Figure 1.
Point-of-care detection of SARS-CoV-2 using RAPID 1.0
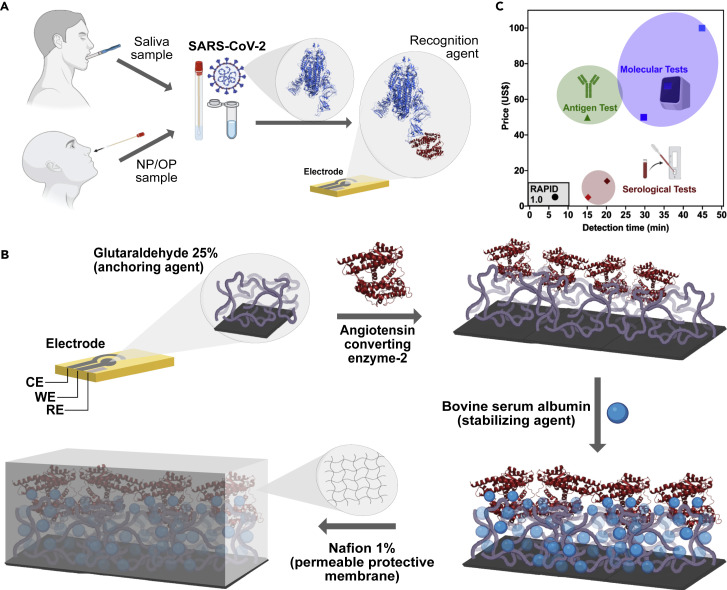
(A) RAPID 1.0 enables diagnosis using neat saliva and nasopharyngeal/oropharyngeal (NP/OP) swab samples infected with SARS-CoV-2.
(B) Schematic for the preparation of the electrodes. Briefly, screen-printed electrodes in a three-electrode configuration cell (CE, counter electrode; WE, working electrode; and RE, reference electrode) were printed on phenolic paper circuit board or filter paper with conductive carbon and Ag/AgCl inks. The WE was functionalized with glutaraldehyde to enable anchoring of ACE2, which was stabilized by the addition of bovine serum albumin. Detection was improved by adding a Nafion permeable membrane, enabling chemical preconcentration of cation species and protecting the electrode's surface against biofouling with proteins, lipids, and other macromolecules present in the biological sample matrix.
(C) Cost and detection time comparison between RAPID 1.0 and existing FDA-approved antigen, serological, and molecular tests.2, 3, 4 Note that comparisons were made for a single test of each of the different technologies.