:1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacDougall CR, Hill CE, Jahren AH, Savla J, Riebl SK, Hedrick VE, Raynor HA, Dunsmore JC, Frisard MI, Davy BM. The delta13C value of fingerstick blood is a valid, reliable, and sensitive biomarker of sugar-sweetened beverage intake in children and adolescents. J Nutr. 2018;148:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Leary MH. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–36. [Google Scholar]
- 18. Jahren AH, Saudek C, Yeung EH, Kao WH, Kraft RA, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84:1380–4. [DOI] [PubMed] [Google Scholar]
- 19. O'Brien DM. Stable isotope ratios as biomarkers of diet for health research. Annu Rev Nutr. 2015;35:565–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nash SH, Kristal AR, Bersamin A, Hopkins SE, Boyer BB, O'Brien DM. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr. 2013;143:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yun HY, Lampe JW, Tinker LF, Neuhouser ML, Beresford SAA, Niles KR, Mossavar-Rahmani Y, Snetselaar LG, Van Horn L, Prentice RL. Serum nitrogen and carbon stable isotope ratios meet biomarker criteria for fish and animal protein intake in a controlled feeding study of a Women's Health Initiative cohort. J Nutr. 2018;148:1931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Votruba SB, Shaw PA, Oh EJ, Venti CA, Bonfiglio S, Krakoff J, O'Brien DM. Associations of plasma, RBCs, and hair carbon and nitrogen isotope ratios with fish, meat, and sugar-sweetened beverage intake in a 12-wk inpatient feeding study. Am J Clin Nutr. 2019;110:1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schoeller DA, Minagawa M, Slater R, Kaplan IR. Stable isotopes of carbon, nitrogen and hydrogen in the contemporary North American human food web. Ecol Food Nutr. 1986;18:159–70. [Google Scholar]
- 24. Jahren AH, Kraft RA. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc Natl Acad Sci. 2008;105:17855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perriello G, Jorde R, Nurjhan N, Stumvoll M, Dailey G, Jenssen T, Bier DM, Gerich JE. Estimation of glucose-alanine-lactate-glutamine cycles in postabsorptive humans: role of skeletal muscle. Am J Physiol Metab. 1995;269:E443–50. [DOI] [PubMed] [Google Scholar]
- 26. Waterhouse C, Keilson J. The contribution of glucose to alanine metabolism in man. J Lab Clin Med. 1978;92:803–12. [PubMed] [Google Scholar]
- 27. Choy K, Nash SH, Kristal AR, Hopkins S, Boyer BB, O'Brien DM. The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. J Nutr. 2013;143:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–4. [DOI] [PubMed] [Google Scholar]
- 29. Reinhardt M, Piaggi P, DeMers B, Trinidad C, Krakoff J. Cross calibration of two dual‐energy X‐ray densitometers and comparison of visceral adipose tissue measurements by iDXA and MRI. Obesity. 2017;25:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corr LT, Berstan R, Evershed RP. Development of N-acetyl methyl ester derivatives for the determination of δ13C values of amino acids using gas chromatography-combustion-isotope ratio mass spectrometry. Anal Chem. 2007;79:9082–90. [DOI] [PubMed] [Google Scholar]
- 31. Ventura LM, Andersen N, O'Brien DM, Piatkowski U, McCarthy MD. Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting. PLoS One. 2013;8:e73441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang YV, Wan AHL, Lock E-J, Andersen N, Winter-Schuh C, Larsen T. Know your fish: a novel compound-specific isotope approach for tracing wild and farmed salmon. Food Chem. 2018;256:380–9. [DOI] [PubMed] [Google Scholar]
- 33. Silfer JA, Engel MH, Macko SA, Jumeau EJ. Stable carbon isotope analysis of amino acid enantiomers by conventional isotope ratio mass spectrometry and combined gas chromatography/isotope ratio mass spectrometry. Anal Chem. 1991;63:370–4. [Google Scholar]
- 34. O'Brien DM, Fogel ML, Boggs CL. Renewable and nonrenewable resources: amino acid turnover and allocation to reproduction in Lepidoptera. Proc Natl Acad Sci. 2002;99:4413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med. 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 36. Martínez del Rio C, Carleton SA. How fast and how faithful: the dynamics of isotopic incorporation into animal tissues. J Mammal. 2012;93:353–9. [Google Scholar]
- 37. Yun HY, Tinker LF, Neuhouser ML, Schoeller DA, Mossavar-Rahmani Y, Snetselaar LG, Van Horn LV, Eaton CB, Prentice RL, Lampe JWet al. . The carbon isotope ratios of serum amino acids in combination with participant characteristics can be used to estimate added sugar intake in a controlled feeding study of US postmenopausal women. J Nutr, 2020, 150, 2764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dragsted LO, Gao Q, Scalbert A, Vergeres G, Kolehmainen M, Manach C, Brennan L, Afman LA, Wishart DS, Andres Lacueva Cet al. . Validation of biomarkers of food intake—critical assessment of candidate biomarkers. Genes Nutr. 2018;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr. 2005;135:1515–20. [DOI] [PubMed] [Google Scholar]
- 40. Petzke KJ, Lemke S. Hair protein and amino acid 13C and 15N abundances take more than 4 weeks to clearly prove influences of animal protein intake in young women with a habitual daily protein consumption of more than 1 g per kg body weight. Rapid Commun Mass Spectrom. 2009;23:2411–20. [DOI] [PubMed] [Google Scholar]
- 41. Tasevska N. Urinary sugars—a biomarker of total sugars intake. Nutrients. 2015;7:5816–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost Get al. . A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017;105:600–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.