Abstract
Background
Primary pancreatic lymphoma (PPL) is a rare disease representing 0.1% of all malignant lymphomas, which lacks well-defined diagnostic and therapeutic protocols. We conducted a systematic review to analyze demographic, diagnostic and therapeutic features of PPL.
Methods
This review identified small series and single case reports. Sources were MEDLINE, PubMed, and the Cochrane library from January 2001 to December 2020. Data were screened, extracted and the risk of bias analyzed by three independent reviewers.
Results
A total of 107 eligible papers (17 small series, 90 single case reports) describing 266 patients were identified. Patients had a median age of 53.1 (range 3–86) years and were males in 64.6% of cases. Abdominal pain and jaundice were the most common presenting symptoms, affecting 75.3% and 41.8% of patients, respectively. PPL had a median size of 60.6 mm (range 16–200) and it was localized in the pancreatic head in 63.7% of cases. At diagnosis most patients underwent ultrasonography followed by computed tomography. PPL typically showed low echogenicity, and lower contrast enhancement than solid tumors. Histopathological specimens were obtained by percutaneous or endoscopic biopsies in 47.7% of patients; abdominal surgery was performed in 33.5% of cases. Overall, diffuse large B-cell lymphoma was the most frequent histological diagnosis (53.6%). However, patients aged <18 years were affected by Burkitt lymphoma in 52.4% of cases. Most patients (53.6%) received immunochemotherapy (IC) or IC plus radiotherapy (14%). Demolitive surgery appeared to be associated with impaired survival. Central nervous system (CNS) relapse or progression was observed in 20% of patients.
Conclusion
PPL is a rare entity, with some peculiar features at modern imaging. For diagnostic purposes percutaneous or endoscopic biopsies might be preferable, as opposed to surgery. No definite data is available about the optimal treatment, which should be tailored on the histological type and associated with CNS prophylaxis.
Keywords: primary pancreatic lymphoma, diffuse large B cell lymphoma, Burkitt lymphoma
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Primary Pancreatic Lymphoma (PPL) is a rare disease representing only 0.1% of malignant lymphomas, 0.6% of extranodal lymphomas, and 0.2% of all pancreatic tumors.1,2 On the contrary, secondary pancreatic involvement occurs quite commonly in lymphomas, especially in the presence of widespread nodal or extranodal disease, and may be observed in up to 30% of cases.3
Over the years, different definitions of PPL have been suggested.3,4 More recently, the World Health Organization (WHO) has provided the following diagnostic criteria: i) the bulk of disease has to be located in the pancreas, ii) although adjacent lymph nodes involvement and distant spread may exist, the primary clinical presentation has to involve the pancreatic gland.5
PPL can develop at any age, but usually affects elderly patients, with a male prevalence. Immunosuppression, related to HIV infection or solid organ transplantation, may favor its development.6 From a clinical standpoint, the main presenting symptom of PPL is the abdominal pain. However, other common clinical findings include systemic symptoms (fever, night sweats and weight loss), jaundice, pancreatitis and/or gastric or duodenal obstruction.2,7,8 Overall, these symptoms resemble those of other pancreatic diseases, often resulting in diagnostic problems.9,10 PPL can be located in any portion of the gland, but it mainly involves the pancreatic head, which contains the greatest amount of lymphoid tissue.9 Histopathological analysis is usually consistent with diffuse large B cell lymphoma (DLBCL) not otherwise specified (NOS); nevertheless, other subtypes of lymphomas, including marginal zone lymphoma (MZL) or follicular lymphoma (FL), may be detected.5,9,10 Anecdotal cases of Hodgkin (HL) and T cell non Hodgkin lymphoma (T-NHL) have been also described.11,12
A diagnosis of PPL can be obtained through percutaneous/endoscopic biopsy, exploratory laparotomy or demolition surgery.7 With regard to treatment, no definite guidelines can be drawn from literature. In fact, most reports on PPL are retrospective and describe undersized and heterogeneously treated groups of patients.
The aim of this review was to retrieve, analyze and summarize data obtained from case collections and single case reports published in the last two decades.
The final goal was to identify specific characteristics of this rare lymphoma entity in order to provide evidence to establish future diagnostic and therapeutic guidelines.
Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines.13 We conducted an extensive systematic literature search since 01 January 2000 through 31 December 2020. Sources were PubMed, Medline, and Cochrane library. We deemed eligible all types of reports (case collections and single cases). This search was limited to studies published in English.
The eligibility criteria were (a) adult and pediatric population (b) pancreatic lymphomas fulfilling diagnostic criteria for PPL according to WHO.5
All titles were downloaded into an Endnote library and duplicated removed automatically. Three reviewers (DF, EB, CV) screened the titles and abstracts for eligibility. Then full text was screened again. A senior reviewer (CT) resolved any disagreement. For each article, at least one reviewer extracted the following information: demographics, presenting symptoms, diagnostic methods, and histological classification. Whenever available data about treatment and outcome were also retrieved.
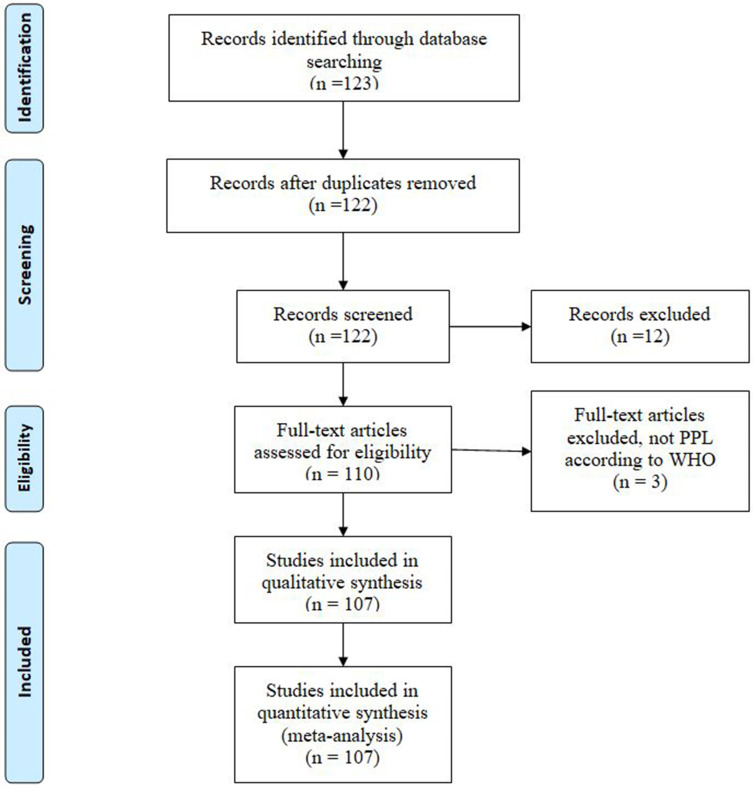
A total of 107 papers reporting on 266 patients were deemed eligible.10–12,14–113 Figure 1 provides the PRISMA flow diagram.
Figure 1.
PRISMA flow diagram.
Percentages regarding each item analyzed were calculated based on the number of available data as specified in each table.
Results
Demographics and Clinical Characteristics
Among the 266 patients retrieved from literature, 172 (64.6%) were males, 94 (35.4%) females. The mean age was 53.1 (range 3–86) years. Twenty-one (7.9%) patients were aged less than 18 years.
As reported in Table 1, abdominal pain (75.4%) was the most common presenting symptom, followed by jaundice (41.8%), and B symptoms such as fever, night sweats and weight loss (31.9%). Acute pancreatitis and gastric or duodenal obstruction were the first clinical presentation in 25.9% and 10.7% of patients, respectively. Two patients were immunosuppressed after a simultaneous pancreas-kidney transplant.61,107
Table 1.
Patient Characteristics
| Characteristics | n (%) |
|---|---|
| Presenting symptoms | |
| Abdominal pain | 175/232 (75.4) |
| Jaundice | 100/239 (41.8) |
| B symptoms | 77/241 (31.9) |
| GI obstruction | 24/225 (10.7) |
| Acute pancreatitis | 56/218 (25.9) |
| Laboratory values | |
| LDH > upper limits | 65/129 (50.4) |
| CA 19–9 > upper limits | 32/119 (26.9) |
| Anemia | 19/91 (20.9) |
| Thrombocytopenia | 4/84 (4.8) |
| WBC > 10.000/mmc | 17/93 (18.3) |
Abbreviations: GI, gastrointestinal; LDH, lactate dehydrogenase; CA, carbohydrate antigen; WBC, white blood cells.
Laboratory Tests
Pretreatment laboratory tests were available for a minority of patients. Serum levels of lactate dehydrogenase (LDH) were elevated in 50.4% of patients, carbohydrate antigen 19–9 (CA 19–9) in 26.9%. The blood count showed anemia in 20.9% of patients, leukocytosis (>10.000/mmc) in 18.3%, and thrombocytopenia in 4.8%. Laboratory tests are reported in Table 1.
Imaging
Imaging features were available in 256 patients (Table 2). PPL had a mean size of 60.6 mm (range 16–200) and it was located in the pancreatic head in 163 (63.7%) cases. Early-stage Ann-Arbor disease (I–II) was diagnosed in 76% of patients, advanced (III–IV) in the remaining 24%.
Table 2.
Imaging Characteristics
| Imaging | n (%) |
|---|---|
| Imaging method | |
| US | 214/256 (83.6) |
| CT | 249 (97.3) |
| MRI | 36 (14.1) |
| PET-CT | 51 (19.9) |
| Pancreatic localization | |
| Head | 163/256 (63.6) |
| Body-tail | 76 (29.6) |
| All gland | 17 (6.6) |
| Tumor diameter, mean (range) | 60.6 mm (range 16–200 mm) |
Abbreviations: US, ultrasonography; CT, computed tomography; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-CT.
Ultrasonography (US) was performed in 214/256 patients (83.6%) at disease onset. Computed tomography (CT) was performed in 249/256 cases (97.3%), often following US; multidetector technology and contrast agent enhanced protocols were always used.
Magnetic Resonance Imaging (MRI) was performed in 36/256 cases (14.0%), nuclear medicine, in particular positron emission tomography-CT (PET-CT) with evaluation of 18fluoro-2-deoxy-d-glucose (18FDG) intake, in 51/256 patients (19.9%).
US is extensively used since it enables a rapid evaluation of pancreas size, borders, echostructure and vessels.54,92 The most common finding is enlargement of the parenchyma, focal or diffuse; the affected pancreas presents also lower echogenicity, appearing darker than normal. At color-Doppler evaluation major vessels can be surrounded by pathological tissue, but always patent (contrary to other solid neoplasms like adenocarcinoma).110,114 When a pancreatic disease is identified by US a second line radiological examination, usually CT, is mandatory.
At CT, PPL presents as a large solid lesion, potentially involving the whole gland; contrast enhancement is lower than healthy pancreas. Fat stranding in the peri-pancreatic region is typical; dilation of the bile and pancreatic duct is not common, contrary to pancreatic adenocarcinoma. Vascular encasement can be observed, without irregularities in vessels wall.51,88,114
MRI can be used if CT is inconclusive or to avoid radiation exposure in younger patients. Involved parenchyma shows lower signal intensity on T1-weighted images and higher signal on T2-weighetd images compared to healthy pancreas; diffusion-weighted sequences present very high sensitivity to depict lymphomatous tissue and lymph nodes.114 PET-CT is indicated to assess the metabolic activity of the primary neoplasm and to depict involved lymph nodes in the whole body.98
Diagnostic Procedures and Histological Assessment
Information about diagnostic procedures was available in 224 patients (Table 3). Histopathological specimens were obtained mostly by percutaneous or endoscopic biopsies in 68 (30.3%) and 39 (17.4%) cases, respectively. Fine needle aspiration (FNA) was performed in 33 (14.8%) cases, abdominal surgery in 75 (33.5%) (26 surgical biopsy, 49 demolition surgery). Demolition surgery consisted mainly of Whipple procedure and spleno-pancreasectomy. In the remaining 9 (4.0%) patients the diagnosis was autoptic. The main histological diagnosis was DLBCL in 143 (53.6%) patients, followed by FL in 26 (9.8%) and BL in 20 (7.5%). In 31 (11.6%) cases the histology was not specified. Overall, this data is in agreement with that reported by a recent paper by Mukhija et al115 evaluating 835 patients affected by pancreatic lymphomas, though the analysis was not restricted to the PPLs only.
Table 3.
Diagnostic Methods, Histology and Clinical Stage
| Diagnosis | n (%) |
|---|---|
| Diagnostic method | |
| Percutaneous biopsy | 68/224 (30.3) |
| Endoscopic biopsy | 39 (17.4) |
| FNA | 33 (14.8) |
| Surgical biopsy | 26 (11.6) |
| Demolition surgery | 49 (21.9) |
| Autopsy | 9 (4.0) |
| Histology | |
| DLBCL | 143/266 (53.6) |
| FL | 26 (9.8) |
| BL | 20 (7.5) |
| High grade B cell lymphoma | 14 (5.2) |
| T-NHL | 18 (6.7) |
| HL | 4 (1.5) |
| LPL | 4 (1.5) |
| MZL | 2 (0.7) |
| SLL | 2 (0.7) |
| PTLD | 2 (0.7) |
| Lymphoblastic lymphoma | 1 (0.4) |
| Unknown | 31 (11.6) |
| Clinical stage (Ann Arbor) | |
| I–II | 152/200 (76) |
| III–IV | 48/200 (26) |
Abbreviations: FNA, fine needle aspiration; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; BL, Burkitt lymphoma; NHL, non Hodgkin lymphoma; HL, Hodgkin lymphoma; LPL, lymphoplasmacytic lymphoma; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma; PTLD, post-transplant lymphoproliferative disorder.
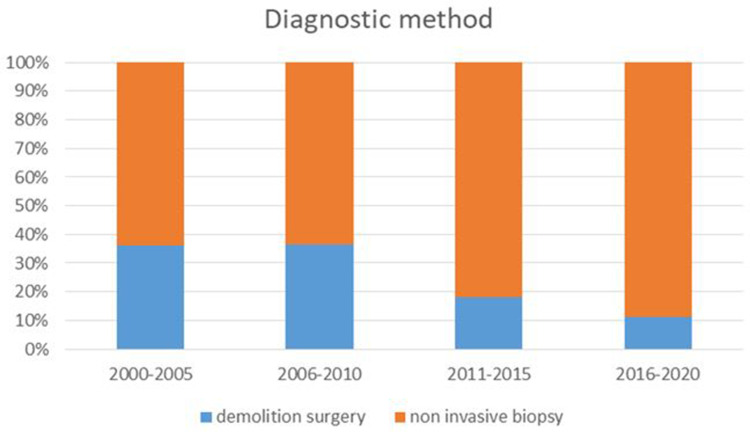
According to data published in literature, percutaneous or endoscopic biopsies are reliable and scarcely invasive. On the other hand, despite providing a significant diagnostic accuracy for adenocarcinoma (sensitivity of 86.8% [95% CI 85.5–87.9] and specificity of 95.8% [95% CI 94-6-96.7]),116 endoscopic ultrasound guided fine needle aspiration (EUS-FNA) is a poor diagnostic tool for lymphoma.117 Immunophenotypic analysis may be useful to integrate cytology.118,119 In a study involving 11 patients with PPL, cytology alone revealed a correct diagnosis in 28% compared to 100% by integrated immunophenotype.118 However, tissue architecture in addition to cytomorphology is crucial in the diagnosis of lymphoma. Overall, a non-invasive diagnostic approach is preferable to demolition surgery, which was associated with a higher mortality rate.10,34,51,120 Figure 2 shows the temporal variation in biopsy techniques.
Figure 2.
Temporal variation in biopsy techniques.
Treatment and Pattern of Relapse
Information about treatment was available in 207 of the 266 patients retrieved from literature (Table 4). The initial treatment consisted of immunochemotherapy (IC) alone in 111 patients (53.6%), IC plus radiotherapy (RT) in 29 (14.0%), RT alone in 1 case (0.5%), and demolition surgery in 59 (28.5%). Surgery alone was reserved to 15 patients, it was followed by IC in 39, RT in 3 and both IC and RT in 2 cases. Seven patients (3.4%) were not treated at all.
Table 4.
Patient Survival According to Treatment and Histology
| Alive | Deceased | |||
|---|---|---|---|---|
| n (%) | OS, Months (Range) | n (%) | TTD, Months (Range) | |
| Treatment* | ||||
| DS | 4 (50) | 11 (3–19) | 4 (50) | 2.5 (1–6) |
| DS-IC | 13 (76.47) | 37.8 (4–160) | 4 (23.53) | 13.5 (8–27) |
| DS-RT | 2 (100) | 34 (6–62) | / | / |
| DS-IC-RT | 2 (100) | 44.5 (24–65) | / | / |
| IC | 26 (74.28) | 34.7 (5–192) | 9 (25.72) | 13.4 (1–88) |
| IC-RT | 15 (78.94) | 45.4 (2–128) | 4 (21.06) | 36.5 (9–67) |
| Histology** | ||||
| DLBCL | 48 (68.57) | 39.3 (2–132) | 22 (31.43) | 18.9 (1–88) |
| FL | 11 (84.61) | 31.4 (6–62) | 2 (15.39) | 67 (63–72) |
| T-NHL | 3 (23.07) | 10.3 (4–15) | 10 (76.93) | 4.3 (0–8) |
| BL | 8 (66.66) | 63.25 (8–192) | 4 (33.34) | 2.9 (1–7) |
| HGBCL | 3 (50) | 34 (3–94) | 3 (50) | 10.3 (8–12) |
Notes: *Data available in 83 patients; **data available in 113 patients.
Abbreviations: OS, overall survival; TTD, time to death; DS, demolition surgery; IC, Immuno-chemotherapy; RT, radiotherapy; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; NHL, non Hodgkin lymphoma; BL, Burkitt lymphoma; HGBCL, high grade B cell lymphoma.
There is no consensus on the ideal treatment approach for PPL patients; studies from the pre-rituximab era suggested an aggressive local management of disease with demolition surgery.3,34 In contrast, more recent papers suggested a less invasive approach with IC as the cornerstone of treatment.121,122 There is no consensus on the role of RT in patients affected by PPL.39
Among 169 patients for whom a follow up was provided, 30 (17.7%) presented a disease relapse after a median time of 15 months (range 2–108 months).10,19,28,33,49,51,53,64,73,101 Interestingly, 6 relapses (20%) (2 DLBCL, 2 BL, 1 T-NHL, and 1 high grade B cell lymphoma) involved the CNS.
PPL in the Pediatric Population
Our analysis identified 21 cases of PPL in patients under 18 years of age.15,24,30,35,37,51,55,67,70,81,82,84,87,92,96,99–102 The mean age was 10.3 (range 3–16) years, with most of patients (19, 90.5%) being males (Table 5).
Table 5.
PPL Characteristics in Pediatric vs Adult Patients
| Characteristics | Patients <18 Year n (%) |
Patients >18 Years n (%) |
|---|---|---|
| Presenting symptoms | ||
| Abdominal pain | 16/21 (76.2) | 159/211 (75.4) |
| Jaundice | 13 (61.9) | 87/218 (39.9) |
| B symptoms | 2 (9.52) | 75/220 (34.1) |
| GI obstruction | 1 (4.76) | 23/204 (11.3) |
| Acute pancreatitis | 10 (47.6) | 55/197 (27.9) |
| Laboratory values | ||
| LDH > upper limits | 8/10 (80) | 57/119 (47.9) |
| CA 19–9 > upper limits | 0/3 (0) | 32/116 (27.6) |
| Pancreatic localization | ||
| Head | 13/21 (61.9) | 150/235 (63.8) |
| Body-tail | 2 (9.5) | 74 (31.5) |
| All gland | 6 (28.6) | 11 (4.7) |
| Histology | ||
| DLBCL | 5/21 (23.8) | 138/245 (56.3) |
| BL | 11 (52.4) | 9 (3.7) |
| High grade B cell lymphoma | 1 (4.8) | 13 (5.3) |
| T-NHL | 2 (9.5) | 17 (6.9) |
| Unknown | 2 (9.5) | 29 (11.8) |
| Clinical stage (Ann Arbor) | ||
| I–II | 5/13 (38.5) | 147/187 (78.6) |
| III–IV | 8 (61.5) | 40/187 (21.4) |
Abbreviations: GI, gastrointestinal; LDH, lactate dehydrogenase; CA 19–9, carbohydrate antigen; WBC, white blood cells; DLBCL, diffuse large B cell lymphoma; BL, Burkitt lymphoma; NHL, non Hodgkin lymphoma.
As in adults, the main presenting symptom was abdominal pain. The percentage of jaundice was higher than in adults (61.9% vs 39.9%), while the number of patients aged <18 years with B symptoms was very low (9.5% vs 34.1%). Interestingly 10 patients (47.6%) had an acute pancreatitis at disease onset.
Laboratory tests, compared to adults, showed elevated LDH values in the large majority of patients (80% vs 47.9%), while CA 19–9 never increased (0% vs 27.6%).
The mean size was similar (56.6 mm vs 61.1 mm) and also the pancreatic location, with a predilection for the pancreatic head (61.9% vs 63.8%).
Interestingly, in the pediatric cohort the main histological diagnosis was BL in 11 (52.4%), DLBCL in 5 (23.8%) patients.
Ng et al123 reviewed the imaging findings of 80 children affected by NHL, with pancreatic involvement being present in 3 cases (3.75%). Similarly, Vade and Blane124 reviewed the diagnostic imaging of 19 pediatric patients with BL and found 2 children with pancreatic involvement (10%).
The initial treatment consisted of IC alone in 16 patients (88.9%), and chemotherapy after demolition surgery in 2 (11.2%). Information about follow-up was available in 15 patients only. Fourteen/15 (93.3) reached a complete remission (CR) with first line therapy and one patient had a CNS relapse during the observation period.101 These 2 patients were rescued with high dose chemotherapy plus autologous stem cell transplantation. With a median follow-up of 56.43 (range 8–132) months all patients were alive and in CR.
Discussion
This study presents data (diagnosis, histology, treatment and outcome) retrieved from 107 papers published from 2000 to 2020 on PPL.
As compared to pancreatic adenocarcinoma, which usually manifests in the 60- to 80-year-old age group,125 PPL is usually diagnosed in younger adults (mean age 53 years). As previously described, the presentation of PPL may overlap with the onset of other neoplastic or inflammatory pancreatic diseases.126,127
Interestingly, in spite of symptoms and radiological findings (pancreatic head involvement) overlapping those of pancreatic ductal adenocarcinoma and autoimmune pancreatitis, some findings may indicate a diagnosis of PPL. For instance, a relatively large tumor size (>60 mm) together with the presence of distant lymph nodes at radiological assessment may suggest a diagnosis of lymphoma.114 Unfortunately, few reports reported laboratory data, so it is not clear whether a elevate LDH value associated with CA 19–9 within the normal range may suggest a diagnosis of PPL, as previously indicated by our group.51 Concerning the diagnostic approach, in more than half of cases reported in literature, tumor samples were collected through a noninvasive procedure: transcutaneous biopsy in 30.3%, endoscopic in 17.4% and FNA in 14.8%. However, in a significant number of patients, a surgical biopsy (11.6%) or a demolition surgery (21.9%) were performed. Worth of note, demolitive surgery was more frequently performed in the earlier time frame of our analysis, while noninvasive methods were preferred in most recent years (Figure 2). Finally, in 4.0% the diagnosis was obtained post-mortem.
In regard to histology, PPL was mostly classified as DLBCL (53.6%), FL (9.8%) and BL (7.5%). However, other types of lymphoma were described, including a non-negligible number of T-NHL (6.7%) and 5.2% of high-grade B cell lymphomas. Therefore, an accurate histological diagnosis should be obtained in order to provide patients with the best available treatment. It is interesting to note that among the 21 (7.9%) patients aged <18 years, BL was the prevalent histological type (52.4%). Unfortunately, a detailed histological classification according to the new 2016 WHO classification of lymphomas128 was difficult to establish based on data reported by most of the articles here revised.
The lack of large PPL study series hampers definitive conclusion about the optimal treatment, which should rely on the histological subtype. According to our revision, chemotherapy with or without rituximab was the standard of care in most patients (53.6%), sometimes associated to RT (14.0%) or following diagnostic debulking surgery (18.9%). Overall, 26.1% of patients underwent a probably unnecessary surgical treatment.
Similarly to what has been described in the literature for other extranodal lymphomas,129 the recurrence rate was high, at least according to the larger case series (23.52% and 34.21%, respectively).10,51 Extranodal lymphomas may have different patterns of relapse depending on the tissue/organ involved. The CNS-International Prognostic Index (IPI) considers some primitive lymphoma sites (kidney and adrenal gland) as at increased risk of CNS relapse without taking into account pancreas.130 Importantly, according to this literature search, in agreement with our previous findings,51 patients affected by PPL had a relatively high incidence of CNS relapses (20% of the relapses reported). Obviously, no definitive conclusions can be drawn given the relatively small number of patients considered, and their heterogeneity in regard to histology and therapy received.
In conclusion, PPL represents a rare and challenging disease with relatively non-specific symptoms. Most PPLs are DLBCL in adults, BL in children. According to literature, a noninvasive approach should be the preferred diagnostic method. The diagnosis of PPL should be suspected also in pediatric cases. Because reported studies have been retrospective, used undersized patient groups and involved heterogenous regimens, no conclusions can be drawn for an optimal regimen, that should be tailored on histological subtype. The high CNS relapse rate reported in available literature, suggests that patients with PPL may benefit from a CNS directed prophylaxis.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Baylor SM, Berg JW. Cross-classification and survival characteristics of 5,000 cases of cancer of the pancreas. J Surg Oncol. 1973;5(4):335–358. doi: 10.1002/jso.2930050410 [DOI] [PubMed] [Google Scholar]
- 2.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29(1):252–260. [DOI] [PubMed] [Google Scholar]
- 3.Behrns KE, Sarr MG, Strickler JG. Pancreatic lymphoma: is it a surgical disease? Pancreas. 1994;9(5):662–667. doi: 10.1097/00006676-199409000-00019 [DOI] [PubMed] [Google Scholar]
- 4.Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80–89. doi: 10.1002/bjs.18004921319 [DOI] [PubMed] [Google Scholar]
- 5.Fléjou JF. [WHO Classification of digestive tumors: the fourth edition]. Ann Pathol. 2011;31(5 Suppl):S27–31. France. doi: 10.1016/j.annpat.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 6.Jones WF, Sheikh MY, McClave SA. AIDS-related non-Hodgkin’s lymphoma of the pancreas. Am J Gastroenterol. 1997;92(2):335–338. [PubMed] [Google Scholar]
- 7.Rad N, Khafaf A, Alizadeh AHM. Primary pancreatic lymphoma: what we need to know. J Gastrointest Oncol. 2017;8(4):749–757. doi: 10.21037/jgo.2017.06.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning MA, Paal EE, Srivastava A, Mortele KJ. Nonepithelial neoplasms of the pancreas, part 2: malignant tumors and tumors of uncertain malignant potential from the radiologic pathology archives. RadioGraphics. 2018;38(4):1047–1072. doi: 10.1148/rg.2018170201 [DOI] [PubMed] [Google Scholar]
- 9.Mishra MV, Keith SW, Shen X, Ad VB, Champ CE, Biswas T. Primary pancreatic lymphoma. Am J Clin Oncol. 2013;36(1):38–43. doi: 10.1097/COC.0b013e3182354bbb [DOI] [PubMed] [Google Scholar]
- 10.Sadot E, Yahalom J, Do RKG, et al. Clinical features and outcome of primary pancreatic lymphoma. Ann Surg Oncol. 2015;22(4):1176–1184. doi: 10.1245/s10434-014-4176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamadnejad M, Khosravi P, Khani M, Nikmanesh A, Eloubeidi MA. Primary pancreatic Hodgkin’s lymphoma diagnosed on EUS-guided FNA. Gastrointest Endosc. 2016;83(4):844–845. doi: 10.1016/j.gie.2015.09.028 [DOI] [PubMed] [Google Scholar]
- 12.Matsubayashi H, Takagaki S, Otsubo T, et al. Pancreatic T-cell lymphoma with high level of soluble interleukin-2 receptor. J Gastroenterol. 2002;37(10):863–867. doi: 10.1007/s005350200143 [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boni L, Benevento A, Dionigi G, Cabrini L, Dionigi R. Primary pancreatic lymphoma. Surg Endosc. 2002;16(7):1107–1108. doi: 10.1007/s00464-001-4247-1 [DOI] [PubMed] [Google Scholar]
- 15.Bozzoli V, Tisi MC, Pianese L, et al. Primary pancreatic lymphoma in a patient with maturity onset diabetes of the young type 3. Mediterr J Hematol Infect Dis. 2012;4(1):e2012005. doi: 10.4084/MJHID.2012.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghobakhlou M, Mohammad alizadeh AH, Naderi N, et al. A patient with chronic hepatitis C and a pancreatic mass in endoscopic ultrasound. Case Rep Gastroenterol. 2012;6(2):387–393. doi: 10.1159/000339693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Zhang S, Vasdani N, Castillo E. Clues for diagnosing primary pancreatic lymphoma. Case Rep Gastroenterol. 2012;6(2):438–445. doi: 10.1159/000339968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Hua R, Zhang JF, Huo YM, Liu DJ, Sun YW. First report of primary pancreatic natural killer/T-cell nasal type lymphoma. Eur Rev Med Pharmacol Sci. 2013;17(3):318–322. [PubMed] [Google Scholar]
- 19.Carbonetti F, Iannicelli E, Federici M, et al. Primary pancreatic burkitt lymphoma presenting as acute pancreatitis. J Gastrointest Cancer. 2014;45(1):265–269. doi: 10.1007/s12029-014-9657-0 [DOI] [PubMed] [Google Scholar]
- 20.Abedi SH, Ahmadzadeh A, Nikmanesh A, et al. The role of endoscopic ultrasound in primary pancreatic lymphoma presented with acute pancreatitis: a case report. JOP. 2014;15(5):493–496. doi: 10.6092/1590-8577/2637 [DOI] [PubMed] [Google Scholar]
- 21.Nakaji S, Hirata N, Shiratori T, et al. A case of primary pancreatic lymphoblastic lymphoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Clin J Gastroenterol. 2014;7(2):180–184. doi: 10.1007/s12328-014-0462-x [DOI] [PubMed] [Google Scholar]
- 22.Knibbeler-van Rossum CTAM, Peters FJP, Erdkamp FLG, Bos LP. Unusual presentation of Hodgkin’s lymphoma. Ann Oncol. 2002;13(4):637. doi: 10.1093/annonc/mdf148 [DOI] [PubMed] [Google Scholar]
- 23.Levy MJ, Wiersema MJ. Endoscopic removal of a Biliary Wallstent with a suture-cutting device in a patient with primary pancreatic lymphoma. Endoscopy. 2002;34(10):835–837. doi: 10.1055/s-2002-34258 [DOI] [PubMed] [Google Scholar]
- 24.Kurosawa H, Matsunaga T, Shimaoka H, et al. Burkitt lymphoma associated with large gastric folds, pancreatic involvement, and biliary tract obstruction. J Pediatr Hematol Oncol. 2002;24(4):310–312. doi: 10.1097/00043426-200205000-00018 [DOI] [PubMed] [Google Scholar]
- 25.Cohen Y, Libster D, Amir G, et al. Primary ALK positive anaplastic large cell lymphoma of the pancreas. Leuk Lymphoma. 2003;44(1):205–207. doi: 10.1080/1042819021000054715 [DOI] [PubMed] [Google Scholar]
- 26.Yoshida N, Nakamura M, Yamada H. Primary pancreatic lymphoma forming a giant mass in a short period: a case report. J Med Ultrason. 1999;26(12):1205–1210. doi: 10.1007/bf02481373 [DOI] [PubMed] [Google Scholar]
- 27.Reddy D, Gumaste V, Benisovich V. Primary pancreatic lymphoma presenting as acute pancreatitis. Pancreatology. 2003;3(5):403–405. doi: 10.1159/000073656 [DOI] [PubMed] [Google Scholar]
- 28.Nayer H, Weir EG, Sheth S, Ali SZ. Primary pancreatic lymphomas: a cytopathologic analysis of a rare malignancy. Cancer. 2004;102(5):315–321. doi: 10.1002/cncr.20488 [DOI] [PubMed] [Google Scholar]
- 29.Volmar KE, Routbort MJ, Jones CK, Xie HB. Primary pancreatic lymphoma evaluated by fine-needle aspiration: findings in 14 cases. Am J Clin Pathol. 2004;121(6):898–903. doi: 10.1309/UAD9PYFUA82X9R9U [DOI] [PubMed] [Google Scholar]
- 30.Lee ACW, Li CH. Burkitt lymphoma presenting as acute pancreatitis: report of 3 cases and review of the literature. J Pediatr Hematol Oncol. 2020;42(8):e830–e834. doi: 10.1097/MPH.0000000000001630 [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Cheng X, Huo L, Li F. Primary pancreatic lymphoma on FDG PET/CT. Clin Nucl Med. 2020;45(10):830–832. doi: 10.1097/RLU.0000000000003205 [DOI] [PubMed] [Google Scholar]
- 32.Chon HK, Choi KH, Kim TH. Primary pancreatic natural killer/T-cell lymphoma. Pancreas. 2020;49(3):E21–E23. doi: 10.1097/MPA.0000000000001508 [DOI] [PubMed] [Google Scholar]
- 33.Pezzilli R, De Giorgio R, Ceciliato R, et al. A case of primary pancreatic lymphoma. JOP. 2004;5(2):105–106. [PubMed] [Google Scholar]
- 34.Koniaris LG, Lillemoe KD, Yeo CJ, et al. Is there a role for surgical resection in the treatment of early-stage pancreatic lymphoma? J Am Coll Surg. 2000;190(3):319–330. doi: 10.1016/S1072-7515(99)00291-4 [DOI] [PubMed] [Google Scholar]
- 35.Fraser CJ, Chan YF, Heath JA. Anaplastic large cell lymphoma of the pancreas: a pediatric case and literature review. J Pediatr Hematol Oncol. 2004;26(12):840–842. [PubMed] [Google Scholar]
- 36.Yoon SN, Lee MH, Yoon JK. F-18 FDG positron emission tomography findings in primary pancreatic lymphoma. Clin Nucl Med. 2004;29(9):574–575. doi: 10.1097/01.rlu.0000135269.00531.f8 [DOI] [PubMed] [Google Scholar]
- 37.Turkish A, Levy J, Kato M, et al. Pancreatitis and probable paraneoplastic cholestasis as presenting manifestations of pancreatic lymphoma in a child. J Pediatr Gastroenterol Nutr. 2004;39(5):552–556. doi: 10.1097/00005176-200411000-00020 [DOI] [PubMed] [Google Scholar]
- 38.Arcari A, Anselmi E, Bernuzzi P, et al. Primary pancreatic lymphoma. A report of five cases. Haematologica. 2005;90(1):7–9. [PubMed] [Google Scholar]
- 39.Shahar KH, Carpenter LS, Jorgensen J, Truong L, Baker K, Teh BS. Role of radiation therapy in a patient with primary pancreatic lymphoma. Clin Lymphoma Myeloma. 2005;6(2):143–145. doi: 10.3816/CLM.2005.n.042 [DOI] [PubMed] [Google Scholar]
- 40.Chim CS, Ho J, Ooi GC, Choy C, Liang R. Primary anaplastic large cell lymphoma of the pancreas. Leuk Lymphoma. 2005;46(3):457–459. doi: 10.1080/1042819040007474 [DOI] [PubMed] [Google Scholar]
- 41.Ji Y, Kuang TT, Tan YS, Chen Y, Zeng H-Y, Jin DY. Pancreatic primary lymphoma: a case report and review of the literature. Hepatobiliary Pancreat Dis Int. 2005;4(4):622–626. [PubMed] [Google Scholar]
- 42.Savopoulos CG, Tsesmeli NE, Kaiafa GD, et al. Primary pancreatic anaplastic large cell lymphoma, ALK negative: a case report. World J Gastroenterol. 2005;11(39):6221–6224. doi: 10.3748/wjg.v11.i39.6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mimery A, De Clercq S. Primary pancreatic lymphoma presenting as recurrent idiopathic pancreatitis: a diagnostic dilemma. ANZ J Surg. 2020;90(4):E81–E82. doi: 10.1111/ans.15309 [DOI] [PubMed] [Google Scholar]
- 44.Tikue A, Bedanie G, Brandi L, Islam S, Nugent K. Primary pancreatic large B-cell lymphoma presenting as acute pancreatitis. Cureus. 2020;12(8). doi: 10.7759/cureus.9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueda K, Nagayama Y, Narita K, Kusano M, Mernyei M, Kamiya M. Pancreatic involvement by non-Hodgkin’s lymphoma. J Hepatobiliary Pancreat Surg. 2000;7(6):610–613. doi: 10.1007/s005340070013 [DOI] [PubMed] [Google Scholar]
- 46.Kopel J, Swarup K, Thein K, Swarup S. Primary B cell lymphoma of the pancreas. J Gastrointest Cancer. 2020;51(3):1077–1080. doi: 10.1007/s12029-020-00400-4 [DOI] [PubMed] [Google Scholar]
- 47.McCullagh D, McNeice A, Rice P, Mainie I. Primary pancreatic lymphoma. Ulster Med J. 2020 Jan;89(1):41-42. Available from: https://pubmed.ncbi.nlm.nih.gov/32218630/. Accessed January13, 2021. [PMC free article] [PubMed]
- 48.Alzerwi NAN. Primary pancreatic lymphoma masquerading as carcinoma. Case Rep Oncol Med. 2020;2020:1–6. doi: 10.1155/2020/5160545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunphy L, Abbas SH, Al Shoek I, Al-Salti W. Primary pancreatic lymphoma: a rare clinical entity. BMJ Case Rep. 2020;13(1):e231292. doi: 10.1136/bcr-2019-231292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savari O, Al-Duwal Z, Wang Z, Ganesan S, Danan-Rayes R, Ayub S. Pancreatic lymphoma: a cytologic diagnosis challenge. Diagn Cytopathol. 2020;48(4):350–355. doi: 10.1002/dc.24349 [DOI] [PubMed] [Google Scholar]
- 51.Facchinelli D, Sina S, Boninsegna E, et al. Primary pancreatic lymphoma: clinical presentation, diagnosis, treatment, and outcome. Eur J Haematol. 2020;105(4):468–475. doi: 10.1111/ejh.13468 [DOI] [PubMed] [Google Scholar]
- 52.Lin H, Li SD, Hu XG, Li ZS. Primary pancreatic lymphoma: report of six cases. World J Gastroenterol. 2006;12(31):5064–5067. doi: 10.3748/wjg.v12.i31.5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grimison PS, Chin MT, Harrison ML, Goldstein D. Primary pancreatic lymphoma - pancreatic tumours that are potentially curable without resection, a retrospective review of four cases. BMC Cancer. 2006;6:1–9. doi: 10.1186/1471-2407-6-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battula N, Srinivasan P, Prachalias A, Rela M, Heaton N. Primary pancreatic lymphoma: diagnostic and therapeutic dilemma. Pancreas. 2006;33(2):192–194. doi: 10.1097/01.mpa.0000227910.63579.15 [DOI] [PubMed] [Google Scholar]
- 55.Meier C, Kapellen T, Tröbs RB, et al. Temporary diabetes mellitus secondary to a primary pancreatic Burkitt lymphoma. Pediatr Blood Cancer. 2006;47(1):94–96. doi: 10.1002/pbc.20752 [DOI] [PubMed] [Google Scholar]
- 56.Islam S, Callery MP. Primary pancreatic lymphoma—a diagnosis to remember. Surgery. 2001;129(3):380–382. doi: 10.1067/msy.2001.113284 [DOI] [PubMed] [Google Scholar]
- 57.Saif MW. Primary pancreatic lymphomas. JOP. 2006;7(3):262–273. [PubMed] [Google Scholar]
- 58.Lee MK, Jeon SW, Lee YD, et al. A case of primary pancreatic non-Hodgkin’s lymphoma. Korean J Intern Med. 2006;21(2):123–126. doi: 10.3904/kjim.2006.21.2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YJ, Jeng CM, Wang YC, Chang PP, Wang TH. Primary pancreatic Burkitt’s lymphoma mimicking carcinoma with obstructive jaundice and very high CA19-9. Eur J Gastroenterol Hepatol. 2006;18(5):537–540. doi: 10.1097/00042737-200605000-00014 [DOI] [PubMed] [Google Scholar]
- 60.Alldinger I, Peiper M, Diallo R, et al. Primary pancreatic lymphoma - A rare cause of cholestasis leading to surgical treatment [1]. Ann Hematol. 2006;85(7):485–486. doi: 10.1007/s00277-006-0102-8 [DOI] [PubMed] [Google Scholar]
- 61.Dyckmans K, Lerut E, Gillard P, et al. Post-transplant lymphoma of the pancreatic allograft in a kidney-pancreas transplant recipient: a misleading presentation. Nephrol Dial Transplant. 2006;21(11):3306–3310. doi: 10.1093/ndt/gfl382 [DOI] [PubMed] [Google Scholar]
- 62.Sallapan S, Abu Bakar NZ, Jarmin R, et al. Primary follicular lymphoma of the pancreas: a rare tumour mimicking pancreatic carcinoma. Malaysian J Pathol .2018; 40(3) : 359 – 371. Available from: https://pubmed.ncbi.nlm.nih.gov/30580370/. Accessed January13, 2021. [PubMed]
- 63.Ito H, Hiraiwa SI, Sugiyama T, et al. An autopsy case of primary extranodal NK/T cell lymphoma (extranodal NK/T-cell lymphoma) of the bile duct. Clin J Gastroenterol. 2019;12(3):209–212. doi: 10.1007/s12328-018-00931-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez-Infante A, Fernández-Martínez D, Iglesias-García E, García-Flórez LJ. Linfoma pancreático primario como causa de ictericia obstructiva. Rev Gastroenterol Mex. 2018;4–5. doi: 10.1016/j.rgmx.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 65.Badrinath M, Tambe A, Mandru R, Saleem S, Heisig D. Large pancreatic mass with chylous ascites. Baylor Univ Med Cent Proc. 2020;33(1):53–54. doi: 10.1080/08998280.2019.1668661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Z, Li M, He D, et al. Two-dimensional texture analysis based on CT images to differentiate pancreatic lymphoma and pancreatic adenocarcinoma: a preliminary study. Acad Radiol. 2019;26(8):e189–e195. doi: 10.1016/j.acra.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 67.Eisenhuber E, Schoefl R, Wiesbauer P, Bankier AA. Primary pancreatic lymphoma presenting as acute pancreatitis in a child. Med Pediatr Oncol. 2001;37(1):53–54. doi: 10.1002/mpo.1163 [DOI] [PubMed] [Google Scholar]
- 68.Hughes B, Habib N, Chuang KY. Primary anaplastic large cell lymphoma of the pancreas. ACG Case Reports J. 2019;6(10):e00231. doi: 10.14309/crj.0000000000000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zafar Y, Kaur A, Banno F, Anuj S. Primary pancreatic lymphoma: an uncommon presentation in the pancreatic tail. Cureus. 2019;11(8). doi: 10.7759/cureus.5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim GE, Bingham S, Gariepy C. Pancreatic Non-Hodgkin lymphoma presenting as pancreatitis and duodenal polyps in a pediatric patient. J Pediatr Gastroenterol Nutr. 2019;69(5):e146–e147. doi: 10.1097/MPG.0000000000002463 [DOI] [PubMed] [Google Scholar]
- 71.Yu L, Chen Y, Xing L. Primary pancreatic lymphoma: two case reports and a literature review. Onco Targets Ther. 2017;10:1687–1694. doi: 10.2147/OTT.S121521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhagat VH, Sepe T. Pancreatic lymphoma complicating early stage chronic hepatitis C. BMJ Case Rep. 2017;2017:bcr2016216698. doi: 10.1136/bcr-2016-216698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amine B, Najat L, Ilias T. Epigastralgia revealing primary pancreatic large B-cell lymphoma in a young patient: about a case. Pan Afr Med J. 2018;31:161. doi: 10.11604/pamj.2018.31.161.16850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blouhos K, Boulas KA, Paraskeva A, Kariotis I, Barettas N, Hatzigeorgiadis A. Obstructive jaundice as primary presentation of a stage IIE Non-Hodgkin lymphoma: a decision making process between advanced lymphoma and locally advanced/metastatic pancreatic adenocarcinoma. Int J Surg Case Rep. 2018;44:226–229. doi: 10.1016/j.ijscr.2018.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konjeti VR, Hefferman GM, Paluri S, Ganjoo P. Primary pancreatic Burkitt’s lymphoma: a case report and review of the literature. Case Rep Gastrointest Med. 2018;2018:5952315. doi: 10.1155/2018/5952315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshizawa N, Inoue H, Yamada R, Takei Y. Pancreatic Burkitt’s lymphoma presenting as an unusual cause of obstructive jaundice. J Dig Dis. 2018;19(8):508–510. doi: 10.1111/1751-2980.12601 [DOI] [PubMed] [Google Scholar]
- 77.Cagle BA, Holbert BL, Wolanin S, Tappouni R, Lalwani N. Knife wielding radiologist: a case report of primary pancreatic lymphoma. Eur J Radiol Open. 2018;5:141–146. doi: 10.1016/j.ejro.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishimura R, Tsujimoto M, Nakao K, et al. Usefulness of intraoperative cytology for the diagnosis of primary pancreatic lymphoma. Acta Cytol. 2001;45(1):104–108. doi: 10.1159/000327196 [DOI] [PubMed] [Google Scholar]
- 79.Magalhães-Costa P, Brito MJ, Pinto-Marques P. A diffusely enlarged pancreas: the (un)usual suspect. Rev Esp Enfermedades Dig. 2016;108(12):809–811. [PubMed] [Google Scholar]
- 80.Inoue A, Sawada Y, Ohmori S, et al. Erythema papulosa semicircularis recidivans associated with primary pancreas B cell lymphoma. Eur J Dermatol. 2016;26(3):306–307. doi: 10.1684/ejd.2016.2749 [DOI] [PubMed] [Google Scholar]
- 81.Lachter J, Balanson S, Ilivitzki A. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of Burkitt’s lymphoma in the pancreas of a 3 year old. Dig Endosc. 2016;28(5):613. doi: 10.1111/den.12648 [DOI] [PubMed] [Google Scholar]
- 82.Shapira G, Fisher Y, Ilivitzki A. Bifocal primary pancreatic Burkitt’s lymphoma in a 4-year-old child. J Clin Ultrasound. 2017;45(3):171–174. doi: 10.1002/jcu.22372 [DOI] [PubMed] [Google Scholar]
- 83.Zheng S-M, Zhou D-J, Chen Y-H, et al. Pancreatic T/histiocyte-rich large B-cell lymphoma: a case report and review of literature. World J Gastroenterol. 2017;23(24):4467. doi: 10.3748/wjg.v23.i24.4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Araújo J, Sampaio Macedo C, Sousa L. Pancreas Burkitt primary lymphoma in pediatric age. Rev Esp Enferm Dig. 2017;109(6):451. [PubMed] [Google Scholar]
- 85.Qiu T, Li W, Geng H, Shi S. Clinicopathological characteristics of primary pancreatic lymphoma: report of two cases. Int J Clin Exp Pathol. 2017;10(11):10941–10946. [PMC free article] [PubMed] [Google Scholar]
- 86.Gleeson FC, Zhang L, Levy MJ. Primary pancreatic lymphoma: endoscopic ultrasound-guided Trucut biopsy to the rescue! Endoscopy. 2008;40(Suppl 2):E23–E24. doi: 10.1055/s-2007-966960 [DOI] [PubMed] [Google Scholar]
- 87.Fernández-Plaza S, Sevilla J, Diaz MA, Madero L, Tamariz-Martel A. Dyspnea as the first manifestation of primary pancreatic lymphoma. Pediatr Blood Cancer. 2008;50(2):434. doi: 10.1002/pbc.21242 [DOI] [PubMed] [Google Scholar]
- 88.Sağlam M, Yilmaz MI, Mas MR, et al. A case of pancreatic Burkitt lymphoma: radiological findings. Diagn Interv Radiol. 2009;15(1):39–42. [PubMed] [Google Scholar]
- 89.Nishimura R, Takakuwa T, Hoshida Y, Tsujimoto M, Aozasa K. Primary pancreatic lymphoma: clinicopathological analysis of 19 cases from Japan and review of the literature. Oncology. 2001;60(4):322–329. doi: 10.1159/000058528 [DOI] [PubMed] [Google Scholar]
- 90.Nistala SS, Sawalakhe NR, Thiruvengadam NR, Rathi PM. A rare case of primary pancreatic Burkitt lymphoma in a young Indian male. Case report and review of the literature. JOP. 2009;10(6):686–689. [PubMed] [Google Scholar]
- 91.Haji AG, Sharma S, Majeed KA, Vijaykumar DK, Pavithran K, Dinesh M. Primary pancreatic lymphoma: report of three cases with review of literature. Indian J Med Paediatr Oncol. 2009;30(1):20–23. doi: 10.4103/0971-5851.56331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Francisco B, Lucas M, Del Mar AM, Maria FNJ, Manuel FS, Amparo V. Abdominal pain as the first manifestation of primary pancreatic lymphoma. J Pediatr Hematol Oncol. 2009;31(3):222–223. doi: 10.1097/MPH.0b013e3181979e28 [DOI] [PubMed] [Google Scholar]
- 93.Miwa I, Maruyama Y, Kageoka M, et al. A case of pancreatic mucosa-associated lymphoid tissue (MALT) lymphoma. Japanese J Gastroenterol. 2008;105(12):1794–1801. doi: 10.11405/nisshoshi.105.1794 [DOI] [PubMed] [Google Scholar]
- 94.Baysal B, Kayar Y, Ince AT, Arici S, Türkmen I, Şentürk H. Primary pancreatic lymphoma is a rare cause of pancreatic mass: a case report. Oncol Lett. 2015;10(3):1701–1703. doi: 10.3892/ol.2015.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shirai Y, Okamoto T, Kanehira M, et al. Pancreatic follicular lymphoma presenting as acute pancreatitis: report of a case. Int Surg. 2015;100(6):1078–1083. doi: 10.9738/INTSURG-D-14-00132.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koca T, Aslan N, Dereci S, Akcam M. Burkitt lymphoma with unusual presentation: acute pancreatitis. Pediatr Int. 2015;57(4):775–777. doi: 10.1111/ped.12640 [DOI] [PubMed] [Google Scholar]
- 97.Fukuba N, Moriyama I, Ishihara S, et al. Primary pancreatic malignant lymphoma diagnosed from endoscopic ultrasound-guided fine-needle aspiration findings. Intern Med. 2016;55(1):31–35. doi: 10.2169/internalmedicine.55.5749 [DOI] [PubMed] [Google Scholar]
- 98.Abe Y, Tamura K, Sakata I, et al. Unique intense uptake demonstrated by 18F-FDG positron emission tomography/computed tomography in primary pancreatic lymphoma: a case report. Oncol Lett. 2010;1(4):605–607. doi: 10.3892/ol_00000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sugishita H, Watanabe Y, Yamamoto Y, et al. Primary pancreatic lymphoma: the role of surgical treatment. Case Rep Gastroenterol. 2010;4(1):104–110. doi: 10.1159/000283405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pietsch JB, Shankar S, Ford C, Johnson JE. Obstructive jaundice secondary to lymphoma in childhood. J Pediatr Surg. 2001;36(12):1792–1795. doi: 10.1053/jpsu.2001.28840 [DOI] [PubMed] [Google Scholar]
- 101.Yoon WJ, Yoon YB, Kim YJ, Ryu JK, Kim YT. Primary pancreatic lymphoma in Korea-a single center experience. J Korean Med Sci. 2010;25(4):536–540. doi: 10.3346/jkms.2010.25.4.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rossi ED, Larghi A, Verna EC, et al. Endoscopic ultrasound-guided fine-needle aspiration with liquid-based cytologic preparation in the diagnosis of primary pancreatic lymphoma. Pancreas. 2010;39(8):1299–1302. doi: 10.1097/MPA.0b013e3181dc694e [DOI] [PubMed] [Google Scholar]
- 103.Kav T, Soyuoz AG, Altundag K, Bayraktar Y. Primary pancreatic lymphoma or secondary involvement: what is the difference? JOP. 2010;11:482–483. doi: 10.6092/1590-8577/3442 [DOI] [PubMed] [Google Scholar]
- 104.Ravi K, Sanchez W, Sweetser S. Primary pancreatic follicular lymphoma mimicking adenocarcinoma. Clin Gastroenterol Hepatol. 2010;8(10):e101–e102. doi: 10.1016/j.cgh.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 105.Alexander RE, Nakeeb A, Sandrasegaran K, et al. Primary pancreatic follicle center-derived lymphoma masquerading as carcinoma. Gastroenterol Hepatol. 2011;7(12):834–838. [PMC free article] [PubMed] [Google Scholar]
- 106.Federico E, Falconi M, Zuodar G, Falconieri G, Puglisi F. B-cell lymphoma presenting as acute pancreatitis. Pancreatology. 2011;11(6):553–556. doi: 10.1159/000332038 [DOI] [PubMed] [Google Scholar]
- 107.Rehbinder B, Wullstein C, Bechstein WO, et al. Epstein-barr virus-associated posttransplant lymphoproliferative disorder of donor origin after simultaneous pancreas-kidney transplantation limited to pancreas allograft. Am J Transplant. 2006;6(10):2506–2511. doi: 10.1111/j.1600-6143.2006.01464.x [DOI] [PubMed] [Google Scholar]
- 108.Sata N, Kurogochi A, Endo K, Shimura K, Koizumi M, Nagai H. Follicular lymphoma of the pancreas: a case report and proposed new strategies for diagnosis and surgery of benign or low-grade malignant lesions of the head of the pancreas. JOP. 2007;8(1):44–49. [PubMed] [Google Scholar]
- 109.Basu A, Patil N, Mohindra P, et al. Isolated non-Hodgkin’s lymphoma of the pancreas: case report and review of literature. J Cancer Res Ther. 2007;3(4):236–239. doi: 10.4103/0973-1482.39000 [DOI] [PubMed] [Google Scholar]
- 110.Jayanthi V, Randhir J, Rajesh N. Problems in diagnosing lymphoma of the pancreas with computed tomography. A case report. J Gastrointest Liver Dis. 2007;16(1):101–103. [PubMed] [Google Scholar]
- 111.Piesman M, Forcione DG, Carr-Locke DL. A case of pancreatic lymphoma. Med Gen Med Medscape Gen Med. 2007;9(3). [PMC free article] [PubMed] [Google Scholar]
- 112.Liakakos T, Misiakos EP, Tsapralis D, Nikolaou I, Karatzas G, Macheras A. A role for surgery in primary pancreatic B-cell lymphoma: a case report. J Med Case Rep. 2008;2:1–6. doi: 10.1186/1752-1947-2-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakamura T, Ito T, Abe Y, et al. Primary pancreatic low-grade mucosa-associated lymphoid tissue lymphoma presenting with multiple masses. Clin J Gastroenterol. 2008;1(4):168–173. doi: 10.1007/s12328-008-0028-x [DOI] [PubMed] [Google Scholar]
- 114.Boninsegna E, Zamboni GA, Facchinelli D, et al. CT imaging of primary pancreatic lymphoma: experience from three referral centres for pancreatic diseases. Insights Imaging. 2018;9(1):17–24. doi: 10.1007/s13244-017-0585-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mukhija D, Nagpal SJS, Sohal DPS. Epidemiology, tumor characteristics, and survival in patients with primary pancreatic lymphoma: a large population-based study using the seer database. Am J Clin Oncol Cancer Clin Trials. 2019;42(5):454–458. doi: 10.1097/COC.0000000000000544 [DOI] [PubMed] [Google Scholar]
- 116.Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42(1):20–26. doi: 10.1097/MPA.0b013e3182546e79 [DOI] [PubMed] [Google Scholar]
- 117.Ramesh J, Hebert-Magee S, Kim H, Trevino J, Varadarajulu S. Frequency of occurrence and characteristics of primary pancreatic lymphoma during endoscopic ultrasound guided fine needle aspiration: a retrospective study. Dig Liver Dis. 2014;46(5):470–473. doi: 10.1016/j.dld.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson EA, Benson ME, Guda N, Pfau PR, Frick TJ, Gopal DV. Differentiating primary pancreatic lymphoma from adenocarcinoma using endoscopic ultrasound characteristics and flow cytometry: a case-control study. Endosc Ultrasound. 2014;3(4):221–225. doi: 10.4103/2303-9027.144530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khashab M, Mokadem M, Dewitt J, et al. Endoscopic ultrasound-guided fine-needle aspiration with or without flow cytometry for the diagnosis of primary pancreatic lymphoma a case series. Endoscopy. 2010;42(3):228–231. doi: 10.1055/s-0029-1243859 [DOI] [PubMed] [Google Scholar]
- 120.Anand D, Lall C, Bhosale P, Ganeshan D, Qayyum A. Current update on primary pancreatic lymphoma. Abdom Radiol. 2016;41(2):347–355. doi: 10.1007/s00261-015-0620-8 [DOI] [PubMed] [Google Scholar]
- 121.Webb TH, Lillemoe KD, Pitt HA, Jones RJ, Cameron JL. Pancreatic lymphoma. Is surgery mandatory for diagnosis or treatment? Ann Surg. 1989;209(1):25–30. doi: 10.1097/00000658-198901000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zucca E, Roggero E, Bertoni F, Cavalli F. Primary extranodal non-Hodgkin’s lymphomas. Part 1: gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol. 1997;8(8):727–737. doi: 10.1023/A:1008282818705 [DOI] [PubMed] [Google Scholar]
- 123.Ng YY, Healy JC, Vincent JM, Kingston JE, Armstrong P, Reznek RH. The radiology of non-Hodgkin’s lymphoma in childhood: a review of 80 cases. Clin Radiol. 1994;49(9):594–600. doi: 10.1016/S0009-9260(05)81874-4 [DOI] [PubMed] [Google Scholar]
- 124.Vade A, Blane CE. Imaging of Burkitt lymphoma in pediatric patients. Pediatr Radiol. 1985;15(2):123–126. doi: 10.1007/BF02388718 [DOI] [PubMed] [Google Scholar]
- 125.Klöppel G, Solcia E, Sobin LH, et al. Histological Typing of Tumours of the Exocrine Pancreas. Springer Science & Business Media; 1996. doi: 10.1007/978-3-642-61024-0_2 [DOI] [Google Scholar]
- 126.Kanno A, Masamune A, Hanada K, Kikuyama M, Kitano M. Advances in early detection of pancreatic cancer. Diagnostics. 2019;9(1):18. doi: 10.3390/diagnostics9010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Pretis N, Amodio A, Frulloni L. Updates in the field of autoimmune pancreatitis: a clinical guide. Expert Rev Gastroenterol Hepatol. 2018;12(7):705–709. doi: 10.1080/17474124.2018.1489230 [DOI] [PubMed] [Google Scholar]
- 128.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vitolo U, Seymour JF, Martelli M, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl5):mdw175. doi: 10.1093/annonc/mdw175 [DOI] [PubMed] [Google Scholar]
- 130.Ollila TA, Olszewski AJ. Extranodal diffuse large B cell lymphoma: molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol. 2018;19(8):38. doi: 10.1007/s11864-018-0555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]