Abstract
Background:
During murine kidney development, new cortical blood vessels form and pattern in cycles that coincide with cycles of collecting duct branching and the accompanying splitting of the cap mesenchyme (nephron progenitor cell populations that ‘cap’ collecting duct ends). At no point in the patterning cycle do blood vessels enter the cap mesenchyme. We hypothesised that the exclusion of blood vessels from the cap mesenchyme may be controlled, at least in part, by an anti-angiogenic signal expressed by the cap mesenchyme cells.
Results:
We show that semaphorin-3f (Sema3f), a known anti-angiogenic factor, is expressed in cap mesenchymal cells and its receptor, neuropilin-2 (Nrp2), is expressed by newly forming blood vessels in the cortex of the developing kidney. We hypothesised that Sema3f/Nrp2 signalling excludes vessels from the cap mesenchyme. Genetic ablation of Sema3f and of Nrp2, however, failed to result in vessels invading the cap mesenchyme.
Conclusions:
Despite their complementary expression patterns, our data suggest that Sema3f and Nrp2 are dispensable for the exclusion of vessels from the cap mesenchyme during kidney development. These results should provoke additional experiments to ascertain the biological significance of Sema3f/Nrp2 expression in the developing kidney.
Keywords: metanephros, renal, anti-angiogenic, endothelial, vasculature, lymphatic
Introduction
In his Collected Essays, T.H. Huxley referred to ‘The great tragedy of Science – the slaying of a beautiful hypothesis by an ugly fact’. This report describes just such a slaying, of a hypothesis about how the arrangement of blood vessels is controlled in the developing kidney.
The major function of the adult kidney is to filter blood. Blood enters the kidneys via the renal arteries before being transported to the glomerular capillaries where small molecules are filtered into the Bowman’s capsule. After filtration, useful molecules are reabsorbed by tubular cells and collected by peritubular capillaries, while waste and toxins are excreted as urine. This task requires a complex vasculature that must be assembled accurately during development.
Mouse metanephric (permanent) kidney development begins at E10.5 when the ureteric bud, the precursor of the collecting duct and ureter, evaginates from the caudal end of the Wolffian/nephric duct in response to glial cell-line-derived neurotrophic factor (GDNF; Sainio et al., 1997; Costantini & Kopan, 2010). The ureteric bud invades a mass of undifferentiated cells, the metanephrogenic mesenchyme, and begins to branch. As the ureteric bud branches, it induces nephron formation by provoking groups of nephron progenitor cells to undergo mesenchymal-to-epithelial transitions (Carroll et al., 2005). The nephron progenitor cells reciprocate by inducing branching of the ureteric bud (Majumdar et al., 2003; Costantini & Shakya, 2006; Cebrian et al., 2014). Prior to their differentiation, nephron progenitor cells situate around ureteric bud tips in cell populations known as the cap mesenchyme (they ‘cap’ the ureteric tips: Reinhoff, 1922). As the ureteric bud bifurcates, the overlaying cap mesenchymal population splits so that each new ureteric tip inherits a population of cap mesenchymal cells; this occurs in cycles throughout kidney development (Short et al., 2014).
Recently, we have shown that blood vessels pattern in cycles alongside the splitting of the cap mesenchyme and the branching of the ureteric bud in the developing kidney (Munro et al., 2017). Vascular plexuses form around the cap mesenchyme, but they do not enter it. As the cap splits, endothelia migrate through the fissure to form new vascular plexuses. The mechanism by which this happens is not understood, but we hypothesised that at least part of the pattern, the avoidance of the cap mesenchyme by the vasculature, may arise through the cap mesenchyme expressing anti-angiogenic signalling molecules.
Certain class-3 semaphorins have a known capacity to act as anti-angiogenic signalling molecules. An example is semaphorin-3f (Sema3f; Bielenberg et al., 2004; Guttmann-Raviv et al., 2007; Parker et al., 2010; Buehler et al., 2013; Guo et al., 2013). Although classically thought of as an axonal guidance molecule, Sema3f is highly expressed in multiple non-neuronal organs during development, such as the skin, lung, and kidney (Giger et al., 1998). Its functions in these tissue types are poorly understood.
In vascular development, Sema3f inhibits angiogenesis by interacting with its receptors on the endothelial cell membrane, neuropilin-1 (Nrp1; its low-affinity receptor) and neuropilin-2 (Nrp2; its high-affinity receptor; Guo et al., 2013; Sakurai et al., 2012). Sema3f/Nrp signalling results in the suppression of endothelial cell survival, proliferation, and migration (Guttmann-Raviv et al., 2007; Procaccia et al., 2014; Nakayama et al., 2015).
Here, we characterise the expression patterns of Sema3f, Nrp1, and Nrp2 in the developing kidney. We show that they are expressed in a manner perfectly compatible with the hypothesis that they mediate exclusion of vasculature from caps, but go on to show that they are not, in fact, required for this aspect of vascular plexus patterning.
Results and Discussion
Selection of a cap mesenchyme-expressed, anti-angiogenic candidate gene
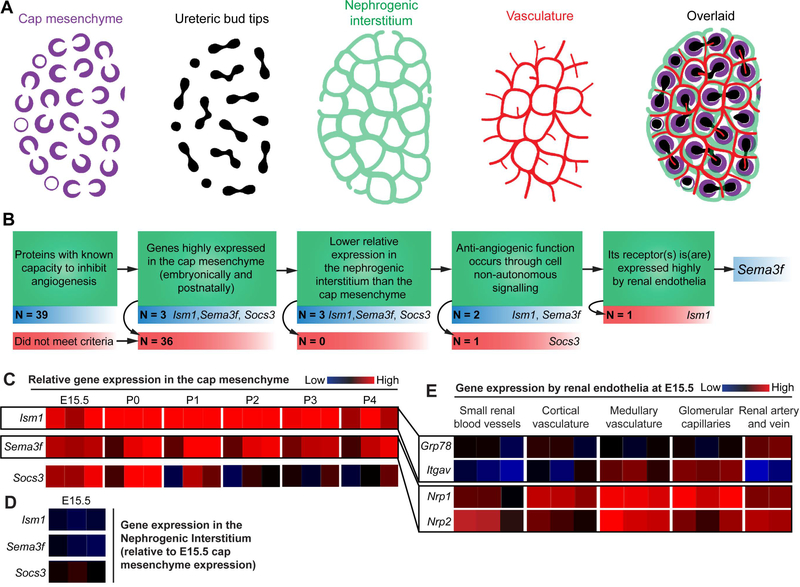
We previously demonstrated that renal endothelia pattern around, but do not enter, the cap mesenchyme in the nephrogenic zone of the developing kidney (Munro et al., 2017; Fig. 1A). Patterning occurs in cycles throughout most of embryonic kidney development, and we hypothesised that anti-angiogenic signals produced by the cap mesenchyme might be responsible for it being avascular. To test this, we generated a list of criteria to be met by cap mesenchyme-expressed candidate genes whose proteins might inhibit angiogenesis (Fig. 1B). We reviewed the literature to produce a list of 39 known anti-angiogenic genes and used microarray data from the GenitoUrinary Development Molecular Anatomy Project (GUDMAP) database (Brunskill et al., 2009; Harding et al., 2011) to assess their relative expression in the cap mesenchyme (Fig. 2). Of the genes investigated, isthmin-1 (Ism1), semaphorin-3f (Sema3f), and suppressor of cytokine signalling-3 (Socs3) met our initial criteria as candidate genes as they are highly expressed in the cap mesenchyme during the embryonic (E15.5) and early postnatal phase (at least at one age between P0-P4; Fig. 1C and Fig. 2).
Figure 1. Selection of Sema3f as a candidate gene for vascular plexus patterning in kidney development.
(A) Cartoons portraying the relevant cell populations in the nephrogenic zone of the kidney (modified GUDMAP cartoons). Note that the vasculature forms within the nephrogenic interstitium and around the cap mesenchyme. (B) Criteria for candidate gene selection. (C) GUDMAP microarray heatmap data showing the expression of the top three anti-angiogenic candidate genes by the cap mesenchyme at E15.5 and P0-P4: reproduced under the terms of the GUDMAP licence for re-use. (D) Expression of the top three candidate genes in the nephrogenic interstitium at E15.5 (relative to the cap mesenchyme). (E) Expression of the receptors for Ism1 (Grp78 and Itgav) and Sema3f (Nrp1 and Nrp2) by various renal endothelial beds at E15.5.
Figure 2. Microarray heatmap data from GUDMAP showing the relative expression of anti-angiogenic genes by the cap mesenchyme at E15.5 and P0-P4.
This list of anti-angiogenic genes is not exhaustive. Heatmap data is reproduced under the terms of the GUDMAP licence for re-use.
The next condition to be met was that the relative expression of the gene had to be lower in the nephrogenic interstitium (which becomes vascularized) than the cap mesenchyme (which does not): this was true for all three of these candidate genes at E15.5 (Fig. 1D). The anti-angiogenic function of the protein also had to be mediated through cell non-autonomous signalling (i.e. via secretion from the cap mesenchyme or presentation on cap mesenchyme membranes so it can exert its effect on the nearby endothelia). Socs3 is a Stat-inhibiting, intracellular protein (Starr et al., 1997) and its anti-angiogenic effect is endothelial-cell autonomous (Stahl et al., 2012; Rao et al., 2015): it was therefore discounted as a possible candidate gene.
Ism1 and Sema3f are secreted proteins, and their anti-angiogenic properties are exerted when they bind to their receptors on the endothelial cell membrane: receptor-ligand binding leads to the activation of signalling pathways that prevent endothelial cell survival and proliferation (Guttmann-Raviv et al., 2007; Xiang et al., 2011; Rao et al., 2015). Consequently, the receptors for Ism1 and Sema3f would need to be expressed in the plexus endothelia for them to transduce their signals. The receptors for Ism1 are integrin alpha-V (Itgav; Zhang et al., 2011) and 78 kDa glucose-regulated protein homolog (Grp78; Chen et al., 2014), and, based on microarray data, they were expressed weakly in renal endothelia (Fig. 1E). In contrast, the expression of the Sema3f receptors, neuropilin-1 and neuropilin-2 (Nrp1 and Nrp2, respectively), was high in renal endothelia (Fig. 1E). Based on these data, we further investigated the Sema3f/Nrp signalling pathway in vascular patterning in the nephrogenic zone.
Complementary expression pattern of Sema3f and Nrp2 in the developing kidney
The GUDMAP microarray data indicating that Sema3f is highly expressed in the cap mesenchyme at E15.5 has been validated at the RNA level by in situ hybridisation of whole-mount kidneys (data made available on GUDMAP by the McMahon lab; Fig 3A-A’). Although GUDMAP has little data on renal Sema3f expression prior to E15.5, data from the Allen Brain Atlas (Lein et al., 2007) show that Sema3f is also expressed in the kidney at E11.5 and E13.5 (Fig 3B-C’). At the earliest stages of renal organogenesis (E10.5-E11.5), the kidney is predominantly avascular, despite being surrounded by blood vessels (Munro et al., 2017). As Sema3f is expressed throughout the metanephrogenic mesenchyme at E11.5 (Fig. 3B-B’), its anti-angiogenic function could prevent premature renal vascularisation.
Figure 3. Confirmation that Sema3f is expressed in the cap mesenchyme of the developing kidney.
(A-A’) In situ hybridisation image showing Sema3f expression in the cap mesenchyme of the E15.5 kidney (from GUDMAP; submitted by the McMahon lab). (B-C’) In situ hybridisation images showing Sema3f expression in the (B-B’) E11.5 and (C-C’) E13.5 kidney (image credit: Allen Institute). (D) E12.5 kidney co-stained with anti-Sema3f and anti-Gata3. Red blood cells are yellow (due to heme auto-fluorescence in the red and green channels as a result of tissue clearing). (E) E15.5 kidney co-stained with anti-Sema3f and anti-integrin alpha-8 (ITGA8). Negative control (secondary only) image was taken using identical microscope settings. Scale bars = 100 μm (except from B-C’ where scale bar values are stated).
To investigate the localisation of the Sema3f protein, we stained whole-mount kidneys at E12.5 and E15.5 with anti-Sema3f. Kidneys were cleared with benzyl alcohol/benzyl benzoate (BABB) prior to imaging. At E12.5, Sema3f localises in tubular epithelia (in agreement with Villegas and Tufro, 2002) and in the presumptive cap mesenchyme (Fig. 3D). At E15.5, we assessed co-localisation of Sema3f with the cap mesenchyme marker, integrin alpha-8 (Müller et al., 1997). Sema3f staining was not exclusive to the cap mesenchyme: it was observed in tubular epithelia, the cap mesenchyme, and the nephrogenic interstitium (Fig 3E). This is unsurprising as Sema3f is a secreted protein whose localisation is not restricted to the cell that produces it. Indeed, Sema3f would be required to exit the cap to bind to, and inhibit, endothelia in the nephrogenic interstitium.
To respond to Sema3f, the endothelia in the nephrogenic interstitium would have to express either Nrp1, Nrp2, or both. Confocal z-stack images of an E15.5 Nrp2-EGFP transgenic mouse kidney, made available on GUDMAP by Steven Potter’s lab, indicated that Nrp2 is expressed in proximal tubules and by an unknown cell type within the nephrogenic interstitium (Supplementary movie 1; adapted with permission from the Potter Lab). We examined whether the Nrp2+ cells within the nephrogenic interstitium were endothelial by co-staining with anti-Nrp2 and anti-CD31 (an endothelial marker). The Nrp2+ cells were endothelial and were always present across the bifurcation site of the ureteric bud (42/42, 100%, CI95% ± 1.19%; n = 42 bifurcation sites analysed in 1 kidney; Fig. 4A-C). Because new vascular plexuses form when endothelial cells migrate away from the bifurcation site (Munro et al., 2017; Fig. 4D), these Nrp2+ endothelia were situated in the precise location we would anticipate for them to be responding to Sema3f.
Figure 4. Expression of Nrp2 by endothelial cells in the nephrogenic interstitium.
(A) Nrp2+ cells within the nephrogenic interstitium are endothelial. (B) As the cap mesenchyme splits, Nrp2+ endothelia are present at the bifurcation site where new vascular plexuses form (white arrowhead shows an example). (C) Nrp2+ endothelia are positioned around the cap mesenchyme. (D) Cartoon illustrating the complementary expression pattern of Nrp2 and Sema3f in the nephrogenic zone. Green arrowheads indicate the direction of endothelial migration as cap mesenchymal populations split. Scale bars = 100 μm.
Expression of Nrp1 and Nrp2 in the developing kidney and ureter
Studies suggest that Nrp1 and Nrp2 are differentially expressed in arterial, venous, and lymphatic endothelia (Herzog et al., 2001). Nrp2 is preferentially expressed in lymphatic vessels and veins, whereas Nrp1 is expressed in arteries. We corroborated these findings by co-staining Nrp1+ and Nrp2+ endothelia with venous and lymphatic markers in the ureter (Fig. 5A-C’). The Nrp2+ endothelia in the ureter are lymphatic and venous (based on morphology [Fig. 5A] and as shown by co-staining with anti-Lyve-1 [Fig. 5B] and anti-EphB4 [Fig. 5C], markers for lymph and vein respectively). The Nrp1+ endothelia in the ureter are arterial (as shown by lack of co-staining with anti-Nrp2 and anti-EphB4; Fig. 5C’).
Figure 5. Expression of Nrp1 and Nrp2 in the endothelia of the ureter.
(A) Based on vessel morphology in the E18.5 ureter, veins (V; larger diameter vessel with loosely packed endothelia) are CD31+Nrp2+, whereas arteries (A; smaller diameter vessel with tightly packed endothelia) are CD31+Nrp2-. (B-C’) Based on co-staining in the E18.5 ureter, (B) lymphatic vessels are Nrp2+Lyve-1+CD31+, (C-C’) veins are Nrp2+EphB4+Nrp1-, and arteries are Nrp1+Nrp2-EphB4-. Macrophages (MΦ) are also present in B. A, artery; V, vein; LV, lymphatic vessel; MΦ, macrophages. Scale bars = 100 μm.
In the E11.5 and E12.5 kidney, almost all renal blood vessels expressed Nrp2, but few vessels expressed Nrp1 (Fig. 6A, B). By E13.5, Nrp1 had become highly expressed in renal arteries (Fig. 6C-E). The endothelial plexuses in the E13.5 kidney had many Nrp2+ cells, but few discernible Nrp1+ cells. In contrast, by E18.5 the plexus endothelia expressed Nrp2 as well as Nrp1 (Fig 6E, F). Nrp2 is highly enriched in endothelial tip cells (Siemerink et al., 2012). Based on the location of the Nrp2+ plexus endothelia at the vascular front (the site of new blood vessel formation), we speculate that these Nrp2+ cells are the endothelial tip cells in the kidney; however, there are no known specific endothelial tip cell markers to verify this hypothesis.
Figure 6. Nrp1 and Nrp2 expression in the developing kidney.
(A-C) Expression of Nrp1 and Nrp2 by renal endothelia at E11.5-E13.5. (D) Cartoons illustrating the expression patterns of Nrp1 and Nrp2 and the position of the cap mesenchyme (CM) in the E11.5-E13.5 kidney. (E-F) Nrp1 and Nrp2 expression in plexus endothelia at (E) E13.5 and (F) E18.5. Scale bars = 100 μm.
In the ureter, anti-Nrp2 staining is very strong in lymphatic vessels (Fig 5A-C). We next explored whether Nrp2+ lymphatic vessels form within the kidney. To do this, we cleared whole-mount E15.5 kidneys with BABB prior to imaging. We demonstrate that Nrp2+ lymphatic vessels form rings around the ureter and that these vessels connect with the lymphatic vessels in the renal pelvis (Fig 7A-B). In agreement with previous studies (Tanabe et al., 2012), renal Nrp2+ lymphatic vessels extend along the segmental vasculature from the renal pelvis (Fig 7C). The Nrp2+ lymphatic vessels in the kidney and ureter are in connection with a network of Nrp2+ lymphatic vessels that supply the adrenal gland, gonad, mesonephros, and ureter (Fig 7A; Supplementary movie 2).
Figure 7. Nrp2 expression in lymphatic endothelia.
(A) A network of Nrp2+ lymphatic vessels surround the major arteries in the kidney, mesonephros, gonad, and adrenal gland (stitched confocal z-stacks; 2×2 tiles; 57 images per tile). (B) Renal Nrp2+ lymphatic vessels connect with the Nrp2+ lymphatic vessels in the ureter (representative image of n = 4 confocal z-stack images). (C) Lymphatic vessels form alongside the segmental vasculature. Red blood cells are magenta (due to heme auto-fluorescence in the red and blue channels as a result of tissue clearing). AG, adrenal gland. Scale bars = 100 μm.
Sema3f and Nrp2 are not required for exclusion of endothelia from the cap mesenchyme
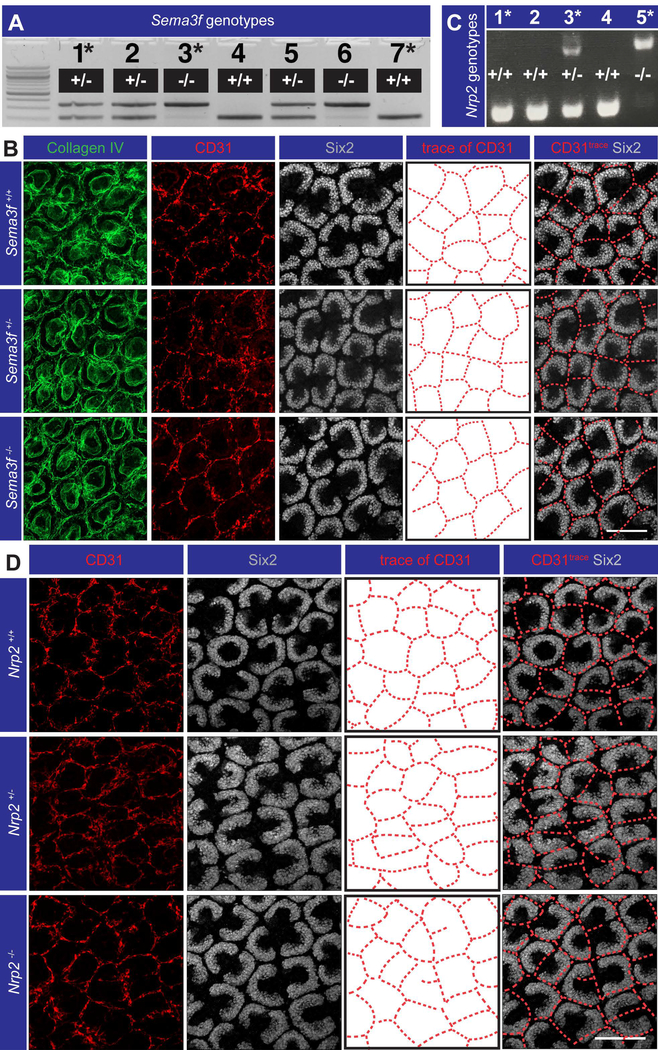
After confirming the complementary expression pattern of Sema3f and Nrp2 in the nephrogenic zone, we tested the hypothesis that their interaction repels endothelia from the caps. Genetic ablation of Sema3f did not result in vessels entering the cap mesenchyme, as shown by co-staining for anti-Six2 (cap mesenchyme marker), anti-collagen IV (blood vessel and ureteric bud basement membrane marker), and anti-CD31 (endothelial marker; Fig. 8A, C). Endothelial patterning was also normal in Nrp2 null mouse kidneys (Fig. 8B, D), providing validation that signalling through the Sema3f/Nrp2 pathway is not required for normal vascular plexus patterning around the cap mesenchyme.
Figure 8. Sema3f and Nrp2 are not required for exclusion of blood vessels from the cap mesenchyme in the nephrogenic zone.
(A-B) Genetic ablation of Sema3f had no effect on vascular patterning around the caps (representative images from n = 2–3 P0 mice; 4–6 kidneys per genotype; 9–12 total fields of staining per genotype). (C-D) Genetic ablation of Nrp2 had no effect on vascular patterning around the caps (representative images from n = 1–3 E18.5 embryos; 2–6 total fields of staining per genotype). Asterisks in the genotyping gel images represent the embryos/pups whose kidneys are shown in the representative images. Scale bars = 100 μm.
In conclusion, we have demonstrated that, although Sema3f and its receptors (Nrp1/2) are expressed in a manner compatible with the hypothesis that their interaction excludes endothelia from the cap mesenchyme, signalling through the Sema3f/Nrp pathway is not required for vasculature repulsion from the cap. This lack of requirement for Sema3f and Nrp2 could be the result of redundancy with another pathway, the result of compensatory expression of other genes (such as other class-3 semaphorins) in response to their loss, or simply that they are not involved in this aspect of vascular patterning.
We investigated the consequences of Sema3f and Nrp2 deletion on vascular plexus patterning around the cap mesenchyme; however, Sema3f is also expressed by tubular cells in the kidney (Villegas and Tufro, 2002), and we have not eliminated the possibility that tubular expression of Sema3f could guide blood vessel patterning around nephrons/collecting ducts. Future studies should aim to reveal the significance of Sema3f and Nrp2 expression in the development of the kidney.
Experimental procedures
Databases
Cap mesenchyme-expression of anti-angiogenic genes was examined using GUDMAP’s gene expression search tool (http://www.gudmap.org/gudmap/pages/database_homepage.html). The microarray heatmap data for each gene was taken from the ‘Developing Kidney (MOE430)’ and ‘Developing Kidney (ST1)’ data sets (data retrieved May, 2017). The normalised heatmap data (red indicating high expression, blue indicating low expression, and black indicating expression that is close to the median) represents the binary logarithm of the average expression value of a given gene across an entire data set. For each probe set, experiments were performed in triplicate. Where there were two probe sets available for the expression of a given gene, the probe set with the most consistent data between replicate samples were shown. Where there were more than two probe sets available for the expression of a given gene, the probe set with the median values and/or colour were chosen for the analysis. The in situ hybridisation images showing Sema3f expression in the E15.5 kidney was submitted by the McMahon group (data retrieved from GUDMAP in May, 2017) and can be found at http://www.gudmap.org/gudmap/pages/gene.html?geneId=MGI%3A1096347. The confocal z-stack movie of the Nrp2-EGFP kidney was produced by the Potter group and can be found at http://www.gudmap.org/Resources/MouseStrains/Mouse_Strains_Summary.html under the heading ‘Mouse strains generated by external sources’ (data retrieved from GUDMAP in May, 2017). The in situ hybridisation images from the Allen Brain Atlas showing Sema3f expression at E11.5 can be found at http://developingmouse.brain-map.org/experiment/show/100093437 (section 12/20; accessed May, 2017), and for E13.5 can be found at http://developingmouse.brain-map.org/experiment/show/100076578 (section 7/16; accessed May, 2017). Using the above Allen Brain Atlas links, renal Sema3f expression can be observed in sections 11–13 and 16–17 of the E11.5 mouse (out of 20 sections), and in sections 6–10 and 15–16 of the E13.5 mouse (out of 16 sections).
Mice
Wild-type embryonic tissues were obtained from CD-1 mice that were killed by qualified staff of a UK Home Office-licenced animal house following the guidelines set under Schedule 1 of the UK Animals (Scientific Procedures) Act 1986. Experiments were approved and performed in accordance with the institutional guidelines and regulations as set by the University of Edinburgh. Tissue from P0 Sema3f knockout embryos was obtained from Georgetown University facilities according to locally approved procedures. The Sema3f mutant line was maintained in accordance with the NIH Animal Care and Use Committee. The caudal portions of euthanized P0 Sema3f mice were sent at 4°C to the University of Edinburgh where the kidneys were dissected and immunostained (for details, see Davies, 2006). The Sema3f knockout mouse line has previously been described (Walz et al., 2007). Sema3f mutant mice were bred for over 9 generations into a C57BL/6 genetic background (Charles River Laboratories, Frederick, MD). Tissue from E18.5 Nrp2 transgenic mouse embryos was kindly provided by Alex Kolodkin and Qiang Wang (Johns Hopkins University). Nrp2 mutant mice were bred into the C57BL/6 genetic background, and this mouse line has previously been described (Giger et al., 2000).
Immunohistochemistry
Embryonic kidneys were dissected as previously described (Davies, 2010). Kidneys were then fixed in methanol (pre-cooled at −20°C) for 1 hr. Fixed kidneys were either stored in methanol at −20°C (for up to 6 months) or directly processed following fixation. PBS was used to rinse off the methanol from the kidneys (3 × 20 mins) and the kidneys were blocked with 1x PBS with 5% donkey serum (Sigma, D9663) and 2.5% BSA (Sigma, A9647) for 1 hr-overnight. Kidneys were incubated with the following primary antibodies overnight at 4°C: rat-anti-CD31 (1:100; BD Pharmingen; 550274), goat-anti-collagen IV (1:100; Merckmillipore; AB769), rat-anti-EphB4 (1:100; abcam; ab73259), rabbit-anti-laminin (1:100; Sigma; L9393), rabbit-anti-Lyve1 (1:100; Abcam; ab33682), rabbit-anti-Nrp1 (1:100; abcam; ab81321), goat-anti-Nrp2 (1:100; R&D systems; AF567), mouse-anti-pan cytokeratin (1:100; Sigma; C2562), rabbit-anti-Sema3f (1:100; abcam; ab39956), rabbit-anti-Six2 (1:250; Proteintech; 11562–1-AP), goat-anti-mouse-integrin alpha 8 (1:100; R&D systems; AF4076). Kidneys were then rinsed in 1x PBS (3 × 5 mins, then 3 × 1 hr) and subsequently incubated in Alexa Fluor conjugated secondary antibodies overnight at 4°C (emission λ of 350, 488, 594, 647; dilution of 1:200; Thermo Fisher Scientific). Finally, kidneys were washed in 1x PBS (3 × 5 mins, then 4 × 1 hr), before being mounted onto glass slides using Vectashield (Vectorlabs, H1000) as a mounting medium. Benzyl alcohol/benzyl benzoate (BABB) clearing whole-mount immunofluorescence was performed as previously described (Munro et al., 2017). BABB clearing was used for Fig. 3D-E and Fig. 7.
Microscopy
Images were captured using the Nikon A1R and Zeiss LSM800 confocal microscopes. Objective lenses of 4–60x were used (lenses were oil-immersed from 40x upwards).
Statistics and data presentation
95% confidence intervals for binary (yes/no) data were calculated using the formula t=1.64SQRT(pq/n)+1/2n, where t is the 95% confidence interval, p is the proportion positive, q is 1-p, and n is the number examined. IMARIS (version 8.3.1) and Adobe premiere pro CC (2015) were used to prepare the supplementary movies.
Supplementary Material
Supplementary Movie 1. Confocal z-stack images of an E15.5 Nrp2-EGFP kidney. White arrows show examples of Nrp2+ cells in the nephrogenic interstitium. White arrowheads show examples of Nrp2+ proximal tubules. Video adapted from data generated by the Potter lab.
Supplementary Movie 2. Tiled z-stack images of the Nrp2+ network of lymphatic vessels in the E15.5 kidney and adjacent organs. Vessels were rendered using the volume tool in IMARIS.
Key findings:
Using gene expression data from the GUDMAP database, we selected Sema3f as an anti-angiogenic candidate gene that may exclude developing blood vessels from the cap mesenchyme (groups of nephron progenitor cells) in the murine metanephric (permanent) kidney.
In the developing kidney, Sema3f is expressed in the cap mesenchyme, and its receptor, Nrp2, is expressed in endothelia that pattern in cycles around the caps in the nephrogenic zone.
Genetic ablation of Sema3f and of Nrp2 in mice demonstrated that they are not required for vascular exclusion from the cap mesenchyme.
Acknowledgements
We are extremely grateful to Prof. Alex Kolodkin and Dr Qiang Wang for supplying us with tissue from Nrp2 knockout mouse embryos and for providing us with genotyping information. We are thankful to Dr Jane Armstrong, Prof Karen Chapman, Dr Melanie Lawrence, and Mr Christopher Mills for useful comments and advice. We would also like to express our gratitude to Dr Anisha Kubasik-Thayil of the IMPACT imaging facility at the University of Edinburgh for assistance with microscopy.
Grant sponsor: Medical Research Council, Biotechnology and Biological Sciences Research Council, and National Institutes of Health. Grant numbers: MR/K501293/1, MR/K010735/1, BB/P013732/1, and NIH DC13107.
Footnotes
Conflict of interest disclosure: None to declare.
References
- Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. 2004. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 114: 1260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. 2008. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell. 15: 781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler A, Sitaras N, Favret S, Bucher F, Berger S, Pielen A, Joyal JS, Juan AM, Martin G, Schlunck G, Agostini HT, Klagsbrun M, Smith LE, Sapieha P, Stahl A. 2013. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS Lett. 587: 1650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. 2005. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 9: 283–92. [DOI] [PubMed] [Google Scholar]
- Cebrian C, Asai N, D’Agati V, Costantini F. 2014. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep. 7: 127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhang Y, Yu VC, Chong YS, Yoshioka T, Ge R. 2014. Isthmin targets cell-surface GRP78 and triggers apoptosis via induction of mitochondrial dysfunction. Cell Death Differ. 21: 797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F, Kopan R. 2010. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 18, 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F, Shakya R. 2006. GDNF/Ret signaling and the development of the kidney. Bioessays. 28: 117–27. [DOI] [PubMed] [Google Scholar]
- Davies JA. 2006. A method for cold storage and transport of viable embryonic kidney rudiments. Kidney Int. 70: 2031–4. [DOI] [PubMed] [Google Scholar]
- Davies JA. 2010. The embryonic kidney: isolation, organ culture, immunostaining and RNA interference. Method Mol Biol. 633: 57–69. [DOI] [PubMed] [Google Scholar]
- Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, Herbert JM, Bono F. 2006. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Angiogenesis. 108: 1243–50. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. 1998. Neuropilin-2 Is a Receptor for Semaphorin IV: Insight into the Structural Basis of Receptor Function and Specificity. Neuron. 21: 1079–92. [DOI] [PubMed] [Google Scholar]
- Guo HF, Li X, Parker MW, Waltenberger J, Becker PM, Vander Kooi CW. 2013. Mechanistic basis for the potent anti-angiogenic activity of semaphorin 3F. Biochemistry. 29: 7551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. 2007. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 7: 26294–305. [DOI] [PubMed] [Google Scholar]
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, Sharghi M, Tindal C, McMahon AP, Gottesman B, Little MH, Georgas K, Aronow BJ, Potter SS, Brunskill EW, Southard-Smith EM, Mendelsohn C, Baldock RA, Davies JA, Davidson D. 2011. The GUDMAP database - an online resource for genitourinary research. Development. 138: 2845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. 2001. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 109: 115–9. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 445: 168–176. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. 2003. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 130: 3175–85. [DOI] [PubMed] [Google Scholar]
- Müller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. 1997. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 88: 603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro DAD, Hohenstein P, Davies JA. 2017. Cycles of vascular plexus formation within the nephrogenic zone of the developing kidney. Sci Rep. 7: 3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Bruneau S, Kochupurakkal N, Coma S, Briscoe DM, Klagsbrun M. 2015. Regulation of mTOR Signaling by Semaphorin 3F-Neuropilin 2 Interactions In Vitro and In Vivo. Sci Rep. 5: 11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW. 2010. Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry. 18: 4068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccia V, Nakayama H, Shimizu A, Klagsbrun M. 2014. Gleevec/imatinib, an ABL2 kinase inhibitor, protects tumor and endothelial cells from semaphorin-induced cytoskeleton collapse and loss of cell motility. Biochem Biophys Res Commun. 448: 134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N, Lee YF, Ge R. 2015. Novel endogenous angiogenesis inhibitors and their therapeutic potential. Acta Pharmacol Sin. 36: 1177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhoff WF. 1922. Development and growth of the metanephros or permanent kidney in chick embryos. Johns Hopkins Hospital Bulletin. 33: 392–406. [Google Scholar]
- Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumäe U, Meng X, Lindahl M, Pachnis V, Sariola H. 1997. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 124: 4077–87. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Doçi CL, Gutkind JS. 2012. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 22: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KM, Combes AN, Lefevre J, Ju AL, Georgas KM, Lamberton T, Cairncross O, Rumballe BA, McMahon AP, Hamilton NA, Smyth IM, Little MH. 2014. Global quantification of tissue dynamics in the developing mouse kidney. Dev Cell. 29: 188–202. [DOI] [PubMed] [Google Scholar]
- Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, Schlingemann RO. 2012. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 15: 151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Joyal JS, Chen J, Sapieha P, Juan AM, Hatton CJ, Pei DT, Hurst CG, Seaward MR, Krah NM, Dennison RJ, Greene ER, Boscolo E, Panigrahy D, Smith LE. 2012. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood. 120: 2925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. 1997. A family of cytokine-inducible inhibitors of signalling. Nature. 387: 917–21. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Shimizu A, Masuda Y, Kataoka M, Ishikawa A, Wakamatsu K, Mii A, Fujita E, Higo S, Kaneko T, Kawachi H, Fukuda Y. 2012. Development of lymphatic vasculature and morphological characterization in rat kidney. Clin Exp Nephrol. 16: 833–42. [DOI] [PubMed] [Google Scholar]
- Villegas G, Tufro A. 2002. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech Dev. 119: S149–53. [DOI] [PubMed] [Google Scholar]
- Walz A, Feinstein P, Khan M, Mombaerts P. 2007. Axonal wiring of guanylate cyclase-D-expressing olfactory neurons is dependent on neuropilin 2 and semaphorin 3F. Development. 134: 4063–72. [DOI] [PubMed] [Google Scholar]
- Xiang W, Ke Z, Zhang Y, Cheng GH, Irwan ID, Sulochana KN, Potturi P, Wang Z, Yang H, Wang J, Zhuo L, Kini RM, Ge R. 2011. Isthmin is a novel secreted angiogenesis inhibitor that inhibits tumour growth in mice. J Cell Mol Med. 15: 359–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen M, Venugopal S, Zhou Y, Xiang W, Li YH, Lin Q, Kini RM, Chong YS, Ge R. 2011. Isthmin exerts pro-survival and death-promoting effect on endothelial cells through alphavbeta5 integrin depending on its physical state. Cell Death Dis. 2: e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1. Confocal z-stack images of an E15.5 Nrp2-EGFP kidney. White arrows show examples of Nrp2+ cells in the nephrogenic interstitium. White arrowheads show examples of Nrp2+ proximal tubules. Video adapted from data generated by the Potter lab.
Supplementary Movie 2. Tiled z-stack images of the Nrp2+ network of lymphatic vessels in the E15.5 kidney and adjacent organs. Vessels were rendered using the volume tool in IMARIS.