Abstract
To date, drugs to attenuate cytokine storm in severe cases of Corona Virus Disease 2019 (COVID-19) are not available. In this study, we investigated the effects of intragastric and atomized administration of canagliflozin (CAN) on cytokine storm in lung tissues of lipopolysaccharides (LPS)-induced mice. Results showed that intragastric administration of CAN significantly and widely inhibited the production of inflammatory cytokines in lung tissues of LPS-induced sepsis mice. Simultaneously, intragastric administration of CAN significantly improved inflammatory pathological changes of lung tissues. Atomized administration of CAN also exhibited similar effects in LPS-induced sepsis mice. Furthermore, CAN significantly inhibited hypoxia inducible factor 1α (HIF-1α) and phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3) protein levels in LPS-treated lung tissues. These results indicated that CAN might attenuate cytokine storm and reduce the inflammatory symptoms in critical cases in COVID-19. Its action mechanism might involve the regulation of HIF-1α and glycolysis in vivo. However, further studies about clinical application and mechanism analysis should be validated in the future.
Keywords: Canagliflozin, COVID-19, Cytokine storm, Inflammation
Abbreviations: CAN, Canagliflozin; CMC, sodium carboxymethylcellulose; COVID-19, Corona Virus Disease 2019; DXM, dexamethasone; GM-CSF, granulocyte- macrophage colony stimulating factor; HIF-1α, hypoxia inducible factor 1α; IFN-γ, interferon γ; interleukin, IL; KC, keratinocyte-derived chemokine; LIX, LPS-induced CXC Chemokine; LPS, lipopolysaccharides; MIP-2, Macrophage inflammatory protein 2-α; MCP-1, monocyte chemotactic protein 1; PFKFB3, phosphofructokinase-2/fructose-2,6-bisphosphatase 3; SGLT2, sodium glucose transporter 2; TNF-α, tumor necrosis factor α
1. Introduction
COVID-19 patients with pneumonia caused by new coronavirus known as SARS-Cov-2 by WHO was first found in Wuhan, China, which leads to a pandemic primarily because of rapid human-to-human transmission by respiratory tract infections and high mortality after lung infection [1], [2]. The initial mortality rate of COVID-19 declared by WHO was 2% [3]. In particular, COVID-19 has higher severity and has higher mortality in elderly patients when they have comorbidities with diabetes and cardiovascular diseases [3], [4], [5], [6], [7].
COVID-19 pathogenesis could occur in three sequential phases: pulmonary, proinflammatory, and prothrombic[8]. Severe inflammatory lung injury is an important cause for critical cases of COVID-19 [9]. COVID-19 in critically ill patients accompanies an unprecedented spike in cytokines levels known as cytokine release syndrome and results in inflammation, infiltration of macrophages, neutrophils and lung injury in patients [10]. COVID-19 patients with basic diseases such as diabetes and cardiovascular diseases are easily subjected to this lung injury [10]. Anti-inflammatory strategies have emerged to serve as an important method to combat the cytokine storm and improve clinical outcomes in severe COVID-19 patients. However, ideal drugs are still not available to date.
Canagliflozin (CAN) is a low-molecular-weight hypoglycemic compound. It can inhibit the reabsorption of glucose by inhibiting sodium glucose cotransporter 2 (SGLT2) in renal proximal convoluted tubules; thus, glucose can be excreted in urine, and blood glucose can be reduced [11]. New studies have found that CAN has been clinically found to significantly reduce the incidence of diabetic cardiovascular events such as heart failure [12]. Considerable studies have found that CAN has new SGLT2-independent activities [13], [14]. In our previous study, we found that CAN significantly inhibited the production of inflammatory interleukin 6 (IL-6), interleukin 1 (IL-1), and tumor necrosis factor α (TNF-α) and the pathological changes of lung inflammation induced by LPS in mice [15]. However, we did not systematically evaluate the effects of CAN on inflammatory cytokine storm.
Considering that unavailability of specific drugs to combat cytokine storm in COVID-19 patients, in this study, we would systematically investigate whether intragastric and atomized administration of CAN inhibited inflammatory cytokine storm in lung tissues of LPS-induced mouse model, which was frequently found in COVID-19 patients.
2. Materials and methods
2.1. Animal protocol
Four-week-old NIH male mice were purchased from Guangdong Medical Laboratory Animal Center and housed under controlled conditions (constant temperature: 22 °C ± 2 °C; constant humidity: 60%±5%; 12 h dark/light cycle). The study was performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the protocol was approved by the Bioethics Committee of Shenzhen International Graduate School, Tsinghua University, China. For mice with intragastric administration of drugs, the mice were divided into the following groups: normal control group, untreated LPS control group (10 mg/kg, Sigma-Aldrich, USA), CAN group (5–20 mg/kg, BioChemPartner, Shanghai, China), and dexamethasone group (DXM, 2 mg/kg, Sangon Biotech, Shanghai, China). The drugs dissolved in 0.5% sodium carboxymethylcellulose (CMC, Sangon Biotech, Shanghai, China) aqueous solution were intragastrically administrated, and the normal and model controls were given the same amount of 0.5% CMC aqueous solution.
The other administration was performed by a simple atomized drug delivery device. The mice were divided into two groups. We dissolved 4 mg/mL of CAN in 50% (v/v) ethanol. The mice were placed in a sealed 1.5 L container and CAN was sprayed for 20 min at a dose of 0.4 mL/mouse. Normal control mice were given 50% ethanol at identical volume.
All mice were treated with drugs or vehicles for 3 days. After treatment, the mice were intraperitoneally injected with LPS at a dosage of 10 mg/kg for systematic inflammatory stimulation. After 4 h of LPS induction, anesthesia was induced with isoflurane and blood was collected. Immediately, the mice were sacrificed by cervical dislocation. The lung tissues were removed and weighed. Part of lung tissue aliquots were stored at −80 °C for further biochemical analysis after instantaneous freezing by liquid nitrogen. The remaining lung tissue aliquots were soaked in formalin solution and sliced for hematoxylin − eosin (H & E) staining, immunohistochemistry staining against CD11b antibody (Servicebio, Wuhan, China) and pathological analysis.
2.2. Evans blue staining
After 3 days of intragastric administration of drugs, LPS (10 mg/kg) was injected intraperitoneally in mice. After 4 h of LPS stimulation, Evans blue (1%, g/mL, Sigma Aldrich, USA) was intravenously injected at a dose of 1 mL/10 g body weight). After 10 min of Evans blue injection, lung tissue perfusion was conducted with PBS and whole lung tissues were weighed and immersed in 2 mL of formamide solution. After 24 h, the Evans blue absorbance values were detected at 620 nm. The Evans blue absorbance value and lung weight ratio were compared among different groups.
2.3. Multiplex cytokine assay
Multiplex cytokine assay (EMD Millipore’s MILLIPLEX® MAP Mouse High Sensitivity T Cell Magnetic Bead Panel, Millipore Corp., Billerica, MA) was used for the simultaneous quantification of any of the following 18 mouse cytokines: granulocyte- macrophage colony stimulating factor (GM-CSF), interferon γ (IFN-γ), interleukin 1α (IL-1α), interleukin 1β (IL-1β), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 10 (IL-10), interleukin 12 (IL-12 (p70)), interleukin 13 (IL-13), interleukin 17A (IL-17A), keratinocyte-derived chemokine (KC), LPS-induced CXC Chemokine (LIX), monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 2-α (MIP-2) and TNF-α in mouse serum and lung tissue supernatant samples according to the kit-specific protocols. More details can be found in previous studies [16], [17].
2.4. Western blotting analysis
Lung tissues (approximately 100 mg) from mice were homogenized in 1 mL of ice-cold tissue lysis buffer (Beyotime Biotechnology, Shanghai, China). The protein concentration was determined by the Bradford protein assay kit (Beyotime Biotechnology, Shanghai, China). Protein samples were run by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Epizyme Biotech, China), and the separated proteins in the gel were transferred to nitrocellulose transfer membranes (Bio Trace, New Zealand) through further electrophoresis. The membranes were blocked with freshly prepared 5% (g/mL) non-fat dry milk (Anchor, New Zealand) dissolved in Tris buffered saline with 0.1% Tween 20 (TBST) for 2 h and incubated overnight at 4 °C with primary antibodies dissolved in 3% bovine serum albumin (BIOFROXX, Germany). After rinsing three times with TBST, the membrane was incubated with respective second antibody for 2 h at room temperature and washed with TBST again. Protein bands were visualized by enhanced chemiluminescence solution (ThermoFisher Scientific, United States). Finally, relative grey density values of protein bands were quantified using ImageJ 1.45 software. The specific primary antibodies used were anti-HIF-1α (Abbkine, United States, ABP51513, 1:1000), anti-PFKFB3 (ABCAM, United Kingdom, ab181861,1:2000) and anti-β-actin (Sigma-Aldrich, United States, 1:50000 dilution). The respective second antibody was goat polyclonal antibody to rabbit IgG H&L HRP (Cell Signal Technology, United States, 1:5000) or goat polyclonal antibody to mouse IgG H&L HRP (Cell Signal Technology, United States, 1:5000).
2.5. Statistical analysis
All data were presented as mean ± SD. Unpaired t-test (two tailed) was used when comparing two groups whereas one-way ANOVA with Tukey’s post hoc test was used for multiple comparisons by using GraphPad Prism v8 software. P < 0.05 indicated a statistically significant difference.
3. Results
3.1. Pulmonary inflammatory edema and permeability
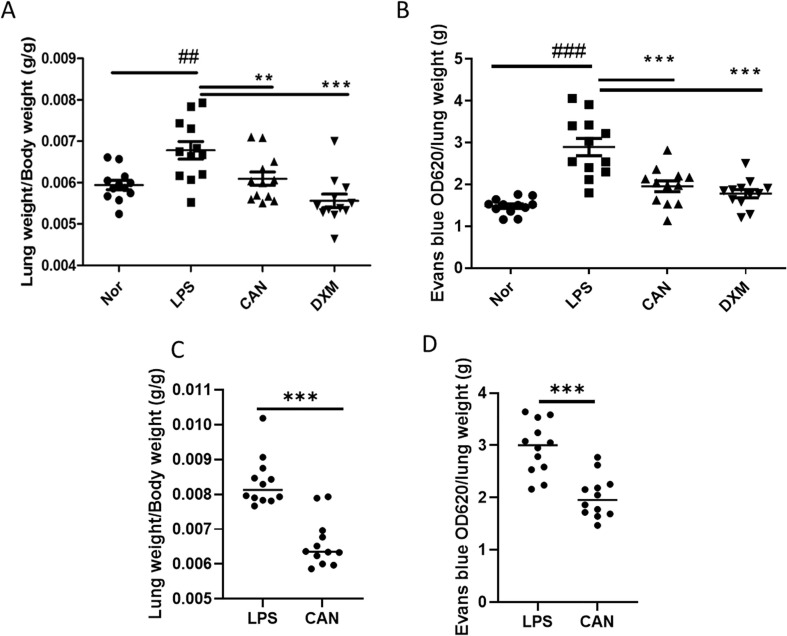
LPS can activate systematic inflammation and pulmonary edema. In this study, after 4 h of LPS injection (10 mg/mL), lung weight was significantly increased by 14.2% in LPS-induced mice compared with that in normal controls (Fig. 1 A). However, CAN significantly inhibited the increase by −10.1% in lung tissues, whereas DXM inhibited the increase by −18.0%. The effects of CAN were comparable to that of DXM.
Fig. 1.
Effects of intragastric and atomized administration of CAN on pulmonary edema and capillary permeability. A) Lung weight index (for intragastric administration, n = 10–12); B) Evans blue levels in lung tissues (for intragastric administration, n = 12). C) lung weight index (for atomized administration, n = 12); D) Evans blue levels in lung tissues (for atomized administration, n = 12). Nor, normal control group; LPS, untreated LPS control group; CAN, CAN-treated LPS group; DXM, dexamethasone-treated LPS group. Data were expressed as mean ± SD, ##P < 0.01, ###P < 0.001 vs Nor; **P < 0.01, ***P < 0.001 vs LPS.
Systematic inflammation may increase lung capillary permeability. Evans blue was used to evaluate this permeability after intravenous injection. We found that LPS significantly increased the Evans blue level by 95.6% in lung tissues compared with the normal control (Fig. 1B). However, after intragastric administration, CAN significantly inhibited the increase of Evans blue concentration by −32.4% in lung tissues compared with the untreated LPS control, whereas DXM inhibited the increase by −38.5%. The effects of CAN were comparable to that of DXM.
Similarly, after atomized administration, CAN also significantly inhibited the lung weight index by −31.5% (Fig. 1C) and Evans blue concentration by −31.6% in lung tissues (Fig. 1D). Collectively, these results indicate that CAN could attenuate inflammatory lung injury.
3.2. Pathological changes in lung tissues
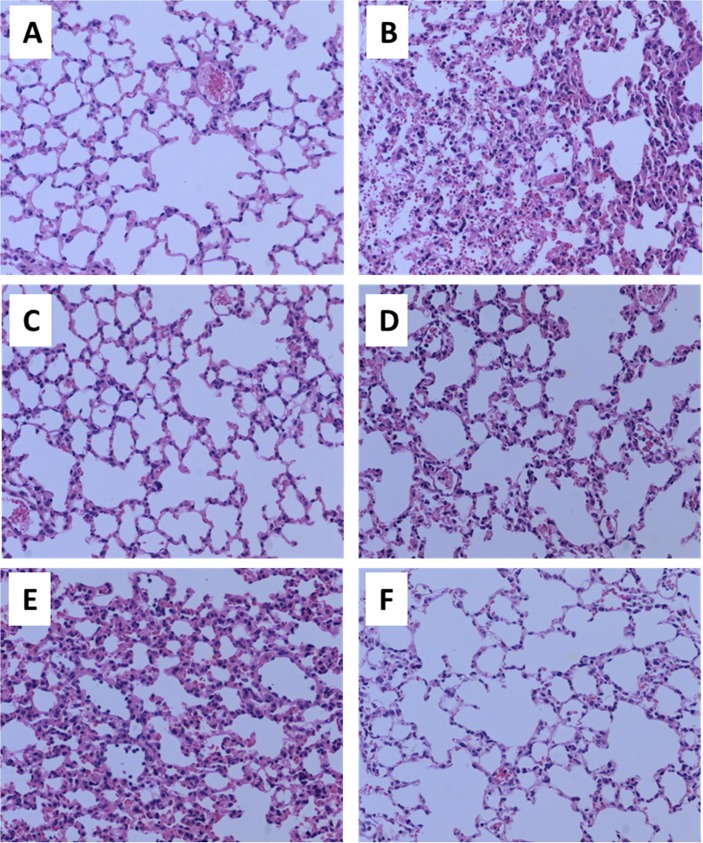
We assayed the pathological changes in lung tissues. The results showed that LPS significantly increased pathological changes (inflammatory cell or blood cell infiltration, alveolar septum thickening) but intragastric administration of CAN significantly inhibited the pathological changes (Fig. 2 A−D). The effects of CAN were comparable to those of DXM. After atomized administration, CAN also significantly inhibited pathological changes (Fig. 2E−F). These results indicated that CAN may reduce inflammatory injury induced by LPS in lung tissues.
Fig. 2.
Representative pathological slices in mouse lung tissues after H&E staining (magnify by 200-fold). A) Normal control group (for intragastric administration); B) untreated LPS control group (for intragastric administration); C) CAN-treated group (for intragastric administration); D) DXM-treated group (for intragastric administration); E) untreated LPS control group (for atomized administration); F) CAN-treated group (for atomized administration).
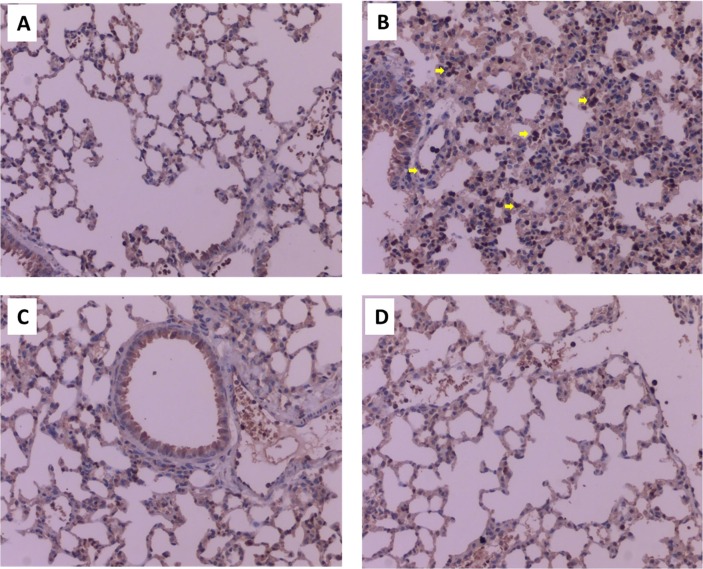
Furthermore, we stained the pathological slices with CD11b antibody, a specific antibody for recognizing surface markers of macrophages. LPS significantly increased infiltration of macrophages in lung tissues of mice (Fig. 3 A-B). However, the increase was significantly attenuated by CAN or DXM (Fig. 3C-D).
Fig. 3.
Representative pathological slices in mouse lung tissues after immunohistochemistry analysis by staining with CD11b antibody (magnify by 200-fold). A) Normal control group (for intragastric administration); B) untreated LPS control group (for intragastric administration); C) CAN-treated group (for intragastric administration); D) DXM-treated group (for intragastric administration).
3.3. Multiplex cytokine assay
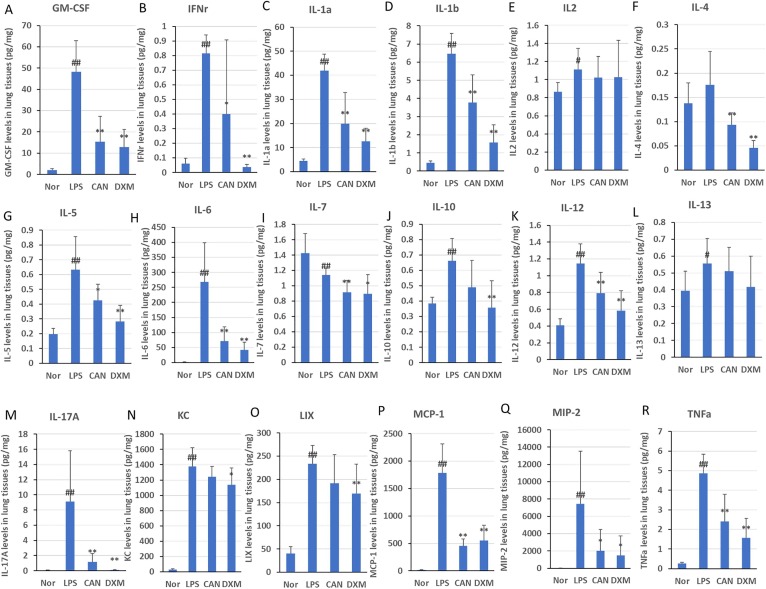
Considering that cytokine storm played an important role in the pathological changes after LPS stimulation, we systematically evaluated the effects of CAN on 18 inflammatory factor levels in lung tissues of LPS-induced mice detected through multiplex cytokine assay. In this study, LPS-treated mice had significantly increased levels of inflammatory factors in the lung tissues compared with those in normal controls (Fig. 4 ): GM-CSF (2364.0%), IFN-γ (1228.5%), IL-1α (845.2%), IL-1β (1294.0%), IL-5 (224.2%), IL-6 (18906.3%), IL-10 (71.9%), IL-12 (182.0%), IL-13 (40.6%), IL-17a (13296.1%), KC (5227.4%), LIX (478.9%), MCP-1 (12939.8%), MIP-2 (73497.4%) and TNF-α (1734.3%). However, intragastric administration of CAN significantly inhibited the protein levels of inflammatory cytokines induced by LPS in lung tissues, such as GM-CSF (−68.1%), IFN-γ (−51.1%), IL-1α (−52.3%), IL-1β (−41.8%), IL-4 (−46.8%), IL-5 (−33.1%), IL-6 (−73.5%), IL-7 (−19.9%), IL-12 (−30.7%), IL-17a (−87.2%), MCP-1 (−74.6%), MIP-2 (−73.3%) and TNF-α (−50.5%). Intragastric administration of DXM significantly reduced the protein levels of GM-CSF (−73.4%), IFN-γ (−95.4%), IL-1α (−69.8%), IL-1β (−75.6%), IL-4 (−73.7%), IL-5 (−55.3%), IL-6 (−84.4%), IL-7 (−21.5%), IL-10 (−46.1%), IL-12 (−49.1%), IL-17a (−98.5%), LIX (−36.6%), MCP-1 (−69.2%), MIP-2 ((−80.4%) and TNF-α (−67.8%) induced by LPS in lung tissues. Most of the effects of CAN were comparable to those of DXM. Collectively, these results indicated that CAN significantly inhibited inflammatory cytokine storm.
Fig. 4.
Effects of CAN on 18 inflammatory factors in lung tissues after intragastric administration. Nor, normal control group; LPS, untreated LPS control group; CAN, CAN-treated LPS group; DXM, dexamethasone-treated LPS group. Data were expressed as mean ± SD (n = 8), #P < 0.05, ##P < 0.01 vs Nor; *P < 0.05, **P < 0.01 vs LPS.
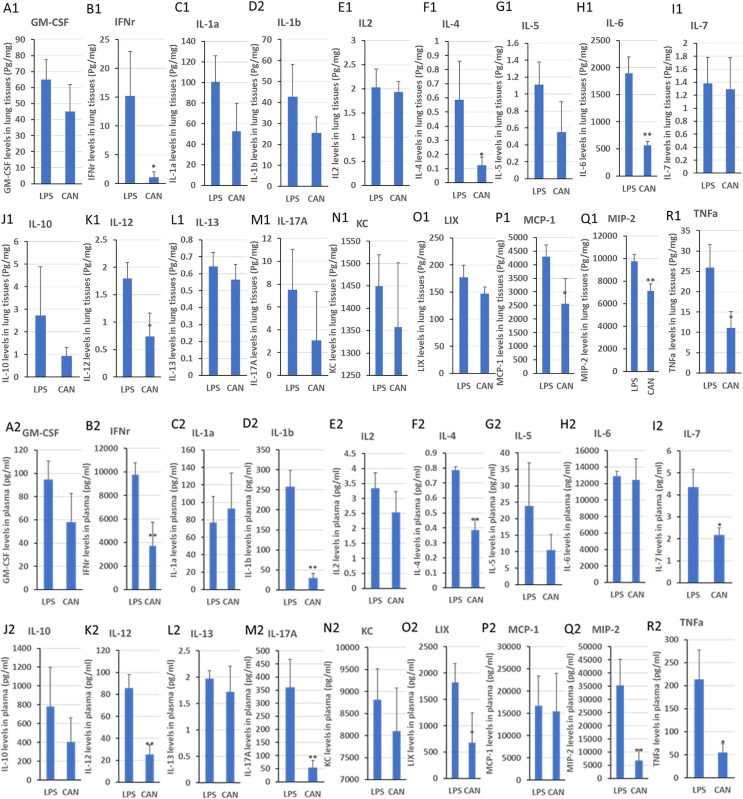
After atomized therapy, CAN significantly inhibited the levels of IFN-γ (−92.6%), IL-4 (−79.0%), IL-6 (−70.1%), IL-12 (−58.7%), MCP-1 (−40.4%), MIP-2 (−26.8%) and TNF-α (−57.1%) in lung tissues of mice compared with the LPS control (Fig. 5 A1−R1). In addition, CAN significantly inhibited the levels of IFN-γ (−62.0%), IL-1β (−88.3%), IL-4 (−50.8%), IL-7 (−50.3%), IL-12 (−70.4%), IL-17a (−85.0%), LIX (−62.7%), MIP-2 (−80.7%) and TNF-α (−74.2%) in the serum (Fig. 5A2−R2). These results indicated that CAN might be used to attenuate inflammatory cytokine storm, inhibit inflammatory cytokine release, and reduce inflammatory lung injury through local airway application. However, more studies should be validated.
Fig. 5.
Effects of CAN on 18 inflammatory factors in lung tissues (A1-R1) and plasma (A2-R2) after atomized administration. LPS, untreated LPS control group; CAN, CAN-treated LPS group. Data were expressed as mean ± SD (n = 3), *P < 0.05, **P < 0.01 vs LPS.
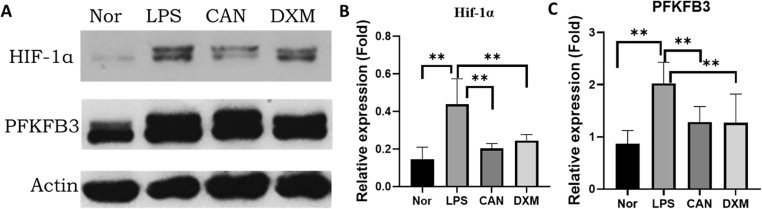
3.4. HIF-1α and PFKFB3 protein expressions in lung tissues
Glycolysis plays an important role in regulating immune cell activation and inflammatory cytokine release [18]. HIF-1α is a key factor to control glycolysis and inflammatory cytokine storm [19]. In this study, we determined the protein levels of HIF-1α and its downstream protein PFKFB3 through Western blotting. We found that LPS significantly increased HIF-1α and PFKFB3 protein levels by 203.4% and 133.4%, respectively (Fig. 6 ). However, CAN significantly attenuated the increase of HIF-1α and PFKFB3 protein levels by −53.4% and −36.6%, respectively. DXM also significantly attenuated the increase of HIF-1α and PFKFB3 protein levels by −44.1% and −37.3%, respectively.
Fig. 6.
HIF-1α and PFKFB3 protein levels in lung tissues assayed by Western blotting (A-C). Nor, normal control group; LPS, untreated LPS control group; CAN, CAN-treated LPS group; DXM, dexamethasone-treated LPS group. Data were expressed as mean ± SD (n = 6), #P < 0.05, ##P < 0.01 vs Nor; *P < 0.05, **P < 0.01 vs LPS.
4. Discussion
In COVID-19 patients, bilateral alveoli showed diffuse changes with fibrous mucoid exudates and both lungs showed interstitial lung inflammatory infiltrates with mononuclear cells and lymphocyte [20]. Intensive care unit (ICU) cases showed a significant increase of IL-6, IL-2, IL-7, IL-10, GSCF, IP-10, MCP1, MIP-1A and TNF-α levels compared with non-ICU controls [21], which be known as “cytokines storm”.
Cytokine storm indicates that the production of inflammatory cytokines such as IL-1, IL-6, and TNF-α is not controlled by immune cells [22]. Such sytokines can cause injury to all cells, causing extensive injury to the body; thus, patients will have multiple organ failure and severe diseases. COVID-19 patients may show a cytokine storm and inflammatory injuries in multiple organs, for example, the lungs, hearts, livers, and kidneys. Cytokine storm plays an important role in the switching of disease from slight to severe [23]. Early inhibition of the production of inflammatory cytokines is an important strategy for the prevention and treatment of severe diseases. Clinical immunologists try to use glucocorticoids, IL-6 inhibitors, IL-1 inhibitors, TNF inhibitors, JAK inhibitors, chloroquine and hydroxychloroquine to suppress inflammation caused by SARS-COV-2 [24].
However, at present, cytokine storm has no ideal drug [22]. Glucocorticoid has a significant anti-inflammatory effect, which can save the lives of many patients with COVID-19 [25]. It also plays an important role during SARS in 2003, but the related side effects are also worthy of attention [26]. The application of glucocorticoid may delay the clearance of SARS-COV-2, and lead to asymptomatic infection and secondary spread of the disease [27]. Furthermore, glucocorticoid can increase blood glucose, blood pressure, and osteoporosis. It is not suitable for some elderly patients with basic diseases such as diabetes, hypertension and osteoporosis. It may easily induce drug-induced diseases and death. A non-hormonal anti-inflammatory drug is required for the treatment of COVID-19 patients.
Recent studies have found that IL-6 is the key inflammatory factor in COVID-19 patients with inflammatory storm [28]. Therefore, blocking the IL-6 pathway is an important treatment strategy to inhibit cytokine storm [29]. Torzumab, produced by Roche, Switzerland, is an antibody against IL6 receptor, which can inhibit the inflammatory signal pathway caused by IL-6 [30]. In addition, inflammatory cytokine storm involves the release of many inflammatory factors, such as IL-1, TNF-α, and CCL-2. These factors can directly cause inflammation independent of IL6 pathway. Therefore, in theory, controlling inflammation by blocking a certain inflammatory cytokine pathway is difficult. Our research idea should anchor the master switch or more upstream link of the production control of all inflammatory factors, which may be the key to the control of systemic inflammatory storm.
Glycolysis plays a key role in the control of the production of inflammatory factors in immune cells [18]. 2-DG, a small molecule inhibiting glycolysis, significantly inhibited the production of inflammatory factors [15], [31]. In our previous study, CAN may inhibit the production of inflammatory factors by inhibiting the aerobic glycolysis metabolism of immune cells using PFKFB3 [15], [32]. Here, we systematically evaluate the effects of intragastric or atomized administration of CAN on multiple inflammatory factors and found that a significant and wide inhibition effect was found on these inflammatory factors, for example, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-4, IL-6, IL-7, IL-12, IL-17a, LIX, MCP-1, MIP-2 and TNF-α in lung tissues or serum. However, whether such potential mechanisms in vivo were related to the regulation of glycolysis remained unclear.
HIF-1α is a key factor involved in the regulation of glycolysis [33]. PFKFB3 is a key factor involved in glycolysis and is a downstream factor regulated by HIF-1α [34]. HIF-1α promotes glycolysis and activation of macrophages [19]. In addition, HIF-1α directly mediates the production of inflammatory factors such as IL-1β [35]. HIF-1α deficiency attenuates inflammatory damage [36]. In COVID-19, the virus causes the inflammatory sites to produce a hypoxic microenvironment and induce HIF-1α production, which may activate cytokine storm [37]. HIF-1α is used as a potential therapeutic target for acute lung injury [38]. HIF-1α/glycolysis involve SARS-CoV-2 infection and monocyte response [39]. Therefore, HIF-1α is a potential target for the inflammatory control for COVID-19. In this study, we found that CAN significantly attenuated the increased levels of HIF-1α and its downstream PFKFB3 proteins induced by LPS. DXM also significantly inhibited the HIF-1α level as described previously [40]. These results indicated that CAN might attenuate the inflammatory cytokine storm by inhibiting the HIF-1α levels in vivo and then downregulating glycolysis in lung tissues. However, the mechanism of CAN in inhibiting HIF-1α levels should be further investigated in the future.
In this study, CAN significantly improved acute lung injures induced by LPS (increased lung edema and lung capillary penetration) in mice. The pathological slice results also indicated this improvement. Immunohistochemistry analysis further indicated that CAN attenuated macrophage (identified by CD11b antibody) infiltration in lung tissues induced by LPS, which may be associated with the decreased chemotactic cytokines, for example, MCP-1 and MIP-2 in lung tissues of mice. In blood samples, CAN significantly reduced the inflammatory factors, which suggested that CAN had a systematic inhibition on immune cell activation in vivo after acute treatment. In this study, although we used CAN or DXM only for 3 days, more COVID-19 patients may require longer administration of these anti-inflammatory drugs and a safety concern could be considered. In our preliminary experiment, chronic administration (about 3 months) of CAN (25 mg/kg) did not affect the spleen weight in mice compared with untreated controls, whereas 1-month administration of dexamethasone (2 mg/kg) significantly lowered the spleen weight in mice (supplemental Fig. 1) compared with untreated controls. These results indicated that CAN might not significantly affect immune organ development similar to glucocorticoids if patients required a long-term administration. However, more studies should be validated in the future. Collectively, CAN could serve as a good inhibitor of inflammatory cytokine storm in a non-cortisone manner.
This study provided a valuable reference for the treatment of severe cases of COVID-19 and inflammatory cytokine storm. At present, the drug has been on the market for the treatment of diabetes, which has a cardiovascular protective effect, with relatively few side effects. COVID-19 patients with cytokine storm and basic diseases (diabetes, hypertension and cardiovascular diseases) might benefit more from CAN treatment than other drugs. However, given the lack of ideal animal models for COVID-19, here, we induced cytokine storm in animal lungs only by LPS stimulation to simulate the clinical “cytokine storm” of COVID-19. Clinical trials should be conducted in COVID-19 patients with or without diabetes, hypertension and cardiovascular disease because CAN has direct anti-inflammatory effects in vivo.
We found that intragastric administration of CAN attenuated lung tissue injury and inflammatory cytokine storm in LPS-induced mice. Atomized administration also significantly inhibited the production of inflammatory cytokines. These results indicated that CAN had a wide, non-cortisone anti-inflammatory effect, which might inhibit inflammatory cytokine storm and serve as a candidate drug to treat COVID-19 patients. The action mechanism of CAN might be associated with the inhibition of HIF-1α/glycolysis in lung tissues. Considering that this drug could treat some basic diseases, for example, diabetes, hypertension and cardiovascular diseases, CAN might be used for COVID-19 patients with basic diseases; however, further validation should be conducted in the future.
Funding sources
This work was supported by Shenzhen Science and Technology Innovation Committee (JSGG20200519160752002) and the National Natural Science Foundation of China (No. 81373460).
CRediT authorship contribution statement
Yaoyun Niu: Data curation, Formal analysis, Investigation, Methodology. Yang Chen: Investigation, Validation. Pengbo Sun: Investigation. Yangyang Wang: Investigation. Jingyi Luo: Investigation. Yipei Ding: Investigation. Weidong Xie: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing - original draft, Writing - review &editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.107773.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Zhu N.a., Zhang D., Wang W., Li X., Yang B.o., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhroo A.D., Al Thani A.A., Yassine H.M. Markers Associated with COVID-19 Susceptibility, Resistance, and Severity. Viruses. 2020;13(1):45. doi: 10.3390/v13010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasmi A., Peana M., Pivina L., Srinath S., Gasmi Benahmed A., Semenova Y., Menzel A., Dadar M., Bjørklund G. Interrelations between COVID-19 and other disorders. Clin. Immunol. 2021;224:108651. doi: 10.1016/j.clim.2020.108651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal G., Lippi G., Lavie C.J., Henry B.M., Sanchis-Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. J. Diabetes. 2020;12(11):851–855. doi: 10.1111/1753-0407.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., Khare S., Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J. Stroke Cerebrovasc. Dis. 2020;29(8):104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C., Choi W.J. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch. Pharm. Res. 2021;44(1):99–116. doi: 10.1007/s12272-020-01301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A.C. Borczuk, Pulmonary pathology of COVID-19: a review of autopsy studies, Curr. Opin. Pulm Med Publish Ahead of Print (2021). [DOI] [PubMed]
- 10.Pasrija R., Naime M. The deregulated immune reaction and cytokines release storm (CRS) in COVID-19 disease. Int. Immunopharmacol. 2020;90 doi: 10.1016/j.intimp.2020.107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura S., Sakamaki S., Hongu M., Kawanishi E., Koga Y., Sakamoto T., Yamamoto Y., Ueta K., Kimata H., Nakayama K., Tsuda-Tsukimoto M. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J. Med. Chem. 2010;53(17):6355–6360. doi: 10.1021/jm100332n. [DOI] [PubMed] [Google Scholar]
- 12.Rådholm K., Figtree G., Perkovic V., Solomon S.D., Mahaffey K.W., de Zeeuw D., Fulcher G., Barrett T.D., Shaw W., Desai M., Matthews D.R., Neal B. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results From the CANVAS Program. Circulation. 2018;138(5):458–468. doi: 10.1161/CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secker P.F., Beneke S., Schlichenmaier N., Delp J., Gutbier S., Leist M., Dietrich D.R. Canagliflozin mediated dual inhibition of mitochondrial glutamate dehydrogenase and complex I: an off-target adverse effect. Cell Death Dis. 2018;9(2):226. doi: 10.1038/s41419-018-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough P.A., Kluger A.Y., Tecson K.M., Barbin C.M., Lee A.Y., Lerma E.V., Rosol Z.P., Kluger S.L., Rangaswami J. Inhibition of the Sodium-Proton Antiporter (Exchanger) is a Plausible Mechanism of Potential Benefit and Harm for Drugs Designed to Block Sodium Glucose Co-transporter 2. Rev. Cardiovasc. Med. 2018;19(2):51–63. doi: 10.31083/j.rcm.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Xu C., Wang W., Zhong J., Lei F., Xu N., Zhang Y., Xie W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem. Pharmacol. 2018;152:45–59. doi: 10.1016/j.bcp.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira A.P., Ayo C.M., Mimura K.K.O., Oliani S.M., Bernardo C.R., Camargo A.V.S., Ronchi L.S., Borim A.A., de Campos Júnior E., Brandão de Mattos C.C., Castiglioni L., Bestetti R.B., Cavasini C.E., de Mattos L.C. Plasma concentrations of CCL3 and CCL4 in the cardiac and digestive clinical forms of chronic Chagas disease. Cytokine. 2017;91:51–56. doi: 10.1016/j.cyto.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Shetty G., Beasley G.M., Sparks S., Barfield M., Masoud M., Mosca P.J., Pruitt S.K., Salama A.K.S., Chan C., Tyler D.S., Weinhold K.J. Plasma cytokine analysis in patients with advanced extremity melanoma undergoing isolated limb infusion. Ann. Surg. Oncol. 2013;20(4):1128–1135. doi: 10.1245/s10434-012-2785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghesquière B., Wong B.W., Kuchnio A., Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511(7508):167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 19.Lin S., Jin P., Shao C., Lu W., Xiang Q., Jiang Z., Zhang Y., Bian J. Lidocaine attenuates lipopolysaccharide-induced inflammatory responses and protects against endotoxemia in mice by suppressing HIF1α-induced glycolysis. Int. Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2019.106150. [DOI] [PubMed] [Google Scholar]
- 20.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L.i., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Seminars Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 23.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Z., Wang Y., Colunga-Lozano L.E., Prasad M., Tangamornsuksan W., Rochwerg B., Yao L., Motaghi S., Couban R.J., Ghadimi M., Bala M.M., Gomaa H., Fang F., Xiao Y., Guyatt G.H. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192(27):E756–E767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao R., Wang H., Wang X., Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporosis Int. : A J. Established as Result Cooperation Between Eur. Foundation Osteoporosis Natl. Osteoporosis Foundation USA. 2017;28(3):1027–1034. doi: 10.1007/s00198-016-3824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Y., Jiang M., Xia D., He L., Lv X., Liao X., Meng J. COVID-19 in a patient with long-term use of glucocorticoids: A study of a familial cluster. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2019;2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka Toshio, Narazaki Masashi, Kishimoto Tadamitsu. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;105954 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong Wen-Jing, Yang Hui-Hui, Guan Xin-Xin, Xiong Jian-Bing, Sun Chen-Chen, Zhang Chen-Yu, Luo Xiao-Qin, Zhang Yan-Feng, Zhang Jun, Duan Jia-Xi, Zhou Yong, Guan Cha-Xiang. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J. Cell. Physiol. 2019;234(4):4641–4654. doi: 10.1002/jcp.27261. [DOI] [PubMed] [Google Scholar]
- 32.Zhong J., Sun P., Xu N., Liao M., Xu C., Ding Y., Cai J., Zhang Y., Xie W. Canagliflozin inhibits p-gp function and early autophagy and improves the sensitivity to the antitumor effect of doxorubicin. Biochem. Pharmacol. 2020;175 doi: 10.1016/j.bcp.2020.113856. [DOI] [PubMed] [Google Scholar]
- 33.Meng Qingyuan, Guo Pinhao, Jiang Zhengyu, Bo Lulong, Bian Jinjun. Dexmedetomidine inhibits LPS-induced proinflammatory responses via suppressing HIF1α-dependent glycolysis in macrophages. Aging (Albany NY) 2020;12(10):9534–9548. doi: 10.18632/aging.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu L., Zeng Z., Xia Q., Liu Z., Feng X., Chen J., Huang M., Chen L., Fang Z., Liu Q., Zeng H., Zhou X., Liu J. Metformin attenuates hepatoma cell proliferation by decreasing glycolytic flux through the HIF-1α/PFKFB3/PFK1 pathway. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.116966. [DOI] [PubMed] [Google Scholar]
- 35.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., Zheng L., Gardet A., Tong Z., Jany S.S., Corr S.C., Haneklaus M., Caffrey B.E., Pierce K., Walmsley S., Beasley F.C., Cummins E., Nizet V., Whyte M., Taylor C.T., Lin H., Masters S.L., Gottlieb E., Kelly V.P., Clish C., Auron P.E., Xavier R.J., O’Neill L.A.J. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu Yanliu, Wang Jianquan, Li Zhen, Yao Keqing, Wang Liangliang, Song Jingjing. HIF1α Deficiency in Dendritic Cells Attenuates Symptoms and Inflammatory Indicators of Allergic Rhinitis in a SIRT1-Dependent Manner. Int. Arch. Allergy Immunol. 2020;181(8):585–593. doi: 10.1159/000506862. [DOI] [PubMed] [Google Scholar]
- 37.Jahani M., Dokaneheifard S., Mansouri K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J. Inflamm. (Lond.) 2020;17(1):33. doi: 10.1186/s12950-020-00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Xiang D., Zhang H., Yao H., Wang Y. Hypoxia-Inducible Factor-1: A Potential Target to Treat Acute Lung Injury. Oxid Med Cell Longev. 2020;2020:8871476. doi: 10.1155/2020/8871476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., Jimenez Restrepo J.L., Vendramini P.H., Reis-de-Oliveira G., Bispo Dos Santos K., Toledo-Teixeira D.A., Parise P.L., Martini M.C., Marques R.E., Carmo H.R., Borin A., Coimbra L.D., Boldrini V.O., Brunetti N.S., Vieira A.S., Mansour E., Ulaf R.G., Bernardes A.F., Nunes T.A., Ribeiro L.C., Palma A.C., Agrela M.V., Moretti M.L., Sposito A.C., Pereira F.B., Velloso L.A., Vinolo M.A.R., Damasio A., Proença-Módena J.L., Carvalho R.F., Mori M.A., Martins-de-Souza D., Nakaya H.I., Farias A.S., Moraes-Vieira P.M. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32(3):437–446.e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim W., Park C., Shim M.K., Lee Y.H., Lee Y.M., Lee Y. Glucocorticoids suppress hypoxia-induced COX-2 and hypoxia inducible factor-1α expression through the induction of glucocorticoid-induced leucine zipper. Br. J. Pharmacol. 2014;171(3):735–745. doi: 10.1111/bph.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.