Abstract
The mature mammalian brain has long been thought to be a structurally rigid, static organ since the era of Ramón y Cajal in the early 20th century. Evidence accumulated over the past three decades, however, has completely overturned this long-held view. We now know that new neurons and glia are continuously added to the brain at postnatal stages, even in mature adults of various mammalian species, including humans. Moreover, these newly added cells contribute to structural plasticity and play important roles in higher-order brain function, as well as repair after damage. A major source of these new neurons and glia is neural stem cells (NSCs) that persist in specialized niches in the brain throughout life. With this new view, our understanding of normal brain physiology and interventional approaches to various brain disorders has changed markedly in recent years. This article provides a brief overview on the historical changes in our understanding of the developmental dynamics of neurogenesis and gliogenesis in the postnatal and adult mammalian brain and discusses the roles of NSCs and other progenitor populations in such cellular dynamics in health and disease of the postnatal mammalian brain.
Keywords: Neural stem cell, neurogenesis, gliogenesis, neuron, astrocyte, oligodendrocyte, development, mammalian brain, injury, neurodegeneration
INTRODUCTION
The Spanish neuropathologist Santiago Ramón y Cajal, often called “the father of modern neuroscience,” wrote in his publication in 1914, “Once the development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably. In the adult centres, the nerve paths are something fixed, ended, immutable. Everything may die, nothing may be regenerated” [1]. Although this Cajal’s notion was based mainly on his histological observations of damaged nerve tracts, he also left a vast amount of work on the regeneration (and the lack thereof) of nerve cells (neurons and glia in contemporary terms) in injured brains of various animal species. Since then, it has long been thought that little structural changes occur in the brain in mature animals, in particular, those in mammals.
Remarkable advances of molecular and cellular neurobiology over the past three decades, however, have completely changed such a grim view. First starting from demonstrations of changes in fine subcellular structures at the level of dendrites and synapses in the 70’s and 80’s [2] to more recent discoveries of neural stem cells (NSCs) in the 90’s [3, 4], recent new findings have let us realize that the mature mammalian brain is actually quite plastic and dynamically changes over time. In particular, the discovery of continuous neurogenesis and gliogenesis (i.e., production of new neurons and glia) in the adult brain has drawn renewed attention to the developmental dynamics of the mammalian brain at the cellular level [3, 4]. Now we know that new neurons and glia are continuously produced from various types of undifferentiated progenitors, including NSCs, beyond birth, and that such an activity persists throughout life in some areas of the adult brain [3, 4]. In this review, we summarize our current understanding of the developmental dynamics of the three major neural cell types in the mammalian brain, i.e., neurons, oligodendrocytes, and astrocytes, in the postnatal and adult brain, and discuss implications of recent findings for future development of new intervention strategies for various brain disorders.
Neurogenesis in the postnatal and adult brain
In the developing rodent (mouse and rat) telencephalon (the most anterior part of the brain), differentiation of a variety of neuronal subtypes begins at mid-gestation stages [embryonic (E) day 9 to E10 in mouse] and mostly complete before birth [5, 6] (Figure 1A–B). There are, however, two notable exceptions: One is various interneurons in the olfactory bulb (OB), and the other is granule cells in the hippocampal dentate gyrus (DG) (Figure 1C–H). The peak of the generation of these two neuronal subtypes lies at an early postnatal stage [postnatal (P) day 7 to P14], and, although gradually wearing down by P30, it continues at the adult stage and sustains even in aged animals [7–10]. It has been estimated that approximately 9,000 new neurons are added to the hippocampal DG each day, accounting for about 0.6% of the total DG granule cells in rat and monkey [11, 12]. A larger number of neurons, 10,000 to 30,000 cells per day, corresponding to 1% of the total population, are thought to be continuously supplied to the mouse OB [13, 14]. Moreover, in the OB, more than twice as many cells are initially generated, but not all these newborn cells survive to become mature neurons [14, 15]. Such production of new neurons continues even in aged animals albeit at a lower level [16]. Thus, although the daily addition of new neurons is small in number relative to the total neuronal population in the adult brain as a whole, their cumulative contribution to the respective structures over the entire life span is substantial.
Figure 1.
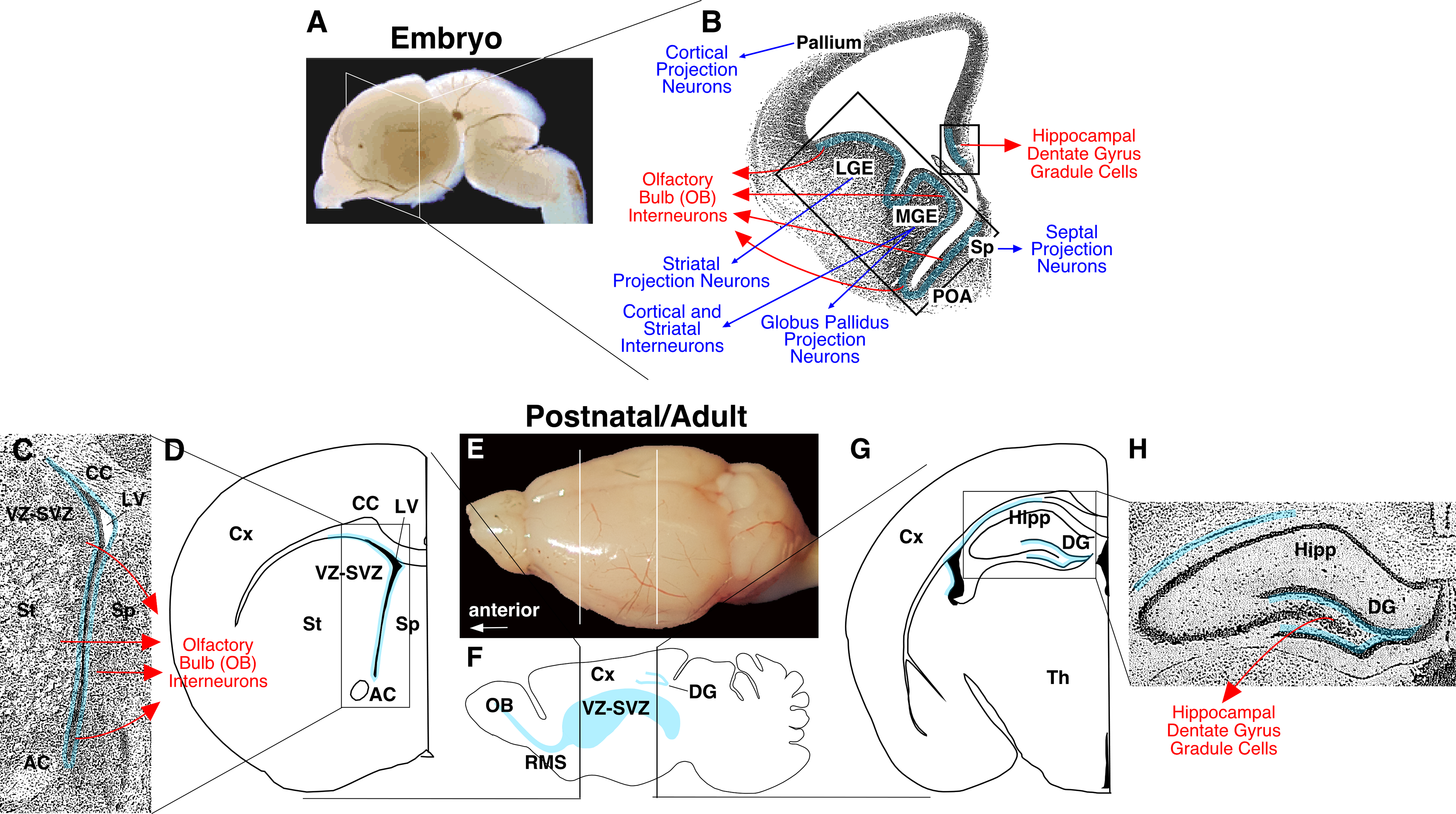
Continuous production of new neurons from embryonic to adult stages in the mammalian brain. (A, B) Production of different subtypes of neurons in distinct progenitor domains of the embryonic mouse telencephalon. Panel A shows a representative lateral view of the embryonic brain, and panel B shows its view at a coronal plane (indicated by a while square in A). The light blue line in B indicates the areas where progenitors of new neurons reside. The embryonic primordia of the pallium, lateral ganglionic eminence (LGE), medial ganglionic eminence (MGE), and septum produce region-specific projection neurons (blue arrows). The MGE also serves as the source of pallial (cortical) and striatal interneurons. The generation of these projection neurons and interneurons ceases by the end of embryogenesis. By contrast, the production of olfactory bulb (OB) interneurons and hippocampal dentate gyrus (DG) granule cells (red arrows) begins at embryonic stages and continues beyond birth in the postnatal and adult brain in rodents. Importantly, progenitor domains that produce OB interneurons partially overlap with those for region-specific projection neurons. (C-H) Neurogenic/stem cell niches in the postnatal and adult mouse brain. Panel E shows a dorsolateral view of the adult mouse brain (anterior to the left). Panel F shows the locations of two neurogenic/stem cell niches, the VZ-SVZ and hippocampal DG (areas indicated by light blue shades), in a parasagittal view. Panels C and D schematically show the anatomical relationships of the VZ-SVZ with other brain structures in a coronal plane indicated by the left horizontal lines in E and F. Panels G and H show the location of the hippocampal DG in a coronal plane indicated by the right horizontal lines in E and F. Panels C and H are magnified photographic views of the boxed areas in D and G, respectively. In all panels, areas shaded with light blue are where NSCs reside and produce new OB interneurons and DG granule cells (red arrows in C and H, respectively). Abbreviations: AC, anterior commissure; CC, corpus callosum; DG, dentate gyrus; LV, lateral ventricle; LGE, lateral ganglionic eminence; Hipp, hippocampus; MGE, medial ganglionic eminence; OB, olfactory bulb; POA, preoptic area; RMS, rostral migratory stream; Sp, septum; St, striatum; Th, thalamus; VZ-SVZ, ventricular-subventricular zone.
This phenomenon, so-called “adult neurogenesis” in the mammalian brain, is now widely recognized and accepted [3, 4]. It is noteworthy, however, that there have been interesting twists of our view on this matter in the history of neuroscience. Although experimental evidence for adult neurogenesis was repeatedly reported by several pioneering researcher groups, including Altman and Bayer, and Hiss, such findings were largely ignored at the time of their publications [for details, see refs. 17, 18, and references therein]. In the 80’s, however, a breakthrough came from an unexpected direction. Fernando Nottebohm and his colleagues in New York reported a series of studies that investigated the neural mechanisms underlying the seasonal acquisition of new courtship/territorial songs by adult male canaries and zebra finches [19]. Surprisingly, they found that this sophisticated learning-memory function in birds involves an addition of a large number of new neurons followed by a loss of existing neurons in specific brain nuclei [19, 20]. This seminal finding has completely changed our view on neuronal cell genesis and replacement in adult animals. Although amazing functional restoration of a part of the central nervous system (CNS) after injury have long been recognized through studies on the regeneration of the eyes and spinal cord in newts and other amphibian species, a major view in the field had been that such cases are rather exceptional for so-called “lower vertebrates” and restricted merely to specialized or “peculiar pathological” conditions [21]. The new finding discovered in songbirds has dampened down such a long-held view and firmly established the case that new neurons added to pre-existing, mature neural circuitries significantly contribute to higher brain function under physiological conditions. Following these studies on songbirds, many groups have “re-discovered” sustained production of new neurons in the adult brain, now commonly called “adult neurogenesis,” first in rodents, and later in many other mammalian species, including large mammals such as sheep and marmoset, as well as non-human primates in the late 90’s and early 2000’s [22–28]. Later, these studies were further expanded to include the human brain [29], and the occurrence of postnatal and adult neurogenesis across all mammalian species, including humans, has become a widely accepted view by the beginning of the 21st century [3, 4].
How active and widespread is adult neurogenesis?
It should be noted, however, that several important questions still remain unanswered regarding adult neurogenesis. First, to what extent neurogenesis actually occurs in the postnatal human brain is still under hot debates [30–40]. One school of investigators propose that significant neurogenesis persists even in aged human brains [30–35], whereas others argue that production of new neurons become undetectable in early postnatal life and does not sustain in adult humans [36–40]. Such discrepancies are based on different results obtained using distinct methods, and further investigations are necessary to reconcile the differences [for details, see refs. 30 and 40].
Another unsettled issue is how spatially widespread neurogenesis is in the adult mammalian CNS. Although neurogenesis in the OB and DG regions of the adult brain is commonly detected across mammalian species under physiological conditions, whether a similar cell genesis also occurs in other parts of the CNS has been highly controversial [reviewed in detail in ref. 41]. For example, a recent study has provided evidence for the production of a small but significant number of new neurons in the adult human striatum [42]. Similar neuronal addition in the adult striatum has also been reported in rodents and non-human primates [43–46]. Likewise, detection of similar low-level production of new neurons has been reported in many other parts of the adult CNS, notably the cerebral cortex [46–50] and hypothalamus [51–54]. Many other studies, however, do not support such findings [55, 56]. Thus, how widespread neurogenesis is in the intact postnatal mammalian CNS is still an unsolved issue [for more details, see ref. 41].
Physiological roles of adult neurogenesis in brain function
Another unanswered important issue is that despite extensive studies over the past three decades, what roles new neurons actually play in the adult mammalian brain is still unclear. In an analogy with the case of adult neurogenesis in songbirds, it is widely thought that newly produced neurons in the mammalian brain play important roles in the plasticity of brain function through the modulation of the activity of the existing neural circuitry in certain ways, and results reported in many published studies support this idea in one way or another [reviewed extensively in refs. 57–59]. In fact, one notable feature common to the two neural circuitries to which new neurons are constantly added, i.e., the hippocampal and olfactory circuitries, is that they both exhibit high functional plasticity and play important roles in learning and memory [57]. Moreover, it has been shown that the level of the production of new neurons in these two regions significantly changes in response to a variety of environmental stimuli such as exposure to new odors and spatial/acoustic cues, exercise, stress, and fear [57]. Various neurological and psychiatric disease conditions are also known to alter the level of neurogenesis in these regions, and in certain cases, including epilepsy and depression, such changes are implicated in disease pathogenesis [58, 59]. Yet, conclusions drawn in different studies are surprisingly different from each other on the actual role of new neurons in these brain functions [reviewed in detail in refs. 3 and 4]. Thus, a better understanding of how adult neurogenesis contributes to the plasticity of higher-order brain function is an outstanding important issue in future studies.
NSCs as the source of new neurons in the postnatal and adult brain
These new findings also raise the issue of the cell of origin of new neurons in the adult mammalian brain. In the early 90’s, around the same time when adult neurogenesis was re-discovered, another breakthrough occurred and quickly answered this question. Two groups of researchers, Sally Temple and her colleagues in New York and Samuel Weiss and his group in Calgary, have independently developed new cell culture methods that allow researchers to examine the properties of each individual neuronal progenitor cell in the embryonic and adult brains at the single cell level [60, 61]. Studies using these new techniques have demonstrated the occurrence of specialized progenitor cells that retain the capacity of both self-renewal and multi-lineage-differentiation, first in the embryonic and later in the adult mouse brain. These progenitors are now collectively called NSCs given their similarities with so-called “tissue-specific” or “adult” stem cells in other organs and tissues [3, 4].
Subsequent studies have identified specialized regions where these NSCs reside in the adult rodent brain (Figure 1C–H). In the hippocampal DG, the subgranular zone (SGZ), a narrow layer of cells beneath the granule cell layer, is the area where NSCs and their early progeny reside, and new neurons produced by them migrate in a short distance to the overlaying granule cell layer [3] (Figure 2G–H). In contrast, new neurons in the OB originate from NSCs that reside in the so-called ventricular-subventricular zone (VZ-SVZ) or ependymal-subependymal layer, a thin strip of tissue lining the lateral ventricle (LV) of the telencephalon (for reasoning of the nomenclature of this structure, see below) [66, 67] (Figure 2C–D). New OB neurons are first produced by NSCs within this VZ-SVZ region, and subsequently migrate anteriorly through a specialized narrow path called the rostral migratory stream (RMS) that physically connects the VZ-SVZ and the OB [4] (Figure 2F). These two stem cell residences, i.e., the SGZ and VZ-SVZ, are collective called ‘neurogenic’ or “stem cell” niches in the adult mammalian brain and are distinguished from the remaining ‘non-neurogenic’ brain parenchyma [41]. Histological evidence has also shown the presence of similar neurogenic/stem cell niches in the brain of humans and non-human primates [30–40].
Figure 2.
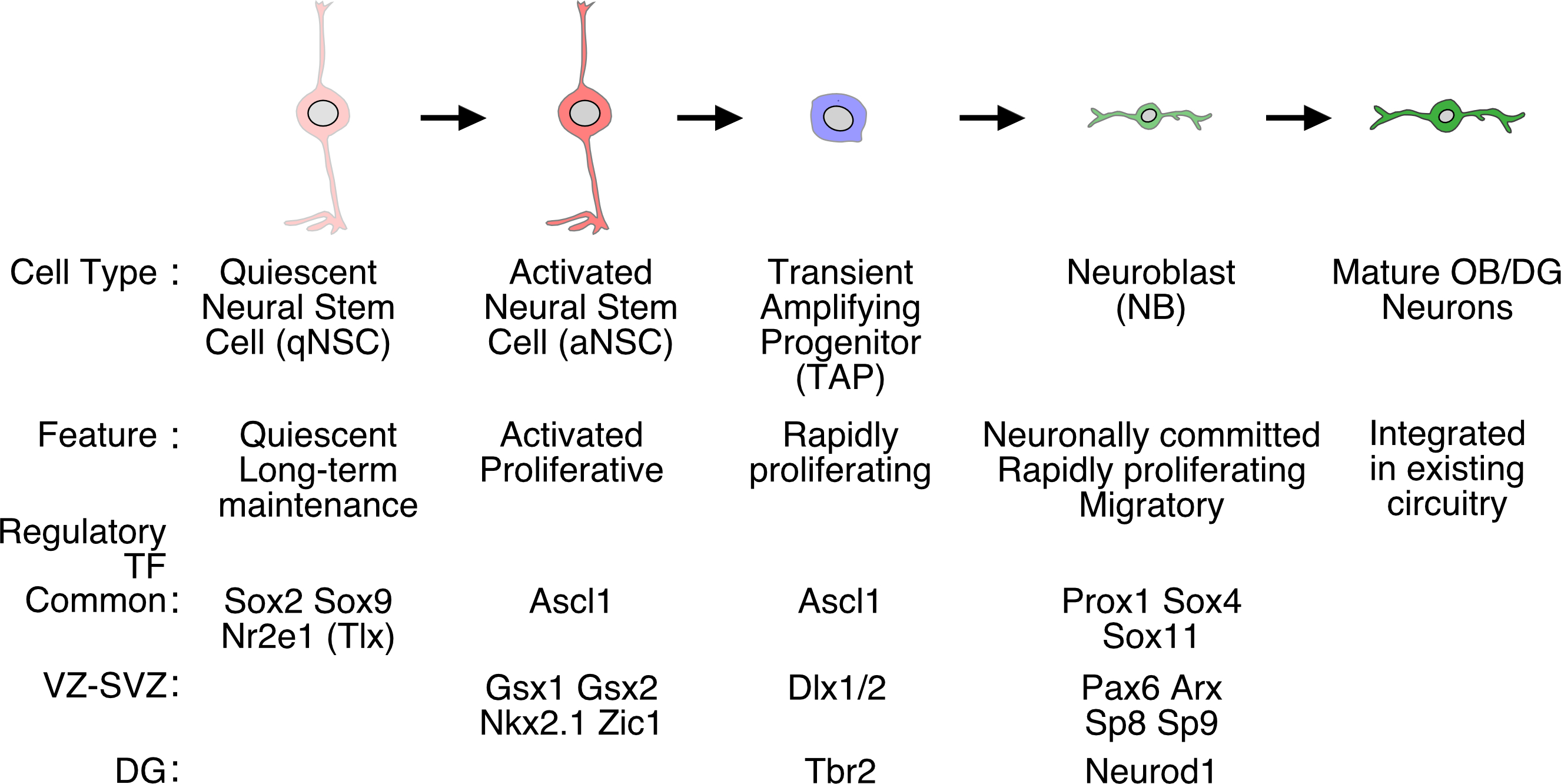
Stepwise differentiation of NSCs into new neurons through intermediate cell types in the postnatal and adult brain. Intermediate progenitor cell types, and their features and representative regulatory transcription factor (TF) genes are schematically shown. Note that some TFs regulate NSCs in both the VZ-SVZ and DG, whereas others operate in one stem niche but not in the other. For details, see text. Abbreviations: aNSCs, activated neural stem cells; NB, neuroblast; qNSCs, quiescent neural stem cells; TAP, transient amplifying progenitor. Others are the same as Figure 1.
Similarities and differences between neurogenesis and NSCs in two neurogenic regions in the postnatal brain
There are a number of interesting similarities and differences between these two adult neurogenic/stem cell niches. First, NSCs are preserved throughout life mostly as quiescent, non-dividing cells (qNSCs) in both regions (Figure 2). When these qNSCs are activated and begin to proliferate (aNSCs), they produce transient amplifying progenitors (TAPs), which in turn undergo rapid cell divisions a few times and subsequently differentiate into immature neurons called neuroblasts (NBs) (Figure 2). Thus, the capacity of stem cells to self-renew, either through symmetric or asymmetric divisions, and rapid and transient amplification of TAPs and NBs are key mechanisms to maintain a relatively small number of NSCs locally while generating a much larger number of new neurons continuously over a prolonged period of time [62, 63].
These NSCs also share some morphological and molecular features with specialized glial cells such as radial glial cells (RGCs) in embryos and astrocytes in the adult brain [63, 64] (see Figure 4). One notable example is that all these cells commonly express glial fibrillary acidic protein (Gfap) and the glial high affinity glutamate transporter (Slc1a3 or Glast) [67, 68]. Second, recent studies have revealed many common molecular players and mechanisms that control the maintenance and differentiation of NSCs in both regions [see refs. 62, 62, and references therein]. For example, in both the VZ-SVZ and SGZ, the Sox family of high-mobility-group-containing TFs Sox2 and Sox9, as well as the orphan nuclear receptor Tlx (Nr2e1) have been shown to be crucial for the long-term maintenance of adult NSCs, whereas the basic helix-loop-helix transcription factor (TF) Ascl1, the homeodomain factor Prox1, and Sox family factors Sox4 and Sox11commonly play important roles in the neuronal differentiation of NSCs (Figure 2) [62, 63, 68–72].
Figure 4.
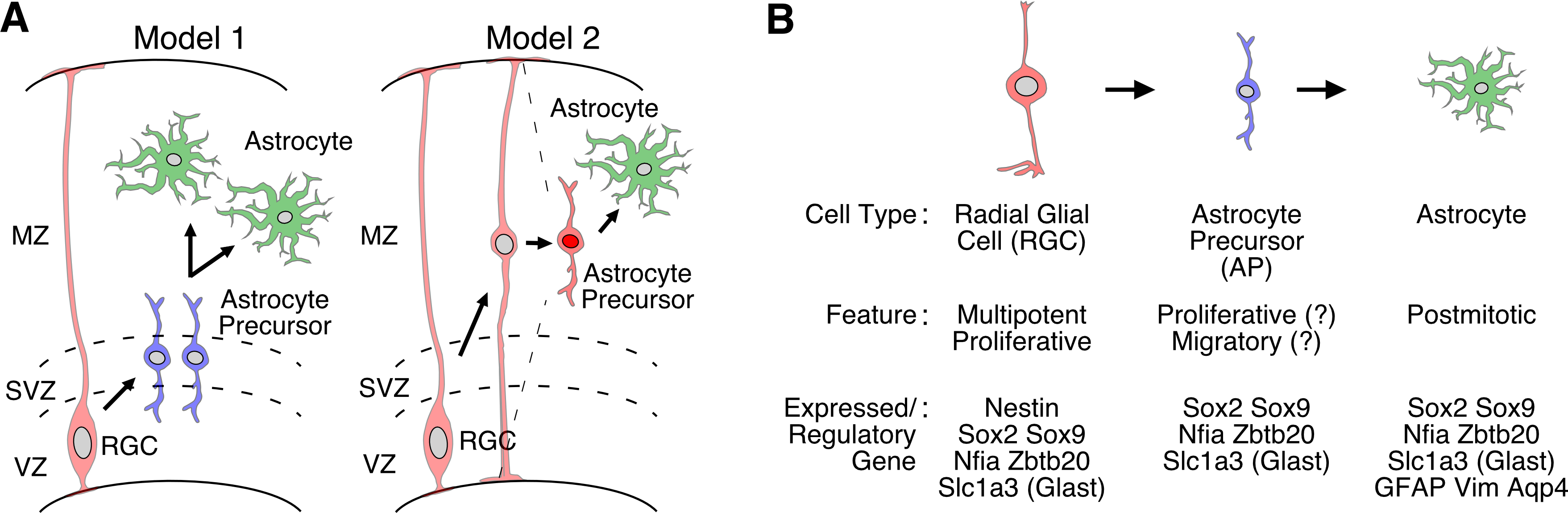
Production of astrocytes in the mammalian brain. (A) Two proposed models for the generation of astrocytes from radial glial progenitors (RGCs) in the developing cerebral cortex (boxed area). (B) Proposed stepwise differentiation of astrocytes from NSCs. The features and representative expressed/regulatory genes of RGCs, astrocyte precursors (APs), and astrocytes are schematically shown. Like NBs and OPCs in the neuronal and oligodendrocyte lineages, the occurrence of an intermediate proliferative precursor cell type [astrocyte precursor (AP)] is speculated in the astrocyte lineage, but their exact identity is currently unknown. For details, see text. Abbreviations: MZ, mantle zone. Others are same as in Figure 1.
Yet, some important differences also exist between NSCs in the VZ-SVZ and SGZ. Granule cells produced in the adult DG are glutamatergic projection neurons, whereas newly generated OB neurons are interneurons that use γ-aminobutyric acid (GABA) or dopamine as neurotransmitters [4]. Moreover, newly produced DG granule cells are generally thought to be homogeneous and belong to a single neuronal subtype, and no subtype heterogeneity has so far been identified among them [4]. By contrast, OB interneurons produced in the adult SVZ include diverse neuronal subtypes that settle and integrate into different laminae of the mature OB [73]. Recent studies have demonstrated that these diverse subtypes of neurons are generated by NSCs that reside in distinct subdomains within the VZ-SVZ and retain different molecular identities [74–77]. Such heterogeneity has not yet been identified in NSCs in the DG. Reflecting these differences, distinct sets of TFs, including Gsx1, Gsx2, Nkx2.1, Zic1, Pax6, Arx, and Dlx2 in the VZ-SVZ] and Tbr2 and Neurod1 in the SGZ, have been shown to play important roles in the neuronal differentiation of NSCs in respective regions (Figure 2) [refs. 62, 63, and references therein.
The developmental time course of neurogenesis is also different between the two regions. The production of OB interneurons in mice begins very early (as early as E10) in embryos and continues throughout the rest of the embryonic and postnatal development [7, 8]. Yet, distinct interneuron subtypes have different peak stages for their production, and some are mostly generated before birth, whereas others are most actively produced in the first and second postnatal weeks [7, 8]. Subsequently, the overall level of OB neurogenesis gradually declines and reaches a steady-state level by P30 and sustains for the rest of life. Such differential kinetics among distinct neuronal subtypes are explained by the idea that NSCs residing in distinct embryonic primordia produce different neuronal subtypes at different stages, and that these distinct NSC populations gradually mature and settle in distinct subdomains of the adult VZ-SVZ niche with different time courses [74–77]. In this scenario, the OB neurogenesis can be seen as a life-long activity of diverse NSC populations that sustain from early embryogenesis to the end of life. By contrast, the production of DG granule cells is mostly a postnatal event, and the formation of the mature form of the DG completes by P30 in mice [9, 10]. Interestingly, a recent study has proposed that progenitors that are responsible for the generation of the major part of the DG in the first three postnatal weeks and those that become NSCs in the SGZ and continuously supply new granule cells at the adult stage have topologically distinct embryonic origins [78]. This new scenario suggests that the initial development of the DG and its subsequent modulations by new neurons supplied by adult NSCs are separable developmental events. The significance of this finding remains to be further investigated.
Finally, extensive studies over the past three decades have identified a variety of extracellular signaling mechanisms that regulate the behavior of NSCs in the VZ-SVZ and DG either commonly or differentially [for details, see refs. 3, 4, 41]. An important concept drawn from these studies is that NSCs in the postnatal brain act as sensitive responders to various physiological stimuli. This property of NSCs also plays an important role in regenerative and restorative responses after injury (see below).
Prolonged development of oligodendrocytes
Oligodendrocytes are myelin-forming glia in the mammalian CNS. In the rodent brain, myelinated axons are scarce at birth, and the full complement of myelination in the whole brain does not occur until the fourth week of postnatal development [79, 80]. Full myelination in the human brain is further delayed to an age of 10 to 12 years in some cortical regions responsible for higher cognitive function such as the prefrontal cortex, and such a delay is considered to be a notable example of the so-called neoteny feature of the human development [81]. Therefore, oligodendrocytes have long been thought to be the last cell types to be generated during development among the three major cell types in the mammalian CNS.
Studies over the past three decades, however, have totally changed this view. It has turned out that the delayed appearance of oligodendrocytes examined in old studies was simply because the cells had been detected based on either the formation of myelin sheets in electron microscopic (EM) studies or the expression of major myelin proteins such as myelin basic protein (MBP) and myelin glycoprotein (MAG) in conventional histology. Since the early 90’s, many studies have identified a variety of molecular markers for progenitor/precursor cells that stay undifferentiated for a long period of time during embryonic and early postnatal stages and eventually become myelin-expressing mature oligodendrocytes at later stages [82, 83]. For instance, platelet-derived growth factor receptor α (PDGFRα) and chondroitin sulfate proteoglycan 4 (Cspg4) (also known as NG2) are selectively expressed in early-stage proliferative cells called oligodendrocyte progenitor cells (OPCs) [82, 83] (Figure 3B). These OPCs also express the basic helix-loop-helix TFs Olig1, Olig2, and Ascl1, as well as the Sox family of high-mobility-group-containing TFs Sox8, Sox9, and Sox10 [82, 83]. These OPCs cease cell divisions and begin to differentiate first into pre-myelinating immature oligodendrocytes (Pre-OL) at early postnatal stages, and subsequently gradually undergo maturation as myelin-expressing oligodendrocytes (OLs) (Figure 3B). The stage of pre-OLs is defined by the expression of the homeodomain TFs NKx2–2 and Nkx6–2, and various zinc-finger TFs such as Myt1 (Nzf2), Zfp24 (ZFP191), and Zfp488 [83]. In myelinating OLs, the TF MYRF (Gm98) directly regulates the expression of various myelin proteins including MBP and MAG. Studies on the expression and function of these stage-specific regulatory genes have now revealed details of the developmental time course of oligodendrogenesis. In mouse embryonic telencephalon, cells in the oligodendrocyte lineage begin to emerge as early as E11.5, and these OPCs are continuously produced throughout the rest of embryogenesis and increase their number while migrating toward various brain regions, thereby becoming abundant and widespread already at birth [83, 84] (Figure 3A). Generation of new OPCs further continue during early postnatal stages, but begin to cease cell divisions from the second postnatal week, and eventually differentiate into Pre-OLs and OLs by the end of the first postnatal month [84–90]. In general, the transition from OPCs to Pre-OLs and OLs proceeds in a ventro-caudal to dorso-rostral gradient in the mouse telencephalon, but the precise mechanisms that control the timing of their transitions in different regions of the brain remain largely unknown.
Figure 3.
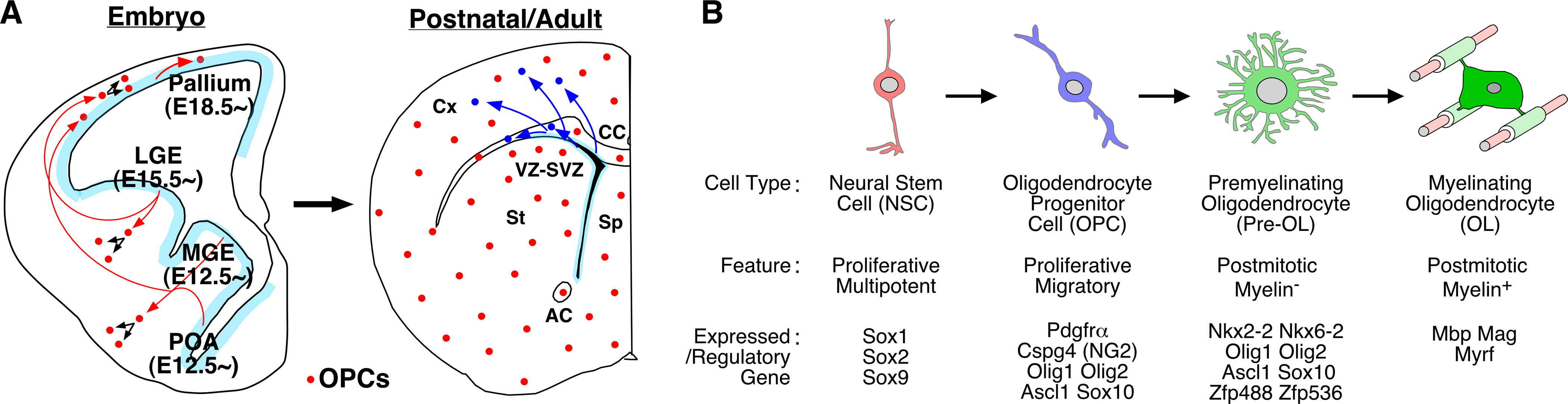
Continuous production of oligodendrocytes from embryonic to adult stages in the mammalian brain. (A) The left panel schematically shows that multiple progenitor domains (POA, MGE, LGE, and pallium) generate oligodendrocyte progenitor cells (OPCs) (red circles) at distinct embryonic stages in the developing mouse telencephalon. Red arrows indicate their migratory routes. The right panel schematically shows the sustained production of new OPCs (blue circles) in the VZ-SVZ in the postnatal and adult brain, which exist as a population separate from embryonically produced OPCs. Note that OPCs continue cell divisions and increase in number while migrating toward widespread regions of the brain parenchyma (black arrows). (B) Stepwise differentiation of oligodendrocytes from NSCs. Intermediate progenitor cell types in the oligodendrocyte lineage, and their features and representative expressed/regulatory genes are schematically shown. For details, see text. Abbreviations: NSC, neural stem cell; OL, oligodendrocyte; OPC, oligodendrocyte progenitor cell; Pre-OL, pre-myelinating oligodendrocyte. Others are same as in Figure 1.
Importantly, similarly to neurogenesis of OB interneurons, there is evidence that production of new OPCs in the VZ-SVZ sustains albeit at a low level in the fully matured adult brain under both physiological and pathological conditions [91–99]. Moreover, a significant number of OPCs generated during embryonic and early postnatal periods remain undifferentiated and sustain in the widespread regions of the mature brain parenchyma throughout life [79, 80]. A small fraction of these pre-existing and newly generated OPCs in the adult brain become mature oligodendrocytes and replace existing OLs that are lost physiologically or after injury in both rodents [84–98] and humans [99]. Thus, the development of oligodendrocytes from their generation to maturation proceeds on an extremely protracted schedule in the mammalian CNS.
Interestingly, recent lineage-tracing studies have shown that the generation of OPCs from NSCs in the telencephalon occurs in spatially distinct progenitor domains at different stages. Early born OCPs start to emerge E12.5 onward from ventral progenitor domains such as the preoptic area (POA) and median ganglionic eminence (MGE), and their generation subsequently spreads to a more dorsal domain called the lateral ganglionic eminence (LGE) at later embryonic stages (by E15.5) [84, 85] (Figure 3A). In addition to these ventral domains, progenitors in the pallium (dorsal telencephalon) become a major source of OPCs at late embryonic (E18.5 onward) and early postnatal stages [86–90]. OPCs derived from ventral progenitor domains mainly populate the ventral aspect of the telencephalon, while those from both ventral and dorsal domains contribute to oligodendrocytes that later myelinate axons in the dorsal telencephalon such as the neocortex and corpus callosum [84–90]. Thus, the origin of oligodendrocytes is diverse both spatially and temporary. It is currently unknown, however, whether oligodendrocytes originating from distinct domains at different stages exhibit any functional differences.
Development of astrocytes
Astrocytes are among the most abundant and widely distributed cell types in the mammalian CNS and exert multiple important functions [100, 101]. Nevertheless, our current understanding of the development of astrocytes remains surprisingly sketchy. As in the case of oligodendrocytes, the lack of appropriate molecular markers had long hampered detailed analysis of astrocyte development until the late 80’s [101]. By then, studies on the ontogeny of astrocytes heavily relied on the detection of so-called glial filaments, which are composed of unique intermediate filament proteins such as glial fibrillary acidic protein (GFAP) and vimentin (Vim), by either EM or conventional histology. Like myelin proteins in oligodendrocytes, the expression of GFAP and Vim is a relatively late event in the developing brain so that earlier-stage precursors of astrocytes were difficult to identify [101]. More recent studies have begun to identify several molecular regulators of early-stage astrocyte development such as the TFs Sox2, Sox9, Nf1a, and Zbtb20 [102–108] (Figure 4B). These TFs, however, are also expressed in NSCs and other progenitors in the germinal zones of both embryonic and postnatal brains [102–108]. In fact, when the in vivo identity of NSCs in the adult mouse brain was first identified, a surprising finding was that astrocytes and adult NSCs share many features [64, 65]. For example, not only GFAP and Vim, but also the glutamate transporter Glast (Slc1a3) and the water-selective channel aquaporin 4 (Aqp4) are all important regulators of certain aspects of astrocyte functions, but they are also expressed in adult NSCs [68, 69]. Likewise, the TFs Sox2, Sox9, and Nfia have been shown to play crucial roles in both differentiation of astrocytes in embryos and maintenance of NSCs in adults [102–107]. Thus, adult NSCs and astrocytes share many common gene expression profiles and regulatory mechanisms. Nevertheless, only NSCs, but not astrocytes, retain bona fide capacities as stem cells, and we do not fully understand yet what molecular programs actually distinguish these two cell types in the adult brain. This similarity between astrocytes and NSCs also makes studies on astrocyte development even more difficult than that of oligodendrocytes.
Thus, based mostly on indirect morphological and marker analysis, two ontogenic pathways to generate astrocytes have been speculated: One is that germinal zone immature progenitors such as NSCs produce precursors committed to an astrocyte lineage, and these precursors migrate to the brain parenchyma and differentiate into mature astrocytes later in development [109] (Figure 4A, left). This mode of astrocyte generation is in an analogy with the specification of oligodendrocytes and consistent with the observation that some scattered cells expressing Sox2, Sox9, Nfia, and/or Zbtb20 begin to emerge outside the germinal zone in the late embryonic and early postnatal brain [102–108]. Another possible pathway is transformation of so-called radial glial cells (RGCs) into astrocytes. In mid-gestation embryos, RCGs, which are thought to serve as the origin of both neurons and glia, exhibit elongated morphology, and their radial process spans across the entire apico-basal axis of the developing brain [65] (Figure 4A, right). At late embryonic stages, however, their apical and basal processes gradually retract, and they appear to transform into cells that populate widespread regions of the brain parenchyma in a scattered manner. Based on their morphology and marker expression, these cells are thought to become astrocytes during the postnatal period [65]. This second scenario supports the idea that both postnatal NSCs and astrocytes are descents of embryonic RGCs, and, thus, share common features [64, 65]. Recent studies using various transgenic mice, in which RGCs and their progeny can be genetically labeled and their fates can be tracked, have provide evidence that supports both of these two scenarios, but do not necessarily proves one idea over the other [48, 109–111]. Thus, it remains unknown whether either pathway is correct, or both pathways co-exist in astrocyte development. In the mature adult brain, there is evidence that a small number of new astrocytes are generated by NSCs in the hippocampal DG, although their number appears to be quite small [114–116]. NSCs in the adult VZ-SVZ region have also been demonstrated to generate new astrocytes in the corpus callosum and RMS [117], although whether such NSCs are the same as those for new neurons and oligodendrocytes at the clonal level remain uncertain [118, 119].
Relationship between neurogenesis and gliogenesis in the postnatal brain
It is noteworthy here that the spatiotemporal dynamics of the development of OB interneurons and oligodendrocytes look very similar. In fact, both cell types are continuously generated from multiple progenitor domains from early embryonic to postnatal stages in the mouse telencephalon. This raises the question of whether or not these two cell types originate from common progenitors. In particular, the dorsal progenitor domain that covers the roof region of the LV in the postnatal and adult brain has been identified as the source of both OB interneurons and oligodendrocytes in different studies [91–98]. A cell culture experiment has provided evidence that NSCs in this region differentiate into oligodendrocytes only but not neurons, whereas those derived from more ventral regions preferentially generate neurons only [93]. Other studies, however, have reported that the same region contains NSCs that produce new OB neurons in vivo and in vitro [74, 77]. Thus, it is currently unknown whether the same pool of NSCs produces both cell types simultaneously, or separate pools of NSCs co-exist in the same region. This question is related to a more general issue regarding the distinction between the in vivo fate and capacity of NSCs in the postnatal and adult NSCs. Lineage-tracing studies have demonstrated that NSCs in the adult DG generate both neurons and astrocytes [115, 116]. Moreover, a recent clonal level analysis of the fate of single NSCs has shown that some NSCs produce both neurons and astrocytes, while other cells produce only one of these two cell types over a period of one year [114]. The same study, however, did not detect any NSCs that produced oligodendrocytes in the same period. It remains unknown whether these neuron-astrocyte bi-potent NSCs and those that are mono-potent and produce neurons or astrocytes only have intrinsically different capacities, or they all are multipotent but choose particular fates stochastically. Likewise, although NSCs derived from various VZ-SVZ subdomains in the adult brain often behave as multipotent progenitors that produce all three neural lineages when cultured in vitro, whether each individual cell indeed produces both neurons and glia in vivo has not been experimentally determined.
Another important point is the ontogenic relationship between oligodendrocytes and astrocytes. When OPCs were first identified in the 80’s, cell culture experiments demonstrated that they behave as bi-potent progenitors that can differentiate into both oligodendrocytes and astrocytes in vitro [120]. Subsequent in vivo studies, however, have demonstrated that the same cells become almost exclusively oligodendrocytes only, but not astrocytes [82, 83]. Thus, a current consensus view is that the so-called OPCs are progenitors committed to an oligodendrocyte lineage. Yet, some recent studies using genetic lineage-tracing methods have provided evidence that a fraction of PDGFRα/NG2-expressing OPCs differentiate not only into oligodendrocytes, but also into astrocytes and/or neurons in certain regions of the brain in vivo, although such cells seems to be relatively rare [121–124]. Other studies, however, argues that OPCs are committed exclusively to an oligodendrocyte lineage [125, 126]. Thus, the actual potency and fate of OPCs in vivo still needs to be further investigated.
Impacts of non-neuronal/glial cell types on postnatal brain development
There are several structurally and functionally important non-neuronal/glial cell types in the postnatal mammalian brain. Ependymal cells form a single-cell layer epithelium that separates the brain parenchyma from the LV and other ventricular systems [127]. Although ependymal cells lining the LV have long been thought to develop postnatally, recent studies have demonstrated that they start to emerge as early as E13 in mice near the posterior edge of the LV and progressively spread over the entire LV during late embryonic and early postnatal stages [128, 129]. Importantly, while embryonic RGCs in the germinal zone transform into postnatal NSCs in the VZ-SVZ region, they also generate ependymal cells in the same region [130]. Thus, postnatal NSCs are intercalated with ependymal cells within the ependymal layer, and they together confer unique pinwheel architecture to the ventricular surface of the LV [131]. Such a pinwheel structure is not evident in non-neurogenic parts of the ependymal layer such as those in the fourth ventricle of the hindbrain or the central canal of the spinal cord.
Because of this unique microarchitecture, there have been intense debates over the identity of adult NSCs within the VZ-SVZ region over the past two decades [132]. When the cellular identity of NSCs in the adult mouse brain was first reported, one group proposed that a pool of NSCs reside in the ependymal layer, whereas another group argued that NSCs are strictly confined to the subependymal region [66, 67]. Since then, there have been a number of studies that support both ideas [131, 134–136]. At the end, it has turned out that the soma and/or apical processes of NSCs actually reside in both the ependymal and subependymal regions, and, thus, cells with a capacity as NSCs and typical ependymal cells with motile multi cilia co-exist in the ependymal layer [124–131]. Yet, there have been revived conflicting views in recent years regarding the exact identity and functional properties of individual cells that exist in the ependymal layer of the LV [134–136]. This issue is related to the question of why NSCs that actively produce new neurons are detected only in the telencephalon, but not in other parts of the CNS, although the so-called ependymal cells are ubiquitous all along the ventricular system. Importantly, a number of in vitro culture studies have identified the occurrence of cells with properties similar to NSCs in widespread regions of the adult mammalian CNS [for details, see ref. 41, 132]. Therefore, the issues of the exact identity of NSCs and the reason why active neurogenesis is confined to specialized regions in the adult mammalian CNS remain to be further investigated. It also remains unknown whether any functional crosstalk between NSCs and ependymal cells exits within the ependymal layer.
The choroid plexus is a single-cell layer epithelial structure that protrudes from the ependymal layer into the lateral, third, and fourth ventricles [136]. Together with some underlying mesenchymal components, it is thought to be responsible for the production and absorption of the CSF [136]. The epithelial cells in the choroid plexus are morphologically similar to ependymal cells, and both cell types are thought to differentiate from embryonic RGCs in a similar way. However, ependymal cells are ubiquitously present throughout the ventricular system of the CNS, whereas choroid plexus cells develop only in a few restricted regions [137]. The mechanisms underlying such region-selective generation of the choroid plexus remains largely unknown [137]. Importantly, recent studies have demonstrated that certain secreted signaling molecules from choroid plexus cells regulate proliferation of NSCs in the adult VZ-SVZ [138]. It has also been shown that the flow of the CSF generated by the choroid plexus and ependymal cells regulates both proliferation NSCs and directional migration of their neuronal progeny in the VZ-SVZ niche [139, 140]. Meningeal fibroblastic cells, which cover the entire outer surface of the CNS, are also an important part of the CNS ventricular system. They are thought to be mainly involved in the production and absorption of the CSF, but a recent study has revealed their surprising function in development. Evidence has demonstrated that meningeal cells overlaying the dorsal aspects of the embryonic telencephalon produce retinoic acid, which in turn controls the elongation of the RGCs along the apico-basal axis and subsequent temporary regulated production of cortical pyramidal neurons with specific laminar phenotypes [141]. Whether the meninge in other parts of the CNS has similar function is currently unknown.
The blood vessels are also an important cellular component in the CNS. In particular, pericytes are a unique cell type that participates into the formation and maintenance of the BBB, together with vascular endothelial cells and astrocytes, at the border between the brain parenchyma and a dense network of microvasculature within the brain [142]. Recent studies have proposed that a subset of pericytes derive from peripheral macrophages migrating into the brain [142, 143]. Yet, given the recent discovery of the molecular heterogeneity of pericytes, the origin of pericytes in various brain regions need to be further investigated. Importantly, recent studies have demonstrated intricate communications between the brain vasculature and various neural cell types, including NSCs [144]. For example, it has been shown that angiogenesis in the mouse embryonic telencephalon progresses in a ventral-to-dorsal gradient, apparently coinciding with the progression of neurogenesis, and that such temporal gradient is regulated by various homeodomain TFs that are expressed commonly in both embryonic NSCs and vascular endothelial cells in a region-specific manner [145]. Moreover, blood vessels actively participate in controlling various aspects of the development of neurons and glia [144]. A notable example is crosstalk between NSCs and blood vessels in the postnatal and adult VZ-SVZ, in which NSCs and their progeny physically interact with specialized blood vessels called the SVZ vasculature that forms a dense and widespread vascular network just beneath the ependymal layer of the LV [146, 147]. Through this interaction, blood vessels are thought to send various signals to postnatal NSCs that control their proliferation and differentiation.
Microglia are another cell type that comes into the postnatal CNS from the periphery [148]. As major immune-competent cells in the brain, microglia exert multiple functions under various physiological and pathological conditions [148]. Notably, recent studies have demonstrated that microglia regulate proliferation and differentiation of NSCs and OPCs either positively or negatively depending on conditions, thereby affecting the cellular dynamics of the postnatal brain in both the VZ-SVZ and DG [150–153]. Yet, what impact microglia have on normal brain development remains to be further investigated. Taken together, it is important to note that these vascular-NSC and microglia-NSC interactions serve as important mechanisms by which various systemic and local environmental signals regulate the behavior of NSCs and other CNS cell types, thereby affecting the cellular dynamics of the postnatal and adult brain under various physiological and pathological conditions.
Neurogenesis and gliogenesis in the injured brain
As described above, many lines of recent studies have collectively established that production of new neurons and glia is active beyond embryogenesis, and continues, albeit at a lower level, even at the fully mature stage in the mammalian brain under physiological conditions [3, 4]. Importantly, numerous studies in the past two decades have further demonstrated that such cell genesis occurs at an elevated level under various pathological conditions [41, 154]. For example, acute brain injury such as ischemia, trauma, and epilepsy stimulates the net production of new neurons in both the VZ-SVZ and hippocampal DG [41, 154] (Figure 5). Chronic neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases also lead to a sustained high level of neurogenesis in these regions [41]. Although most of these studies used rodent models, several studies have demonstrated evidence for similar enhancement of endogenous neurogenesis in the injured brain of adult humans and non-human primates [for example, see refs. 155–160].
Figure 5.
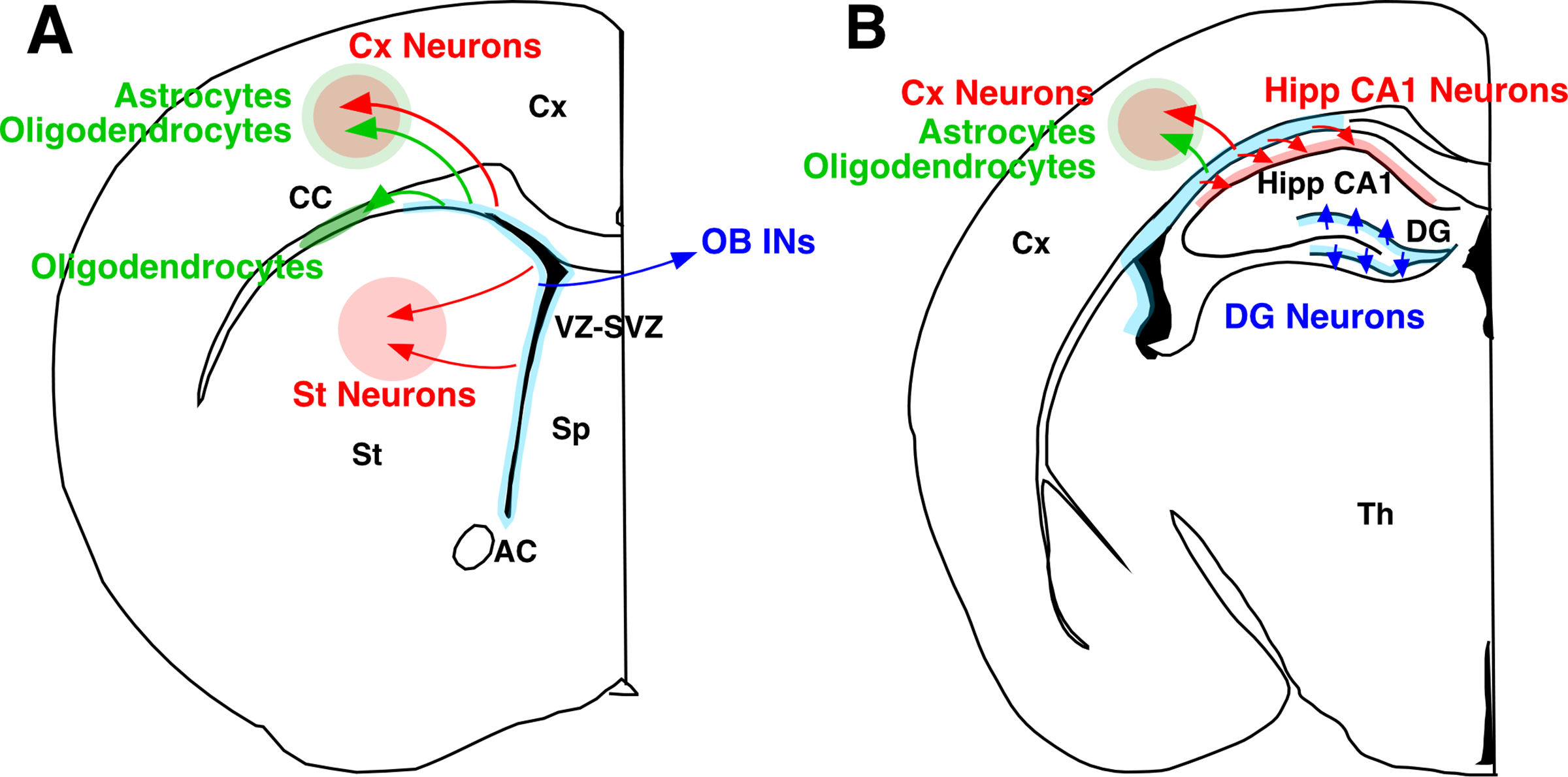
Injury-induced neurogenesis and gliogenesis in the postnatal and adult mammalian brain. In the intact brain, NSCs in the VZ-SVZ and DG selectively produce OB interneurons (A) or DG granule cells (B), respectively (blue arrows). In response to various insults, however, those in the VZ-SVZ are thought to produce new neurons and glia (astrocytes and/or oligodendrocytes) that migrate to damaged areas such as the corpus callosum (CC), cerebral cortex (Cx), striatum (St), and hippocampal CA1 region (highlighted in red and green shades), and replace cells lost to insult (red arrows). The exact identity and functional properties of these cells, however, remain poorly understood. Abbreviations are the same as in Figure 1.
It is important to note, however, that such increased production of neurons in these so-called neurogenic regions do not necessary lead to a replacement of neurons lost to insult in damaged areas. It appears that most of the newly produced neurons after injury stay within the normally neurogenic areas and are considered as a part of compensatory responses to injury [41]. In certain cases, however, there is clear evidence that neurons newly produced after injury migrate toward lesion and replace lost cells. For example, new neurons that resemble striatal and cortical projection neurons have been detected in the adult brain after ischemic and hemorrhagic injury in rodents, humans, and non-human primates [155–164] (Figure 5A). Replacement of hippocampal CA1 pyramidal neurons after ischemic injury has also been reported in rats [165] (Figure 5B). These studies have, thus, demonstrated that neuronal regeneration indeed occurs under certain pathological conditions in the mammalian brain. Given that the production of these types of projection neurons is normally undetectable or negligible in the adult brain under physiological conditions, it is speculated that NSCs that normally produce OB interneurons in the VZ-SVZ region under physiological conditions change their properties after injury to produce these neuronal subtypes, or, alternatively, normally dormant NSCs are activated by injury to give rise to new neurons [discussed in detail in ref. 41]. In fact, recent single-cell transcriptome analysis of NSCs have demonstrated injury-induced changes in the properties of otherwise dormant NSCs in normally non-neurogenic regions of the adult CNS [134, 166].
Studies using different injury models have shown that such increased neurogenesis after injury occurs through multiple different mechanisms such as recruitment of more NSCs into cell divisions, stimulation of proliferation of intermediate progenitors, and/or survival and maturation of new neurons depending on injury types [for details, see ref. 41]. Recent studies have also begun to reveal the molecular mechanisms underlying these regulations. For example, it has been shown that the TFs Gsx2 and Ascl1, which are essential for neurogenesis in the intact brain, also play crucial roles in injury-induced neurogenesis [70, 75]. In addition, a variety of exogenous manipulations, including systemic and local administration of growth factors, cytokines, and hormones, have been shown to augment the extent of such injury-induced neurogenesis [for details see ref. 41]. By contrast, both acute and chronic inflammation and treatment with corticosteroids significantly attenuate injury-induced neurogenesis [167–171]. An important unsolved issue is how much contribution these newly generated neurons have to the restoration of function lost to insult. Some studies present evidence that new neurons are integrated into existing circuitry and exhibit properties similar to lost cells, thereby contributing to functional repair [154, 165]. Other studies, however, argue that new neurons detected in damaged areas do not show region-appropriate phenotypes and have little functional impact [171]. Further studies are necessary to address this issue in order to harness the endogenous regenerative capacity for brain repair.
The production of glial cells is also stimulated after injury. As mentioned above, a low level of oligodendrogenesis continues in the postnatal brain [91–95]. A number of recent studies have shown that its level is significantly increased in response to injury [96–98]. In particular, in the early postnatal brain, in which production of new oligodendrocytes is very active, demyelinating injury of the white matter markedly stimulates the production of OPCs in the VZ-SVZ region, and these new OPCs are recruited to demyelinated lesion and enhance re-myelination [96–98]. Whether similar re-myelination also occurs in the damaged gray matter remains to be investigated. Studies using various animal models of ischemic and traumatic injury have also shown that new astrocytes are produced following insult [110, 111]. These studies have demonstrated that reactive astrocytes originate from NSCs in the VZ-SVZ and participate in the formation of so-called glial scar, which physically seals off lesion and prevents the spreading of secondary tissue damage [110, 111]. On the other hands, other studies have reported that after localized injury such as stab wound in the cerebral cortex, reactive astrocytes are generated mainly through proliferative divisions of local resident astrocytes, but not by NSCs in the VZ-SVZ [112, 113]. Thus, it could be that new astrocytes originate from distinct cellular sources depending on the type of injury and location.
Interestingly, some recent studies suggest that reactive astrocytes in injured areas acquire certain properties of NSCs and produce new neurons locally under certain pathological conditions [112, 113, 173]. Moreover, recent studies have demonstrated that many non-neuronal cell types in the adult brain, including astrocytes, OPCs, and pericytes, can be directly converted into functional neurons in vivo by targeted genetic manipulations, offering a new strategy to regenerate neurons in injured brains [174]. How widespread such a phenomenon of cell type conversion or reprogramming is in injured brains, and whether converted/reprogrammed NSCs and neurons have any significant contributions to tissue repair need to be further investigated.
All together an important message drawn from these studies is that the postnatal and adult mammalian brain has a significant intrinsic capacity for tissue repair. Such a finding has raised the possibility that their endogenous capacity can be further harnessed for better regeneration and repair of the damaged brain by certain means [41]. Yet, the extent of the replacement of lost neurons and glia revealed in these studies so far is rather small with a few exceptions [41]. Therefore, further studies are necessary to develop more effective strategies to augment the endogenous regenerative capacity to achieve functionally meaningful repair after injury.
CONCLUSION AND FUTURE PERSPECTIVES
As described in detail above, studies in recent years have revealed that dynamic changes in the cellular composition continues beyond birth, and even sustains to some extent throughout life in the mammalian CNS. This is a significant departure from the long-held static view on the postnatal brain since the era of Ramón y Cajal and has led us to understand the overall picture of the development of the mammalian brain very differently. Such a new view also lets us take new approaches to understand the pathophysiology of various neurological and psychiatric disorders such as autism spectrum disorders and developmental disabilities that affect the postnatal brain of humans, and to develop new interventional strategies to treat these diseases.
Many important basic developmental biology questions, however, remain unanswered. For instance, although a number of important regulators of postnatal and adult NSCs have so far been identified, our current understanding of how their long-term maintenance and commitment to particular lineages are controlled is still sketchy [62, 63]. In addition, although generation of new neurons and glia continues from embryonic to postnatal periods, the mode of cell genesis markedly changes over time. Little has so far been known about details of such temporal changes and underlying control mechanisms. We need to better understand the similarities and differences between NSCs in embryonic, early postnatal, and adult brains at the molecular level to better understand postnatal brain development [62].
These issues are also important for future advancement of restorative neurology aiming at regeneration and repair of damaged/diseased brains. Although active neurogenesis and gliogenesis is confined to a few restricted regions in the adult mammalian brain under physiological conditions, injury not only accelerates such ongoing cell production, but also induces new neurons and glia in more widespread regions, and, importantly, some of these new cells exhibit properties not seen in the intact brain. Whether the same NSC pool is responsible for such new cell genesis under physiological and pathological conditions is currently unknown. It could be that new neurons and glia produced in injured brain originate from a unique NSC pool that normally remains dormant in the intact brain but is activated by injury. In fact, some recent studies have provided evidence for the occurrence of such dormant NSCs outside the normally neurogenic regions [113, 133, 134, 173]. Alternatively, injury may alter the properties of existing NSCs and change the fate of their progeny. In order to harness the intrinsic regenerative capacity of NSCs for better functional restoration after injury and disease, we need to understand what stimuli regulate their proliferation and differentiation in vivo, and how we can manipulate their responses for better repair. Obviously, such mechanisms and effective interventional manipulations could be significantly different between early postnatal and adult stages, as well as under various pathological conditions.
One thing that has become clear over the past three decades, however, is that unlike in Cajal’s and subsequent eras when people considered the postnatal mammalian brain as a static, rigid structure, we now know that it is actually quite a dynamic and plastic organ. In this regard, it is noteworthy that Cajal himself wrote, after the famous quote, “It is for the science of the future to change, if possible, this harsh decree. Inspired with high ideas, it must work to impede or moderate the gradual decay of the neurons, ….. We must recognize that, in the matter of neurogenesis and nerve regeneration, we are still in the phase of collection of materials” [1]. We are indeed still in the phase of collection of materials, but with much better tools and methods in our hands now compared with Cajal’s era. Further advances in molecular and cellular neurobiology will certainly lead us to better understand the development of the mammalian brain and to develop effective strategies for regeneration and repair of the damaged brain in the future.
ACKNOWLEDGEMENTS
We apologize to authors whose relevant studies we could not cite in this article because of space limitation. We thank the current and former members of our laboratory for their contributions to studies mentioned in this article. Our research mentioned in this article is supported by the National Institute of Health (R01NS069893 and R01NS044080 to M.N.), Ohio Eminent Scholar Fund, and Cincinnati Children’s Research Foundation.
Contributor Information
Masato Nakafuku, Divisions of Developmental Biology, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Avenue, Cincinnati, OH 45229-3039, U.S.A..
Ángela del Águila, Department of Pediatrics, University of Cincinnati College of Medicine.
REFERENCES
- 1.DeFelipe J, Jones EG, May RM. Cajal’s Degeneration and regeneration of the nervous system, transl., In: History of Neuroscience No. 5, New York, Oxford University Press; (1991). ISBN-13: 9780195065169 (DOI: 10.1093/acprof:oso/9780195065169.001.0001). [DOI] [Google Scholar]
- 2.Oberman L, Pascual-Leone A. Changes in plasticity across the lifespan: cause of disease and target for intervention. Prog. Brain Res, 207, 91–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci, 28, 223–250 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron, 30, 588–601 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Hu JS, Vogt D, Sandberg M, Rubenstein JL . Cortical interneuron development: a tale of time and space. Development, November 144, 3867–3878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci, 8, 427–437 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Lemasson M, Saghatelyan A, Olivo-Marin JC, Lledo PM. Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J. Neurosci, 25, 6816–6825 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci, 28, 3966–3975 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Pleasure SJ. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Dev. Neurosci, 27, 93–99 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Hochgerner H, Zeisel A, Lönnerberg P, Linnarsson S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci, 21, 290–299 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol, 435, 406–417 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Jabès A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur. J. Neurosci, 31, 273–285 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA, 90, 2074–2077 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. Petreanu L, Alvarez-Buylla A. J. Neurosci, 22, 6106–6113 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J. Neurosci, 29, 12302–12314 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Wijngaarden P, Franklin RJ. Ageing stem and progenitor cells: implications for rejuvenation of the central nervous system. Development, 140, 2562–2575 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Altman J Are new neurons formed in the brains of adult mammals? Science, 135, 1127–1128 (1962). [DOI] [PubMed] [Google Scholar]
- 18.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat. Rev. Neurosci, 1, 67–73 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Nottebohm F Neuronal replacement in adult brain. Brain Res. Bull, 57, 737–49 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron, 25, 481–492 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Grandel H, Brand M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol, 223, 131–147 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc. Natl. Acad. Sci. USA, 98, 10910–10917 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp. Neurol, 172, 1–16 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Bedard A, Levesque M, Bernier PJ, Parent A. The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur. J. Neurosci, 16, 1917–1924 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl. Acad. Sci. USA, 104, 17169–17173 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp. Anim, 58, 403–407 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Bunk EC, Stelzer S, Hermann S, Schäfers M, Schlatt S, Schwamborn JC. Cellular organization of adult neurogenesis in the common marmoset. Aging Cell, 10, 28–38 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Brus M, Meurisse M, Gheusi G, Keller M, Lledo PM, Lévy F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J. Comp. Neurol, 521, 169–188 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Eriksson PS, Perfilieva E., Bjork-Eriksson T, Alborn AM, Nordborg C, PPeterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med, 4, 1313–1317 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, Hen R, Abrous DN, Toni N, Schinder AF, Zhao X, Lucassen PJ, Frisén J. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell, 23, 25–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell, 122, 133–143 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtås S, van Roon-Mom WM, Björk-Eriksson T, Nordborg C, Frisén J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science, 315, 1243–1249 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science, 318, 980–985 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell, 153, 1219–1227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell, 22, 589–599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-García Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature, 427, 740–744 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature, 478, 382–386 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paredes MF, James D, Gil-Perotin S, Kim H, Cotter JA, Ng C, Sandoval K, Rowitch DH, Xu D, McQuillen PS, Garcia-Verdugo JM, Huang EJ, Alvarez-Buylla A. Extensive migration of young neurons into the infant human frontal lobe. Science, 354, 6308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature, 555, 377–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paredes MF, Sorrells SF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez Martin AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Does Adult Neurogenesis Persist in the Human Hippocampus? Cell Stem Cell, 23, 780–781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakafuku M, Grande A. Neurogenesis in the Damaged Mammalian Brain. In: 70, 71, Comprehensive Developmental Neuroscience: Patterning and cell type specification in the developing CNS and PNS. Vol. 1, Ch 29: 551–608. J.L. Rubenstein and Pasko Rakic, eds. in chief. Academic Press, Elsevier, New York (2013). [Google Scholar]
- 42.Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. Neurogenesis in the striatum of the adult human brain. Cell, 156, 1072–1083 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Bédard A, Cossette M, Lévesque M, Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci. Lett, 328, 213–216 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Bédard A, Gravel C, Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp. Brain Res, 170, 501–512 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Inta D, Cameron HA, Gass P. New neurons in the adult striatum: from rodents to humans. Trends Neurosci, 38, 517–523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated fromdifferent precursors. J. Cell Biol, 168, 415–427 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron HA, Dayer AG. New interneurons in the adult neocortex: small, sparse, but significant? Biol. Psychiatry, 63, 650–655 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J. Neurosci, 26, 8609–8621 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science, 286, 548–552 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. USA, 99, 11464–11469 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science, 310, 679–683 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J. Comp. Neurol, 505, 209–220 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Pérez-Martín M, Cifuentes M, Grondona JM, López-Avalos MD, Gómez-Pinedo U, García-Verdugo JM, Fernández-Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur. J. Neurosci, 31, 1533–1548 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J. Neurosci, 30, 723–730 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science, 294, 2127–2130 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Koketsu D, Mikami A, Miyamoto Y, Hisatsune T. Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J. Neurosci, 23, 937–942 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev, 94, 991–1026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danzer SC. Postnatal and adult neurogenesis in the development of human disease. Neuroscientist, 14, 446–458 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Bowers M, Jessberger S. Linking adult hippocampal neurogenesis with human physiology and disease. Dev. Dyn, 245, 702–709 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Temple S Division and differentiation of isolated CNS blast cells in microculture. Nature, 340, 471–473 (1989). [DOI] [PubMed] [Google Scholar]
- 61.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science, 255, 1707–1710 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Götz M, Nakafuku M, Petrik D. Neurogenesis in the developing and adult brain-Similarities and key differences. Cold Spring Harb. Perspect. Biol, 8, pii: a018853 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsieh J Orchestrating transcriptional control of adult neurogenesis. Genes Dev, 26, 1010–1021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doetsch F The glial identity of neural stem cells. Nat. Neurosci, 6, 1127–1134 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci, 32, 149–184 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell, 96, 25–34 (1999) [DOI] [PubMed] [Google Scholar]
- 67.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell, 97, 703–716 (1999). [DOI] [PubMed] [Google Scholar]
- 68.Beckervordersandforth R, Tripathi P, Ninkovic J, Bayam E, Lepier A, Stempfhuber B, Kirchhoff F, Hirrlinger J, Haslinger A, Lie DC et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell, 7, 744–758 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Codega P, Silva-Vargas V, Paul A, Maldonado-Soto AR, Deleo AM, Pastrana E, Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron, 82, 545–559 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andersen J, Urbán N, Achimastou A, Ito A, Simic M, Ullom K, Martynoga B, Lebel M, Göritz C, Frisén J, Nakafuku M, Guillemot F. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron, 83, 1085–1097 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schütz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev, 22, 2473–2478 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol, 12, 31–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lledo PM, Merkle FT, and Alvarez-Buylla A Origin and function of olfactory bulb interneuron diversity. Trends Neurosci, 31, 392–400 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merkle FT, Mirzadeh Z, and Alvarez-Buylla A Mosaic organization of neural stem cells in the adult brain. Science, 317, 381–384 (2007). [DOI] [PubMed] [Google Scholar]
- 75.López-Juárez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun YY, Yang D, Kuan CY, Campbell K, Nakafuku M. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes Dev, 27, 1272–1287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci, 17, 207–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brill MS, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick GS, Ashery-Padan R, Saghatelyan A, Berninger B, Götz M. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J. Neurosci, 28, 6439–6452 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li G, Fang L, Fernández G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron, 78, 658–672 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci, 28,10434–10442 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol., 5, 57–67 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skulachev VP, Holtze S, Vyssokikh MY, Bakeeva LE, Skulachev MV, Markov AV, Hildebrandt TB, Sadovnichii VA. Neoteny, prolongation of youth: From naked mole rats to “naked apes” (Humans). Physiol. Rev, 97, 699–720 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Emery B, Lu QR. Transcriptional and Epigenetic Regulation of Oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb. Perspect. Biol, 7(9), a020461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development, 142, 3983–3995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci, 9, 173–179 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parras CM, Hunt C, Sugimori M, Nakafuku M, Rowitch D, Guillemot F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J. Neurosci, 27, 4233–4242 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yue T, Xian K, Hurlock E, Xin M, Kernie SG, Parada LF, Lu QR. A critical role for dorsal progenitors in cortical myelination. J. Neurosci, 26, 1275–1280 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tripathi RB, Clarke LE, Burzomato V, Kessaris N, Anderson PN, Attwell D, Richardson WD. Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts. J. Neurosci, 31, 6809–6819 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crawford AH, Tripathi RB, Richardson WD, Franklin RJM. Developmental origin of oligodendrocyte lineage cells determines response to demyelination and susceptibility to age-associated functional decline. Cell Rep, 15, 761–773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winkler CC, Yabut OR, Fregoso SP, Gomez HG, Dwyer BE, Pleasure SJ, Franco SJ. The Dorsal Wave of Neocortical Oligodendrogenesis Begins Embryonically and Requires Multiple Sources of Sonic Hedgehog. J. Neurosci, 38, 5237–5250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J. Neurosci, 25, 7289–7298 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Götz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci, 5, 308–315 (2002). [DOI] [PubMed] [Google Scholar]
- 92.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci, 26, 7907–7918 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat. Cell Biol, 15, 602–613 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Azim K, Fischer B, Hurtado-Chong A, Draganova K, Cantù C, Zemke M, Sommer L, Butt A, Raineteau O. Persistent Wnt/β-catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells, 32, 1301–1312 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Tong CK, Fuentealba LC, Shah JK, Lindquist RA, Ihrie RA, Guinto CD, Rodas-Rodriguez JL, Alvarez-Buylla A. A dorsal SHH-dependent domain in the V-SVZ produces large numbers of oligodendroglial lineage cells in the postnatal brain. Stem Cell Reports, 5, 461–470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Baron-Van Evercooren A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci, 11, 4357–4366 (1999). [DOI] [PubMed] [Google Scholar]
- 97.Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Höglinger GU, Hirsch EC, Reynolds R, Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA, 104, 4694–4699 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci, 13, 541–550 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisén. Dynamics of oligodendrocyte generation in the human brain. Cell, 159, 766–774 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol, 20, 588–594 (2010). [DOI] [PubMed] [Google Scholar]
- 101.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb. Perspect. Biol, 7, a020362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell, 1, 515–528 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang W, Hébert JM. A Sox2 BAC transgenic approach for targeting adult neural stem cells. PLoS One, 7, e49038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang P, Lee HK, Glasgow SM, Finley M, Donti T, Gaber ZB, Graham BH, Foster AE, Novitch BG, Gronostajski RM, Deneen B. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron, 74, 79–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glasgow SM, Carlson JC, Zhu W, Chaboub LS, Kang P, Lee HK, Clovis YM, Lozzi BE, McEvilly RJ, Rosenfeld MG, Creighton CJ, Lee SK, Mohila CA, Deneen B. Glia-specific enhancers and chromatin structure regulate NFIA expression and glioma tumorigenesis. Nat. Neurosci, 20, 1520–1528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun W, Cornwell A, Li J, Peng S, Osorio MJ, Aalling N, Wang S, Benraiss A, Lou N, Goldman SA, Nedergaard M. SOX9 Is an Astrocyte-Specific Nuclear Marker in the Adult Brain Outside the Neurogenic Regions. J. Neurosci, 37, 4493–4507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, Gomez Gaviro MV, Booth S, Gao B, Cheah KS, Lovell-Badge R, Briscoe J. SOX9 induces and maintains neural stem cells. Nat. Neurosci,13, 1181–1189 (2010). [DOI] [PubMed] [Google Scholar]
- 108.Nagao M, Ogata T, Sawada Y, Gotoh Y. Zbtb20 promotes astrocytogenesis during neocortical development. Nat. Commun, 7, 11102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature, 484, 376–80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature, 497, 369–373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Faiz M, Sachewsky N, Gascón S, Bang KW1, Morshead CM, Nagy A. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell, 17, 624–634 (2015). [DOI] [PubMed] [Google Scholar]
- 112.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA, 105, 3581–3586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer IR, Plesnila N, Staufenbiel M, Haass C, Snapyan M, Saghatelyan A, Tsai LH, Fischer A, Grobe K, Dimou L, Götz M. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell, 12, 426–439 (2013). [DOI] [PubMed] [Google Scholar]
- 114.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell, 145, 1142–1155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell, 8, 566–579 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologiesrespond selectively to physiological and pathological stimuli and aging. Cell Stem Cell, 6, 445–456 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Sohn J, Orosco L, Guo F, Chung SH, Bannerman P, Mills Ko E, Zarbalis K, Deng W, Pleasure D. The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice. J. Neurosci, 35, 3756–3763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Calzolari F, Michel J, Baumgart EV, Theis F, Götz M, Ninkovic J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci, 18, 490–492 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Basak O, Krieger TG, Muraro MJ, Wiebrands K, Stange DE, Frias-Aldeguer J, Rivron NC, van de Wetering M, van Es JH, van Oudenaarden A, Simons BD, Clevers H. Troy+ brain stem cells cycle through quiescence and regulate their number by sensingnice occupancy. Proc. Natl. Acad. Sci. USA, 115, E610–E619 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raff MC. Glial cell diversification in the rat optic nerve. Science, 243, 1450–1455 (1989). [DOI] [PubMed] [Google Scholar]
- 121.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development, 135, 145–157(2008). [DOI] [PubMed] [Google Scholar]
- 122.Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A. Olig2-dependent developmental fate switch of NG2 cells. Development, 139, 2299–2307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRα/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci, 11, 1392–1401 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J. Neurosci, 30, 12036–12049 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J. Neurosci, 30, 16383–16390 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron, 68, 668–681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol, 119, 55–73 (2010). [DOI] [PubMed] [Google Scholar]
- 128.Abdelhamed Z, Vuong SM, Hill L, Shula C, Timms A, Beier D, Campbell K, Mangano FT, Stottmann RW, Goto J. A mutation in Ccdc39 causes neonatal hydrocephalus with abnormal motile cilia development in mice. Development, 145, pii: dev154500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Coletti AM, Singh D, Kumar S, Shafin TN, Briody PJ, Babbitt BF, Pan D, Norton ES, Brown EC, Kahle KT, Del Bigio MR, Conover JC. Characterization of the ventricular-subventricular stem cell niche during human brain development. Development, 145, pii: dev170100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, Bennett V, Garcia-Verdugo JM, Kuo CT. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron, 71, 61–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell, 3, 265–278 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakafuku M, Nagao M, Grande A, Cancelliere A. Revisiting neural stem cell identity. Proc. Natl. Acad. Sci. USA, 105, 829–830 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC Jr, Fan G, de Vellis J, Sun YE. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc. Natl. Acad. Sci. USA, 105, 1026–1031 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luo Y, Coskun V, Liang A, Yu J, Cheng L, Ge W, Shi Z, Zhang K, Li C, Cui Y, Lin H, Luo D, Wang J, Lin C, Dai Z, Zhu H, Zhang J, Liu J, Liu H, deVellis J, Horvath S, Sun YE, Li S. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell, 161, 1175–1186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shah PT, Stratton JA, Stykel MG, Abbasi S, Sharma S, Mayr KA, Koblinger K, Whelan PJ, Biernaskie J. Single cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell. 173, 1045–1057 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Kaur C, Rathnasamy G, Ling EA. The Choroid Plexus in Healthy and Diseased Brain. J. Neuropathol. Exp. Neurol, 75, 198–213 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Johansson PA, Irmler M, Acampora D, Beckers J, Simeone A, Götz M. The transcription factor Otx2 regulates choroid plexus development and function. Development, 140, 1055–1066 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell, 19, 643–652 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science, 311, 629–632 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Petrik D, Myoga MH, Grade S, Gerkau NJ, Pusch M, Rose CR, Grothe B, Götz M. Epithelial sodium channel regulates adult neural stem cell proliferation in a flow-dependent manner. Cell Stem Cell, 22, 865–878 (2018). [DOI] [PubMed] [Google Scholar]
- 141.Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. Retinoic acid from the meninges regulates cortical neuron generation. Cell, 139, 597–609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci, 19, 771–783 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamamoto S, Muramatsu M, Azuma E, Ikutani M, Nagai Y, Sagara H, Koo BN, Kita S, O’Donnell E, Osawa T, Takahashi H, Takano KI, Dohmoto M, Sugimori M, Usui I, Watanabe Y, Hatakeyama N, Iwamoto T, Komuro I, Takatsu K, Tobe K, Niida S, Matsuda N, Shibuya M, Sasahara M. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci. Rep, 7, 3855 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paredes I, Himmels P, Ruiz de Almodóvar C. Neurovascular communication during CNS development. Dev. Cell, 45, 10–32 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci, 11, 429–439 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell, 3, 279–288 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell, 3, 289–300 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Microglia in Physiology and Disease. Wolf SA, Boddeke HW, Kettenmann H. Annu. Rev. Physiol, 79, 619–643 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia, 54, 815–825 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Morton MC, Neckles VN, Seluzicki CM, Holmberg JC, Feliciano DM. Neonatal subventricular zone neural stem cells release extracellular vesicles that act as a microglial morphogen. Cell Rep, 23, 78–89 (2018). [DOI] [PubMed] [Google Scholar]
- 151.Naruse M, Shibasaki K, Shimauchi-Ohtaki H, Ishizaki Y. Microglial activation induces generation of oligodendrocyte progenitor cells from the subventricular zone after focal demyelination in the corpus callosum. Dev. Neurosci, 40, 54–63 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Vay SU, Flitsch LJ, Rabenstein M, Rogall R, Blaschke S, Kleinhaus J, Reinert N, Bach A, Fink GR, Schroeter M, Rueger MA. The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo. J. Neuroinflammation, 15, 226. doi: 10.1186/s12974-018-1261-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kreisel T, Wolf B, Keshet E, Licht T. Unique role for dentate gyrus microglia in neuroblast survival and in VEGF-induced activation. Glia. 2018. November 19. doi: 10.1002/glia.23505. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 154.Goldman SA. Stem and progenitor cell-based therapy of the central nervous system: Hopes, hype, and wishful thinking. Cell Stem Cell, 18, 174–188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA, 101, 343–347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA, 103, 13198–13202 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J. Neurosci, 26, 13114–13119 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen J, Xie L, Mao X, Zhou Y, Zhan R, Greenberg DA, Jin K. Neurogenesis after primary intracerebral hemorrhage in adult human brain. J. Cereb. Blood Flow Metab, 28,1460–1468 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nakayama D, Matsuyama T, Ishibashi-Ueda H, Nakagomi T, Kasahara Y, Hirose H, Kikuchi-Taura A, Stern DM, Mori H, Taguchi A. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur. J. Neurosci, 31, 90–98 (2010). [DOI] [PubMed] [Google Scholar]
- 160.Zheng W, ZhuGe Q, Zhong M, Chen G, Shao B, Wang H, Mao X, Xie L, Jin K. Neurogenesis in adult human brain after traumatic brain injury. J. Neurotrauma, 30, 1872–1880 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koketsu D, Furuichi Y, Maeda M, Matsuoka N, Miyamoto Y, Hisatsune T. Increased number of new neurons in the olfactory bulb and hippocampus of adult non-human primates after focal ischemia. Exp. Neurol, 199, 92–102 (2006). [DOI] [PubMed] [Google Scholar]
- 162.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med, 8, 963–970 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol, 52, 802–813 (2002). [DOI] [PubMed] [Google Scholar]
- 164.Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, Ment LR, Vaccarino FM. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp. Neurol, 199, 77–91 (2006). [DOI] [PubMed] [Google Scholar]
- 165.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell, 110, 429–441 (2002). [DOI] [PubMed] [Google Scholar]
- 166.Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain Injury. Cell Stem Cell, 17, 329–340 (2015). [DOI] [PubMed] [Google Scholar]
- 167.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA, 100, 13632–13637 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science, 302, 1760–1765 (2003). [DOI] [PubMed] [Google Scholar]
- 169.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience, 61, 203–209 (1994) [DOI] [PubMed] [Google Scholar]
- 170.Charalampopoulos I, Remboutsika E, Margioris AN, Gravanis A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends Endocrinol. Metab, 19, 300–307 (2008) [DOI] [PubMed] [Google Scholar]
- 171.Yang Z, You Y, Levison SW. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J. Comp. Neurol, 511, 19–33 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grande A, Sumiyoshi K, López-Juárez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun, 4, 2373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen G, Wernig M, Berninger B, Nakafuku M, Parmar M, Zhang CL. In Vivo Reprogramming for Brain and Spinal Cord Repair. eNeuro, 2(5) pii: ENEURO.0106–15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


