ABSTRACT
Background
It is unclear whether breakfast consumption and breakfast composition are independently associated with changes in cognition over a long-term period in older adults.
Objectives
We aimed to examine the associations between energy and macronutrient intakes at breakfast and cognitive declines.
Methods
We included 2935 participants aged 55–93 y at baseline from the China Health and Nutrition Survey in our analysis. Cognition was assessed in 1997, 2000, 2004, 2006, and 2015. Dietary intake was assessed using weighing methods in combination with 24-h food records.
Results
Breakfast contributed to 25.9% of total energy intake of the day and percentages of breakfast energy intake from protein, fat, and carbohydrates were 12.8%, 11.5%, and 75.7%, respectively. During a median follow-up of 9 y, the β values for changes in global cognitive z-scores for Quintile 5 of protein and fat intakes at breakfast, with Quintile 1 as the reference, were 0.13 (95% CI: 0.01–0.25) and 0.17 (95% CI: 0.04–0.30), respectively. Substitution of 5% energy from carbohydrates with equivalent energy from protein (β, 0.06; 95% CI: 0.01–0.11) or fat (β, 0.05; 95% CI: 0.02–0.08) at breakfast was positively associated with the change in the global cognitive z-score. Energy intake at breakfast was not significantly associated with the global cognitive z-score. Similar results were found for the verbal memory z-score. The positive association of breakfast fat intake and the inverse association of breakfast carbohydrate intake with cognitive declines were stronger in urban residents.
Conclusions
Higher intakes of protein and fat and lower intake of carbohydrates at breakfast were associated with a lower rate of cognitive decline in older adults. Substitution of carbohydrates with protein or fat intake at breakfast may help to delay or prevent cognitive declines.
Keywords: dietary protein, dietary fat, dietary carbohydrates, energy, breakfast, cognitive decline
Introduction
Breakfast is the first meal of the day and usually considered as the most important meal (1, 2). Breakfast consumption was associated with higher intakes of fiber, vitamins, calcium, iron, and magnesium (3, 4) and lower intakes of red and processed meat, appetizers, sugar-sweetened beverages, and alcohol in middle-aged and older adults (5), thus probably leading to beneficial effects on health. Previous prospective studies have shown an inverse association between breakfast consumption and well-known dementia risks, including obesity (6), diabetes (7), heart disease (8), atherosclerosis (5), and hypertension (9), in later life. A regular meal pattern is associated with improved circadian rhythmicity (10, 11), resulting in better cognitive health (12, 13).
Increasingly, studies have investigated the beneficial effects of breakfast on postprandial cognitive performance, but the results remain inconsistent, probably due to different breakfast compositions (14–16). A recent systematic review of clinical trials found a small, beneficial, immediate effect (postprandial cognitive responses) of breakfast consumption on memory in healthy adults (17). Some studies demonstrate that protein intake enhances postprandial delayed memory performance in older adults, but others found no such effect (18–20). A cross-sectional analysis of 800 middle-aged adults demonstrated that a higher frequency of breakfast consumption was associated with fewer cognitive failures (21). In contrast, a prospective study of older adults did not observe a favorable association between daily breakfast cereal consumption and cognitive performance (22). To our knowledge, no longitudinal studies have reported the association between breakfast consumption and breakfast composition and long-term changes in cognition. Therefore, it is imperative to explore this association in older adults based on longitudinal data, given the increasing prevalence of dementia and the increasing number of deaths caused by dementia globally (23, 24).
We aimed to examine whether baseline energy and macronutrient intakes at breakfast were independently associated with changes in cognition over 9 y in a large sample of older adults.
Methods
Participants
The present analysis was based on the China Health and Nutrition Survey (CHNS), an ongoing open-cohort study initiated in 1989 and followed up in 1991, 1993, 1997, 2000, 2004, 2006, 2009, 2011, and 2015 (25, 26). The study was conducted in 9 provinces from the northeast to southwest across the whole of China. We randomly selected 2 cities (urban areas) and 4 counties (rural areas) from each province. We then randomly selected 4 communities in each selected city or county and 20 households from each community, and all selected household members were interviewed. Cognitive assessments were performed in 1997, 2000, 2004, 2006, and 2015. Of the 20,254 individuals who participated in any of the 5 surveys, the following were excluded from the present analysis: those aged less than 55 y at baseline (n = 11,923), those who completed the cognitive assessment at only 1 survey (n = 5213), those who had missing values in meals or who fell in the top or bottom percentile of total energy intake (n = 44), or those who had diabetes, stroke, heart disease, or cancer at baseline (n = 139). A total of 2935 participants were included in the final analysis (Supplemental Figure 1).
The survey was approved by the institutional review committees of the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention. Informed consent was obtained from all participants prior to any study procedure.
Dietary assessment
Weighing methods were used to assess diet intake at the household level for 3 consecutive days at each survey. All foods, beverages, and condiments were measured using scales by trained interviewers at the beginning and end of the 3-day survey period. The 3-day 24-h dietary recalls were used to assess diet intake at the individual level. Proportions of foods, beverages, and condiments consumed at the household level were allocated to individuals based on the data they reported (27). Nutrient and energy intakes were calculated based on the China Food Composition tables (28). Energy and macronutrient intakes at breakfast were also computed by summing the intakes from all food items consumed at breakfast. The average annual consumption of energy and nutrient intakes was calculated from surveys completed before and until the first cognitive assessment.
The doubly labeled water method was used to validate the assessment of energy intake, with a correlation coefficient of 0.56 for men and 0.60 for women (29). Another validation study showed that the correlation coefficients between dietary intake estimated by weighing with 24-h recalls and urine excretions measured from 24-h urine samples for sodium and potassium were 0.58 and 0.59, respectively (30).
Cognitive function test
The primary outcome variables in the present study were the global cognitive score and the verbal memory score. We used a subset of the items from the Telephone Interview for Cognitive Status (modified version) to assess cognitive function in 1997, 2000, 2004, 2006, and 2015 (31). The tool has been used in other population studies in China (32, 33). Cognitive screening through a face-to-face interview included 3 tasks, including immediate and delayed recall of a 10-word list, counting backward from 20, and serial 7 subtraction 5 times. The total score for immediate and delayed recall ranged from 0 to 20, with each correctly recalled word assigned a score of 1. For counting backward, a score of 2 was given to those who counted backwards correctly on the first try and a score of 1 was given to those only counted backward correctly on the second try. The total score for serial 7 subtraction ranged from 0 to 5, with a score of 1 assigned to each of the 5 serial 7 subtractions.
The global cognitive score was computed by summing the scores of all 3 tasks, with a total score ranging from 0 to 27. A total verbal memory score was computed as the sum of the immediate and delayed 10-word recalls. The distributions of the global cognitive score and verbal memory score at baseline, as well as the changes in these scores, were not highly skewed (Supplemental Figures 2–5). All scores were analyzed as age- and gender-standardized z-scores, and a higher score represented better cognitive function. The cognitive decline was computed by subtracting the scores at baseline from those at follow-up. Follow-up was computed by subtracting the date of the first cognitive assessment from that of the last cognitive assessment.
Physical examinations
Height was measured to the nearest 0.1 centimeter using a Seca stadiometer, and weight was measured to the nearest 0.1 kg on a calibrated beam scale. BMI was computed as weight in kilograms divided by squared height in meters. Systolic and diastolic blood pressure were measured in the seated position using a standard mercury sphygmomanometer by trained nurses: 3 measurements were taken to the nearest 2 mmHg, and the average of the last 2 measurements was used for analysis.
Confounders
Age, gender, education, smoking, and alcohol consumption were self-reported. Physical activity was assessed based on hours per week spent in different occupational, household, transportation, and leisure-time activities using a validated questionnaire, from which the metabolic equivalent of task (MET) was calculated (34). Any history of hypertension, diabetes, stroke, myocardial infarction, or cancer was also collected based on the diagnosis (self-reported) of physicians.
Statistical analysis
We performed an ANOVA for continuous variables and the chi-square test for categorical variables to compare the differences in baseline characteristics across the quintiles of energy intake at breakfast.
General linear models (GLMs) with identity links and normally distributed responses were used to test the differences in cognitive score changes between individuals in different quintiles of baseline energy and macronutrient intakes at breakfast. We tested 3 models: 1) Model 1 was adjusted for communities in cities or counties as clustering effects and for characteristics of individuals, including age (continuous) and gender (women and men), as fixed effects; 2) Model 1 was adjusted for all variables in Model 1 plus education, urbanicity (rural and urban areas), years of follow-up, smoking (never, former, and current smokers), alcohol intake (yes, no), physical activity (continuous), global cognitive score (continuous), BMI (continuous), and systolic and diastolic blood pressure (continuous) at baseline as fixed effects; and 3) Model 3 was adjusted for all variables in Model 2 plus intakes of total energy, breakfast energy, fiber, sodium, potassium, grains, vegetables, fruits, red meat, processed meat, fish, and poultry (continuous) at baseline as fixed effects. A GLM was also used to test the association of changes in cognitive scores with each 5% energy increment from energy and macronutrient intakes at breakfast. A nonlinear association between energy and macronutrient intakes at breakfast and changes in global cognitive scores was analyzed using a GLM. A repeated-measures analysis of the association between breakfast energy and macronutrient intakes and changes in cognitive scores was also conducted.
Changes in cognitive scores associated with substitution of 5% energy (breakfast) from the carbohydrate intake with equivalent energy from the protein and fat intakes were estimated using a GLM. We simultaneously included energy intake at breakfast and percentages of energy (breakfast) from carbohydrate and other specific macronutrients at breakfast (continuous), as well as other potential confounders, in the GLM. The difference in beta coefficients and 95% CIs between the 2 macronutrients of interest was then computed as the coefficients associated with the substitution of 1 macronutrient for another (35).
An interaction analysis was conducted to explore whether the association of energy and macronutrient intakes with changes in cognitive scores differed between subgroups of age, gender, education, urbanicity, follow-up period, physical activity, BMI, and blood pressure. A stratified analysis was then performed for those factors with significant interactions. We did a further analysis of the association between breakfast consumption and changes in cognitive scores, stratified by participants who did and did not develop chronic conditions (obesity, hypertension, diabetes, stroke, heart disease, or cancer) during follow-up, as these conditions might mediate the associations.
The numbers of participants who had missing data on education, physical activity, BMI, and blood pressure were 95 (3.2%), 190 (6.5%), 31 (1.1%), and 81 (2.8%), respectively. Missing values for education were assigned as a single category. Multiple imputations (5 iterations) were conducted for missing values of continuous covariates using a Markov Chain Monte Carlo–based method. All covariates in the primary analysis were included in the multiple imputation procedures.
Data analyses were conducted using SAS 9.4 for Windows (SAS Institute Inc.), and 2-sided P values < 0.05 were considered statistically significant.
Results
Participant characteristics
We included 2935 participants (51.4% women) aged 55–93 y (mean ± SD, 61.8 ± 6.6 y) at baseline with complete data on variables of interest in the analysis. Individuals living in rural areas or those with high occupational activity levels were more likely to consume more energy from breakfast (Table 1). Participants in the fifth quintile of breakfast energy intake had higher BMIs and blood pressure and were more likely to be physically active compared with those in the first quintile. Breakfast energy intake was associated with higher 3-day average intakes of total energy and carbohydrates and lower intakes of fat and protein at baseline. The global cognitive z-scores and verbal memory z-scores at baseline did not differ between quintiles of breakfast energy intake.
TABLE 1.
Baseline characteristics according to quintiles of breakfast energy intake
| Breakfast energy intake, % | ||||||
|---|---|---|---|---|---|---|
| Quintile 1, <20.7, n = 587 | Quintile 2, 20.7–24.2, n = 587 | Quintile 3, 24.3–27.3, n = 587 | Quintile 4, 27.4–31.2, n = 587 | Quintile 5, >31.2, n = 587 | P value1 | |
| Age, y | 61.58 ± 6.392 | 61.82 ± 6.53 | 62.38 ± 7.04 | 61.73 ± 6.37 | 61.66 ± 6.45 | 0.9287 |
| Gender | — | — | — | — | — | 0.9168 |
| Men | 296 (50.4)3 | 265 (45.1) | 301 (51.3) | 269 (45.8) | 296 (50.4) | |
| Women | 291 (49.6) | 322 (54.9) | 286 (48.7) | 318 (54.2) | 291 (49.6) | |
| Education | — | — | — | — | — | 0.7571 |
| Low, 0 y | 290 (49.4) | 242 (41.2) | 256 (43.6) | 273 (46.5) | 250 (42.6) | |
| Moderate, 1–12 y | 244 (41.6) | 269 (45.8) | 272 (46.4) | 252 (42.9) | 276 (47.0) | |
| High, ≥13 y | 37 (6.3) | 56 (9.6) | 46 (7.9) | 45 (7.6) | 32 (5.5) | |
| Missing | 16 (2.7) | 20 (3.4) | 13 (2.2) | 17 (2.9) | 29 (4.9) | |
| Living area | — | — | — | — | — | <0.0001 |
| Urban | 221 (37.6) | 278 (47.4) | 249 (42.4) | 211 (35.9) | 115 (19.6) | |
| Rural | 366 (62.4) | 309 (52.6) | 338 (57.6) | 376 (64.1) | 472 (80.4) | |
| Smoking | — | — | — | — | — | 0.7993 |
| Never | 368 (62.7) | 409 (69.7) | 394 (67.1) | 420 (71.6) | 364 (62.0) | |
| Former | 27 (4.6) | 23 (3.9) | 21 (3.6) | 10 (1.7) | 15 (2.6) | |
| Current | 192 (32.7) | 155 (26.4) | 172 (29.3) | 157 (26.7) | 208 (35.4) | |
| Alcohol intake | — | — | — | — | — | 0.3041 |
| None | 381 (64.9) | 403 (68.7) | 381 (64.9) | 406 (69.2) | 398 (67.8) | |
| Yes | 206 (35.1) | 184 (31.3) | 206 (35.1) | 181 (30.8) | 189 (32.2) | |
| Global cognitive score, z-score | 0.09 ± 1.17 | −0.14 ± 1.15 | −0.08 ± 1.12 | −0.10 ± 1.19 | 0.00 ± 1.11 | 0.3302 |
| Verbal memory score, z-score | −0.03 ± 0.90 | 0.17 ± 0.88 | 0.08 ± 0.85 | 0.20 ± 0.86 | 0.05 ± 0.84 | 0.0748 |
| Memory recall–delayed score, z-score | −0.22 ± 1.00 | 0.00 ± 0.98 | −0.06 ± 0.95 | 0.06 ± 0.96 | −0.12 ± 0.96 | 0.0581 |
| Follow-up period, y | 9.3 ± 4.8 | 8.8 ± 4.9 | 8.9 ± 4.8 | 9.0 ± 4.9 | 8.3 ± 4.7 | 0.0025 |
| Physical activity, MET-h/wk | 191.66 ± 117.78 | 176.57 ± 116.14 | 182.91 ± 114.92 | 190.24 ± 120.25 | 213.38 ± 123.17 | 0.0002 |
| Occupational activity | — | — | — | — | — | 0.0005 |
| Very light | 84 (14.3) | 119 (20.3) | 102 (17.4) | 109 (18.6) | 70 (11.9) | |
| Light | 126 (21.5) | 122 (20.8) | 119 (20.3) | 104 (17.7) | 87 (14.8) | |
| Moderate | 141 (24.0) | 140 (23.9) | 164 (27.9) | 139 (23.7) | 131 (22.3) | |
| Heavy | 173 (29.5) | 140 (23.9) | 144 (24.5) | 179 (30.5) | 237 (40.4) | |
| Very heavy | 3 (0.5) | 4 (0.7) | 1 (0.2) | 1 (0.2) | 2 (0.3) | |
| No working ability | 40 (6.8) | 41 (7.0) | 40 (6.8) | 34 (5.8) | 35 (6.0) | |
| Missing | 20 (3.4) | 21 (3.6) | 17 (2.9) | 21 (3.6) | 25 (4.3) | |
| BMI, kg/m2 | 22.7 ± 3.4 | 23.1 ± 3.5 | 22.7 ± 3.6 | 23.4 ± 3.6 | 23.2 ± 3.7 | 0.0024 |
| Diastolic blood pressure, mmHg | 80.4 ± 11.3 | 80.6 ± 11.6 | 80.5 ± 10.9 | 82.0 ± 11.8 | 82.2 ± 12.3 | 0.0008 |
| Systolic blood pressure, mmHg | 126.6 ± 19.8 | 128.59 ± 19.1 | 128.9 ± 18.9 | 129.48 ± 19.5 | 131.0 ± 20.4 | 0.0002 |
| Dietary intake | ||||||
| Total energy, kcal/d | 1975.1 ± 612.5 | 1908.1 ± 573.1 | 2005.6 ± 634.1 | 2114.8 ± 719.8 | 2166.6 ± 805.6 | <0.0001 |
| Fiber, g/d | 26.65 ± 23.39 | 27.66 ± 25.06 | 30.23 ± 28.65 | 23.79 ± 23.41 | 20.67 ± 21.30 | <0.0001 |
| Sodium, mg/d | 7350.9 ± 4987.8 | 7165.13 ± 4293.5 | 7499.4 ± 4647.9 | 7249.6 ± 4460.4 | 7466.6 ± 4907.3 | 0.6043 |
| Potassium, mg/d | 2289.8 ± 1655.3 | 2153.9 ± 1637.1 | 2471.6 ± 2133.56 | 2219.6 ± 1629.6 | 2034.1 ± 1514.4 | 0.0486 |
| Protein,4 % energy/d | 14.20 ± 3.37 | 14.52 ± 3.16 | 14.59 ± 3.28 | 14.02 ± 3.07 | 13.55 ± 2.66 | <0.0001 |
| Fat, % energy/d | 16.90 ± 10.47 | 18.09 ± 9.53 | 17.26 ± 9.13 | 14.47 ± 9.11 | 12.15 ± 9.05 | <0.0001 |
| Carbohydrate, % energy/d | 67.82 ± 13.40 | 66.72 ± 11.80 | 67.92 ± 11.26 | 71.52 ± 11.61 | 74.89 ± 11.06 | <0.0001 |
| Protein at breakfast, % breakfast energy | 12.52 ± 2.68 | 12.61 ± 2.19 | 12.91 ± 2.39 | 13.06 ± 2.07 | 12.91 ± 1.88 | <0.0001 |
| Fat at breakfast, % breakfast energy | 9.64 ± 7.73 | 11.13 ± 7.61 | 12.10 ± 7.35 | 12.50 ± 8.01 | 11.95 ± 7.57 | <0.0001 |
| Carbohydrate at breakfast, % breakfast energy | 77.84 ± 9.61 | 76.27 ± 9.16 | 74.99 ± 8.94 | 74.44 ± 9.35 | 75.14 ± 8.63 | <0.0001 |
| Grains, g/2000 kcal | 474.32 ± 153.17 | 497.64 ± 144.13 | 497.66 ± 125.39 | 500.07 ± 120.78 | 502.95 ± 114.70 | <0.0001 |
| Vegetables, g/2000 kcal | 291.18 ± 182.74 | 285.32 ± 173.81 | 276.54 ± 172.84 | 253.15 ± 184.68 | 231.81 ± 218.87 | <0.0001 |
| Fruit, g/2000 kcal | 11.95 ± 61.98 | 15.22 ± 70.67 | 8.36 ± 44.48 | 10.91 ± 48.12 | 6.49 ± 34.26 | 0.0292 |
| Red meat, g/2000 kcal | 70.32 ± 67.50 | 73.27 ± 65.31 | 59.57 ± 60.71 | 47.20 ± 65.42 | 30.52 ± 49.72 | <0.0001 |
| Processed meat, g/2000 kcal | 40.02 ± 56.70 | 37.93 ± 50.90 | 34.04 ± 50.29 | 24.63 ± 52.44 | 13.04 ± 35.51 | <0.0001 |
| Fish, g/2000 kcal | 14.57 ± 44.75 | 14.11 ± 41.98 | 17.61 ± 60.19 | 11.56 ± 35.55 | 12.88 ± 45.18 | 0.3268 |
| Poultry, g/2000 kcal | 6.60 ± 25.17 | 5.34 ± 20.93 | 4.50 ± 18.78 | 5.18 ± 22.12 | 3.32 ± 19.05 | 0.0160 |
Abbreviation: MET, metabolic equivalent of task.
An ANOVA was used to test the differences in continuous variables across quintiles of breakfast energy intake and a chi-square test was used for categorical variables.
All such data were means ± SDs.
All such data were frequencies (%).
Refers to % energy of total daily intake, while those for macronutrients at breakfast refer to % breakfast energy intake.
Changes in cognitive scores
During a median follow-up of 9 y (range, 2–19 y; mean ± SD, 8.9 ± 4.8 y), the global cognitive raw score decreased by 2.2 ± 6.8. The global cognitive z-score decreased from 0.11 ± 0.90 at baseline to 0.06 ± 0.94 at follow-up (mean difference, −0.04 ± 1.15). The verbal memory raw score decreased by 1.5 ± 5.7. The verbal memory z-score decreased from 0.10 ± 0.87 at baseline to 0.07 ± 0.92 at follow-up (mean difference, −0.03 ± 1.17).
Energy and macronutrient intakes
Percentages of daily energy intake from protein, fat, and carbohydrates were 14.4%, 15.8%, and 69.8%, respectively (Supplemental Figure 6). Breakfast contributed to 25.9% of the total energy intake of the day. Percentages of breakfast energy intake from protein, fat, and carbohydrates were 12.8%, 11.5%, and 75.7%, respectively.
Energy and macronutrient intakes at breakfast and the change in global cognitive score
The energy intake at breakfast was not significantly associated with the change in the global cognitive z-score (Table 2). In the multivariable analysis, the β for the change in the global cognitive z-score was 0.13 (95% CI: 0.01–0.25) for the fifth quintile of protein intake at breakfast. Breakfast protein intake (each 5% breakfast energy increment) was positively associated with the change in the global cognitive z-score (β, 0.08; 95% CI: 0.01–0.16). Individuals in the fourth and fifth quintiles of fat intake at breakfast had relatively higher increases in the global cognitive z-score [β, 0.15 (95% CI: 0.03–0.27) for the fourth quintile; 0.17 (95% CI: 0.04–0.30) for the fifth quintile] compared with those in the first quintile after adjustment for confounders. Fat intake at breakfast (each 5% breakfast energy increment) was positively associated with the change in the global cognitive z-score (0.06; 95% CI: 0.03–0.09). Carbohydrate intake at breakfast was inversely associated with the change in the global cognitive z-score before (P-trend = 0.0382) but not after adjustment for confounders (P-trend = 0.2094; Model 2). A marginal, nonlinear association between breakfast fat intake and the change in the global cognitive z-score was observed (P = 0.0710; Supplemental Table 1).
TABLE 2.
The change in global cognitive score associated with energy and macronutrient intakes at breakfast
| Consumption level | |||||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend | 5% energy increment | |
| Breakfast energy intake | |||||||
| Range, % total daily energy | <20.7 | 20.7−24.2 | 24.3–27.3 | 27.4–31.2 | >31.2 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | 0.02 ± 0.05 | 0.10 ± 0.04 | 0.13 ± 0.04 | 0.14 ± 0.04 | 0.09 ± 0.05 | 0.1893 | 0.01 (−0.02 to 0.03) |
| Change in cognitive score | −0.01 ± 0.06 | 0.01 ± 0.06 | 0.05 ± 0.05 | 0.02 ± 0.05 | −0.01 ± 0.06 | 0.8752 | — |
| β (95% CI), Model 11 | 0 | 0.02 (−0.09 to 0.12) | 0.06 (−0.05 to 0.17) | 0.03 (−0.08 to 0.15) | −0.00 (−0.13 to 0.12) | 0.8752 | −0.00 (−0.03 to 0.02) |
| β (95% CI), Model 22 | 0 | 0.01 (−0.10 to 0.11) | 0.04 (−0.07 to 0.15) | 0.03 (−0.09 to 0.14) | 0.01 (−0.12 to 0.13) | 0.8027 | −0.01 (−0.03 to 0.02) |
| β (95% CI), Model 33 | 0 | 0.00 (−0.10 to 0.11) | 0.03 (−0.08 to 0.14) | 0.00 (−0.12 to 0.12) | −0.05 (−0.20 to 0.09) | 0.6309 | −0.00 (−0.03 to 0.03) |
| Breakfast protein intake | |||||||
| Range (% breakfast energy) | <10.8 | 10.8–11.7 | 11.8–12.6 | 12.7–14.0 | >14.0 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | −0.07 ± 0.05 | 0.06 ± 0.04 | 0.15 ± 0.04 | 0.14 ± 0.04 | 0.21 ± 0.05 | <0.0001 | 0.15 (0.08–0.22) |
| Change in cognitive score | −0.11 ± 0.05 | −0.06 ± 0.04 | −0.13 ± 0.04 | −0.04 ± 0.04 | 0.07 ± 0.05 | 0.0071 | — |
| β (95% CI), Model 1 | 0 | 0.06 (−0.05 to 0.16) | −0.01 (−0.13 to 0.10) | 0.07 (−0.04 to 0.18) | 0.18 (0.06–0.30) | 0.0071 | 0.09 (0.02–0.15) |
| β (95% CI), Model 2 | 0 | 0.05 (−0.06 to 0.15) | −0.03 (−0.14 to 0.08) | 0.05 (−0.06 to 0.16) | 0.13 (0.01–0.25) | 0.0600 | 0.08 (0.01–0.16) |
| β (95% CI), Model 3 | 0 | 0.05 (−0.06 to 0.16) | −0.02 (−0.13 to 0.09) | 0.06 (−0.05 to 0.17) | 0.13 (0.01–0.25) | 0.0544 | 0.08 (0.01–0.16) |
| Breakfast fat intake | |||||||
| Range, % breakfast energy | <4.6 | 4.6–7.3 | 7.4–11.1 | 11.2–17.2 | >17.2 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | −0.13 ± 0.04 | 0.08 ± 0.04 | 0.13 ± 0.04 | 0.14 ± 0.04 | 0.28 ± 0.05 | <0.0001 | 0.07 (0.04–0.10) |
| Change in cognitive score | −0.12 ± 0.05 | −0.20 ± 0.04 | −0.10 ± 0.04 | 0.06 ± 0.04 | 0.11 ± 0.05 | <0.0001 | — |
| β (95% CI), Model 1 | 0 | −0.08 (−0.19 to 0.02) | 0.02 (−0.09 to 0.13) | 0.18 (0.07–0.29) | 0.23 (0.10–0.35) | <0.0001 | 0.09 (0.07–0.11) |
| β (95% CI), Model 2 | 0 | −0.08 (−0.19 to 0.02) | 0.00 (−0.11 to 0.11) | 0.14 (0.03–0.25) | 0.17 (0.04–0.29) | 0.0003 | 0.05 (0.03–0.08) |
| β (95% CI), Model 3 | 0 | −0.08 (−0.19 to 0.03) | 0.02 (−0.09 to 0.13) | 0.15 (0.03–0.27) | 0.17 (0.04–0.30) | 0.0003 | 0.06 (0.03–0.09) |
| Breakfast carbohydrates intake | |||||||
| Range, % breakfast energy | <64.6 | 64.6–73.2 | 73.3–78.7 | 78.7–82.8 | >82.8 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | 0.10 ± 0.05 | 0.13 ± 0.04 | 0.09 ± 0.04 | 0.17 ± 0.04 | −0.01 ± 0.05 | 0.1292 | −0.00 (−0.02 to 0.02) |
| Change in cognitive score | −0.03 ± 0.05 | 0.03 ± 0.04 | −0.02 ± 0.04 | −0.16 ± 0.04 | −0.09 ± 0.05 | 0.0382 | — |
| β (95% CI), Model 1 | 0 | 0.06 (−0.05 to 0.17) | 0.01 (−0.10 to 0.13) | −0.13 (−0.24 to −0.01) | −0.06 (−0.18 to 0.07) | 0.0382 | −0.02 (−0.03 to −0.01) |
| β (95% CI), Model 2 | 0 | 0.08 (−0.03 to 0.18) | 0.04 (−0.08 to 0.15) | −0.08 (−0.20 to 0.04) | −0.01 (−0.13 to 0.11) | 0.2094 | −0.00 (−0.02 to 0.01) |
| β (95% CI), Model 3 | 0 | 0.08 (−0.03 to 0.19) | 0.05 (−0.07 to 0.16) | −0.07 (−0.19 to 0.05) | −0.01 (−0.13 to 0.11) | 0.2242 | −0.00 (−0.02 to 0.01) |
General linear regression models were used to obtain coefficients (95% CIs) for the changes in global cognitive scores for Quintiles 2–5 versus Quintile 1 of energy and macronutrient intakes at breakfast. Each 5% energy increment from breakfast energy and macronutrient intakes was associated with the change in the global cognitive score. The change in global cognitive scores was computed as the score at baseline subtracted from that at follow-up.
Model 1 was adjusted for communities in cities or counties as random effects and for age and gender as fixed effects.
Model 2 was adjusted for the variables from Model 1 plus education, urbanicity, years of follow-up, smoking, alcohol intake, physical activity, global cognitive score, BMI, and systolic and diastolic blood pressure at baseline as fixed effects.
Model 3 was adjusted for the variables from Model 2 plus intakes of total energy, breakfast energy, fiber, sodium, potassium, grains, vegetables, fruits, red meat, processed meat, fish, and poultry at baseline as fixed effects.
Energy and macronutrient intakes at breakfast and the change in verbal memory score
No significant association between breakfast energy intake and the change in the verbal memory z-score was observed (Table 3). Protein intake at breakfast was positively associated with a change in the verbal memory z-score before but not after adjustment for confounders. Fat intake at breakfast was positively associated with the change in the verbal memory z-score [0.14 (95% CI: 0.01–0.27) for the fifth quintile versus the first quintile] after adjustment for confounders. Fat intake at breakfast (each 5% breakfast energy increment) was positively associated with the change in the verbal memory z-score (β, 0.05; 95% CI: 0.03–0.08).
TABLE 3.
The change in verbal memory score associated with energy and macronutrient intakes at breakfast
| Consumption level | Each 5% energy increment | ||||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend | ||
| Breakfast energy intake | |||||||
| Range (% total daily energy) | <20.7 | 20.7–24.2 | 24.3–27.3 | 27.4–31.2 | >31.2 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | 0.01 ± 0.05 | 0.09 ± 0.04 | 0.13 ± 0.04 | 0.13 ± 0.04 | 0.08 ± 0.05 | 0.1748 | 0.01 (−0.02 to 0.04) |
| Change in cognitive score | 0.01 ± 0.06 | 0.04 ± 0.06 | 0.06 ± 0.05 | 0.00 ± 0.05 | 0.03 ± 0.06 | 0.7983 | — |
| β (95% CI), Model 11 | 0 | 0.03 (−0.08 to 0.13) | 0.05 (−0.06 to 0.16) | 0.01 (−0.10 to 0.13) | 0.02 (−0.10 to 0.15) | 0.7983 | 0.00 (−0.02 to 0.03) |
| β (95% CI), Model 22 | 0 | 0.02 (−0.09 to 0.13) | 0.04 (−0.07 to 0.15) | 0.00 (−0.11 to 0.12) | 0.03 (−0.10 to 0.15) | 0.8119 | −0.00 (−0.02 to 0.02) |
| β (95% CI), Model 33 | 0 | 0.02 (−0.09 to 0.12) | 0.02 (−0.09 to 0.13) | −0.02 (−0.14 to 0.10) | −0.04 (−0.18 to 0.11) | 0.5478 | 0.01 (−0.02 to 0.03) |
| Breakfast protein intake | |||||||
| Range (% breakfast energy) | <10.8 | 10.8–11.7 | 11.8–12.6 | 12.7–14.0 | >14.0 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | −0.04 ± 0.04 | 0.05 ± 0.04 | 0.14 ± 0.04 | 0.12 ± 0.04 | 0.17 ± 0.04 | 0.0001 | 0.11 (0.05–0.18) |
| Change in cognitive score | −0.07 ± 0.05 | −0.03 ± 0.04 | −0.08 ± 0.04 | −0.02 ± 0.04 | 0.04 ± 0.05 | 0.0001 | — |
| β (95% CI), Model 1 | 0 | 0.05 (−0.06 to 0.16) | −0.01 (−0.12 to 0.10) | 0.05 (−0.06 to 0.16) | 0.11 (0.00–0.22) | 0.0001 | 0.06 (0.01–0.11) |
| β (95% CI), Model 2 | 0 | 0.04 (−0.07 to 0.15) | −0.02 (−0.13 to 0.09) | 0.04 (−0.08 to 0.15) | 0.07 (−0.05 to 0.19) | 0.0645 | 0.05 (−0.01 to 0.12) |
| β (95% CI), Model 3 | 0 | 0.04 (−0.07 to 0.15) | −0.02 (−0.13 to 0.10) | 0.04 (−0.07 to 0.16) | 0.07 (−0.05 to 0.19) | 0.0992 | 0.05 (−0.02 to 0.13) |
| Breakfast fat intake | |||||||
| Range, % breakfast energy | <4.6 | 4.6–7.3 | 7.4–11.1 | 11.2–17.2 | >17.2 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | −0.11 ± 0.04 | 0.10 ± 0.04 | 0.09 ± 0.04 | 0.13 ± 0.04 | 0.25 ± 0.05 | <0.0001 | 0.06 (0.03–0.08) |
| Change in cognitive score | −0.07 ± 0.05 | −0.16 ± 0.04 | −0.09 ± 0.04 | 0.08 ± 0.04 | 0.10 ± 0.05 | 0.0001 | — |
| β (95% CI), Model 1 | 0 | −0.09 (−0.20 to 0.02) | −0.02 (−0.13 to 0.09) | 0.15 (0.04–0.26) | 0.17 (0.04–0.29) | 0.0001 | 0.07 (0.05–0.10) |
| β (95% CI), Model 2 | 0 | −0.09 (−0.20 to 0.01) | −0.04 (−0.15 to 0.07) | 0.13 (0.01–0.24) | 0.13 (0.00–0.26) | 0.0018 | 0.05 (0.02–0.07) |
| β (95% CI), Model 3 | 0 | −0.09 (−0.19 to 0.02) | −0.02 (−0.13 to 0.09) | 0.14 (0.02–0.25) | 0.14 (0.01–0.27) | 0.0014 | 0.05 (0.03–0.08) |
| Breakfast carbohydrates intake | |||||||
| Range, % breakfast energy | <64.6 | 64.6–73.2 | 73.3–78.7 | 78.7–82.8 | >82.8 | — | |
| Participants | 587 | 587 | 587 | 587 | 587 | — | |
| Cognitive score at baseline | 0.10 ± 0.05 | 0.12 ± 0.04 | 0.08 ± 0.04 | 0.15 ± 0.04 | −0.01 ± 0.04 | 0.1211 | −0.00 (−0.02 to 0.01) |
| Change in cognitive score | −0.00 ± 0.05 | 0.04 ± 0.04 | −0.00 ± 0.04 | −0.15 ± 0.04 | −0.05 ± 0.05 | 0.0520 | — |
| β (95% CI), Model 1 | 0 | 0.04 (−0.07 to 0.15) | 0.00 (−0.11 to 0.12) | −0.15 (−0.27 to −0.03) | −0.05 (−0.17 to 0.07) | 0.0520 | −0.02 (−0.03 to −0.01) |
| β (95% CI), Model 2 | 0 | 0.05 (−0.06 to 0.16) | 0.01 (−0.10 to 0.13) | −0.12 (−0.24 to −0.00) | −0.02 (−0.14 to 0.10) | 0.1442 | −0.01 (−0.02 to 0.01) |
| β (95% CI), Model 3 | 0 | 0.05 (−0.06 to 0.16) | 0.02 (−0.10 to 0.13) | −0.12 (−0.24 to 0.00) | −0.03 (−0.15 to 0.09) | 0.1225 | −0.01 (−0.03 to 0.00) |
General linear regression models were used to obtain coefficients (95% CIs) for the changes in verbal memory score for Quintiles 2–5 versus Quintile 1 of energy and macronutrient intakes at breakfast. Each 5% energy increment from breakfast energy and macronutrient intakes was associated with the change in the global cognitive score. The change in verbal memory score was computed as the score at baseline subtracted from that at follow-up.
Model 1 was adjusted for communities in cities or counties as random effects and for age and gender as fixed effects.
Model 2 was adjusted for the variables in Model 1 plus education, urbanicity, years of follow-up, smoking, alcohol intake, physical activity, verbal memory score, BMI, and systolic and diastolic blood pressure at baseline as fixed effects.
Model 3 was adjusted for the variables in Model 2 plus intakes of total energy, breakfast energy, fiber, sodium, potassium, grains, vegetables, fruits, red meat, processed meat, fish, and poultry at baseline as fixed effects.
Repeated-measures analysis of the association between breakfast energy and macronutrient intakes and changes in cognitive scores
Each 5% increment change in energy intake from breakfast protein was associated with a 0.05 unit (95% CI: 0.03–0.08 unit) increase in the global cognitive z-score (Supplemental Table 2). The corresponding number for breakfast fat was 0.02 units (95% CI: 0.01–0.03 units). Similar results were observed for the verbal memory z-score.
Substitution of carbohydrate intake with protein and fat intake at breakfast and the change in cognition
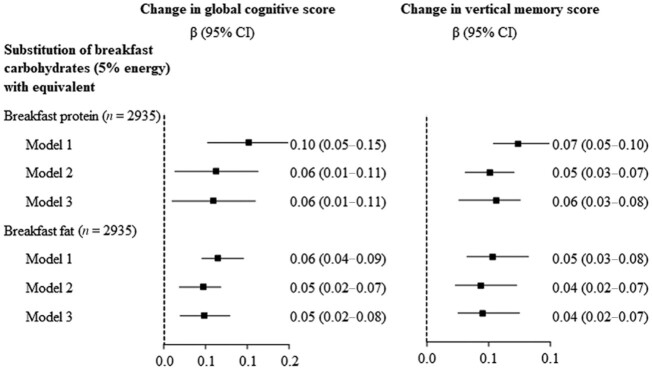
The substitution of 5% energy from carbohydrates at breakfast with equivalent energy from protein (β, 0.06; 95% CI: 0.01–0.11) or fat (β, 0.05; 95% CI: 0.02–0.08) at breakfast was associated with a relative increase in the global cognitive z-score (Figure 1). Likewise, substitution of 5% energy from carbohydrates at breakfast with equivalent energy from protein (0.06; 95% CI: 0.03–0.08) or fat (0.04; 95% CI: 0.02–0.07) at breakfast was associated with a relative increase in the verbal memory z-score.
FIGURE 1.
Substitution of the carbohydrate intake with equivalent energy from the protein and fat intakes and the change in cognition. Changes in cognitive scores associated with substitution of 5% energy (breakfast) from the carbohydrate intake with equivalent energy from the protein and fat intakes were estimated using GLM. We simultaneously included energy intake at breakfast and percentages of energy (breakfast) from carbohydrates and other specific macronutrients at breakfast (continuous), as well as other potential confounders in the GLM. The differences in coefficients and 95% CIs for the 2 macronutrients of interest were then computed. The change in cognitive scores was computed as the scores at baseline subtracted from those at follow-up. Model 1 was adjusted for age and gender; Model 2 was adjusted for the same variables in Model 1 plus education, urbanicity, years of follow-up, smoking, alcohol intake, physical activity, global cognitive score, BMI, and systolic and diastolic blood pressure at baseline; and Model 3 was adjusted for the same variables in Model 2 plus intakes of total energy, breakfast energy, fiber, sodium, potassium, grains, vegetables, fruits, red meat, processed meat, fish, and poultry at baseline. Abbreviation: GLM, general linear regression model.
Moderation analysis
The positive association between fat intake at breakfast and the change in the global cognitive z-score was more evident in urban residents [β, 0.42 (95% CI: 0.20–0.64) for urban areas and 0.09 (95% CI: −0.09 to 0.27) for rural areas for the fifth quintile versus the first quintile; P-value for interaction < 0.0001; Table 4]. The inverse association between carbohydrate intake at breakfast and the change in the global cognitive z-score was observed in urban residents only [β, −0.29 (95% CI: −0.50 to −0.09) for urban areas and 0.06 (95% CI: −0.11 to 0.23) for rural areas for the fifth quintile versus the first quintile; P value for interaction < 0.0001]. Similar results were found for the change in the verbal memory z-score. As shown in Supplemental Tables 3 and 4, the association between breakfast energy and macronutrient intakes and a change in cognitive scores did not differ between individuals who did and did not develop main chronic conditions during follow-up.
TABLE 4.
The association of fat and carbohydrate intakes at breakfast with the change in cognition, stratified by urbanicity
| Consumption level | |||||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend | P-interaction1 | |
| Breakfast fat intake | |||||||
| Participants | |||||||
| Urban | 118 | 147 | 186 | 234 | 389 | ||
| Rural | 469 | 440 | 401 | 353 | 198 | ||
| Change in global cognitive score, β (95% CI)2 | — | — | — | — | — | <0.0001 | |
| Urban | 0 | −0.00 (−0.22 to 0.22) | 0.18 (−0.03 to 0.40) | 0.38 (0.16–0.59) | 0.42 (0.20–0.64) | <0.0001 | |
| Rural | 0 | −0.10 (−0.23 to 0.02) | −0.01 (−0.15 to 0.12) | 0.12 (−0.02 to 0.26) | 0.09 (−0.09 to 0.27) | 0.0438 | |
| Change in verbal memory score, β (95% CI) | — | — | — | — | — | <0.0001 | |
| Urban | 0 | −0.00 (−0.22 to 0.22) | 0.14 (−0.07 to 0.36) | 0.35 (0.14–0.57) | 0.42 (0.20–0.65) | <0.0001 | |
| Rural | 0 | −0.11 (−0.23 to 0.02) | −0.05 (−0.18 to 0.08) | 0.11 (−0.03 to 0.25) | 0.01 (−0.16 to 0.19) | 0.1836 | |
| Breakfast carbohydrates intake | |||||||
| Participants | |||||||
| Urban | 319 | 275 | 185 | 164 | 131 | ||
| Rural | 268 | 312 | 402 | 423 | 456 | ||
| Change in global cognitive score, β (95% CI) | — | — | — | — | — | <0.0001 | |
| Urban | 0 | 0.03 (−0.12 to 0.18) | 0.01 (−0.16 to 0.19) | −0.11 (−0.30 to 0.08) | −0.29 (−0.50 to −0.09) | 0.0075 | |
| Rural | 0 | 0.11 (−0.06 to 0.27) | 0.06 (−0.10 to 0.22) | −0.09 (−0.26 to 0.08) | 0.06 (−0.11 to 0.23) | 0.6565 | |
| Change in verbal memory score, β (95% CI) | — | — | — | — | — | <0.0001 | |
| Urban | 0 | 0.01 (−0.14 to 0.16) | −0.03 (−0.21 to 0.15) | −0.19 (−0.38 to 0.00) | −0.32 (−0.53 to −0.11) | 0.0016 | |
| Rural | 0 | 0.09 (−0.08 to 0.25) | 0.06 (−0.11 to 0.22) | −0.10 (−0.27 to 0.07) | 0.06 (−0.10 to 0.23) | 0.7994 | |
An interaction analysis was conducted to explore whether the associations of energy and macronutrient intakes with changes in cognitive scores differed between subgroups of age, gender, education, urbanicity, follow-up period, physical activity, BMI, and blood pressure using general linear regression models. A significant interaction was only observed for urbanicity. General linear regression models were used to obtain coefficients (95% CIs) for the changes in cognition associated with breakfast fat and carbohydrate intakes. The changes in cognitive scores were computed as the scores at baseline subtracted from those at follow-up.
A multivariable analysis was adjusted for communities in cities or counties as random effects and age, gender, education, years of follow-up, smoking, alcohol intake, physical activity, cognitive score (baseline), BMI, and systolic and diastolic blood pressure, as well as intakes of total energy, breakfast energy, fiber, sodium, potassium, grains, vegetables, fruits, red meat, processed meat, fish, and poultry at baseline as fixed effects.
Discussion
Breakfast energy intake was not significantly associated with cognitive declines. A second primary finding was that a higher intake of protein and fat at breakfast was associated with a lower rate of cognitive decline. A large proportion of total energy was consumed from breakfast, with carbohydrates as the predominant source, and a significant decrease was seen in the global cognitive z-score during follow-up in this large population of older adults. The substitution of carbohydrate intake at breakfast with equivalent energy from protein or fat intake at breakfast was associated with a lower rate of cognitive decline. The association between fat and carbohydrate intakes at breakfast and cognitive declines was stronger in urban residents.
Breakfast consumption has not been linked to cognitive impairments or dementia in previous observational studies. As cardiovascular and metabolic disorders have been demonstrated to be important risk factors for dementia (36), the inverse association between breakfast consumption and cardiovascular and metabolic disorders, as demonstrated in studies in Western countries (5–9), may suggest that breakfast consumption is associated with a lower rate of cognitive decline. Our study showed that breakfast energy intake was not significantly associated with cognitive declines. This may be attributed to the much lower prevalence of breakfast skippers and much higher breakfast energy intake in older adults in China as compared to Western countries. The percentage of energy consumed from breakfast in our study (25.9%) was even greater than the upper end of the range (15% to 25%) recommended by dietary guidelines in Western countries (2). Data from the Progression of Early Subclinical Atherosclerosis study from Spain showed that >20% energy intake from breakfast was associated with a lower prevalence of atherosclerosis (5). However, breakfast contributed to a small proportion of energy intake, with 27% of the population consuming >20% of their total energy from breakfast and 3% consuming <5% of their total energy from breakfast (skippers) in the Spanish study (5). In our study, the percentages of the population consuming >20% and <5% of their energy from breakfast were 82.9% and 0.7%, respectively. A recent meta-analysis of clinical trials showed that habitual breakfast eaters had a small but significant weight gain compared to breakfast skippers (37), which suggests a large breakfast may not be beneficial for cognitive health.
High-protein diets may be beneficial for reductions in weight, fat mass, and lipids (38, 39), because of their potential effects on enhancing energy expenditure, substrate oxidation, and satiety (40). We found that a higher protein intake at breakfast was associated with a lower rate of cognitive decline over the long term. Our findings were supported by a cross-sectional study of 162 older adults showing a potentially protective impact of high dietary protein intake on the brain amyloid-β burden (41); a high amyloid-β burden was associated with accelerated cognitive declines (42). In contrast, a cross-sectional study of 661 participants aged <65 y found that a higher protein intake was associated with a higher prevalence of mild cognitive impairment (43). A possible explanation for this inconsistency is that total protein intake over the day was analyzed in this study, whereas breakfast protein intake was linked to cognitive declines in our study. Participants of the Ding et al. (43) study were young and middle-aged adults, but those included in our study were older adults aged ≥55 y who might require higher levels of protein to maintain health. The study was also limited by the cross-sectional design and the small sample size compared with our study. Our findings suggest a relatively high protein intake at breakfast may prevent or delay cognitive declines in an older population with diets high in carbohydrates.
Previous studies have demonstrated the importance of dietary fat for cognitive health. A recent meta-analysis of prospective studies showed that the total daily fat intake was not significantly associated with cognitive declines (44). We found that a higher breakfast fat intake was associated with a lower rate of cognitive decline. This association was independent of socioeconomic status, lifestyle factors, and intakes of total energy, breakfast energy, fiber, sodium, grains, vegetables, fruits, red meat, fish, and poultry. Inconsistent with our findings, a recent clinical trial of 1606 women aged ≥65 y suggests that a low-fat eating pattern resulted in a decreased risk of cognitive impairment (45). However, fat intake contributed to 20% of energy in the low-fat intervention group, which was much higher than the mean breakfast fat intake (11.5%) in our study. This suggests that a higher breakfast fat intake may have the potential to prevent cognitive declines in a population with low levels of fat intake.
Carbohydrates are the main source of energy and glucose in our body, and the US dietary guidelines recommend that carbohydrates provide 45–65% of total energy intake (46). In our study, carbohydrates contributed to 69.8% of total energy intake and accounted for 75.7% of breakfast energy intake, which was even higher than the upper recommended range. Since carbohydrates are less functional in the formation and regulation of the body's tissues than protein and fat, diets low in carbohydrates and high in protein or fat have been recommended for promoting metabolic health. This is consistent with our study, which demonstrated that a higher breakfast carbohydrate intake was associated with a higher rate of cognitive decline. Similarly, a prospective study of 3831 older adults reported that those with daily consumption of breakfast cereal (mainly carbohydrates) had poorer cognitive performance at baseline and over 11 y of follow-up compared to those who consumed cereal more or less frequently (22). The high content of simple sugars and high glycemic index of diets high in carbohydrates are associated with increased risks of obesity, hypertension, and diabetes (47), which are known to be important risk factors of dementia and cognitive decline. This may partly explain why a higher breakfast carbohydrates intake was associated with an accelerated cognitive decline. Our findings, together with previous studies, suggest the substitution of carbohydrates with protein or fat consumed at breakfast is associated with a lower rate of cognitive decline. Our further analysis shows that older, urban residents with a high fat intake and a low carbohydrate intake at breakfast are more likely to experience cognitive declines during follow-up.
The strengths of the present study include the large sample size, long-term follow-up, and the measurement of dietary intake using weighing methods for 3 consecutive days. To our knowledge, this is the first longitudinal study to investigate the association between energy and macronutrient intakes at breakfast and changes in cognition. The present study also has several limitations. First, older adults aged ≥55 y contributed to a small proportion of the total sample of the CHNS; therefore, our findings may not be generalized to the whole population of China. Although socioeconomic and lifestyle factors and intakes of energy, fiber sodium, and main food groups were adjusted for in our analysis, unknown and unmeasured confounding is possible. Thirdly, time-dependent covariates were not taken into consideration, which might bias the association between breakfast intake and cognitive declines.
In conclusion, macronutrient composition but not energy intake at breakfast was associated with changes in cognition over the long term. Substitution of carbohydrates with protein or fat intake at breakfast may help to delay or prevent cognitive declines.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows – XS, MH: conceived and designed the study; XS, YL: conducted the data analysis; XS: drafted the initial manuscript; and all authors: made critical revisions to the manuscript for important intellectual content and read and approved the final manuscript.
Author disclosures: XS, EH, YL, and MH, no conflicts of interest.
Notes
Support was provided by the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Carolina Population Center (P2C HD050924, T32 HD007168), the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, R24 HD050924, and R01-HD38700), and the NIH Fogarty International Center (D43 TW009077, D43 TW007709) for the China Health and Nutrition Survey (CHNS) data collection and analysis files from 1989 to 2015 and for future surveys; the China-Japan Friendship Hospital, Ministry of Health, for CHNS 2009; the Chinese National Human Genome Center at Shanghai has provided funding since 2009; and Beijing Municipal Center for Disease Prevention and Control has provided funding since 2011. The publication of this article was supported by Fundamental Research Funds of the State Key Laboratory of Ophthalmology.
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplemental Tables 1–4 and Supplemental Figures 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CHNS, China Health and Nutrition Survey; GLM, general linear model; MET, metabolic equivalent of task.
Contributor Information
Xianwen Shang, Department of Ophthalmology, Guandong Eye Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China; School of Behavioural and Health Sciences, Australian Catholic University, Melbourne, Australia; Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Melbourne, Australia; Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, Melbourne, Australia.
Edward Hill, Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Melbourne, Australia; Wicking Dementia Research and Education Centre, University of Tasmania, Hobart, Australia.
Yanping Li, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Mingguang He, Department of Ophthalmology, Guandong Eye Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China; Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, Melbourne, Australia; State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China.
Data Availability
This research uses data from the China Health and Nutrition Survey (CHNS). Data described in the manuscript, code book, and analytic code are made publicly and freely available without restriction at https://www.cpc.unc.edu/projects/china.
References
- 1. Affenito SG. Breakfast: A missed opportunity. J Am Diet Assoc. 2007;107(4):565–9. [DOI] [PubMed] [Google Scholar]
- 2. O'Neil CE, Byrd-Bredbenner C, Hayes D, Jana L, Klinger SE, Stephenson-Martin S. The role of breakfast in health: Definition and criteria for a quality breakfast. J Acad Nutr Diet. 2014;114(Suppl 12):S8–S26. [DOI] [PubMed] [Google Scholar]
- 3. Gaal S, Kerr MA, Ward M, McNulty H, Livingstone MBE. Breakfast consumption in the UK: Patterns, nutrient intake and diet quality. A study from the International Breakfast Research Initiative Group. Nutrients. 2018;10(8):999. doi: 10.3390/nu10080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uzhova I, Mullally D, Peñalvo JL, Gibney ER. Regularity of breakfast consumption and diet: Insights from National Adult Nutrition Survey. Nutrients. 2018;10(11):1578. doi: 10.3390/nu10111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uzhova I, Fuster V, Fernandez-Ortiz A, Ordovas JM, Sanz J, Fernandez-Friera L, Lopez-Melgar B, Mendiguren JM, Ibanez B, Bueno Het al. . The importance of breakfast in atherosclerosis disease: Insights from the PESA study. J Am Coll Cardiol. 2017;70(15):1833–42. [DOI] [PubMed] [Google Scholar]
- 6. Purslow LR, Sandhu MS, Forouhi N, Young EH, Luben RN, Welch AA, Khaw KT, Bingham SA, Wareham NJ. Energy intake at breakfast and weight change: Prospective study of 6,764 middle-aged men and women. Am J Epidemiol. 2007;167(2):188–92. [DOI] [PubMed] [Google Scholar]
- 7. Ballon A, Neuenschwander M, Schlesinger S. Breakfast skipping is associated with increased risk of type 2 diabetes among adults: A systematic review and meta-analysis of prospective cohort studies. J Nutr. 2019;149(1):106–13. [DOI] [PubMed] [Google Scholar]
- 8. Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB, Rimm EB. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation. 2013;128(4):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K. Meal timing and frequency: Implications for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation. 2017;135(9):e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paoli A, Tinsley G, Bianco A, Moro T. The influence of meal frequency and timing on health in humans: The role of fasting. Nutrients. 2019;11(4):719. doi: 10.3390/nu11040719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci. 2009;106(50):21453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rijo-Ferreira F, Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Med. 2019;11(1):82. doi: 10.1186/s13073-019-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benton D, Parker PY. Breakfast, blood glucose, and cognition. Am J Clin Nutr. 1998;67(4):772S–778S. [DOI] [PubMed] [Google Scholar]
- 15. Lloyd HM, Rogers PJ, Hedderley DI, Walker AF. Acute effects on mood and cognitive performance of breakfasts differing in fat and carbohydrate content. Appetite. 1996;27(2):151–64. [DOI] [PubMed] [Google Scholar]
- 16. Benton D, Slater O, Donohoe RT. The influence of breakfast and a snack on psychological functioning. Physiol Behav. 2001;74(4–5):559–71. [DOI] [PubMed] [Google Scholar]
- 17. Galioto R, Spitznagel MB. The effects of breakfast and breakfast composition on cognition in adults. Adv Nutr. 2016;7(3):576S–89S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Dietary protein, carbohydrate, and fat enhance memory performance in the healthy elderly. Am J Clin Nutr. 2001;74(5):687–93. [DOI] [PubMed] [Google Scholar]
- 19. Jones EK, Sünram-Lea SI, Wesnes KA. Acute ingestion of different macronutrients differentially enhances aspects of memory and attention in healthy young adults. Biol Psychol. 2012;89(2):477–86. [DOI] [PubMed] [Google Scholar]
- 20. Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr. 2000;72(3):825–36. [DOI] [PubMed] [Google Scholar]
- 21. Chaplin K, Smith AP. Breakfast and snacks: Associations with cognitive failures, minor injuries, accidents and stress. Nutrients. 2011;3(5):515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wengreen H, Nelson C, Munger RG, Corcoran C. Prospective study of ready-to-eat breakfast cereal consumption and cognitive decline among elderly men and women. J Nutr Health Aging. 2011;15(3):202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jia J, Wang F, Wei C, Zhou A, Jia X, Li F, Tang M, Chu L, Zhou Y, Zhou Cet al. . The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014;10(1):1–9. [DOI] [PubMed] [Google Scholar]
- 24. Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer report 2015-The global impact of dementia: An analysis of prevalence, incidence, cost and trends. London, UK: Alzheimer's Disease International; 2015. [Google Scholar]
- 25. Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: The China Health and Nutrition Survey–Monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol. 2010;39(6):1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang B, Zhai FY, Du SF, Popkin BM. The China Health and Nutrition Survey, 1989–2011. Obes Rev. 2014;15:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du S, Batis C, Wang H, Zhang B, Zhang J, Popkin BM. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr. 2014;99(2):334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Wang G, Pan X. China food composition. Beijing, China:Peking Medical University; 2009. [Google Scholar]
- 29. Yao M, McCrory MA, Ma G, Tucker KL, Gao S, Fuss P, Roberts SB. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am J Clin Nutr. 2003;77(6):1409–16. [DOI] [PubMed] [Google Scholar]
- 30. He K, Du S, Xun P, Sharma S, Wang H, Zhai F, Popkin B. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am J Clin Nutr. 2011;93(6):1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plassman BL, Welsh KA, Helms M, Brandt J, Page WF, Breitner JC. Intelligence and education as predictors of cognitive state in late life: A 50-year follow-up. Neurology. 1995;45(8):1446–50. [DOI] [PubMed] [Google Scholar]
- 32. Lei X, Hu Y, McArdle JJ, Smith JP, Zhao Y. Gender differences in cognition among older adults in China. J Hum Resour. 2012;47(4):951–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strauss J, Lei X, Park A, Shen Y, Smith JP, Yang Z, Zhao Y. Health outcomes and socio-economic status among the elderly in Gansu and Zhejiang Provinces, China: Evidence from the CHARLS pilot. J Popul Ageing. 2010;3(3–4):111–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR Jr., Schmitz KH, Emplaincourt POet al. . Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl 9):S498–504. [DOI] [PubMed] [Google Scholar]
- 35. Willet W. Nutritional epidemiology: 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 36. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper Cet al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–16.. doi:10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sievert K, Hussain SM, Page MJ, Wang Y, Hughes HJ, Malek M, Cicuttini FM. Effect of breakfast on weight and energy intake: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;364:142. doi: 10.1136/bmj.l42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98. [DOI] [PubMed] [Google Scholar]
- 39. Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18(3):248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilbert JA, Bendsen NT, Tremblay A, Astrup A. Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis. 2011;21:B16–31. [DOI] [PubMed] [Google Scholar]
- 41. Fernando W, Rainey-Smith SR, Gardener SL, Villemagne VL, Burnham SC, Macaulay SL, Brown BM, Gupta VB, Sohrabi HR, Weinborn Met al. . Associations of dietary protein and fiber intake with brain and blood amyloid-β. J Alzheimers Dis. 2018;61(4):1589–98. [DOI] [PubMed] [Google Scholar]
- 42. Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, Maruff P. Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: A meta-analysis. Alzheimers Dement. 2017;6:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ding B, Xiao R, Ma W, Zhao L, Bi Y, Zhang Y. The association between macronutrient intake and cognition in individuals aged under 65 in China: A cross-sectional study. BMJ Open. 2018;8(1):e018573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao GY, Li M, Han L, Tayie F, Yao SS, Huang Z, Ai P, Liu YZ, Hu YH, Xu B. Dietary fat intake and cognitive function among older populations: A systematic review and meta-analysis. J Prevent Alzheimers Dis. 2019;6(3):204–11. [DOI] [PubMed] [Google Scholar]
- 45. Chlebowski RT, Rapp S, Aragaki AK, Pan K, Neuhouser ML, Snetselaar LG, Manson JE, Wactawski-Wende J, Johnson KC, Hayden Ket al. . Low-fat dietary pattern and global cognitive function: Exploratory analyses of the Women's Health Initiative (WHI) randomized dietary modification trial. EClinicalMedicine. 2020;18:100240. doi: 10.1016/j.eclinm.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. [DOI] [PubMed] [Google Scholar]
- 47. Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, Björck I, Brand-Miller JC, Brighenti F, Buyken AEet al. . Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research uses data from the China Health and Nutrition Survey (CHNS). Data described in the manuscript, code book, and analytic code are made publicly and freely available without restriction at https://www.cpc.unc.edu/projects/china.