SUMMARY
Acute respiratory distress syndrome (ARDS) is a life-threatening form of acute lung injury (ALI) associated with hypoxemic lung damage and inflammation. Matrix metalloproteinase protein-3 (MMP3 or Stromelysin-1) is known to promote vascular injury in ALI/ARDS. Cisatracurium, a nicotinic neuromuscular blocker, is used in ARDS patients to decrease mechanical ventilator dyssynchrony, increase oxygenation, and improve mortality. However, the magnitude and the underlying mechanisms of these potential benefits of cisatracurium remains unclear. We investigated the effect of cisatracurium on lipopolysaccharide-induced MMP3 expression in human microvascular endothelial cells. In our results, cisatracurium treatment significantly decreased LPS-induced MMP3 expression and increased expression of cell junction proteins such as vascular endothelial cadherin (VE-cadherin) and claudin-5.
Keywords: Cisatracurium, MMP3, stromelysin-1, lipopolysaccharide, cell junction
1. Introduction
Acute respiratory distress syndrome (ARDS) results in life-threatening hypoxemia secondary to lung injury from diffuse alveolar damage and subsequent edema (1). A myriad of clinical trials have been conducted to develop strategies to manage ARDS patients with minimal success (2), and the 2018 guidelines for ARDS management recommend only supportive care treatments (e.g., mechanical ventilation) (3). Neuromuscular blocking agents (NMBAs) are used in supportive care to decrease patient-ventilator dyssynchrony (and the associated lung damage) in mechanically ventilated ARDS patients (4). The most common NMBA used in ARDS is cisatracurium as it has been evaluated in two large-scale clinical trials (ACURASYS and ROSE) (5–7).
At the cellular level, the exudative stage of ARDS results from the extensive damage to the alveolar-capillary unit (8), which in turn, results in neutrophil infiltration and edema (9). Hence, preventing injury to the alveolar-capillary unit may be a potential strategy to halt ARDS disease progression. We have demonstrated the integral role of the Akt pathway in endothelial-barrier protection (10,11), which acts by removing the transcriptional suppression by FoxO and β-catenin necessary for barrier function (11–13). Inhibition of the Akt pathway resulted in the reduced expression of tight-junction (TJ) proteins, mainly claudin-5 (14) in the endothelial cells and the lung blood vessels, leading to lung injury and edema (15) and ultimately endothelial-to-mesenchymal transition and lung scarring (16). Apart from the transcriptional suppression of TJ proteins, activated FoxO was also observed to increase the expression of matrix metalloproteinases-3 (MMP-3/stromelysin-1) (15) a protease previously known to break up the junctional protein complexes (17). The potential therapeutic role of MMP3 was further confirmed with increased expression and activity of MMP3 in the LPS-injured lungs and bronchoalveolar lavage fluid (BALF) in mice (15) and ARDS patient plasma samples (18). In the current study, we investigated the direct effect of cisatracurium to suppress LPS-induced MMP3 expression in human microvascular endothelial cells.
2. Materials and Methods
2.1. Cell culture
Human dermal (Telomerase-immortalized) microvascular ECs (HMECs) (CRL-4025; ATCC, Manassas, VA, USA) were maintained in EC Basal Medium-2 with a Growth Medium-2 Bullet Kit (Lonza; Walkersville, MD, USA). Cells were maintained in a humidified 5% CO2 incubator at 37°C and routinely passaged when 80–90% confluent. Cisatracurium (cat. No S2113, Selleckchem) was reconstituted according to the manufacturer’s protocol. Cells were treated with 100 ng/mL LPS and different doses of cisatracurium 0.32, 0.64, and 1.28 μM and PBS (vehicle), respectively, in a 5% serum-containing medium for 24 hours. The optimal doses of cisatracurium were determined based on a similar study performed previously (19).
2.2. Western blot analysis
Western analysis was performed as described previously (20). Cell lysates were prepared using complete lysis buffer (EMD Millipore, San Diego, CA) with protease and phosphatase inhibitor cocktails (Roche Diagnostics, Indianapolis, IN). Protein quantification was performed using DC protein assay from Bio-Rad (Hercules, and CA). Western blot analysis was performed as described previously (21,22). Antibodies used include stromelysin1 (cat. No. 14351-S) dilution 1:1,000 in milk, vascular endothelial cadherin (VE-cadherins; cat No. 2158) dilution 1:1,000 in BSA, P-P38 MAPK (cat No. 9112-S) dilution 1:1,000 in BSA, T-P38MAPK (cat No. 9212-S) dilution 1:1,000 in BSA, P-SRC Tyr-416 (cat No. 6943-S) dilution 1:1,000 in BSA and T-SRC (cat No. 2109-S) dilution 1:1,000 in BSA all from Cell Signaling Technology (Danvers, MA). β-actin (dilution in milk, primary antibodies 1:10,000 and secondary antibodies 1:20,000) from Sigma (St. Louis, MO) and Claudin-5 antibodies (cat No. ab15106) 1:1,000 and secondary antibodies 1:5,000 dilution in milk from Abcam (Cambridge, MA). Band densitometry was done using NIH Image J software.
2.3. Immunofluorescence staining
Immunofluorescent staining of HMEC monolayers was performed using the 8-well chamber slides as described previously (23). Cells were then washed twice with PBS, fixed using 2% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 for 15 min, and blocked with 4% BSA in sterile PBS. Cell monolayers were then incubated with antibody against VE-cadherin (1:100, Catalog# 2158S, Rabbit antibody, Cell Signaling Technology, Danvers, MA) at 4°C overnight. Immunofluorescence was revealed using Alexa Fluor 488 secondary antibody (1:1,000, Goat anti-Rabbit, Life Technologies, Grand Island, NY). Cells were mounted onto a glass slide using DAPI containing mounting medium (Vector Laboratories). Samples were observed under KEYENCE Fluorescence Microscope BZ-X800 (Itasca, IL). Controls were performed by omitting primary antibodies and all controls gave negative results with no detectable non-specific labeling.
2.4. Statistical analysis
All the data are presented as mean ± SD and were calculated from multiple independent experiments performed in triplicates. The ‘n’ value for each figure implies the multiple independent experiments performed. All the data were analyzed by parametric testing using the Student’s unpaired t-test or one-way ANOVA, followed by the posthoc test using the GraphPad Prism 6.01 software. Data with p < 0.05 were considered significant.
3. Results and Discussion
3.1. Treatment with cisatracurium reduced LPS-induced MMP3 expression in HMECs
Our results indicated a significantly higher MMP3 expression in HMECs with LPS treatment for 24 hours, which was blunted by co-treatment with cisatracurium (Figures 1A and 1B) indicating that cisatracurium reduces LPS-induced MMP3 expression in HMECs.
Figure 1. Cisatracurium inhibited LPS-induced increase in MMP3 expression and Src phosphorylation.
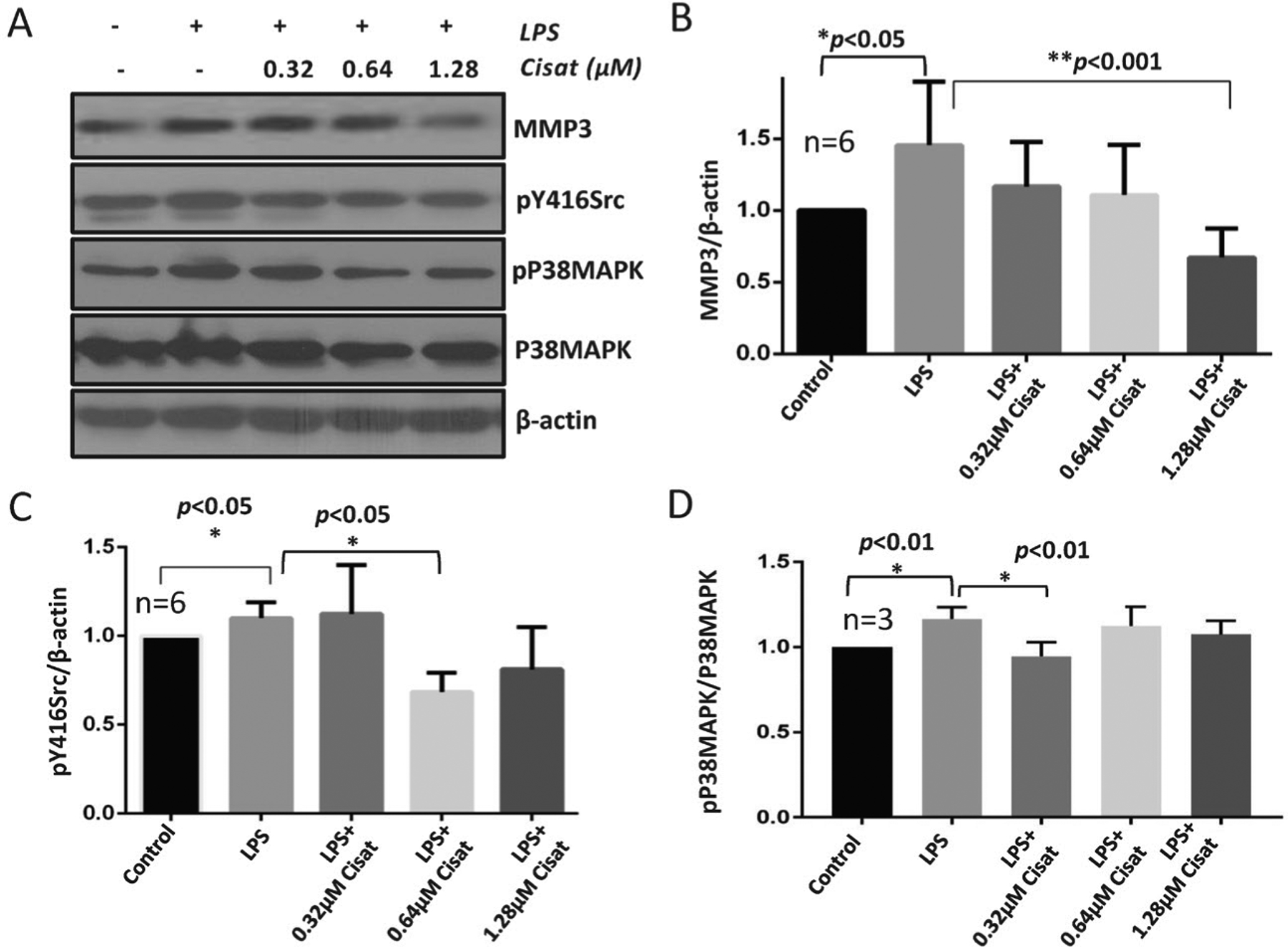
(A-B) Representative Western blot images and a bar graph with band densitometry analysis indicating increased MMP3 expression in HMECs with LPS treatment and its reversal by co-treatment with cisatracurium after 24 hours of incubation. (C) Bar graph with band densitometry analysis indicating increased pY416Src expression in HMECs with LPS treatment and its reversal by co-treatment with cisatracurium after 24 hours of incubation. (D) Bar graph with band densitometry analysis indicating a modest increase in pP38MAPK expression in HMECs with LPS treatment and its partial inhibition by co-treatment with a very low dose of cisatracurium after 24 hours of incubation. Data are shown as Mean + SD.
3.2. Cisatracurium inhibits LPS-induced Src and P38MAPK phosphorylation in HMECs
Since Src has been demonstrated to break up adherens junction (AJ) cell interactions through the cadherins (24), we determined how LPS and cisatracurium treatment affect Src activating phosphorylation at Tyrosine-416 residue. In HMECs, treatment with LPS for 24 hours resulted in increased pY416Src, which was significantly inhibited by co-treatment with cisatracurium (Figures 1A and 1C), thus indicating that cisatracurium protects the cell-barriers by inhibiting Src activity.
Since P38MAPK is a stress and inflammation associated kinase, we next determined if cisatracurium protects the endothelial cells from cell stress associated with pro-inflammatory stimuli such as the bacterial LPS. Our results indicated that although LPS stimulation of HMECs modestly increased phosphorylated P38MAPK, the effect was not reversed upon co-treatment with cisatracurium, except at a lower dose (Figures 1A and 1D). Overall, our data suggested that cisatracurium does not affect P38 MAPK activity in endothelial cells.
3.3. Cisatracurium rescued LPS-induced loss of claudin-5 and VE-cadherin
To determine if cisatracurium could preserve AJ and TJ complexes in HMECs, we treated them with LPS alone or in combination with cisatracurium for 24 hours and subjected them to the Western analysis of VE-cadherin and claudin-5. Our analysis indicated that 24 hours of treatment with LPS significantly reduced VE-cadherin expression in HMECs, which was reversed upon co-treatment with cisatracurium (Figures 2A and 2B). Akin to VE-cadherin, treatment with LPS significantly reduced claudin-5 expression in HMECs, which was reversed upon co-treatment with cisatracurium (Figures 2A and 2C). Together, these results indicated that cisatracurium modulates AJ and TJ protein expression in endothelial cells.
Figure 2. Cisatracurium prevented LPS-induced loss of VE-cadherin and claudin-5 in HMECs.

(A) Representative Western blot images showing reduced VE-cadherin and claudin-5 expressions in HMECs with LPS treatment and its reversal by co-treatment with cisatracurium after 24 hours of incubation. (B) Bar graph with band densitometry analysis indicating reduced VE-cadherin expression in HMECs with LPS treatment and its reversal by co-treatment with cisatracurium after 24 hours of incubation. (C) Bar graph with band densitometry analysis indicating reduced claudin-5 expression in HMECs with LPS treatment and reversal by co-treatment with cisatracurium after 24 hours of incubation. Data are shown as Mean + SD.
3.4. Cisatracurium preserves HMEC monolayer integrity upon LPS insult
Immunofluorescence staining on HMEC monolayer in LPS alone or combination with cisatracurium for 24 hours was performed to determine the sub-cellular compartment involved in the VE-cadherin alterations witnessed at the protein level (Figure 2A and 2B). The drug was used at a dose of 0.64 μM since it resulted in a significant increase in protein expression on Western blot analysis (Figure 3). As anticipated, we observed disruption of VE-cadherin distribution in HMEC monolayers by LPS insult thereby disturbing the continuity of the AJ bands in the cell junctions. Treatment with cisatracurium, however, prevented the LPS-induced disruption of VE-cadherin cell-cell contacts to preserve cell cohesion thus maintaining integrity. These findings confirm the beneficial effects of cisatracurium in HMECs during LPS-induced injury.
Figure 3.

Representative images of VE-cadherin staining in human microvascular endothelial cells post LPS treatment alone, or in combination with cisatracurium at 24 hours.
NMBA, particularly cisatracurium, is recommended by the guidelines for severe ARDS and is thought to improve mortality by optimizing pulmonary airflow mechanics and oxygenation (3,5). Cisatracurium has also been shown to exert anti-inflammatory effects and may play a role in mitigating the deleterious effects of inflammation in the early stages of ARDS, with support largely based on the results of the ACURASYS study, which showed reduced 90-day mortality (6,7,25–27). In 2019, the efficacy of NMBA in ARDS was called into question by the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial, which evaluated cisatracurium versus no cisatracurium in moderate-to-severe ARDS and observed no difference in 90-day mortality (6). These conflicting results have prompted the scientific community to evaluate the potential confounders in these studies and further investigate the molecular mechanisms of cisatracurium-induced effects. Our study demonstrated the ability of cisatracurium to reduce the expression of LPS-induced MMP3 by the endothelial cells. Although the direct effects of cisatracurium on the endothelial barrier protein expression were modest, these were significant.
Preclinical studies in our laboratory demonstrated that ARDS/ALI was associated with elevated expression and activity of MMP3 (15), a matricellular protease that is known to breakdown the endothelial barrier junctions and promote vascular permeability (28). MMP3 is also known to have an essential role in the innate immunity and inflammatory response, and cause degradation for the extracellular matrix (ECM) (17). Several inflammatory lung diseases such as ARDS, asthma, and pulmonary fibrosis are characterized by an increase in the expression of one or more of the MMPs (29,30). Recent studies from our laboratory have identified that pharmacological inhibition of MMP3 or its upstream regulator, FoxO transcription factors, has been demonstrated to reverse LPS-induced lung injury and edema (15). The studies also demonstrated elevated MMP3 expression and activity in LPS-administered mouse BALF. Another study demonstrated increased MMP3 expression/activity in the plasma samples collected from human ARDS patients compared to healthy subjects (18). Increased MMP3 expression was correlated with reduced expression of claudin-5 in endothelial cells and mouse lung lysates, and increased neutrophil activity in the lungs (15) indicating the importance of MMP3 in the promotion of lung inflammation and ARDS disease progression. The disruption of the ECM by MMPs intra- and intercellularly in the ALI experimental model has also reported disruption of the TJ and AJ complexes that preserve the lung vascular integrity (31). Studies conducted on cisatracurium in different disease models have shown its effects on decreasing inflammation and cell migration involving various pathways (19,32).
In the current study, we investigated the effect of cisatracurium to modulate the pro-inflammatory and cell-barrier modulating pathways in HMECs and determined its efficacy to inhibit the injury-response elicited by bacterial LPS treatment. The ability of cisatracurium to reverse the LPS-induced increase in MMP3 expression and Src phosphorylation and preventing the loss of AJ protein VE-cadherin and TJ protein claudin-5 in HMECs are indications of its potential benefits in preventing pathological vascular permeability and inflammation. However, the fact that cisatracurium had no direct effect on the activity of pro-inflammatory P38MAPK suggests that cisatracurium has no direct effect on the HMECs in reducing the inflammatory response. However, the study has limitations as it has been conducted in individual cell lines in vitro that a clear understanding of the collective effect and molecular mechanisms of cisatracurium in a disease model is lacking. Further studies will be required to fully understand the benefits and mechanisms of cisatracurium in the treatment of ARDS patients.
Funding:
Funds were provided by the NHLBI (R01HL103952), NCATS (UL1TR002378), Georgia-CTSA (KL2TR002381), and Wilson Pharmacy Foundation (intramural). This work has been accomplished using the resources and facilities at the VA Medical Center in Augusta, GA.
Footnotes
Conflict of Interest: Authors declare that there are no financial or other conflicts of interest exist.
References
- 1.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006; 21:119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019; 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall JB. Point: Should paralytic agents be routinely used in severe ARDS? Yes. Chest 2013; 144:1440–1442. [DOI] [PubMed] [Google Scholar]
- 5.Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016; 44:2079–2103. [DOI] [PubMed] [Google Scholar]
- 6.National Heart L, Blood Institute PCTN, Moss M, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019; 380:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363:1107–1116. [DOI] [PubMed] [Google Scholar]
- 8.Herrero R, Sanchez G, Lorente JA. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann Transl Med. 2018; 6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015; 194:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005; 11:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao F, Sabbineni H, Artham S, Somanath PR. Modulation of long-term endothelial-barrier integrity is conditional to the cross-talk between Akt and Src signaling. J Cell Physiol. 2017; 232:2599–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alwhaibi A, Verma A, Adil MS, Somanath PR. The unconventional role of Akt1 in the advanced cancers and in diabetes-promoted carcinogenesis. Pharmacol Res. 2019; 145:104270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adil MS, Narayanan SP, Somanath PR. Cell-cell junctions: structure and regulation in physiology and pathology. Tissue Barriers. 2020;1848212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Artham S, Sabbineni H, Al-Azayzih A, Peng XD, Hay N, Adams RH, Byzova TV, Somanath PR. Akt1 promotes stimuli-induced endothelial-barrier protection through FoxO-mediated tight-junction protein turnover. Cell Mol Life Sci. 2016; 73:3917–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artham S, Gao F, Verma A, Alwhaibi A, Sabbineni H, Hafez S, Ergul A, Somanath PR. Endothelial stromelysin1 regulation by the forkhead box-O transcription factors is crucial in the exudative phase of acute lung injury. Pharmacol Res. 2019; 141:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabbineni H, Verma A, Artham S, Anderson D, Amaka O, Liu F, Narayanan SP, Somanath PR. Pharmacological inhibition of beta-catenin prevents EndMT in vitro and vascular remodeling in vivo resulting from endothelial Akt1 suppression. Biochem Pharmacol. 2019; 164:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006; 69:562–573. [DOI] [PubMed] [Google Scholar]
- 18.Artham S, Verma A, Newsome AS, Somanath PR. Patients with acute respiratory distress syndrome exhibit increased stromelysin1 activity in the blood samples. Cytokine. 2020; 131:155086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanelli V, Morita Y, Cappello P, Ghazarian M, Sugumar B, Delsedime L, Batt J, Ranieri VM, Zhang H, Slutsky AS. Neuromuscular blocking agent cisatracurium attenuates lung injury by inhibition of nicotinic acetylcholine receptor-alpha1. Anesthesiology. 2016; 124:132–140. [DOI] [PubMed] [Google Scholar]
- 20.Abdalla M, Sabbineni H, Prakash R, Ergul A, Fagan SC, Somanath PR. The Akt inhibitor, triciribine, ameliorates chronic hypoxia-induced vascular pruning and TGFbeta-induced pulmonary fibrosis. Br J Pharmacol. 2015; 172:4173–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabbineni H, Verma A, Somanath PR. Isoform-specific effects of transforming growth factor beta on endothelial-to-mesenchymal transition. J Cell Physiol. 2018; 233:8418–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goc A, Al-Azayzih A, Abdalla M, Al-Husein B, Kavuri S, Lee J, Moses K, Somanath PR. P21 activated kinase-1 (Pak1) promotes prostate tumor growth and microinvasion via inhibition of transforming growth factor beta expression and enhanced matrix metalloproteinase 9 secretion. J Biol Chem. 2013; 288:3025–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao F, Al-Azayzih A, Somanath PR. Discrete functions of GSK3alpha and GSK3beta isoforms in prostate tumor growth and micrometastasis. Oncotarget. 2015; 6:5947–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008; 121:2115–2122. [DOI] [PubMed] [Google Scholar]
- 25.Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, Perrin G, Gainnier M, Bongrand P, Papazian L. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006; 34:2749–2757. [DOI] [PubMed] [Google Scholar]
- 26.Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, Papazian L. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004; 32:113–119. [DOI] [PubMed] [Google Scholar]
- 27.Paton WD. Mode of action of neuromuscular blocking agents. Br J Anaesth. 1956; 28:470–480. [DOI] [PubMed] [Google Scholar]
- 28.McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J. 2009; 33:77–84. [DOI] [PubMed] [Google Scholar]
- 29.Ito JT, Lourenco JD, Righetti RF, Tiberio I, Prado CM, Lopes F. Extracellular matrix component remodeling in respiratory diseases: What has been found in clinical and experimental studies? Cells. 2019; 8:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandenbroucke RE, Dejonckheere E, Libert C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur Respir J. 2011; 38:1200–1214. [DOI] [PubMed] [Google Scholar]
- 31.Schlingmann B, Molina SA, Koval M. Claudins: Gatekeepers of lung epithelial function. Semin Cell Dev Biol. 2015; 42:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabasin IB, Sanches JGP, Ibrahim MM, Huidan J, Williams W, Lu ZL, Wen Q. Cisatracurium retards cell migration and invasion upon upregulation of p53 and inhibits the aggressiveness of colorectal cancer. Front Physiol. 2018; 9:941. [DOI] [PMC free article] [PubMed] [Google Scholar]


