Abstract
Rationale and Objectives:
The present study characterized the behavioral pharmacology of a novel, mixed-action delta-selective (78:1) opioid receptor agonist, BBI-11008. This glycopeptide drug candidate was tested in assays assessing antinociception (acute, inflammatory, and neuropathic pain-like conditions), and side-effect endpoints (respiratory depression and drug self-administration).
Results:
BBI-11008 had a 78-fold greater affinity for the delta opioid receptor than the mu receptor and there was no binding to the kappa opioid receptor. BBI-11008 (3.2 – 100; 10 – 32 mg/kg, i.v.) and morphine (1 – 10; 1 – 3.2 mg/kg, i.v.) produced antinociceptive and anti-allodynic effects in assays of acute thermal nociception and complete Freund’s adjuvant (CFA)-induced inflammatory pain, with BBI-11008 being less potent than morphine in both assays. BBI-11008 (1 – 18 mg/kg, i.v.) had similar efficacy to gabapentin (10 – 56 mg/kg, i.v.) in a spinal nerve ligation (SNL) model of neuropathic pain. In the respiration assay, with increasing %CO2 exposure, BBI-11008 produced an initial increase (32 mg/kg, s.c.) and then decrease (56 mg/kg, s.c.) in minute volume (MV) whereas morphine (3.2 – 32 mg/kg, s.c.) produced dose-dependent decreases in MV. In the drug self-administration procedure, BBI-11008 did not maintain self-administration at any dose tested.
Conclusions:
These results suggest that the glycopeptide drug candidate possesses broad-spectrum antinociceptive and anti-allodynic activity across a range of pain-like conditions. Relative to morphine or fentanyl, the profile for BBI-11008 in the respiration and drug self-administration assays suggests that BBI-11008 may have less pronounced deleterious side effects. Continued assessment of this compound is warranted.
Keywords: Antinociception, Antiallodynia, Respiratory depression, GI transit, Drug self-administration, Delta opioid receptor, Delta/mu opioid agonist, Glycopeptide, Rats, Mice
1. Introduction
Chronic pain remains a major public health concern and represents a spectrum of clinical conditions that are challenging to diagnose and treat (IOM 2011). Standard opioid, steroid, or NSAID drugs used to treat chronic pain show undesirable side effects, and inconsistent analgesic efficacy, and it is clear that efficacious and safer drugs are still needed (Antman 2017; Godfrey 1996; Kaye et al. 2017; Krashin et al. 2015). Strategies for drug discovery in the pain field include the elucidation of novel non-opioid targets as well as development of effective but safer mixed-action opioid analgesics that target both mu and delta receptors (Fischer 2011; Yekkirala et al. 2017). One particular method of improved opioid drug design has been the synthesis of bivalent compounds that bind to and activate or block both mu and delta opioid receptors. This particular mixed structure activity has resulted in lead candidates with maintained or enhanced analgesic efficacy and attenuated side effects relative to typical prescription mu opioid analgesics (Anand et al. 2016; Bilsky et al. 2000; Elmagbari et al. 2004; Mosberg et al. 2014; Schiller et al. 1995; Stevenson et al. 2015).
Our laboratories and others have synthesized and tested novel mixed-action delta/mu opioid agonists, in an effort to develop effective yet safer opioid analgesics (Li et al. 2012; Schiller et al. 1995; Yamamoto et al. 2007). As an example of this, we have reported that the mixed-action delta/mu opioid MMP-2200 has antinociceptive efficacy, with attenuated side effects, including abuse liability and tolerance/dependence, relative to standard mu receptor prescription opioids (Lowery et al. 2011; Stevenson et al. 2015). In a continued effort to improve upon mixed action ligands, a novel series of compounds was recently developed with greater activity at delta versus mu receptors. Here we report on the lead candidate BBI-11008 (amino acid sequence: [H]-YaFE-Nle-Nle-T(b-Glu)-[NH2]) for analgesic efficacy in assays of acute, inflammatory and neuropathic pain, as well as receptor mediation, respiratory depression, and drug self-administration. BBI-11008 is a bivalent compound based on the dermorphins and deltorphins. Glycosylation of these compounds results in enhanced penetration of the blood-brain barrier (Erspamer et al. 1989; Heck et al. 1996; Kreil et al. 1989; Melchiorri et al. 1996).
To determine the capacity for broad spectrum analgesic efficacy, the delta-selective glycopeptide BBI-11008 was tested in an assay of acute thermal nociception, as well as complete Freund’s adjuvant (CFA)-induced inflammatory (Taurog et al. 1988), and spinal nerve ligation (SNL)-induced neuropathic-like pain models (Colburn et al. 1999). Delta vs. mu receptor mediation, and central vs. peripheral activity for BBI-11008 were determined in the acute thermal nociception assay. In order to characterize opioid side effects, BBI-11008 was also assessed for efficacy to depress respiration and maintain drug self-administration. BBI-11008 was initially tested by the i.v. route of administration to determine potency and efficacy, followed by p.o. and s.c. routes, in order to assess systemic bioavailability. The analgesic and respiratory effects of BBI-11008 were compared to the standard prescription opioid agonist morphine, and for the SNL procedure, BBI-11008 was compared to the voltage-gated calcium channel α2 subunit modulator, gabapentin (Taylor 2009). In the self-administration procedure BBI-11008 was compared to the selective mu opioid agonist, and training drug fentanyl.
Materials and Methods
Subjects.
Male CD-1 mice (25–40g, Charles River Laboratories) were used for all acute thermal nociception and CFA experiments, and male Sprague Dawley rats (250–350g, Charles River Laboratories) were used for all SNL, respiration, and drug self-administration experiments. Mice were housed in groups of n=4, and rats in groups of n=2–3, in the University of New England Animal Care Facility. All animals received food and water available ad libitum and were maintained in a temperature and humidity controlled colony on a 12-h light/dark cycle (lights on at 0700 and off at 1900). Animals were acclimated in the animal facility for at least 5 days prior to use. All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and procedures were approved by the University of New England Institutional Animal Care and Use Committee (IACUC). The health of the animals was assessed daily by laboratory technicians and animal care staff.
Opioid Receptor Binding Studies.
Receptor binding studies were performed by radioligand displacement studies following protocols previously published (Lowery et al. 2011). Chinese hamster ovary (CHO) cells stably transfected with the human delta opioid receptor (DOP), mu opioid receptor (MOP), or kappa opioid receptor (KOP) were obtained from Drs. Larry Toll (SRI International, Palo Alto, CA USA) and George Uhl (NIDA Intramural Program, Bethesda, MD USA). The cells were grown in 100 mm dishes in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% fetal bovine serum and penicillin-streptomycin (10,000 units/mL) at 37°C in a 5% CO2 atmosphere. CHO cells, expressing either the human MOP, DOP, KOP were incubated with 12 different concentrations of each drug to be assayed, and the radiolabeled ligand in 50 mM Tris-HCL, pH 7.5, at a final volume of 1 ml. Nonspecific binding was measured by inclusion of 10 μM naloxone. Data were taken as the mean Ki values ± S.E.M. from three separate experiments performed in triplicate. Incubation times of 60 min were used for the MOP-selective peptide [3H]DAMGO and the KOP-selective ligand [3H]U69,593. A 3-hour incubation time was used for the DOP-selective antagonist [3H]naltrindole. The IC50 values for the glycopeptides were determined using final concentrations of [3H]DAMGO, [3H]naltrindole and [3H]U69,593 or 0.25 nM, 0.2nM and 1 nM, respectively. Nonspecific binding was measured by the use of 10 μM naloxone to block all opioid binding. The Ki values of unlabeled compounds were calculated from the equation Ki = IC50/(1+S), where S = (concentration of radioligand) / (KD of radioligand) (Cheng and Prusoff 1973).
Assay of Thermal Nociception.
Antinociception was assessed using a 55°C warm-water tail-withdrawal test. The latency to the first sign of a rapid tail withdrawal was defined as the behavioral endpoint (Janssen et al. 1963). Initial baseline latency was characterized by tail immersion in the warm water and recording latency to tail withdrawal. Mice not responding within 5 sec were excluded from further testing. Mice (n = 9 per group; 1 cohort per dose) were administered either saline, or a single dose of BBI-11008 (3.2–100mg/kg i.v.) or morphine (1–10mg/kg i.v.) and tested for antinociception at time points, 10, 20, 30, 45, 60, 90, 120, 150 and 180 min post drug administration. Antinociception was calculated using the following formula:
To avoid tissue damage, if a subject did not withdraw its tail within 10 sec, the tail was removed from the water by the experimenter, and a latency of 10 sec was recorded for that measurement.
Antagonist Studies.
To determine opioid receptor mediation for the antinociceptive effects of BBI-11008 in the thermal tail withdrawal assay, mice (n = 5 – 9 per group; 1 cohort for each agonist + antagonist combination) were pretreated with the following antagonists: the nonselective opioid antagonist naloxone (1 mg/kg, s.c.), the peripherally selective opioid antagonist naloxone-methiodide (3.2 mg/kg, s.c.), the mu-selective antagonist β-funaltrexamine (β-FNA) (19 nmol, i.c.v.), and the delta-selective antagonist naltrindole (10 mg/kg s.c.). Doses and pre-treatment times were based on previous experiments from our laboratories (Lowery et al., 2011). For all pretreatment studies, BBI was injected at t=0 and mice were tested in the tail withdrawal assay at the 20 min mark. To determine central vs. peripheral opioid receptor mediation, the opioid antagonist naloxone HCL (1mg/kg, i.p., −10 min,) and the perpipherally restricted opioid antagonist naloxone-methiodide (3mg/kg, i.p., −10 min) were delivered separately prior to BBI-11008. To determine the degree of MOP mediation, the highly selective mu opioid antagonist β-FNA (19nmol, i.c.v., −24 hr) was delivered prior to BBI-11008. The highly selective, high-efficacy mu agonist LYM-100 (1mg/kg, i.v.) was administered alone following administration of β-FNA (−24hr), as a positive control. To determine the degree of DOP mediation, the selective delta opioid antagonist naltrindole (10mg/kg, s.c., −10min) was delivered prior to BBI-11008. The highly selective, high-efficacy DOP agonist DPDPE (30nmol, i.c.v.) was administered alone and administered following naltrindole (10mg/kg, s.c., −10 min), as a positive control.
CFA Model.
Inflammation was induced by administration of 20μl CFA (EMD Chemicals, 0.1% dry Mycobacterium butyricum dissolved in 85% Drakeol 5NF and 15% Arlacel A), using a 50μl luer tip glass syringe (Hamilton) with a 30 gauge ½ inch beveled needle, into the subcutaneous space of the plantar surface of the left hind paw in the center of the pads following light anesthesia. Mice were anesthetized with 3% isoflurane at a flow rate of 0.8–1 L/min with oxygen, for approximately 60–90 sec, until a light depth of anesthesia was attained.
Assay of CFA-induced Tactile Allodynia.
To assess tactile allodynia, mice (n=7 per group; 1 cohort per drug dose) were individually placed into Plexiglas chambers (16 chambers at: 4”×3”×3”, Marine Ecological Habitats) with a wire mesh bottom. This structure was suspended on a PVC pipe frame (designed and built by author E.J.B). Mice were allowed to acclimate for 30–60 min, or until exploratory and grooming behavior declined to a level compatible with behavioral testing. A series of monofilaments were applied to the mid-plantar left hind paw (ipsilateral side of CFA injection) that ranged in stiffness from 0.04 to 4 g (0.04, 0.07, 0.16, 0.40, 1, 2, 4). Filaments were applied once for 5 sec with inter-stimulus intervals of 1 min. Mice were tested using the up-down method (Chaplan et al. 1994). For example, mice were first tested with the 0.40 g monofilament. A positive response was operationally defined as a rapid withdrawal of the left hind paw or licking of the paw. If the 0.40 g monofilament did not elicit a positive response, the next highest filament in the sequence was tested until the mouse showed a positive response. If the 0.40 g monofilament did elicit a response, the next lowest filament was used until the mouse stopped emitting a positive response. All von Frey experiments were conducted 24 hr after CFA administration with a CFA baseline reading taken prior to drug administration. A decrease in von Frey threshold following CFA relative to control latencies indicated an allodynia-like response. Either BBI-11008, morphine, or saline was administered after the 24 hr post-CFA administration baseline. Immediately after drug administration, animals were returned to their assigned Plexiglas test chambers and a complete time course for each drug dose was characterized at times 15, 30, 45, 60, 90, and 120 min (or until the test latency approached post-CFA baseline latency) post drug administration.
Neuropathic Pain Model.
Rats were anesthetized in an induction chamber with 3–5% isoflurane at a flow rate of 0.8–1L/min with oxygen, for approximately 60–90 sec. Once anesthetized, rats were shaved with hair clippers from the dorsal pelvic area to shoulder blades and placed on a nosecone with 1.5–2% isoflurane. A surgical scrub of alcohol and betadine was applied to the shaven area. Using the level of the posterior iliac crest as midpoint, a 4 cm incision was made in the dorsal midline using a #10 scalpel blade. A midsacral incision was then made and slid approx. 1.5–2 cm along the left side of the spinal wall. Muscle and ligaments were then blunt dissected and retracted, exposing a portion of the spine down to the level of the transverse process. The transverse process of the left L6 vertebra was then carefully nipped off using bone rongeurs (Fine Science Tools #16015–17) to expose the L4/L5 spinal nerves. The L5 nerve was slightly elevated and separated from the L4 nerve using a small custom glass hook (“Chung rod”). The L5 nerve was then ligated using a 4–0 silk suture that was maneuvered around the nerve using a slip-knot that secured the suture on the glass hook. The L6 nerve was hooked from under the medial edge of the sacrum and gently lifted and ligated in the same manner as the previous L5 ligation. Damaged tissue was debrided and fascia and muscle were sutured with 3–0 Vicryl suture. The skin was closed using wound clips and topical triple antibiotic was applied to the wound. The rat also received 100 μl i.p. injection of Gentamicin at 10 mg/ml and was allowed to recover from anesthesia before being placed into a new single housing container where it was allowed to recover for 7 days.
SNL-induced tactile allodynia.
To assess tactile allodynia, rats (n=8 per group; 1 cohort per drug dose) were individually placed into Plexiglas chambers (6 chambers at: 10”×4.5”×6”, Marine Ecological Habitats, Biddeford, ME) with a wire mesh bottom. This structure was suspended on a PVC pipe frame designed and built in house (E.J.B). Rats were allowed to acclimate for 30–60 min, or until exploratory and grooming behavior declined to a level compatible with behavioral testing. A series of monofilaments were applied to the mid-plantar left hind paw (ipsilateral side of CFA injection) that ranged in stiffness from 0.40 to 15 g (0.40, 0.60, 1, 2, 4, 6, 8, 15). Filaments were applied once for 5 sec with inter-stimulus intervals of 1 min. Rats were tested using the up-down method (Chaplan et al. 1994). Specifically, rats were first tested with the 2 g monofilament. A positive response was operationally defined as a rapid withdrawal of the left hind paw or licking of the paw. If the 2 g monofilament did not elicit a positive response, the next highest filament in the sequence was tested until the mouse showed a positive response. If the 2 g monofilament did elicit a response, the next lowest filament was used until the rat stopped emitting a positive response. All von Frey experiments were conducted 7 days after SNL surgery with a new baseline reading taken prior to drug administration. Drug or saline was administered after the 7-day post-SNL baseline. Immediately after drug administration, animals were returned to their assigned Plexiglas test chambers and a complete time course for each drug dose was characterized at times 15, 30, 45, 60, 90, 120, 150, and 180 min (or until the test latency approached post-SNL baseline latency) post drug administration.
Respiration Studies
Apparatus and Procedure.
Whole body plethysmography equipment consisted of a Buxco Bias Flow Regulator, Buxco Gas Analyzer, and Buxco Max II. Rats were initially placed in the respiration chambers for 30 min to allow for habituation. Following the 30-min habituation, rats (n=6 per group; 1 cohort per drug dose) were injected with either saline (s.c.), morphine (3.2 – 32 mg/kg, s.c.), or BBI-11008 (32 – 56 mg/kg, s.c.). Total session duration was 115 minutes. Respiratory parameters recorded included respiratory frequency (breaths per minute = fR), tidal volume (volume inhaled = VT), and minute volume (MV = fR × VT). Minute volume (MV) = the rate of ventilation and represents the amount of gas exhaled by each rat, per minute. CO2 was raised to 4% at 75 minutes, 6% at 90 minutes, and 8% at 105 minutes, with 5 min CO2 purge immediately prior to each increase (eg: CO2 purge at 70 – 75 min; 85 – 90 min; 100 – 105 min).
Respiration data were averaged over 50 breaths and stored on the computer for analysis. The slope of the hypercapnic ventilatory response was determined from the slope of the relationship between V and the four levels of inspired carbon dioxide using least-squares regression analysis.
Drug Self-Administration.
All studies were conducted in drug self-administration operant conditioning chambers (Med Associates, model MED-008-CT-B1) placed within sound-attenuating cubicles equipped with a house light and exhaust fan. Each chamber contained two response levers situated on the front wall of the chamber. A shallow steel cup situated between the two levers, and just above the floor, contained a reservoir for consumption of food pellets. A pellet dispenser delivered 45 mg food pellets (see food training). Stimulus lights were situated above each response lever and were programmed to signal the availability of drug or food. A drug infusion pump was mounted outside each individual chamber to deliver intravenous drug via Tygon tubing. A complete swivel system with tether (Camcaths, Cambridgeshire, GB) was mounted inside each chamber to allow for unconstrained movement of the animal.
Food training.
Lever pressing was initially shaped during daily training sessions (30min day 1; 15 min days 2–8). Food training involved reinforcement of successive approximations of lever-press behavior with delivery of a food pellet. Once shaping was complete, 45 mg food pellets (Noyes brand) were available under a Fixed Ratio (FR) 1 schedule of reinforcement. Illumination of the stimulus light above the active lever served as a discriminative stimulus that the response-food delivery contingency was in place. Responding on the other inactive lever was counted but had no programmed consequences (animals were counterbalanced such that the left lever was active for half the rats and the right lever was active for the other half). A maximum of 50 food reinforcers was available during each daily training session. Once responding stabilized under the FR1 schedule (three consecutive days in which response rates varied by no more than 20% and at least 80% of responses emitted on the active lever), the FR requirement was raised to FR5 over the course of 3–6 sessions. After responding stabilized under the FR5 schedule, using the same criteria as above, rats underwent surgery for implantation of an i.v. catheter.
Surgery.
Rats underwent surgery using aseptic techniques. In brief, animals were sedated with a 10-minute pretreatment of midazolam (5mg/ml i.p.) and then anesthetized with an isoflurane/oxygen vapor mixture and implanted with a chronic indwelling i.v. catheter into the right external jugular vein. The surgical procedure was based on methods described elsewhere (Thomsen and Caine 2005). A single dose of the analgesic non-steroidal-anti-inflammatory drug ketoprofen (5 mg/kg s.c.) as well as the antibiotic amikacin (10 mg/kg s.c.) was administered immediately prior to surgery. Following surgery, animals were allowed to recover for seven days before training was resumed.
Drug self-administration training.
After 7 days recovery from surgery, the high efficacy MOP agonist fentanyl was available as the reinforcer (training drug) during three-hour sessions five days/week. Sessions began with a noncontingent “priming” infusion of the available drug dose in a 56μl volume. Responding on the initial food-trained lever was reinforced under a FR1/time out 20 sec schedule with an i.v. infusion of fentanyl (0.0032 mg/kg/infusion). Fentanyl was available until baseline criteria were met (three consecutive sessions with a minimum of 15 reinforcers earned, no more than 20% variation in number of reinforcers earned between three sessions, at least 80% responses on the active lever). Once responding stabilized under the FR1 schedule, the response requirement was raised to FR5 over the course of 4–7 sessions. Following stable fentanyl self-administration under the FR5 schedule, saline substitution was initiated for 1–3 sessions until responding decreased to at least 50% of fentanyl-maintained baseline rates. Baseline fentanyl responding was then re-established followed by determination of a full dose-effect function (see below) using a within-subjects design.
Drug testing.
All test sessions began with a non-contingent “priming” infusion of the available drug dose. Test sessions were conducted no more than twice each week and were separated by at least 48 hr. On Mondays, Wednesdays and Fridays, the solution available for self-administration was either saline or the training dose of fentanyl (0.0032 mg/kg/infusion), and substitution doses were tested on Tuesdays and Thursdays. Following determination of the fentanyl dose-effect function (0.00032 – 0.01 mg/kg/infusion), a range of doses of the mixed action delta/mu opioid agonist BBI-11008 (0.032 – 3.2 mg/kg/infusion) was tested (n=6 per group; single cohort). The primary dependent variable for drug self-administration was number of drug infusions. Statistical analysis was accomplished with one-factor ANOVA. A significant ANOVA was followed by the Duncan post hoc test. Significance was set a priori at p ≤ 0.05.
Drugs.
BBI-11008 and LYM 100 were synthesized in the Polt laboratory. Morphine sulfate was generously provided by Mallinckrodt (St. Louis, MO). Naloxone, β-funaltrexamine (β-FNA), naltrindole (NTI), and fentanyl were generously provided by National Institute on Drug Abuse, Drug Supply Program. Naloxone-methiodide, [D-Pen2,D-Pen5]enkephalin (DPDPE), and gabapentin were purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in sterile saline (0.9% NaCl). Midazolam, ketoprofen, gentamycin and amikacin were generously donated by Dr. S. Barak Caine. All radiolabels were obtained from PerkinElmer (Waltham, MA).
Results
Structure and binding affinity of BBI-11008.
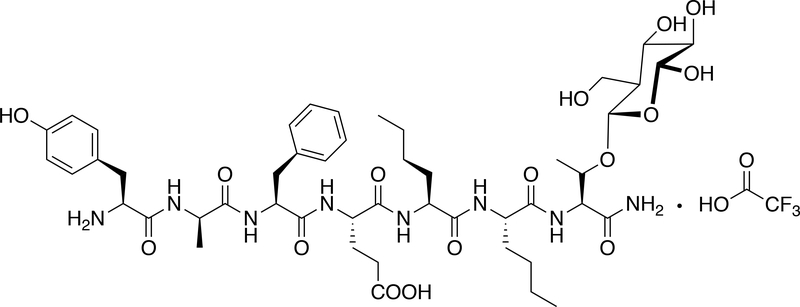
Figure 1 shows the structure of BBI-11008, and Figure 2 shows the binding affinities of BBI-11008 at DOP, MOP, and KOP. BBI-11008 displayed preferential binding to DOP over MOP and KOP, with approximately 78-fold higher affinity for DOP vs. MOP, and no apparent binding to KOP at concentrations up to 10 μM. Kd values were as follows: [3H]Naltrindole = 0.096 nM (DOP), [3H]DAMGO = 0.556 nM (MOP), [3H]U69,593 = 0.344 nM (KOP). Specific Activity were as follows: [3H]Naltrindole = 20 Ci/mmol (DOP), [3H]DAMGO = 59 Ci/mmol (MOP), [3H]U69,593 = 57 Ci/mmol (KOP).
Fig. 1.
Structure of BBI-11008
Fig. 2.
Binding affinity of BBI-11008. Membranes from CHO cells stably expressing either the DOP, MOP, or KOP were incubated with 12 different concentrations of BBI-11008 and 0.2nM [3H]naltrindole (DOP), 0.25 nM [3H]DAMGO (MOP), or 1 nM [3H]U69,593 (KOP) as described in Materials and Methods. Data are the mean values ±SEM from three independent experiments performed in triplicate.
Effects of BBI-11008 and morphine on acute thermal nociception.
Figure 3 shows the effects of BBI-11008 (3.2 – 100 mg/kg, i.v.) and morphine (1–10 mg/kg, i.v.) in the thermal tail withdrawal assay in mice. Both compounds produced dose- and time-dependent thermal antinociception. Within the dose ranges tested, peak antinociception for BBI-11008 occurred for ~30 min, whereas peak antinociception for morphine occurred for ~ 60 min, with overall duration of effect lasting ~90 min and ~120 min, respectively. Duration of effect for peak doses was quantified by Area under the Curve and BBI-11008 100 mg/kg (AUC = 5936) was ~54% of morphine 10 mg/kg (AUC = 10855). For BBI-11008, there were significant main effects of Time [F(5,145) = 114.82, p < 0.0001] and Dose [F(3,145) = 8.98, p = 0.0002] and a significant Interaction [F(15,145) = 3.88, p < 0.0001]. For morphine, there were significant main effects of Time [F(6,138) = 155.40, p < 0.0001] and Dose [F(2,138) = 134.42, p < 0.0001] and a significant Interaction [F(12,138) = 22.95, p < 0.0001].
Fig. 3.
Effects of BBI-11008 and morphine in a warm water (50˚) tail withdrawal assay in mice. Abscissae: Time in minutes. Ordinates: Mean percent antinociception. Data represent mean ± S.E.M., n = 8 – 10 mice/dose group. Left Panel: **, *** = significantly different from 3.2 mg/kg at p < 0.01, 0.001, respectively. Significant main effects of Time [F(5,145) = 114.82, p < 0.0001] and Dose [F(3,145) = 8.98, p = 0.0002] and a significant Interaction [F(15,145) = 3.88, p < 0.0001]. Right Panel: **, *** = significantly different from 1 mg/kg at p < 0.01, 0.001, respectively. Significant main effects of Time [F(6,138) = 155.40, p < 0.0001] and Dose [F(2,138) = 134.42, p < 0.0001] and a significant Interaction [F(12,138) = 22.95, p < 0.0001].
Antagonism studies.
Figure 4 shows antagonism studies in the tail withdrawal assay in mice. BBI-11008 alone produced ~97% maximum antinociception. The left-most panel shows central vs. peripheral receptor mediation of BBI-11008. The opioid antagonist naloxone (1mg/kg) blocked BBI-induced thermal antinociception [F(2,18) = 101.7, p < 0.001]. In contrast, the peripherally restricted opioid antagonist naloxone methiodide (3mg/kg) was ineffective in blocking BBI-11008 effects. The middle panel shows mu receptor mediation. The selective mu opioid antagonist β-FNA (0.1 mg/kg) partially blocked the antinociceptive effects of BBI-11008, and as expected, fully blocked the effects of the positive control and selective mu agonist LYM-100, 1 mg/kg [F(3,35) = 129.7, p < 0.001]. The right-most panel shows delta receptor mediation. The selective delta antagonist NTI (10 mg/kg) fully blocked the antinociceptive effects of BBI-11008 as well as the positive control and selective delta agonist, DPDPE [F(3,34) = 267.5, p < 0.001).
Fig. 4.
Receptor mediation in a warm water tail withdrawal assay in mice. Left panel shows (left to right) BBI-11008 alone, mu antagonist naloxone + BBI-11008, and peripherally restricted naloxone methiodide + BBI-11008. *** indicates significantly decreased compared to BBI-11008 alone (p ≤ 0.0001). Middle panel shows (left to right) BBI-11008 alone, selective mu antagonist βFNA + BBI-11008, selective mu agonist LYM 100 alone, and βFNA + LYM 100. *** indicates significantly decreased compared to BBI-11008 alone (p ≤ 0.0001). ^^^ indicates significantly less than βFNA + BBI-11008 (p ≤ 0.0001). Right panel shows (left to right) BBI-11008 alone, selective delta antagonist NTI + BBI-11008, selective delta agonist DPDPE alone, and NTI + DPDPE. *** indicates significantly decreased compared to BBI-11008 alone or DPDPE alone (p ≤ 0.0001).
Effects of BBI-11008 and morphine on CFA-induced tactile allodynia.
Figure 5 shows the effects of BBI-11008 (10–32 mg/kg, i.v.) and morphine (1–3.2 mg/kg, i.v.) on CFA-induced tactile allodynia in mice. Both compounds produced dose- and time-dependent reversal of tactile allodynia in the von Frey assay, and produced peak effects at 15–30 min, with the duration of action slightly longer for BBI (compare 32 mg/kg BBI with 3.2 mg/kg morphine at 45 min). Duration of effect for peak doses was quantified by Area under the Curve and BBI-11008, 32 mg/kg (AUC = 18.18) was approximately 1.4-fold greater than morphine, 3.2 mg/kg (AUC = 12.85). For BBI-11008, there were significant main effects of Time [F(6,156) = 45.7, p < 0.0001] and Dose [F(3,156) = 5.89, p = 0.0033] and a significant Interaction [F(18,156) = 2.56, p = 0.0010]. For morphine, there were significant main effects of Time [F(6,132) = 38.20, p < 0.0001] and Dose [F(3,132) = 3.91, p = 0.0222] and a significant Interaction [F(18,132) = 2.53, p = 0.0013].
Fig. 5.
Effects of BBI-11008 and morphine in a von Frey test of tactile allodynia following CFA administration in mice. BL = baseline; CFA = complete Freund’s adjuvant. Abscissae: Time in minutes. Ordinates: Mean withdrawal threshold in grams. Data represent mean ± S.E.M., n = 6 – 8 mice/dose group. Left Panel: **, *** = significantly different from saline at p < 0.01, 0.001, respectively. Significant main effects of Time [F(6,156) = 45.7, p < 0.0001] and Dose [F(3,156) = 5.89, p = 0.0033] and a significant Interaction [F(18,156) = 2.56, p = 0.0010]. Right Panel: **, *** = significantly different from saline at p < 0.01, 0.001, respectively. Significant main effects of Time [F(6,132) = 38.20, p < 0.0001] and Dose [F(3,132) = 3.91, p = 0.0222] and a significant Interaction [F(18,132) = 2.53, p = 0.0013].
Effects of BBI-11008 and morphine on SNL-induced tactile allodynia.
Figure 6 shows the effects of BBI-11008 (1–18 mg/kg, i.v.) and Gabapentin (10–56 mg/kg, i.v.) on SNL-induced tactile allodynia in rats. Both compounds produced dose- and time-dependent reversal of tactile allodynia in the von Frey assay with BBI-11008 showing an earlier onset of action relative to gabapentin (compare at 30-min mark), but gabapentin showing longer duration of action (compare 90–150 min). Duration of effect for peak doses was quantified by Area under the Curve and BBI-11008, 18 mg/kg (AUC = 116.8) was ~90% of gabapentin, 56 mg/kg (AUC = 130.2). For BBI-11008, there were significant main effects of Time [F(9,324) = 81.00, p < 0.0001] and Dose [F(4,324) = 9.98, p < 0.0001] and a significant Interaction [F(36,324) = 4.61, p < 0.0001]. For gabapentin, there were significant main effects of Time [F(9,270) = 65.37, p < 0.0001] and Dose [F(3,270) = 43.30, p < 0.0001] and a significant Interaction [F(27,270) = 5.49, p < 0.0001].
Fig. 6.
Effects of BBI-11008 and gabapentin in a von Frey test of tactile allodynia following SNL surgery in rats. BL = baseline; L = spinal nerve ligation surgery. Abscissae: Time in minutes. Ordinates: Mean withdrawal threshold in grams. Data represent mean ± S.E.M., n = 6 – 9 rats/dose group. Left Panel: *, *** = significantly different from saline at p < 0.05, 0.001, respectively. Significant main effects of Time [F(9,324) = 81.00, p < 0.0001] and Dose [F(4,324) = 9.98, p < 0.0001] and a significant Interaction [F(36,324) = 4.61, p < 0.0001]. Right Panel: **, *** = significantly different from saline at p < 0.01, 0.001, respectively. Significant main effects of Time [F(9,270) = 65.37, p < 0.0001] and Dose [F(3,270) = 43.30, p < 0.0001] and a significant Interaction [F(27,270) = 5.49, p < 0.0001].
Effects of oral administration of BBI-11008 and morphine on acute thermal nociception.
To demonstrate systemic availability of BBI-11008, the i.v. data in Figure 3 were replicated using the p.o. route of administration such that Figure 7 shows the effects of BBI-11008 (3.2 – 320 mg/kg, p.o.) and morphine (18–100 mg/kg, p.o.) in the thermal tail withdrawal assay in mice. Both compounds produced dose- and time-dependent thermal antinociception. Within the dose ranges tested, peak antinociception for BBI-11008 occurred for ~25 min, whereas peak antinociception for morphine occurred for ~ 75 min, with overall duration of effect lasting ~120 min and ~180 min, respectively. Duration of effect for peak doses was quantified by Area under the Curve and BBI-11008, 100 mg/kg (AUC = 8786) was ~54% of morphine, 10 mg/kg (AUC = 14774). For BBI-11008, there were significant main effects of Time [F(6,270) = 98.09, p < 0.0001] and Dose [F(4,270) = 53.70, p < 0.0001] and a significant Interaction [F(24,270) = 20.58, p < 0.0001]. For morphine, there were significant main effects of Time [F(6,216) = 55.84, p < 0.0001] and Dose [F(3,216) = 9.12, p = 0.0001] and a significant Interaction [F(18,216) = 5.71, p < 0.0001].
Fig. 7.
Effects of oral (p.o.) administration of BBI-11008 and morphine in a warm water (50˚) tail withdrawal assay in mice. Abscissae: Time in minutes. Ordinates: Mean percent antinociception. Data represent mean ± S.E.M., n = 10 mice/dose group. Left Panel: **, *** = significantly different from 3.2 mg/kg at p < 0.01, 0.001, respectively. Significant main effects of Time [F(6,270) = 98.09, p < 0.0001] and Dose [F(4,270) = 53.70, p < 0.0001] and a significant Interaction [F(24,270) = 20.58, p < 0.0001]. Right Panel: **, *** = significantly different from 18 mg/kg at p < 0.01, 0.001, respectively. Significant main effects of Time [F(6,216) = 55.84, p < 0.0001] and Dose [F(3,216) = 9.12, p = 0.0001] and a significant Interaction [F(18,216) = 5.71, p < 0.0001].
Effects of BBI-11008 and morphine on minute volume with increasing CO2 exposure.
Figure 8 shows the effects of BBI-11008 (32, 56 mg/kg, i.v.) and morphine (3.2–32 mg/kg, i.v.) on % control minute ventilation (MV) under control conditions (0), and at 4, 6, and 8% CO2 exposure in rats. For both compounds, minute ventilation was increased with increasing CO2 exposure. The left panel shows that, with increasing CO2 exposure, 32 mg/kg BBI-11008 produced increases in MV relative to saline controls, and a higher dose of 56 mg/kg produced decreases in MV relative to saline controls For BBI-11008, there were significant main effects of %CO2 [F(3,60) = 148.10, p < 0.0001], and Dose [F(2,60) = 74.40, p < 0.0001] and a significant Interaction [F(6,60) = 10.97, p < 0.0001). The right panel shows that, with increasing CO2 exposure, morphine produced dose-dependent decreases in MV relative to saline controls. For morphine, there was a significant main effect of %CO2 [F(3,80) = 279.23, p < 0.0001] and Dose [F(3,80) = 70.02, p < 0.0001] and a significant Interaction [F(9,80) = 15.62, p < 0.0001).
Fig. 8.
Mean % control minute ventilation under increasing CO2 exposure for BBI-11008 (left panel) and morphine (right panel) in rats. * indicates significantly different compared to saline (p ≤ 0.05). ** indicates significantly different compared to saline (p ≤ 0.001). *** indicates significantly different compared to saline (p ≤ 0.0001).
Self-Administration of Fentanyl and BBI-11008.
Figure 9 shows drug self-administration data for the reference mu opioid agonist fentanyl (0.00032 – 0.01 mg/kg/infusion) and BBI-11008 (0.032 – 3.2 mg/kg/infusion). Fentanyl produced a characteristic inverted U-shaped dose-effect function. ANOVA revealed that fentanyl (0.001 and 0.0032 mg/kg/infusion) maintained significantly greater responding than saline [F(4,42) = 7.39, p < 0.001]. In contrast, BBI-11008 did not maintain drug self-administration at any dose tested and the full range of BBI 11008 doses were not significantly different from saline [F(5,32) = 0.888, p = 0.486].
Fig. 9.
Dose-effect curves for i.v. self-administration of BBI-11008 and fentanyl under a FR5-TO-20 sec schedule of reinforcement in rats. For fentanyl, one-way ANOVA [F(4,46) = 7.39, p < 0.0001]. ** indicates significantly greater than saline (p ≤ 0.001). *** indicates significantly greater than saline (p ≤ 0.0001). For BBI-11008, one-way ANOVA [F(5,49) = 1.03, p = 0.2623].
Irwin Test to determine seizure activity.
Table 1 shows safety pharmacology for BBI-11008 using the Primary Observation (Irwin) Test in the rat. BBI-11008 did not produce any behaviorally observable changes in the dose range of 0.32 to 10 mg/kg. At 32 mg/kg, BBI produced abnormal gait (rolling) and decreased respiration in 1 rat from 0 to 15 min. Abdominal muscle tone was also decreased in a different rat at 15 min. At 100 mg/kg i.v., it induced death in all treated rats within the first 10 min following administration. Seizure activity was not observed in the dose range tested. Death was preceded by abnormal gait (rolling) and decreased respiration.
Table 1:
Irwin Test following individual i.v. doses of BBI-11008 (0.32 – 100 mg/kg). BBI showed no untoward behavioral signs up to 10mg/kg. At 32 mg/kg, 1 of 3 mice showed abnormal gait, decreased respiration and abdominal tone, within 15 min of administration. At 100 mg/kg 2 of 3 mice died at the 9 min mark and the 3rd mouse at the 10 min mark. No seizure-like activity was observed at any dose tested. ’ = minutes. (x/y) = proportion of animals showing reported effects. ↓ = decreased.
| 0.32 | 1 | 3.2 | 10 | 32 | 100 |
|---|---|---|---|---|---|
| No change | No change | No change | No change | Abnormal gait (rolling) (1/3) 0–15’ |
Death (2/3) at 9’ (1/3) at 10’ |
| ↓ Respiration (1/3) 0–15’ |
|||||
| ↓ Abdominal muscle tone (1/3) at 15’ |
|||||
4. Discussion
The present paper represents an assessment of the behavioral pharmacology of the novel, mixed-action delta/mu opioid receptor agonist, BBI-11008 in rodents. The main findings were that BBI demonstrated analgesic efficacy across a broad range of pain-like conditions, was less potent relative to the prescription opioid agonist morphine, and showed a slightly improved side effect profile relative to morphine and fentanyl. The reduction in side effects were manifested as attenuated respiratory depression, and absence of drug self-administration. An additional finding was that the receptor binding assay and in vivo antagonism studies indicated that BBI-11008 showed affinity for and efficacy at both DOP and MOP. Finally, the mu-mediated effects were determined to be centrally, but not peripherally, mediated.
The analgesic efficacy of BBI-11008 was tested in an assay of acute thermal nociception, and in models of CFA-induced inflammatory pain (Taurog et al. 1988), and SNL-induced neuropathic pain (Colburn et al. 1999). In the thermal nociception tail withdrawal test, BBI displayed full antinociceptive efficacy that was less potent and with shorter duration of peak effect (~30 min) than morphine (~1 hr). BBI also demonstrated anti-allodynic efficacy in the CFA model of inflammatory pain that was less potent and with similar duration of peak effect to morphine (~30–45 min). In the SNL model of neuropathic pain, BBI produced comparable anti-allodynic effects to the α2 sub-unit modulator, Gabapentin, with similar peak effects at ~90 min, and BBI showing a longer duration of action in the SNL model relative to the CFA model. Overall, peak effects of BBI were ~10-fold less potent than morphine in the acute thermal and CFA model, and ~5-fold less potent than morphine in the SNL model. In summary, BBI-11008 demonstrates antinociceptive and anti-allodynic efficacy in thermal acute, inflammatory, and neuropathic pain-like conditions, and is less potent in producing these effects relative to morphine or gabapentin. In order to test if BBI-11008 was systemically bioavailable, both BBI-11008 and morphine were re-tested in the acute thermal tail withdrawal assay, with both compounds delivered by the oral route of administration. Oral administration of BBI demonstrated antinociceptive efficacy, was less potent than morphine, and had a shorter duration of peak effect (~40 min) compared to morphine (~2 hr). The finding that BBI by the oral route of administration demonstrated full efficacy in the tail withdrawal procedure indicates that this mixed-action compound is systemically viable and that future studies may be undertaken using systemic routes of drug administration.
The behavioral pharmacology data for BBI-11008 reported here, are consistent with the therapeutic/side-effect profiles of mixed-action delta/mu opioid agonists in the literature. For example, our laboratories have previously shown that the delta/mu opioid glycopeptide agonist, MMP-2200 has antinociceptive efficacy similar to morphine in the acute thermal tail withdrawal assay, and that this effect was blocked by selective mu or delta antagonists, suggesting a combined mu/delta mechanism of action. In contrast to morphine, MMP-2200 produced less robust locomotor activity activation, less pronounced naloxone-precipitated withdrawal behaviors, and lower rates of drug self-administration (Lowery et al. 2011; Stevenson et al. 2015). Similarly, Jutkiewicz and colleagues have shown that VRP26, a mixed-action mu agonist/delta antagonist produced equivalent antinociceptive efficacy to fentanyl in the warm water tail withdrawal assay, but in contrast to fentanyl showed significantly less behavioral signs of withdrawal, and no conditioned place preference (Anand et al. 2016). The underlying mechanism(s) for why both delta agonists + mu agonists as well as delta antagonists + mu agonists produce similar profiles as defined by enhanced or maintained therapeutic effects with reduced side effects, are unknown. A logically possible mechanism may include a delta receptor binding requirement (i.e.: allosteric) being a necessary and sufficient condition, whereas a delta receptor efficacy requirement being merely a sufficient condition (i.e.: not required). A binding requirement interpretation is consistent with the in vivo pharmacology as well as the molecular opioid heteromer literature, the latter showing that delta/mu heteromers induced by mu agonists + delta agonists or antagonists generally produce a greater therapeutic index than mu agonists delivered alone (reviewed in Anand & Montgomery, 2018).
The receptor mediation of BBI-11008 for thermal antinociception was characterized using the highly selective MOP antagonist β-FNA, and the selective DOP antagonist, NTI. β-FNA produced partial reversal of BBI-induced acute antinociception compared to NTI, suggesting that the acute antinociceptive effects of the compound are mediated by both DOP and MOP, with perhaps greater efficacy derived from DOP activation. However, multiple doses of antagonist were not tested, and thus this interpretation, is preliminary at best. Additionally, the 10 mg/kg dose of NTI is within the range of doses tested in mice (Bilsky et al., 1995; Broom et al., 2002; Jutkiewicz et al., 2004; Lei et al., 2019; Suzuki et al., 1995), but is on the high end of the dose range typically reported in the literature (1 – 20 mg/kg). Finally, determination of a peripheral vs. central mechanism was accomplished by pretreatments with the centrally penetrant MOP blocker naloxone, as well as the peripherally restricted MOP blocker naloxone-methiodide. Results indicated that antinociceptive effects were centrally mediated given the effectiveness of naloxone, but not it’s methiodide analogue, in reversing BBI-induced antinociception. The central mediation suggests that other routes of administration (other than i.v., and p.o. reported here) may produce therapeutic effects, and thus further testing of this compound is warranted.
Within the range of doses tested in respiration under normal O2 levels, BBI-11008 showed comparable respiratory depression to the prescription drug morphine. However, the two compounds showed divergent dose-dependent effects when tested in an environment with increasing % CO2, with BBI showing increased respiration at one dose, and morphine showing no change or decreased respiration, relative to vehicle controls. The respiration data for BBI add to the complex opioid respiration literature that includes reports of mixed-action delta/mu drugs, dose addition studies using combinations of delta + mu ligands, and separate mu or delta compounds administered alone. Overall, delta agonism as well as antagonism have been reported to attenuate or mediate respiratory depression and hypercapnia induced by selective mu or delta agonists (Dahan et al. 2001; Lonergan et al. 2003; Morin-Surun et al. 1984; Pazos et al. 1984; Su et al. 1998; Wojciechowski et al. 2011).
The abuse liability of BBI-11008 was quantified and compared to the mu opioid prescription agonists fentanyl using a drug self-administration procedure. Fentanyl produced the characteristic inverted U-shaped dose-response function seen with selective MOR and prescription opioids (Mello and Negus 1996). In contrast BBI did not produce self-administration across a broad range of doses. Although preliminary, these data suggest that BBI-11008 may not have rewarding or has abuse liability in humans. However, it is worth noting that a more complete assessment of abuse liability may require monitoring the subjective effects of BBI in humans after clinically relevant route and formulation are determined and/or further preclinical work using drug discrimination learning procedures (Lynch et al. 2010).
A frequently reported side effect of delta agonists is seizure activity (Broom et al., 2002; Jutkiewicz et al., 2004). To determine the frequency of any potential seizure activity, BBI-11008 was assessed using the Primary Observation (Irwin) Test. Results indicated an absence of behavioral effects in the dose range 0.32 – 10 mg/kg i.v. However, at a higher dose of 32 mg/kg i.v., there was evidence of abnormal gait, decreased respiration, and decreased abdominal tone, but a lack of any noticeable seizure activity. At 100 mg/kg i.v., BBI had lethal effects in all rats tested. The 100 mg/kg i.v. lethal dose in rats was ¾ log higher than the peak anti-allodynic dose of 18 mg/kg in rats in the SNL model suggesting a narrow therapeutic index. Thus, although the absence of drug self-administration and slight reduction in respiratory depression in BBI-11008 are promising findings, the narrow therapeutic index is a concern.
There are a number of limitations to these studies. First, only males were tested. Although current studies in our laboratories are incorporating females as subjects, the present results cannot be extrapolated to females. Second, there is an extensive literature on sex differences in rodents and humans (Craft, 2007; Fillingim et al., 2009; Greenspan et al., 2007) including reports that females have higher prevalence and incidence of chronic pain in human populations. Third, all studies reported herein were run during the light cycle, and given that rodents are most active during the dark cycle, results obtained may have been different if experiments were run when rodents are most active. Finally, the pain-related behavioral endpoints utilized represented assays of pain-stimulated responding only, and there are several limitations to these kinds of tests: 1) they are subject to false positive analgesic effects, 2) they are limited in clinical relevance, particularly for chronic pain conditions, and 3) there is a great deal of experimenter interpretation associated with defining occurrence of pain-stimulated behavioral endpoints (Negus et al., 2006, Negus, 2019; Stevenson et al., 2006; Yezierski and Hansson, 2018).
Taken together, BBI-11008 represents a mixed-action delta/mu opioid receptor agonist with efficacy in assays or models of acute, inflammatory and neuropathic pain. The respiration, GI and self-administration data suggest that BBI-11008 may be safer, relative to prescription opioids such as morphine or fentanyl (Fishman et al. 2004), but the Irwin Test data on lethal effects at high doses indicate that further structure-activity characterization may be required in order to advance this lead candidate.
Acknowledgments
We would like to thank Drs. Mark S. Kleven and Vincent Castagné for running the Primary Observation (Irwin) Test. This research was supported by a NIDA/SBIR R43 DA026653-01A1 to Biousian Biosystems, Inc. (R.P. and E.J.B.) and an NIAMS AR054975 to G.W.S.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: Edward J. Bilsky is co-owner of BBI. Robin Polt is co-owner of BBI.
References
- Anand JP, Boyer BT, Mosberg HI, Jutkiewicz EM (2016) The behavioral effects of a mixed efficacy antinociceptive peptide, VRP26, following chronic administration in mice. Psychopharm 233:2479–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand JP and Montgomery D (2018) Multifunctional opioid ligands. In: Jutkiewicz EM, editor. Delta Opioid Receptor Pharmacology and Therapeutic Applications, Handbook of Experimental Pharmacology 247. Springer; pp 21–52 [DOI] [PubMed] [Google Scholar]
- Antman EM (2017) Evaluating the cardiovascular safety of nonsteroidal anti-inflammatory drugs. Circul 135(21):2062–2072 [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F (1995) SNC80 a selective nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther 273:359–366 [PubMed] [Google Scholar]
- Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R (2000) Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem 43(13):2586–2590 [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR (2002) Comparison of receptor mechanism and efficacy requirements for δ-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther 303:723–729 [DOI] [PubMed] [Google Scholar]
- Cheng Y and Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22(23):3099–3108 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Back FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth 53:55–63 [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA (1999) The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol 157:289–304 [DOI] [PubMed] [Google Scholar]
- Craft RM (2007) Modulation of pain by estrogens. Pain 132:S3–S12 [DOI] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL (2001) Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesth 94(5):824–832 [DOI] [PubMed] [Google Scholar]
- Elmagbari NO, Egleton RD, Palian MM, Lowery JJ, Schmid WR, Davis P, Navratilova E, Dhanasekaran M, Keyari CM, Yamamura HI, Porreca F, Hruby VJ, Polt R, Bilsky EJ (2004) Antinociceptive structure-activity studies with enkephalin-based opioid glycopeptides. J Pharmacol Exp Ther 311:290–297 [DOI] [PubMed] [Google Scholar]
- Erspamer V, Melchiorri P, Falconieir-Erspamer G, et al. (1989) Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci USA 86(13):5188–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL (2009) Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain 10(5):447–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD (2011) Preclinical assessment of drug combinations for the treatment of pain: isobolographic and dose-addition analysis of the opioidergic system. CNS Neurol Disord Drug Targets 10(5):529–535 [DOI] [PubMed] [Google Scholar]
- Fishman SM, Condon J, Holtsman M (2004) Common opioid-related side effects. In: Warfield CA, Bajwa ZH, editors. Principles and practice of pain medicine. 3rd ed. McGraw-Hill; pp 612–615 [Google Scholar]
- Godfrey RG (1996) A guide to the understanding and use of tricyclic antidepressants in the overall management of fibromyalgia and other chronic pain syndromes. Arch Intern Med 156(10):1047–1052 [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L,… et al. (2007) Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132:S26–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck SD, Faraci WS, Kelbaugh PR, Saccomano NA, Thadeio PF, Volkmann RA (1996) Posttranslational amino acid epimerization: enzyme-catalyzed isomerization of amino acid residues in peptide chains. Proc Natl Acad Sci USA 93(9):4036–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) (2011) Relieving pain in America: A blueprint for transforming prevention, care, education, and research, The National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH (2004) δ-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther 309:173–181 [DOI] [PubMed] [Google Scholar]
- Kaye AD, Cornett EM, Helander E, Menard B, Hsu E, Hart B, Brunk A (2017) An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin 35(2):55–71 [DOI] [PubMed] [Google Scholar]
- Krashin D, Murinova N, Jumelle P, Ballantyne J (2015) Opioid risk assessment in palliative medicine. Expert Opin Drug Saf 14(7):1023–1033 [DOI] [PubMed] [Google Scholar]
- Kreil G, Barra D, Simmaco M, et al. (1989) Deltorphin, a novel amphibian skin peptide with high selectivity and affinity for delta opioid receptors. Eur J Pharm 162(1):123–128 [DOI] [PubMed] [Google Scholar]
- Lei W, Vekariya RH, Subramaniam A, Streicher JM (2019) A novel mu-delta opioid agonist demonstrates enhanced efficacy with reduced tolerance and dependence in mouse neuropathic pain models. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lefever MR, Muthu D, Bidlack JM, Bilsky EJ, Polt R (2012) Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins. Future Med Chem 4(2):205–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM (2003) Presynaptic delta opioid receptors differentially modulate rhythm and pattern generation in the ventral respiratory group of the rat. Neurosci 121(4):959–973 [DOI] [PubMed] [Google Scholar]
- Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ (2011) In vivo characterization of MMP-2200, a mixed δ/μ opioid agonist, in mice. J Pharmacol Exp Ther 336(3):767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL (2010) Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comp Med 60(3):177–188 [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Narita M, Muramatsu N, Nakayama T, Misawa K, Kitajima M, Tashima K, Devi L, Suzuki T, Takayama H, Horie S (2014) Orally active opioid μ/δ dual agonist MGM-16, a derivative of the indole alkaloid mitragynine, exhibits potent antiallodynic effect on neuropathic pain in mice. J Pharmacol Exp Ther 348:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorri P, Negri L (1996) The dermorphin peptide family. Gen Pharmacol 27(7):1099–1107 [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424 [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Boudinot E, Gacel G, Champagnat J, Roques BP, Denavit-Saubie M (1984) Different effects of mu and delta opiate agonists on respiration. Eur J Pharmacol 98(2):235–240 [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, Jutkiewicz EM (2014) Development of a bioavailable μ opioid receptor (MOPr) agonist, δ opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J Med Chem 57:3148–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky ER, Becerra L, Borsook D (2006) Preclinical assessment of candidate analgesic drugs: recent advances and future directions. J Pharmacol Exp Ther 319:507–514 [DOI] [PubMed] [Google Scholar]
- Negus SS (2019) Core outcome measures in preclinical assessment of candidate analgesics. Pharmacol Rev 71:225–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Florez J (1984) A comparative study in rats of the respiratory depression and analgesia induced by mu- and delta-opioid agonists. Eur J Pharmacol 99(1):15–21 [DOI] [PubMed] [Google Scholar]
- Schiller PW, Weltrowska G, Schmidt R, Nguyen TMD, Berezowska I, Lemieux C, Chunb NN, Carpenter KA, Wilkes BC (1995) Four different kinds of opioid peptides with mixed μ agonist/δ antagonist properties. Analgesia 1(4–6):703–706 [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS (2006) Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: Effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain 7(6):408–416 [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Luginbuhl A, Dunbar C, LaVigne J, Dutra J, Atherton P, Bell B, Cone K, Giuvelis D, Polt R, Streicher JM, Bilsky EJ (2015) The mixed-action delta/mu opioid agonist MMP-2200 does not produce conditioned place preference but does maintain drug self-administration in rats, and induces in vitro markers of tolerance and dependence. Pharmacol Biochem Behav 132:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YF, McNutt RW, Chang KJ (1998) Delta-opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J Pharmacol Exp Ther 287(3):815–823 [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Misawa M, Endoh T, Nagase H (1995) Effects of a highly selective nonpeptide δ opioid receptor agonist, TAN-67, on morphine-induced antinociception in mice. Life Sciences 57(2):155–168 [DOI] [PubMed] [Google Scholar]
- Taylor CP (2009) Mechanisms of analgesia by gabapentin and pregabalin – calcium channel α2-δ [Cavα2-δ] ligands. Pain 142:13–16 [DOI] [PubMed] [Google Scholar]
- Taurog JD, ARgentieri DC, McReynolds RA (1988) Adjuvant arthritis. Meth Enzymol 162:339–355 [DOI] [PubMed] [Google Scholar]
- Thomsen M and Caine SB (2005) Chronic intravenous drug self-administration in rodents. Curr Protoc Neurosci (Suppl):9.20.1–9.20.40 [DOI] [PubMed] [Google Scholar]
- Wojciechowski P, Szereda-Przestaszewska M, Lipkowski AW (2011) Delta opioid receptors contribute to the cardiorespiratory effects of biphalin in anesthetized rats. Pharmacol Rep 63(5):1235–1242 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nair P, Davis P, Ma SW, Navratilova E, Moye S, Tumati S, Lai J, Vanderah TW, Yamamura HI, Porreca F, Hruby VJ (2007) Design, synthesis, and biological evaluation of novel bifunctional C-terminal-modified peptides for delta/mu opioid receptor agonists and neurokinin-1 receptor antagonists. J Med Chem 50(12):2779–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala AS, Roberson DP, Bean BP, Woolf CJ (2017) Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 16(8):545–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezierski RP and Hansson P (2018) Inflammatory and neuropathic pain from bench to bedside: what went wrong? J Pain 19(6):571–588 [DOI] [PubMed] [Google Scholar]