Abstract
Oxidative stress, a term that describes the imbalance between oxidants and antioxidants, leads to the disruption of redox signals and causes molecular damage. Increased oxidative stress from diverse sources has been implicated in most senescence-related diseases and in aging itself. The Kelch-like ECH-associated protein 1- (Keap1-) nuclear factor-erythroid 2-related factor 2 (Nrf2) system can be used to monitor oxidative stress; Keap1-Nrf2 is closely associated with aging and controls the transcription of multiple antioxidant enzymes. Simultaneously, Keap1-Nrf2 signaling is also modulated by a more complex regulatory network, including phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), protein kinase C, and mitogen-activated protein kinase. This review presents more information on aging-related molecular mechanisms involving Keap1-Nrf2. Furthermore, we highlight several major signals involved in Nrf2 unbinding from Keap1, including cysteine modification of Keap1 and phosphorylation of Nrf2, PI3K/Akt/glycogen synthase kinase 3β, sequestosome 1, Bach1, and c-Myc. Additionally, we discuss the direct interaction between Keap1-Nrf2 and the mammalian target of rapamycin pathway. In summary, we focus on recent progress in research on the Keap1-Nrf2 system involving oxidative stress and aging, providing an empirical basis for the development of antiaging drugs.
1. Introduction
Aging is a fundamental and complex physiological process that compromises health, causing multiple chronic diseases. Aging and antiaging are universal concerns in the life sciences. Over the past few decades, researchers have continuously explored the underlying mechanisms of aging and antiaging interventions. Among the theories proposed to explain aging, damage accumulation driven by oxidative stress is one of the most accepted ones [1].
The concept of oxidative stress can be traced back to the 1950s and has been studied since the 1970s [2]. The term “oxidative stress,” popularized by Sies and Cadenas [3], was defined as “a disturbance in the prooxidant-antioxidant balance in favor of the former, leading to potential damage,” and later, Jones [4] introduced a new definition of oxidative stress as “a disruption of redox signaling and control.” It involves the excessive generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as peroxynitrite (ONOO−), hydrogen peroxide (H2O2), nitric oxide (NO), hydroxyl radicals (HO·), and superoxide anion radicals (O2·-) [4]. Sources of ROS and RNS in organisms are abundant under physiological and pathological conditions. Cells contain multiple sources for ROS, including the mitochondria, endoplasmic reticulum, peroxisomes, NAD(P)H oxidases, and monoamine oxidases [5, 6]. Of these, complexes I and III, involved in mitochondrial oxidative phosphorylation, are the largest contributors to cellular ROS production [7, 8]. High cellular ROS levels result from an imbalance between ROS production and the antioxidant system. This increase in ROS damages DNA, cell membranes, and proteins, leading to the development of aging-related and neurodegenerative diseases [9, 10]. Recent studies have corroborated the idea that DNA damage has a significant effect on aging [11, 12]. Over the past two decades, we have rapidly increased our understanding of the role of DNA damage response in cancer and aging [13]. Research in a wide variety of animal models from Caenorhabditis elegans to mammals has shown that cellular autonomy and systematic DNA damage response mechanisms coordinate adaptive responses, thereby enhancing maintenance in aging organisms and accumulating DNA damage more gradually [14, 15]. Interestingly, recent evidence has shown that routine exercise attenuates aging-induced oxidative stress [16]. Similarly, constraint-induced movement in mice effectively inhibits aging, whereas aging is accelerated with greater cellular oxidative stress [17]. Thus, an increasing body of evidence suggests that accumulating ROS, dysfunctional mitochondria, and DNA damage play an important role in the aging process.
To deal with oxidative damage, the body is equipped with an efficient defense system that detoxifies and eliminates harmful chemicals and inactivates ROS. Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a master regulator of multiple antioxidant enzymes; it modulates cell redox balance and senses the status of cellular oxidative stress. This is done by stimulating the activity of components of antioxidant defense, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), heme oxygenase-1 (HO-1), glutathione reductase, thioredoxin reductase, ferritin, and NAD(P)H:quinone oxidoreductase 1 (NQO1) [18, 19]. Therefore, Nrf2 is a key regulator in the signaling pathway for lifespan extension because of its role in regulating antioxidant expression [20]. Activation of the Nrf2-related antioxidant defense system prevents cell senescence, while inhibiting Nrf2 activity significantly promotes cell senescence [21], indicating that Nrf2 has a protective effect on aging. Furthermore, Nrf2 expression and activity decrease during aging [22, 23].
Kelch-like ECH-associated protein 1 (Keap1), a master negative regulator of Nrf2 discovered in 1999 [24], has a broad, complex, tram-track, bric-a-brac structure at its N-terminus. Keap1 is the site of action for cullin-dependent E3 ubiquitin ligase, which degrades Nrf2 [25, 26]. Most Nrf2 inducers, such as tert-butylhydroquinone (tBHQ) and itaconate [27, 28], are electrophilic and readily react with cysteine thiol groups in Keap1, activating the Keap1-Nrf2 signaling pathway and triggering a protective antioxidant response. Under oxidative stress, Keap1 undergoes a conformational change that causes Nrf2 to dissociate. The latter protein then aggregates into the nucleus and forms a heterodimer with musculoaponeurotic fibrosarcoma (Maf), which combines with antioxidant response elements (AREs) to initiate the transcription of multiple antioxidants [29].
The Keap1-Nrf2 system has been widely studied and is considered a key cellular defense mechanism. However, multiple factors regulate the Keap1-Nrf2 system, posing a formidable challenge to unraveling the underlying mechanism linking the Keap1-Nrf2 signaling pathway to aging. Researchers are currently working to identify candidate antioxidant-related agents that activate Nrf2 for development into antiaging drugs and for treating aging-related diseases. These efforts are largely dependent on elucidating the correlation between Keap1-Nrf2 signaling and oxidative stress. Therefore, in this review, we present a compilation of the regulation of Keap1-Nrf2 signaling to provide new ideas for the identification and development of antiaging agents.
2. Activation of Nrf2 and Aging
The role of Nrf2 in antioxidative stress is well defined [30]. Only within the last few years, however, evidence demonstrates that Nrf2 activity is repressed in aging. A recent study showed that Nrf2 deletion caused widespread aging-related transcriptomic changes in age-related diseases [31]. Nrf2 signaling was impaired in the retinal pigment epithelium in aging mice [32]. Furthermore, aging was associated with a loss of Nrf2 activity, particularly from regions that had high Nrf2 activity in young animals [33]. Interestingly, acute aerobic exercise activates Nrf2 on peripheral blood mononuclear cells in vivo in both the old and young adults, but the nuclear accumulation of Nrf2 was attenuated in older adults [34]. These findings suggest that Nrf2 signaling is closely associated with aging and age-related diseases. However, how does Nrf2 signaling influence aging and age-associated diseases?
As mentioned above, Nrf2 levels have been shown to decrease with aging, and there is a positive relationship between Nrf2 activity and species longevity. Therefore, the regulation and downstream pathways of Nrf2 have received special attention. Nrf2 regulates various downstream antioxidation and detoxification enzymes such as NQO1, HO-1, SOD, catalase (CAT), and glutamate-cysteine ligase catalytic subunit (GCLC) by binding to the antioxidant response element in their promoter regions [35]. For example, trehalose and chitosan oligosaccharide all showed an antiaging effect by promoting the nuclear translocation of Nrf2 and subsequently activating the expression of downstream target genes HO-1, NQO1, and CAT in aging mice [36, 37]. Furthermore, recent studies showed that the activation of multiple signaling pathways, such as phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK), facilitates the release of Nrf2 from Keap1 and subsequent translocation for the induction of various antioxidant and detoxification enzyme expressions [19, 38, 39]. Intriguingly, Lewis et al. found that high levels of Nrf2 signaling activity in naturally long-lived naked mole rats are not due to increased expression of Nrf2 protein but rather are due to reduced expression of its negative regulators Keap1 and β-transducin repeat-containing protein (β-TrCP) [40]. Rapamycin also shows a similar mechanism to inhibit cell senescence and increase longevity [41]. In addition, Nrf2 activation or overexpression might not be enough to prolong healthy life in aging models. The constitutive activation or overexpression of Nrf2 even can cause deleterious outcomes. Rajasekaran et al. found that chronic activation of Nrf2 causes a hyperreductive state and leads to hypertrophic cardiomyopathy in the cardiac-specific transgenic mice [42]. Nrf2 overexpression increases the risk of high tumor mutation and induces drug resistance in cancer patients [43, 44].
Taken together, accumulating evidence suggests that the Nrf2 plays a key role in oxidative stress resistance and may be directly linked to the healthspan and lifespan of the organism (Figure 1). However, there are numerous targets on the Nrf2 signaling that may show positive effects on delaying aging. Thus, there is still a long way ahead to understand Nrf2 signaling during aging. Here, we provide a review of that area in order to provide the context for our discussion of how Nrf2 activation affects aging.
Figure 1.

Simplistic schematic of the relationship between Nrf2 and aging.
3. Mechanisms of Nrf2 Dissolution from Keap1
3.1. Modification of Keap1 Cysteine Residues
Keap1, a ubiquitous Nrf2 regulator, is a major target in the discovery of antiaging drugs. Keap1 comprises two canonical domains: the N-terminal BTB and the C-terminal DC (or Kelch) domains, connected by an intervening region (IVR) [24]. Keap1 contains highly conserved, reactive cysteine residues that act as electrophilic sensors responding to endogenous and exogenous ROS. Studies have revealed three major half-cysteine residues of Keap1: Cys151 in the BTB domain and Cys273 and Cys288 in the IVR. These residues are critical for modulating the E3 ubiquitin ligase activity of the Keap1-Cul3 complex [45, 46]. Of the three half-cysteines, Cys151 is the main sensor of oxidative stress that disrupts the Keap1-Cul3 interaction and causes Nrf2 dissolution from Keap1 [47, 48]. In contrast, Cys273 and Cys288 play a crucial role in the Keap1 conformational change under oxidative modification [49]. Most importantly, while specific cysteine sensors have evolved in response to different inducers, there is redundancy between sensors, enabling continued oxidant accumulation even with a loss-of-function mutation in a given Keap1 sensor [28, 45, 46, 49]. This flexibility is extremely important and ensures cell survival by preventing excessive glutathione consumption and subsequent oxidative stress. Furthermore, Saito and colleagues have confirmed that Cys151, Cys273, and Cys288 function collaboratively in sensing 9-nitro-octadec-9-enoic acid, sodium meta-arsenite, and 4-hydroxy-nonenal [49]. Their findings also suggest that S-nitroso-N-acetylpenicillamine, 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole, tBHQ, diethylmaleate, sulforaphane, and dimethylfumarate are Cys151-preferring inducers [49]. Furthermore, other cysteine residues (e.g., Cys226, Cys613, and His225) are involved in the sensing function of Keap1 and are necessary for detecting Cd2+, As3+, Se4+, and Zn2+ [45, 50]. The H2O2 sensing ability was originally thought to be dependent on a conformational change in Keap1, induced through a disulfide bond forming between Cys226 and Cys613 [51]. However, a recent study has found that the conformational change occurs via the synergy of Cys226, Cys613, and Cys622/Cys624, inactivating Keap1 and stabilizing Nrf2 [52]. In addition, H2O2 mediates Keap1 phosphorylation at Ser104, Ser53, and Ser293 in response to oxidative stress [53, 54]. Given that different chemicals can target different cysteine sites in Keap1 to regulate Nrf2, chemical Nrf2 inducers have been divided into at least five categories as shown in Figure 2: I (Cys151 preferring), II (Cys288 preferring), III (Cys151/Cys273/Cys288 collaboration preferring), IV (Cys151/Cys273/Cys288 independent), and V (Keap1-Nrf2 protein-protein interaction) [55].
Figure 2.
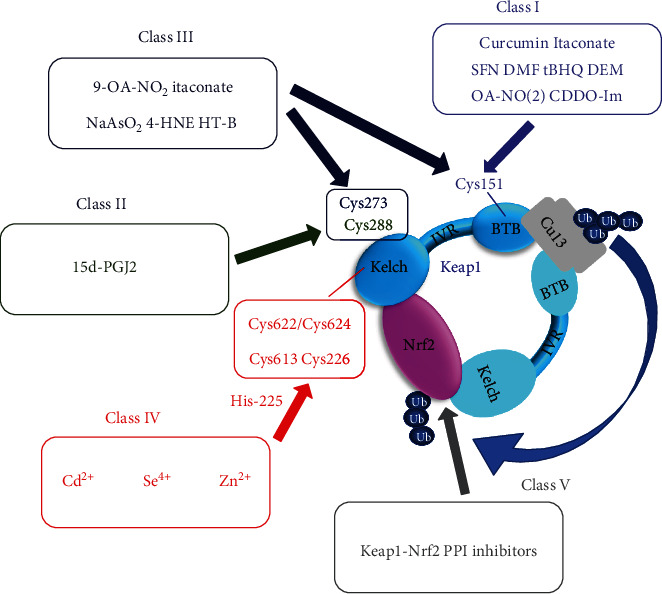
Different classes of activators of Keap1. Several representative compounds are shown and are divided into Classes I–V (further classified using the criteria established by Suzuki and Yamamoto [55] and based on current studies). 9-OA-NO2: 9-nitro-octadec-9-enoic acid; NaAsO2: sodium meta-arsenite; 4-HNE: 4-hydroxy-nonenal; SNAP: S-nitroso-N-acetylpenicillamine; 15d-PGJ2: 15-deoxy-prostaglandin J2; DMF: dimethylfumarate; CDDO-Im: 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole; tBHQ: tert-butylhydroquinone; DEM: diethylmaleate; SFN: sulforaphane; HT-B: hydroxytyrosol butyrate; PPI: protein-protein interaction.
Inflammation is closely associated with aging [56]. Inflammation produces large amounts of ROS that induce oxidative damage in DNA, membrane lipids, and proteins, further contributing to aging [57]. Itaconate is a novel and potent Nrf2 activator that plays an anti-inflammatory role through alkylating Cys151, Cys257, Cys288, Cys273, and Cys297 in Keap1, enabling Nrf2 to increase the expression of downstream antioxidant and anti-inflammatory genes [27]. The itaconate derivative, 4-octyl itaconate, protects neuronal cells from H2O2 by targeting Keap1 [58]. The discovery of three important cysteine residues of Keap1 and several other half-cysteine sensors elucidates the oxidative stress-induced transcription of Nrf2-dependent genes, providing a reliable, theoretical basis for unveiling the aging mechanism and the development of antiaging agents. Of note, the structural distinction among compounds and combinations of cysteine residues in Keap1 has generated different modifications of cysteine residues. To interpret this mechanism, researchers have proposed the “cysteine code” hypothesis [59], which transforms cysteine modifications into specific biological effects. Deciphering the cysteine code of each Nrf2-activating compound will increase our understanding of its antioxidant function.
Collectively, these findings suggest that Keap1 is an important unit of the Keap1-Nrf2 system that protects cells from oxidative damage through sensing oxidative stress and regulating Nrf2 activity. Studies on the underlying mechanism of Keap1 play a critical role in revealing aging mechanisms and developing antiaging agents.
3.2. Competitive Interaction in the Keap1-Nrf2 Axis
Apart from oxidative modifications in the cysteine residues in Keap1, the Keap1 and Nrf2 interaction can also be disrupted via competitive binding. The multifunctional autophagy adapter, sequestosome 1 (p62/SQSTM1), participates in noncanonical activation of Nrf2 [60]. Being involved in the regulation of diverse biological processes, including inflammation, oxidative stress, tumorigenesis, and misfolded protein degradation [61, 62], p62 is a possible initial target of aging interventions, especially because it interacts with the Keap1-Nrf2 pathway. The interaction between Keap1 and p62 was first revealed in 2010 by five independent research groups [63–67]. Like the Keap1-interacting Neh2 domain of Nrf2, p62 contains a KIR motif that allows it to directly interact with Keap1 [63–67]. This competitive interaction leads to sustained accumulation of nonubiquitinated Nrf2 and activation of the antioxidant response [68].
As an important selective substrate for autophagy, p62 is implicated in many models of aging and aging-related diseases [69, 70]. Although p62-promoting autophagy has been linked to a longer lifespan [71–73], direct evidence is very limited. Perhaps, the clearest evidence of its importance in longevity is the premature aging of p62−/− mice [74]. Kwon and colleagues have demonstrated that p62−/− mice exhibit a significantly shortened lifespan and accelerated aging phenotypes. Importantly, the mice also exhibited attenuated NQO1 expression and higher intracellular oxidant levels [74]. These results suggest that p62 may exert its antiaging effects through Keap1-Nrf2 signaling. Recently, one study demonstrated that upregulation of dp62 (Drosophila p62 homolog ref(2)P) from midlife onward significantly increases fly lifespan and reduces mitochondrial ROS levels in flight muscles at 37 days old [75]. However, we do not know whether the decline in ROS levels is related to the competitive interaction between p62 and Keap1. Lamin C (LamC) is one of two nuclear membrane proteins encoded by the LMNA gene [76]. Recently, researchers demonstrated that LamC mutations lead to a shorter lifespan in Drosophila [77]. Furthermore, mutant LamC caused Nrf2 mislocalization and increased p62 levels, generating oxidative stress. In addition, Wei et al. have demonstrated that Keap1/Nrf2/p62 signaling plays an important role in alleviating high inorganic phosphate-induced oxidative stress and subsequent vascular calcification, the latter a complication of aging [78]. Collectively, Keap1/Nrf2/p62 signaling links autophagy to aging, making the pathway a promising antiaging target. Given the complexity of interactions among the Keap1/Nrf2/p62 axis, oxidative stress, and aging, further research is needed to elucidate underlying mechanisms.
In addition to p62, several competing binding proteins of Nrf2 have been reported in recent years. For instance, minichromosome maintenance protein 3 (MCM3) regulates genome replication and redox homeostasis by competing with Nrf2 for Keap1 [79]. Likewise, other Nrf2 competitors can be potential targets for the development of antiaging and anticancer drugs, such as atypical protein kinase Cι (aPKCι) [80], inhibitor of apoptosis stimulating protein p53 (iASPP) [81], and family with sequence similarity 129, member B (FAM129B) [82].
Strikingly, both p62 accumulation and Keap1 inhibition mediate Nrf2 activation and participate in aging and aging-related diseases. Additionally, the Keap1 mutation is one of the mechanisms allowing Nrf2 to escape Keap1-mediated repression [83]. These findings indicate that regulation of the Keap1-Nrf2 signaling pathway in higher organisms is a means to promote longevity.
4. Nrf2 Phosphorylation
Thus far, the discussed regulatory mechanisms that activate Nrf2 depend on direct interaction with Keap1. However, numerous recent studies have revealed Keap1-independent mechanisms of Nrf2 regulation, including phosphorylation by multiple protein kinases (protein kinase C (PKC), phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), and glycogen synthase kinase 3β (GSK-3β)) [84]. Therefore, these protein kinases are part of the aging process. Here, we elaborate and discuss the importance of Nrf2 phosphorylation in aging.
4.1. PI3K/Akt/Nrf2
PI3Ks are a family of lipid kinases that play a pivotal role in intracellular signal transduction, controlling many physiological functions and cell processes [85]. The serine/threonine kinase Akt, known as protein kinase B (PKB), is involved in multiple cellular processes, including proliferation, growth, survival, migration, and metabolism [86]. Akt/PKB has emerged as an important signal transduction node in higher eukaryotic cells and is one of the most important and versatile protein kinases at the core of human physiology [87]. Accumulating evidence over the past 20 years suggests that Akt regulates a variety of functions related to longevity and senescence [88, 89]. A downstream target of PI3K/Akt signaling is Nrf2 [90]. Given its well-studied involvement in Nrf2 phosphorylation, the PI3K/Akt/Nrf2 pathway is considered a major way for cells to resist oxidative stress [91]. Thus, the PI3K/Akt/Nrf2 pathway may be a therapeutic target for aging and other conditions related to oxidative stress.
Indeed, PI3K/Akt/Nrf2 signaling plays a central role in aging-related diseases [92, 93]. Many natural compounds exert antioxidant and antiaging effects through the PI3K/Akt/Nrf2 pathway [94, 95]. For instance, naringenin and bungeanum ameliorate behavioral and neurological dysfunction in a D-galactose-induced mouse model of aging through activating this pathway [96, 97]. Through the same pathway, curcumin increases SOD levels and improves premature ovarian failure in mice [98], while anthocyanins potentially target the pathway to ameliorate neurodegenerative diseases [92]. Certain endogenous substances such as thyroid hormone T3 act on the PI3K/Akt/Nrf2 pathway to increase HO-1 [99], whereas erythropoietin and fibroblast growth factors do the same to increase SOD [100, 101]. Because Akt has multiple downstream targets, whether this pathway involves the participation of other signaling molecules remains an open question.
4.2. GSK-3β
PI3K/Akt signaling regulates Nrf2 activity through GSK-3β, a widely distributed serine/threonine kinase encoded by two different genes, alpha (α) and beta (β) [102, 103]. As a major Akt target, GSK-3β is involved in various signaling pathways regulating cell proliferation, apoptosis, glycogen metabolism, and stem cell renewal [104]. Activated Akt phosphorylates and inactivates two GSK-3 subtypes at the N-terminus: for GSK-3α, position S21 is targeted, whereas GSK-3β is phosphorylated at position S9 [105]. GSK-3β has many phosphorylation targets and thereby regulates a variety of biological processes involved in several human aging-related diseases, such as cancer, diabetes, and Alzheimer's disease [106]. GSK-3β also regulates Nrf2 relocation to the cytosol [107]. When the PI3K/Akt pathway is initiated, Akt first activates GSK-3β. The latter then phosphorylates Fyn at threonine residues, leading to Fyn nuclear accumulation [108]. Finally, Fyn phosphorylates Nrf2 at Tyr568, allowing Nrf2 to bind with Crm1 and degrade in a Keap1-independent manner [109]. GSK-3β also phosphorylates serine residues (334-338) in the Neh6 region of Nrf2 to form a structural motif recognized by SCF/β-TrCP E3 ubiquitin ligase, leading to Nrf2 degradation [110].
Increasing evidence demonstrates that GSK-3β plays a critical role in aging. GSK-3α knockout mice have shorter lifespans than wild-type mice [111]. As a downstream target of GSK-3β, Nrf2 is a pivotal molecule in PI3K/Akt/GSK-3β signaling [109, 112]. Unlike Akt, GSK-3β is active in resting, unstimulated cells [113], and aberrant activation of GSK-3β is implicated in aging and neurodegenerative diseases [114, 115]. Inhibition of GSK-3β attenuates H2O2-induced oxidative damage through mediating Nrf2-ARE signaling activation [116, 117]. Xin et al. show that activation of the Akt/GSK-3β/Fyn signaling pathway prevents cardiomyopathy through upregulating Nrf2 [108]. Furthermore, adding antisense oligonucleotides targeted to GSK-3β improves memory and learning deficits in mice, an effect related to increased nuclear Nrf2, indicating that GSK-3β helps maintain healthy aging in rodents [118].
In recent years, the mammalian target of rapamycin complex 2 (mTORC2) has also been found to interact with GSK-3β [119]. The mTOR is a highly conserved serine/threonine kinase that responds to changes in energy balance and regulates many cellular functions [120]. There are currently two known mTOR complexes: rapamycin-sensitive mTOR complex 1 (mTORC1) and mTORC2 [121]; the latter is less sensitive to rapamycin and less studied. Among the mTORC2-phosphorylated kinases, such as the protein kinase A/protein kinase G/PKC family, Akt is the most important substrate because of its role in insulin/PI3K signaling [122]. Activation of mTORC2/Akt signaling by 14,15-epoxyeicosatrienoic acid delays endothelial senescence and restores age-dependent endothelial dysfunction [123]. Recently, Yang et al. demonstrated that the mTORC2/Akt/GSK-3β pathway is a potential therapeutic target in endothelial senescence [124]. The pathway also plays a major role in cognitive dysfunction [125]. Another protein that inactivates GSK-3β and promotes Nrf2 activation is AMP-activated protein kinase (AMPK), involved in cell survival under stress [126]. AMPK also directly phosphorylates Nrf2 at Ser558 (Ser550 in mice), promoting Nrf2 nuclear accumulation [127].
Collectively, existing research shows that GSK-3β is a key node related to aging and aging-related diseases, particularly in terms of the regulatory network centered on the GSK-3β/Nrf2 axis. However, the interaction and complexity of multiple signaling pathways currently obscure our understanding of how the GSK-3β/Nrf2 axis affects aging. Nevertheless, the characteristics of GSK-3β make it an attractive target for the development of antiaging agents.
4.3. Other Protein Kinases Involved in Nrf2 Phosphorylation
PKC is a family of serine/threonine kinases that regulates cell proliferation, survival, apoptosis, and migration [128]. Huang and colleagues have found that PKC phosphorylates Nrf2 at Ser40 in the Neh2 domain, leading to the dissociation of Nrf2 from Keap1 and consequent Nrf2 nuclear translocation [129]. PKC-δ is a novel PKC that is 676-amino acid long [130], has multiple functions in cell signal transduction, and regulates the effects of several different molecules. PKC-δ/Nrf2 signaling is a potential therapeutic target because of its antioxidant effect in aging-related diseases, such as osteoarthritis [131] and diabetes [132].
Protein kinase CK2 is involved in a diverse array of biological processes [133], including the cell cycle and cell survival [134, 135]. CK2 is an important regulator of Nrf2 activity [136]. Apopa and colleagues first identified transcription activation domains of Nrf2, Neh4 and Neh5, and then demonstrated that CK2 phosphorylates Nrf2 to trigger the latter's nuclear accumulation [137]. Importantly, downregulation of CK2 activity is closely related to cellular senescence and organismal aging [138, 139], suggesting that the CK2-Nrf2 pathway may be a novel, antiaging target.
Similarly, extracellular signal-regulated kinase (ERK) and mitogen-activated protein kinases, such as p38, both promote Nrf2 activation and upregulate Nrf2 target genes [140, 141]. In particular, p38 phosphorylates Nrf2 at three serine residues (Ser215, Ser408, and Ser577), facilitating a decrease in Nrf2 nuclear accumulation [142]. Modulating this effect through drugs is a potential method for prolonging lifespan [143].
5. Crosstalk between mTOR and Nrf2
mTOR, a relatively large (259 kDa) and highly conserved serine/threonine protein kinase regulated mainly by the PI3K/Akt signaling pathway [144], plays a central role in the aging process [121, 122, 145]. Research in the nematode C. elegans and the fruit fly Drosophila melanogaster was the first to show that mTOR regulates lifespan [146, 147]. Nrf2 regulates mTOR through intermediate proteins acting on mTOR posttranslationally [148] and also indirectly upregulates mTOR activity through increasing the expression of the mTOR activator, RagD [149]. However, Bendavit et al. have proposed that Nrf2 directly binds to and thus regulates the mTOR promoter [150]. Furthermore, β-TrCP degrades Nrf2 through interaction with the Neh6 region, a structural motif recognized by β-TrCP E3 ligase [110]. Qiao and colleagues have shown that mTOR and Nrf2 also interact via the β-TrCP/Nrf2 pathway [151], wherein mTOR promotes Nrf2 nuclear translocation through inhibiting β-TrCP expression; this process is critical to the initiation and progression of diabetic nephropathy [151]. The Akt/mTORC1/Nrf2 signaling pathway is therefore a valuable therapeutic target for AMD [152, 153], while the Nrf2-miR-129-3p-mTOR axis may be a therapeutic target for autophagy and tumor resistance [154].
Although both mTOR and Nrf2 play an essential role in aging, evidence demonstrating a direct regulatory effect between the two is very limited. Here, we present available studies showing a direct regulatory effect rather than interaction through other signaling pathways. Clarifying the relationship between mTOR and Nrf2 will help elucidate the underlying mechanisms of aging and aging-related diseases.
6. Bach1 and c-Myc
Bach1 (BTB and CNC homolog 1) and c-Myc (a proto-oncogene) are the main negative regulators of Nrf2. During aging, both Nrf2 inhibitors increase [155, 156], suggestive of the mechanism by which age compromises adaptive homeostasis. The ARE motif is recognized by Bach1, best known as a repressor of NQO1 expression [157]. Basally, the Bach1/Maf heterodimer competes with the Nrf2/Maf heterodimer for binding to ARE, resulting in the repression or activation of target genes under different cellular conditions [157, 158]. Higher heme levels inhibit Bach1 DNA-binding activity, leading to Bach1 dissociation from enhancers and activation of target genes [159]. Bach1 downregulation protects cells from oxidative stress through enhancing Nrf2/ARE signaling [160]. Thus, Bach1 plays an important role in the redox induction of HO-1 and NQO1 [157, 161]. Recently, Pomatto and colleagues demonstrated in vitro that hyperoxia strongly elevates Bach1 levels and the competitive effect of Bach1 and Nrf2 increases in a time-dependent manner [162]. Additionally, silencing Bach1 increases basal expression of Nrf2-regulated antioxidant genes in both the young and older human bronchial epithelial cells [163], suggesting that Bach1 is a potential target for antiaging intervention. In addition to its role in aging, the Bach1 negative regulation of Nrf2 is also important in lung cancer metastasis [164, 165].
A transcription factor in the basic helix-loop-helix-leucine zipper (bHLH-LZ) family, c-Myc regulates the expression of many genes involved in cellular growth and proliferation, as well as DNA damage and genomic instability [166, 167]. Interestingly, Myc+/− mice exhibit an increased lifespan [168]. The c-Myc is a binding competitor of Nrf2 for ARE sites and regulates Nrf2/ARE signaling through interaction with the ARE-binding complex that increases Nrf2 degradation [169, 170], but until recently, the specific mechanism of c-Myc effects on aging was unknown.
Collectively, as known inhibitors of Nrf2-regulated transcription, increases in Bach1 and c-Myc reflect declining Nrf2 effectiveness in adaptive homeostasis during aging. Davies and Forman have proposed that although Bach1 and c-Myc expression increased with age, continual adaptive homeostasis contributes to lower cancer risk [171]. Additionally, Pomatto et al. believe that changes in the balance of Nrf2, Bach1, and c-Myc levels may be the cause of serious disturbances to the stress response and adaptive homeostasis during chronic hyperoxia and aging [162]. Nevertheless, research has mainly focused on quantitative changes when examining the interaction between the two inhibitors, Nrf2, and aging. We require further exploration on how increased Bach1 and c-Myc expression generates aging-related changes through Nrf2 signaling.
7. MicroRNAs (miRNAs) in the Regulation of Keap1-Nrf2 Signaling
The miRNAs are small noncoding RNA sequences containing approximately 22-24 nucleotides. They inhibit gene expression by binding to complementary sequences in the 3′-untranslated region (UTR) of target mRNA and modulate many biological functions [172]. A growing body of evidence shows that miRNAs play key roles in and may thus be drug discovery targets for aging and age-related diseases. miR-34a, miR-100, and miR-21 are involved in endothelial senescence and upregulated in senescent cells [173, 174]. Through downregulation of GSK-3β and upregulation of Nrf2 signaling, miR-135a and miR-135b-5p, respectively, alleviate neuronal damage from oxygen-glucose deprivation and reoxygenation [175, 176]. Additionally, high expression of miR-200a inhibits Keap1 and activates the Nrf2 antioxidant pathway, thereby preventing lipids from accumulating oxidative damage [177]. Moreover, miR-941 is a novel Keap1-targeting miRNA that protects cells from oxidative stress through inhibition of Keap1 3′-UTR expression, thus activating the Nrf2 cascade [178]. miR-144 is a therapeutic option for retinal degenerative diseases [179]. Numerous miRNAs participate in the regulation of the Keap1-Nrf2 pathway for oxidative stress. Additional studies will likely uncover more miRNAs that are involved in regulating Nrf2/ARE signaling through targeting Nrf2, Keap1, or associated proteins [180]. Currently, less information is available on aging-dependent miRNA changes that target Nrf2 or Keap1 signaling. Interactions involving miRNA are complex because each miRNA has a variety of target genes, while being controlled by multiple upstream signals. Moreover, miRNA expression can differ across tissues or species. Despite the potential difficulties in untangling these networks, miRNAs are clearly an attractive field for studies on aging-related disease therapy.
8. Antioxidant Ingredients in the Diet
Diet is an important regulatory factor during the aging process [181]. Dietary calorie restriction and dietary antioxidants are advantageous for healthy aging and longevity by decreasing oxidative stress and modulating age-related signaling pathways [182]. Dietary polyphenols (phenolics), the general term for a variety of chemical substances found in plants, have been considered potential antiaging compounds due to their prominent antioxidant capacity [182, 183]. For example, curcumin, a polyphenolic compound isolated from Curcuma longa, has been shown to exert antiaging characteristics [184]. Research has shown that curcumin is a hormetic agent that stabilizes Nrf2 and enhances the expression of HO-1 [185, 186]. Furthermore, curcumin upregulates the antiapoptotic Bcl-2 protein and downregulates the proapoptotic proteins, Bax and caspase-3 [187]. The oxidative stress biomarkers, including MDA (malondialdehyde), SOD, and GSH, are upregulated (SOD, GSH) or downregulated (MDA) by curcumin [188]. The antioxidant activities of catechins have also been extensively studied [189]. Of these, (−)-epigallocatechin-3-gallate (EGCG), the principal catechin in green tea, has received the most attention. Sun and colleagues demonstrated that EGCG can activate Nrf2 by binding to Keap1, which plays a key role in preventing diabetic nephropathy [190]. Additionally, EGCG can activate other factors, such as Akt and ERK, which may also result in Nrf2 activation [191]. Resveratrol is the most widely studied polyphenol and is considered an important compound for life extension and anticancer and cardioprotection treatments. These three properties are inseparable from its ability to reduce oxidative stress [192]. Resveratrol can stimulate the activity of Nrf2, upregulate a variety of antioxidant enzymes such as NQO1 and HO-1, and control γ-glutamylcysteine synthetase (GCLC), the enzyme that regulates glutathione synthesis [193]. On the one hand, resveratrol can regulate Nrf2 by activating the PI3K/Akt signaling pathway to increase Nrf2 stabilization, and on the other hand, it can also upregulate the expression of p62, which can compete with Nrf2 to bind to the Keap1-Kelch domain, thereby promoting Nrf2 nuclear translocation [194]. Other bioactive polyphenols including tannic acid, wogonin, ampelopsin, (-)epicatechin, ellagic acids, lignans, rosmarinic acid, and their derivatives are also potentially important for healthy human aging and longevity.
Dietary flavonoids, also major antioxidant phytochemicals, are closely associated with antiaging due to their direct antioxidant capacity [195]. More than 8000 naturally occurring flavonoids have been identified from various vegetables, fruits, and plants [196]. Many natural flavonoids, including flavones, flavonols, chalcones, and isoflavones, have been identified to be Nrf2 activators and are thus regarded to be potential antiaging agents [195, 197]. For instance, quercetin upregulates the expression of Nrf2, HO-1, NQO1, and GCLC to resist oxidative stress. Quercetin could restore the serum and tissue activities of SOD, GSH-Px, and CAT and reduce ROS and MDA levels to different extents in Nrf2 wild-type model mice of dry, age-related macular degeneration [198]. Moreover, a recent study showed that quercetin and catechin can not only directly but also indirectly regulate the expression of Nrf2, HO-1, and NQO1 via two miRNA molecules, miR-25-3p and let-7a-5p [199]. Similarly, other widely investigated flavonoids, such as apigenin [200], baicalin [201], kaempferol [202], genistein [203], dihydroquercetin [204], and procyanidins [205], protect cells and tissues against oxidative injury via activation of the Nrf2 signaling pathway. Notably, baicalin and baicalein can increase Nrf2 protein levels by upregulating the expression of p62 protein and phosphorylating ERK1/2 and PKC [206].
Some triterpenoids also have obvious antioxidant and antiaging activity. For instance, Xu et al. demonstrated that ganoderic acid D exhibits potent antisenescence effects against H2O2-induced premature senescence of human amniotic mesenchymal stem cells through the activation of PERK/Nrf2 signals [141]. Additionally, Lefaki and colleagues demonstrated that 18α-glycyrrhetinic acid can activate the Nrf2/SKN-1 pathway against DNA damage [207]. These findings suggest that dietary triterpenoids could be used in a prophylactic or therapeutic strategy against aging or aging-related diseases.
Dietary constituents activate the Nrf2/ARE pathway by a variety of mechanisms, but most of the activators act by stimulating the phosphorylation of Nrf2, leading to the dissociation of Nrf2 from Keap1. For example, curcumin and resveratrol can phosphorylate Nrf2 through the PI3K/Akt signaling pathway. Phosphorylated Nrf2 translocates into the nucleus and binds to ARE/sMaf, promoting the transcription of ARE-driven genes and thereby alleviating oxidative stress-mediated damage [187, 191, 194]. Similarly, dietary components can also activate AMPK, CK2, PKC, ERK, and p38 signaling pathways to cause Nrf2 phosphorylation and nuclear translocation, thus promoting the transcription of antioxidant genes [141, 204, 206, 208]. Additionally, certain dietary phytochemicals such as resveratrol can react with the cysteine residues of Keap1 (i.e., Cys151, Cys257, Cys273, Cys288, and Cys297) via oxidation or alkylation to dissociate Nrf2 from Keap1 [208, 209]. Flavonoids baicalin and baicalein can increase Nrf2 protein levels by upregulating the expression of p62 protein [206], which contains a KIR motif that allows it to compete with Nrf2 to bind to Keap1 and obstruct the formation of Nrf2-Keap1 complex [210]. Other dietary constituents such as EGCG promote Nrf2 into the nucleus by competing with Keap1 [190]. Therefore, exploring the effects of dietary components on the activation of the Nrf2/ARE pathway is crucial to elucidate the mechanisms of these dietary antioxidants in exerting chemopreventive effects.
Taken together, there is increasing evidence that dietary antioxidant phytochemicals exert positive effects against age and age-related diseases. The intracellular Keap1/Nrf2/ARE signaling pathway plays a vital role in this activation process. Generally, these dietary antioxidant phytochemicals exert their antiaging effects by targeting Nrf2 signals. However, most studies mainly focus on in vitro experimental models. So far, only a few in vivo studies have been conducted on the antiaging effects. Moreover, the activation of the Nrf2/ARE pathway is not always beneficial because certain flavonoids may also promote the growth of cancer cells [208]. Therefore, the relationship between the influence of dietary antioxidant intake on the Nrf2/ARE pathway and the physiological effect still remains unclear. In addition, some dietary antioxidants such as flavonoids undergo sulfation, glucuronic acid oxidation, and methylation in the intestinal cells of the small intestine and liver, thereby producing different metabolites [211]. Therefore, this stage of metabolism changes the bioavailability of the parent compound. Elucidating these mechanisms will require more in vivo studies.
9. Conclusions
Elevated oxidative stress in older animals is considered a hallmark of aging. The Keap1-Nrf2 signaling pathway is the most important cellular antioxidant system, controlling the expression of various antioxidant enzymes. Therefore, Keap1-Nrf2 signaling is a potential pharmacological target for the development of antiaging therapies. Keap1 regulation, especially via its component cysteines, is mainly restricted to the cytoplasm. Cracking the “cysteine code” in Keap1 is a major undertaking that is expected to clarify underlying mechanisms of action of certain antiaging agents. In contrast, Nrf2 regulation is not limited to the cytoplasm, and nuclear regulation through Bach1 and c-Myc is also very important. Furthermore, various factors regulate Nrf2 signal transduction, including Nrf2 protein stability, phosphorylation, nuclear export, and Nrf2-ARE complex formation (Figure 3). The Keap1-Nrf2 signaling pathway may also closely interact with other signaling pathways, such as PI3K/Akt/GSK-3β and ERK. The direct interaction between Nrf2 and mTOR may be an attractive area of study to uncover antiaging therapeutic targets.
Figure 3.
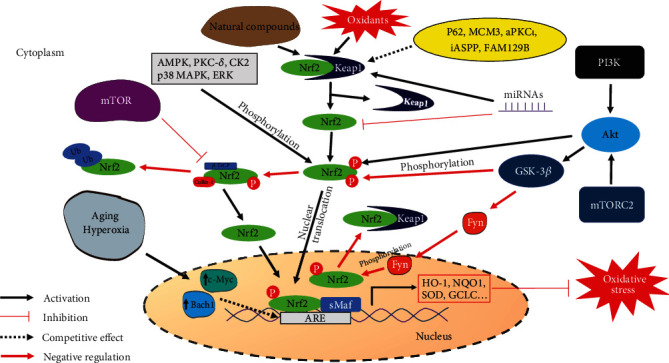
The crosstalk between Keap1/Nrf2 and other signaling pathways involved in oxidative stress and aging.
Although scientists have achieved unprecedented results in elucidating the Keap1-Nrf2 signaling pathway, many key issues remain unresolved. For example, is there a more efficient domain in Keap1 that regulates its conformational changes? Are there other specific molecules involved upstream or downstream of Nrf2? Why does Nrf2 decrease with age? Moreover, we know next to nothing about the possible effects of miRNAs on the Nrf2 pathway in elderly individuals. Additionally, the relationship between the influence of dietary compounds on the Keap1/Nrf2/ARE signaling pathway and its physiological effects and how to improve their bioavailability still needs more research. Understanding these mechanisms should provide new tools for developing interventions that improve the quality of life in older adults.
Acknowledgments
The authors are grateful for financial support from the National Natural Science Foundation of China (No. 31960191), Science and Technology Innovation Leading Academics of National High-level Personnel of Special Support Program (GKFZ-2018-29), Guizhou Provincial S&T Foundation (No. QKHJC-ZK-2021-ZD026), and S&T Foundation of Zunyi Science and Technology Bureau (Nos. ZSKH-HZ-Z-2020212, ZSKH-HZ-Z-2020222, ZSKH-HZ-Z-2020249, and ZSK-RC-2020-1).
Conflicts of Interest
The authors confirm that there are no conflicts of interest.
References
- 1.Finkel T., Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Gerschman R., Gilbert D. L., Nye S. W., Dwyer P., Fenn W. O. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119(3097):623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 3.Sies H., Cadenas E. Oxidative stress: damage to intact cells and organs. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1985;311(1152):617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 4.Jones D. P. Redefining oxidative stress. Antioxidants & Redox Signaling. 2006;8(9-10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 5.Hauptmann N., Grimsby J., Shih J. C., Cadenas E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Archives of Biochemistry and Biophysics. 1996;335(2):295–304. doi: 10.1006/abbi.1996.0510. [DOI] [PubMed] [Google Scholar]
- 6.Di Meo S., Reed T. T., Venditti P., Victor V. M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Medicine and Cellular Longevity. 2016;2016:44. doi: 10.1155/2016/1245049.1245049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray P. D., Huang B. W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinlan C. L., Perevoshchikova I. V., Hey-Mogensen M., Orr A. L., Brand M. D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biology. 2013;1(1):304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shigenaga M. K., Hagen T. M., Ames B. N. Oxidative damage and mitochondrial decay in aging. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojsiat J., Zoltowska K. M., Laskowska-Kaszub K., Wojda U. Oxidant/Antioxidant Imbalance in Alzheimer’s Disease: Therapeutic and Diagnostic Prospects. Oxidative Medicine and Cellular Longevity. 2018;2018:16. doi: 10.1155/2018/6435861.6435861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Z., Dong Q., Liu X., et al. Dynamic transcriptome profiling in DNA damage-induced cellular senescence and transient cell-cycle arrest. Genomics. 2020;112(2):1309–1317. doi: 10.1016/j.ygeno.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Luceri C., Bigagli E., Femia A. P., Caderni G., Giovannelli L., Lodovici M. Aging related changes in circulating reactive oxygen species (ROS) and protein carbonyls are indicative of liver oxidative injury. Toxicology Reports. 2018;5:141–145. doi: 10.1016/j.toxrep.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou H. L., Schumacher B. DNA damage responses and p53 in the aging process. Blood. 2018;131(5):488–495. doi: 10.1182/blood-2017-07-746396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schumacher B., Hanazawa M., Lee M. H., et al. Translational Repression of _C. elegans_ p53 by GLD-1 Regulates DNA Damage- Induced Apoptosis. Cell. 2005;120(3):357–368. doi: 10.1016/j.cell.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Edifizi D., Nolte H., Babu V., et al. Multilayered Reprogramming in Response to Persistent DNA Damage in _C. elegans_. Cell Reports. 2017;20(9):2026–2043. doi: 10.1016/j.celrep.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva Dias D., Moraes-Silva I. C., Bernardes N., et al. Exercise training initiated at old stage of lifespan attenuates aging-and ovariectomy-induced cardiac and renal oxidative stress: role of baroreflex. Experimental Gerontology. 2019;124, article 110635 doi: 10.1016/j.exger.2019.110635. [DOI] [PubMed] [Google Scholar]
- 17.Lee J. Y., Paik I. Y., Kim J. Y. Voluntary exercise reverses immune aging induced by oxidative stress in aging mice. Experimental Gerontology. 2019;115:148–154. doi: 10.1016/j.exger.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Sajadimajd S., Khazaei M. Oxidative stress and cancer: the role of Nrf2. Current Cancer Drug Targets. 2018;18(6):538–557. doi: 10.2174/1568009617666171002144228. [DOI] [PubMed] [Google Scholar]
- 19.Yuan H., Xu Y., Luo Y., Wang N. X., Xiao J. H. Role of Nrf2 in cell senescence regulation. Molecular and Cellular Biochemistry. 2021;476(1):247–259. doi: 10.1007/s11010-020-03901-9. [DOI] [PubMed] [Google Scholar]
- 20.Gorbunova V., Rezazadeh S., Seluanov A. Dangerous entrapment for NRF2. Cell. 2016;165(6):1312–1313. doi: 10.1016/j.cell.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 21.Wati S. M., Matsumaru D., Motohashi H. NRF2 pathway activation by KEAP1 inhibition attenuates the manifestation of aging phenotypes in salivary glands. Redox Biology. 2020;36, article 101603 doi: 10.1016/j.redox.2020.101603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih P. H., Yen G. C. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8(2):71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 23.Kapeta S., Chondrogianni N., Gonos E. S. Nuclear Erythroid Factor 2-mediated Proteasome Activation Delays Senescence in Human Fibroblasts. The Journal of Biological Chemistry. 2010;285(11):8171–8184. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh K., Wakabayashi N., Katoh Y., et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & Development. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furukawa M., Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Molecular and Cellular Biology. 2005;25(1):162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cullinan S. B., Gordan J. D., Jin J., Harper J. W., Diehl J. A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Molecular and Cellular Biology. 2004;24(19):8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills E. L., Ryan D. G., Prag H. A., et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D. D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Molecular and Cellular Biology. 2003;23(22):8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinti L., Dayalan Naidu S., Träger U., et al. KEAP1-modifying small molecule reveals muted NRF2 signaling responses in neural stem cells from Huntington's disease patients. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(23):E4676–E4685. doi: 10.1073/pnas.1614943114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidlin C. J., Dodson M. B., Madhavan L., Zhang D. D. Redox regulation by NRF2 in aging and disease. Free Radical Biology & Medicine. 2019;134:702–707. doi: 10.1016/j.freeradbiomed.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rojo A. I., Pajares M., Rada P., et al. NRF2 deficiency replicates transcriptomic changes in Alzheimer's patients and worsens APP and TAU pathology. Redox Biology. 2017;13:444–451. doi: 10.1016/j.redox.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachdeva M. M., Cano M., Handa J. T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Experimental Eye Research. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liddell J. R. Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration. Antioxidants. 2017;6(3):p. 65. doi: 10.3390/antiox6030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Done A. J., Gage M. J., Nieto N. C., Traustadóttir T. Exercise-induced Nrf2-signaling is impaired in aging. Free Radical Biology & Medicine. 2016;96:130–138. doi: 10.1016/j.freeradbiomed.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Bruns D. R., Drake J. C., Biela L. M., Peelor F. F., 3rd, Miller B. F., Hamilton K. L. Nrf2 signaling and the slowed aging phenotype: evidence from long-lived models. Oxidative Medicine and Cellular Longevity. 2015;2015:15. doi: 10.1155/2015/732596.732596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L., Zhao Q., Xiao Y., et al. Trehalose targets Nrf2 signal to alleviate d-galactose induced aging and improve behavioral ability. Biochemical and Biophysical Research Communications. 2020;521(1):113–119. doi: 10.1016/j.bbrc.2019.10.088. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Xiong Y., Zhang A., et al. Oligosaccharide attenuates aging-related liver dysfunction by activating Nrf2 antioxidant signaling. Food Science & Nutrition. 2020;8(7):3872–3881. doi: 10.1002/fsn3.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuang Y., Wu H., Wang X., He J., He S., Yin Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxidative Medicine and Cellular Longevity. 2019;2019:14. doi: 10.1155/2019/7591840.7591840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao Q., Piao R., Wang H., Li C., Song L. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. International Journal of Biological Macromolecules. 2018;118(Part A):747–755. doi: 10.1016/j.ijbiomac.2018.06.130. [DOI] [PubMed] [Google Scholar]
- 40.Lewis K. N., Wason E., Edrey Y. H., Kristan D. M., Nevo E., Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(12):3722–3727. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prašnikar E., Borišek J., Perdih A. Senescent cells as promising targets to tackle age-related diseases. Ageing Research Reviews. 2021;66, article 101251 doi: 10.1016/j.arr.2020.101251. [DOI] [PubMed] [Google Scholar]
- 42.Rajasekaran N. S., Varadharaj S., Khanderao G. D., et al. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxidants & Redox Signaling. 2011;14(6):957–971. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X. F., Yao J., Gao S. G., et al. Nrf2 overexpression predicts prognosis and 5-FU resistance in gastric cancer. Asian Pacific Journal of Cancer Prevention. 2013;14(9):5231–5235. doi: 10.7314/APJCP.2013.14.9.5231. [DOI] [PubMed] [Google Scholar]
- 44.Liu P., Ma D., Wang P., Pan C., Fang Q., Wang J. Nrf2 overexpression increases risk of high tumor mutation burden in acute myeloid leukemia by inhibiting MSH2. Cell Death & Disease. 2021;12(1):p. 20. doi: 10.1038/s41419-020-03331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon M., Lamont D. J., Beattie K. A., Hayes J. D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(44):18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takaya K., Suzuki T., Motohashi H., et al. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radical Biology & Medicine. 2012;53(4):817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eggler A. L., Small E., Hannink M., Mesecar A. D. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. The Biochemical Journal. 2009;422(1):171–180. doi: 10.1042/BJ20090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dayalan Naidu S., Muramatsu A., Saito R., et al. C151 in KEAP1 is the main cysteine sensor for the cyanoenone class of NRF2 activators, irrespective of molecular size or shape. Scientific Reports. 2018;8(1):p. 8037. doi: 10.1038/s41598-018-26269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito R., Suzuki T., Hiramoto K., et al. Characterizations of three major cysteine sensors of Keap1 in stress response. Molecular and Cellular Biology. 2015;36(2):271–284. doi: 10.1128/MCB.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMahon M., Swift S. R., Hayes J. D. Zinc-binding triggers a conformational-switch in the cullin-3 substrate adaptor protein KEAP1 that controls transcription factor NRF2. Toxicology and Applied Pharmacology. 2018;360:45–57. doi: 10.1016/j.taap.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 51.Hourihan J. M., Kenna J. G., Hayes J. D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxidants & Redox Signaling. 2013;19(5):465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T., Muramatsu A., Saito R., et al. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Reports. 2019;28(3):746–758.e4. doi: 10.1016/j.celrep.2019.06.047. [DOI] [PubMed] [Google Scholar]
- 53.Zipper L. M., Mulcahy R. T. The Keap1 BTB/POZ Dimerization Function Is Required to Sequester Nrf2 in Cytoplasm∗. The Journal of Biological Chemistry. 2002;277(39):36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- 54.Wei S., Pei Y., Wang Y., et al. Role of human Keap1 S53 and S293 residues in modulating the binding of Keap1 to Nrf2. Biochimie. 2019;158:73–81. doi: 10.1016/j.biochi.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki T., Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. The Journal of Biological Chemistry. 2017;292(41):16817–16824. doi: 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shang D., Sun D., Shi C., et al. Activation of epidermal growth factor receptor signaling mediates cellular senescence induced by certain pro-inflammatory cytokines. Aging Cell. 2020;19(5, article e13145) doi: 10.1111/acel.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sendama W. The effect of ageing on the resolution of inflammation. Ageing Research Reviews. 2020;57, article 101000 doi: 10.1016/j.arr.2019.101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H., Feng Y., Xu M., Yang J., Wang Z., Di G. Four-octyl itaconate activates Keap1-Nrf2 signaling to protect neuronal cells from hydrogen peroxide. Cell Communication and Signaling. 2018;16(1):p. 81. doi: 10.1186/s12964-018-0294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi M., Li L., Iwamoto N., et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Molecular and Cellular Biology. 2009;29(2):493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moscat J., Diaz-Meco M. T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137(6):1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao W., Wang Z., Fu Z., et al. p62/SQSTM1 protects against cisplatin-induced oxidative stress in kidneys by mediating the cross talk between autophagy and the Keap1-Nrf2 signalling pathway. Free Radical Research. 2019;53(7):800–814. doi: 10.1080/10715762.2019.1635251. [DOI] [PubMed] [Google Scholar]
- 62.Jena K. K., Mehto S., Kolapalli S. P., et al. TRIM16 governs the biogenesis and disposal of stress-induced protein aggregates to evade cytotoxicity: implication for neurodegeneration and cancer. Autophagy. 2019;15(5):924–926. doi: 10.1080/15548627.2019.1586251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain A., Lamark T., Sjøttem E., et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of Biological Chemistry. 2010;285(29):22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komatsu M., Kurokawa H., Waguri S., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature Cell Biology. 2010;12(3):213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 65.Copple I. M., Lister A., Obeng A. D., et al. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. The Journal of Biological Chemistry. 2010;285(22):16782–16788. doi: 10.1074/jbc.M109.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau A., Wang X. J., Zhao F., et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Molecular and Cellular Biology. 2010;30(13):3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan W., Tang Z., Chen D., et al. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy. 2010;6(5):614–621. doi: 10.4161/auto.6.5.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee D. H., Park J. S., Lee Y. S., et al. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy. 2020;16(11):1949–1973. doi: 10.1080/15548627.2020.1712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen M., Rubinsztein D. C., Walker D. W. Autophagy as a promoter of longevity: insights from model organisms. Nature Reviews. Molecular Cell Biology. 2018;19(9):579–593. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salazar G., Cullen A., Huang J., et al. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy. 2020;16(6):1092–1110. doi: 10.1080/15548627.2019.1659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumsta C., Chang J. T., Lee R., et al. The autophagy receptor p62/SQST-1 promotes proteostasis and longevity in _C. elegans_ by inducing autophagy. Nature Communications. 2019;10(1):p. 5648. doi: 10.1038/s41467-019-13540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aparicio 1R., Hansen M., Walker D. W., Kumsta C. The selective autophagy receptor SQSTM1/p62 improves lifespan and proteostasis in an evolutionarily conserved manner. Autophagy. 2020;16(4):772–774. doi: 10.1080/15548627.2020.1725404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang C., Xu Q., Martin T. D., et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349(6255, article aaa5612) doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwon J., Han E., Bui C. B., et al. Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Reports. 2012;13(2):150–156. doi: 10.1038/embor.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aparicio R., Rana A., Walker D. W. Upregulation of the Autophagy Adaptor p62/SQSTM1 Prolongs Health and Lifespan in Middle-Aged _Drosophila_. Cell Reports. 2019;28(4):1029–1040.e5. doi: 10.1016/j.celrep.2019.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larrieu D., Britton S., Demir M., Rodriguez R., Jackson S. P. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science. 2014;344(6183):527–532. doi: 10.1126/science.1252651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhide S., Trujillo A. S., O'Connor M. T., et al. Increasing autophagy and blocking Nrf2 suppress laminopathy-induced age-dependent cardiac dysfunction and shortened lifespan. Aging Cell. 2018;17(3, article e12747) doi: 10.1111/acel.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei R., Enaka M., Muragaki Y. Activation of KEAP1/NRF2/P62 signaling alleviates high phosphate-induced calcification of vascular smooth muscle cells by suppressing reactive oxygen species production. Scientific Reports. 2019;9(1, article 10366) doi: 10.1038/s41598-019-46824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamberg N., Tahk S., Koit S., et al. Keap1-MCM3 interaction is a potential coordinator of molecular machineries of antioxidant response and genomic DNA replication in metazoa. Scientific Reports. 2018;8(1, article 12136) doi: 10.1038/s41598-018-30562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian L., Lu Y., Yang T., et al. aPKCι promotes gallbladder cancer tumorigenesis and gemcitabine resistance by competing with Nrf2 for binding to Keap1. Redox Biology. 2019;22, article 101149 doi: 10.1016/j.redox.2019.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ge W., Zhao K., Wang X., et al. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap1 binding. Cancer Cell. 2017;32(5):561–573.e6. doi: 10.1016/j.ccell.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 82.Cheng K. C., Lin R. J., Cheng J. Y., et al. FAM129B, an antioxidative protein, reduces chemosensitivity by competing with Nrf2 for Keap1 binding. eBioMedicine. 2019;45:25–38. doi: 10.1016/j.ebiom.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao S., Song T., Gu Y., et al. Hydrogen sulfide alleviates liver injury Through the S‐Sulfhydrated‐Kelch‐Like ECH‐Associated Protein 1/Nuclear Erythroid 2–Related Factor 2/Low‐Density Lipoprotein Receptor–Related Protein 1 pathway. Hepatology. 2021;73(1):282–302. doi: 10.1002/hep.31247. [DOI] [PubMed] [Google Scholar]
- 84.Tonelli C., Chio I., Tuveson D. A. Transcriptional regulation by Nrf2. Antioxidants & Redox Signaling. 2018;29(17):1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engelman J. A., Luo J., Cantley L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Reviews Genetics. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 86.Marte B. M., Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends in Biochemical Sciences. 1997;22(9):355–358. doi: 10.1016/S0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 87.Manning B. D., Cantley L. C. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Méndez-Pertuz M., Martínez P., Blanco-Aparicio C., et al. Modulation of telomere protection by the PI3K/AKT pathway. Nature Communications. 2017;8(1):p. 1278. doi: 10.1038/s41467-017-01329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jing L., Jiang J. R., Liu D. M., et al. Structural characterization and antioxidant activity of polysaccharides from Athyrium multidentatum (Doll.) Ching in d-galactose-induced aging mice via PI3K/AKT pathway. Molecules. 2019;24(18):p. 3364. doi: 10.3390/molecules24183364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin D., Rojo A. I., Salinas M., et al. Regulation of Heme Oxygenase-1 Expression through the Phosphatidylinositol 3-Kinase/Akt Pathway and the Nrf2 Transcription Factor in Response to the Antioxidant Phytochemical Carnosol∗. The Journal of Biological Chemistry. 2004;279(10):8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 91.Lai T. T., Yang C. M., Yang C. H. Astaxanthin protects retinal photoreceptor cells against high glucose-induced oxidative stress by induction of antioxidant enzymes via the PI3K/Akt/Nrf2 pathway. Antioxidants. 2020;9(8):p. 729. doi: 10.3390/antiox9080729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ali T., Kim T., Rehman S. U., et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer's disease. Molecular Neurobiology. 2018;55(7):6076–6093. doi: 10.1007/s12035-017-0798-6. [DOI] [PubMed] [Google Scholar]
- 93.Li H., Tang Z., Chu P., et al. Neuroprotective effect of phosphocreatine on oxidative stress and mitochondrial dysfunction induced apoptosis in vitro and in vivo: involvement of dual PI3K/Akt and Nrf2/HO-1 pathways. Free Radical Biology & Medicine. 2018;120:228–238. doi: 10.1016/j.freeradbiomed.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Papaiahgari S., Zhang Q., Kleeberger S. R., Cho H. Y., Reddy S. P. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxidants & Redox Signaling. 2006;8(1-2):43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- 95.de Oliveira M. R., Ferreira G. C., Schuck P. F., Dal Bosco S. M. Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chemico-Biological Interactions. 2015;242:396–406. doi: 10.1016/j.cbi.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y., Liu B., Chen X., et al. Naringenin ameliorates behavioral dysfunction and neurological deficits in a D-galactose-induced aging mouse model through activation of PI3K/Akt/Nrf2 pathway. Rejuvenation Research. 2017;20(6):462–472. doi: 10.1089/rej.2017.1960. [DOI] [PubMed] [Google Scholar]
- 97.Zhao M., Tang X., Gong D., Xia P., Wang F., Xu S. Bungeanum improves cognitive dysfunction and neurological deficits in D-galactose-induced aging mice via activating PI3K/Akt/Nrf2 signaling pathway. Frontiers in Pharmacology. 2020;11:p. 71. doi: 10.3389/fphar.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan Z., Dai Y., Fu H., et al. Curcumin exerts a protective effect against premature ovarian failure in mice. Journal of Molecular Endocrinology. 2018;60(3):261–271. doi: 10.1530/JME-17-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng B., Liu L., Liao X., Zhang C., Ruan H. Thyroid hormone protects cardiomyocytes from H2O2-induced oxidative stress via the PI3K-AKT signaling pathway. Experimental Cell Research. 2019;380(2):205–215. doi: 10.1016/j.yexcr.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Wu H., Chen M., Yan P., et al. Erythropoietin suppresses D-galactose-induced aging of rats via the PI3K/Akt/Nrf2-ARE pathway. International Journal of Clinical and Experimental Pathology. 2018;11(4):2227–2240. [PMC free article] [PubMed] [Google Scholar]
- 101.Yu Y., Bai F., Liu Y., et al. Fibroblast growth factor (FGF21) protects mouse liver against D-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Molecular and Cellular Biochemistry. 2015;403(1-2, article 2358):287–299. doi: 10.1007/s11010-015-2358-6. [DOI] [PubMed] [Google Scholar]
- 102.Grimes C. A., Jope R. S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Progress in Neurobiology. 2001;65(4):391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 103.Woodgett J. R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. The EMBO Journal. 1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jellusova J., Cato M. H., Apgar J. R., et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nature Immunology. 2017;18(3):303–312. doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 106.Juhaszova M., Zorov D. B., Yaniv Y., Nuss H. B., Wang S., Sollott S. J. Role of glycogen synthase kinase-3β in cardioprotection. Circulation Research. 2009;104(11):1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salazar M., Rojo A. I., Velasco D., de Sagarra R. M., Cuadrado A. Glycogen Synthase Kinase-3β Inhibits the Xenobiotic and Antioxidant Cell Response by Direct Phosphorylation and Nuclear Exclusion of the Transcription Factor Nrf2∗. The Journal of Biological Chemistry. 2006;281(21):14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 108.Xin Y., Bai Y., Jiang X., et al. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biology. 2018;15:405–417. doi: 10.1016/j.redox.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rojo A. I., Sagarra M. R., Cuadrado A. GSK-3β down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. Journal of Neurochemistry. 2008;105(1):192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 110.Rada P., Rojo A. I., Chowdhry S., McMahon M., Hayes J. D., Cuadrado A. SCF/ -TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Molecular and Cellular Biology. 2011;31(6):1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou J., Freeman T. A., Ahmad F., et al. GSK-3α is a central regulator of age-related pathologies in mice. The Journal of Clinical Investigation. 2013;123(4):1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu M., Wang P., Qiao Y., et al. GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: a molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biology. 2019;26, article 101275 doi: 10.1016/j.redox.2019.101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim H. S., Skurk C., Thomas S. R., et al. Regulation of Angiogenesis by Glycogen Synthase Kinase-3β∗. The Journal of Biological Chemistry. 2002;277(44):41888–41896. doi: 10.1074/jbc.M206657200. [DOI] [PubMed] [Google Scholar]
- 114.Morroni F., Sita G., Graziosi A., et al. Neuroprotective effect of caffeic acid phenethyl ester in a mouse model of Alzheimer's disease involves Nrf2/HO-1 pathway. Aging and Disease. 2018;9(4):605–622. doi: 10.14336/AD.2017.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lavu N., Richardson L., Radnaa E., et al. Oxidative stress-induced downregulation of glycogen synthase kinase 3 beta in fetal membranes promotes cellular senescence†. Biology of Reproduction. 2019;101(5):1018–1030. doi: 10.1093/biolre/ioz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan C., Zhang X., Miao J., et al. Farrerol directly targets GSK-3β to activate Nrf2-ARE pathway and protect EA.hy926 cells against oxidative stress-induced injuries. Oxidative Medicine and Cellular Longevity. 2020;2020:17. doi: 10.1155/2020/5967434.5967434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sklirou A. D., Gaboriaud-Kolar N., Papassideri I., Skaltsounis A. L., Trougakos I. P. 6-bromo-indirubin-3′-oxime (6BIO), a Glycogen synthase kinase-3β inhibitor, activates cytoprotective cellular modules and suppresses cellular senescence- mediated biomolecular damage in human fibroblasts. Scientific Reports. 2017;7(1, article 11713) doi: 10.1038/s41598-017-11662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farr S. A., Ripley J. L., Sultana R., et al. Antisense oligonucleotide against GSK-3β in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: involvement of transcription factor Nrf2 and implications for Alzheimer disease. Free Radical Biology & Medicine. 2014;67:387–395. doi: 10.1016/j.freeradbiomed.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen C.-H., Shaikenov T., Peterson T. R., et al. ER stress inhibits mTORC2 and Akt signaling through GSK-3β-mediated phosphorylation of rictor. Science Signaling. 2011;4(161, article ra10) doi: 10.1126/scisignal.2001731. [DOI] [PubMed] [Google Scholar]
- 120.Donato A. J., Machin D. R., Lesniewski L. A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circulation Research. 2018;123(7):825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harrison D. E., Strong R., Sharp Z. D., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saxton R. A., Sabatini D. M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang C., Pan S., Yan S., et al. Inhibitory effect of 14,15-EET on endothelial senescence through activation of mTOR complex 2/Akt signaling pathways. The International Journal of Biochemistry & Cell Biology. 2014;50:93–100. doi: 10.1016/j.biocel.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 124.Yang H. W., Hong H. L., Luo W. W., et al. mTORC2 facilitates endothelial cell senescence by suppressing Nrf2 expression via the Akt/GSK-3β/C/EBPα signaling pathway. Acta Pharmacologica Sinica. 2018;39(12):1837–1846. doi: 10.1038/s41401-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu Z.-X., Tan J.-W., Xu H., et al. Caspase-2 promotes AMPA receptor internalization and cognitive flexibility via mTORC2-AKT-GSK3β signaling. Nature Communications. 2019;10(1, article 3622) doi: 10.1038/s41467-019-11575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duan J., Cui J., Yang Z., et al. Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3β/Nrf2 signaling. Journal of Neuroinflammation. 2019;16(1, article 1406):p. 24. doi: 10.1186/s12974-019-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Joo M. S., Kim W. D., Lee K. Y., Kim J. H., Koo J. H., Kim S. G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Molecular and Cellular Biology. 2016;36(14):1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Griner E. M., Kazanietz M. G. Protein kinase C and other diacylglycerol effectors in cancer. Nature Reviews. Cancer. 2007;7(4):281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 129.Huang H. C., Nguyen T., Pickett C. B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. The Journal of Biological Chemistry. 2002;277(45):42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 130.Kikkawa U., Matsuzaki H., Yamamoto T. Protein kinase C (PKC ): activation mechanisms and functions. Journal of Biochemistry. 2002;132(6):831–839. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- 131.Yang X., Teguh D., Wu J. P., et al. Protein kinase C delta null mice exhibit structural alterations in articular surface, intra-articular and subchondral compartments. Arthritis Research & Therapy. 2015;17(1):p. 210. doi: 10.1186/s13075-015-0720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Klymenko K., Novokhatska T., Kizub I., Parshikov A., Dosenko V., Soloviev A. PKC-δ isozyme gene silencing restores vascular function in diabetic rats. Journal of Basic and Clinical Physiology and Pharmacology. 2014;25(4):1–9. doi: 10.1515/jbcpp-2013-0147. [DOI] [PubMed] [Google Scholar]
- 133.Litchfield D. W. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. The Biochemical Journal. 2003;369(1):1–15. doi: 10.1042/bj20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Homma M. K., Homma Y. Cell cycle and activation of CK2. Molecular and Cellular Biochemistry. 2008;316(1-2):49–55. doi: 10.1007/s11010-008-9823-4. [DOI] [PubMed] [Google Scholar]
- 135.Piazza F. A., Ruzzene M., Gurrieri C., et al. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood. 2006;108(5):1698–1707. doi: 10.1182/blood-2005-11-013672. [DOI] [PubMed] [Google Scholar]
- 136.Pi J., Bai Y., Reece J. M., et al. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radical Biology & Medicine. 2007;42(12):1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Apopa P. L., He X., Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. Journal of Biochemical and Molecular Toxicology. 2008;22(1):63–76. doi: 10.1002/jbt.20212. [DOI] [PubMed] [Google Scholar]
- 138.Ryu S. W., Woo J. H., Kim Y. H., Lee Y. S., Park J. W., Bae Y. S. Downregulation of protein kinase CKII is associated with cellular senescence. FEBS Letters. 2006;580(3):988–994. doi: 10.1016/j.febslet.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 139.Wang D., Jang D. J. Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Cancer Research. 2009;69(20):8200–8207. doi: 10.1158/0008-5472.CAN-09-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keum Y.-S., Yu S., Chang P. P.-J., et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Research. 2006;66(17):8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 141.Xu Y., Yuan H., Luo Y., Zhao Y. J., Xiao J. H. Ganoderic acid D protects human amniotic mesenchymal stem cells against oxidative stress-induced senescence through the PERK/NRF2 signaling pathway. Oxidative Medicine and Cellular Longevity. 2020;2020:18. doi: 10.1155/2020/8291413.8291413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Song C., Heping H., Shen Y., et al. AMPK/p38/Nrf2 activation as a protective feedback to restrain oxidative stress and inflammation in microglia stimulated with sodium fluoride. Chemosphere. 2020;244, article 125495 doi: 10.1016/j.chemosphere.2019.125495. [DOI] [PubMed] [Google Scholar]
- 143.Vrailas-Mortimer A., del Rivero T., Mukherjee S., et al. A Muscle-Specific p38 MAPK/Mef2/MnSOD Pathway Regulates Stress, Motor Function, and Life Span in _Drosophila_. Developmental Cell. 2011;21(4):783–795. doi: 10.1016/j.devcel.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu F., Na L., Li Y., Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell & Bioscience. 2020;10(1):p. 54. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Shin M.-G., Lee J.-W., Han J.-S., et al. Bacteria-derived metabolite, methylglyoxal, modulates the longevity ofC. elegansthrough TORC2/SGK-1/DAF-16 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(29):17142–17150. doi: 10.1073/pnas.1915719117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A. L., Orosz L., Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):p. 620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 147.Kapahi P., Zid B. M., Harper T., Koslover D., Sapin V., Benzer S. Regulation of Lifespan in _Drosophila_ by Modulation of Genes in the TOR Signaling Pathway. Current Biology. 2004;14(10):885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jia Y., Wang H., Wang Q., Ding H., Wu H., Pan H. Silencing Nrf2 impairs glioma cell proliferation via AMPK-activated mTOR inhibition. Biochemical and Biophysical Research Communications. 2016;469(3):665–671. doi: 10.1016/j.bbrc.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 149.Sancak Y., Peterson T. R., Shaul Y. D., et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bendavit G., Aboulkassim T., Hilmi K., Shah S., Batist G. Nrf2 Transcription Factor Can Directly Regulate mTOR: The Journal of Biological Chemistry. 2016;291(49):25476–25488. doi: 10.1074/jbc.M116.760249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qiao S., Liu R., Lv C., et al. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress _via_ the mTOR/ β-TrcP/Nrf2 pathway. Free Radical Biology & Medicine. 2019;145:118–135. doi: 10.1016/j.freeradbiomed.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 152.Li K. R., Yang S. Q., Gong Y. Q., et al. 3H-1,2-Dithiole-3-thione protects retinal pigment epithelium cells against ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling. Scientific Reports. 2016;6(1, article 25525) doi: 10.1038/srep25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu H., Hao L., Tang C., Zhu Y., Jiang Q., Yao J. Activation of KGFR-Akt-mTOR-Nrf2 signaling protects human retinal pigment epithelium cells from ultra-violet. Biochemical and Biophysical Research Communications. 2018;495(3):2171–2177. doi: 10.1016/j.bbrc.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 154.Sun W., Yi Y., Xia G., et al. Nrf2-miR-129-3p-mTOR axis controls an miRNA regulatory network involved in HDACi-induced autophagy. Molecular Therapy. 2019;27(5):1039–1050. doi: 10.1016/j.ymthe.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pomatto L., Cline M., Woodward N., et al. Aging attenuates redox adaptive homeostasis and proteostasis in female mice exposed to traffic-derived nanoparticles (‘vehicular smog’) Free Radical Biology & Medicine. 2018;121:86–97. doi: 10.1016/j.freeradbiomed.2018.04.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou L., Zhang H., Davies K., Forman H. J. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biology. 2018;14:35–40. doi: 10.1016/j.redox.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dhakshinamoorthy S., Jain A. K., Bloom D. A., Jaiswal A. K. Bach1 Competes with Nrf2 Leading to Negative Regulation of the Antioxidant Response Element (ARE)-mediated NAD(P)H:Quinone Oxidoreductase 1 Gene Expression and Induction in Response to Antioxidants∗. The Journal of Biological Chemistry. 2005;280(17):16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 158.Reichard J. F., Motz G. T., Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Research. 2007;35(21):7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ogawa K., Sun J., Taketani S., et al. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. The EMBO Journal. 2001;20(11):2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tian X., Cong F., Guo H., Fan J., Chao G., Song T. Downregulation of Bach1 protects osteoblasts against hydrogen peroxide-induced oxidative damage _in vitro_ by enhancing the activation of Nrf2/ARE signaling. Chemico-Biological Interactions. 2019;309, article 108706 doi: 10.1016/j.cbi.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 161.Sudan K., Vijayan V., Madyaningrana K., et al. TLR4 activation alters labile heme levels to regulate BACH1 and heme oxygenase-1 expression in macrophages. Free Radical Biology & Medicine. 2019;137:131–142. doi: 10.1016/j.freeradbiomed.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 162.Pomatto L., Sun P. Y., Yu K., et al. Limitations to adaptive homeostasis in an hyperoxia-induced model of accelerated ageing. Redox Biology. 2019;24, article 101194 doi: 10.1016/j.redox.2019.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang H., Zhou L., Davies K., Forman H. J. Silencing Bach1 alters aging-related changes in the expression of Nrf2-regulated genes in primary human bronchial epithelial cells. Archives of Biochemistry and Biophysics. 2019;672, article 108074 doi: 10.1016/j.abb.2019.108074. [DOI] [PubMed] [Google Scholar]
- 164.Lignitto L., LeBoeuf S. E., Homer H., et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell. 2019;178(2):316–329.e18. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wiel C., le Gal K., Ibrahim M. X., et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178(2):330–345.e22. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 166.Oster S. K., Ho C. S., Soucie E. L., Penn L. Z. The myc oncogene: MarvelouslY Complex. Advances in Cancer Research. 2002;84:81–154. doi: 10.1016/S0065-230X(02)84004-0. [DOI] [PubMed] [Google Scholar]
- 167.Kretzner L., Blackwood E. M., Eisenman R. N. Myc and Max proteins possess distinct transcriptional activities. Nature. 1992;359(6394):426–429. doi: 10.1038/359426a0. [DOI] [PubMed] [Google Scholar]
- 168.Hofmann J. W., Zhao X., de Cecco M., et al. Reduced expression of MYC increases longevity and enhances healthspan. Cell. 2015;160(3):477–488. doi: 10.1016/j.cell.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Levy S., Forman H. J. C-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62(3):237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang H., Li T. W. H., Zhou Y., et al. Activation of a novel c-Myc-miR27-prohibitin 1 circuitry in cholestatic liver injury inhibits glutathione synthesis in mice. Antioxidants & Redox Signaling. 2015;22(3):259–274. doi: 10.1089/ars.2014.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davies K., Forman H. J. Does Bach1 & c-Myc dependent redox dysregulation of Nrf2 & adaptive homeostasis decrease cancer risk in ageing? Free Radical Biology & Medicine. 2019;134:708–714. doi: 10.1016/j.freeradbiomed.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 173.Li N., Muthusamy S., Liang R., Sarojini H., Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mechanisms of Ageing and Development. 2011;132(3):75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 174.Kuosmanen S. M., Kansanen E., Sihvola V., Levonen A. L. MicroRNA profiling reveals distinct profiles for tissue-derived and cultured endothelial cells. Scientific Reports. 2017;7(1, article 10943) doi: 10.1038/s41598-017-11487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu X., Li M., Hou M., Huang W., Song J. MicroRNA-135a alleviates oxygen-glucose deprivation and reoxygenation-induced injury in neurons through regulation of GSK-3β/Nrf2 signaling. Journal of Biochemical and Molecular Toxicology. 2018;32(7, article e22159) doi: 10.1002/jbt.22159. [DOI] [PubMed] [Google Scholar]
- 176.Duan Q., Sun W., Yuan H., Mu X. MicroRNA-135b-5p prevents oxygen-glucose deprivation and reoxygenation-induced neuronal injury through regulation of the GSK-3β/Nrf2/ARE signaling pathway. Archives of medical science : AMS. 2018;14(4):735–744. doi: 10.5114/aoms.2017.71076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhao X. J., Yu H. W., Yang Y. Z., et al. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biology. 2018;18:124–137. doi: 10.1016/j.redox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li S.-p., Cheng W.-n., Li Y., et al. Keap1-targeting microRNA-941 protects endometrial cells from oxygen and glucose deprivation-re-oxygenation via activation of Nrf2 signaling. Cell Communication and Signaling. 2020;18(1):p. 32. doi: 10.1186/s12964-020-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jadeja R. N., Jones M. A., Abdelrahman A. A., et al. Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biology. 2020;28, article 101336 doi: 10.1016/j.redox.2019.101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kurinna S., Werner S. NRF2 and microRNAs: new but awaited relations. Biochemical Society Transactions. 2015;43(4):595–601. doi: 10.1042/BST20140317. [DOI] [PubMed] [Google Scholar]
- 181.Piper M. D., Bartke A. Diet and aging. Cell Metabolism. 2008;8(2):99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 182.Khurana S., Venkataraman K., Hollingsworth A., Piche M., Tai T. C. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5(10):3779–3827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pawlowska E., Szczepanska J., Koskela A., Kaarniranta K., Blasiak J. Dietary polyphenols in age-related macular degeneration: protection against oxidative stress and beyond. Oxidative Medicine and Cellular Longevity. 2019;2019:13. doi: 10.1155/2019/9682318.9682318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sundar Dhilip Kumar S., Houreld N. N., Abrahamse H. Therapeutic potential and recent advances of curcumin in the treatment of aging-associated diseases. Molecules. 2018;23(4):p. 835. doi: 10.3390/molecules23040835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Serafini M. M., Catanzaro M., Fagiani F., et al. Modulation of Keap1/Nrf2/ARE signaling pathway by Curcuma- and garlic-derived hybrids. Frontiers in Pharmacology. 2020;10:p. 1597. doi: 10.3389/fphar.2019.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Méndez-García L. A., Martínez-Castillo M., Villegas-Sepúlveda N., Orozco L., Córdova E. J. Curcumin induces p53-independent inactivation of Nrf2 during oxidative stress-induced apoptosis. Human & Experimental Toxicology. 2019;38(8):951–961. doi: 10.1177/0960327119845035. [DOI] [PubMed] [Google Scholar]
- 187.Wu L., Jiang C., Kang Y., Dai Y., Fang W., Huang P. Curcumin exerts protective effects against hypoxia-reoxygenation injury via the enhancement of apurinic/apyrimidinic endonuclease 1 in SH-SY5Y cells: involvement of the PI3K/AKT pathway. International Journal of Molecular Medicine. 2020;45(4):993–1004. doi: 10.3892/ijmm.2020.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lu J., Zhu W., Wu Y., et al. Effect of curcumin on aging retinal pigment epithelial cells. Drug Design, Development and Therapy. 2015;9:5337–5344. doi: 10.2147/dddt.s84979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bernatoniene J., Kopustinskiene D. M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23(4):p. 965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sun W., Liu X., Zhang H., et al. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radical Biology & Medicine. 2017;108:840–857. doi: 10.1016/j.freeradbiomed.2017.04.365. [DOI] [PubMed] [Google Scholar]
- 191.Na H. K., Kim E. H., Jung J. H., Lee H. H., Hyun J. W., Surh Y. J. (-)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Archives of Biochemistry and Biophysics. 2008;476(2):171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 192.Kim E. N., Lim J. H., Kim M. Y., et al. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging. 2018;10(1):83–99. doi: 10.18632/aging.101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gao Y., Fu R., Wang J., Yang X., Wen L., Feng J. Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway. Pharmaceutical Biology. 2018;56(1):440–449. doi: 10.1080/13880209.2018.1502326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhao Y., Song W., Wang Z., et al. Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biology. 2018;14:609–617. doi: 10.1016/j.redox.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pallauf K., Duckstein N., Rimbach G. A literature review of flavonoids and lifespan in model organisms. The Proceedings of the Nutrition Society. 2017;76(2):145–162. doi: 10.1017/S0029665116000720. [DOI] [PubMed] [Google Scholar]
- 196.Faggio C., Sureda A., Morabito S., et al. Flavonoids and platelet aggregation: a brief review. European Journal of Pharmacology. 2017;807:91–101. doi: 10.1016/j.ejphar.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 197.Pallauf K., Duckstein N., Hasler M., Klotz L. O., Rimbach G. Flavonoids as putative inducers of the transcription factors Nrf2, FoxO, and PPARγ. Oxidative Medicine and Cellular Longevity. 2017;2017:11. doi: 10.1155/2017/4397340.4397340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shao Y., Yu H., Yang Y., Li M., Hang L., Xu X. A solid dispersion of quercetin shows enhanced Nrf2 activation and protective effects against oxidative injury in a mouse model of dry age-related macular degeneration. Oxidative Medicine and Cellular Longevity. 2019;2019:12. doi: 10.1155/2019/1479571.1479571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang D., Jiang Y., Sun-Waterhouse D. X., et al. MicroRNA-based regulatory mechanisms underlying the synergistic antioxidant action of quercetin and catechin in H2O2-stimulated HepG2 cells: roles of BACH1 in Nrf2-dependent pathways. Free Radical Biology & Medicine. 2020;153:122–131. doi: 10.1016/j.freeradbiomed.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 200.Zhang Y., Yang Y., Yu H., Li M., Hang L., Xu X. Apigenin protects mouse retina against oxidative damage by regulating the Nrf2 pathway and autophagy. Oxidative Medicine and Cellular Longevity. 2020;2020:14. doi: 10.1155/2020/9420704.9420704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.He P., Wu Y., Shun J., Liang Y., Cheng M., Wang Y. Baicalin ameliorates liver injury induced by chronic plus binge ethanol feeding by modulating oxidative stress and inflammation via CYP2E1 and NRF2 in mice. Oxidative Medicine and Cellular Longevity. 2017;2017:11. doi: 10.1155/2017/4820414.4820414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Du W., An Y., He X., Zhang D., He W. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxidative Medicine and Cellular Longevity. 2018;2018:14. doi: 10.1155/2018/1610751.1610751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang L., Li H., Gao M., et al. Genistein attenuates di-(2-ethylhexyl) phthalate-induced testicular injuries via activation of Nrf2/HO-1 following prepubertal exposure. International Journal of Molecular Medicine. 2018;41(3):1437–1446. doi: 10.3892/ijmm.2018.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lei L., Chai Y., Lin H., et al. Dihydroquercetin activates AMPK/Nrf2/HO-1 signaling in macrophages and attenuates inflammation in LPS-induced endotoxemic mice. Frontiers in Pharmacology. 2020;11:p. 662. doi: 10.3389/fphar.2020.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Z., Liu Y., Zhao X., Liu S., Liu Y., Wang D. Aronia melanocarpa prevents alcohol-induced chronic liver injury via regulation of Nrf2 signaling in C57BL/6 mice. Oxidative Medicine and Cellular Longevity. 2020;2020:13. doi: 10.1155/2020/4054520.4054520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shi L., Hao Z., Zhang S., et al. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: the involvement of ERK1/2 and PKC. Biochemical Pharmacology. 2018;150:9–23. doi: 10.1016/j.bcp.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 207.Lefaki M., Papaevgeniou N., Tur J. A., Vorgias C. E., Sykiotis G. P., Chondrogianni N. The dietary triterpenoid 18α-glycyrrhetinic acid protects from MMC-induced genotoxicity through the ERK/Nrf2 pathway. Redox Biology. 2020;28, article 101317 doi: 10.1016/j.redox.2019.101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Suraweera T. L., Rupasinghe H., Dellaire G., Xu Z. Regulation of Nrf2/ARE pathway by dietary flavonoids: a friend or foe for cancer management. Antioxidants. 2020;9(10):p. 973. doi: 10.3390/antiox9100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxidative Medicine and Cellular Longevity. 2019;2019:20. doi: 10.1155/2019/9372182.9372182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang W., Feng C., Jiang H. Novel target for treating Alzheimer's diseases: crosstalk between the Nrf2 pathway and autophagy. Ageing Research Reviews. 2021;65, article 101207 doi: 10.1016/j.arr.2020.101207. [DOI] [PubMed] [Google Scholar]
- 211.Thilakarathna S. H., Rupasinghe H. P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5(9):3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]


