Abstract
Over the past several decades, it has become increasingly clear that women have distinct cardiovascular profiles compared to men. In this review, our goal is to provide an overview of the literature regarding the influences of female sex and reproductive hormones (primarily estradiol) on mechanisms of cardiovascular control relevant to regulation of blood pressure, body temperature and cerebral blood flow. Young women tend to have lower resting blood pressure compared with men. This sex difference is reversed at menopause, when women develop higher sympathetic nerve activity and the risk of systemic hypertension increases sharply as postmenopausal women age. Vascular responses to thermal stress, including cutaneous vasodilation and vasoconstriction, are also affected by reproductive hormones in women, where estradiol appears to promote vasodilation and heat dissipation. The influence of reproductive hormones on cerebral blood flow and sex differences in the ability of the cerebral vasculature to increase its blood flow (cerebrovascular reactivity) are a relatively new area of investigation. Sex and hormonal influences on integrative blood flow regulation have further implications during challenges to physiological homeostasis, including exercise. We propose that increasing awareness of these sex-specific mechanisms is important for optimizing healthcare and promotion of wellness in women across the lifespan.
Keywords: thermoregulation, cerebral blood flow, sex differences, vasodilation, estradiol
Introduction
The cardiovascular and autonomic nervous systems work together as major integrators of physiological homeostasis in humans. The regulation of blood flow throughout the tissues of the organism is central to the maintenance of integrative homeostasis, including optimizing the levels of blood pressure and body temperature. In the present discussion, our goal is to provide a brief review and update of these mechanisms with a specific focus on cardiovascular control mechanisms in women, and the impact of the female sex on cardiovascular function and cerebrovascular responsiveness across the lifespan.
Autonomic nervous system and cardiovascular control
The autonomic nervous system regulates blood pressure via central and peripheral mechanisms. At the level of the heart, autonomic nerves control both heart rate and cardiac contractility. Sympathetic post-ganglionic nerves increase heart rate and cardiac contractility via β-1 adrenergic receptor binding. Thus, increased cardiac sympathetic activity results in increased cardiac output (CO) by both increases in heart rate and stroke volume. In humans, the primary cardiovascular effect of parasympathetic activity is via vagal innervation of the heart. The vagus nerve innervates the sinoatrial (SA) and atrioventricular (AV) nodes and has the effect of slowing down heart rate. Since the vagus nerve is tonically active in healthy humans, decreases in its activity can increase heart rate relatively rapidly.
Sympathetic nerve activity (SNA) also influences the state of constriction or dilation of peripheral blood vessels, thus controlling peripheral vascular resistance. SNA is an important contributor to peripheral vascular resistance. Post-ganglionic vascular sympathetic nerves release noradrenaline, which causes vasoconstriction via binding to α-adrenergic receptors at the vascular smooth muscle, thus increasing vascular resistance in the area of innervation. Because most vascular sympathetic nerves exhibit tonic baseline activity, these nerves have the ability to either increase or decrease peripheral vascular resistance by increasing or decreasing, respectively, their activity. A detailed discussion of the integrative mechanisms of sympathetic and parasympathetic neural control of blood pressure is beyond the scope of the present review; the interested reader is referred to several comprehensive reviews (1-3).
The autonomic nervous system has important roles in the regulation of both blood pressure and body temperature in humans, and often these two sets of mechanisms overlap. Changes in blood pressure are sensed by arterial baroreceptors, located at the carotid sinuses and the aortic arch, which send information to central autonomic nuclei including the nucleus tractus solitarii (NTS) of the medulla and the paraventricular nucleus (PVN) of the hypothalamus (2, 4). The central autonomic nuclei then activate efferent autonomic pathways, which work to “buffer”, or minimize, changes in blood pressure by activating or de-activating sympathetic and parasympathetic pathways. The baroreflex responsiveness, or sensitivity, can be measured as the response of an output variable (such as heart rate, sympathetic nerve activity or vascular resistance) to a given change in blood pressure. Baroreflex sensitivity in control of heart rate (cardiovagal sensitivity) is decreased in young women compared to men (5), whereas sympathetic baroreflex sensitivity tends to be similar between the sexes until after menopause, when sensitivity decreases in women (5).
Changes in body temperature are primarily integrated at the preoptic area of the anterior hypothalamus (PO/AH), which receives afferent information from peripheral thermoreceptors (6). The PO/AH also responds to its own (local) temperature, which is reflective of the temperature of the body core (7, 8). Increases in body temperature elicit heat dissipation responses which, in humans, are primarily sweating and cutaneous vasodilation. Decreases in body temperature elicit cutaneous vasoconstriction and shivering, which act to decrease heat dissipation and increase heat generation, respectively (6, 8, 9). As discussed below, the female sex hormones estradiol and progesterone have significant influences on control of human body temperature (10, 11). However, the overall capacity for thermoregulation (maintenance of body temperature) does not appear to be different between the sexes, with a possible exception of very high intensities of exercise in very hot and humid environments (12).
Important examples of overlap in autonomic regulation of blood pressure and body temperature can occur at both extremes of the thermal environment. For example, in hot environments, large amounts of blood flow are redirected to the skin circulation to aid in heat dissipation. Because the cutaneous circulation is relatively compliant, and contains venous plexuses which are designed to increase the volume of blood in the skin at any given time, the redirection of blood flow to the skin can cause a marked decrease in the amount of venous return for a given level of CO (13, 14). Reduced venous return can lead to a marked decrease in orthostatic tolerance and thus, the experience of feeling dizzy or fainting while upright becomes much more common during exposure to hot environments (15, 16).
The converse situation is also true – the cutaneous vasoconstriction that occurs with cold exposure is effective in increasing the insulative properties of the skin and decreasing heat loss to the environment. However, the same vasoconstriction might increase cardiac afterload in a manner that would predispose someone to an increase in cardiovascular risk (risk of acute hypertensive events), particularly in older individuals or in those already being treated for systemic hypertension. This integrative pathophysiology likely contributes to the increased risk of cardiovascular events in cold environments, for example, when older people are shoveling snow in the winter (17, 18).
Neural-hemodynamic balance and blood pressure regulation in men and women
The female sex is associated with some specific biological differences in neurovascular control mechanisms, many of which appear to be dependent on the reproductive hormone estradiol. For example, the extent to which β-2 adrenergic vasodilation contributes to total peripheral resistance (TPR) in women is quantitatively greater in young women compared to young men (1, 19), a phenomenon which has extensive implications for cardiovascular health in both young and older men and women.
Over the last 2 decades, we and others have been interested in evaluating the mechanisms for, and implications of, potential differences between men and women in the regulation of arterial blood pressure. Our original questions stemmed from the classic observation that there is significant inter-individual variability in resting levels of vascular SNA across young, healthy subjects with similar, normal blood pressure values (20). Furthermore, even though vascular SNA itself can cause large increases in blood pressure, there is no relationship between the resting levels of this activity and blood pressure. In investigating potential mechanisms for this apparent paradox, we noted that in young, healthy men, there was an inverse relationship between resting CO and resting vascular SNA (21). Vascular SNA was positively related to TPR, and it appeared that the pressor effects of higher SNA were offset by lower levels of CO. Since women tend to have lower resting levels of both CO and SNA, we hypothesized that women would have a similar inverse CO-SNA relationship, but shifted to lower values.
We were surprised, therefore, to see no relationship between CO and SNA or between SNA and TPR in our groups of young healthy women (22). We noted an earlier study (23) in which young women were shown to have blunted forearm vasoconstrictor responses to intra-brachial infusions of noradrenaline compared to young men. In that study, blockade of β-adrenergic receptors eliminated the sex differences, suggesting β-mediated vasodilation was offsetting some of the vasoconstriction in the women (23). We therefore followed up on those observations to test whether systemic β-blockade via propranolol would have similar effects on the whole-body SNA-TPR relationship that local β-blockade had in the forearm. Systemic β-adrenergic blockade with propranolol caused the SNA-TPR relationships in young women to look more similar to those seen in young men and older women, suggesting that estrogen and β-adrenergic vasodilation are major factors that minimize the pressor effects of vascular sympathetic adrenergic neural activity (24).
In a follow up study, intravenous trimethaphan was used to systemically block autonomic ganglia in order with to evaluate the extent to which SNA supports blood pressure in young and older women (25). We found that blood pressure dropped about 3 times more in older women compared to younger women (~29 vs ~9 mmHg) during trimethaphan infusion. Furthermore, the extent of the drop in blood pressure was significantly related to the resting activity of sympathetic nerves across subjects. This finding supports the idea that the higher SNA seen in post-menopausal women is a contributor to the higher risk of systemic hypertension in this group (25).
In young women, the balance between SNA and TPR relationship may be altered by oral contraceptives. Harvey and colleagues reported similar muscle SNA between a group of young women taking oral contraceptives and a group with regular menstrual cycles, but found that arterial pressure was significantly (~4 mmHg) higher in the oral contraceptive group (26). There is also evidence that autonomic support of blood pressure is influenced by cardiorespiratory fitness in young women, such that those with higher aerobic fitness have an attenuated response to ganglionic blockade using trimethaphan; interestingly, the association with cardiorespiratory fitness is not observed in older women (27). Taken together, these results give insight into the complex interactions among sex hormones, cardiorespiratory fitness, and autonomic regulation of blood pressure.
Although outside the scope of the present discussion, female reproductive hormones also exert important influences on fluid volume regulation, an additional variable important to the integrative control of systemic blood pressure in humans. For example, both estrogen and progesterone alter osmotic regulation of vasopressin, which can lead to fluid retention in some circumstances (28). The interested reader is referred to comprehensive reviews on this topic (29, 30).
Responses to thermal stress in young and older women
In young women, body temperature is ~0.5 °C higher in the mid-luteal phase of the menstrual cycle, when progesterone and estrogen are elevated, compared to the early follicular phase, when hormone levels are relatively low. Studies from the 1980s and 90s showed that this shift in body temperature is part of a larger, integrative shift in thermoregulation. Cutaneous vasodilation, sweating, cutaneous vasoconstriction and shivering are all shifted to higher body temperatures during the mid-luteal compared to the early follicular phase (31-33). When women take exogenous hormones in the form of oral contraceptive pills, similar shifts are observed, particularly when so called “combination” oral contraceptive pills that include both estrogen and progestin are taken (34-36).
The shift in thermoregulation to higher body temperatures seems to be specific to progesterone or the combination of progesterone plus estrogen, since estrogen alone appears to have a pro-vasodilation, pro-heat dissipation effect. In post-menopausal women, for example, addition of estrogen-only hormone replacement therapy (HRT) shifted cutaneous vasodilation and sweating to lower body temperatures, such that heat dissipation was initiated earlier (37). Furthermore, addition of progesterone to the HRT reversed the effect (38). Thus, the pro-vasodilator effects of estrogen that are seen in many other areas of cardiovascular function are also seen in the skin circulation as related to regulation of body temperature.
Whether the influences of female hormones extend to a broader sex difference in thermoregulation in the heat is a separate question. Overall, there is no evidence to suggest that thermoregulation is impaired in women relative to men in most environmental conditions when factors such as aerobic capacity, heat acclimation status and hydration are appropriately accounted for (reviewed in (39, 40)). On average, women sweat less than men, but also have higher surface area to mass ratios. Thus, women’s blood flow responses may be more optimized in some circumstances compared to similar environmental heat exposure in men. Ultimately, the net effect of anatomical and/or physiological differences between the sexes on thermoregulation appears to be minimal in most “real-life” scenarios.
Thermal stress and cerebral blood flow
Observational and experimental thermoregulation studies have revealed symptoms suggestive of changes in cerebral blood flow (including dizziness, confusion) and symptoms known to alter cerebral blood flow (hyperventilation, respiratory-induced alkalosis, dehydration, central hypovolemia, increased SNA). Passive heat stress experiments have demonstrated reductions in cerebral blood flow, especially when combined with other physiological or environmental challenges (41).
The majority of studies that investigate passive heat stress and cerebrovascular control have evaluated men only, making it difficult to determine if the known influence of sex hormones on thermoregulation, also impacts the cerebral circulation. In the available studies in men, there have been contrasting results for the effect of aging on the cerebral blood flow responses to heat stress. For example, passive heat stress resulted in a slight reduction in cerebral blood flow in older men, but this was only apparent during upright posture (42). Lucas et al. reported minimal differences in middle cerebral artery (MCA) blood velocity during hyperthermia in either young or older men (43). However, as mentioned earlier, the influence of other physiological factors associated with passive heat stress on cerebral blood flow regulation further complicates these assessments (see Bain et al. for review (41)). For example, during passive heat stress, the increases in the external carotid artery (ECA) blood flow was not associated with the changes in the internal carotid artery (ICA) blood flow to (44). During exercise-induced hyperthermia, there are reciprocal shifts in regional blood flow distribution, such that flow through the ICA is decreased, but balanced by the increased flow through the ECA which may help dissipate excess heat to the skin on the face and protect the brain (45).
In addition to examining the impact of passive heat stress on cerebral perfusion, cerebral autoregulation and cerebrovascular reactivity have been evaluated. Cerebral autoregulation is the ability of the cerebral circulation to maintain blood flow despite fluctuations in systemic perfusion pressure. Cerebral autoregulation is reportedly unaltered by thermal stress (46, 47), but there is little agreement regarding the best method to use to quantify cerebral autoregulation, especially during physiological perturbations (48). Cerebrovascular reactivity is often defined as the ability of the cerebral vasculature to respond to vasoactive stimuli. When using changes in arterial CO2 as the stimulus to measure cerebrovascular reactivity, many studies show no change in cerebrovascular reactivity (49, 50), whereas others report increases (43) or even decreases in cerebrovascular reactivity (51). Thus, the existing data suggests that thermal stress challenges cerebral blood flow regulatory mechanisms, but more research is needed to understand the interaction of thermoregulation and cerebrovascular regulation.
Cerebrovascular responsiveness in young and older men and women
Global cerebral blood flow at rest is reduced in older adults(52), but the age-associated decline in global cerebral blood flow is only significant in women (53). Therefore, these age-associated declines in cerebral blood flow have a greater impact on women and may, in part, underlie the higher risk of stroke and cognitive decline in women relative to age-matched men (54, 55). In addition to resting measures of cerebral blood flow, cerebrovascular responsiveness can be quantified by evaluating the cerebral blood flow response to a stimulus to quantify cerebrovascular health. Reduced cerebrovascular reactivity to arterial CO2 is associated with future risk of cerebrovascular disease (56). Previous studies have shown that cerebrovascular reactivity to hypercapnia is impaired with advancing age (57-59). Similar to cerebral blood flow changes, it has been suggested that this age-associated decline in cerebrovascular reactivity may be driven by the decreases occurring primarily in women (60), with young women having higher cerebrovascular reactivity compared with age-matched men (61).
The age-associated changes in cerebral blood flow and cerebrovascular reactivity occur around the age of menopause (53, 60). Yet, within postmenopausal women, cerebrovascular reactivity was higher in women who were taking menopausal HRT (60). There have also been reports that both acute estrogen (62, 63), and 12 months of menopausal HRT, increase regional and global cerebral blood flow (64). Collectively, this suggests that treatment with sex hormones (primarily estrogen and progesterone) might offer a neuroprotective effect, at least on the cerebral vasculature. Remaining unanswered questions are whether the higher cerebrovascular reactivity measured during menopausal HRT is maintained after cessation of hormone treatment or what formulation of menopausal HRT is most beneficial for brain health.
Many reports on the effects of menopausal HRT come from short-term interventions, non-randomized studies, or employ a variety of hormonal formulations (65). Even after the Women’s Health Initiative study, which included a clinical trial, there were conflicting recommendations regarding the use of menopausal HRT to prevent cardiovascular disease. The major criticism of this study was that HRT was initiated late in the menopausal transition. Therefore, the influences of long-term menopausal HRT use, when initiated shortly after the onset of menopause, are not clear (66). Furthermore, whether menopausal HRT use is beneficial for the cerebral circulation and brain health was unknown. In order to address this question, women who had previously participated in the Kronos Early Estrogen Replacement Study (KEEPS), where they were randomized to receive either oral conjugated equine estrogen, transdermal 17ß-estradiol, or placebo for four years, were re-evaluated 3 years after the trial ended (67). Three years after cessation of hormone or placebo treatment, there were no significant group differences in aortic hemodynamics (68) or cerebrovascular reactivity (69). However, there was a trend for higher cerebral artery blood velocity and cerebrovascular conductance at rest, which could reflect higher global cerebral blood flow in the combined menopausal hormone groups (oral conjugated equine estrogen and transdermal 17ß-estradiol). Additionally, repeated measures analysis revealed a marginal sustained effect of higher cerebral artery blood velocity during hypercapnia and a trend for greater cerebrovascular reactivity in the combined hormone group (69). Importantly, these data are the first to report on the potential sustained effects of long-term menopausal HRT three years after cessation of treatment. While menopausal HRT use in postmenopausal women is associated with elevated cerebrovascular reactivity, comparable to values in premenopausal women (60), the effect is only marginal in the years after cessation of menopausal HRT. Likewise, menopausal HRT initiated early in menopause did not reduce cerebrovascular reactivity, although more research is necessary to explore the potential underlying mechanisms of the responses to menopausal HRT. These data are further supported by studies showing no differences between treatment groups in global brain volume and cognitive function 3 years after menopausal HRT cessation (70).
Prior studies have shown an age-related decline in cerebrovascular reactivity in women, compared with men, and that current treatment with exogenous sex hormones in postmenopausal women is associated with higher cerebrovascular reactivity (60). One question that remains is whether young premenopausal women have better cerebrovascular control mechanisms compared to men due to the availability of estrogen? Because young women have higher global cerebral blood flow at rest compared with age-matched young men (53), estrogen availability is often cited as having a mechanistic role in the differences in cerebral circulatory control between males and females (71).
Pre-clinical studies in animal models consistently shows several sex differences in the cerebral blood vessels. For example, isolated cerebral arteries from rats demonstrate sex differences in wall structure, as well as a shift in stress-strain curves and a lower autoregulatory range in female vessels (72). Other studies have shown that isolated cerebral vessels from females exhibit greater vasodilation compared with male vessels (73, 74). Yet, both estrogen and androgen receptors are present in both male and female cerebral arteries (75) and may influence vascular tone in isolated middle cerebral artery segments (76). Estrogen can augment the production and activity of nitric oxide (NO), vasodilating prostaglandins and endothelial-derived hyperpolarizing factor (EDHF) (77), whereas androgens reduce EDHF and increase the vasoconstrictor thromboxane (78, 79). Therefore, higher circulating estrogen and estrogen receptor signaling may contribute to lower cerebrovascular tone and greater cerebrovascular dilation in response to vasoactive stimuli.
The above discussion suggests that sex influences cerebral artery structure and function, but sex differences in cerebrovascular control mechanisms in men and women are less clear. Some studies have reported higher cerebrovascular reactivity to hypercapnia in young women compared with young men (60, 61); yet, more recent studies have reported higher cerebrovascular reactivity in young men compared with young women (58, 77, 80) or no change in cerebrovascular reactivity (81). It is important to note that any potential sex differences in cerebrovascular reactivity may be influenced by alterations in chemosensitivity to hypercapnia between men and women (82). Sex differences in other aspects of cerebrovascular control have been reported, including cerebral blood velocity responses to changes in posture (83, 84). These studies suggest that young men demonstrate an increase in cerebrovascular resistance (vasoconstriction) compared with age-matched premenopausal women in response to postural changes, which is consistent with reports of more frequent orthostatic intolerance in women. Along these lines, sex differences in young adults in measures of cerebral autoregulation have also been reported (85-87), but results are inconsistent, making it difficult to determine possible underlying mechanisms. In older adults, Deegan et al. reported higher cerebral autoregulation in postmenopausal women compared with age-matched men (88). Additionally, it is important to note that sex differences in cerebrovascular responses to physiological challenges (including postural changes or hypercapnia) may be influenced by SNA. Controversy remains regarding the role and influence of sympathetic innervation of the cerebral vessels on these measurements, and experiments are confounded by cerebrovascular sensitivity to CO2 and cerebral autoregulation (for further review see (89, 90)). These uncertainties add to the complexity of understanding the influence of SNA on sex differences or age-related changes in cerebrovascular responsiveness.
Another factor that contributes to the lack of consensus regarding sex differences in the cerebral circulation in humans is that the ability to accurately quantify cerebral blood flow responses in humans is inherently difficult. Often, quantifying cerebral blood flow or cerebrovascular responsiveness is based on the blood velocity through a single cerebral artery (often the MCA), and relies on the assumption that vessel diameter remains constant. This assumption has been recently challenged with high resolution MRI data demonstrating that the MCA diameter changes during hypercapnia in some populations of adults (58, 91, 92). Additionally, sex differences could be exaggerated if the vessel sizes are also different between men and women. To address this, Miller et al. recently used state-of-the-art MRI analysis to quantify cerebral blood flow through the MCA at rest, and in response to hypercapnia using 4D flow MRI in healthy humans (58). On average, the cross-sectional area of the MCAs was slightly larger in young men compared with young women. Upon further analysis of the right and left MCA separately, we found that there was a trend for greater cerebral dilation of the left MCA during hypercapnia in men compared with women, although the high variability in cerebrovascular responsiveness may mean that this study could be underpowered to adequately assess sex differences in MCA dilation (93).
In addition to the technique used to measure cerebral blood flow, the conflicting findings may be due to differences in menstrual cycle control in young women, as Krejza et al. reported that cerebrovascular reactivity varied throughout the cycle, with regional differences between the right and left hemispheres (94). In all of our work, women were studied during the same phase of the menstrual cycle (early follicular phase or placebo phase of the menstrual cycle) in attempt to minimize the variation in sex hormones. Other groups have reported that cerebrovascular variables are only minimally influenced by menstrual cycle phase (83, 85). Thus, it is unclear if sex hormone concentrations explain the previously reported differences in cerebrovascular control between young men and women.
As mentioned above, MRI-based studies on cerebral blood flow at rest report greater cerebral perfusion in young women compared with young men (53). This is consistent with the lower cerebrovascular tone observed in female animals compared with male animals and more constricted cerebral arteries found in ovariectomized females (74). Therefore, another interpretation of the findings of that cerebrovascular reactivity in women is lower than men is that young women may exhibit less responsiveness to hypercapnia, which is supported in the rat model (95). This may mean that a greater CO2 stimulus is necessary to initiate vasodilation in females compared with males. Overall, much is unknown regarding the underlying mechanisms of sex differences in control of the cerebral circulation, and more research is necessary to understand the sex-specific risk for stroke and cognitive decline.
Influences of exercise and cardiorespiratory fitness
All of the cardiovascular and cerebrovascular mechanisms discussed here have implications for optimal physiological function during exercise. The influences of sex and reproductive hormones on exercise performance are often viewed from the perspective that women may be “less able” than men to perform certain tasks. For example, it was historically assumed (or estimated based on anecdotal information) that women were less able to thermoregulate effectively during exercise in the heat. This type of assumption led to women being banned from participation in marathons in the early part of the 20th century and were not allowed to “legally” compete until 1976 (96). However, when potential confounders are accounted for, the only scenario in which women appear to be at a slight disadvantage compared to men in terms of ability to dissipate heat (due to lower maximal evaporative sweat loss) appears to occur at very high intensity exercise in the heat (12, 39).
Indeed, women may have thermoregulatory advantages or disadvantages (or both) compared with men depending on the specifics of the circumstances (40). Higher surface area to mass ratio is an advantage for heat dissipation, as long as ambient temperature and humidity allow for heat transfer to the environment at the level of the skin. Interestingly, when behavioral aspects of thermoregulation are included, women appear to have an advantage in thermoregulation during prolonged endurance events. For example, there is some evidence that women follow more effective strategies for pacing and hydration during long distance events, thus optimizing their ability to maintain body temperature within a safe range (97, 98).
The observed sex differences in control of arterial blood pressure may also be influenced by exercise or cardiorespiratory fitness. As previously discussed, our recent work has shown that fitness, measured by maximal aerobic capacity (VO2max) is associated with baseline muscle SNA and influences sympathetic control of blood pressure (27). This is in contrast to previous studies conducted using mostly young men showing no influence of fitness on baseline muscle SNA (99).
In the cerebral circulation, there is conflicting evidence regarding the role of cardiorespiratory fitness. Some studies report that endurance-trained individuals have greater cerebrovascular reactivity (100) or no differences in cerebrovascular reactivity (101) or that cerebrovascular reactivity is positively associated with VO2max (102, 103). In addition, trained men have greater MCA blood velocity throughout the lifespan compared to sedentary men (104). Importantly, all of the aforementioned studies included both men and women in a combined group (100, 102), or only included men (101, 103, 104). Recent work from Labrecque et al. demonstrated that cerebral autoregulation was reduced in highly fit young women, suggesting that cardiorespiratory fitness may impact cerebral autoregulatory mechanisms (86). Therefore, more information is necessary to understand the interaction between sex differences and fitness on the control of the cerebral circulation.
Summary, conclusions and future directions
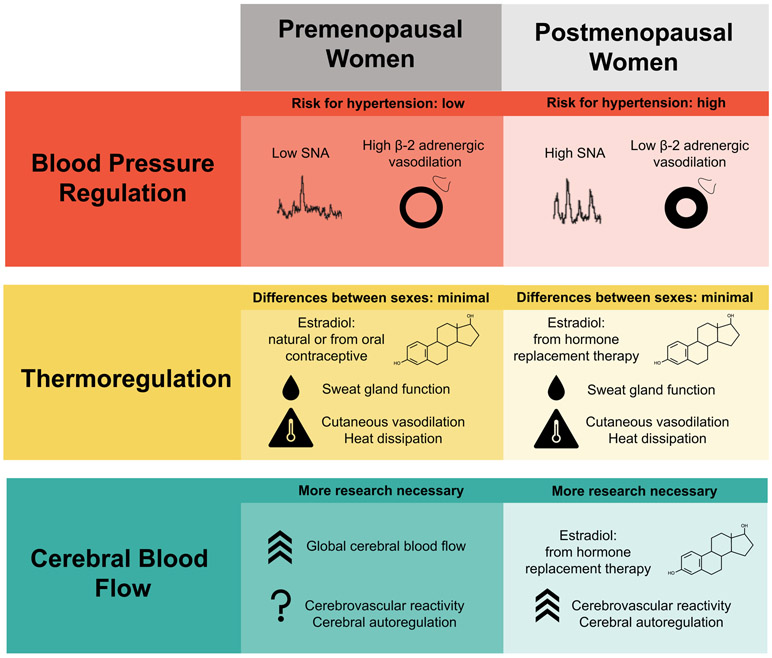
Cardiovascular control mechanisms are central to the integrative regulation of blood pressure, body temperature and brain function (Figure). Prior to menopause, women have significantly lower risk of systemic hypertension compared to men of similar age and health status. This appears to be due to differences in sympathetic control mechanisms, where women tend to have less resting activity of sympathetic vasoconstrictor nerves and noradrenergic vasoconstriction is offset by augmented β-adrenergic vasodilation in young women. Around the time of menopause, with decreasing estrogen levels, this augmented vasodilation is lost, and sympathetic nerve activity increases, leading to increased risk of systemic hypertension in women in older age groups. Thermoregulatory vasodilation (primarily in the skin circulation) is also augmented by estradiol in younger women, and by estrogen replacement therapy in older women. The reproductive hormone effects on thermoregulation do not appear to result in major sex differences in the ability to thermoregulate under most conditions and activity levels.
Figure.
Conceptual overview of the reproductive hormone effects on cardiovascular regulation that may influence integrative physiological regulation of blood pressure, body temperature and cerebral blood flow in women. The vasodilation promoting influence of estradiol is usually an advantage for women in terms of blood pressure regulation, whereas the net effects of female sex or estradiol are less clear with regard to thermoregulation and cerebrovascular control.
Although there are still many aspects that remain to be investigated, the structure and function of the cerebral circulation are clearly affected by sex and circulating estradiol. There is some debate in the literature regarding the extent to which young men and women have different cerebrovascular reactivity to hypercapnia or cerebral autoregulation, but emerging evidence suggests that there are sex differences in the control of the cerebral circulation. The current lack of clarity in the literature is in part because of differences in measurement techniques among studies, and lack of control for menstrual cycle phase in some, but not all, studies in this area.
In broad terms, the pro-vasodilatory effects of estradiol, via β-adrenergic vasodilation, nitric oxide, EDHF and other mechanisms, appear to be protective in young women in terms of ability to prevent systemic hypertension and regulate body temperature. In young women, the influences of estradiol on control of the cerebral circulation may be more subtle and reports are more varied. More consistently, menopausal hormone replacement therapy has a positive effect on cerebrovascular reactivity, which may provide beneficial clinical effects in terms of brain blood flow and cognitive function with increasing age.
Acknowledgements
The authors would like to acknowledge Kathleen Miller for her technical assistance and critical review of this manuscript. The authors also acknowledge their funding sources: NIH HL118154 (JNB) AARG17-499398 (JNB) and USAMRDC MOMRP (NC).
Abbreviations
- AV
atrioventricular
- CO
cardiac output
- ECA
external carotid artery
- EDHF
endothelial-derived hyperpolarizing factor
- HRT
hormone replacement therapy
- ICA
internal carotid artery
- MCA
middle cerebral artery
- NO
nitric oxide
- NTS
nucleus tractus solitarii
- PO/OH
preoptic area of the anterior hypothalamus
- PVN
paraventricular nucleus
- SA
sinoatrial
- SNA
sympathetic nerve activity
- TPR
total peripheral resistance
Footnotes
Disclaimer
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official United States Department of the Army position, or decision, unless so designated by other official documentation. Approved for public release; distribution unlimited.
Citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
References:
- 1.Joyner MJ, Barnes JN, Hart EC, Wallin BG, and Charkoudian N (2015) Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol 5, 193–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charkoudian N, and Wallin BG (2014) Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4, 825–850 [DOI] [PubMed] [Google Scholar]
- 3.Fisher JP (2014) Autonomic control of the heart during exercise in humans: role of skeletal muscle afferents. Exp Physiol 99, 300–305 [DOI] [PubMed] [Google Scholar]
- 4.Benarroch EE (1993) The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68, 988–1001 [DOI] [PubMed] [Google Scholar]
- 5.Fu Q, and Ogoh S (2019) Sex differences in baroreflex function in health and disease. J Physiol Sci 69, 851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura K, and Morrison SF (2010) A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A 107, 8848–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulant JA (2006) Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol (1985) 100, 1347–1354 [DOI] [PubMed] [Google Scholar]
- 8.Madden CJ, and Morrison SF (2019) Central nervous system circuits that control body temperature. Neurosci Lett 696, 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alba BK, Castellani JW, and Charkoudian N (2019) Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp Physiol 104, 1202–1214 [DOI] [PubMed] [Google Scholar]
- 10.Charkoudian N, and Stachenfeld NS (2014) Reproductive hormone influences on thermoregulation in women. Compr Physiol 4, 793–804 [DOI] [PubMed] [Google Scholar]
- 11.Charkoudian N, and Stachenfeld N (2016) Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci 196, 75–80 [DOI] [PubMed] [Google Scholar]
- 12.Gagnon D, and Kenny GP (2012) Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss. J Appl Physiol (1985) 113, 746–757 [DOI] [PubMed] [Google Scholar]
- 13.Rowell LB (1974) Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54, 75–159 [DOI] [PubMed] [Google Scholar]
- 14.Rowell LB (1984) Reflex control of regional circulations in humans. J Auton Nerv Syst 11, 101–114 [DOI] [PubMed] [Google Scholar]
- 15.Schlader ZJ, Wilson TE, and Crandall CG (2016) Mechanisms of orthostatic intolerance during heat stress. Auton Neurosci 196, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crandall CG, and Wilson TE (2015) Human cardiovascular responses to passive heat stress. Compr Physiol 5, 17–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass RI, and Zack MM Jr. (1979) Increase in deaths from ischaemic heart-disease after blizzards. Lancet 1, 485–487 [DOI] [PubMed] [Google Scholar]
- 18.Auger N, Potter BJ, Smargiassi A, Bilodeau-Bertrand M, Paris C, and Kosatsky T (2017) Association between quantity and duration of snowfall and risk of myocardial infarction. CMAJ 189, E235–E242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charkoudian N, Hart ECJ, Barnes JN, and Joyner MJ (2017) Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin Auton Res [DOI] [PubMed] [Google Scholar]
- 20.Fagius J, and Wallin BG (1993) Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res 3, 201–205 [DOI] [PubMed] [Google Scholar]
- 21.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, and Wallin BG (2005) Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568, 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, and Joyner MJ (2009) Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, and Ritter JM (2000) Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. Journal of the American College of Cardiology 36, 1233–1238 [DOI] [PubMed] [Google Scholar]
- 24.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, and Joyner MJ (2011) Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. The Journal of physiology 589, 5285–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, and Joyner MJ (2014) Aging enhances autonomic support of blood pressure in women. Hypertension 63, 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey RE, Hart EC, Charkoudian N, Curry TB, Carter JR, Fu Q, Minson CT, Joyner MJ, and Barnes JN (2015) Oral Contraceptive Use, Muscle Sympathetic Nerve Activity, and Systemic Hemodynamics in Young Women. Hypertension 66, 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker SE, Limberg JK, Scruggs ZM, Curry TB, Nicholson WT, Barnes JN, and Joyner MJ (2020) Greater Influence of Aerobic Fitness on Autonomic Support of Blood Pressure in Young Women Than in Older Women. Hypertension 75, 1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stachenfeld NS, and Keefe DL (2002) Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab 283, E711–721 [DOI] [PubMed] [Google Scholar]
- 29.Wenner MM, and Stachenfeld NS (2012) Blood pressure and water regulation: understanding sex hormone effects within and between men and women. The Journal of physiology 590, 5949–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stachenfeld NS (2008) Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev 36, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson LA, and Kolka MA (1985) Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol 249, R186–191 [DOI] [PubMed] [Google Scholar]
- 32.Kolka MA, and Stephenson LA (1997) Resetting the thermoregulatory set-point by endogenous estradiol or progesterone in women. Ann N Y Acad Sci 813, 204–206 [DOI] [PubMed] [Google Scholar]
- 33.Hessemer V, and Bruck K (1985) Influence of menstrual cycle on shivering, skin blood flow, and sweating responses measured at night. J Appl Physiol (1985) 59, 1902–1910 [DOI] [PubMed] [Google Scholar]
- 34.Charkoudian N, and Johnson JM (1997) Modification of active cutaneous vasodilation by oral contraceptive hormones. J Appl Physiol (1985) 83, 2012–2018 [DOI] [PubMed] [Google Scholar]
- 35.Charkoudian N, and Johnson JM (1999) Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. Am J Physiol 276, H1634–1640 [DOI] [PubMed] [Google Scholar]
- 36.Rogers SM, and Baker MA (1997) Thermoregulation during exercise in women who are taking oral contraceptives. Eur J Appl Physiol Occup Physiol 75, 34–38 [DOI] [PubMed] [Google Scholar]
- 37.Tankersley CG, Nicholas WC, Deaver DR, Mikita D, and Kenney WL (1992) Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol (1985) 73, 1238–1245 [DOI] [PubMed] [Google Scholar]
- 38.Brooks EM, Morgan AL, Pierzga JM, Wladkowski SL, O'Gorman JT, Derr JA, and Kenney WL (1997) Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol (1985) 83, 477–484 [DOI] [PubMed] [Google Scholar]
- 39.Gagnon D, and Kenny GP (2012) Does sex have an independent effect on thermoeffector responses during exercise in the heat? J Physiol 590, 5963–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanovich R, Ketko I, and Charkoudian N (2020) Sex Differences in Human Thermoregulation: Relevance for 2020 and Beyond. Physiology (Bethesda) 35, 177–184 [DOI] [PubMed] [Google Scholar]
- 41.Bain AR, Nybo L, and Ainslie PN (2015) Cerebral Vascular Control and Metabolism in Heat Stress. Compr Physiol 5, 1345–1380 [DOI] [PubMed] [Google Scholar]
- 42.Ota A, Takeda R, Imai D, Naghavi N, Kawai E, Saho K, Morita E, Suzuki Y, Yokoyama H, Miyagawa T, and Okazaki K (2019) The effects of aging on the distribution of cerebral blood flow with postural changes and mild hyperthermia. Eur J Appl Physiol 119, 1261–1272 [DOI] [PubMed] [Google Scholar]
- 43.Lucas RA, Cotter JD, Morrison S, and Ainslie PN (2008) The effects of ageing and passive heating on cardiorespiratory and cerebrovascular responses to orthostatic stress in humans. Exp Physiol 93, 1104–1117 [DOI] [PubMed] [Google Scholar]
- 44.Bain AR, Smith KJ, Lewis NC, Foster GE, Wildfong KW, Willie CK, Hartley GL, Cheung SS, and Ainslie PN (2013) Regional changes in brain blood flow during severe passive hyperthermia: effects of PaCO2 and extracranial blood flow. J Appl Physiol (1985) 115, 653–659 [DOI] [PubMed] [Google Scholar]
- 45.Sato K, Ogoh S, Hirasawa A, Oue A, and Sadamoto T (2011) The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. The Journal of physiology 589, 2847–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Low DA, Wingo JE, Keller DM, Davis SL, Cui J, Zhang R, and Crandall CG (2009) Dynamic cerebral autoregulation during passive heat stress in humans. Am J Physiol Regul Integr Comp Physiol 296, R1598–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brothers RM, Zhang R, Wingo JE, Hubing KA, and Crandall CG (2009) Effects of heat stress on dynamic cerebral autoregulation during large fluctuations in arterial blood pressure. J Appl Physiol (1985) 107, 1722–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzeng YC, Ainslie PN, Cooke WH, Peebles KC, Willie CK, MacRae BA, Smirl JD, Horsman HM, and Rickards CA (2012) Assessment of cerebral autoregulation: the quandary of quantification. American journal of physiology Heart and circulatory physiology 303, H658–671 [DOI] [PubMed] [Google Scholar]
- 49.Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, and Crandall CG (2008) Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol (1985) 104, 976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JF, Christmas KM, Harrison ML, Kim K, Hurr C, and Brothers RM (2014) Cerebral vasoreactivity: impact of heat stress and lower body negative pressure. Clin Auton Res 24, 135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, and Shibasaki M (2014) Hyperthermia modulates regional differences in cerebral blood flow to changes in CO2. J Appl Physiol (1985) 117, 46–52 [DOI] [PubMed] [Google Scholar]
- 52.Chen JJ, Rosas HD, and Salat DH (2011) Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 55, 468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W, Lou X, and Ma L (2016) Use of 3D pseudo-continuous arterial spin labeling to characterize sex and age differences in cerebral blood flow. Neuroradiology 58, 943–948 [DOI] [PubMed] [Google Scholar]
- 54.Miller VM, Garovic VD, Kantarci K, Barnes JN, Jayachandran M, Mielke MM, Joyner MJ, Shuster LT, and Rocca WA (2013) Sex-specific risk of cardiovascular disease and cognitive decline: pregnancy and menopause. Biol Sex Differ 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller VM, Jayachandran M, Barnes JN, Mielke MM, Kantarci K, and Rocca WA (2019) Risk factors of neurovascular ageing in women. J Neuroendocrinol, e12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Portegies ML, de Bruijn RF, Hofman A, Koudstaal PJ, and Ikram MA (2014) Cerebral vasomotor reactivity and risk of mortality: the Rotterdam Study. Stroke 45, 42–47 [DOI] [PubMed] [Google Scholar]
- 57.Barnes JN, Schmidt JE, Nicholson WT, and Joyner MJ (2012) Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol (1985) 112, 1884–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O, and Barnes JN (2019) Age-Related Reductions in Cerebrovascular Reactivity Using 4D Flow MRI. Front Aging Neurosci 11, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoiland RL, Fisher JA, and Ainslie PN (2019) Regulation of the Cerebral Circulation by Arterial Carbon Dioxide. Compr Physiol 9, 1101–1154 [DOI] [PubMed] [Google Scholar]
- 60.Kastrup A, Dichgans J, Niemeier M, and Schabet M (1998) Changes of cerebrovascular CO2 reactivity during normal aging. Stroke 29, 1311–1314 [DOI] [PubMed] [Google Scholar]
- 61.Kastrup A, Thomas C, Hartmann C, and Schabet M (1997) Sex dependency of cerebrovascular CO2 reactivity in normal subjects. Stroke 28, 2353–2356 [DOI] [PubMed] [Google Scholar]
- 62.Acar M, Cevrioglu AS, Haktanir A, Demirel R, Albayrak R, Degirmenci B, Yucel A, and Akyol AM (2005) Effect of Aerodiol administration on cerebral blood flow volume in postmenopausal women. Maturitas 52, 127–133 [DOI] [PubMed] [Google Scholar]
- 63.Kaya E, Sahin FK, Koken G, Kose M, and Cevrioglu AS (2008) Acute effect of intranasal estrogen on cerebral and cerebellar perfusion in postmenopausal women. Maturitas 59, 72–82 [DOI] [PubMed] [Google Scholar]
- 64.Slopien R, Junik R, Meczekalski B, Halerz-Nowakowska B, Maciejewska M, Warenik-Szymankiewicz A, and Sowinski J (2003) Influence of hormonal replacement therapy on the regional cerebral blood flow in postmenopausal women. Maturitas 46, 255–262 [DOI] [PubMed] [Google Scholar]
- 65.Bain CA, Walters MR, Lees KR, and Lumsden MA (2004) The effect of HRT on cerebral haemodynamics and cerebral vasomotor reactivity in post-menopausal women. Hum Reprod 19, 2411–2414 [DOI] [PubMed] [Google Scholar]
- 66.Rocca WA, Grossardt BR, and Shuster LT (2010) Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis 7, 163–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller VM, Naftolin F, Asthana S, Black DM, Brinton EA, Budoff MJ, Cedars MI, Dowling NM, Gleason CE, Hodis HN, Jayachandran M, Kantarci K, Lobo RA, Manson JE, Pal L, Santoro NF, Taylor HS, and Harman SM (2019) The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause 26, 1071–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harvey RE, Johnson MC, Ranadive SM, Joyner MJ, Lahr BD, Miller VM, and Barnes JN (2017) Aortic hemodynamics in postmenopausal women following cessation of hormone therapy. Physiol Rep 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnes JN, Harvey RE, Eisenmann NA, Miller KB, Johnson MC, Kruse SM, Lahr BD, Joyner MJ, and Miller VM (2019) Cerebrovascular reactivity after cessation of menopausal hormone treatment. Climacteric 22, 182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Lowe VJ, Fields JA, Gunter JL, Senjem ML, Settell ML, Gleason CE, Shuster LT, Bailey KR, Dowling NM, Asthana S, Jack CR Jr., Rocca WA, and Miller VM (2018) Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology 90, e1404–e1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krause DN, Duckles SP, and Pelligrino DA (2006) Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol (1985) 101, 1252–1261 [DOI] [PubMed] [Google Scholar]
- 72.Wang S, Zhang H, Liu Y, Li L, Guo Y, Jiao F, Fang X, Jefferson JR, Li M, Gao W, Gonzalez-Fernandez E, Maranon RO, Pabbidi MR, Liu R, Alexander BT, Roman RJ, and Fan F (2020) Sex differences in the structure and function of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 318, H1219–H1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geary GG, Krause DN, and Duckles SP (2000) Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol 279, H511–519 [DOI] [PubMed] [Google Scholar]
- 74.Geary GG, Krause DN, and Duckles SP (1998) Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol 275, H292–300 [DOI] [PubMed] [Google Scholar]
- 75.Robison LS, Gannon OJ, Salinero AE, and Zuloaga KL (2019) Contributions of sex to cerebrovascular function and pathology. Brain Res 1710, 43–60 [DOI] [PubMed] [Google Scholar]
- 76.Krause DN, Duckles SP, and Gonzales RJ (2011) Local oestrogenic/androgenic balance in the cerebral vasculature. Acta Physiol (Oxf) 203, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnes JN (2017) Sex-specific factors regulating pressure and flow. Exp Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzales RJ, Ansar S, Duckles SP, and Krause DN (2007) Androgenic/estrogenic balance in the male rat cerebral circulation: metabolic enzymes and sex steroid receptors. J Cereb Blood Flow Metab 27, 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzales RJ, Ghaffari AA, Duckles SP, and Krause DN (2005) Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol Heart Circ Physiol 289, H578–585 [DOI] [PubMed] [Google Scholar]
- 80.Kassner A, Winter JD, Poublanc J, Mikulis DJ, and Crawley AP (2010) Blood-oxygen level dependent MRI measures of cerebrovascular reactivity using a controlled respiratory challenge: reproducibility and gender differences. J Magn Reson Imaging 31, 298–304 [DOI] [PubMed] [Google Scholar]
- 81.Madureira J, Castro P, and Azevedo E (2017) Demographic and Systemic Hemodynamic Influences in Mechanisms of Cerebrovascular Regulation in Healthy Adults. J Stroke Cerebrovasc Dis 26, 500–508 [DOI] [PubMed] [Google Scholar]
- 82.Usselman CW, Gimon TI, Nielson CA, Luchyshyn TA, Coverdale NS, Van Uum SH, and Shoemaker JK (2015) Menstrual cycle and sex effects on sympathetic responses to acute chemoreflex stress. Am J Physiol Heart Circ Physiol 308, H664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abidi S, Nili M, Serna S, Kim S, Hazlett C, and Edgell H (2017) Influence of sex, menstrual cycle, and oral contraceptives on cerebrovascular resistance and cardiorespiratory function during Valsalva or standing. J Appl Physiol (1985) 123, 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edgell H, Robertson AD, and Hughson RL (2012) Hemodynamics and brain blood flow during posture change in younger women and postmenopausal women compared with age-matched men. J Appl Physiol (1985) 112, 1482–1493 [DOI] [PubMed] [Google Scholar]
- 85.Favre ME, and Serrador JM (2019) Sex differences in cerebral autoregulation are unaffected by menstrual cycle phase in young, healthy women. Am J Physiol Heart Circ Physiol 316, H920–H933 [DOI] [PubMed] [Google Scholar]
- 86.Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Drapeau A, Smirl JD, Bailey DM, and Brassard P (2019) Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep 7, e13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Favre ME, Lim V, Falvo MJ, and Serrador JM (2020) Cerebrovascular reactivity and cerebral autoregulation are improved in the supine posture compared to upright in healthy men and women. PLoS One 15, e0229049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deegan BM, Sorond FA, Galica A, Lipsitz LA, O'Laighin G, and Serrador JM (2011) Elderly women regulate brain blood flow better than men do. Stroke; a journal of cerebral circulation 42, 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.ter Laan M, van Dijk JM, Elting JW, Staal MJ, and Absalom AR (2013) Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 111, 361–367 [DOI] [PubMed] [Google Scholar]
- 90.Brassard P, Tymko MM, and Ainslie PN (2017) Sympathetic control of the brain circulation: Appreciating the complexities to better understand the controversy. Auton Neurosci 207, 37–47 [DOI] [PubMed] [Google Scholar]
- 91.Coverdale NS, Gati JS, Opalevych O, Perrotta A, and Shoemaker JK (2014) Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117, 1090–1096 [DOI] [PubMed] [Google Scholar]
- 92.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, and van Osch MJ (2014) Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117, 1084–1089 [DOI] [PubMed] [Google Scholar]
- 93.Miller KB, Howery AJ, Rivera-Rivera LA, Wieben O, and Barnes JN (2020) Sex Differences in the Cerebral Hemodynamic Response to Hypercapnia in Young Adults. FASEB J 34, 1–1 [Google Scholar]
- 94.Krejza J, Rudzinski W, Arkuszewski M, Onuoha O, and Melhem ER (2013) Cerebrovascular reactivity across the menstrual cycle in young healthy women. Neuroradiol J 26, 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ances BM, Greenbe6rg JH, and Detre JA (2001) Sex differences in the cerebral blood flow response after brief hypercapnia in the rat. Neurosci Lett 304, 57–60 [DOI] [PubMed] [Google Scholar]
- 96.Joyner MJ (2017) Physiological limits to endurance exercise performance: influence of sex. J Physiol 595, 2949–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trubee NW, Vanderburgh PM, Diestelkamp WS, and Jackson KJ (2014) Effects of heat stress and sex on pacing in marathon runners. J Strength Cond Res 28, 1673–1678 [DOI] [PubMed] [Google Scholar]
- 98.Periard JD, Racinais S, Timpka T, Dahlstrom O, Spreco A, Jacobsson J, Bargoria V, Halje K, and Alonso JM (2017) Strategies and factors associated with preparing for competing in the heat: a cohort study at the 2015 IAAF World Athletics Championships. Br J Sports Med 51, 264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seals DR (1991) Sympathetic neural adjustments to stress in physically trained and untrained humans. Hypertension 17, 36–43 [DOI] [PubMed] [Google Scholar]
- 100.Tarumi T, Gonzales MM, Fallow B, Nualnim N, Lee J, Pyron M, Tanaka H, and Haley AP (2015) Cerebral/Peripheral Vascular Reactivity and Neurocognition in Middle-Age Athletes. Med Sci Sports Exerc 47, 2595–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Braz ID, Fluck D, Lip GYH, Lundby C, and Fisher JP (2017) Impact of aerobic fitness on cerebral blood flow and cerebral vascular responsiveness to CO2 in young and older men. Scand J Med Sci Sports 27, 634–642 [DOI] [PubMed] [Google Scholar]
- 102.Barnes JN, Taylor JL, Kluck BN, Johnson CP, and Joyner MJ (2013) Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol (1985) 114, 1383–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, and Ainslie PN (2013) Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke; a journal of cerebral circulation 44, 3235–3238 [DOI] [PubMed] [Google Scholar]
- 104.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, and Atkinson G (2008) Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586, 4005–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]