Abstract
Background
Although perineural invasion is a well known prognostic factor used in several cancers, its prognostic role in esophageal squamous cell carcinoma remains controversial. Here, we investigated the prognostic role of perineural invasion in surgically treated esophageal squamous cell carcinoma.
Methods
We retrospectively reviewed the medical records of 316 patients who underwent esophagectomy and lymph node dissection for esophageal squamous cell carcinoma between 2007 and 2016.
Results
Overall, 287 men (mean age: 62.73 ± 7.97 years) were included in the study. The median follow‐up period was 35.97 ± 30.99 months, perineural invasion was confirmed in 25 patients, and three‐year overall and disease‐free survival were significantly lower in the perineural invasion group than in the no‐perineural invasion group (75.9% vs. 40.0%, p < 0.001; 70.3% vs. 21.6%, p < 0.001). Cumulative incidences of locoregional recurrence and distant metastasis over three years were higher in the perineural invasion group (13.8% vs. 9.6%, p = 0.009 and 52.8% vs. 14.6%, p < 0.001). On performing multivariable analysis, perineural invasion, pathological stage, incomplete resection, and neoadjuvant therapy were adverse risk factors for disease‐free survival. The concordance index increased when perineural invasion was included in the model (0.712 vs. 0.723). On subgroup analysis, perineural invasion demonstrated a prognostic value in node‐negative patients (79.4% vs. 35.7%, p = 0.012).
Conclusions
Perineural invasion was found to be an adverse risk factor for disease‐free survival in surgically treated patients with esophageal squamous cell carcinoma. Close observation and individualized adjuvant therapy may be helpful for patients with perineural invasion.
Keywords: esophageal squamous cell carcinoma, perineural invasion, prognosis
Perineural invasion is an independent prognostic factor for shorter disease‐free survival in patients treated surgically for esophageal squamous cell carcinoma. Documenting and closely following up with the perineural invasion status may significantly help physicians in devising therapeutic modalities to improve oncological outcome.
INTRODUCTION
Esophageal cancer is one of the most invasive cancers worldwide, with the highest incidence rates found in Eastern Asia and Southern and Eastern Africa. 1 The dominant type of esophageal cancer in Eastern Asia is esophageal squamous cell carcinoma (ESCC). Despite recent improvements in diagnostic and therapeutic modalities, its prognosis remains poor, with an overall survival rate of 15%–25% and a high risk of metastases and recurrence. 2 , 3 Prognostication of ESCC using the Tumor, Node and Metastasis (TNM) classification system of the American Joint Committee on Cancer is widely accepted. 4 Although the TNM system can provide a satisfactory prediction of survival, its accuracy is variable; for example, even for superficial tumors, ESCC showed significant rates of metastases and recurrences, which is related to poor outcomes. 3 , 5 Therefore, identifying new prognostic factors for ESCC in addition to the current staging system is important for optimizing treatment and improving outcomes.
Esophageal cancer cells spread through direct infiltration of vascular or lymphatic channels. This pattern of invasion is well characterized and precedes metastases and disease recurrence in ESCC. In addition to these metastatic routes, perineural invasion (PNI) can occur, which is the neoplastic invasion of nerve cells. 6 , 7 PNI is most commonly reported in head and neck cancers, with an incidence as high as 80%; it is considered a significant risk factor for invasion and metastasis and is therefore regarded as a negative prognostic factor. 8 However, the significance of PNI in ESCC remains controversial; while some studies suggest that PNI status is related to a poor prognosis in ESCC, others question its role as a predictor of prognosis. 6 , 9 , 10 , 11 Therefore, this retrospective study investigated the prognostic effect of PNI in surgically treated ESCC patients. We present the following article in accordance with the STROBE reporting checklist.
METHODS
Patients
We retrospectively reviewed the medical records of patients with ESCC who were surgically treated between January 2007 and December 2016. Patients with operative mortality (n = 15) and who underwent a procedure with noncurative intent (n = 12) were excluded. A total of 316 patients underwent esophagectomy and lymph node dissection for thoracic ESCC. The clinicopathological data of the patients were recorded in a prospectively established institutional database. This study was approved by the Institutional Review Board of Yonsei University College of Medicine (IRB No: 4–2020‐0770). The requirement to obtain informed consent was waived owing to the retrospective nature of the study by the aforementioned Institutional Review Board.
Preoperative endoscopic biopsy confirmed histological diagnosis. The staging workup consisted of ultrasonography, chest and abdominopelvic computed tomography (CT), and positron emission tomography. The lymph node (LN) station was utilized by the 11th Japanese Guidelines for Clinical Pathological Studies on Carcinoma of the Esophagus. 12 After the workup, patients with locally advanced cancers (T3 or T4a) or multiple LN metastases were assigned to neoadjuvant chemoradiation or chemotherapy following institutional policy. Upfront McKeown transthoracic esophagectomy was performed for clinically resectable T1N1–2 ESCC following institutional guidelines. Total mediastinal lymphadenectomy comprising dissection of bilateral recurrent laryngeal nerve nodes was routinely performed, except for patients with significant comorbidities. For a minimally invasive approach, thoracoscopic or robot‐assisted esophagectomies were performed. Bilateral neck dissection under the collar incision was performed in patients with upper esophageal cancer, and with middle or lower esophageal cancer when clinically evident metastases at the upper mediastinum or cervical area were suspected. Usually, a gastric tube was used to reconstruct the esophagus. Gastric mobilization and upper abdominal LN dissection were performed using a laparoscopic or laparotomy approach based on the surgeon's preferences. Esophagogastrostomy was performed with a circular stapler for intrathoracic anastomoses, and circular stapling or the two‐layered hand‐sewn method was employed for cervical anastomoses.
Chest and abdominopelvic CT scans were obtained postoperatively at six‐month intervals to detect recurrence. Locoregional recurrence was defined as disease occurring at the anastomosis site, or at sites where LN dissection had been performed. Distant recurrence was defined as recurrence outside the operative field, such as the lung, brain, liver, and bone. Recurrence was confirmed on tissue biopsy if clinically indicated.
Pathological evaluation
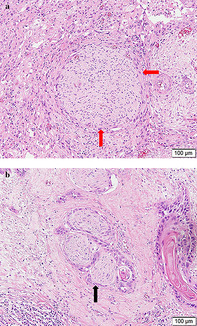
Histopathological slides were reviewed by an expert pathologist in esophageal cancer from our institution who was blinded to postoperative outcome and prognosis. Specimens were stained with hematoxylin and eosin (H&E), and the presence of PNI was carefully examined. When viable tumor cells were identified within any of the three layers of the nerve sheath, PNI was considered to be present. In cases where tumor cells were not located within the nerve sheath but close to the perineural environment, the criterion for PNI diagnosis included involvement of tumor cells around ≥33% of the nerve circumference (Figure 1(a) and (b)). 8 , 13
FIGURE 1.
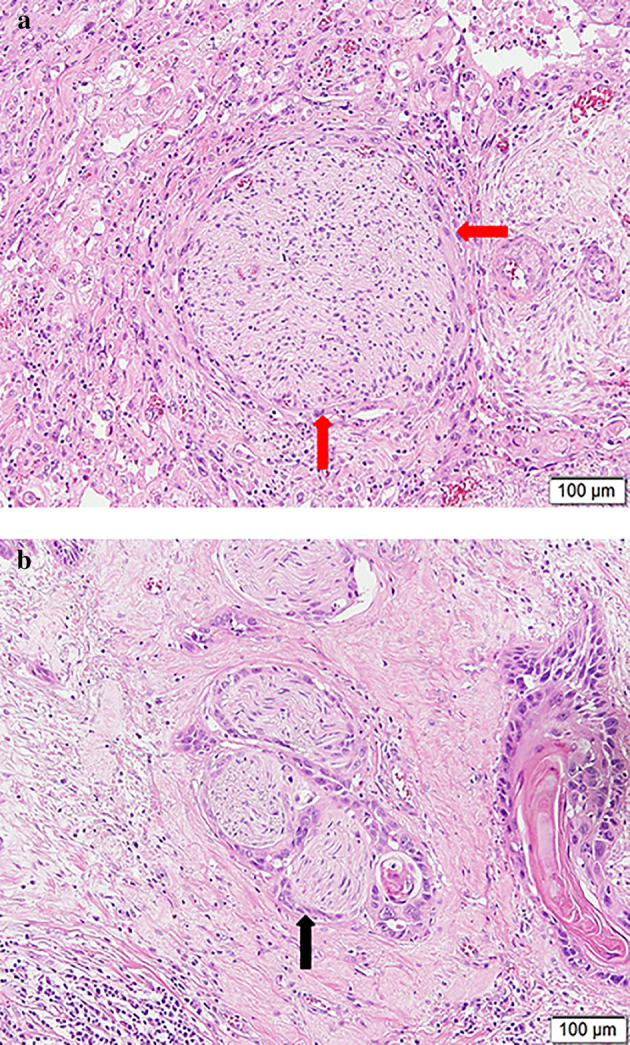
The presence of perineural invasion in an esophageal squamous cell carcinoma specimen stained with hematoxylin and eosin. (a) The nerve fiber was partially surrounded by tumor cells (red arrow). (b) Tumor cells embedded in the perineurium (black arrow)
Statistical analysis
Clinicopathological parameters are described as means ± standard deviations for continuous variables and as frequencies (%) for categorical variables. Student's t‐test, chi‐square, or Fisher's exact tests were applied for the assessment of differences between groups. Overall survival (OS) was estimated from the date of surgery to the date of the last follow‐up or death from any cause. Disease‐free survival (DFS) extended from the day of surgery to the date of the first recurrence, last follow‐up, or death. We applied the Kaplan–Meier method to analyze survival rates and cumulative hazard. Risk factors were analyzed using logistic regression analysis. Because of concern for multicollinearity, we calculated variance inflation factors (VIFs) among potential predictors. 14 Following the survival and risk factor analysis, we calculated Harrell's concordance index for two separate models to determine the explanatory ability of PNI; model 1 without PNI and model 2 with PNI. A p‐value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS (ver.25.0; IBM Corp.), R version 4.0.2 (R Foundation for Statistical Computing), and STATA (ver.15.0; STATA Corp.).
RESULTS
Patient demographics
The patient demographics are presented in Table 1. The mean age was 62.73 ± 7.97 years, and 287 (90.8%) patients were male, while 70 (22.2%) underwent neoadjuvant therapy. Most patients (93.4%) had SCC in the middle or lower esophagus, and 51.8% presented with clinical stages 0 or 1 of the disease. R0 resection was achieved in 306 patients (96.8%); the mean number of dissected LNs was 48.93 ± 26.89; PNI was identified in 25 (7.9%) patients.
TABLE 1.
Patient demographics
| Variable | Value |
|---|---|
| Age (years) | 62.73 ± 7.97 |
| Sex, male (n, %) | 287 (90.8%) |
| Location (n, %) | |
| Upper | 21 (6.6%) |
| Mid | 169 (53.5%) |
| Lower | 126 (39.9%) |
| Tumor differentiation (n, %) | |
| Well differentiated (G1) | 43 (13.6%) |
| Moderately differentiated (G2) | 179 (56.7%) |
| Poorly differentiated (G3) | 94 (29.7%) |
| Neoadjuvant treatment (n, %) | 70 (22.2%) |
| Three‐field dissection (n, %) | 20 (6.3%) |
| R0 resection (n, %) | 306 (96.8%) |
| Number of total dissected lymph nodes (n) | 48.93 ± 26.89 |
| Depth of invasion (T stage) (n, %) | |
| 0 and 1 | 216 (68.4%) |
| 2 | 33 (10.4%) |
| 3 | 64 (20.3%) |
| 4 | 3 (0.9%) |
| Nodal stage (n, %) | |
| 0 | 194 (61.4%) |
| 1 | 66 (20.9%) |
| 2 | 45 (14.2%) |
| 3 | 11 (3.5%) |
| Pathological stage (n, %) | |
| 0 and I | 164 (51.8%) |
| II | 83 (26.3%) |
| III | 69 (21.9%) |
| Perineural invasion (n, %) | 25 (7.9%) |
Note: Data are shown as either mean ± standard deviation, or as n (%).
Correlation between perineural invasion and pathological features
The pathological features with and without PNI are presented in Table 2. The clinical stage tended to be higher in the PNI group, with 88% of patients presenting with pathological T stage of ≥3 compared to the 45% of patients in the no‐PNI group (p < 0.001). LN metastases were confirmed in 72% in the PNI group when compared to 35.8% of patients in the no‐PNI group (p = 0.001). There was no difference observed in the tumor differentiation between both groups.
TABLE 2.
Pathological features and recurrence patterns according to perineural invasion
| Variable | No perineural invasion | Perineural invasion | p‐value |
|---|---|---|---|
| (n = 291) | (n = 25) | ||
| Depth of invasion (T stage) | < 0.001 | ||
| 1 | 214 (72.6%) | 2 (8.0%) | |
| 2 | 32 (12.0%) | 1 (4.0%) | |
| 3 | 43 (14.8%) | 21 (84.0%) | |
| 4 | 2 (0.6%) | 1 (4.0%) | |
| Nodal metastases | 0.001 | ||
| Absent | 187 (64.2%) | 7 (28%) | |
| Present | 104 (35.8%) | 18 (72%) | |
| Tumor differentiation | 0.342 | ||
| Well differentiated (G1) | 42 (14.4%) | 1 (4%) | |
| Moderately differentiated (G2) | 163 (56.1%) | 16 (64%) | |
| Poorly differentiated (G3) | 86 (29.5%) | 8 (32%) |
Note: Data are shown as n (%).
Survival and recurrence pattern
The median follow‐up period was 35.97 ± 30.99 months. The three‐year OS and DFS of all patients were 73.5% and 66.4%, respectively (Figure 2(a) and (b)). The three‐year OS and DFS were significantly lower in the PNI group (75.9% vs. 40.0%, p < 0.001; 70.3% vs. 21.6%, p < 0.001) (Figure 2(c) and (d)).
FIGURE 2.
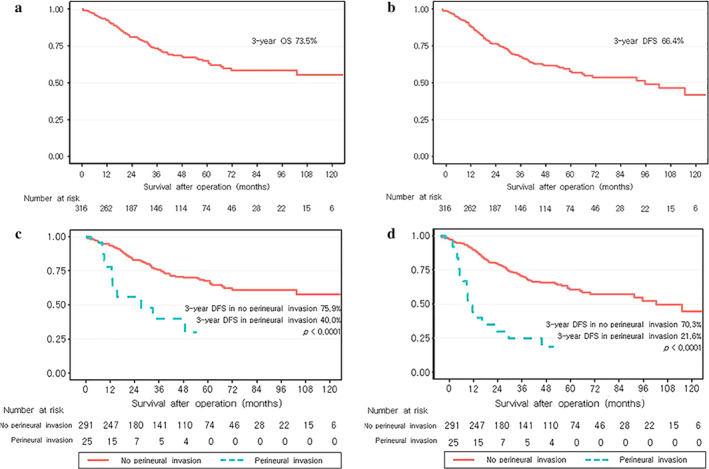
Survival curves showing the (a) overall survival (OS) and (b) disease‐free survival (DFS) of the entire sample. Survival curves showing (c) OS and (d) DFS according to perineural invasion
Recurrence of ESCC was detected in 80 (25.3%) of the 316 patients; locoregional recurrence occurred in 44 (13.9%) and distant metastases occurred in 45 (14.2%) patients. Nine patients (2.8%) had recurrence at multiple sites (locoregional and/or distant recurrence). The most common site of locoregional recurrence was the mediastinal LNs (4.7%) followed by the cervical LNs (4.1%). The liver (3.2%) was the most common site of distant metastases followed by the lungs (2.8%). In the no‐PNI group, locoregional recurrence was observed in 31 (10.7%) patients. Recurrence at an adjacent LN occurred in 27 patients, and recurrence at the anastomosis site was observed in two patients. Distant metastasis was observed in 35 (12.0%) patients in the no‐PNI group. A total of 13 patients had liver metastasis, while 11 showed lung metastasis, and seven patients showed bone metastasis. Distant LN metastasis was observed in 11 patients. In the PNI group, locoregional recurrence was observed in four patients (16%). Among them, three showed recurrence at the mediastinal LNs and one showed recurrence at the anastomosis site. A total of 10 patients (40%) in the PNI group had distant metastases. Three showed metastases at an upper abdominal LN (No.16), two presented with multiple lung metastases, two showed bone metastases, and two had metastases in abdominal organs such as the liver and colon.
Figure 3 shows cumulative incidence curves of locoregional recurrence and distant metastasis of the no‐PNI and PNI groups. The cumulative incidences of locoregional recurrence over the three‐year period were 9.6% in the no‐PNI and 18.3% in the PNI group (p = 0.009, Figure 3(a)), and the cumulative incidences of distant metastases over the three‐year period were 14.6% in the no‐PNI and 52.8% in the PNI group (p < 0.001, Figure 3(b)).
FIGURE 3.
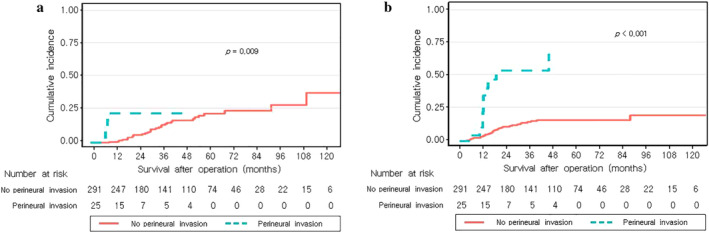
Cumulative incidence curves of recurrence showing (a) locoregional recurrence and (b) distant metastases
Risk factors for disease‐free survival
Age, neoadjuvant therapy, incomplete resection, stage III, and PNI were risk factors for DFS on the univariable analysis (Table 3). We performed the multivariable analysis with two models, model 1 without PNI and model 2 with PNI. The multivariable analysis of model 2 showed neoadjuvant therapy (hazard ratio [HR]: 2.436, 95% confidence interval (CI): 1.589–3.735, p < 0.001), incomplete resection (HR: 2.114, 95% CI: 1.166–3.835, p = 0.014), stage III (HR: 4.987, 95% CI: 3.114–7.987, p < 0.001), and PNI (HR: 1.890, 95% CI: 1.088–3.282, p = 0.024) as risk factors for DFS. The VIFs of the predictors mentioned above varied from 1.008–1.164, suggesting no collinearity in both models. The concordance index was calculated for each model. The concordance indices for models 1 and 2 were 0.712 (95% CI: 0.638–0.786) and 0.723 (95% CI: 0.649–0.798), respectively. Model 2 appeared to have a better prognostic ability with a slightly increased concordance index.
TABLE 3.
Risk factors for three‐year disease‐free survival
| Multivariate analysis | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Model 1 | Model 2, PNI | ||||
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age | 1.008 (0.983–1.033) | 0.551 | 1.024 (0.991–1.058) | 0.163 | 1.011 (0.988–1.035) | 0.339 |
| Sex | 0.772 (0.376–1.586) | 0.481 | 0.858 (0.339–2.175) | 0.747 | 0.777 (0.377–1.603) | 0.495 |
| Neoadjuvant Tx. | 2.037 (1.346–3.083) | 0.001 | 0.741 (0.382–1.435) | 0.373 | 2.436 (1.589–3.735) | < 0.001 |
| Incomplete resection | 4.467 (2.614–7.631) | < 0.001 | 1.167 (0.476–2.858) | 0.020 | 2.114 (1.166–3.835) | 0.014 |
| Stage II (vs. 0 and I) | 1.553 (0.937–2.573) | 0.087 | 0.736 (0.329–1.647) | 0.456 | 1.489 (0.896–2.474) | 0.124 |
| Stage III (vs. 0 and I) | 5.736 (3.689–8.918) | < 0.001 | 3.860 (1.620–9.193) | 0.002 | 4.987 (3.114–7.987) | < 0.001 |
| Perineural invasion | 4.000 (2.393–6.688) | < 0.001 | 1.890 (1.088–3.282) | 0.024 | ||
| Concordance index | 0.712 (0.638–0.786) | 0.723 (0.649–0.798) | ||||
Abbreviations: CI, confidence interval; PNI, perineural invasion.
Subgroup analysis
Subgroup analysis was performed to determine the prognostic role of PNI in specific situations. The three‐year DFS was significantly lower in the PNI group in patients with and without LN metastasis (Figure 4(a) and (b)). The three‐year DFS of the PNI group was 35.7% and that of the no‐PNI group was 79.3% (p = 0.012) in the subgroup of node‐negative patients. The three‐year DFS of patients with grades 1 and 2 tumor differentiation was significantly lower in the PNI group (29.9% vs. 72.5%, p < 0.001) (Figure 4(c) and (d)). Among patients with grade 3 differentiated tumors, the three‐year DFS was also lower in the PNI group (0% vs. 64.8%, p < 0.001). We also conducted subgroup analysis according to neoadjuvant treatment and found that the 3‐year DFS was lower in the PNI group (Figure 4(e) and (f)). PNI presence was related to DFS regardless of nodal status, tumor differentiation, and neoadjuvant therapy.
FIGURE 4.
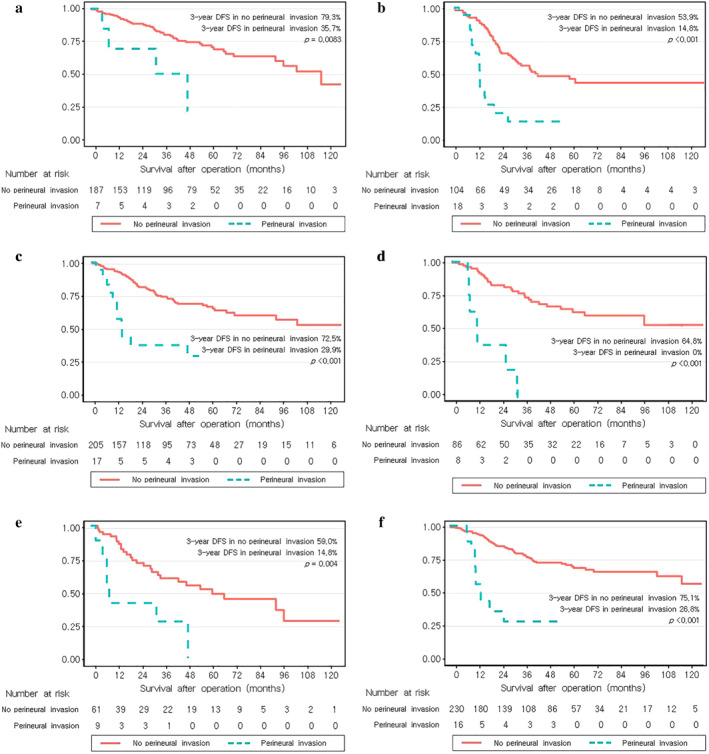
Survival curves of disease‐free survival showing (a) node‐negative patients, (b) node‐positive patients, (c) tumor differentiation grade 1 and 2, (d) grade 3, (e) patients who underwent neoadjuvant treatment, and (f) those who did not undergo neoadjuvant treatment
DISCUSSION
Our retrospective analysis revealed that PNI following esophageal cancer surgery correlated with aggressive features, such as higher T stage and LN metastasis, and was an unfavorable prognostic factor for DFS.
Our findings were consistent with those of previous studies on the clinical implications of PNI. Chen et al. analyzed 430 patients with ESCC and reported that PNI can be an independent prognostic factor and that PNI status in primary ESCC should be considered for therapy stratification. 6 Guo et al. also analyzed 162 patients with pN0M0 ESCC and concluded that PNI is an important risk factor to determine outcomes and that it can be used for risk assessment and to tailor adjuvant radiotherapy. 15 Our data also showed poor survival in patients with PNI, including in node‐negative patients (Figure 4(a)); hence, it could be an indicator for adjuvant therapy. The latest National Comprehensive Cancer Network guidelines do not recommend adjuvant treatment for ESCC with PNI, 16 whereas in head and neck cancer, PNI is usually regarded as an indicator for adjuvant radiotherapy. 17 Notably, the cumulative incidences of both locoregional recurrence and distant metastasis over three years were higher in the PNI group, meaning that PNI was related to both locoregional and distant failures. Although Guo et al. reported that PNI could be an indicator for adjuvant radiotherapy, 15 we considered that both adjuvant chemotherapy for distant metastasis and adjuvant radiotherapy for local control may be beneficial for patients with PNI. The appropriate modality of adjuvant therapy should be studied in further analyses. Another strength of this study is its statistical analysis. We calculated the concordance index of two multivariable models and showed that the inclusion of PNI increased the concordance index, which indicates the predictive ability of a survival model. 18 , 19 Therefore, combining PNI with the TNM staging system could increase the accuracy of survival prediction.
Although the importance of PNI is evident, its definition and detection in ESCC have not been uniformly established. In our study, PNI was observed in 7.9% of ESCC patients who underwent surgery, whereas other studies have reported incidences ranging from 22.2%–48.3%. 20 These differences in its incidence may have originated from the absence of standardized detection methods for PNI in ESCC. Detecting PNI with H&E staining is difficult if the nerve fibers are damaged by invading tumor cells or if the nerve fibers are extremely thin. 9 Thus, other methods, such as keratin or S‐100 protein detection, which has been implicated in colorectal carcinogenesis, may improve the accuracy of PNI detection. 21 When reporting the pathology of head and neck cancer, the presence of PNI is usually documented; 20 , 22 , 23 however, we suggest that it should also be documented for ESCC.
The metastatic mechanism of PNI has been explained in several ways. PNI is not an extension of lymphovascular metastasis because lymphatics do not penetrate the inner layer of the nerve sheath. 24 Recently, reciprocal signaling between tumor cells and nerves were reported in vitro. 7 , 9 , 13 The receptors for nerve growth factors (NGFs) such as NTRK1 and NGFR are expressed on nerves, while NGF production in esophageal cancer cells is shown in vitro; further, esophageal cancer cells may induce neurite outgrowth of neuronal cells. 2 In these biological processes, neurotransmitters and neuropeptides secreted by nerves act as molecular determinants and promote invasion and metastasis. 25 PNI comprises both infiltration and invasion of tumor cells towards nerve sheaths and a dynamic signaling process for metastases. As a result, PNI correlated with poor pathologic features, showing that PNI reflects a more malignant behavior in ESCC.
This study has certain limitations. First, this was a retrospective study carried out at a single institution, with a relatively small number of patients and a short period of follow‐up. Future studies should enroll a larger number of patients who are followed‐up over a longer period. Second, the guidelines for detecting PNI in ESCC are yet to be standardized. We did not perform immunohistochemical staining, such as anti‐S‐100, to detect nerve fibers that could improve the accuracy of PNI detection. 8 The low incidence of PNI in this study may be explained by underestimation owing to a lack of precise protocols.
In conclusion, PNI was an independent prognostic factor for shorter DFS in patients with surgically treated ESCC as assessed by multivariable analyses. The prognostic effect of PNI was not affected by nodal status, tumor differentiation, or neoadjuvant treatment. Given the prognostic implications of the PNI status, it should be clearly documented on the pathologic report to identify patients who require a closer follow‐up. Additionally, PNI could also be an important indicator for postoperative adjuvant therapy.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) which is funded by the Ministry of Education (2019 R1I1A1A01055513).
Kim HE, Park SY, Kim H, Kim DJ, Kim SI. Prognostic effect of perineural invasion in surgically treated esophageal squamous cell carcinoma. Thorac Cancer. 2021;12:1605–1612. 10.1111/1759-7714.13960
Seong Yong Park and Hyunki Kim are contributed equally to this work
Funding information National Research Foundation of Korea, Grant/Award Number: 2019 R1I1A1A01055513
Contributor Information
Seong Yong Park, Email: syparkcs@yuhs.ac.
Hyunki Kim, Email: kimhyunki@yuhs.ac.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2. Wang Q, Zhang W, Liu X, Zhang X, He J, Feng Q, et al. Prognosis of esophageal squamous cell carcinoma patients with preoperative radiotherapy: comparison of different cancer staging systems. Thorac Cancer. 2014;5:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. [DOI] [PubMed] [Google Scholar]
- 4. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and Esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai WJ, Xin PL. Pattern of relapse in surgical treated patients with thoracic esophageal squamous cell carcinoma and its possible impact on target delineation for postoperative radiotherapy. Radiother Oncol. 2010;96:104–7. [DOI] [PubMed] [Google Scholar]
- 6. Chen JW, Xie JD, Ling YH, Li P, Yan SM, et al. The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmitd LB, Scanlon CS, D'Silva NJ. Perineural invasion in head and neck cancer. J Dent Res. 2018;97:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91. [DOI] [PubMed] [Google Scholar]
- 9. Xu G, Feng F, Liu Z, Liu S, Zheng G, Xiao S, et al. Prognosis and progression of ESCC patients with Perineural invasion. Sci Rep. 2017;7:43828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsai CY, Yeh CJ, Chao YK, Chang HK, Tseng CK, Liu YH. Perineural invasion through the sheath in posttherapy esophagectomy specimens predicts poor survival in patients with esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017;43:1970–6. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka A, Matsumura E, Yosikawa H, Uchida T, Machidera N, Kubo R, et al. An evaluation of neural invasion in esophageal cancer. Surg Today. 1998;28:873–8. [DOI] [PubMed] [Google Scholar]
- 12. Japan Esophageal Society . Japanese classification of esophageal cancer, 11th edition: part 1. Esophagus. 2017;14:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chatterjee D, Katz MH, Rashid A, Wang H, Iuga AC, Varadhachary GR, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Senaviratna NAMR, Cooray TMJA. Diagnosing multicollinearity of logistic regression model. AJPAS. 2019;5(2):1–9. [Google Scholar]
- 15. Guo YN, Tian DP, Gong QY, Huang H, Yang P, Chen SB, et al. Perineural invasion is a better prognostic indicator than lymphovascular invasion and a potential adjuvant therapy indicator for pN0M0 esophageal squamous cell carcinoma. Ann Surg Oncol. 2020;27:4371–81. [DOI] [PubMed] [Google Scholar]
- 16. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:855–83. [DOI] [PubMed] [Google Scholar]
- 17. Bakst RL, Glastonbury CM, Parvathaneni U, Katabi N, Hu KS, Yom SS. Perineural invasion and Perineural tumor spread in head and neck cancer. Int J Radiat Oncol Biol Phys. 2019;103:1109–24. [DOI] [PubMed] [Google Scholar]
- 18. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 19. Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C‐statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ning ZH, Zhao W, Li XD, Chen LJ, Xu B, Gu WD, et al. The status of perineural invasion predicts the outcomes of postoperative radiotherapy in locally advanced esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:6881–90. [PMC free article] [PubMed] [Google Scholar]
- 21. van Wyk HC, Going J, Horgan P, McMillan DC. The role of perineural invasion in predicting survival in patients with primary operable colorectal cancer: a systematic review. Crit Rev Oncol Hematol. 2017;112:11–20. [DOI] [PubMed] [Google Scholar]
- 22. Müller S, Boy SC, Day TA, Magliocca KR, Richardson MS, Sloan P, et al. Data set for the reporting of oral cavity carcinomas: explanations and recommendations of the guidelines from the international collaboration of cancer reporting. Arch Pathol Lab Med. 2019;143:439–46. [DOI] [PubMed] [Google Scholar]
- 23. Moonis G, Cunnane MB, Emerick K, Curtin H. Patterns of perineural tumor spread in head and neck cancer. Magn Reson Imaging Clin N Am. 2012;20:435–46. [DOI] [PubMed] [Google Scholar]
- 24. Larson DL, Rodin AE, Roberts DK, O'Steen WK, Rapperport AS, Lewis SR. Perineural lymphatics: myth or fact. Am J Surg. 1966;112:488–92. [DOI] [PubMed] [Google Scholar]
- 25. Gao A, Wang L, Li J, Li H, Han Y, Ma X, et al. Prognostic value of perineural invasion in esophageal and esophagogastric junction carcinoma: a meta‐analysis. Dis Markers. 2016;2016:7340180. [DOI] [PMC free article] [PubMed] [Google Scholar]


