Abstract
OBJECTIVES
The objective of this study was to assess our 43-year experience with arterial switch operation (ASO) for transposition of the great arteries (TGA) by analysing cardiac outcome measures (hospital and late mortality, reoperations and catheter interventions, significant coronary artery obstruction) and to identify risk factors for reoperation and catheter interventions.
METHODS
A total of 490 patients who underwent ASO for TGA from 1977 to 2020 were included in this retrospective, single-centre study. Data on reoperation and catheter intervention of hospital survivors were estimated by the Kaplan–Meier method and compared using a long-rank test. Risk factors for reoperation and/or catheter intervention were assessed by multivariate Cox regression analysis.
RESULTS
Hospital mortality occurred in 43 patients (8.8%), late death in 12 patients (2.9%) and 43 patients were lost to follow-up. Median follow-up time of 413 hospital survivors was 15.6 (interquartile range 7.0–22.4) years. Reoperations were performed in 83 patients (117 reoperations). Neoaortic valve regurgitation with root dilatation was the second most common indication for reoperation (15/83 patients, 18.1%) after right ventricular outflow tract obstruction (50/83 patients, 60.2%). Risk factors for any reoperation on multivariable analysis were: TGA morphological subtype [TGA with ventricular septal defect: hazard ratio (HR) = 1.99, 95% confidence interval (CI) 1.18–3.36; P = 0.010 and Taussig-Bing: HR = 2.17, 95% CI 1.02–4.64; P = 0.045], aortic arch repair associated with ASO (HR = 3.03, 95% CI 1.62–5.69; P = 0.001) and a non-usual coronary artery anatomy (HR = 2.41, 95% CI 1.45–4.00; P = 0.001). One hundred and one catheter interventions were performed in 54 patients, usually for relief of supravalvular pulmonary stenosis (44/54 patients, 81.5%) or arch obstruction (10/54 patients, 18.5%). Main risk factor for catheter intervention on multivariable analysis was aortic arch repair associated with ASO (HR = 2.95, 95% CI 1.37–6.36; P = 0.006). Significant coronary artery stenosis was relatively uncommon (9/413 patients, 2.2%) but may be underrepresented.
CONCLUSIONS
Patients after ASO typically have good long-term clinical outcomes but reoperations and interventions remain necessary in some patients. Neoaortic valve regurgitation with root dilatation is the second most common indication for reoperation after right ventricular outflow tract obstruction and an increasing need for neoaortic valve and root redo surgery in future is to be expected.
Keywords: Transposition of the great arteries, Arterial switch operation, Reoperation, Intervention
INTRODUCTION
For more than 40 years, the arterial switch operation (ASO) is the standard surgical treatment for transposition of the great arteries (TGA). Surgical results with low perioperative mortality over the last decades have now led to focus on the long-term outcomes. Health condition and quality-of-life assessments recently showed that most adult patients perceive normal functional health status, similar to healthy peers [1]. Cardiovascular outcome and survival from mid- and long-term follow-up studies are based on data from 20- up to 30-year post-ASO. In these reports, residual lesions are reported such as supravalvular pulmonary stenosis [2–4], neoaortic root dilatation, neoaortic valve insufficiency [5, 6] and coronary artery problems [7–9]. The purpose of this study was to assess our 43-year experience with ASO and to determine late mortality and morbidity, including coronary artery stenosis, arrhythmias and cardiac device implantations, as well as the need for reoperation and catheter intervention, and finally, to identify risk factors for cardiovascular reoperation and catheter intervention.
METHODS
Study population
Patients who underwent ASO for TGA at the Leiden University Medical Center, Leiden, between January 1977 and January 2020 were included in this study. TGA subgroups based on anatomic differences included: TGA with intact ventricular septum (TGA-IVS); TGA with ventricular septal defect (TGA-VSD) and double outlet right ventricle with subpulmonary VSD (i.e. Taussig-Bing anomaly, TBA). Data on demographics, morphologic and surgical details, reoperations, catheter interventions, coronary artery problems, atrial or ventricular arrhythmias, the need for cardiac devices (pacemaker and implantable cardioverter-defibrillator) and mortality were collected from hospital and outpatient records. Hospital mortality was defined as death occurring before hospital discharge; late mortality was defined as death after hospital discharge. Reoperations and catheter interventions were subdivided in early (<30 days post-ASO) and late reoperations/interventions (>30 days post-ASO). Re-explorations for perioperative complications (e.g. for bleeding) were not included. Catheter ablations for arrhythmias were not included as catheter intervention but were reported separately. Rhythm disorders after hospital discharge were assessed. Left ventricular outflow tract obstruction was included as a separate variable; patients with TGA-VSD and (sub)pulmonary stenosis were only included when ASO had been performed (n = 4) and were classified into the TGA-VSD group. The local Committee for Medical Ethics of the Amsterdam and Leiden University Medical Centers approved the study and waived the need for informed consent. Data collection was completed in January 2020.
Surgical procedure
A description of the ASO procedure in our centre and indications for two-stage correction for TGA have been reported previously [10]: coronary artery translocation was performed by the button technique whenever feasible or by trap-door technique otherwise. In case of posterior common ostium (Yacoub types B and C), the technique as described by Yacoub was used [11]. The neopulmonary artery (PA) was reconstructed by using a pantaloon-shaped patch of fresh autologous pericardium. The Lecompte manoeuvre has been applied whenever possible since 1981; before that time, a classical Jatene procedure with implantation of a conduit between the right ventricle and PA was performed. A Lecompte procedure was avoided when a side-to-side relationship of the great arteries was found, as this would lead to stretching and narrowing of the left PA. VSDs were closed via the right atrium or the aortic valve, and in TBA, the approach to the VSD was often from multiple sides sometimes including a limited right ventriculotomy. Arch repair was only by extended side-to-side anastomosis when a discrete coarctation was present; in all other cases, the arch was repaired with a xenopericardial curved patch which has the additional advantage of a better fit to the wider PA base.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 23.0. Clinical characteristics were presented as frequency (%) for categorical variables or as median (interquartile range, IQR or range). The normality of the distribution was tested using the Shapiro–Wilk test. Differences in frequencies of baseline characteristics and outcome parameters between TGA subgroups were assessed by the Fisher–Freeman–Halton exact test. A Kruskal–Wallis test was performed to test for differences in continuous data between the TGA subgroups. Primary outcome parameters were reoperations and catheter interventions. Secondary outcome parameters were hospital and late mortality, arrhythmias and coronary artery problems.
Kaplan–Meier analyses were performed to estimate overall survival and reoperation and catheter intervention-free survival of hospital survivors, and to estimate differences in reoperation rate and type based on era. Patients with late mortality, without having had reoperation (7/12 patients) or catheter intervention (10/12 patients) before death occurred, were censored at the age of death in this analysis (considering late death as competing risk resulted in similar percentages of freedom from reoperation/catheter intervention). The log-rank test was used to test for differences between intervention-free (reoperation, catheter intervention or both) survival curves among morphological TGA subtypes. A Cox regression model was used to assess the independent predictive values of different variables on primary outcome measures reoperation and/or catheter intervention, resulting in cause-specific hazard ratios (HRs) for the variables. Risk factors known from literature and potential explanatory variables were entered into the equation at the same time. Included risk factors from literature were: ‘TGA morphological subtype’; ‘arch abnormality’, for which surgical repair was performed; ‘left ventricular outflow tract obstruction’; PA banding prior to ASO, ‘prior PA banding’; ‘coronary artery anatomy’, divided into 2 categories: (i) usual coronary artery anatomy: 1LCx-2R, 1L-2CxR and 1L-2R, no Cx and (ii) non-usual coronary artery anatomy: other than (i); ‘weight at ASO’. Potential explanatory variables included in the models were: ‘sex’; use of ‘Lecompte manoeuvre’ with the ASO. A P-value <0.05 (2-sided) was considered statistically significant.
RESULTS
Patient characteristics and hospital mortality
During the study period, 490 patients underwent ASO. Thirty-four overseas patients were lost to follow-up. Hospital mortality was 8.8% (43 patients), highest from 1977 to 1987, and decreased to 3.3% between 2000 and 2020. Baseline patient characteristics of remaining 413 hospital survivors with follow-up are shown in Table 1. The morphological TGA subtypes were: TGA-IVS in 258 (62.5%), TGA-VSD in 117 (28.4%) and TBA in 38 (9.2%) patients. Arch anomalies for which surgical repair before or during ASO was necessary were present in 35 (8.5%) patients and consisted of hypoplastic aortic arch with (n = 25) or without (n = 2) coarctation, interrupted aortic arch (n = 7) and double aortic arch with coarctation (n = 1) (Table 1). The majority of the arch abnormalities were present in TBA patients (n = 18, 47.4%), in 14 (12.0%) of the TGA-VSD patients and in 3 (1.2%) of the TGA-IVS patients (P = 5.5*10−16). Left ventricular outflow tract obstruction was not equally distributed across the TGA subgroups and present in 5 (13.2%) of the TBA patients, in 10 (8.5%) of the TGA-VSD patients and in 1 (0.4%) of the TGA-IVS patients (P = 3.0*10−6). Median age at ASO was 8 days for the patients who underwent one-stage ASO. Forty-two patients had prior surgery before ASO (i.e. two-stage ASO). Median follow-up was 15.6 (IQR 7.0–22.4) years post-ASO, with a maximum follow-up of 41.1 years.
Table 1:
Demographics and preoperative anatomy
| Study cohort (N = 413) | TGA-IVS (N = 258) | TGA-VSD (N = 117) | TBA (N = 38) | P-value | |
|---|---|---|---|---|---|
| Male, n (%) | 280 (67.8) | 184 (71.3) | 72 (61.5) | 24 (63.2) | 0.133 |
| Age follow-up (years)a | 15.6 (0.1–41.1) | 15.7 (0.1–40.1) | 15.8 (0.2–41.1) | 12.3 (0.1–36.3) | 0.661 |
| Age follow-up (years)b | 15.6 (0.1–45.8) | 15.7 (0.1–40.6) | 15.8 (0.2–45.8) | 12.4 (0.1–36.9) | 0.706 |
| Coexisting findings, n (%) | |||||
| Arch abnormality | 35 (8.5) | 3 (1.2) | 14 (12.0) | 18 (47.4) | 5.5*10−16 |
| Bicuspid pulmonary valve | 27 (6.5) | 16 (6.2) | 10 (8.5) | 1 (2.6) | 0.337 |
| LVOTO | 16 (3.9) | 1 (0.4) | 10 (8.5) | 5 (13.2) | 3.0*10−6 |
| Coronary anatomy,cn (%) | |||||
| Usual (1LCx-2R, 1L-2CxR, 1L-2R-noCx) | 341 (82.6) | 224 (86.8) | 94 (80.3) | 23 (60.5) | 3.1*10−4 |
| Other | 67 (16.4) | 31 (12.0) | 21 (17.9) | 15 (3.9) | |
| Unknown | 5 (1.2) | 3 (1.2) | 2 (1.7) | 0 (0) | |
| Intramural course of LAD | 12 (2.9) | 8 (3.1) | 4 (3.4) | 0 (0) | 0.812 |
| Preoperative procedures, n (%) | |||||
| Balloon atrial septostomy | 216 (52.3) | 151 (58.5) | 57 (48.7) | 8 (21.1) | 4.0*10−5 |
| Previous PAB | 30 (7.2) | 11 (4.3) | 15 (12.8) | 4 (10.5) | 0.007 |
| Arterial switch operation | |||||
| One stage, n (%) | 371 (89.8) | 246 (95.3) | 94 (80.3) | 31 (81.6) | 1.0*10−5 |
| Median age | 8 days | 7 days | 11 days | 23 days | 9.2*10−13 |
| (0 day–0.9 years) | (0 day–0.5 years) | (1 day–0.9 years) | (4 days–0.6 years) | ||
| Two stage, n (%) | 42 (10.2) | 12 (4.7) | 23 (19.7) | 7 (18.4) | 1.0*10−5 |
| Median age | 166 days | 107 days | 238 days | 286 days | 0.256 |
| (7 days–12.7 years) | (7 days–5.1 years) | (46 days–12.7 years) | (32 days–1.7 years) | ||
| Lecompte manoeuvre, n (%) | 376 (91.0) | 243 (94.2) | 108 (92.3) | 25 (65.8) | 2.1*10−7 |
Median age (range) post-ASO.
Median age (range) post-birth.
Coronary anatomy description according to the Leiden Convention.
P-values based on Fisher–Freeman–Halton exact test for frequencies and Kruskal–Wallis test for median values of continue data.
ASO: arterial switch operation; IVS: intact ventricular septum; L or LAD: left anterior descending coronary artery; LVOTO: left ventricular outflow tract obstruction; PAB: pulmonary artery banding; R: right coronary artery; TBA: Taussig-Bing anomaly; TGA: transposition of the great arteries; VSD: ventricular septal defect.
Late mortality
Twelve patients died late during follow-up (2.9%). Median age of death was 10.6 (IQR 0.9–21.9) years. TGA morphology in these patients was as follows: TGA-IVS in 3 (1.2%), TGA-VSD in 5 (4.3%) and TBA in 4 (10.5%) patients (P = 0.006). In 7 patients, the exact cause of death could not be retrieved retrospectively. Causes of mortality of the other patients were: recurrent Staphylococcus aureus endocarditis of a Bentall prosthesis with severe neurological complication (n = 1, age 27.9 years); lymphocytic myocarditis with sudden cardiac death (n = 1, age 22.8 years); acute cardiac failure after PA embolism from a calcified in-stent vegetation by catheter intervention (n = 1, age 19.0 years); cardiac failure after reoperation for right ventricular outflow tract (RVOT) obstruction (RVOTO) (n = 1, age 0.4 years); and mediastinitis after pacemaker implantation (n = 1, age 0.2 years). The overall survival rate of the ASO hospital survivors was 97.9% at 10 years, 97.3% at 20 years and 94.5% at 30 and 40 years; the survival curve was significantly different between TGA subgroups (P = 0.005) (Fig. 1).
Figure 1:
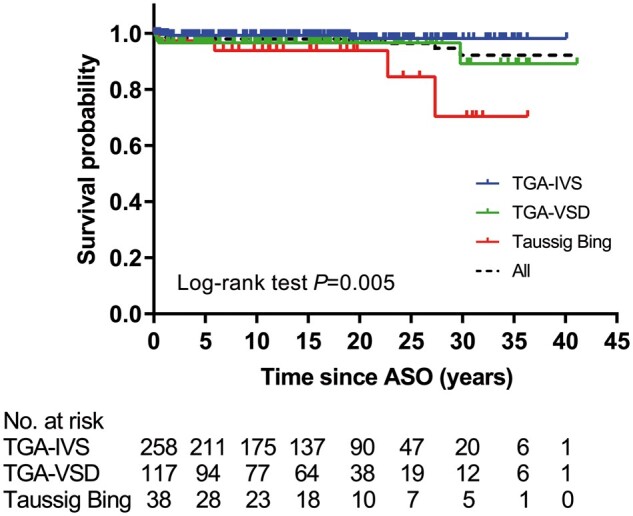
Kaplan–Meier estimates of overall survival of ASO hospital survivors by transposition of the great arteries morphology subgroups. ASO: arterial switch operation; TGA-IVS: TGA with intact ventricular septum; TGA-VSD: TGA with ventricular septal defect.
Arrhythmias, ablation and cardiac devices
Conduction disorders or late arrhythmias appeared in 26 patients post-ASO (6.3%). Details are presented in Supplementary Material, Table S1. In short, 8 patients had a complete atrioventricular block requiring pacemaker implantation (2 after reoperation). Eight patients presented with ventricular tachyarrhythmias at a median follow-up of 15.4 (IQR 12.3–18.5) years post-ASO. Supraventricular arrhythmias were diagnosed in 12 patients at a median age of 17.8 (IQR 8.1–24.1) years.
Reoperations
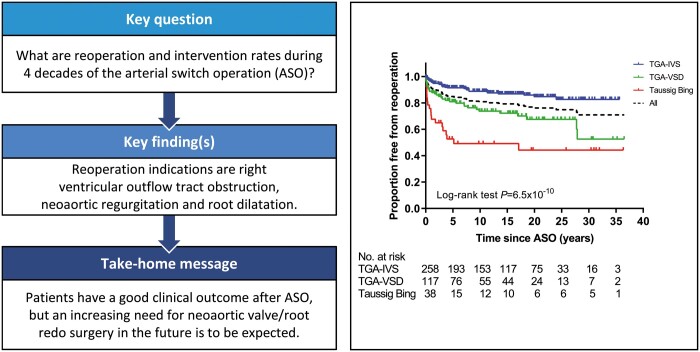
In total, 117 reoperations (137 procedures) were performed in 83 patients (20.1%) (Table 2). Twenty-two patients needed 2 or more reoperations (Fig. 2). Seven reoperations were performed within 30 days post-ASO (1.6%, 7/447 patients). Median age at reoperation was 2.0 (IQR 0.3–7.1) years post-ASO. Distribution of TGA morphology among patients who underwent reoperation was: TGA-IVS in 32 (32/258, 12.4%), TGA-VSD in 32 (32/117, 27.4%) and TBA in 19 (19/38, 50.0%) and reoperation-free survival differed significantly between TGA subgroups (P = 6.5*10−10) (Fig. 3A). Overall freedom from reoperation for the entire TGA population was 85% at 5 years, decreasing to 81%, 79%, 76%, 75%, 71% and 71% at 10, 15, 20, 25, 30 and 35 years after ASO, respectively (Fig. 3A).
Table 2:
Reoperation and catheter intervention procedures
| Reoperation (n = 83) | Number of procedures (%) 137 procedures 117 reoperations | Number of patients |
|---|---|---|
| RVOTO relief | 68 (49.6%) | 50 |
| Neoaortic valve and root surgery | 21 (15.3%) | 15 |
| Neoaortic valve plasty | 1 | |
| Neoaortic valve replacement | 3 | |
| Bentall operation | 12 | |
| David operation | 2 | |
| Switchback Ross | 1 | |
| Replacement ascending aorta | 2 | |
| Coronary revascularisation | 8 (5.8%) | 8 |
| Ostial plasty | 4 | |
| CABG | 4 | |
| Relief arch obstruction | 12 (8.8%) | 12 |
| LVOTO relief | 4 (2.9%) | 4 |
| Miscellaneousa | 24 (17.5%) | 22 |
| Catheter intervention (n = 54) | Number of procedures (%) 104 procedures 101 interventions | Number of patients |
|---|---|---|
| Relief supravalvular PS | 80 (76.9%) | 44 |
| Balloon angioplastyb | 27 | |
| MPA | 5 | |
| RPA | 8 | |
| LPA | 5 | |
| Bilateral PA | 9 | |
| Stent implantation | 39 | |
| MPA only | 1 | |
| RPA only | 13 | |
| LPA only | 11 | |
| Bilateral PA | 13 | |
| MPA + RPA | 1 | |
| Relief arch obstruction | 15 (14.4%) | 10 |
| Balloon angioplasty | 14 | |
| Stent implantation | 1 | |
| Coronary artery intervention | 2 (1.9%) | 2 |
| PTCA | 1 | |
| Stent implantation | 1 | |
| Neoaortic valve intervention | 1 (1.0%) | 1 |
| TAVI | 1 | |
| Closure shunts | 6 (5.8%) | 5 |
| Atrial septal defect | 5 | |
| Aorta-pulmonary collaterals | 1 |
AV-valve plasty (n = 11), closure residual shunt (ASD/VSD) (n = 5), relief supravalvular aortic stenosis (n = 1), relief MPA aneurysm (n = 3), relief pulmonary vein obstruction (n = 1), pulmonary artery endarterectomy (n = 1).
Dilatation of native/in-stent PA (branch) stenosis.
ASD/VSD: atrial septal defect/ventricular septal defect; CABG: coronary artery bypass grafting; LPA: left pulmonary artery; LVOTO: left ventricular outflow tract obstruction; MPA: main pulmonary artery; PA: pulmonary artery; PTCA: percutaneous transluminal coronary angioplasty; RPA: right pulmonary artery; RVOTO: right ventricular outflow tract obstruction; TAVI: transcatheter aortic valve implantation.
Figure 2:
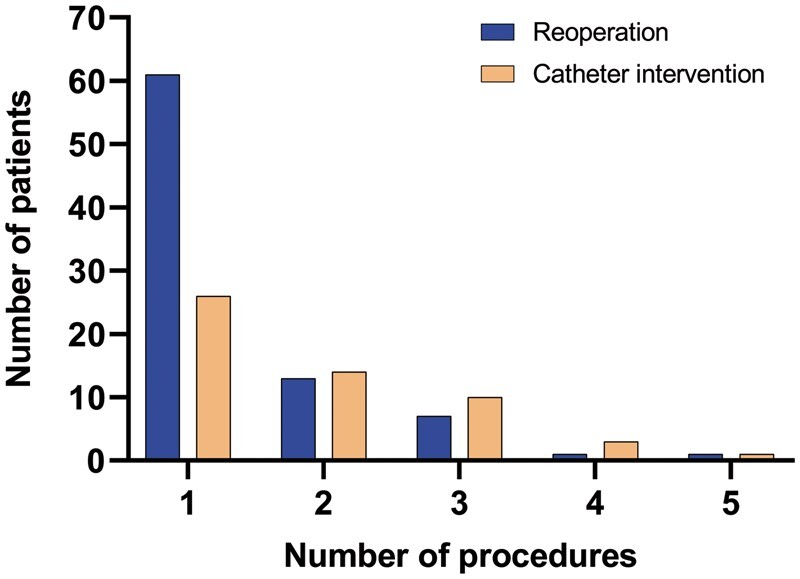
Histogram on the number of reoperations and catheter interventions.
Figure 3:
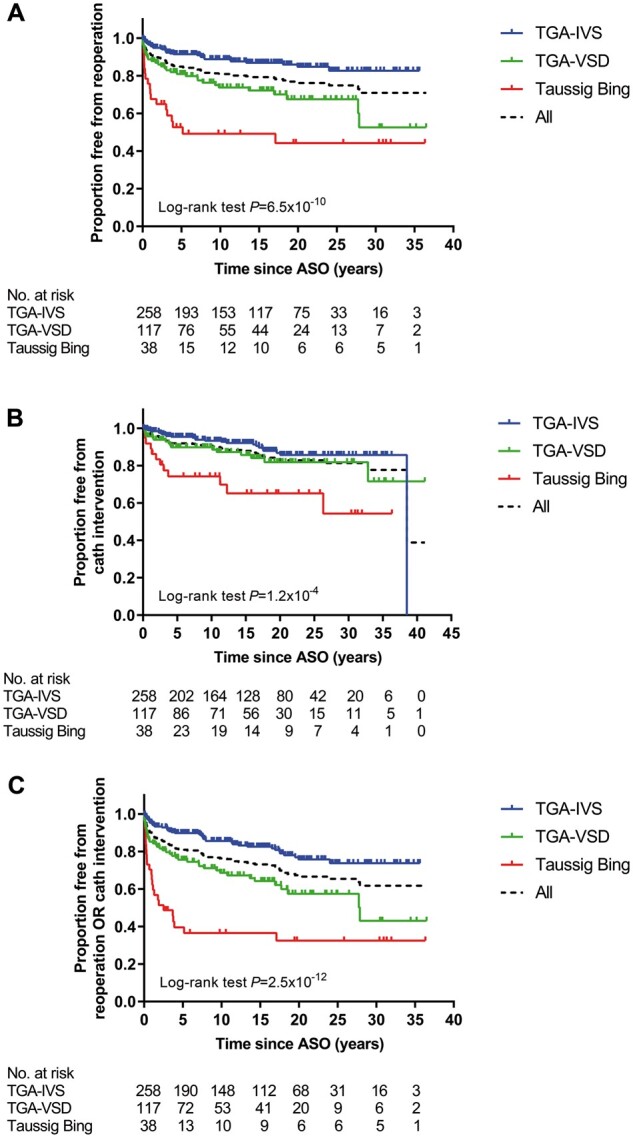
Kaplan–Meier estimates of freedom from reoperation (A), catheter intervention (B) and both reoperation and catheter intervention (C) by transposition of the great arteries morphology subgroups. ASO: arterial switch operation; TGA-IVS: TGA with intact ventricular septum; TGA-VSD: TGA with ventricular septal defect.
The most common indication for reoperation was RVOTO (n = 50, 68 procedures) (Table 2). A hybrid procedure with intraoperative stent implantation for relief of PA branch stenosis was performed in 7 of 50 patients. Median age at reoperation for RVOTO was 1.3 (IQR 0.3–4.5) years. Twenty-two of these patients had TGA-IVS (8.5%), 15 TGA-VSD (12.8%) and 13 TBA (34.2%) (P = 2.2*10−4).
Neoaortic valve, root or ascending aorta reoperations for neoaortic valve insufficiency and/or root dilatation were performed 15 patients (3.6%) and include 18% of all reoperations (21/117 reoperations). Median age at reoperation for these indications was 15.7 (IQR 11.4–20.0) years post-ASO. Ten patients underwent a Bentall operation, 2 patients had a valve-sparing root replacement, 1 patient had neoaortic valve repair followed by later valve replacement, 1 patient underwent replacement of the neoaortic root/ascending aorta by a supracoronary tubular prosthesis and in 1 patient, a switch back Ross operation was performed. Seven of these patients had TGA-IVS (2.7%), 5 TGA-VSD (4.3%) and 3 TBA (7.9%) (P = 0.193).
Aortic arch repair after ASO was necessary in 12 of the 83 reoperated patients (14.5%) and consisted of either reoperations for recoarctation (in 7 patients, after ASO with arch repair) or reoperations for neocoarctation (in 5 patients; i.e. aortic coarctation following ASO in patients who were not diagnosed with coarctation before or during initial ASO).
Reoperations for coronary artery problems were needed in 8 patients (1.9%) at median age of 11.5 (IQR 1.0–24.1) years. Patient-specific and surgical characteristics are summarized in Supplementary Material, Table S2. Number of coronary artery reoperations differed significantly between TGA subgroups: 2 patients had TGA-IVS (0.8%), 2 TGA-VSD (1.7%) and 4 TBA (10.5%) (P = 0.004). Usual coronary artery anatomy (1LCx-2R) was present in 4 patients (50%).
Era-specific impact on reoperation rate and common reoperation types could not be detected, except for aortic arch repair due to recoarctation (Supplementary Material, Figs S1–S3). Patients who have been operated on for TGA with arch obstruction in the era ‘1977–1989’ were more likely to require an aortic arch reoperation for recoarctation compared to patients with ASO and aortic arch repair performed in later eras (P = 0.005; Supplementary Material, Fig. S3).
Univariable Cox regression analysis showed following factors associated with reoperation for any indication: TGA morphological subtype (TGA-VSD and TBA), weight at ASO, aortic arch repair associated with ASO, not having had a Lecompte manoeuvre and a non-usual coronary artery anatomy. On multivariate analysis, TGA morphological subtype (TGA-VSD: HR = 1.99, 95% confidence interval (CI) 1.18–3.36; P = 0.010 and TBA: HR = 2.17, 95% CI 1.02–4.64; P = 0.045), aortic arch repair associated with ASO (HR = 3.03, 95% CI 1.62–5.69; P = 0.001) and a non-usual coronary artery anatomy (HR = 2.41, 95% CI 1.45–4.00; P = 0.001) were identified as independent risk factors for reoperation (Supplementary Material, Table S3).
Catheter interventions
In total, 101 interventions were performed in 54 patients (13.1%) (Table 2). Twenty-eight patients needed 2 or more interventions (Fig. 2). Median age at first catheter intervention was 3.7 (IQR 1.3–12.8) years. Distribution of TGA morphology among patients who underwent catheter interventions was: TGA-IVS in 25 (25/258, 9.7%) patients, TGA-VSD in 17 (17/117, 14.5%) patients, TBA in 12 (12/38, 31.6%) patients and reoperation-free survival differed significantly between TGA subgroups (P = 1.2*10−4) (Fig. 3B). Overall freedom from catheter intervention for the entire TGA population was 92% at 5 years, decreasing to 91%, 88%, 84%, 83%, 81% and 78% at 10, 15, 20, 25, 30 and 35 years after ASO, respectively (Fig. 3B).
The major indication for catheter intervention was relief of RVOTO (n = 44, 80 procedures). In 39 patients (9.4%), 1 or more stent implantation(s) in the PA system were performed to treat supravalvular pulmonary stenosis (including aforementioned intraoperative stent implantations in 7 patients). Distribution of TGA morphology among patients with stent implantations was: TGA-IVS in 21 (21/258, 8.1%) patients, TGA-VSD in 13 (13/117, 11.1%) patients and TBA in 5 (5/38, 13.2%) patients (P = 0.413). One patient received a transcatheter pulmonary valve implantation (Melody®) in neopulmonary valve position.
Balloon angioplasty for aortic recoarctation was performed in 10 patients. Catheter interventions for significant coronary artery stenosis were done in 3 patients. As summarized in Supplementary Material, Table S2, percutaneous transluminal coronary angioplasty was performed in 2 patients (prior to or after coronary revascularization); in 1 patient, a drug-eluding stent was implanted for left anterior descending coronary artery ostial stenosis. A transcatheter aortic valve implantation (TAVI) was performed in a single TBA patient (with monocoronary ostium) for recurrent severe neoaortic valve regurgitation, 1.5 year after a valve-sparing root replacement. Although originally developed for the treatment of aortic stenosis, TAVI has emerged as a viable alternative to surgical aortic valve replacement for patients with pure aortic regurgitation (AR) with a high-risk profile for surgery. Furthermore, this technique has proven to be technically feasible and successful for severe AR after David valve-sparing root replacement. With the large experience of TAVI for pure AR in our centre and the patient’s explicit wish to be treated via transcatheter procedure, TAVI was chosen over redo surgery.
Univariable Cox regression analysis showed following factors associated with catheter intervention: TGA morphological subtype (TBA), aortic arch repair associated with ASO and a non-usual coronary artery anatomy. On multivariable analysis, aortic arch repair associated with ASO (HR = 2.95, 95% CI 1.37–6.36; P = 0.006) was identified as independent risk factor for catheter intervention (Supplementary Material, Table S4).
Risk factors for any intervention—reoperations or catheter interventions
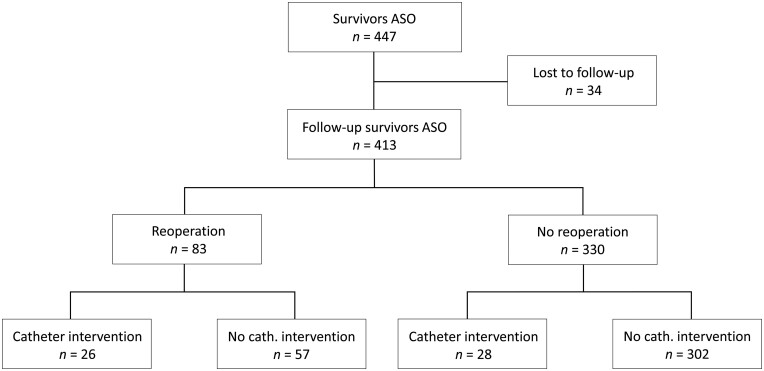
Of the 413 survivors with follow-up, 302 patients did not receive any reoperation or catheter intervention (73.1%) during follow-up (Fig. 4). One hundred and eleven patients (26.9%) underwent at least 1 reoperation or catheter intervention; 26 patients (6.3%) underwent at least both a reoperation and catheter intervention. Distribution of the 111 patients over the TGA subgroups was: TGA-IVS in 46 (17.8%), TGA-VSD in 41 (35.0%), TBA in 24 (63.2%) patients and the combined reoperation/catheter intervention-free survival differed significantly between TGA subgroups (P = 2.5*10−12) (Fig. 3C). Overall freedom from reoperation or catheter intervention for the entire TGA population was 81% at 5 years, decreasing to 76%, 73%, 65%, 63%, 61% and 61% at 10, 15, 20, 25, 30 and 35 years after ASO, respectively (Fig. 3C).
Figure 4:
Flow diagram of reoperations and catheter interventions in ASO hospital survivors. ASO: arterial switch operation.
Risk analysis for the need of any reoperation(s) or catheter intervention(s) is presented in Table 3. TGA morphological subtype (TGA-VSD and TBA), aortic arch repair associated with ASO, weight at ASO, not having had a Lecompte manoeuvre, prior PA banding and a non-usual coronary artery anatomy were univariable risk factors for the risk of reoperation or catheter intervention. Independent risk factors for either reoperation or catheter intervention for all indications were TGA morphological subtype (TGA-VSD: HR = 1.84, 95% CI 1.18–2.89; P = 0.008), aortic arch repair associated with ASO (HR = 3.72, 95% CI 2.13–6.50; P = 4.0*10−6) and a non-usual coronary artery anatomy (HR = 1.92, 95% CI 1.20–3.07; P = 0.007).
Table 3:
Univariable and multivariable Cox proportional hazard analysis for reoperation or catheter interventions
| Univariable |
Multivariable* |
||||||
|---|---|---|---|---|---|---|---|
| Risk factors | Events/total | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| Morphological subtype | 1.35*10−10 | 0.023 | |||||
| TGA-IVSa | 46/258 | 1 | 1 | ||||
| TGA-VSD | 41/117 | 2.30 | 1.51–3.51 | 1.06*10−4 | 1.84 | 1.18–2.89 | 0.008 |
| TBA | 24/38 | 5.33 | 3.25–8.76 | 3.91*10−11 | 1.92 | 0.95–3.91 | 0.071 |
| Gender | |||||||
| Maleb | 72/280 | 1 | 1 | ||||
| Female | 39/133 | 1.19 | 0.80–1.75 | 0.39 | 1.18 | 0.79–1.77 | 0.41 |
| Weight at ASO (per 1 kg) | 1.10 | 1.04–1.17 | 4.49*10−4 | 1.03 | 0.95–1.12 | 0.41 | |
| LVOTO | |||||||
| Noc | 106/397 | 1 | 1 | ||||
| Yes | 5/16 | 1.13 | 0.54–3.23 | 0.55 | 1.01 | 0.39–2.62 | 0.99 |
| Aortic arch repair | |||||||
| Nod | 84/378 | 1 | 1 | ||||
| Yes | 27/35 | 5.75 | 3.69–8.95 | 9.02*10−15 | 3.72 | 2.13–6.50 | 4.0*10−6 |
| Lecompte | |||||||
| Noe | 17/29 | 1 | 1 | ||||
| Yes | 93/384 | 0.29 | 0.17–0.48 | 3.00*10−6 | 0.74 | 0.38–1.43 | 0.37 |
| Prior PAB | |||||||
| Nof | 99/383 | 1 | 1 | ||||
| Yes | 12/30 | 2.00 | 1.10–3.65 | 0.023 | 1.24 | 0.59–2.61 | 0.57 |
| Coronary artery anatomy | |||||||
| Usual (1LCx-2R, 1L-2CxR, 1L-2R, noCx)g | 79/341 | 1 | 1 | ||||
| Other (non-usual) | 31/67 | 2.65 | 1.74–4.02 | 5.00*10−6 | 1.92 | 1.20–3.07 | 0.007 |
Reference categories of covariate:
morphological subtype ‘TGA-IVS’,
‘male sex’,
‘no left ventricular outflow tract obstruction’,
‘no aortic arch repair associated with ASO’,
‘no Lecompte manoeuvre’,
‘no pulmonary artery banding prior to ASO’,
‘usual coronary artery anatomy’. *Variables included for MV analysis: morphological subtype, sex, weight, aortic arch repair, pulmonary artery banding prior to ASO and coronary artery anatomy.
ASO: arterial switch operation; CI: confidence interval; L: left coronary artery; LVOTO: left ventricular outflow tract obstruction; PAB: pulmonary artery banding; R: right coronary artery; TBA: Taussig-Bing anomaly; TGA: transposition of the great arteries; TGA-IVS: TGA with intact ventricular septum; TGA-VSD: TGA with ventricular septal defect.
DISCUSSION
This study evaluated the long-term cardiac outcome in an ASO cohort with one of the longest reported follow-up and focused on late mortality, reoperations and catheter interventions, coronary problems and late arrhythmias. During 43-year experience of ASO, overall survival significantly improved over time, with a late mortality of 2.9% and a decline in hospital mortality to 3.3% for the past 2 decades. The need for 1 or more reoperations and 1 or more catheter interventions was 20% and 13%, respectively, and most reoperations and interventions were performed for RVOT problems. A more complex TGA morphology (TGA-VSD), aortic arch repair associated with ASO, and the presence of a non-usual coronary artery anatomy were found to be independent risk factors for the need for reoperation or intervention for any indication.
In 4 decades of ASO, a significant decrease in perioperative mortality to rates below 5% in high-volume cardiothoracic surgical centres has been achieved [2, 12, 13]. Although survival has greatly improved, recurrent and new lesions remain and require reoperations or catheter interventions. The reoperation rate in this study was 20% which is relatively high compared to other studies. These studies reported reoperation rates between 5% and 20%, but with shorter duration of follow-up [12–17]. Reoperation rates between studies may vary depending on the complexity of the included TGA patients (TGA-VSD and TBA included or not). Moreover, our early adoption of ASO (1977) has probably influenced the number of reinterventions. With longer follow-up, it is expected that more patients will need a reoperation or catheter intervention, confirmed by the 11% reoperation rate beyond childhood in a study of adult TGA patients post-ASO [17].
Right ventricular outflow tract procedures
RVOT problems are the most common indication for reoperations or catheter interventions and often occur in the first years after ASO, as already known from short- and intermediate-term follow-up studies [2–4]. Our data are in line with these reports, with a median age of 1.3 (IQR 0.3–4.5) years at the time of first RVOT reoperation. The overall surgical and percutaneous intervention rates for RVOTO varied from 5% to 28% between the different studies with at least 20-year follow-up [2–4, 14–16], which is also similar to the results of this study.
Approximately 75% of the reoperations and all catheter interventions for RVOTO were performed for supravalvular obstruction in this study. Anatomical and surgical components may play a role in the development of PA stenosis. Patients with concomitant aortic arch obstruction (often TBA patients) are at increased risk for RVOTO [18, 19]. Moreover, the surgical technique and the material used for PA reconstruction, application of Lecompte manoeuvre, extent of mobilization of the PAs, and the age at operation impact the risk of RVOTO [2–4].
Balloon angioplasty for supravalvular pulmonary stenosis is the least invasive option but usually has limited effect and stent implantation is eventually required in most cases, preferably performed at an older age. Although we have not seen complications of branch PA stenting after ASO (first procedures 1994), these procedures should be performed with restraint because severe complications such as coronary artery compression and especially aortic erosion and aorto-pulmonary fistulas have been reported [20].
Neoaortic reoperations
Twenty-one surgical procedures and 1 catheter intervention for neoaortic valve regurgitation and/or root dilatation were performed in 15 patients (3.6%), approximately 20% of all reoperations. It is the second most common indication for reoperation, as has been reported in other studies with long follow-up duration [12, 16]. The main indication for neoaortic root reoperation in this study was root dilatation with or without significant neoaortic regurgitation [6, 21]. In two-thirds of cases, surgery was performed in the last decade (2009–2020). Although the total amount of reoperations on the neoaortic valve/root is still low and comparable to other surgical centres [15, 16, 22], we expect that with longer follow-up this may increase. Longitudinal follow-up studies on the neoaortic valve function confirm this concern as root dilatation progresses over time and proved to be an independent risk factor for neoaortic valve impairment [5, 6]. Recently, we showed in a subset of this cohort that 14% of patients had developed root diameters ≥40mm, with moderate AR in about 25% of them and one-quarter of these patients being below 18 years of age [6].
Thresholds for root replacement in TGA patients after ASO are adapted from international guidelines on bicuspid aortic valve and connective-tissue disease-related aortopathy and are based on absolute diameters. No aortic dissections or ruptures have been reported after ASO to date. In our centre root replacement is indicated if the neoaortic root diameter reaches 55 mm according to the guidelines [23], and at smaller sizes if significant neoaortic valve regurgitation is present or if root diameter has rapidly progressed over time. A Bentall operation has been performed in most patients [6, 21], but valve-sparing operation should be considered whenever possible.
Coronary artery lesions
In our series, coronary artery reoperations or interventions for partial or total occlusion were required in only 9 patients (2.2%), suggesting that coronary problems do not frequently occur during follow-up so far. Moreover, late mortality was not related to acute coronary artery problems in the patients with identified causes of death. One patient had a near-fatal ventricular fibrillation due to ischaemia based on coronary ostial stenosis. These findings are in line with the results of a systematic review on sudden cardiac death due to coronary complications late after ASO (at least more than 5 years) [24]. From that review, sudden cardiac death was extremely rare with only a few possible cases and no proven cases of coronary artery-related sudden death. In our centre, we do not systematically perform coronary surveillance imaging during childhood which might have influenced numbers of coronary artery problems. In general, children with complex coronary morphology or those with intraoperative difficulties of coronary artery transfer receive coronary computed tomography angiography (CTA) in the first year after ASO. Furthermore, patients undergo exercise tests in childhood and all asymptomatic patients underwent a coronary CTA at least once in early adolescence [25].
The detection of coronary artery abnormalities in the patients who underwent intervention was based on symptoms, abnormal findings from echocardiography or an accidental finding by a surveillance CTA or catheter coronary angiography (all representing one-third of the causes of detection). Treatment for partial or total coronary occlusion was performed by either ostial plasty or by coronary artery bypass graft. Taussig-Bing anomaly patients had the highest prevalence of coronary artery reoperations in this study (4/38 patients, 10.5%). Remarkably, all coronary reoperations in this subgroup were performed during childhood, unlike most coronary procedures in the patients with other TGA subtypes. Given the small number of coronary reoperations, it is unclear whether TBA patients are at increased risk or whether this is related to the prevalent non-usual coronary artery pattern in TBA. Although abnormal coronary artery patterns have been reported as risk factor for early coronary artery obstruction [8, 9, 26], it is suggested that the acquired coronary anatomy after ASO may be more important as a risk factor for late coronary artery obstruction [27]. Correspondingly, half of the patients necessitating coronary reoperation from this study had a usual coronary artery pattern without problems during coronary artery transfer. Acquired abnormal coronary anatomy may result from the surgically created pattern after reimplantation and changes by growth, arterial distortion or root dilatation [25, 27].
Risk factors for reoperation, intervention or combined
Independent risk factors for reoperation in this study were: TGA morphological subtype (TGA-VSD), aortic arch repair associated with ASO and a non-usual coronary artery anatomy. More complex TGA anatomy have been reported to be a risk factor for reoperation in previous studies [12–14]. Not surprisingly, aortic arch repair associated with ASO was reported as independent risk factor for surgical or catheter reintervention. A higher rate of reinterventions was previously described in TGA patients with arch obstruction by our group [18] and in other large series as well [12, 19, 22], particularly indicated for right-sided obstructions next to residual arch obstruction. A non-usual coronary artery anatomy showed to be an independent risk factor for both reoperation and any reoperation or intervention for any indication in this study. Vargo et al. [28] hypothesized that this could be related to the fact that an abnormal coronary artery anatomy sometimes requires an unusual reconstructive technique to avoid compression of the coronary artery button. However, we were not able to prove this in our series. Differences in reoperation rate or type based on era of ASO could not be detected in this series, except for aortic arch reoperation due to recoarctation. Although these are relatively small numbers, patients operated on for TGA with arch obstruction in the early years after start of the ASO program (era ‘1977–1989’) were more likely to require an aortic arch reoperation for recoarctation compared to patients operated on in later eras.
Rhythm disorders
Arrhythmias requiring ablative therapy or device implantation are fairly uncommon after ASO in this study, which is in concordance with other studies [12, 14]. Most sustained ventricular arrhythmias are related to coronary ischaemia or post myocardial infarction necessitating preventive ventricular tachycardia ablation or implantable cardioverter-defibrillator implantation and, if present, relief of coronary artery obstruction. Supraventricular tachycardia episodes are usually more prevalent in the newborn period and directly after ASO and only very rarely occur later, as was also shown in this study. Pacemaker need for atrioventricular block was low and related to VSD closure (in TGA-VSD and TBA) from early surgical eras (before 2000).
Limitations
Limitations of this study are related to the retrospective design from a single centre. The prevalence of coronary artery stenosis or occlusion identified in this study may be underestimated because coronary surveillance imaging by CTA or coronary angiogram was not performed routinely in all cases.
CONCLUSION
Patients after ASO typically have good clinical outcomes in the long term but reoperations and interventions remain necessary in some patients. RVOT problems are the main indication for surgical and percutaneous interventions with neoaortic valve or root reoperations as second most common reoperation indication. More complex TGA anatomy (TGA-VSD and TBA), aortic arch repair associated with ASO and non-usual coronary artery anatomy were independent risk factors for any reoperations or interventions of any indication. Reoperations for neoaortic valve insufficiency and/or root dilatation are relatively low but revealed an increased incidence over the last decade. With the reported ongoing neoaortic root dilatation in adulthood, this observation is of major concern for the future, as this implicates an expected increased need for neoaortic valve or root redo surgery.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This work was supported by the Netherlands Heart Foundation grant 2014T087 to R.L.F.v.d.P.
Conflict of interest: none declared.
Author contributions
Roel L.F. van der Palen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Supervision; Validation; Writing—original draft; Writing—review & editing. Nico A. Blom: Conceptualization; Investigation; Methodology; Resources; Supervision; Writing—review & editing. Irene M. Kuipers: Investigation; Supervision; Validation; Writing—review & editing. Lukas A.J. Rammeloo: Investigation; Resources; Supervision; Validation; Writing—review & editing. Monique R.M. Jongbloed: Investigation; Resources; Supervision; Validation; Writing—review & editing. Thelma C. Konings: Investigation; Supervision; Validation; Writing—review & editing. Berto J. Bouma: Investigation; Resources; Supervision; Validation; Writing—review & editing. David R. Koolbergen: Investigation; Supervision; Validation; Writing—review & editing. Mark G. Hazekamp: Conceptualization; Investigation; Resources; Supervision; Validation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Filippo Rapetto and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- AR
Aortic regurgitation
- ASO
Arterial switch operation
- CI
Confidence interval
- CTA
Computed tomography angiography
- HR
Hazard ratio
- IQR
Interquartile range
- PA
Pulmonary artery
- RVOT
Right ventricular outflow tract
- RVOTO
Right ventricular outflow tract obstruction
- TAVI
Transcatheter aortic valve implantation
- TBA
Taussig-Bing anomaly
- TGA
Transposition of the great arteries
- TGA-IVS
TGA with intact ventricular septum
- TGA-VSD
TGA with ventricular septal defect
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
REFERENCES
- 1. Jegatheeswaran A, Devlin PJ, DeCampli WM, Welke KF, Williams WG, Blackstone EH. et al. Longitudinal functional health status in young adults with repaired dextro-transposition of the great arteries: a Congenital Heart Surgeons' Society study. J Thorac Cardiovasc Surg 2020;159:604–14.e3. [DOI] [PubMed] [Google Scholar]
- 2. Cleuziou J, Vitanova K, Pabst von Ohain J, Ono M, Tanase D, Burri M. et al. Incidence and risk factors for right ventricular outflow tract obstruction after the arterial switch operation. Thorac Cardiovasc Surg 2019;67:37–43. [DOI] [PubMed] [Google Scholar]
- 3. Delmo Walter EM, Miera O, Nasseri B, Huebler M, Alexi-Meskishvili V, Berger F. et al. Onset of pulmonary stenosis after arterial switch operation for transposition of great arteries with intact ventricular septum. HSR Proc Intensive Care Cardiovasc Anesth 2011;3:177–87. [PMC free article] [PubMed] [Google Scholar]
- 4. Nellis JR, Turek JW, Aldoss OT, Atkins DL, Ng BY.. Intervention for supravalvar pulmonary stenosis after the arterial switch operation. Ann Thorac Surg 2016;102:154–62. [DOI] [PubMed] [Google Scholar]
- 5. Shepard CW, Germanakis I, White MT, Powell AJ, Co-Vu J, Geva T.. Cardiovascular magnetic resonance findings late after the arterial switch operation. Circ Cardiovasc Imaging 2016;9:e004618. [DOI] [PubMed] [Google Scholar]
- 6. van der Palen RLF, van der Bom T, Dekker A, Tsonaka R, van Geloven N, Kuipers IM. et al. Progression of aortic root dilatation and aortic valve regurgitation after the arterial switch operation. Heart 2019;105:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasquali SK, Hasselblad V, Li JS, Kong DF, Sanders SP.. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: a meta-analysis. Circulation 2002;106:2575–80. [DOI] [PubMed] [Google Scholar]
- 8. Ou P, Khraiche D, Celermajer DS, Agnoletti G, Le Quan Sang KH, Thalabard JC. et al. Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. J Thorac Cardiovasc Surg 2013;145:1263–9. [DOI] [PubMed] [Google Scholar]
- 9. Angeli E, Formigari R, Pace Napoleone C, Oppido G, Ragni L, Picchio FM. et al. Long-term coronary artery outcome after arterial switch operation for transposition of the great arteries. Eur J Cardiothorac Surg 2010;38:714–20. [DOI] [PubMed] [Google Scholar]
- 10. Lalezari S, Bruggemans EF, Blom NA, Hazekamp MG.. Thirty-year experience with the arterial switch operation. Ann Thorac Surg 2011;92:973–9. [DOI] [PubMed] [Google Scholar]
- 11. Yacoub MH, Radley-Smith R.. Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax 1978;33:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khairy P, Clair M, Fernandes SM, Blume ED, Powell AJ, Newburger JW. et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 2013;127:331–9. [DOI] [PubMed] [Google Scholar]
- 13. Losay J, Touchot A, Serraf A, Litvinova A, Lambert V, Piot JD. et al. Late outcome after arterial switch operation for transposition of the great arteries. Circulation 2001;104:I121–6. [DOI] [PubMed] [Google Scholar]
- 14. Fricke TA, d'Udekem Y, Richardson M, Thuys C, Dronavalli M, Ramsay JM. et al. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg 2012;94:139–45. [DOI] [PubMed] [Google Scholar]
- 15. Oda S, Nakano T, Sugiura J, Fusazaki N, Ishikawa S, Kado H.. Twenty-eight years' experience of arterial switch operation for transposition of the great arteries in a single institution. Eur J Cardiothorac Surg 2012;42:674–9. [DOI] [PubMed] [Google Scholar]
- 16. Raju V, Burkhart HM, Durham LA 3rd, Eidem BW, Phillips SD, Li Z. et al. Reoperation after arterial switch: a 27-year experience. Ann Thorac Surg 2013;95:2105–12. [DOI] [PubMed] [Google Scholar]
- 17. Tobler D, Williams WG, Jegatheeswaran A, Van Arsdell GS, McCrindle BW, Greutmann M. et al. Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol 2010;56:58–64. [DOI] [PubMed] [Google Scholar]
- 18. Bokenkamp R, Aguilar E, van der Palen RL, Sojak V, Bruggemans EF, Hruda J. et al. Reoperation for right ventricular outflow tract obstruction after arterial switch operation for transposition of the great arteries and aortic arch obstruction. Eur J Cardiothorac Surg 2016;49:e91–6. [DOI] [PubMed] [Google Scholar]
- 19. Fricke TA, Donaldson S, Schneider JR, Menahem S, d'Udekem Y, Brizard CP. et al. Outcomes of the arterial switch operation in patients with aortic arch obstruction. J Thorac Cardiovasc Surg 2020;159:592–9. [DOI] [PubMed] [Google Scholar]
- 20. Vida VL, Biffanti R, Stellin G, Milanesi O.. Iatrogenic aortopulmonary fistula occurring after pulmonary artery balloon angioplasty: a word of caution. Pediatr Cardiol 2013;34:1267–8. [DOI] [PubMed] [Google Scholar]
- 21. Koolbergen DR, Manshanden JS, Yazdanbakhsh AP, Bouma BJ, Blom NA, de Mol BA. et al. Reoperation for neoaortic root pathology after the arterial switch operation. Eur J Cardiothorac Surg 2014;46:474–9. [DOI] [PubMed] [Google Scholar]
- 22. Baruteau AE, Vergnat M, Kalfa D, Delpey JG, Ly M, Capderou A. et al. Long-term outcomes of the arterial switch operation for transposition of the great arteries and ventricular septal defect and/or aortic arch obstruction. Interact CardioVasc Thorac Surg 2016;23:240–6. [DOI] [PubMed] [Google Scholar]
- 23. Baumgartner H, Backer Babu-Narayan Sv DJ, Budts W, Chessa M, Diller GP. et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2020; doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 24. van Wijk SWH, van der Stelt F, Ter Heide H, Schoof PH, Doevendans P, Meijboom FJ. et al. Sudden death due to coronary artery lesions long-term after the arterial switch operation: a systematic review. Can J Cardiol 2017;33:1180–7. [DOI] [PubMed] [Google Scholar]
- 25. Veltman CE, Beeres SL, Kalkman DN, Kelder TP, Kies P, Vliegen HW. et al. Variation in coronary anatomy in adult patients late after arterial switch operation: a computed tomography coronary angiography study. Ann Thorac Surg 2013;96:1390–7. [DOI] [PubMed] [Google Scholar]
- 26. Pretre R, Tamisier D, Bonhoeffer P, Mauriat P, Pouard P, Sidi D. et al. Results of the arterial switch operation in neonates with transposed great arteries. Lancet 2001;357:1826–30. [DOI] [PubMed] [Google Scholar]
- 27. Michalak KW, Sobczak-Budlewska K, Moll JJ, Szymczyk K, Moll JA, Niwald M. et al. Can we predict potentially dangerous coronary patterns in patients with transposition of the great arteries after an arterial switch operation? Cardiol Young 2019;29:1350–5. [DOI] [PubMed] [Google Scholar]
- 28. Vargo P, Mavroudis C, Stewart RD, Backer CL.. Late complications following the arterial switch operation. World J Pediatr Congenit Heart Surg 2011;2:37–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.