Abstract
Purpose
To investigate, in a meta-analysis, the frequency of pulmonary embolism (PE) in patients with COVID-19 and whether D-dimer assessment may be useful to select patients for computed tomography pulmonary angiography (CTPA).
Methods
A systematic literature search was performed for original studies which reported the frequency of PE on CTPA in patients with COVID-19. The frequency of PE, the location of PE, and the standardized mean difference (SMD) of D-dimer levels between patients with and without PE were pooled by random effects models.
Results
Seventy-one studies were included. Pooled frequencies of PE in patients with COVID-19 at the emergency department (ED), general wards, and intensive care unit (ICU) were 17.9% (95% CI: 12.0–23.8%), 23.9% (95% CI: 15.2–32.7%), and 48.6% (95% CI: 41.0–56.1%), respectively. PE was more commonly located in peripheral than in main pulmonary arteries (pooled frequency of 65.3% [95% CI: 60.0–70.1%] vs. 32.9% [95% CI: 26.7–39.0%]; OR = 3.540 [95% CI: 2.308–5.431%]). Patients with PE had significantly higher D-dimer levels (pooled SMD of 1.096 [95% CI, 0.844–1.349]). D-dimer cutoff levels which have been used to identify patients with PE varied between 1000 and 4800 μg/L.
Conclusion
The frequency of PE in patients with COVID-19 is highest in the ICU, followed by general wards and the ED. PE in COVID-19 is more commonly located in peripheral than in central pulmonary arteries, which suggests local thrombosis to play a major role. D-dimer assessment may help to select patients with COVID-19 for CTPA, using D-dimer cutoff levels of at least 1000 μg/L.
Key Points
• The frequency of PE in patients with COVID-19 is highest in the ICU, followed by general wards and the ED.
• PE in COVID-19 is more commonly located in peripheral than in central pulmonary arteries.
• D-dimer levels are significantly higher in patients with COVID-19 who have PE.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-021-08003-8.
Keywords: Coronavirus, Pulmonary embolism, Coagulation, Tomography, Diagnosis
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic has caused dramatic effects on society. On March 21, 2021, more than 122 million people have been infected and more than 2.7 million people have died of the disease worldwide [1]. Although approximately 80% of patients with COVID-19 have a favorable clinical course without hospitalization [2], approximately 20% of patients experiences severe respiratory disease [2]. A high incidence of thromboembolic events, including pulmonary embolism (PE), has been observed in COVID-19, which suggests that COVID-19 may induce intravascular coagulopathy [3–6]. PE may be a direct cause of death in patients with COVID-19, despite the use of antithrombotic prophylaxis [4, 6, 7]. Patients with COVID-19 who experience PE should be managed in a timely manner with therapeutic doses of anticoagulant therapy [8]. Computed tomography pulmonary angiography (CTPA) is the preferred imaging modality to detect PE [9]. To date, the frequency of PE in patients with COVID-19 is not completely clear. As such, it is still unclear which patients should undergo CTPA to detect PE. Unfortunately, clinical pretest probability scores, such as the Wells criteria [10], are unreliable to predict the occurrence of PE in patients with COVID-19 [11–14]. It has been suggested that assessment of D-dimer levels may help to improve risk stratification for PE [5, 15], but the exact value is also not completely clear. In order to overcome these gaps in knowledge, it was our purpose to investigate, in a meta-analysis, the frequency of PE in patients with COVID-19, and whether D-dimer assessment may be useful to select patients with COVID-19 for CTPA.
Materials and methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline [16]. Institutional review board approval was not applicable.
Study retrieval and selection
MEDLINE and Embase were searched using the following search string: (Corona OR Coronavirus OR Covid-19 OR SARS-Cov-2 OR 2019nCoV OR Wuhan-virus) AND (Computed tomography OR Computerized tomography OR Computed tomographic OR CT OR CAT OR CTPA) AND (pulmonary embolism OR PE OR pulmonary thromboembolism OR PTE OR pulmonary thrombosis). Furthermore, the journal Radiology: Cardiothoracic Imaging was manually searched for relevant studies (articles published by this journal were not listed yet in MEDLINE and Embase). The search was updated until March 14, 2021. Bibliographies of studies which were included in our meta-analysis were screened for potentially suitable references.
Original studies which reported the frequency of PE on CTPA scans performed in at least 10 patients with COVID-19 (regardless of PE frequency) were eligible for inclusion. Review articles, abstracts, and studies involving fewer than 10 patients were excluded. Using these selection criteria, titles and abstracts of studies were reviewed. The full text versions of potentially relevant studies were then reviewed to determine whether studies could be included in our meta-analysis. Bibliographies of included studies were screened for other potential relevant studies. Two reviewers (R.M.K. and H.J.A.A.) independently performed the study selection. Any discrepancies were solved by consensus with a third reviewer (T.C.K.).
Study data extraction and study quality assessment
Main study characteristics (country of origin, patient inclusion period, number of patients, age and gender of patients, indication for CTPA, use of antithrombotic prophylaxis before CTPA, and CT interpreter(s)) were extracted for each included study. The proportions of patients with and without PE were extracted. If reported, data were extracted for patients with COVID-19 who presented at the emergency department (ED), for patients with COVID-19 who had been admitted to general wards, and for patients with COVID-19 who had been admitted to the intensive care unit (ICU). Furthermore, we extracted the association between severity of COVID-19 at chest CT and PE, if reported by the included studies. We also extracted the locations of PEs (i.e., main, lobar, segmental, and subsegmental pulmonary arteries) on a per-patient basis if reported by the included studies. In addition, for studies which reported D-dimer levels for patients with and without PE, we extracted the mean values and standard deviations (SDs). Corresponding authors of studies which did not report mean values and SDs were contacted and requested to provide these values. We also extracted sensitivity and specificity values for different D-dimer cutoff levels, if reported by the included studies.
Study quality aspects were assessed by two independent reviewers (R.M.K. and H.J.A.A.) using items from the Newcastle-Ottawa quality assessment scale [17] which were adapted to our meta-analysis (Table 1). Any discrepancies were solved by consensus with a third reviewer (T.C.K.).
Table 1.
Criteria to evaluate the quality of included studies
| Quality items | Signaling questions |
|---|---|
| Type of cohort study | Does the study have a prospective or retrospective design? |
| Method of patient selection | Was a consecutive, randomly selected, or obviously representative series of patients included? |
| Patient spectrum | Were selection criteria for CTPA reported? |
| Blinded assessment of outcome | Were CTPA interpreters blinded to clinical information (i.e., study purpose or COVID-19 status of patients)? |
Statistical analyses
The frequencies of PE in patients with COVID-19 were determined for each included study and pooled with a random effects model. For studies which reported the frequency of PE in both patients with COVID-19 and those without COVID-19, differences were assessed by a chi-square test [18]. The frequencies of central (main and lobar) and peripheral (segmental and subsegmental) PEs were also pooled with a random effects model and the odds ratio (OR) of peripheral vs. central PEs was calculated. Differences in D-dimer levels between patients with COVID-19 vs. those without PE were assessed by calculating the standardized mean difference (SMD). The pooled SMD was estimated by a random effects model. Heterogeneity was tested by the I2 statistic [19]. If significant heterogeneity was present (defined as I2 > 40% [20]), subgroup analyses were performed to explore potential sources of heterogeneity. Predefined covariates were “indication for CTPA” (study reported that was CTPA only performed if PE was clinically suspected vs. study reported that CTPA was performed for triaging or on a routine basis, but not necessarily because of clinically suspected PE), “use of antithrombotic prophylaxis before CTPA” (100% vs. < 100% of included patients who used antithrombotic prophylaxis before CTPA), and “way of CTPA interpretation” (blinding vs. no blinding of CTPA interpreters to clinical information). Statistical analyses were performed using the Open Meta-Analyst software package [21], and MedCalc Statistical Software (MedCalc Software) [22]. p values < 0.05 were considered statistically significant for all analyses.
Results
Study retrieval and selection
The study selection process is summarized in Fig. 1. After screening titles and abstracts, 130 potentially relevant studies remained and were retrieved in full text. After reviewing the full text, 52 studies were excluded because there was no reporting of PE frequency data with respect to the number of CTPA scans performed in patients with COVID-19, 6 studies were excluded because these studies did not allow separate data extraction of patients with and without COVID-19, 2 studies were excluded because they comprised fewer than 10 patients with COVID-19, and 1 study was excluded because in this study PE was also determined based on clinical grounds rather than by CTPA only. Two additional references were found by screening bibliographies of remaining studies. Finally, 71 studies [11, 23–92], which comprised a total of 8086 patients with COVID-19 who underwent CTPA to evaluate for PE (median of 55 patients per study, range 10–1240), were included in our meta-analysis. Main study characteristics are displayed in Table 2.
Fig. 1.
Flow diagram of the study selection process
Table 2.
Main characteristics of the included studies
| Study | Country | Inclusion period (2020) | -Number of patients with COVID-19 who underwent CTPA -Age -Gender |
Selection criteria for CTPA | Antithrombotic prophylaxis before CTPA (% of all included patients) | CTPA interpreter(s) |
|---|---|---|---|---|---|---|
| Alharthy et al [23] | Saudi Arabia | May |
-25 -NR -NR |
Resistant hypoxemia | All patients (100%) | NR |
| Alonso-Fernández et al [24] | Spain | April 6–April 17 |
-30 -Median 64.5 years (IQR 55.8–71.3) -19 males |
Suspected PE | 26 patients (86.7%) | An expert radiologist |
| Artifoni et al [25] | France | March 25–April 10 |
-34 -NR -NR |
Suspected PE | All patients (100%) | NR |
| Baccellieri et al [26] | Italy | April 2–April 18 |
-87 -NR -NR |
NR | All patients (100%) | NR |
| Bellmunt-Montoya et al [27] | Spain | April 2020 |
-38 -NR -NR |
Sudden respiratory or cardiovascular deterioration and signs of pulmonary hypertension, right ventricular dilatation or dysfunction on transthoracic echocardiography | NR | NR |
| Benito et al [28] | Spain | March 9–April 15 |
-76 -Median 60–66 years -51 males |
Patients whose partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2:FiO2) ratio worsened or failed to improve, associated with an increasing or persistently high D-dimer level (> 3,000 ng/mL) and/or hemodynamic deterioration or other “classic” symptoms of PE, such as pleuritic chest pain, hemoptysis, syncope, and/or signs of right ventricular strain. | All patients (100%) | NR |
| Birk et al [29] | UK | March 25–April 30 |
-48 -NR -NR |
All patients underwent CTPA for COVID-19 triage | No patients (0%) | Consultant radiologists specialized in body imaging |
| Bompard et al [30] | France | March 1–April 16 |
-135 -Median 64 years (IQR 54–76) -94 males |
In case of doubt between COVID-19 pneumonia and PE, after clinical probability assessment and D-dimer assessment | All patients 100%) | Two experienced radiologists |
| Brüggemann et al [31] | The Netherlands | April 6–May 3 |
-60 -Mean 68 years (± 11.7) -42 males |
Respiratory deterioration or clinical suspicion of PE | 23 patients (38.3%) | Attending chest radiologist |
| Cavagna et al [32] | Italy | March 20–May 3 |
-101 -Mean 64.1 years (± 15.0) -82 males |
Sudden onset of clinical deterioration with unexplained worsening of dyspnea, symptoms suggestive for PE, D-dimer elevation, or in case of mismatch between clinical worsening and chest radiograph stability | All patients (100%) | Two radiologists with > 5 years, and > 20 years of experience in chest imaging, in consensus |
| Cerda et al [33] | Spain | March 1–April 24 |
-92 -Mean 68.1 years (± 13.2) -68 males |
New or worsening dyspnea or oxygen desaturation, syncope or hemodynamic instability, or chest pain |
All patients (100%) | Two expert thoracic radiologists |
| Chen J et al [34] | China | January–February |
-25 -Median 65 years (range 36–78) -15 males |
Elevated D-dimer level or accompanying symptom(s), including chest pain, hemoptysis, and dyspnea | NR | Two radiologists experienced in thoracic radiology with 20 and 22 years of experience |
| Contou et al [35] | France | March 13–April 24 |
-26 -Mean 63 years -22 males |
Sudden circulatory (introduction or significant increase of the dose of vasopressor) or/and respiratory (significant increase of FiO2 requirement) worsening with no obvious explanation such as ventilatory associated pneumonia or other source of sepsis | NR | NR |
| Darwish et al [36] | Saudi Arabia | May 1–July 14 |
-25 -Mean 49 years (± 11) -NR |
Suspected PE | NR | NR |
| De Cobelli et al [37] | Italy | March 29–April 9 |
-55 -Median 62 years (IQR 56–71) -39 males |
Suspected PE | NR | Two radiologists experienced in thoracic imaging, with 28 and five years of experience |
| Espallargas et al [38] | Spain | March 18–April 11 |
-47 -Median 65 years (range 30–94) -30 males |
Suspected PE | 36 patients (76.6%) | Radiologists with 12 and 29 years of experience |
| Fang et al [39] | UK | March 23–April 19 |
-93 -Median 57 and 62 years -60 males |
NR | NR | Two radiologists with 4 years of experience in cancer imaging and with 6 years of experience in thoracic imaging |
| Fauvel et al [40] | France | February 26–April 20 |
-1240 -Mean 64 years (± 17) -721 males |
If supplementary oxygen was needed in COVID-19 patients with limited disease extension, or when unenhanced CT findings could not explain the severity of respiratory failure. | 837 patients (67.5%) | A senior radiologist |
| Freund et al [41] | France, Spain, Belgium, Italy, Chile, and Canada | February 1–April 10 |
-974 -NR -NR |
Suspected PE | NR | NR |
| García-Ortega et al [42] | Spain | March 8–April 25 |
-73 -Mean 65.4 years (± 16) -52 males |
Age ≥ 18 years and elevated D-dimer levels | 68 patients (93.2%) | Two independent experienced thoracic radiologists |
| Gervaise et al [43] | France | March 14–April 6 |
-72 -Mean 62.3 years (range 22–92) -54 males |
Mainly based on a worsening of the patient’s clinical condition with new onset of dyspnea, desaturation, or chest pain and also an increase in D-dimer levels | NR | Two radiologists with 10 and 12 years of experience in thoracic imaging |
| Grillet et al [44] | France | March 15–April 14 |
-100 -Mean 66 years (± 13) -70 males |
Patients with severe clinical features of COVID-19 infection | NR | Two chest radiologists with 11 and 6 years of experience |
| Grillet et al [45] | France | March 16–April 22 |
-85 -Mean 65 years (± 13) -55 males |
Clinical signs of severe grade infection were present (oxygen saturation below 92%, polypnea over 25 cycles per minute, fever > 40 °C, increasing oxygen needs), need for invasive mechanical ventilation, or when the patient suffered from comorbidities of active neoplasia, immunosuppression, history of organ or bone-marrow transplantation. | NR | Two chest radiologists with 11 and 2 years of experience in chest imaging |
| Hamadé et al [46] | France | March 25–April 8 |
-12 -NR -NR |
Suspected PE | NR | NR |
| Hammer et al [47] | USA | March 1–May 1 |
-17 -NR -NR |
NR | All patients (100%) | NR |
| Helms et al [48] | France | March 3–March 31 |
-99 -NR -NR |
Based on clinical parameters (worse PaO2/FiO2 despite inhaled nitric oxide or after prone positioning or hemodynamic impairment requiring fluid challenge and/or increased norepinephrine infusion rate, dilated right ventricle-even without acute cor pulmonale) or evolution of laboratory parameters (a rapid elevation of D-dimer levels despite anticoagulation) | All patients (100%) | Consultant radiologists specialized in emergency radiology |
| Ippolito et al [49] | Italy | March 5–April 24 |
-170 -Mean 63 years (± 12) -116 males |
Chest pain, worsening of respiratory symptoms, irregular or new-onset rapid heartbeat, worsening of fever, aggravation of arterial blood gas parameters, and a marked increase over time of D-dimer and/or fibrinogen values | NR |
A radiologist with 15 years of experience in chest imaging and a resident radiologist with 4 years of experience, in consensus |
| Jalaber et al [50] | France | March 26–April 17 |
-70 -Mean 65 years (range 21–97) -44 males |
All patients suspected of COVID-19 | NR | Experienced chest radiologist |
| Jevnikar et al [51] | France | April 15–May 23 |
-106 -Median 63 years (range 53–82) -48 males |
All adult patients with a diagnosis of COVID-19 at the time of hospital admission | NR | A senior radiologist and a pulmonologist |
| Kaminetzky et al [52] | USA | March 13–April 5 |
-62 -Mean 57.8 years (range 28–89) -40 males |
Hypoxia in 17, respiratory distress in 16, elevated D-dimer in 14, tachycardia in 7, chest pain in 4, extremity swelling in 1, and 3 had an indication not specified above | 25 patients (40.3%) | Two board-certified thoracic radiologists with 16 and 22 years of experience in thoracic imaging |
| Khan et al [53] | UK | April 20–May 13 |
-13 -NR -NR |
NR | > 10 patients (> 76.9%) | NR |
| Kirsch et al [54] | USA | February 1–July 15 |
-64 -Mean 55 years (± 16) -35 males |
NR | NR | NR |
| Lang et al [55] | USA | March 23–April 6 |
-48 -Mean 58 years (± 19) -25 males |
NR | NR | Two thoracic radiologists with 11 years and 2 years of thoracic imaging subspecialty experience |
| Larsen et al [56] | France | March 11–April 20 |
-35 -Median 66 years (IQR 56–78) -27 males |
Hypoxemic pneumonia (pneumonia requiring oxygen supplementation to achieve oxyhemoglobin saturation > 94%) | 28 patients (80%) | Two radiologists and at least two pulmonologists |
| Lee et al [57] | USA | March 20–May 3 |
-86 -NR-NR |
NR | NR | NR |
| Lodigiani et al [59] | Italy | February 13–April 10 |
-30 -NR -NR |
NR | NR | NR |
| Loffi et al [60] | Italy | February 22–May 15 |
-333 -Median 67 years (IQR 57–77) -211 males |
Inadequate clinical response to high oxygen flow therapy, elevated D-dimer levels, or signs of right ventricle dysfunction at echocardiography | 223 patients (67%) | One senior radiologist |
| Léonard-Lorant et al [58] | France | March 1–March 31 |
-106 -Median 64 years -70 males |
Suspicion of PE in 67 and other indication in 39 | NR | A single reader |
| Mak et al [61] | UK | March–May |
-51 -Mean 45 years (range 26–66) -38 males |
All patients receiving ECMO | NR | Two cardiothoracic radiologists in consensus (7 and 9 years of experience) |
| Martini et al [63] | Switzerland | February–April |
-38 -Median 59 years (range 32–89) -18 males |
Clinical signs and symptoms of deep vein thrombosis, tachypnea, decreased oxygen saturation, or high oxygen demand | 8 patients (21.1%) | Two radiologists |
| Martínez Chamorro et al [62] | Spain | March 15–April 30 |
-342 -Mean 62.4 years (± 16.8) -58 males |
Clinical deterioration with the appearance or worsening of dyspnea, desaturation, chest pain, and elevated D-dimer. | NR | A third or fourth year radiology resident, supervised by at least one radiologist from the emergency department or from the chest section, with at least 15 years of experience. Discrepancies were resolved by consensus between two of the more experienced radiologists. |
| Meiler et al [64] | Germany | March 1–April 20 |
-50 -Mean 60.4 years (± 10.1) -34 males |
NR | NR | Two junior radiologists with subspeciality training in thoracic radiology, and A senior thoracic radiologist (for equivocal cases) |
| Mestre-Gómez et al [65] | Spain | March 30–April 12 |
-91 -Median 65 years -62 males |
Respiratory deterioration not attributable to other causes, data on acute respiratory distress without improvement despite specific treatment or elevation of D-dimer levels in discordance with other inflammatory parameters |
23 of 29 patients with PE (≥ 25.3%) | NR |
| Minuz et al [66] | Italy | March 30–April 6 |
-10 -NR -NR |
Persistent respiratory impairment and a D-dimer value at least five times the upper reference limit. | NR | NR |
| Mirsadraee et al [68] | UK | March 19–June 23 |
-72 -Mean 52 years (± 10) -53 males |
Routine in all patients who are admitted to ICU |
12 patients (16.7%) |
Two consultant cardiothoracic radiologists, with disagreements resolved by consensus. |
| Miró et al [67] | Spain and France | March 6–April 15 |
-320 -NR -NR |
PE suspected based on patient signs and symptoms | NR | NR |
| Moll et al [69] | USA | March 7–April 13 |
-25 -NR -NR |
NR | NR | NR |
| Monfardini et al [70] | Italy | March 1–March 31 |
-34 -NR -NR |
Sudden oxygen desaturation coupled with a moderate to high risk of PE according to the Wells score and D-dimer levels | 8 patients (23.5%) | Two experienced thoracic radiologist with 15 and 20 years of experience |
| Mouhat et al [71] | France | March 15–April 16 |
-162 -Mean 65.57 years (± 13.00) -109 males |
Oxygen saturation measured by pulse oximetry ≤ 93% in room air, breathing rate of ≥ 30 breaths/minute or rapid clinical worsening | 141 patients (87.0%) | Two chest radiologists |
| Mueller-Peltzer et al [72] | Germany | March 8–April 15 |
-16 -Mean 62.2 years (range 47–77) -10 males |
When likelihood of PE was considered high | 4 patients (25%) | Two radiologists with 6 and 13 years of experience in thoracic radiology |
| O'Shea et al [73] | USA | March 17–April 6 |
-94 -NR -NR |
NR | NR | Radiologist with 7 years of experience in cardiovascular imaging |
| Ooi et al [74] | UK | March 1–April 30 |
-84 -Mean 59.8 years, SD 16.59 -42 males |
High D-dimer level (36), shortness of breath (29), hypoxia or increasing oxygen requirement (27), chest pain, discomfort or tightness (25), hemoptysis (7), tachycardia (6), hypotension (5), abnormal ECG changes (5), fever (4), following beside echocardiogram (3), high Wells score (3), intubated and ventilated (5), not improving on extracorporeal membrane oxygenation (ECMO) (3), recent travel (2) |
NR | NR |
| Parzy et al [75] | France | March 18–May 5 |
-13 -Median 50 years (IQR 43–62) -9 males |
Routinely after veno-venous ECMO retrieval | All patients (100%) | NR |
| Patel et al [76] | UK | March 17–April 10 |
-39 -Median 52.5 years (range 29–79) -32 males |
NR | All patients (100%) | Two thoracic radiologists of 14 and 24 years of experience |
| Planquette et al [78] | France | March 1–April 20 |
-269 -Media 63 years (IQR 53–79) -33 males |
Suspected PE | NR | NR |
| Poissy et al [79] | France | February 27–March 31 |
-34 -Median 57 years (range 29–80) -13 males |
Suspected PE | 20 of 22 patients with PE (≥ 58.8%) | NR |
| Poyiadji et al [80] | USA | March 16–April 18 |
-328 -Mean 61.3 years -140 males |
NR | 122 of 328 patients (37.1%) | Thoracic, abdominal, or emergency radiologists, all with 2-40 years of experience |
| Pérez Dueñas et al [77] | Spain | March 23–April 8 |
-81 -Mean 64 years -64 males |
Clinical suspicion of PE due to presence of sudden dyspnea, chest pain, hemoptysis, respiratory failure severe not corrected with high O2 flow, and/or D-dimer level > 500 ng/mL | NR | Two expert radiologists in thromboembolic lung disease with > 15 years of experience |
| Rali et al [81] | USA | April 1–April 27 |
-49 -NR -NR |
High index of clinical suspicion | All patients (100%) | NR |
| Ramadan et al [82] | USA | March 1–June 1 |
-367 -Mean 59.7 years -145 males |
NR | NR | NR |
| Schiaffino et al [83] | Italy | March 1–April 30 |
-45 -Median 67 years (IQR 60–76) 34 males |
Presence of lower-limb deep vein thrombosis at ultrasound Doppler examination, onset or worsening of dyspnea, and worsening or less-than-expected improvement of the PaO2/FiO2 ratio. | All patients (100%) |
A radiologist with 15 years of experience in body and chest CT |
| Scialpi et al [84] | Italy | March–May |
-10 -NR -NR |
Clinical and laboratory data which were suspicious for PE | NR | Two radiologists with at least 25 years of experience in chest CT |
| Shahin et al [85] | UK | NR |
-10 -Mean 70 years (± 16) -6 males |
Suspected acute PE based on clinical assessment and elevated D-dimer | NR | NR |
| Taccone et al [86] | Belgium | March 10–April 20 |
-40 -Mean 61 years (range 57–66) -28 males |
NR | 22 patients (55.0%) | One radiologist |
| Thomas et al [87] | UK | March 15–NR |
-11 -NR -NR |
Clinical suspicion (e.g., unexplained hypotension or hypoxia felt disproportionate to the pneumonia) | All patients (100%) | NR |
| Tung-Chen et al [88] | Spain | March–April |
-51 -Mean 61.4 years (± 17.7) -28 males |
Suspected PE | NR | Two radiologist trainees with 2-4 years of experience, under the supervision of a senior radiologist with more than 10 y of experience |
| Ventura-Díaz et al [89] | Spain | March 1–April 30 |
-242 -Median 68 years (IQR 55–78) -151 males |
Suspected PE | NR | NR |
| Vlachou et al [90] | UK | March 23–April 5 |
-39 -Mean 62.3 years (± 15) -52 males |
Increasing oxygen requirements or refractory hypoxia, not improving on oxygen, elevated D-dimer, or tachycardia |
NR | NR |
| Whyte et al [11] | UK | March 3–May 7 |
-214 -Mean 61.1 years -129 males |
Patients with suspected PE undergo a two-level PE Wells score. Imaging is not undertaken for those considered “PE unlikely” by the Wells rule (score < 4) in conjunction with a D-dimer level < 500 ng/mL | 206 patients (96.3%) | NR |
| Zhang et al [91] | UK | March 3–May 2 |
-43 -Median 46 years (IQR 35.5–52.5) -33 males |
All patients admitted for veno-venous ECMO | All patients without hemorrhagic complications (≈ 100%) | NR |
| Zotzmann et al [92] | Germany | March 8–May 31 |
-20 -Mean 61.6 years (± 9.9) -14 males |
All patients with ARDS and SARS-CoV2 infection |
5 patients (25%) | NR |
IQR interquartile range, NR not reported
Study quality
Details with regard to individual study quality are displayed in Supplemental Table 1. Eight studies (11.3%) had a prospective design, whereas 58 included studies (81.7%) had a retrospective design, whereas in 5 studies (7.0%) it was not reported whether the study design was prospective or retrospective. All but one of the included studies consecutively or randomly selected patients, or obviously comprised a representative series of patients. In 55 studies (77.5%), patient selection criteria for CTPA were reported, in 15 studies (21.1%), patient selection criteria for CTPA were not reported, whereas in 1 study (1.4%), patient selection criteria for CTPA were only reported for a subset of patients. CTPA interpreters were blinded to clinical information in 15 studies (21.1%), and unblinded in 2 studies (2.8%) whereas this was not clear (not reported) in the remaining 54 studies (76.1%).
Frequency of PE in patients with COVID-19
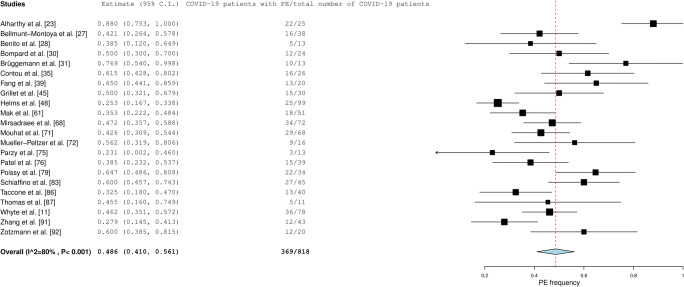
Pooled frequency of PE in all included patients with COVID-19 was 32.1% (95% confidence interval [CI]: 28.5–35.9%). Pooled frequency of PE was lowest in patients who presented at the ED (17.9% [95% CI: 12.0–23.8%]) (Fig. 2), followed by patients who had been admitted to general wards (23.9% [95% CI: 15.2–32.7%] (Fig. 3). In patients with COVID-19 who had been admitted to the ICU, pooled frequency of PE was highest (48.6% [95% CI: 41.0–56.1%]) (Fig. 4).
Fig. 2.
Frequency of PE in patients with COVID-19 who presented at the ED
Fig. 3.
Frequency of PE in patients with COVID-19 who had been admitted to general wards
Fig. 4.
Frequency of PE in patients with COVID-19 who had been admitted to the ICU
Significant heterogeneity was present across the included studies (I2 ≥ 80%). No potential sources of heterogeneity were identified (I2 > 85%) for subgroups according to “indication for CTPA,” “use of antithrombotic prophylaxis before CTPA,” and “way of CTPA interpretation.” In two studies which routinely performed CTPA at the ED (regardless of clinical suspicion of PE), PE frequencies in COVID-19 patients were 2.1% (1/48) and 5.7% (4/70), respectively [29, 50]. In two other studies which routinely performed CTPA at the ICU (regardless of clinical suspicion of PE), PE frequencies in COVID-19 patients were 47.2 % (34/72) and 60.0% (12/20), respectively studies [68, 92]. Six studies reported a significant association between severity of COVID-19 at chest CT and PE, whereas 13 studies did not find a significant association (Supplemental Table 2).
PE location
PE was more commonly located in peripheral than in main pulmonary arteries (pooled frequency of 65.3% [95% CI: 60.0–70.1%] vs. 32.9% [95% CI: 26.7–39.0%]; OR = 3.540 [95% CI: 2.308–5.431%]).
PE in patients with COVID-19 and association with D-dimer levels
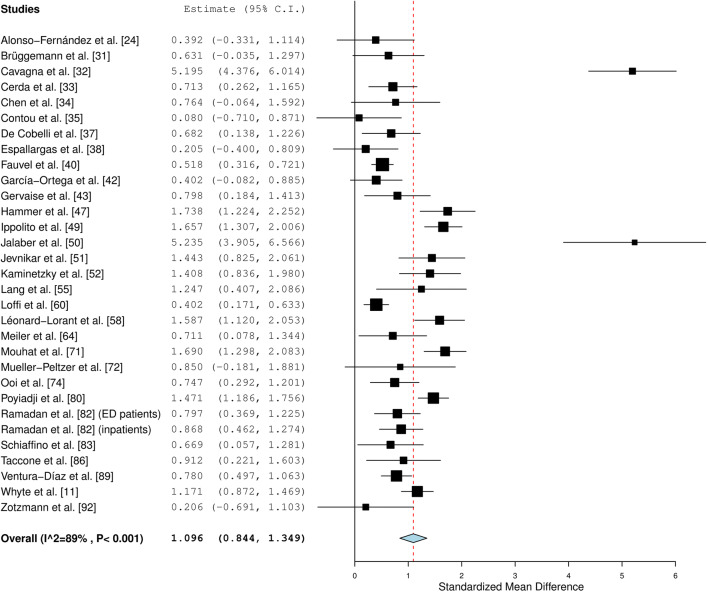
Patients with COVID-19 and PE had significantly higher D-dimer levels than patients with COVID-19 and no PE (pooled SMD of 1.096 [95% CI, 0.844–1.349]; I2 = 89%) (Fig. 5). Sensitivity and specificity values for different D-dimer cutoff levels are displayed in Table 3. D-dimer cutoff levels which have been used to identify patients with PE varied between 1000 and 4800 μg/L. All studies listed in Table 3 used the conventional D-dimer score. Only one study also used age-adjusted D-dimer cutoffs [93], yielding a sensitivity of 94% and a specificity of 35% [33].
Fig. 5.
Association between D-dimer levels and PE in patients with COVID-19
Table 3.
Sensitivity and specificity values for different D-dimer cutoff levels to diagnose PE
| Study | D-dimer cutoff level | Sensitivity | Specificity |
|---|---|---|---|
| Alonso-Fernandez et al [24] | 2500 μg/L | 80% | 51% |
| Cerda et al [33] |
2036 μg/L Age-adjusted cutoff levels |
75%^ 94%^ |
69%^ 35%^ |
| Kaminetzky et al [52] | 1394 μg/L | 95% | 71% |
| Léonard-Lorant et al [58] | 2660 μg/L | 100% | 67% |
| Loffi et al [60] | 2370 μg/L | 70% | 62% |
| Mouhat et al [71] | 2590 μg/L | 83% | 84% |
| Ooi et al [74] | 2247 μg/L | 72% | 74% |
| Planquette et al [78] | 1500 μg/L | 76% | 65% |
| Ramadan et al [82] |
2000 μg/L 1000 μg/L |
78%* 63%# 94%* 89%# |
67%* 66%# 30%* 23%# |
| Taccone et al [86] | 3647 μg/L | 75% | 92% |
| Ventura-Diaz et al [89] | 2903 μg/L | 81% | 59% |
| Whyte et al [11] | 4800 μg/L | 75% | 78% |
*ED patients
#Inpatients
^3 weeks after COVID-19 symptom onset
Discussion
This meta-analysis showed that the frequency of PE in COVID-19 was highest in patients who were in the ICU (pooled frequency of 48.6%), followed by patients who were in general wards (pooled frequency of 23.9%), and by patients who presented at the ED (pooled frequency of 17.9%). PE was more commonly located in peripheral than in main pulmonary arteries (pooled frequency of 65.3% vs. 32.9%). Patients with PE had significantly higher D-dimer levels than patients without PE.
Fifty-eight of the 71 included studies (81.7%) had a retrospective design. However, there was no evidence of selection bias, as all but one of the studies included a consecutive, randomly selected, or obviously representative series of patients. Selection criteria for CTPA were reported in the majority of included studies (77.5%), which benefits the generalizability of study results. In only 21.1% of included studies, it was reported that CTPA interpreters were blinded to clinical information. Non-blinding could have biased the results to either overcalling or undercalling PE frequency on CTPA.
The findings of our meta-analysis suggest that the frequency of PE in patients with COVID-19 increases with increasing disease severity (ICU > general wards and ED). This is supported by six studies which reported a significant association between severity of lung parenchymal abnormalities at CT and PE [32, 40, 44, 45, 71, 74]. However, such an association was not demonstrated in 13 other studies [24, 30, 31, 37, 42, 43, 49, 50, 52, 60, 62, 78]. Therefore, there are probably other COVID-19- and host-related factors that are associated with the occurrence of PE. Further studies are required to improve our understanding of the pathophysiology of PE in COVID-19. Furthermore, the observed frequency of PE depends on the selection criteria for CTPA. In the far majority of included studies, it was reported that CTPA was performed because of clinically suspected PE. In only two studies, CTPA was routinely performed at the ED (regardless of clinical signs of possible PE), with relatively low yields of only 2.1% and 5.7% [29, 50]. In two other studies which routinely performed CTPA in COVID-19 patients at the ICU (regardless of clinical signs of possible PE), PE frequencies were high: 47.2% and 60.0%, respectively [68, 92]. These findings in unselected samples of patients confirm that frequency of PE is higher in ICU patients compared to patients who present at the ED.
Our findings contrast those in patients from the general population without COVID-19, where PE has been reported to occur in main pulmonary arteries as frequent as or more frequently than in peripheral arteries [94–96]. Therefore, the underlying pathomechanisms may be different. The relatively high frequency of peripheral PE suggest that local thrombosis may play a more important role in the development of PE (or pulmonary artery thrombosis) in COVID-19 [37, 55, 97, 98] rather than the classic thromboembolism originating from the leg or pelvic veins in patients without COVID-19 [99]. This hypothesis is supported by in vivo chest CT studies, where vascular thickening, a potential sign of vascular inflammation, endothelial damage, and microthrombosis [55], is observed in most symptomatic patients with COVID-19 [100]. Pathological studies in patients with COVID-19 also confirm the local immunothrombosis hypothesis [97, 98]. Accordingly, the term MicroCLOTS (microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome) has been coined as a new name for severe pulmonary COVID-19, in which alveolar viral damage is followed by an inflammatory reaction and by microthrombosis [97, 101–103]. It may become obsolete to call this pathophysiological disorder PE.
Subgroup analysis did not indicate that the use of antithrombotic prophylaxis was associated with a lower frequency of PE in patients with COVID-19. This implies that physicians should remain alert for the occurrence of PE even in patients who receive antithrombotic prophylaxis. D-dimer levels were found to be significantly higher in patients with PE (pooled SMD of 1.096), which indicates that a rise in D-dimer levels is not only a marker of pneumonia severity but is also associated with a higher risk of PE. Therefore, D-dimer assessment may help to decide which patients with COVID-19 should undergo CTPA to detect PE. However, there is no uniformly accepted D-dimer threshold to discriminate COVID-19 patients with and without PE. Twelve studies used different D-dimer cutoff levels (varying between 1000 and 4800 μg/L), yielding sensitivity and specificity values which varied between 63–100% and 23–84%, respectively [11, 24, 33, 52, 58, 60, 71, 74, 78, 82, 86, 89]. These D-dimer cutoff levels were at least twice as high compared to the conventional D-dimer cutoff level of 500 μg/L, which is usually employed in the general population as a screening test for venous thromboembolism [104, 105]. In non-COVID-19 patients aged 50 or more, the application of age-adjusted D-dimer cutoffs has shown to increase specificity without modifying sensitivity [106]. Only one of the studies included in our meta-analysis also used age-adjusted D-dimer cutoffs, yielding high sensitivity (94%) but poor specificity (35%) [33]. More research is needed to investigate whether the use of age-adjusted D-dimer cutoffs can improve the clinical utility of D-dimer testing in patients with COVID-19.
Our study has some limitations. First, in the far majority of included studies, CTPA was only performed in case of clinically suspected PE. Therefore, the true prevalence of PE in patients with COVID-19 remains to be elucidated. Second, due to incomplete and unstandardized reporting, we could not adjust the frequency of PE for well-known risk factors for PE (such as cancer, history of previous venous thromboembolism, duration of hospitalization, obesity, and cardiovascular disease [107]) and type and dosage of antithrombotic prophylaxis. Third, there was a great deal of heterogeneity in the patient population and the indication for CTPA in each included study. Although we attempted to group the studies into ED, general wards, and ICU patients, this delineation may be problematic due to the unpredictable course of COVID-19 and the fact that a patient discharged from the ED could become an ICU ARDS patient within a matter of a week. Furthermore, statistical heterogeneity still remained in each of these groups. Fourth, PE was determined by CTPA, which has a good but not perfect sensitivity in PE detection [9]. Although they may be clinically less relevant [108], smaller subsegmental PEs may have been missed by CTPA. This could have resulted in an underestimation of PE frequency.
In conclusion, the frequency of PE in patients with COVID-19 is highest in the ICU, followed by general wards and the ED. PE in COVID-19 is more commonly located in peripheral than in central pulmonary arteries, which suggests local thrombosis to play a major role. D-dimer assessment may help to select patients with COVID-19 for CTPA, using D-dimer cutoff levels of at least 1000 μg/L.
Supplementary Information
(DOCX 36 kb)
Abbreviations
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- CTPA
Computed tomography pulmonary angiography
- ED
Emergency department
- ICU
Intensive care unit
- OR
Odds ratio
- PE
Pulmonary embolism
- SD
Standard deviation
- SMD
Standardized mean difference
Funding
The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is Robert Kwee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
The authors have significant statistical expertise.
Informed consent
Written informed consent was not required for this study because of the meta-analysis.
Ethical approval
Institutional Review Board approval was not required because of the meta-analysis.
Methodology
• Multicentre study
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available via https://coronavirus.jhu.edu/. Accessed 21 Mar 2021
- 2.World Health Organization Coronavirus disease (COVID-19) Available via https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19. Accessed 21 Mar 2021
- 3.(2020) COVID-19 and vascular disease. EBioMedicine 58:102966 [DOI] [PMC free article] [PubMed]
- 4.Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudkerk M, Buller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297:E216–E222. doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benzakoun J, Hmeydia G, Delabarde T, et al. Excess out-of-hospital deaths during the COVID-19 outbreak: evidence of pulmonary embolism as a main determinant. Eur J Heart Fail. 2020;22:1046–1047. doi: 10.1002/ejhf.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIH COVID-19 Treatment Guidelines. Antithrombotic therapy in patients with COVID-19. Available via https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/. Accessed 21 Mar 2021
- 9.Moore AJE, Wachsmann J, Chamarthy MR, Panjikaran L, Tanabe Y, Rajiah P. Imaging of acute pulmonary embolism: an update. Cardiovasc Diagn Ther. 2018;8:225–243. doi: 10.21037/cdt.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 11.Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res. 2020;195:95–99. doi: 10.1016/j.thromres.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzo C, Francesca B, Francesco P, Elena C, Luca S, Paolo S. Acute pulmonary embolism in COVID-19 related hypercoagulability. J Thromb Thrombolysis. 2020;50:223–226. doi: 10.1007/s11239-020-02160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez-Guiu X, Huici-Sanchez M, Romera-Villegas A, Izquierdo-Miranda A, Sancho-Cerro A, Vila-Coll R (2020) Deep vein thrombosis in non-critically ill patients with coronavirus disease 2019 pneumonia: deep vein thrombosis in non-intensive care unit patients. J Vasc Surg Venous Lymphat Disord. 10.1016/j.jvsv.2020.08.028 [DOI] [PMC free article] [PubMed]
- 14.Kermani-Alghoraishi M, Ghahramani R. A review of venous thromboembolism phenomena in COVID-19 patients. Curr Probl Cardiol. 2021;46:100692. doi: 10.1016/j.cpcardiol.2020.100692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abernethy K, Sivakumar P, Patrick T, Robbie H, Periselneris J. Coexistent COVID-19 pneumonia and pulmonary embolism: challenges in identifying dual pathology. Thorax. 2020;75:812–814. doi: 10.1136/thoraxjnl-2020-215011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PRISMA Transparent reporting of systematic reviews and meta-analyses. Available via http://www.prisma-statement.org/. Accessed 21 Mar 2021
- 17.Wells G, Shea B, O’Connell D et al The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available via http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 21 Mar 2021
- 18.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochrane Handbook for Systematic Reviews of Interventions
- 21.OpenMeta[Analyst]. Available via http://www.cebm.brown.edu/openmeta/. Accessed 21 Mar 2021
- 22.MedCalc. Available via https://www.medcalc.org/. Accessed 21 Mar 2021
- 23.Alharthy A, Faqihi F, Abuhamdah M, et al. Prospective longitudinal evaluation of point-of-care lung ultrasound in critically ill patients with Severe COVID-19 pneumonia. J Ultrasound Med. 2021;40:443–456. doi: 10.1002/jum.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso-Fernandez A, Toledo-Pons N, Cosio BG, et al. Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: a prospective study. PLoS One. 2020;15:e0238216. doi: 10.1371/journal.pone.0238216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baccellieri D, Bertoglio L, Apruzzi L et al (2020) Incidence of deep venous thrombosis in COVID-19 hospitalized patients during the first peak of the Italian outbreak. Phlebology. 10.1177/0268355520975592:268355520975592 [DOI] [PubMed]
- 27.Bellmunt-Montoya S, Riera C, Gil D et al (2020) COVID-19 infection in critically ill patients carries a high risk of venous thrombo-embolism. Eur J Vasc Endovasc Surg. 10.1016/j.ejvs.2020.12.015 [DOI] [PMC free article] [PubMed]
- 28.Benito N, Filella D, Mateo J, et al. Pulmonary thrombosis or embolism in a large cohort of hospitalized patients with Covid-19. Front Med (Lausanne) 2020;7:557. doi: 10.3389/fmed.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birk R, Shaw D, Kennedy C et al (2020) Low detection rate of pulmonary embolism in patients presenting to the emergency department with suspected coronavirus disease 2019 (COVID-19): A Single-Centre UK Study. Curr Probl Diagn Radiol. 10.1067/j.cpradiol.2020.09.014 [DOI] [PMC free article] [PubMed]
- 30.Bompard F, Monnier H, Saab I et al (2020) Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 56 [DOI] [PMC free article] [PubMed]
- 31.Bruggemann RAG, Spaetgens B, Gietema HA, et al. The prevalence of pulmonary embolism in patients with COVID-19 and respiratory decline: a three-setting comparison. Thromb Res. 2020;196:486–490. doi: 10.1016/j.thromres.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavagna E, Muratore F, Ferrari F. Pulmonary thromboembolism in COVID-19: venous thromboembolism or arterial thrombosis? Radiology: Cardiothoracic Imaging. 2020;2:e200277. doi: 10.1148/ryct.2020200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerda P, Ribas J, Iriarte A, et al. Blood test dynamics in hospitalized COVID-19 patients: potential utility of D-dimer for pulmonary embolism diagnosis. PLoS One. 2020;15:e0243533. doi: 10.1371/journal.pone.0243533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Wang X, Zhang S, et al. Characteristics of acute pulmonary embolism in patients with COVID-19 associated pneumonia from the city of Wuhan. Clin Appl Thromb Hemost. 2020;26:1076029620936772. doi: 10.1177/1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contou D, Pajot O, Cally R, et al. Pulmonary embolism or thrombosis in ARDS COVID-19 patients: a French monocenter retrospective study. PLoS One. 2020;15:e0238413. doi: 10.1371/journal.pone.0238413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darwish HS, Habash MY, Habash WY. COVID-19 Viral pneumonia complicated with acute pulmonary embolism: a descriptive study. Radiol Res Pract. 2021;2021:6649086. doi: 10.1155/2021/6649086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Cobelli F, Palumbo D, Ciceri F et al (2021) Pulmonary vascular thrombosis in COVID-19 pneumonia. J Cardiothorac Vasc Anesth. 10.1053/j.jvca.2021.01.011 [DOI] [PMC free article] [PubMed]
- 38.Espallargas I, Rodriguez Sevilla JJ, Rodriguez Chiaradia DA et al (2020) CT imaging of pulmonary embolism in patients with COVID-19 pneumonia: a retrospective analysis. Eur Radiol. 10.1007/s00330-020-07300-y [DOI] [PMC free article] [PubMed]
- 39.Fang C, Garzillo G, Batohi B, et al. Extent of pulmonary thromboembolic disease in patients with COVID-19 on CT: relationship with pulmonary parenchymal disease. Clin Radiol. 2020;75:780–788. doi: 10.1016/j.crad.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freund Y, Drogrey M, Miro O, et al. association between pulmonary embolism and COVID-19 in emergency department patients undergoing computed tomography pulmonary angiogram: the PEPCOV International Retrospective Study. Acad Emerg Med. 2020;27:811–820. doi: 10.1111/acem.14096. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Ortega A, Oscullo G, Calvillo P, et al. Incidence, risk factors, and thrombotic load of pulmonary embolism in patients hospitalized for COVID-19 infection. J Infect. 2021;82:261–269. doi: 10.1016/j.jinf.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gervaise A, Bouzad C, Peroux E, Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020;30:6170–6177. doi: 10.1007/s00330-020-06977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grillet F, Busse-Cote A, Calame P, Behr J, Delabrousse E, Aubry S. COVID-19 pneumonia: microvascular disease revealed on pulmonary dual-energy computed tomography angiography. Quant Imaging Med Surg. 2020;10:1852–1862. doi: 10.21037/qims-20-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamade A, Jambert L, Tousch J et al (2020) Systematic duplex ultrasound screening in conventional units for COVID-19 patients with follow-up of 5 days. J Vasc Surg Venous Lymphat Disord. 10.1016/j.jvsv.2020.11.019 [DOI] [PMC free article] [PubMed]
- 47.Hammer MM, Hunsaker AR, Gooptu M, Hatabu H. Frequency of pulmonary embolism in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2478–2479. doi: 10.1016/j.jcmg.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ippolito D, Giandola T, Maino C et al (2021) Acute pulmonary embolism in hospitalized patients with SARS-CoV-2-related pneumonia: multicentric experience from Italian endemic area. Radiol Med. 10.1007/s11547-020-01328-2:1-10 [DOI] [PMC free article] [PubMed]
- 50.Jalaber C, Revel MP, Chassagnon G, et al. Role of upfront CT pulmonary angiography at admission in COVID-19 patients. Thromb Res. 2020;196:138–140. doi: 10.1016/j.thromres.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jevnikar M, Sanchez O, Chocron R et al (2021) Prevalence of pulmonary embolism in patients with COVID 19 at the time of hospital admission. Eur Respir J. 10.1183/13993003.00116-2021 [DOI] [PMC free article] [PubMed]
- 52.Kaminetzky M, Moore W, Fansiwala K, et al. Pulmonary embolism at CT pulmonary angiography in patients with COVID-19. Radiology: Cardiothoracic Imaging. 2020;2:e200277. doi: 10.1148/ryct.2020200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan MZ, Jamal Y, Sutton B, Rauf F (2020) Venous thromboembolism in patients with COVID-19 and correlation with D-dimers: a single-centre experience. BMJ Open Respir Res 7 [DOI] [PMC free article] [PubMed]
- 54.Kirsch B, Aziz M, Kumar S et al (2020) Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Am J Med. 10.1016/j.amjmed.2020.10.044 [DOI] [PMC free article] [PubMed]
- 55.Lang M, Som A, Carey D, et al. Pulmonary vascular manifestations of COVID-19 pneumonia. Radiology Cardiothoracic Imaging. 2020;2:200277. doi: 10.1148/ryct.2020200277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen K, Coolen-Allou N, Masse L, et al. Detection of pulmonary embolism in returning travelers with hypoxemic pneumonia due to COVID-19 in Reunion Island. Am J Trop Med Hyg. 2020;103:844–846. doi: 10.4269/ajtmh.20-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee E, Krajewski A, Clarke C, O'Sullivan D, Herbst T, Lee S (2021) Arterial and venous thromboembolic complications of COVID-19 detected by CT angiogram and venous duplex ultrasound. Emerg Radiol. 10.1007/s10140-020-01884-0 [DOI] [PMC free article] [PubMed]
- 58.Leonard-Lorant I, Delabranche X, Severac F, et al. acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loffi M, Regazzoni V, Toselli M, et al. Incidence and characterization of acute pulmonary embolism in patients with SARS-CoV-2 pneumonia: a multicenter Italian experience. PLoS One. 2021;16:e0245565. doi: 10.1371/journal.pone.0245565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mak SM, Mak D, Hodson D, et al. Pulmonary ischaemia without pulmonary arterial thrombus in COVID-19 patients receiving extracorporeal membrane oxygenation: a cohort study. Clin Radiol. 2020;75:795.e791–795.e795. doi: 10.1016/j.crad.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez Chamorro E, Revilla Ostolaza TY, Perez Nunez M, Borruel Nacenta S, Cruz-Conde Rodriguez-Guerra C, Ibanez Sanz L. Pulmonary embolisms in patients with COVID-19: a prevalence study in a tertiary hospital. Radiologia. 2021;63:13–21. doi: 10.1016/j.rx.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martini K, Bluthgen C, Walter JE, Nguyen-Kim TDL, Thienemann F, Frauenfelder T. Patterns of organizing pneumonia and microinfarcts as surrogate for endothelial disruption and microangiopathic thromboembolic events in patients with coronavirus disease 2019. PLoS One. 2020;15:e0240078. doi: 10.1371/journal.pone.0240078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meiler S, Hamer OW, Schaible J, et al. Computed tomography characterization and outcome evaluation of COVID-19 pneumonia complicated by venous thromboembolism. PLoS One. 2020;15:e0242475. doi: 10.1371/journal.pone.0242475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mestre-Gomez B, Lorente-Ramos RM, Rogado J, et al. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis. 2021;51:40–46. doi: 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minuz P, Mansueto G, Mazzaferri F, et al. High rate of pulmonary thromboembolism in patients with SARS-CoV-2 pneumonia. Clin Microbiol Infect. 2020;26:1572–1573. doi: 10.1016/j.cmi.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miro O, Llorens P, Aguirre A, et al. Association between Covid-19 and Pulmonary Embolism (AC-19-PE study) Thromb Res. 2020;196:322–324. doi: 10.1016/j.thromres.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirsadraee S, Gorog DA, Mahon CF et al (2021) Prevalence of thrombotic complications in ICU-treated patients with coronavirus disease 2019 detected with systematic CT scanning. Crit Care Med. 10.1097/CCM.0000000000004890 [DOI] [PubMed]
- 69.Moll M, Zon RL, Sylvester KW, et al. VTE in ICU Patients With COVID-19. Chest. 2020;158:2130–2135. doi: 10.1016/j.chest.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monfardini L, Morassi M, Botti P, et al. Pulmonary thromboembolism in hospitalised COVID-19 patients at moderate to high risk by Wells score: a report from Lombardy, Italy. Br J Radiol. 2020;93:20200407. doi: 10.1259/bjr.20200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mouhat B, Besutti M, Bouiller K et al (2020) Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J 56 [DOI] [PMC free article] [PubMed]
- 72.Mueller-Peltzer K, Krauss T, Benndorf M, et al. Pulmonary artery thrombi are co-located with opacifications in SARS-CoV2 induced ARDS. Respir Med. 2020;172:106135. doi: 10.1016/j.rmed.2020.106135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Shea A, Parakh A, Hedgire S, Lee SI (2021) Multisystem assessment of the imaging manifestations of coagulopathy in hospitalized patients with coronavirus disease (COVID-19). AJR Am J Roentgenol. 10.2214/AJR.20.24132:1-9 [DOI] [PubMed]
- 74.Ooi MWX, Rajai A, Patel R, Gerova N, Godhamgaonkar V, Liong SY. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography - prevalence, pattern of disease and relationship to D-dimer. Eur J Radiol. 2020;132:109336. doi: 10.1016/j.ejrad.2020.109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parzy G, Daviet F, Puech B, et al. Venous thromboembolism events following venovenous extracorporeal membrane oxygenation for severe acute respiratory syndrome coronavirus 2 based on CT scans. Crit Care Med. 2020;48:e971–e975. doi: 10.1097/CCM.0000000000004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel BV, Arachchillage DJ, Ridge CA, et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez Duenas V, Allona Krauel M, Agrela Rojas E, et al. Blue lungs in Covid-19 patients: a step beyond the diagnosis of pulmonary thromboembolism using MDCT with iodine mapping. Arch Bronconeumol. 2021;57(Suppl 1):35–46. doi: 10.1016/j.arbres.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Planquette B, Le Berre A, Khider L, et al. Prevalence and characteristics of pulmonary embolism in 1042 COVID-19 patients with respiratory symptoms: a nested case-control study. Thromb Res. 2021;197:94–99. doi: 10.1016/j.thromres.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poissy J, Goutay J, Caplan M, et al. pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 80.Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID-19. Radiology. 2020;297:E335–E338. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rali P, O'Corragain O, Oresanya L et al (2020) Incidence of venous thromboembolism in coronavirus disease 2019: an experience from a single large academic center. J Vasc Surg Venous Lymphat Disord. 10.1016/j.jvsv.2020.09.006 [DOI] [PMC free article] [PubMed]
- 82.Ramadan L, Koziatek CA, Caldwell JR et al (2020) Pulmonary thromboembolism in COVID-19: evaluating the role of D-dimer and computed tomography pulmonary angiography results. Am J Emerg Med. 10.1016/j.ajem.2020.08.096 [DOI] [PMC free article] [PubMed]
- 83.Schiaffino S, Giacomazzi F, Esseridou A, et al. Pulmonary thromboembolism in coronavirus disease 2019 patients undergoing thromboprophylaxis. Medicine (Baltimore) 2021;100:e24002. doi: 10.1097/MD.0000000000024002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scialpi M, Sielaszuk EB, Vitale ME, Scalera GB, Nicola R, Mancioli FA. Pulmonary embolism in COVID-19: ancillary findings on chest CT angiography. Lung India. 2021;38:S123–S125. doi: 10.4103/lungindia.lungindia_710_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shahin Y, Rajaram S, Parkash V, Wild JM, Kiely DG, Swift AJ. Patterns of thromboembolic pulmonary vascular disease in COVID-19. Pulm Circ. 2021;11:2045894020979198. doi: 10.1177/2045894020979198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taccone FS, Gevenois PA, Peluso L, et al. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. 2020;48:e1087–e1090. doi: 10.1097/CCM.0000000000004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas W, Varley J, Johnston A, et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tung-Chen Y, Martí de Gracia M, Díez-Tascón A, et al. Correlation between chest computed tomography and lung ultrasonography in patients with coronavirus disease 2019 (COVID-19) Ultrasound Med Biol. 2020;46:2918–2926. doi: 10.1016/j.ultrasmedbio.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ventura-Diaz S, Quintana-Perez JV, Gil-Boronat A, et al. A higher D-dimer threshold for predicting pulmonary embolism in patients with COVID-19: a retrospective study. Emerg Radiol. 2020;27:679–689. doi: 10.1007/s10140-020-01859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vlachou M, Drebes A, Candilio L et al (2021) Pulmonary thrombosis in Covid-19: before, during and after hospital admission. J Thromb Thrombolysis. 10.1007/s11239-020-02370-7 [DOI] [PMC free article] [PubMed]
- 91.Zhang J, Merrick B, Correa GL et al (2020) Veno-venous extracorporeal membrane oxygenation in coronavirus disease 2019: a case series. ERJ Open Res 6 [DOI] [PMC free article] [PubMed]
- 92.Zotzmann V, Lang CN, Wengenmayer T et al (2020) Combining lung ultrasound and Wells score for diagnosing pulmonary embolism in critically ill COVID-19 patients. J Thromb Thrombolysis. 10.1007/s11239-020-02323-0 [DOI] [PMC free article] [PubMed]
- 93.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 94.Alonso Martinez JL, Anniccherico Sanchez FJ, Urbieta Echezarreta MA, Garcia IV, Alvaro JR. Central versus peripheral pulmonary embolism: analysis of the impact on the physiological parameters and long-term survival. N Am J Med Sci. 2016;8:134–142. doi: 10.4103/1947-2714.179128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cha SI, Shin KM, Lee JW, et al. Clinical characteristics of patients with peripheral pulmonary embolism. Respiration. 2010;80:500–508. doi: 10.1159/000277929. [DOI] [PubMed] [Google Scholar]
- 96.Jain CC, Chang Y, Kabrhel C, et al. Impact of pulmonary arterial clot location on pulmonary embolism treatment and outcomes (90 days) Am J Cardiol. 2017;119:802–807. doi: 10.1016/j.amjcard.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 97.Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gasecka A, Borovac JA, Guerreiro RA et al (2020) Thrombotic Complications in Patients WITH COVID-19: pathophysiological mechanisms, diagnosis, and treatment. Cardiovasc Drugs Ther. 10.1007/s10557-020-07084-9 [DOI] [PMC free article] [PubMed]
- 99.Turetz M, Sideris AT, Friedman OA, Triphathi N, Horowitz JM. Epidemiology, pathophysiology, and natural history of pulmonary embolism. Semin Intervent Radiol. 2018;35:92–98. doi: 10.1055/s-0038-1642036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams HJA, Kwee TC, Yakar D, Hope MD, Kwee RM. Chest CT imaging signature of coronavirus disease 2019 infection: in pursuit of the scientific evidence. Chest. 2020;158:1885–1895. doi: 10.1016/j.chest.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Renzi S, Landoni G, Zangrillo A, Ciceri F (2020) MicroCLOTS pathophysiology in COVID 19. Korean J Intern Med. 10.3904/kjim.2020.336 [DOI] [PMC free article] [PubMed]
- 102.Turi S, Nardelli P, Landoni G. Anticoagulants and immunosuppressants in COVID-19: bullets to Defeat MicroCLOTS. Ann Card Anaesth. 2020;23:258–259. doi: 10.4103/aca.ACA_126_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piemonti L, Landoni G. COVID-19 and islet transplantation: different twins. Am J Transplant. 2020;20:2983–2988. doi: 10.1111/ajt.16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pulivarthi S, Gurram MK. Effectiveness of d-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci. 2014;6:491–499. doi: 10.4103/1947-2714.143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schols AMR, Meijs E, Dinant GJ, Stoffers H, Krekels MME, Cals JWL. General practitioner use of D-dimer in suspected venous thromboembolism: historical cohort study in one geographical region in the Netherlands. BMJ Open. 2019;9:e026846. doi: 10.1136/bmjopen-2018-026846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492. doi: 10.1136/bmj.f2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Belohlavek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18:129–138. [PMC free article] [PubMed] [Google Scholar]
- 108.Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8:1716–1722. doi: 10.1111/j.1538-7836.2010.03938.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 36 kb)